Abstract
Ca2+ signals controlling a vast array of cell functions involve both Ca2+ store release and external Ca2+ entry. These two events are coordinated through a dynamic intermembrane coupling between two distinct membrane proteins, STIM and Orai. STIM proteins are endoplasmic reticulum (ER) luminal Ca2+ sensors that undergo a profound redistribution into discrete junctional ER domains closely juxtaposed with the plasma membrane (PM). Orai proteins are PM Ca2+ channels that migrate and become tethered by STIM within the ER-PM junctions, where they mediate exceedingly selective Ca2+ entry. We describe a new understanding of the nature of the proteins and how they function to mediate this remarkable intermembrane signaling process controlling Ca2+ signals.
Store-operated Channels: Role and Significance
Cellular Ca2+ homeostasis and Ca2+ signaling are closely entwined processes. Cytoplasmic Ca2+ is tightly controlled at ∼100 nm; elevations to 300–500 nm constitute powerful signals controlling a spectrum of cell functions ranging from short-term contractile, secretory, or metabolic responses to longer term regulation of transcription, growth, and cell division (1). The ER3 has a special role in Ca2+ signaling, accumulating high (∼500 μm) luminal free Ca2+ levels. The luminal Ca2+ serves two roles: maintaining a correct protein folding environment (2) and serving as the major source of Ca2+ for signaling (1). Cell-surface receptors coupled to phospholipase C and inositol 1,4,5-trisphosphate production induce rapid Ca2+ signals by releasing ER-stored Ca2+ through inositol 1,4,5-trisphosphate receptors. This triggers a second Ca2+ signaling pathway through activation of SOCs (3–5). These PM Ca2+ entry channels are activated by decreased ER luminal Ca2+, involving an intricate ER-PM coupling process. SOCs carry a small but highly Ca2+-selective current termed ICRAC (3–5). This movement of Ca2+ ions can be viewed as a tightly regulated “trickle” of Ca2+ into cells (6), crucial in mediating longer term control of both cytoplasmic and ER luminal Ca2+ (5, 7, 8). Since the first description of SOCs (9), the ER-PM coupling has been considered to involve direct protein interactions occurring at close junctions between the ER and PM (10, 11). The function of the newly discovered STIM and Orai proteins fulfills this prediction.
STIM and Orai: The Machinery of SOCs
Recent high-throughput RNA interference screens identified two protein families as being essential for SOC activation: STIM in the ER (12, 13) and Orai in the PM (14–16). STIM proteins are highly dynamic membrane proteins located mostly in the ER and are able to sense luminal Ca2+ changes and undergo rapid translocation into discrete junctional areas of the ER, closely juxtaposed with the PM (7). Orai proteins are PM Ca2+ channels that translocate within the PM to the same ER junctions and become activated through coupling with STIM proteins (7). Although the function of SOCs has been best recognized in hematopoietic cells (3–5), STIM and Orai proteins are widely expressed among tissues (17, 18), representing potentially crucial pharmacological targets for controlling an array of cell functions.
STIM Proteins: Dynamic SOC Intermediaries
The discovery of STIM1 transformed the store-operated hypothesis into an authentic mechanistic paradigm (12, 13). STIM1 was originally identified as a surface membrane protein in stromal cells (19). Highly homologous STIM proteins are expressed in species ranging from Drosophila (12) to Caenorhabditis elegans (20). Vertebrates also express a second gene product, STIM2 (17). The ubiquitously expressed STIM1 and STIM2 proteins are highly similar, varying only at the extreme N and C termini (Fig. 1). Both STIM1 and STIM2 are predominantly in the ER (21–23). Although some STIM1 is also in the PM, where it can influence SOC activation (23, 24), it functions primarily in the ER, coupling to activate SOCs by transfer into ER-PM junctions (7). Store depletion is reported to increase PM STIM1 (25, 26); however, SOC activation does not require PM insertion of STIM1 (23, 27). STIM1 is normally widely distributed through the ER (13, 28, 29) but rapidly oligomerizes and moves into PM junctional regions seconds after emptying stores (13, 29). The N-terminal Ca2+-sensing domain of STIM1 is a tightly clustered group of short α-helices comprising EF-hand and SAM domains. The cytoplasmic C-terminal region contains more extensive α-helical regions sufficiently long to span much of the ER-PM junctional gap, estimated to be 10–20 nm (13, 25, 28–30), and couple with PM Orai channels.
