Abstract
One of the hallmarks of apoptosis is the redistribution of phosphatidylserine (PS) from the inner-to-outer plasma membrane (PM) leaflet, where it functions as a ligand for phagocyte recognition and the suppression of inflammatory responses. The mechanism by which apoptotic cells externalize PS has been assumed to involve “scramblases” that randomize phospholipids across the PM bilayer. These putative activities, however, have not been unequivocally proven to be responsible for the redistribution of lipids. Because elevated cytosolic Ca2+ is critical to this process and is also required for activation of lysosome-PM fusion during membrane repair, we hypothesized that apoptosis could activate a “pseudo”-membrane repair response that results in the fusion of lysosomes with the PM. Using a membrane-specific probe that labels endosomes and lysosomes and fluorescein-labeled annexin 5 that labels PS, we show that the appearance of PS at the cell surface during apoptosis is dependent on the fusion of lysosomes with the PM, a process that is inhibited with the lysosomotrophe, chloroquine. We demonstrate that apoptotic cells evoke a persistent pseudo-membrane repair response that likely redistributes lysosomal-derived PS to the PM outer leaflet that leads to membrane expansion and the formation of apoptotic blebs. Our data suggest that inhibition of lysosome-PM fusion-dependent redistribution of PS that occurs as a result of chemotherapy- and radiotherapy-induced apoptosis will prevent PS-dependent anti-inflammatory responses that preclude the development of tumor- and patient-specific immune responses.
There is increasing evidence that damaged plasma membranes (PM)2 trigger an emergency Ca2+-dependent exocytotic repair response that patches the affected area by adding lysosome-derived membranes at the cell surface disruption site (1–5). Because high cytosolic Ca2+ concentrations trigger lysosome-PM fusion, the elevated cytosolic Ca2+ levels characteristic to apoptotic cells may also evoke a pseudo-repair mechanism that promotes lysosome-PM fusion. Indeed, similar to normal emergency repair responses, apoptosis is characterized by the appearance of organelle proteins and lipids at the PM surface (6–8). One critical distinction between the apoptotic and physiologic repair processes is the preservation of membrane lipid asymmetry. In normal cells, any perturbation in PS sidedness is corrected by restoration of basal cytosolic [Ca2+], reactivation of the Ca2+-inhibited aminophospholipid translocase (9, 10), and subsequent facilitated transport of PS back to the inner membrane leaflet of the cell. In apoptotic cells, however, persistent high cytosolic [Ca2+] precludes reactivation of the aminophospholipid translocase, and the redistributed PS remains in the outer membrane leaflet (11). The apparent similarities in these processes combined with observations that apoptotic cells express PS at the cell surface prompted us to investigate whether lysosome to PM fusion plays a role in the redistribution of PS during apoptosis.
EXPERIMENTAL PROCEDURES
Cells, Cell Lines, and Reagent
Murine embryonic fibroblasts (MEF) were cultured in DMEM (Invitrogen) supplemented with 15% fetal bovine serum, 50 μg/ml uridine, 2 mm glutamine, and 100 units/ml leukemia inhibitory factor at 37 °C in a 5% CO2 incubator. Staurosporine (STS; apoptosis-inducing agent), colchicine (microtubule disruptor), latrunculin (inhibitor of actin polymerization), and chloroquine (CLQ) were purchased from Sigma-Aldrich.
Apoptosis and Measurement of Apoptotic Markers
Cells were plated onto 2.5-cm glass coverslips (∼106 cells) overnight. Apoptosis was initiated by incubating the cells with staurosporine (0.5 μm) for 4 h at 37 °C. The cells were analyzed for PS externalization using FITC-labeled annexin 5 or lactadherin in 0.5 ml of HEPES-buffered Hanks' balanced salt solution (HHBS) containing 2 mm CaCl2 (for the annexin 5 only) that was added for ∼5 min at 20 °C immediately before examination of the cells. For the detection of PS in the lumen of endocytotic vesicles, FITC-annexin 5 was incubated with the cells for 1 h at 37 °C. For DNA fragmentation (propidium iodide/cell cycle analysis), cells were fixed in 70% ethanol, washed with phosphate-buffered saline (20 mm sodium phosphate, 150 mm NaCl, pH 7.2), and incubated for 30 min at 37 °C with RNase (1 μg/ml) followed by propidium iodide (50 μg/ml) prior to suspension and flow cytometry. Caspase 3 activity was determined fluorometrically in 0.1% Triton X-100 cell lysates (4 × 106 cells) using z-DEVD-AMC (0.5 μm) as described previously (11). Caspase activity was expressed as the rate of increase in fluorescent intensity (λex 380 nm, λem 510 nm) at 20 °C.
