Abstract
MC2 (ACTH) receptors require MC2 receptor accessory protein (MRAP) to reach the cell surface. In this study, we show that MRAP has the opposite effect on the closely related MC5 receptor. In enzyme-linked immunosorbent assay and microscopy experiments, MC2 receptor was retained in the endoplasmic reticulum in the absence of MRAP and targeted to the plasma membrane with MRAP. MC5 receptor was at the plasma membrane in the absence of MRAP, but trapped intracellularly when expressed with MRAP. Using bimolecular fluorescence complementation, where one fragment of yellow fluorescent protein (YFP) was fused to receptors and another to MRAP, we showed that MC2 receptor-MRAP dimers were present at the plasma membrane, whereas MC5 receptor-MRAP dimers were intracellular. Both MC2 and MC5 receptors co-precipitated with MRAP. MRAP did not alter expression of β2-adrenergic receptors or co-precipitate with them. To determine if MRAP affects formation of receptor oligomers, we co-expressed MC2 receptors fused to YFP fragments in the presence or absence of MRAP. YFP fluorescence, reporting MC2 receptor homodimers, was readily detectable with or without MRAP. In contrast, MC5 receptor homodimers were visible in the absence of MRAP, but little fluorescence was observed by microscopic analysis when MRAP was co-expressed. Co-precipitation of differentially tagged receptors confirmed that MRAP blocks MC5 receptor dimerization. The regions of MRAP required for its effects on MC2 and MC5 receptors differed. These results establish that MRAP forms stable complexes with two different melanocortin receptors, facilitating surface expression of MC2 receptor but disrupting dimerization and surface localization of MC5 receptor.
In mammals, the five members of the melanocortin (MC2) receptor family play diverse physiological roles. MC1 receptors (melanocyte-stimulating hormone receptors) control pigmentation in many animals, MC2 receptors (ACTH receptors) regulate adrenal corticosteroid synthesis, MC3 and MC4 receptors in brain influence food intake and energy expenditure, and MC5 receptors control exocrine gland secretion (1). Melanocortin receptors (MC1 through MC5) are structurally related G protein-coupled receptors that respond to agonists with an increase in cAMP. The receptors differ in their affinity for physiological agonists (α-, β-, and γ-melanocyte-stimulating hormone and ACTH) and antagonists (agouti and agouti-related protein).
Unlike other melanocortin receptors, MC2 receptors are selectively regulated by ACTH. The MC2 receptor is also unusual in its requirement for an accessory protein, the MC2 receptor accessory protein (MRAP) (2). MRAP must be expressed with the MC2 receptor in order for the receptor to undergo glycosylation, traffic to the plasma membrane, bind ACTH, and stimulate adenylyl cyclase (2–4). Individuals with inactivating mutations of either the MC2 receptor or MRAP suffer from ACTH resistance and severe glucocorticoid deficiency (2).
MRAP is a small protein with a conserved amino terminus, single membrane-spanning domain, and non-conserved carboxyl terminus that can also differ among splice variants. MRAP forms antiparallel homodimers, which are exceedingly rare structures, and it is these dimers that co-precipitate with the MC2 receptor (3). Mutagenic analysis has revealed that the carboxyl-terminal region of MRAP is not essential, but the amino-terminal and transmembrane regions are necessary for function (3–5). Deletion or alanine substitution of a critical four-amino acid segment, LDYI, at residues 18–21 of the mouse sequence, results in an MRAP molecule that facilitates expression of the MC2 receptor on the plasma membrane but does not allow the receptor to bind agonist or signal (4). MRAP is not required for cell surface expression of other melanocortin receptors and can inhibit expression or signaling in some cases (3, 6).
The MC5 receptor was identified on the basis of its homology to other melanocortin receptors and is thought to be the ancestral ACTH (MC2) receptor (7). Analysis of MC5 receptor knock-out mice revealed that the MC5 receptor is important in controlling exocrine gland secretion (8) and behavioral responses depending on pheromones secreted by the preputial gland (9). MC2 and MC5 receptors are closely related, with 46% identity and 67% homology at the amino acid level, respectively. MC2 and MC5 receptors are both found on 3T3-L1 adipocytes and in some adipose tissues in animals (10, 11). Furthermore, MC2 and MC5 receptors are both expressed in adrenal cortex during embryonic development, when the MC5 receptor appears before the MC2 receptor (12). Mammalian MC2 and MC5 receptors are activated by different pro-opiomelanocortin peptides, responding physiologically to ACTH and melanocyte-stimulating hormone, respectively. Here we demonstrate that MC2 and MC5 receptors are also differentially regulated by MRAP, which exerts opposite effects on surface expression and dimerization of the two receptors.
EXPERIMENTAL PROCEDURES
Materials
hMC2, MC5, and β2-adrenergic receptors with three amino-terminal HA tags and RAMP3 were obtained from Missouri S&T cDNA Resource Center and YFP-F1 and YFP-F2 constructs from Dr. Catherine Berlot (Weis Center for Research, Geisinger Clinic, Danville, PA) (13). Construction of mouse MRAP and MRAP2 plasmids has been described before (4). ER-tracker blue-white DPX was purchased from Invitrogen. Antibodies were from AbDSerotec (Kidlington, UK) (monoclonal anti-V5), Covance (Princeton, NJ) (monoclonal HA11 anti-HA), Bio-Rad (Hercules, CA) (horseradish peroxidase-coupled anti-mouse), or Molecular Probes (Carlsbad, CA) (Alexa546-coupled anti-mouse). MC2 and MC5 receptors with amino-terminal V5 tags were made by PCR; primer sequences are available upon request.