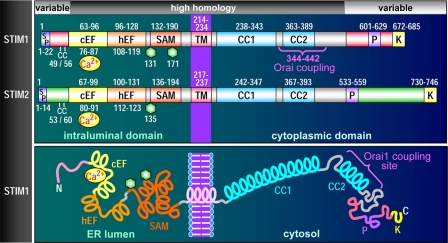
FIGURE 1.
STIM protein domain structures. Upper, domain comparison of STIM1 and STIM2, including signal peptides (SP), a pair of highly conserved cysteines (CC), canonical cEF- and hEF-hands, SAMs, Asn-linked glycosylation sites (hexagons), TMs, coiled-coil regions (CC1 and CC2), proline-rich domains (P), and polybasic-rich domains (K). The minimal region of STIM1 known to be required for coupling to Orai1 is also shown (positions 344–442). Lower, diagrammatic representation of domain topology of STIM1.
Molecular Basis of ER Luminal Ca2+ Sensing
The luminal N terminus of STIM1 includes two EF-hand motifs: a “canonical” cEF-hand (13, 25) with a Kd for Ca2+ in the 0.2–0.6 mm range (31) immediately adjacent to a hEF-hand that does not bind Ca2+ (32). The two EF-hands work in unison tightly associated with the adjoining five-helix SAM domain. This dual EF-hand and SAM domain (EF-SAM) has been carefully studied by Ikura and co-workers (31–33). With Ca2+ bound, there is a tight, stable STIM1 EF-SAM configuration. When Ca2+ dissociates, the EF-SAM domain unfolds and destabilizes due to exposure of hydrophobic residues in both EF-hands and the SAM domain (32). This leads to rapid oligomerization of the EF-SAM regions, the initial response of STIM1 to ER Ca2+ depletion. Further aggregation of STIM1 involves interactions between cytoplasmic C-terminal coiled-coil regions (27, 34, 35). The oligomerization of EF-SAM domains is reversible, with the addition of Ca2+ resulting in stable monomers being re-formed (32). This correlates with STIM1 in vivo refilling of stores causing rapid retreat of STIM1 from puncta and concomitant inactivation of SOCs (13, 28, 36, 37). Mutation of the crucial cEF-hand acidic residues to reduce Ca2+ affinity causes the same oligomerization as reducing Ca2+ (32). Indeed, when expressed in cells, whole STIM1 mutated in the EF-hand (e.g. D76A or E87A) is exclusively punctal, and cells have constitutively activated CRAC channels even though stores are full (13, 22, 24, 25, 38). Remarkably, mutation of the equivalent amino acids in the “shadow” non-Ca2+-binding hEF-hand loop causes the same aggregation and constitutive Ca2+ entry as mutation of the cEF-hand (32). Thus, the two EF-hands function in tandem, but the non-Ca2+ binding property of the hEF-hand ensures that the Kd is low, within the luminal Ca2+ range (400–800 μm) (39).