Endosome Labeling
To simultaneously monitor the endosomal and PM staining, MEF cultured on glass coverslips were washed and resuspended in 1 ml of serum-free DMEM. N-Rhodamine-labeled-phosphatidylethanolamine (N-rho-PE) (5 μl from a 1 mg/ml ethanol solution) (12, 13) was added in the absence or presence of FITC-labeled dextran (200 μg/ml; Invitrogen) or LysoTracker Green (1 μm). After 1 h at 37 °C, the cells were washed with DMEM containing 10% serum (back-exchanged) to remove residual, non-internalized N-rho-PE. The cultures were then resuspended in HHBS or DMEM and further incubated at 37 °C with STS or treated A23187/Ca2+ for the indicated times.
Ionophore-triggered PS Externalization
Back-exchanged N-rho-PE-labeled cells were incubated with A23187 (1 μm) (Sigma) in the presence of 1 mm Ca2+ at 37 °C for 20 min. The cells were then incubated with FITC-annexin 5 and analyzed for the externalization of PS and N-rho-PE by confocal microscopy.
Reversal of Ionophore-triggered PS Externalization
Cytosolic [Ca2+] was restored to normal levels in back-exchanged N-rho-PE-labeled cells after Ca2+ ionophore treatment by incubating the cells in (Ca2+-free) EGTA-containing medium (2 mm) for 30 min at 37 °C. The presence of PS at the cell surface was then monitored with FITC-lactadherin, which binds PS in a Ca2+-independent manner (14).
LAMP-1 Labeling
MEF cells were stained with monoclonal rat anti-LAMP1 (United States Biological) followed by anti-rat cy5-labeled Ig. The cells were also incubated with annexin 5 and monitored for the presence of the fluorescent probes by confocal microscopy.
Confocal Microscopy
Fluorescent images of live cells following the various treatments were recorded with a Zeiss LSM 510 confocal microscope equipped with an Achroplan 63× water immersion lens using appropriate excitation lines and emission filter sets. For presentation purposes, the raw images were organized into plates using PhotoShop.
RESULTS AND DISCUSSION
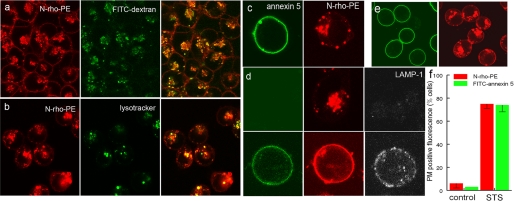
To monitor de novo formation and trafficking of endosomes, MEF were incubated with the aqueous space marker, fluorescein-labeled dextran (FITC-dextran), and the membrane probe, N-rho-PE (12). Endosome formation was then initiated by incubating the cells at 37 °C. Fig. 1a shows that the N-rho-PE that exchanged into the PM of the cell also labeled the membranes of pinocytotic vesicles that also contained lumen-entrapped FITC-dextran. To monitor only the labeled vesicles, residual N-rho-PE remaining in the PM was removed by back-exchange with 10% serum, leaving the red fluorescent label only in the internal organelles (Fig. 1b). To confirm delivery of late endosomes to lysosomes, the maturation of hybrid organelles was established by substituting LysoTracker Green for FITC-dextran. Fig. 1b shows a significant degree of probe colocalization, indicating that many of the rhodamine-labeled endosomes formed hybrid lysosome/endosome organelles.
FIGURE 1.