Cell Culture and Transfection
CHO cells were grown in Dulbecco's modified Eagle's medium/F-12 supplemented with 5% fetal bovine serum. Plasmids were transiently transfected 24 h before experiments using FuGENE HD (Roche Applied Science).
Construction of Split-YFP Vectors
To make 3HA-MC5R-YFP-F1 and 3HA-MC5R-YFP-F2, 3HA-MC5R with exclusion of the stop codon was amplified from the 3HA-MC5R in pcDNA3.1+ plasmid using the following primers: forward, TATATATATAGCTAGCGTTTAAACTTAAGCTTGGTACC and reverse, TATATATATAACGCGTATCCCTTCTGGGAAAGCTGCAGGCG. 3HA-MC2R-YFP-F1 and 3HA-MC2R-YFP-F2 were made by the same strategy using the following primers: forward, TATATATATAGCTAGCGTTTAAACTTAAGCTTGGTACC and reverse, TATATATATAACGCGTCCAGTACCTGCTGCAGAAGATCATC. Products and destination vector (YFP-F1 or YFP-F2 in pciNeo) were digested with NheI and MluI, and the amplified receptor product was inserted 5′ of the coding sequence of YFP-F1 or YFP-F2. To make V5-MRAP-YFP-F2, YFP-F2 was amplified from the YFP-F2stop in pcDNA1 using the following primers: forward, TATACGCGTAAGAACGGCATCAAGGTG and reverse, ATAGAATTCTTACTTGTACAGCTCGTCCAT. The product and destination vector (V5-MRAP-3Flag in pciNeo) were then digested with MluI and NotI (removing the 3 FLAG epitopes and the stop codon from MRAP). YFP-F2 was inserted after the MRAP coding region, producing V5-MRAP-YFP-F2 in pciNeo.
RT-PCR
mRNA extraction from mouse adrenal glands and Y1 cells was performed using the RNeasy kit and RT-PCR using the SuperScript III One-Step RT-PCR system from Invitrogen following the manufacturer's instructions and appropriate primers (primer sequences available on request).
Surface and Total Epitope Detection by Fixed Cell ELISA
To measure epitopes on the extracellular side of the plasma membrane, cells in 12- or 24-well plates were washed with phosphate-buffered saline, fixed for 10 min with 2% paraformaldehyde, washed, blocked in 5% nonfat dry milk in phosphate-buffered saline, incubated with 1:5000 mouse monoclonal anti-HA or anti-V5 antibody in blocking buffer for 2 h at room temperature, washed three times for 5 min in phosphate-buffered saline, and incubated with secondary antibody and processed for ELISA as described (3). The same protocol was performed in permeabilized cells to measure total expression of proteins of interest. In this case the blocking buffer used was 5% milk in radioimmune precipitation assay buffer (150 mm NaCl, 50 mm Tris, 1 mm EDTA, 10 mm NaF, 1% Triton X-100, 0.1% SDS, pH 8.0).
Live Cell Imaging
Cells on glass coverslips were rinsed and incubated with anti-V5 antibody at 1:100 in Dulbecco's modified Eagle's medium/F-12 media supplemented with 5% fetal bovine serum for 1 h at 37 °C, washed, and incubated with 1:100 anti-mouse antibody coupled to Alexa546 for 5 min at room temperature. Where indicated, 1 μm of ER tracker blue-white DPX from Invitrogen was added with the secondary antibody. A Nikon Diaphot inverted microscope with 100×/1.3 numerical aperture oil objective, Photometrics CoolSNAP ES camera, and appropriate filter sets from Chroma were used. Images were captured with Metamorph software from Universal Imaging and transferred to Powerpoint for labeling. Fluorescence intensity measurements were made using Metamorph software. Micrographs displayed in a group were exposed and processed identically.
Immunoprecipitation and Western Blotting
Cells were lysed with 0.1% n-dodecyl-β-maltoside and centrifuged, and supernatants incubated with anti-HA or anti-V5 antibody at 1:5000 overnight at 4 °C, and immunoprecipitates were collected on protein A/G beads. Where noted, deglycosylation was carried out using peptide N-glycosidase from NE Biolabs as recommended. Beads were washed three times, suspended in loading buffer with 100 mm dithiothreitol, boiled 5 min, and centrifuged. Proteins were resolved by SDS-PAGE on 10 or 15% gels from Lonza. Routine Western blotting was performed as previously described (3) using chemiluminescent detection methods. For quantitation, blots were probed with IRDye 800CW goat anti-mouse IgG from LiCor, scanned on a LiCor Imaging System and analyzed using Odyssey software.
RESULTS
Effect of MRAP on MC5 Receptor Expression
Unlike the MC2 receptor, MC5 receptor traffics readily to the plasma membrane when expressed in CHO cells in the absence of MRAP (3, 6, 14). Because MC2 and MC5 receptors are expressed together in several systems, including adipocytes (11), we investigated the effect of MRAP on the MC5 receptor using receptors epitope-tagged at the extracellular N terminus.