STIM2: Similar but Different
Although STIM2 has a structure very similar to STIM1 (Fig. 1), its function has some notable distinctions with important physiological implications. Most cells express both STIM1 and STIM2, although STIM1 levels are generally higher (17, 40). When overexpressed, STIM2 has a strong negative effect on endogenous SOC activity (8, 22, 38), contrasting with the small enhancing effect of STIM1 (22, 24). Moreover, STIM2 mediates slower Orai1 channel activation than comparably expressed STIM1 (41). STIM2 knockdown has a greater effect than STIM1 on basal cytosolic and ER luminal Ca2+ levels, suggesting STIM2 functions as a feedback regulator controlling basal Ca2+ (8). When Ca2+ stores are released gradually with external EGTA, movement of STIM2 into puncta and SOC activation occur with minimal store release, whereas more store release is required for STIM1 activation (8). Indeed, STIM2 overexpression results in high constitutive Ca2+ entry and CRAC channel activity (38, 42), likely reflecting its sensitivity to small luminal Ca2+ changes. The greater sensitivity of STIM2 to store depletion could be related to EF-hand structures. Mutation of three residues in the STIM1 cEF-hand to resemble STIM2 resulted in a more store-sensitive version of STIM1, although the reciprocal exchange mutation did not make a less sensitive STIM2 (8). Direct measurement of Ca2+ binding to the EF-SAM domain of STIM2 revealed a Kd of 0.5 mm, not much different from the Kd of STIM1 (33, 43). Importantly, it was revealed that, compared with STIM1, the STIM2 EF-SAM domain undergoes a 3-fold slower unfolding and 70-fold slower rate of aggregation upon Ca2+ withdrawal (33). Moreover, the short STIM subtype-specific flexible N-terminal sequences upstream of the EF-SAM domains confer large stability changes on the EF-SAM domain, greatly altering the Ca2+ dissociation-induced destabilization that results in STIM protein activation (33). Thus, the much slower rate of Ca2+ dissociation-induced STIM2 unfolding and aggregation may account for the slower kinetics of SOC activation by STIM2 (41) as well as the negative dominance of overexpressed STIM2 on SOC activation (22). Although STIM2 appears to be sensitized to tiny changes in ER Ca2+, the slow unfolding and activation of STIM2 may serve as an important regulatory control to prevent otherwise uncontrolled activation of SOCs (44).
Orai Proteins Are SOCs Carrying ICRAC
The extraordinary characteristics of the CRAC current, the signature of SOC function, predicted a novel channel protein. Despite numerous reports of TRPC and other transient receptor potential channels mediating Ca2+ entry or currents modified by store release, none had CRAC current characteristics. This changed with identification of the three-member Orai family of tetramembrane-spanning proteins (14–16). Their discovery arose from a combination of genome-wide RNA interference screening (14–16) and modified linkage analysis revealing that a mutation in Orai1 (R91W) caused a rare but SCID that ablates T-cell Ca2+ entry (14). This Orai1 mutation eliminated ICRAC (14–16); wild-type Orai1 expression restored CRAC channel activity in cells from SCID patients (14). The Orai1 protein fulfills all the criteria of being the store-operated channel moiety itself, carrying the highly Ca2+-selective CRAC current (16, 38, 45–49). The combined overexpression of STIM1 with Orai1 results in massive levels of authentic CRAC channel activity (16, 38, 45, 46). There are three closely related and widely expressed Orai genes (orai1, orai2, and orai3) (supplemental Fig. S1) (14). Each protein has a set of five conserved acidic residues: four in TM1 and the first extracellular loop region and one in TM3. These are crucial to forming the highly Ca2+-selective filter and channel pore regions (47–49). Conservative mutations (e.g. E106D in Orai1), moving a single pore-contributing carboxyl group away from the pore by just one methylene group, change the strict Ca2+ selectivity of the channel to allow passage of monovalent cations (47–49). Less conservative E106A and E106Q mutations result in a pore-dead protein (18, 48, 49). Overexpressed Orai1 has a strong dominant-negative effect on native SOC activity (38, 45, 46), likely reflecting interference of the required coupling stoichiometry between STIM and Orai. Like Orai1, the Orai2 and Orai3 proteins also couple with STIM1, giving CRAC-like channel activity differing slightly in cation selectivity and inhibition by Ca2+ (50, 51). Orai3 is also distinct in its alteration by the powerful SOC modifier 2-aminoethoxydiphenyl borate, which directly activates and alters its cation selectivity independently of STIM proteins (42, 52–55). Clearly, the Orai channel subtypes can cross-react with each other (18, 50). The negative dominance of the pore-dead Orai1 E106Q mutant over Orai2, Orai3, or endogenous SOC activity indicates that Orai channels can be heteromultimers with possibly multiple channel subtypes in cells (49, 50). Multimerization of Orai subunits appears to involve interactions between the TMs (35, 37). Although Orai dimers are observed in cells (18), the functional Orai channel moiety is a tetramer (56–58). Although dimer-to-tetramer transition was reported during Orai1 activation (59), it appears more likely that Orai channels have a constant tetrameric stoichiometry (57, 58).