Apoptosis-dependent lipid and protein redistribution to the PM. a, MEF were incubated with N-rho-PE and FITC-dextran as described under “Experimental Procedures.” The cells were then washed and photographed. b, cells were incubated together with N-rho-PE and LysoTracker Green for 1 h and then washed with 10% serum for 2 min to remove N-rho-PE remaining in the PM. c, STS-triggered apoptotic cells expressing N-rho-PE and PS (FITC-annexin 5 labeled) at the cell surface were back-exchanged for 2 min with 10% serum (note the absence of N-rho-PE at the cell surface). d, N-rho-PE-labeled cells incubated in the absence (top) and presence of (bottom) of STS for 4 h. The redistribution of LAMP-1 and PS was detected with monoclonal anti-LAMP-1 followed with cy5-labeled anti-rat Ig and FITC-annexin 5, respectively. e, STS-treated cells before serum back-exchange. f, the fraction of control and STS-treated cells containing cell surface N-rho-PE and PS (FITC-annexin 5 staining).
The ability of apoptotic cells to mount a cell death-dependent emergency repair response was assessed by monitoring the migration of rhodamine-labeled lysosomal membranes to the cell surface in response to treatment with STS. In contrast to the preferential perinuclear distribution of the labeled organelles in control cells (Fig. 1a), in apoptotic cells, the organelles migrated toward the subplasmalemmal space (Fig. 1c). Together with the expected finding that these cells stained with FITC-annexin 5, indicating that PS redistributed to the cell surface, most striking was the redistribution of lysosomal-associated membrane protein (LAMP-1) and red organelle fluorescence to the PM (Fig. 1d). Without exception, every apoptotic cell bearing redistributed PS also redistributed vesicular N-rho-PE back to the PM (Fig. 1e). Exhaustive examination of >300 cells revealed that <1% contained single color fluorescent rings.
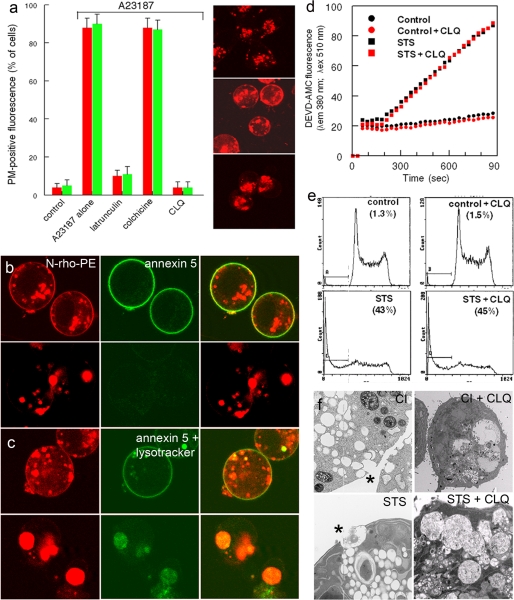
Enforced increases in cytosolic [Ca2+] with Ca2+ ionophore are known to trigger both PS externalization (10, 15) and a membrane repair-dependent response that triggers fusion of lysosomes with the PM (4, 5). Taken together with our results, these studies raise the possibility that PS externalization could be dependent on lysosome fusion to the PM. To determine whether the redistribution of PS is dependent on the migration of lysosomes to the PM, cells were treated with the actin- and microtubule-disrupting reagents, latrunculin and colchicine. Fig. 2a shows that cells incubated with latrunculin, but not colchicine, inhibited both lysosome fusion and the redistribution of PS.
FIGURE 2.
Inhibition of ionophore- and apoptosis-dependent PS externalization and lysosome-PM fusion. a, N-rho-PE-labeled MEF were incubated with the indicated inhibitors for 20 min prior to the addition of ionophore (A23187). Top, middle, and bottom, fluorescent photomicrographs of N-rho-PE-labeled endocytic compartments in control (top), ionophore-treated (middle), and latrunculin/ionophore-treated cells (bottom). b and c, externalization of N-rho-PE and PS was triggered with ionophore (b) or (c) STS in the absence (top) or presence (bottom) of CLQ (10 μm). d and e, apoptosis in control and STS/CLQ-treated cells was verified by caspase activation (d) and DNA fragmentation (e). f, electron micrographs show that organelle fusion to the PM (asterisks) induced with ionophore (CI) and STS was abrogated in the presence of CLQ.