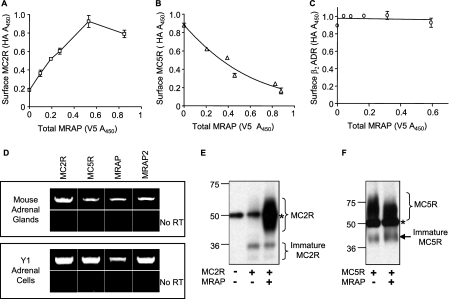
To determine whether MRAP modulates the surface expression of MC5 receptor, we transfected CHO cells with a constant concentration of HA-MC2 receptor or HA-MC5 receptor along with increasing concentrations of MRAP-V5. Surface receptor was quantified by ELISA on non-permeabilized cells and MRAP expression by ELISA in permeabilized cells in parallel dishes. As expected, MC2 receptor expression at the cell surface increased with increasing concentrations of MRAP (Fig. 1A). Surprisingly, we found that MC5 receptor surface expression was greatly decreased by MRAP in a concentration-dependent manner (Fig. 1B). MRAP had no significant effect on the surface expression of the β2-adrenergic receptor (Fig. 1C), establishing that the decrease of MC5 receptor density at the plasma membrane in the presence of MRAP is not due to cell toxicity and that this effect is specific.
FIGURE 1.
Regulation of surface MC2 and MC5 receptors by MRAP. Surface expression of HA-MC2 (A), HA-MC5 (B), or HA-β2-adrenergic receptor (β2ADR) (C) measured by ELISA in non-permeabilized CHO cells using anti-HA antibody, as a function of the total expression of MRAP-V5 measured in parallel dishes by ELISA in permeabilized cells using anti-V5 antibodies. D, detection of MC2R, MC5R, MRAP, and MRAP2 mRNA in mouse adrenal glands or mouse Y1 adrenal cell line mRNA extracts by RT-PCR. Cells expressing HA-MC2 (E) or HA-MC5 (F) receptor in the presence or absence of MRAP-V5 were lysed, and receptors were immunoprecipitated with anti-HA antibody and resolved on SDS-PAGE. Blots were probed with anti-HA antibody. *, IgG heavy chain.
To determine if this effect of MRAP on the MC5 receptor is of possible physiological relevance, we tested the expression of MC2 receptor, MC5 receptor, MRAP, and MRAP2 in mouse adrenal glands and in the Y1 adrenal tumor cell line by RT-PCR. mRNAs coding for all four proteins were detected in mouse adrenal glands and in Y1 cells (Fig. 1D). When the same experiment was performed omitting the reverse transcriptase to verify that the bands observed were due to amplification of cDNA and not contaminating genomic DNA, no amplification was detectable (Fig. 1D).
We speculated that MRAP decreased the amount of MC5 receptor on the plasma membrane by causing it to be retained within intracellular compartments, likely the endoplasmic reticulum (ER) and/or the Golgi apparatus. To test this hypothesis, we expressed MC2 or MC5 receptor in CHO cells with or without MRAP, lysed the cells, immunoprecipitated the receptor, and resolved the proteins by SDS-PAGE. MC2 receptor was detected in the mature glycosylated form only in the presence of MRAP (Fig. 1E), in agreement with published findings (3). In contrast, MC5 receptor was glycosylated in the presence or absence of MRAP (Fig. 1F), showing that MRAP does not prevent post-translational glycosylation of the MC5 receptor. Interestingly, in the presence of MRAP the MC5 receptor ran slightly faster than it did when MRAP was not expressed. To verify that the bands described earlier were indeed representative of glycosylated forms of the MC5 receptor, we expressed MC5 receptor with or without MRAP, immunoprecipitated the receptor, and treated the samples with peptide N-glycosidase F. This experiment confirmed that MC5 receptor was glycosylated even in the presence of MRAP, because the bands hypothesized to be glycosylated forms of MC5 receptor, in both conditions, collapsed to a lower molecular weight band after enzymatic deglycosylation (data not shown). There are four potential glycosylation sites on the MC5 receptor, and faster migration of receptor could be due to the use of fewer glycosylation sites or incomplete maturation of the carbohydrate in the presence of MRAP. Alternatively, MRAP could inhibit formation of homo- or heteromultimers, or alter an unidentified post-translational modification.
Localization of Receptors in Live Cells
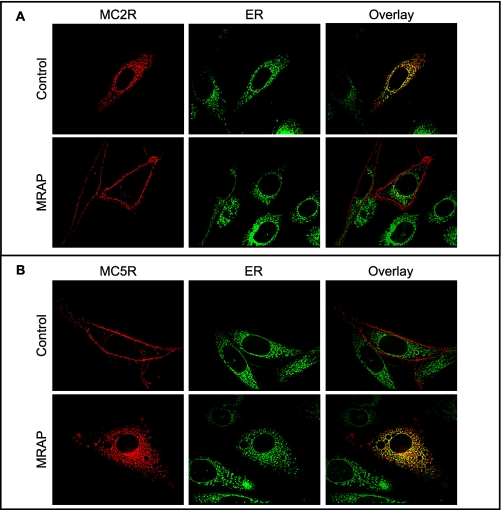
To localize MC2 and MC5 receptors in live cells, we expressed the receptors fused to a red fluorescent protein, tandem (td)-tomato, with or without MRAP. Because the td-tomato reporter contains two copies of the fluorescent protein, it is not prone to form intermolecular dimers (15). In the absence of MRAP, the MC2 receptor was retained in the cell, and the fluorescence largely coincided with a marker for the ER. When MRAP was co-expressed, MC2 receptor was localized at the plasma membrane (Fig. 2A). MC5 receptor demonstrated exactly the opposite behavior. Without MRAP, the receptor was at the plasma membrane, but when MRAP was co-expressed, MC5 receptor was retained within the cell (Fig. 2B) and partially co-localized with the ER marker. It is interesting that much of the MC5 receptor was found in the ER, even though most receptor had undergone N-linked glycosylation, a process that is completed in the Golgi apparatus. These results suggest that MRAP may lead to MC5 receptor retrieval by the ER.
FIGURE 2.