STIM-Orai Coupling Environment
The coupling between STIM and Orai proteins involves a remarkable dynamic convergence. Both STIM and Orai translocate within their respective membranes into discrete, tightly coupled ER-PM junctions (Fig. 2). Aggregated regions of ER STIM proteins observed following store depletion, referred to as “puncta” (13), are areas of STIM proteins undergoing aggregation and accumulation, although not necessarily linked to Orai in ER-PM junctions (28, 36, 57, 60, 61). These regions appear to be largely pre-existing ER-PM junctional domains (29, 36) within which accumulated STIM proteins capture and activate Orai channels. The junctions may dramatically increase in number and size with time (23, 29). Direct interaction between STIM and Orai channels occurring in puncta is now established. Although STIM-Orai pulldown was initially not detected (18), there are now numerous reports revealing direct biochemical association between the proteins (37, 47, 49, 57) and/or Förster resonance energy transfer between appropriately labeled derivatives (37, 42, 62, 63). These interactions are supported by numerous studies revealing that STIM and Orai proteins are superimposably clustered in puncta following store depletion (35, 42, 57–61, 64). Oligomerization between STIM1 molecules is required for accumulation of STIM1 aggregates within puncta. STIM1 with its entire N-terminal domain replaced by a rapalogue-inducible cross-linking domain underwent oligomerization, accumulation in puncta, and coupling to activate CRAC channels (30). However, STIM1 simply missing its entire N terminus (thus with no Ca2+ sensing or oligomerization through the N terminus) still aggregated and moved into puncta to activate CRAC channels (35). Thus, the STIM1 C terminus itself is able to undergo aggregation, a result supported by several studies (27, 34, 35, 57). The elaborate N-terminal Ca2+-sensing and oligomerization process provides the physiological responsiveness to Ca2+ store depletion. Indeed, the N terminus serves to prevent STIM self-association in store-replete cells. When store Ca2+ decreases, STIM oligomerization is triggered, and the process of STIM aggregation and accumulation in junctions is initiated (Fig. 2). Importantly, when stores refill, there is a rapid release of STIM from puncta to become redistributed within the ER (13, 28, 36, 37). Upon re-binding Ca2+, the STIM1 EF-SAM domain aggregates dissociate to become monomers (32). Presumably, this conformational change imparts a decisive alteration in the proximity of aggregated STIM1 molecules that overrides both C-terminal STIM-STIM interactions and STIM-Orai interactions.
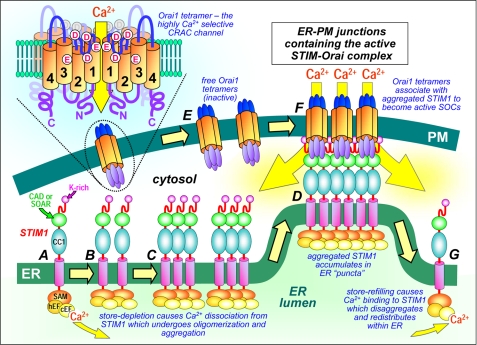
FIGURE 2.