Treatment of cells with the CLQ increases lysosomal pH and inhibits their trafficking and fusion to the PM (16). To determine whether CLQ also affects PS externalization, we tested the ability of the drug to inhibit Ca2+ ionophore- and STS-induced lipid rearrangements. Examination of cells incubated with CLQ revealed the presence of giant N-rho-PE-labeled lysosomes (Fig. 2, b, c, and f) that did not migrate to the subplasmalemmal space (Fig. 2a). Confocal microscopy of Ca2+ ionophore- (Fig. 2b) and STS-treated cells (Fig. 2c) showed that CLQ inhibited both the redistribution of PS and the fusion of N-rho-PE-labeled organelles to the PM. Although CLQ inhibited lipid redistribution and lysosome-PM fusion, it did not affect the ability of the cells to undergo apoptosis because STS-treated cells incubated in the presence and absence of the drug exhibited essentially identical levels of caspase 3 activation and DNA fragmentation (Fig. 2, d and e). Electron microscopic analysis of the CLQ-treated cells revealed that both ionophore-triggered and STS-triggered organelle to PM fusion (asterisks) was inhibited (Fig. 2f).
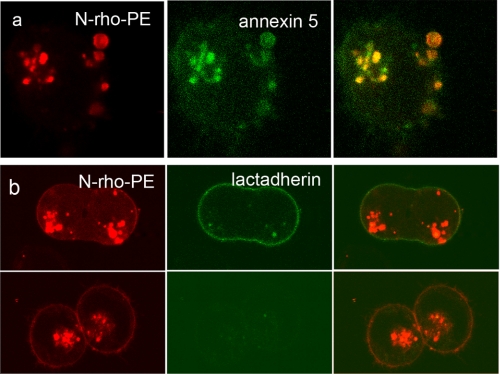
The data presented above suggest that the redistribution of PS to the outer membrane leaflet of the cell is dependent on lysosome to PM trafficking and fusion of the lysosomes with the PM. Because interleaflet lipid distributions appear to be preserved during membrane fusion (17, 18), the fusion process itself is unlikely to contribute to PS externalization. Interestingly, using annexin 5 and PS antibodies, Yeung et al. (18) showed that PS became depleted from the cytosolic face of newly formed phagolysosomes. To determine whether the exclusion of PS might be due to its transport to the outer PM leaflet-derived luminal endosome leaflet, the presence of PS within newly formed endocytotic vesicles was determined after back-exchange of cells incubated with FITC-annexin 5 and N-rho-PE for 1 at 37 °C.3 Surprisingly, the endosomal compartment became intensely labeled with both annexin 5 and N-rho-PE (Fig. 3 a), suggesting that PS redistributed from the PM inner leaflet (18) to the (N-rho-PE labeled) outer PM-derived endosomal luminal leaflet. Although we cannot rule out the contribution of a true scramblase activity that might regulate interleaflet lipid mixing (15), these data raise the possibility that PS externalized to the cell surface during apoptosis originates from the luminal lysosomal leaflet that fuses back to the outer PM leaflet.
FIGURE 3.
Scrambling of PS in endosomes and restoration of PS asymmetry. a, MEF were incubated with N-rho-PE in the presence of FITC-annexin 5 and Ca2+ for 1 h at 37 °C. The cells were then back-exchanged and resuspended in buffer containing the same concentration of annexin 5 and Ca2+. Note that all the N-rho-PE-labeled endosomes became intensely labeled with FITC-annexin 5, whereas the PM remained FITC- and rhodamine-negative. b, back-exchanged, N-rho-PE-labeled cells were incubated with the Ca2+ ionophore A23187 in the presence of 1 mm Ca2+ for 20 min at 37 °C. The coverslips were then washed and labeled with FITC-lactadherin in HHBS (top). A second set of coverslips (bottom) was washed and resuspended in HHBS containing 2 mm EGTA to deplete cytosolic Ca2+. The cells were then incubated at 37 °C for 30 min and stained with FITC-lactadherin. The absence of PS at the cell surface (no FITC-lactadherin labeling) suggests that PS asymmetry was restored.
Our data indicate that the principal difference between PM damage repair in normal and apoptotic cells is the restoration of membrane PS asymmetry. Because Ca2+-dependent cellular responses evoke both lysosomal membrane repair mechanisms and PS externalization, one of the major differences between the responses in normal and apoptotic cells could be related to persistent increases in cytosolic Ca2+ levels in apoptotic cells. If this is the case, then depletion of cytosolic Ca2+ should, in principle, restore a normal phenotype to apoptotic cells. To test this, lysosome to PM fusion and PS externalization was triggered with Ca2+ ionophore (Fig. 3b). Cytosolic Ca2+ was then removed by washing the cells with EGTA-containing medium. The cells were subsequently incubated at 37 °C for an additional 30 min to facilitate reactivation of the aminophospholipid translocase (10). The presence of PS at the cell surface was then monitored with FITC-lactadherin that binds PS in the absence of Ca2+ (14) Fig. 3b shows that although N-rho-PE remained at the cell surface, there was no FITC labeling, indicating that PS asymmetry was re-established.