MRAP-dependent MC2 and MC5 receptor localization. MC2 (A) and MC5 (B) receptors fused to the red fluorescent protein td-Tomato were expressed with or without MRAP and imaged in live CHO cells expressing either receptor in the presence or absence of MRAP. ER was stained with ER-tracker.
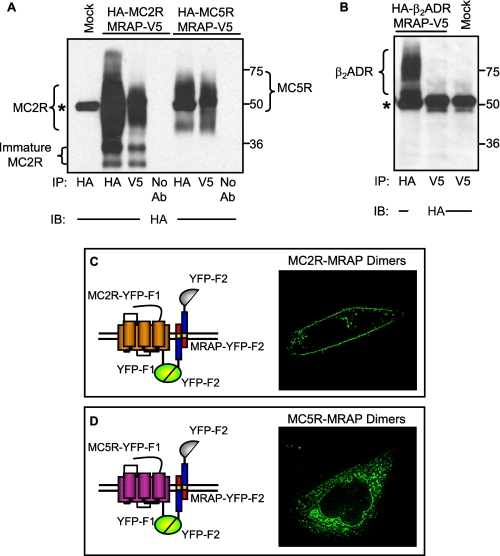
The findings described above suggest that MRAP can, directly or indirectly, differentially regulate MC2 and MC5 receptor surface expression. To determine if this regulation is due to a direct or close interaction between receptors and MRAP, we tested whether each receptor co-immunoprecipitated with MRAP. MC2 receptor co-precipitated with MRAP (Fig. 3A), as reported previously (3). More importantly, MC5 receptor also co-precipitated with MRAP (Fig. 3A). We also showed that the unrelated β2-adrenergic receptor does not co-immunoprecipitate with MRAP to establish that the interactions of MRAP with MC2 and MC5 receptors are specific (Fig. 3B).
FIGURE 3.
MRAP can interact and co-localize with both MC2 and MC5 receptors. A and B, CHO cells expressing MRAP-V5 together with HA-MC2 or HA-MC5 receptor (A) or HA-β2-adrenergic receptor (B) were lysed, and receptors were immunoprecipitated with anti-HA antibody and MRAP with anti-V5 antibody. Proteins were resolved on SDS-PAGE, and receptors were probed with anti-HA antibody. C, live CHO cells transfected with MC2 receptor-YFP-F1 and MRAP-YFP-F2. D, live CHO cells transfected with MC5 receptor-YFP-F1 and MRAP-YFP-F2. *, IgG heavy chain.
To visualize receptor-MRAP complexes in live cells and compare the overall localization of MC2 and MC5 receptors with localization of receptor-MRAP complexes, we used bimolecular fluorescence complementation (16). We fused an amino-terminal fragment of the YFP (YFP-F1) to the C terminus of MC2 and MC5 receptors and the carboxyl-terminal fragment of YFP (YFP-F2) to the C terminus of MRAP. Since YFP fluorescence can be achieved only if the two fragments are close enough to reconstitute the full YFP, fluorescence reports solely the localization of either MC2 receptor-MRAP or MC5 receptor-MRAP complexes, depending on the constructs transfected. The MC2 receptor-MRAP complex was visible mainly at the plasma membrane (Fig. 3C), supporting the idea that MRAP is responsible for the trafficking of the MC2 receptor from the ER to the surface. On the other hand, the MC5 receptor-MRAP complex was localized in intracellular compartments (Fig. 3D), further suggesting that a direct interaction of MRAP with MC5 receptor is responsible for preventing the receptor from trafficking to the plasma membrane. We previously described extensive control experiments to validate the bimolecular fluorescence complementation approach in this model system (4). We obtained negligible fluorescence when both YFP fragments were fused to the C terminus of MRAP (i.e. parallel dimers were not seen), or when YFP fragments were fused to either end of MRAP and expressed with the complementary YFP fragments fused to RAMP3, G protein β or G protein γ subunits. These results show that the split YFPs do not artificially induce significant dimerization.
Effect of MRAP on Receptor Dimerization
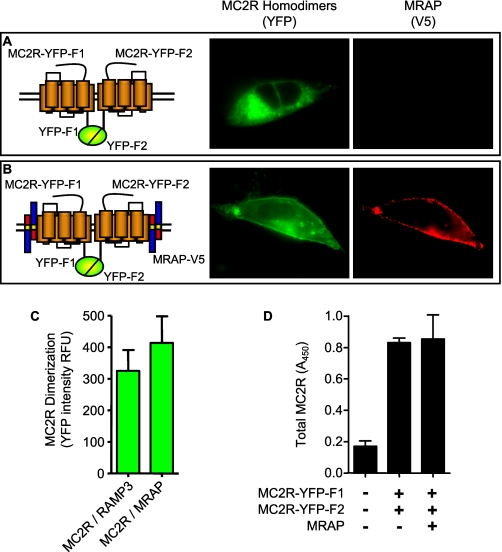
Several of the melanocortin receptors have been reported to form homodimers and heterodimers (17–19). To determine if MC2 or MC5 receptors form homodimers or oligomers and if such complexes are affected by MRAP, we co-expressed MC2 receptor carboxyl-terminally fused to YFP-F1 with MC2 receptor fused to YFP-F2 in the presence or absence of MRAP. In the absence of MRAP, the YFP fluorescence, reporting two MC2 receptors in close proximity, was readily detectable (Fig. 4A). When MRAP was expressed, fluorescence was still detectable and more concentrated on the plasma membrane (Fig. 4B). To quantify bimolecular fluorescence complementation, we measured the average of YFP fluorescence intensity in all successfully transfected cells, which were identified by staining with antibody to the V5 epitope that was present on both RAMP and MRAP. MRAP did not alter the YFP fluorescence intensity of cells expressing MC2 receptors fused to YFP fragments (Fig. 4C), nor did MRAP change the overall expression of MC2 receptor-YFP fragments measured by ELISA in permeabilized cells (Fig. 4D). These results suggest that MC2 receptors form homodimers or higher multimers and that MRAP does not alter the formation of such complexes.