Dynamic molecular coupling between STIM1 and Orai1 within ER-PM junctions. Depletion of ER luminal Ca2+ causes Ca2+ dissociation from the STIM1 N-terminal cEF-hand (A), leading to fast oligomerization of STIM1 due to unfolding and interactions between EF-SAM domains (B). Slower further aggregation of STIM1 through interactions between the C-terminal coiled-coil domain 1 (CC1) and CAD/SOAR domains (C) results in STIM1 diffusion, aggregation, and accumulation of STIM1 in pre-existing ER-PM junctions, stabilized by interactions of lysine-rich (K-rich) STIM1 N termini with the PM (D). Diffusible Orai1 tetramers in the PM (E) are trapped in junctions (F) by interaction with exposed CAD/SOAR domains, which bind to the C and N termini of Orai1 channels and conformationally gate the opening of Orai1 channels and Ca2+ entry. Upon store refilling, Ca2+ association with STIM1 reverses EF-SAM oligomerization, causing uncoupling from and deactivation of Orai1 and release of STIM1 monomers from puncta to redistribute around the ER (G). The topology of the Orai1 tetramer is shown (upper left), depicting the four transmembrane domains (1–4) and clusters of Asp (D) and Glu (E) residues that constitute the Ca2+-selective filter and pore.
Molecular Mechanism of STIM-Orai Coupling
The molecular characteristics of the STIM-Orai interaction are becoming clearer. Within the Orai1 molecule, the C-terminal cytoplasmic coiled-coil region appears key for interaction with and activation by STIM1 (37, 65). The Orai1 L273S mutation, which disrupts this coiled-coil region, blocks STIM1 interaction and channel activation and dominates in blocking wild-type Orai1 (37). The N terminus of Orai1 can be truncated up to residue 73 with little effect on STIM1 coupling (35), but truncation of the following 15 amino acids prevents channel activation while still allowing interaction with STIM1, mimicking the R91W mutation present in SCID patients (37). For STIM1, expression of soluble C-terminal fragments can fully activate Orai1 channels without store emptying (27, 35, 42, 54, 59, 65, 66). Remarkably, a short stretch of just over 100 amino acids including the second coiled-coil domain (Fig. 1) is sufficient for Orai1 activation (57, 65). Thus, SOAR (65) and CAD (57) are potent, full activators of Orai1. The STIM1 CAD fragment undergoes biochemical interactions with the isolated C-terminal domain 254–301 of Orai1 (57) as well as the N-terminal fragment 70–91 (57), suggesting that the interaction with STIM1 involves both termini of Orai1. C-terminal fragments of STIM cause clustering and activation of PM Orai channels (42, 57–59). However, STIM-induced Orai1 clustering is not sufficient for Orai channel activation. Thus, CAD with eight C-terminal residues (positions 441–448) removed still binds and clusters Orai1 yet cannot activate channel activity (57). Moreover, although the two STIM1 truncations 1–448 and 1–440 both enter puncta upon store depletion and cluster Orai1, only construct 1–448 activates Orai1 channel activity (57). Because the SOAR fragment (positions 344–442) activates Orai1 (65), the two missing residues (positions 441 and 442) in the non-activating fragment 340–440 (57) may play a crucial role in gating Orai1. Both CAD and SOAR exist as multimers (57, 65). In solution, CAD is predominantly a tetramer (57) and causes Orai channel particles to cluster in extended multimeric arrays (57). Thus, STIM1 multimers induce a strong cross-linking event resulting in large clustered arrays of Orai channels. The lysine-rich C-terminal extremity (K-region) of STIM1 plays an interesting ancillary function in SOC activation. Although known to play a role (28, 67), the K-region is not essential for SOC coupling (37, 42, 57, 65). Indeed, Drosophila STIM is devoid of this region and still activates SOCs. The K-region appears to enhance PM targeting of STIM1. Hence, although whole STIM1 expressed alone readily forms PM-associated puncta, K-region-deficient STIM1 requires coexpression of Orai1 for punctum formation (57). Thus, the K-region appears to tether oligomeric STIM1 within junctions by binding to an as yet unidentified PM target. The exposed CAD/SOAR domains within STIM are able to trap and conformationally activate Orai channels. Although expressed CAD or SOAR spontaneously binds to and activates Orai channels (57, 65), larger soluble STIM1 C-terminal fragments are poorly active and remain cytoplasmic (42, 57). Hence, the C terminus of STIM1 appears to require unfolding to expose active CAD/SOAR. Interestingly, 2-aminoethoxydiphenyl borate induces rapid binding between soluble whole STIM1 or STIM2 C termini and PM Orai1, causing clustering and full Orai1 channel activation, apparently mimicking the STIM-unfolding and successful Orai-docking events (42).
