Abstract
A central question in Wnt signaling is the regulation of β-catenin phosphorylation and degradation. Multiple kinases, including CKIα and GSK3, are involved in β-catenin phosphorylation. Protein phosphatases such as PP2A and PP1 have been implicated in the regulation of β-catenin. However, which phosphatase dephosphorylates β-catenin in vivo and how the specificity of β-catenin dephosphorylation is regulated are not clear. In this study, we show that PP2A regulates β-catenin phosphorylation and degradation in vivo. We demonstrate that PP2A is required for Wnt/β-catenin signaling in Drosophila. Moreover, we have identified PR55α as the regulatory subunit of PP2A that controls β-catenin phosphorylation and degradation. PR55α, but not the catalytic subunit, PP2Ac, directly interacts with β-catenin. RNA interference knockdown of PR55α elevates β-catenin phosphorylation and decreases Wnt signaling, whereas overexpressing PR55α enhances Wnt signaling. Taken together, our results suggest that PR55α specifically regulates PP2A-mediated β-catenin dephosphorylation and plays an essential role in Wnt signaling.
Wnt/β-catenin signaling plays essential roles in development and tumorigenesis (1–3). Our previous work found that β-catenin is sequentially phosphorylated by CKIα4 and GSK3 (4), which creates a binding site for β-Trcp (5), leading to degradation via the ubiquitination/proteasome machinery (3). Mutations in β-catenin or APC genes that prevent β-catenin phosphorylation or ubiquitination/degradation lead ultimately to cancer (1, 2).
In addition to the involvement of kinases, protein phosphatases, such as PP1, PP2A, and PP2C, are also implicated in Wnt/β-catenin regulation. PP2C and PP1 may regulate dephosphorylation of Axin and play positive roles in Wnt signaling (6, 7). PP2A is a multisubunit enzyme (8–10); it has been reported to play either positive or negative roles in Wnt signaling likely by targeting different components (11–21). Toward the goal of understanding the mechanism of β-catenin phosphorylation, we carried out siRNA screening targeting several major phosphatases, in which we found that PP2A dephosphorylates β-catenin. This is consistent with a recent study where PP2A is shown to dephosphorylate β-catenin in a cell-free system (18).
PP2A consists of a catalytic subunit (PP2Ac), a structure subunit (PR65/A), and variable regulatory B subunits (PR/B, PR/B′, PR/B″, or PR/B‴). The substrate specificity of PP2A is thought to be determined by its B subunit (9). By siRNA screening, we further identified that PR55α, a regulatory subunit of PP2A, specifically regulates β-catenin phosphorylation and degradation. Mechanistically, we found that PR55α directly interacts with β-catenin and regulates PP2A-mediated β-catenin dephosphorylation in Wnt signaling.
EXPERIMENTAL PROCEDURES
Plasmids
Myc-tagged PR55α was cloned into CS2+MT vector. FLAG-tagged PP2Ac was cloned into pRK5 vector. β-Catenin and Axin constructs have been described previously (4). PR55α shRNA constructs were purchased from Sigma. The sequences of shRNA oligos targeting the PR55 gene are: CCGGAGAAACACAAAGCGAGACATACTCGAGTATGTCTCGCTTTGTGTTTCTTTTTT (TRCN0000002489), CCGGGATCCCAGTAACAGGTCATTTCTCGAGAAATGACCTGTTACTGGGATCTTTTT (TRCN0000002490), CCGGTCCTGCTTAGTTGAGATAGTTCTCGAGAACTATCTCAACTAAGCAGGATTTTT (TRCN0000002491), CCGGGTAGATGATGATGTAGCAGAACTCGAGTTCTGCTACATCATCATCTACTTTTT (TRCN0000002492), and CCGGGCAAGTGGCAAGCGAAAGAAACTCGAGTTTCTTTCGCTTGCCACTTGCTTTTT (TRCN0000002493). Clone TRCN0000002493 was used in this study (supplemental Fig. S3). The control shRNA sequence is: CCTAAGGTTAAGTCGCCCTCGCTCTAGCGAGGGCGACTTAACCTTAGG.
siRNA
siRNAs for PP1, PP5, and different subunits of PP2A were purchased from Qiagen (supplemental Fig. S1). The oligonucleotide sequences of these siRNAs are: AACATCGACAGCATTATCCAA (si02225776 for PP1), ATGGAACTTGACGATACTCTA (si02225783 for PP2Ac), CTCGTGGAAACCACACTCAAA (si02225853 for PP5), CTGCAGATGATTTGCGGATTA (si02225825 for PR55α), CAGCGTATTCTGATATAGTAA (si02225846 for PR61α), GAAGTTGTTTATGGAAATGAA (si02653812 for PR61δ), and CCGGTGATATTGACAATAGGA (si02659048 for PR61ϵ). Negative control siRNA was also purchased from Qiagen (1027281). siRNAs were transfected into HEK293T or SW480 cells at a final concentration of 10 nm, using Lipofectamine 2000. Cell lysates were analyzed after 3 days.