FIGURE 4.
Effect of MRAP on MC2 receptor homodimerization. Live CHO cells expressing HA-MC2 receptor-YFP-F1 and HA-MC2 receptor-YFP-F2 without (A) or with (B) MRAP-V5. YFP fluorescence is shown in green (left panel). Surface MRAP in the same cells detected with anti-V5 antibody and secondary anti-mouse Alexa546 is shown in red (right panel). C, average YFP fluorescence intensity was measured in five or more randomly selected fields. All successfully transfected cells, identified by staining with anti-V5 antibody, were analyzed (25–88 cells/condition). YFP fluorescence reports the formation of receptor homodimers. Shown are mean ± S.E. in relative fluorescence units (RFU). D, total expression of HA-MC5 receptor-YFP-F1 and HA-MC5 receptor-YFP-F2 without or with MRAP-V5 measured by ELISA in permeabilized cells using anti-HA antibody.
When the same experiment was performed for the MC5 receptor, YFP fluorescence was detected in the absence of MRAP (Fig. 5A) but was greatly diminished when MRAP was present (Fig. 5B). Quantitative analysis revealed that the average YFP fluorescence was significantly decreased by MRAP (Fig. 5C). These findings indicate that MC5 receptors form homodimers or oligomers in cells, and that MRAP inhibits the formation of multimers. The overall concentration of MC5-YFP fusion proteins, again measured by ELISA in permeabilized cells, was not affected by MRAP, so the loss of fluorescence was not due to an MRAP-mediated decrease in MC5-YFP fusion protein expression (Fig. 5D). Furthermore, as shown above in Fig. 3D, the same MC5 receptor-YFP fusion protein yielded readily measurable fluorescence when it was co-expressed with the complementary MRAP-YFP fragment.
FIGURE 5.
Effect of MRAP on MC5 receptor homodimerization measured by bimolecular fluorescence complementation. A and B, live CHO cells expressing HA-MC5 receptor-YFP-F1 and HA-MC5 receptor-YFP-F2 without (A) or with (B) MRAP-V5. YFP fluorescence is shown in green (left panel). Surface MRAP in the same cells detected with anti-V5 antibody and secondary anti-mouse Alexa546 is shown in red (right panel). C, average YFP fluorescence intensity in relative fluorescence units (RFU). D, total expression of HA-MC5 receptor-YFP-F1 and HA-MC5 receptor-YFP-F2 without or with MRAP-V5. YFP fluorescence and total expression of receptors was quantified as described in the legend to Fig. 4.
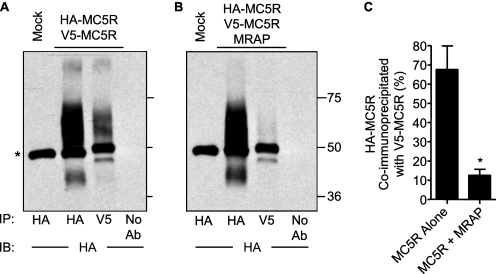
To confirm that MC5 receptors dimerize and that the stability of the receptor dimer is altered by MRAP, we expressed HA-MC5 receptor and V5-MC5 receptor with or without MRAP, immunoprecipitated the V5-tagged receptor, and measured the fraction of HA-tagged receptor co-precipitated with the V5-MC5 receptor by Western blot. A significant fraction of the HA-MC5 receptor was immunoprecipitated along with V5-MC5 receptor in the absence of MRAP (Fig. 6A). In the presence of MRAP, almost no HA-MC5 receptor co-precipitated with V5-MC5 receptor (Fig. 6B). Quantitative analyses confirmed that MRAP caused a very significant decrease in co-immunoprecipitation of HA-MC5 receptor with V5-MC5 receptor (Fig. 6C). The results of both the co-precipitation and bimolecular fluorescence complementation studies indicate that the MC5 receptor homodimer is destabilized by MRAP. We were able to detect dimers of MC2 and MC5 receptors by bimolecular fluorescence complementation but were unable to assess the effect of MRAP because the concentration of heterodimers was low resulting in a very dim signal (data not shown).
FIGURE 6.
Effect of MRAP on MC5 receptor homodimerization measured by co-immunoprecipitation. A and B, cells expressing HA-MC5 receptor and V5-MC5 receptor without (A) or with (B) MRAP were lysed, and MC5 receptor was immunoprecipitated with anti-HA or anti-V5 antibody. Proteins were resolved by SDS-PAGE, and HA-MC5 receptor was detected with anti-HA antibody. *, IgG heavy chain. C, quantitative analysis of HA-MC5 receptor and V5-MC5 receptor co-immunoprecipitation in the presence or absence of MRAP was performed as described under “Experimental Procedures.” It is expected that only a fraction of MC5 receptor dimers will have HA/V5 tags and be visualized, whereas others will have HA/HA or V5/V5 tags. The mean ± S.E. values from three separate experiments are shown. *, p < 0.01 compared with control.