Overall, STIM proteins are remarkably sensitive and dynamic ER Ca2+ sensors, coupling to exceedingly Ca2+-selective PM Orai channels. STIM-Orai coupling is facilitated through ER-PM junctions acting as conduits of close approach between the two membranes. The coordinated function of these two proteins mediates crucial control over cytosolic and luminal Ca2+ homeostasis and the generation of both rapid and long-term Ca2+ signals. Understanding the molecular details of the STIM-Orai transmembrane interaction has great significance as a universally important signaling mechanism.
Supplementary Material
This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ER
- endoplasmic reticulum
- SOC
- store-operated channel
- PM
- plasma membrane
- CRAC
- Ca2+ release-activated Ca2+
- SAM
- sterile α-motif
- cEF-hand
- Ca2+-binding EF-hand
- hEF-hand
- hidden EF-hand
- SCID
- severe combined immune deficiency
- TM
- transmembrane domain
- SOAR
- STIM1 Orai-activating region 344–442
- CAD
- Ca2+-activating domain 340–448.
REFERENCES
- 1.Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4,517–529 [DOI] [PubMed] [Google Scholar]
- 2.Zhang K., Kaufman R. J. (2008) Nature 454,455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parekh A. B., Penner R. (1997) Physiol. Rev. 77,901–930 [DOI] [PubMed] [Google Scholar]
- 4.Venkatachalam K., van Rossum D. B., Patterson R. L., Ma H. T., Gill D. L. (2002) Nat. Cell Biol. 4,E263–E272 [DOI] [PubMed] [Google Scholar]
- 5.Parekh A. B., Putney J. W., Jr. (2005) Physiol. Rev. 85,757–810 [DOI] [PubMed] [Google Scholar]
- 6.Gill D. L., Spassova M. A., Soboloff J. (2006) Science 313,183–184 [DOI] [PubMed] [Google Scholar]
- 7.Lewis R. S. (2007) Nature 446,284–287 [DOI] [PubMed] [Google Scholar]
- 8.Brandman O., Liou J., Park W. S., Meyer T. (2007) Cell 131,1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putney J. W., Jr. (1986) Cell Calcium 7,1–12 [DOI] [PubMed] [Google Scholar]
- 10.Berridge M. J. (1995) Biochem. J. 312,1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson R. L., van Rossum D. B., Gill D. L. (1999) Cell 98,487–499 [DOI] [PubMed] [Google Scholar]
- 12.Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Veliçelebi G., Stauderman K. A. (2005) J. Cell Biol. 169,435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. (2005) Curr. Biol. 15,1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006) Nature 441,179–185 [DOI] [PubMed] [Google Scholar]
- 15.Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., Kinet J. P. (2006) Science 312,1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S. L., Yeromin A. V., Zhang X. H., Yu Y., Safrina O., Penna A., Roos J., Stauderman K. A., Cahalan M. D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103,9357–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams R. T., Manji S. S., Parker N. J., Hancock M. S., Van Stekelenburg L., Eid J. P., Senior P. V., Kazenwadel J. S., Shandala T., Saint R., Smith P. J., Dziadek M. A. (2001) Biochem. J. 357,673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwack Y., Srikanth S., Feske S., Cruz-Guilloty F., Oh-hora M., Neems D. S., Hogan P. G., Rao A. (2007) J. Biol. Chem. 282,16232–16243 [DOI] [PubMed] [Google Scholar]
- 19.Oritani K., Kincade P. W. (1996) J. Cell Biol. 134,771–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strange K., Yan X., Lorin-Nebel C., Xing J. (2007) Cell Calcium 42,193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manji S. S., Parker N. J., Williams R. T., Van Stekelenburg L., Pearson R. B., Dziadek M., Smith P. J. (2000) Biochim. Biophys. Acta 1481,147–155 [DOI] [PubMed] [Google Scholar]