Cell Lines
HEK293T and SW480 cells were maintained in Dulbecco's modified Eagle's medium (Hyclone) containing 10% fetal bovine serum (Hyclone), 1% penicillin/streptomycin, and 1% glutamine at 37 °C in 5% CO2. HEK293 cells were cotransfected with shRNA plasmid, psPAX2 packaging plasmid, and pMD2.G envelope plasmid. Virus-containing media was harvested 48 h after transfection, followed by infection of target HEK293T and SW480 cells. Stabled cells were selected with 2 μg/ml puromycin 48 h after infection. The stabled cells from a mixed population were used in the experiments.
Immunoprecipitation, Western Blot, and Immunostaining
Cells were lysed in 1% Triton buffer or hypotonic buffer as previously described (22). Immunoprecipitation and Western blot were performed as previously described (4, 23). PP2Ac Ab (2038), PR55α Ab (2290), and phospho-β-catenin Abs (9561, 9564, 9566, and 9567) were purchased from Cell Signaling Technology. Total β-catenin Ab was purchased from Sigma (c2206). Immunostaining of mammalian cells was performed as described previously (23).
Reporter Assay
Super8xTOPFlash reporter was kindly provided by Dr. Randall Moon, University of Washington. Super8xTOPFlash reporter and Renilla reporter were cotransfected into HEK293T cells using the calcium transfection method. Luciferase and Renilla activities were analyzed with the Dual-Luciferase® Reporter Assay Kit from Promega.
In Vitro Kinase and in Vitro Phosphatase Assays
Purified GST-β-catenin was phosphorylated by purified GSK3 or CKIα in 1× kinase buffer plus 0.2 mm ATP at 30 °C for 30 min. Phosphorylated β-catenin can also be immunoprecipitated from HCT116 or SW480 cell extracts. Phosphorylated β-catenin was incubated with phosphatase buffer only, or purified PP2A, PP1, or PP5 for 30 min at 37 °C. β-Catenin phosphorylation was analyzed by Western blot with phospho-specific Abs. Purified CKIα and GSK3 have been described (4). PP1 and PP2A were from New England Biolabs (P0754S) and Millipore (14-111), respectively. Recombinant human GST-PP5 was purified from bacteria. PP2A holoenzyme containing PR55α was purified from HEK293T cells as described below.
Protein Purification
GST fusion proteins have been described in our previous study (4). Histidine-tagged PP2Ac and PR55α were cloned in pET-28 vector (Novagen) and transformed into BL21(DE3). A single colony was picked up and cultured at 37 °C. Protein expression was induced by 0.4 mm isopropyl 1-thio-β-d-galactopyranoside. Bacteria was pelleted by a microcentrifuge and resuspended in lysis buffer (50 mm NaH2PO4, 300 mm NaCl, 10 mm imidazole, 0.1% Triton 100, pH 8.0) and sonicated on ice for 5× 15 s. Cleared lysate was incubated with 100 ml of nickel-nitrilotriacetic acid beads (Qiagen 30210) for 1 h at 4 °C. Beads were washed with 3× 1 ml of wash buffer (50 mm NaH2PO4, 300 mm NaCl, 20 mm imidazole, 0.1% Triton 100, pH 8.0). Recombinant protein was eluted by 100 μl of elution buffer (50 mm NaH2PO4, 300 mm NaCl, 250 mm imidazole, 0.1% of Triton 100, pH 8.0). Protein concentration was determined by a Bio-Rad protein assay kit (500-0006).
PP2A holoenzyme containing PR55α was purified according to the method described by Adams and Wadzinski (24). FLAG-tagged PR55α was transcribed into HEK293T cells. PP2A holoenzyme was immunoprecipitated with FLAG-conjugated beads and eluted by FLAG peptides.