The data shown here indicate that although damage to normal and apoptotic cells results in the activation of analogous lysosome-dependent repair mechanisms, normal cells “heal,” whereas apoptotic cells evoke a “suicidal” response. This difference is likely due to inherent deficiencies in the ability of the cell to restore cytosolic [Ca2+] to basal levels. In damaged cells, the membrane disruption site facilitates influx of exogenous Ca2+ that triggers lysosome-PM fusion that patches the damaged area. Once annealed, further Ca2+ entry is blocked, and normal cytosolic [Ca2+] is restored. This results in the reactivation of aminophospholipid translocase activity (9, 10), the restoration of membrane lipid asymmetry, and the cessation of membrane repair. In apoptotic cells, however, persistent elevated cytosolic [Ca2+] results in continuous inhibition of the aminophospholipid translocase and incessant lysosome-PM fusion that leads to exaggerated PM expansion and blebbing.
These findings may have important physiologic implications. Similar to PS functioning as a ligand for the recognition and engulfment of apoptotic cells by phagocytes, PS on viral surfaces is also critical to host infection (19). Because infection triggers virus-induced PS externalization, and in certain instances, host cell apoptosis (20), virions likely acquire PS by budding from their PS-expressing host cells. In principle, therefore, inhibition of PS externalization in the host cell would produce impotent PS-free virions. Moreover, because PS suppresses inflammatory responses, thereby avoiding the development of immunity (21, 22), PS-free virions would be expected to generate more efficient immune responses. Moreover, because conventional radio- and chemotherapies kill tumor cells through apoptosis, suppressing the expression of PS at the cell surface could provide a new therapeutic modality. In principle, inhibition of lysosome-PM fusion and expression of PS with lysosomotrophic agents could shift “silent” physiologic clearance mechanisms (23–25) to phagocyte-processing mechanisms that promote specific antitumor immune responses (26), resulting in more effective tumor therapies. Indeed, there is increasing evidence that the inclusion of lysosomotrophic agents in standard chemotherapeutic protocols results in improved patient outcomes (27–29).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant CA98527 (to A. J. S.). This work was also supported by Department of Defense Grant BCRPW81XWH-06-1-0347 (to B. M.) and a grant from the John Q. Gaines Foundation.
This article was selected as a Paper of the Week.
This experiment is based on the concept that dilute FITC-annexin 5 is transparent under confocal microscopy because its concentration is below detection limits. In the presence of Ca2+ and membrane PS, however, the probe sequesters to the membrane surface, effectively increasing its local concentration above detection limits.
- PM
- plasma membrane
- CLQ
- chloroquine
- MEF
- murine embryonic fibroblasts
- N-rho-PE
- N-rhodamine-labeled phosphatidylethanolamine
- PS
- phosphatidylserine
- STS
- staurosporine
- HHBS
- HEPES-buffered Hanks' balanced salt solution
- FITC
- fluorescein isothiocyanate
- DMEM
- Dulbecco's modified Eagle's medium
- z
- benzyloxycarbonyl
- AMC
- 7-amido-4-methylcoumarin.