- 22.Soboloff J., Spassova M. A., Hewavitharana T., He L. P., Xu W., Johnstone L. S., Dziadek M. A., Gill D. L. (2006) Curr. Biol. 16,1465–1470 [DOI] [PubMed] [Google Scholar]
- 23.Hewavitharana T., Deng X., Wang Y., Ritchie M. F., Girish G. V., Soboloff J., Gill D. L. (2008) J. Biol. Chem. 283,26252–26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spassova M. A., Soboloff J., He L. P., Xu W., Dziadek M. A., Gill D. L. (2006) Proc. Natl. Acad. Sci. U.S.A. 103,4040–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. (2005) Nature 437,902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser C. T., Tsien R. Y. (2007) Proc. Natl. Acad. Sci. U.S.A. 104,3693–3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baba Y., Hayashi K., Fujii Y., Mizushima A., Watarai H., Wakamori M., Numaga T., Mori Y., Iino M., Hikida M., Kurosaki T. (2006) Proc. Natl. Acad. Sci. U.S.A. 103,16704–16709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liou J., Fivaz M., Inoue T., Meyer T. (2007) Proc. Natl. Acad. Sci. U.S.A. 104,9301–9306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu M. M., Buchanan J., Luik R. M., Lewis R. S. (2006) J. Cell Biol. 174,803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luik R. M., Wang B., Prakriya M., Wu M. M., Lewis R. S. (2008) Nature 454,538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stathopulos P. B., Li G. Y., Plevin M. J., Ames J. B., Ikura M. (2006) J. Biol. Chem. 281,35855–35862 [DOI] [PubMed] [Google Scholar]
- 32.Stathopulos P. B., Zheng L., Li G. Y., Plevin M. J., Ikura M. (2008) Cell 135,110–122 [DOI] [PubMed] [Google Scholar]
- 33.Stathopulos P. B., Zheng L., Ikura M. (2009) J. Biol. Chem. 284,728–732 [DOI] [PubMed] [Google Scholar]
- 34.Williams R. T., Senior P. V., Van Stekelenburg L., Layton J. E., Smith P. J., Dziadek M. A. (2002) Biochim. Biophys. Acta 1596,131–137 [DOI] [PubMed] [Google Scholar]
- 35.Li Z., Lu J., Xu P., Xie X., Chen L., Xu T. (2007) J. Biol. Chem. 282,29448–29456 [DOI] [PubMed] [Google Scholar]
- 36.Smyth J. T., Dehaven W. I., Bird G. S., Putney J. W., Jr. (2008) J. Cell Sci. 121,762–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muik M., Frischauf I., Derler I., Fahrner M., Bergsmann J., Eder P., Schindl R., Hesch C., Polzinger B., Fritsch R., Kahr H., Madl J., Gruber H., Groschner K., Romanin C. (2008) J. Biol. Chem. 283,8014–8022 [DOI] [PubMed] [Google Scholar]
- 38.Soboloff J., Spassova M. A., Tang X. D., Hewavitharana T., Xu W., Gill D. L. (2006) J. Biol. Chem. 281,20661–20665 [DOI] [PubMed] [Google Scholar]
- 39.Demaurex N., Frieden M. (2003) Cell Calcium 34,109–119 [DOI] [PubMed] [Google Scholar]
- 40.Oh-Hora M., Yamashita M., Hogan P. G., Sharma S., Lamperti E., Chung W., Prakriya M., Feske S., Rao A. (2008) Nat. Immunol. 9,432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parvez S., Beck A., Peinelt C., Soboloff J., Lis A., Monteilh-Zoller M., Gill D. L., Fleig A., Penner R. (2008) FASEB J. 22,752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y., Deng X., Zhou Y., Hendron E., Ritchie M. F., Tang X. D., Kurosaki T., Mori Y., Soboloff J., Gill D. L. (2009) Proc. Natl. Acad. Sci. U.S.A. 106,7391–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng L., Stathopulos P. B., Li G. Y., Ikura M. (2008) Biochem. Biophys. Res. Commun. 