Regions of MRAP Important for Effects on MC5 Receptors
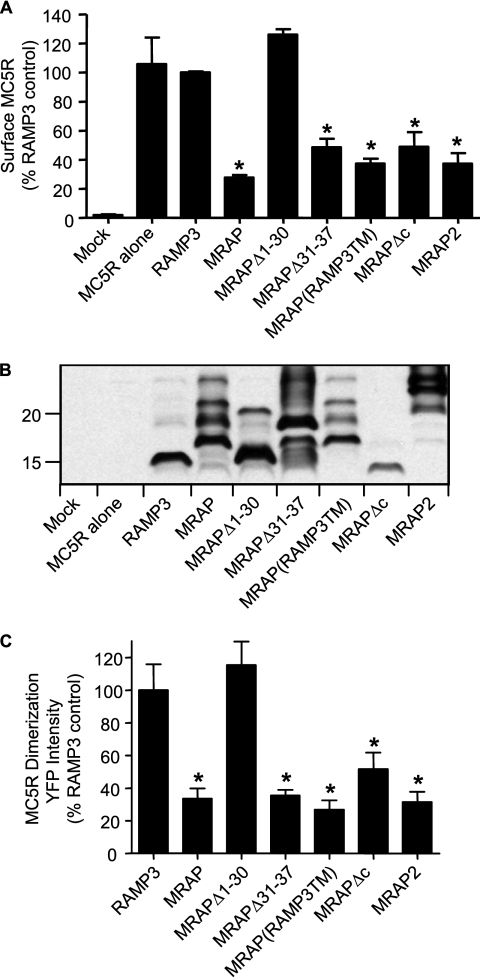
To determine the regions of MRAP that are necessary to retain MC5 receptor in intracellular compartments, we generated several truncation mutants and chimeras of MRAP and RAMP3 and expressed these with MC5 receptor. Surface receptor concentration was measured by ELISA on non-permeabilized cells. MC5 receptor surface expression was greatly reduced when the receptor was expressed with a carboxyl-terminally truncated form of MRAP (MRAPΔc). Surface MC5 receptor was also decreased by an MRAP chimera where the transmembrane domain of MRAP was replaced by the transmembrane region of RAMP3, an accessory protein that does not regulate melanocortin receptor trafficking. Importantly, deletion of the amino acids 31–37, which are responsible for the dual topology of MRAP (4), did not prevent the negative effect of MRAP on surface localization of the MC5 receptor. Finally, deletion of the first 30 amino acids of MRAP (MRAPΔ1–30) completely abolished the effect of MRAP on MC5 receptor trafficking (Fig. 7A). The relative amounts of MRAP and RAMP3 proteins were measured on Western blots (Fig. 7B). The lack of effect of MRAPΔ1–30 on MC5 receptor dimerization was not due to poor expression, because this mutant was expressed at comparable or higher levels than wild type MRAP (Fig. 7B). In fact, the mutant that was expressed at the lowest concentration was MRAPΔc, which still significantly inhibited surface expression of the MC5 receptor.
FIGURE 7.
Regions of MRAP required for regulation of MC5 receptor homodimerization and trafficking. A, surface HA-MC5 receptor was measured by ELISA on non-permeabilized CHO cells transfected with HA-MC5 receptor alone or with RAMP3 or the noted MRAPs. The bar graph represents the mean ± S.E. of three separate experiments, each performed in duplicate or triplicate, for surface expression of MC5 receptor normalized to control expression in the presence of RAMP3. B, Western blot analysis of RAMP3, MRAP, and MRAP mutants or MRAP2 expression in cell lysates from parallel dishes in the experiment depicted in A. All of the accessory proteins were tagged with an amino-terminal V5 epitope and made in the same pCI-Neo vector. C, YFP fluorescence intensity in live CHO cells expressing HA-MC5 receptor-YFP-F1 and HA-MC5 receptor-YFP-F2 with V5-tagged RAMP3 or the noted MRAPs, analyzed as described in the legend to Fig. 4. Sequences of MRAP mutants have been reported previously (3, 4). *, p < 0.05 compared with control.
These results demonstrate that the conserved N terminus of MRAP is required for MRAP to inhibit MC5 receptor localization on the plasma membrane. Surprisingly, the unusual dual topology of MRAP, which appears to be essential for its interactions with the MC2 receptor, does not seem to be necessary for MRAP to restrict MC5 receptor movement to the cell surface. The newly identified mouse MRAP2 protein, which can promote surface expression of MC2 receptor but not efficient ACTH signaling (4), acts like MRAP with the MC5 receptor, preventing its surface expression (Fig. 7).
We used the bimolecular fluorescence complementation approach to test if there was a correlation between the ability of MRAP, MRAP mutants and MRAP2 to prevent MC5 receptor trafficking and prevent receptor dimerization. To quantify MC5 receptor dimer formation, we measured YFP fluorescence intensity in cells expressing MC5R-YFP-F1 and MC5R-YFP-F2 together with V5-tagged RAMP3, MRAP, MRAP mutant, or MRAP2. As described earlier, the average YFP fluorescence intensity of transfected cells, as determined by surface staining with an anti-V5 antibody, was measured (Fig. 7C). In the presence of RAMP3, YFP fluorescence was readily detectable, confirming that RAMP3 does not interfere with MC5 receptor homodimerization. When MRAP, MRAPΔ31–37, MRAP(RAMP3TM), MRAPΔc, or MRAP2 was present, YFP fluorescence was significantly decreased, consistent with a decrease in MC5 receptor homodimer formation. Again, MRAPΔ1–30 did not inhibit MC5 receptor homodimerization. Comparable results were obtained when the intensity of YFP fluorescence in individual cells was analyzed by a fluorescence-activated cell sorter (data not shown). These results show that there is a direct correlation between the ability of MC5 receptor to homodimerize and its presence at the plasma membrane, suggesting that MC5 receptor monomers may be trapped intracellularly.