REFERENCES
- 1.McNeil P. L., Kirchhausen T. (2005) Nat. Rev. Mol. Cell Biol. 6,499–505 [DOI] [PubMed] [Google Scholar]
- 2.Luzio J. P., Poupon V., Lindsay M. R., Mullock B. M., Piper R. C., Pryor P. R. (2003) Mol. Membr. Biol. 20,141–154 [DOI] [PubMed] [Google Scholar]
- 3.Luzio J. P., Pryor P. R., Bright N. A. (2007) Nat. Rev. Mol. Cell Biol. 8,622–632 [DOI] [PubMed] [Google Scholar]
- 4.Pryor P. R., Mullock B. M., Bright N. A., Gray S. R., Luzio J. P. (2000) J. Cell Biol. 149,1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodríguez A., Webster P., Ortego J., Andrews N. W. (1997) J. Cell Biol. 137,93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorice M., Circella A., Misasi R., Pittoni V., Garofalo T., Cirelli A., Pavan A., Pontieri G. M., Valesini G. (2000) Clin. Exp. Immunol. 122,277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franz S., Herrmann K., Fürnrohr B. G., Sheriff A., Frey B., Gaipl U. S., Voll R. E., Kalden J. R., Jäck H. M., Herrmann M. (2007) Cell Death. Differ. 14,733–742 [DOI] [PubMed] [Google Scholar]
- 8.Shiratsuchi A., Mori T., Takahashi Y., Sakai K., Nakanishi Y. (2003) J. Biochem. 133,211–218 [DOI] [PubMed] [Google Scholar]
- 9.Williamson P., Kulick A., Zachowski A., Schlegel R. A., Devaux P. F. (1992) Biochemistry 31,6355–6360 [DOI] [PubMed] [Google Scholar]
- 10.Williamson P., Bevers E. M., Smeets E. F., Comfurius P., Schlegel R. A., Zwaal R. F. (1995) Biochemistry 34,10448–10455 [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanian K., Mirnikjoo B., Schroit A. J. (2007) J. Biol. Chem. 282,18357–18364 [DOI] [PubMed] [Google Scholar]
- 12.Struck D. K., Pagano R. E. (1980) J. Biol. Chem. 255,5404–5410 [PubMed] [Google Scholar]
- 13.Connor J., Schroit A. J. (1987) Biochemistry 26,5099–5105 [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta S. K., Guchhait P., Thiagarajan P. (2006) Transl. Res. 148,19–25 [DOI] [PubMed] [Google Scholar]
- 15.Bevers E. M., Comfurius P., Dekkers D. W., Zwaal R. F. (1999) Biochim. Biophys. Acta 1439,317–330 [DOI] [PubMed] [Google Scholar]
- 16.Hart P. D., Young M. R., Jordan M. M., Perkins W. J., Geisow M. J. (1983) J. Exp. Med. 158,477–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klotz K. H., Bartoldus I., Stegmann T. (1996) J. Biol. Chem. 271,2383–2386 [DOI] [PubMed] [Google Scholar]
- 18.Yeung T., Terebiznik M., Yu L., Silvius J., Abidi W. M., Philips M., Levine T., Kapus A., Grinstein S. (2006) Science 313,347–351 [DOI] [PubMed] [Google Scholar]
- 19.Mercer J., Helenius A. (2008) Science 320,531–535 [DOI] [PubMed] [Google Scholar]
- 20.Soares M. M., King S. W., Thorpe P. E. (2008) Nat. Med. 14,1357–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cvetanovic M., Ucker D. S. (2004) J. Immunol. 172,880–889 [DOI] [PubMed] [Google Scholar]
- 22.Cvetanovic M., Mitchell J. E., Patel V., Avner B. S., Su Y., van der Saag P. T., Witte P. L., Fiore S., Levine J. S., Ucker D. S. (2006) J. Biol. Chem. 281,20055–20067 [DOI] [PubMed] [Google Scholar]
- 23.Zhao H., Cai Y., Santi S., Lafrenie R., Lee H. (2005) Radiat. Res. 164,250–257 [DOI] [PubMed] [Google Scholar]
- 24.Accapezzato D., Visco V., Francavilla V., Molette C., Donato T., Paroli M., Mondelli M. U., Doria M., Torrisi M. R., Barnaba V. (2005) J. Exp. Med. 202,817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiller M., Bekeredjian-Ding I., Heyder P., Blank N., Ho A. D., Lorenz H. M. (2008) Cell Death. Differ. 15,183–191 [DOI] [PubMed] [Google Scholar]
- 26.Cocca B. A., Cline A. M., Radic M. Z. (2002) J. Immunol. 169,159–166 [DOI] [PubMed] [Google Scholar]
- 27.Shoemaker J. P. (1978) Cancer Res. 38,2700–2702 [PubMed] [Google Scholar]
- 28.Savarino A., Lucia M. B., Giordano F., Cauda R. (2006) Lancet Oncol. 7,792–793 [DOI] [PubMed] [Google Scholar]
- 29.Briceño E., Calderon A., Sotelo J. (2007) Surg. Neurol. 67,388–391 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.