369,240–246 [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y., Mancarella S., Wang Y., Yue C., Ritchie M. F., Gill D. L., Soboloff J. (2009) J. Biol. Chem. 284,191864–19168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peinelt C., Vig M., Koomoa D. L., Beck A., Nadler M. J., Koblan-Huberson M., Lis A., Fleig A., Penner R., Kinet J. P. (2006) Nat. Cell Biol. 8,771–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercer J. C., Dehaven W. I., Smyth J. T., Wedel B., Boyles R. R., Bird G. S., Putney J. W., Jr. (2006) J. Biol. Chem. 281,24979–24990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeromin A. V., Zhang S. L., Jiang W., Yu Y., Safrina O., Cahalan M. D. (2006) Nature 443,226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P. G. (2006) Nature 443,230–233 [DOI] [PubMed] [Google Scholar]
- 49.Vig M., Beck A., Billingsley J. M., Lis A., Parvez S., Peinelt C., Koomoa D. L., Soboloff J., Gill D. L., Fleig A., Kinet J. P., Penner R. (2006) Curr. Biol. 16,2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lis A., Peinelt C., Beck A., Parvez S., Monteilh-Zoller M., Fleig A., Penner R. (2007) Curr. Biol. 17,794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeHaven W. I., Smyth J. T., Boyles R. R., Putney J. W., Jr. (2007) J. Biol. Chem. 282,17548–17556 [DOI] [PubMed] [Google Scholar]
- 52.Schindl R., Bergsmann J., Frischauf I., Derler I., Fahrner M., Muik M., Fritsch R., Groschner K., Romanin C. (2008) J. Biol. Chem. 283,20261–20267 [DOI] [PubMed] [Google Scholar]
- 53.DeHaven W. I., Smyth J. T., Boyles R. R., Bird G. S., Putney J. W., Jr. (2008) J. Biol. Chem. 283,19265–19273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S. L., Kozak J. A., Jiang W., Yeromin A. V., Chen J., Yu Y., Penna A., Shen W., Chi V., Cahalan M. D. (2008) J. Biol. Chem. 283,17662–17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peinelt C., Lis A., Beck A., Fleig A., Penner R. (2008) J. Physiol. 586,3061–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mignen O., Thompson J. L., Shuttleworth T. J. (2008) J. Physiol. 586,419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park C. Y., Hoover P. J., Mullins F. M., Bachhawat P., Covington E. D., Raunser S., Walz T., Garcia K. C., Dolmetsch R. E., Lewis R. S. (2009) Cell 136,876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji W., Xu P., Li Z., Lu J., Liu L., Zhan Y., Chen Y., Hille B., Xu T., Chen L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105,13668–13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penna A., Demuro A., Yeromin A. V., Zhang S. L., Safrina O., Parker I., Cahalan M. D. (2008) Nature 456,116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu P., Lu J., Li Z., Yu X., Chen L., Xu T. (2006) Biochem. Biophys. Res. Commun. 350,969–976 [DOI] [PubMed] [Google Scholar]
- 61.Luik R. M., Wu M. M., Buchanan J., Lewis R. S. (2006) J. Cell Biol. 174,815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Navarro-Borelly L., Somasundaram A., Yamashita M., Ren D., Miller R. J., Prakriya M. (2008) J. Physiol. 586,5383–5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calloway N., Vig M., Kinet J. P., Holowka D., Baird B. (2009) Mol. Biol. Cell 20,389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Várnai P., Tóth B., Tóth D. J., Hunyady L., Balla T. (2007) J. Biol. Chem. 282,29678–29690 [DOI] [PubMed] [Google Scholar]
- 65.Yuan J. P., Zeng W., Dorwart M. R., Choi Y. J., Worley P. F., Muallem S. (2009) Nat. Cell Biol. 11,337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muik M., Fahrner M., Derler I., Schindl R., Bergsmann J., Frischauf I., Groschner K., Romanin C. (2009) J. Biol. Chem. 284,8421–8426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang G. N., Zeng W., Kim J. Y., Yuan J. P., Han L., Muallem S., Worley P. F. (2006) Nat. Cell Biol. 8,1003–1010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.