DISCUSSION
The observations described here expand the known functions of accessory proteins in melanocortin receptor biology. We have shown that MRAP has diametrically opposed effects on the structurally similar MC2 and MC5 receptors. The impact of MRAP is substantial. The MC2 receptor requires MRAP to traffic to the plasma membrane, whereas MRAP prevents the MC5 receptor from reaching the cell surface. When MC2 and MC5 receptors were expressed together in the same cells, MRAP stimulated surface expression of MC2 receptors and trapped MC5 receptors intracellularly, so one action does not predominate (data not shown). The absence of receptor on the cell surface with or without MRAP (MC5 and MC2 receptors, respectively) precludes analysis of the influence of MRAP on the structure-activity profile of either receptor. The concentrations of MRAP required for these opposite actions are the same.
MRAP did not modify the surface expression of the β2-adrenergic receptor (Fig. 1C), another Gs-coupled class A GPCR, suggesting that the action of MRAP on MC2 and MC5 receptors is specific. Individuals lacking MRAP protein have severe glucocorticoid deficiency resulting from resistance to ACTH (2) but no additional problems that might indicate a role for MRAP in other receptor systems. The lack of any noticeable MC5 receptor-related phenotype in patients lacking MRAP could be explained by the presence of MRAP2, because the latter is, like MRAP, able to regulate surface expression of the MC5 receptor. The potential redundancy of MRAP and MRAP2 will make it difficult to determine which GPCRs are regulated by these accessory proteins until knock-out animals lacking MRAP, MRAP2, and both are available.
It is not known what characteristics make the MC2 receptor dependent on MRAP for trafficking when the other four melanocortin receptors can function without it. The transmembrane regions of the melanocortin receptors are quite homologous and in several cases have been interchanged with little effect on receptor expression, ligand affinity, or cAMP responses (20, 21). The effects of MRAP on both MC2 and MC5 receptors require the conserved MRAP amino terminus, but because MRAP has dual topology it is unclear whether this domain interacts with MC2 receptor from the exoplasmic or cytoplasmic side of the membrane. For the MC5 receptor, however, our results suggest that the amino-terminal region of MRAP interacts with one of the extracellular domains of the receptor, because the exclusively Nexo-Ccyto mutant MRAPΔ31–37 strongly inhibits MC5 receptor surface expression.
MRAP co-precipitates with both MC2 and MC5 receptors, and forms complexes in which YFP fragments are able to interact, implying direct or at least very close interactions. MRAP interactions with the MC2 receptor have at least two consequences: more efficient receptor trafficking to the plasma membrane and increased ligand binding and signaling. Receptor trafficking may be secondary to proper receptor folding facilitated by MRAP serving as a chaperone, and high affinity ligand binding may be secondary to interaction of the MRAP-MC2 receptor complex with G proteins, which increases affinity of receptor agonists. With the MC2 receptor, the trafficking function of MRAP requires dual topology and the MRAP transmembrane domain, but is retained in MRAP mutants missing much of the amino terminus. The ability of MRAP to enhance ACTH binding to the MC2 receptor requires four amino acids from residues 18–21, LDYI (4). In contrast, MRAP inhibition of MC5 receptor trafficking does not depend on the transmembrane domain of MRAP, dual topology, the LDYI motif (missing in MRAP2) (4) or the carboxyl-terminal region, but it does require the amino-terminal region.
Several lines of evidence show that MRAP powerfully inhibits formation of MC5 receptor dimers. MRAP reduces bimolecular fluorescence complementation between MC5 receptor-YFP fragment fusion proteins. In addition, MRAP reduces co-precipitation of MC5 receptors tagged with different epitopes. The MC2 receptor appears to dimerize equally well with or without MRAP. There is no direct evidence that melanocortin receptors must dimerize to traffic to the cell surface, but homo- and heterodimerization of class A GPCRs is well documented and MC1 and MC3 receptors have been shown to form homo- and heterodimers (17–19). In general, dimerization of GPCRs occurs in the ER at early stages in receptor biosynthesis, and dimerization explains dominant negative effects of some naturally occurring receptor mutations (22, 23), including several in the melanocortin receptor family (24). The data described here can be explained if MRAP disrupts a critical dimerization step in MC5 receptor maturation and trafficking. There is a perfect correlation between the ability of different MRAP mutants and MRAP2 to disrupt MC5 receptor dimerization and to prevent receptor trafficking to the plasma membrane. Interestingly, heterodimerization of μ and δ opioid receptors is promoted by RTP4 (25), a member of the RTP family of accessory proteins that facilitate odorant and taste receptor expression (26, 27).
Because suitable antibodies are not available, there is little information about where MRAP, MRAP2, MC2, and MC5 receptor proteins are expressed in vivo. Analysis of mRNA levels by Northern blot, RT-PCR, and in situ hybridization suggests that both receptors are expressed in certain adipose tissues, immune system cells, and adrenal cortex, although it is not clear if they reside in the same cells. We showed that the mRNA coding for all four proteins is present in normal mouse adrenal glands as well as in the tumor-derived Y1 adrenal cell line, demonstrating that MRAP, MRAP2, MC2, and MC5 receptor may be expressed together under physiological conditions. Endogenous agonists and antagonists regulate melanocortin receptors physiologically. Such dual regulation is very rare, even in the large and diverse GPCR family. Another unusual feature of the melanocortin system is the ability of different peptides derived from a common precursor, pro-opiomelanocortin, to activate the five receptors with varying degrees of selectivity. The work described here shows that an increase in MRAP expression will favor responsiveness to ACTH and decrease responsiveness to melanocyte stimulating hormone in any tissue expressing MC2 and MC5 receptors, whereas a fall in MRAP will shift responses in the opposite direction. In this way the accessory proteins MRAP and MRAP2 add a new dimension to the melanocortin signaling systems.
This work was supported, in whole or in part, by National Institutes of Health Grant DK19974 (to P. M. H.).
- MC
- melanocortin
- ACTH
- adrenocorticotropic hormone
- ER
- endoplasmic reticulum
- GPCR
- G protein-coupled receptor
- MRAP
- MC2-receptor accessory protein
- RAMP
- receptor activity-modifying protein
- YFP
- yellow fluorescent protein
- HA
- hamagglutinin
- CHO
- Chinese hamster ovary
- RT
- reverse transcription
- ELISA
- enzyme-linked immunosorbent assay
- td
- tandem.
REFERENCES
- 1.Abdel-Malek Z. A. (2001) Cell Mol. Life Sci. 58,434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metherell L. A., Chapple J. P., Cooray S., David A., Becker C., Rüschendorf F., Naville D., Begeot M., Khoo B., Nürnberg P., Huebner A., Cheetham M. E., Clark A. J. (2005) Nat. Genet. 37,166–170 [DOI] [PubMed] [Google Scholar]
- 3.Sebag J. A., Hinkle P. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104,20244–20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sebag J. A., Hinkle P. M. (2009) J. Biol. Chem. 284,610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb T. R., Chan L., Cooray S. N., Cheetham M. E., Chapple J. P., Clark A. J. (2009) Endocrinology 150,720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan L. F., Webb T. R., Chung T. T., Meimaridou E., Cooray S. N., Guasti L., Chapple J. P., Egertová M., Elphick M. R., Cheetham M. E., Metherell L. A., Clark A. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106,6146–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron A., Veo K., Angleson J., Dores R. M. (2009) Gen. Comp. Endocrinol. 161,13–19 [DOI] [PubMed] [Google Scholar]
- 8.Chen W., Kelly M. A., Opitz-Araya X., Thomas R. E., Low M. J., Cone R. D. (1997) Cell 91,789–798 [DOI] [PubMed] [Google Scholar]
- 9.Morgan C., Cone R. D. (2006) Behav. Genet 36,291–300 [DOI] [PubMed] [Google Scholar]
- 10.Boston B. A. (1999) Ann. N.Y. Acad. Sci. 885,75–84 [DOI] [PubMed] [Google Scholar]
- 11.Boston B. A., Cone R. D. (1996) Endocrinology 137,2043–2050 [DOI] [PubMed] [Google Scholar]
- 12.Nimura M., Udagawa J., Hatta T., Hashimoto R., Otani H. (2006) Anat. Embryol. (Berl.) 211,109–117 [DOI] [PubMed] [Google Scholar]
- 13.Hynes T. R., Tang L., Mervine S. M., Sabo J. L., Yost E. A., Devreotes P. N., Berlot C. H. (2004) J. Biol. Chem. 279,30279–30286 [DOI] [PubMed] [Google Scholar]
- 14.Grieco P., Balse-Srinivasan P., Han G., Weinberg D., MacNeil T., Van der Ploeg L. H., Hruby V. J. (2002) J. Pept. Res. 59,203–210 [DOI] [PubMed] [Google Scholar]
- 15.Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004) Nat. Biotechnol. 22,1567–1572 [DOI] [PubMed] [Google Scholar]
- 16.Kerppola T. K. (2006) Nat. Rev. Mol. Cell Biol. 7,449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickolls S. A., Maki R. A. (2006) Peptides 27,380–387 [DOI] [PubMed] [Google Scholar]
- 18.Zanna P. T., Sánchez-Laorden B. L., Pérez-Oliva A. B., Turpín M. C., Herraiz C., Jiménez-Cervantes C., García-Borrón J. C. (2008) Biochem. Biophys. Res. Commun. 368,211–216 [DOI] [PubMed] [Google Scholar]
- 19.Mandrika I., Petrovska R., Wikberg J. (2005) Biochem. Biophys. Res. Commun. 326,349–354 [DOI] [PubMed] [Google Scholar]
- 20.Chen M., Cai M., Aprahamian C. J., Georgeson K. E., Hruby V., Harmon C. M., Yang Y. (2007) J. Biol. Chem. 282,21712–21719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M., Cai M., McPherson D., Hruby V., Harmon C. M., Yang Y. (2009) Biochem. Pharmacol. 77,114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulenger S., Marullo S., Bouvier M. (2005) Trends Pharmacol. Sci. 26,131–137 [DOI] [PubMed] [Google Scholar]
- 23.Terrillon S., Bouvier M. (2004) EMBO Rep. 5,30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Laorden B. L., Sánchez-Más J., Martínez-Alonso E., Martínez-Menárguez J. A., García-Borrón J. C., Jiménez-Cervantes C. (2006) J. Invest. Dermatol. 126,172–181 [DOI] [PubMed] [Google Scholar]
- 25.Décaillot F. M., Rozenfeld R., Gupta A., Devi L. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105,16045–16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behrens M., Bartelt J., Reichling C., Winnig M., Kuhn C., Meyerhof W. (2006) J. Biol. Chem. 281,20650–20659 [DOI] [PubMed] [Google Scholar]
- 27.Saito H., Kubota M., Roberts R. W., Chi Q., Matsunami H. (2004) Cell 119,679–691 [DOI] [PubMed] [Google Scholar]