Drosophila Stocks and Immunostaining of Wing Discs
MS1096, act>CD2>gal4, ptc-gal4, UAS-DN-mts, UAS-mtsRNAi, UAS-mts has been described (25, 26). Standard protocols for immunofluorescence staining were used. Wing imaginal discs from late third instar larvae were immunostained with mouse anti-Arm (Developmental Studies Hybridoma Bank), rabbit anti-Dll (Dr. Sean Carroll), and goat anti-Sen (Dr. Hugo Bellen) Abs.
RESULTS
PP2A Regulates β-Catenin Dephosphorylation
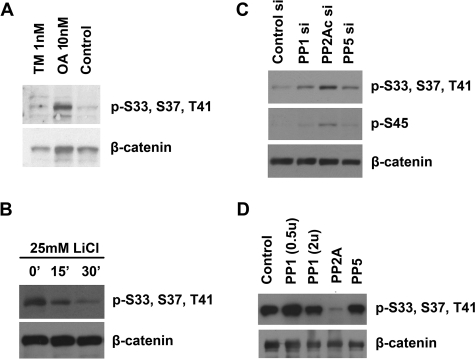
Our previous study (4) showed that the N terminus of β-catenin is phosphorylated by CKIα and GSK3. We further showed that Wnt inhibits GSK3 activity, resulting in a dramatic decrease of β-catenin phosphorylation and increase of stabilization (4). Because protein kinases and phosphatases often have opposing actions, to investigate whether phosphatases are involved in β-catenin dephosphorylation, and if so which phosphatases are responsible, we treated HEK293T cells with 1 nm tautomycin and 10 nm okadaic acid (OA), which preferentially inhibits PP1 and PP2A, respectively. We found that the abundance of phosphorylated β-catenin is elevated by OA but not tautomycin, suggesting that PP2A may regulate β-catenin phosphorylation (Fig. 1A, top panel).
FIGURE 1.
Screening of phosphatases that regulate β-catenin phosphorylation. A, HEK293T cells were treated with 1 nm tautomycine (TM), 10 nm okadaic acid (OA), and dimethyl sulfoxide (Control), respectively. Cytoplasmic fractions were isolated and analyzed by Western blot using Abs that recognize total β-catenin or GSK3-phosphorylated β-catenin (p-S33, S37, T41). B, SW480 cells were treated with 25 mm LiCl for 0, 15, and 30 min. Cell lysates were analyzed by Western blot using Abs that recognize total β-catenin or phosphorylated β-catenin. C, SW480 cell were transfected with control siRNA and siRNAs for the PP1, PP5, and the catalytic subunit of PP2A (PP2Ac). Cell lysates were analyzed by Western blot using Abs that recognize total β-catenin, CKIα-phosphorylated β-catenin (p-S45), or GSK3-phosphorylated β-catenin. D, phosphorylated β-catenin was immunoprecipitated from HCT116 cells. Equal amounts of β-catenin were incubated with purified PP1 (0.5 and 2 units), PP2A (0.5 unit), and PP5 for 30 min. Phosphatase buffer alone was used as a control. β-Catenin dephosphorylation was analyzed by Western blot using Abs that recognize total β-catenin or phosphorylated β-catenin.
To determine which phosphatase is responsible for β-catenin dephosphorylation, we decided to use siRNA to knockdown each of these phosphatases. However, phosphorylated β-catenin is rapidly degraded in HEK293T cells. Thus, it is difficult to analyze β-catenin phosphorylation in these cells. Recently, we noted that β-catenin degradation but not phosphorylation is inhibited in the colon cancer cell line SW480 (22). When SW480 cells were treated with LiCl, which inhibits GSK3 activity, the levels of phosphorylated β-catenin were significantly decreased (Fig. 1B, top panel). Because β-catenin degradation is blocked in SW480 cells, we reasoned that the decrease in β-catenin phosphorylation is caused by phosphatase activity but not protein turnover. SW480 cells provide a good model to analyze β-catenin phosphorylation without the interference of β-catenin degradation.
To identify the phosphatase that specifically dephosphorylates β-catenin, we performed siRNA screening in SW480 cells. PP1, PP5, and the catalytic subunit of PP2A (PP2Ac) were knocked down by siRNAs (supplemental Fig. S1, A–C). The levels of both CKIα- and GSK3-phosphorylated β-catenin were significantly increased in the PP2Ac-depleted cells (Fig. 1C, top and middle panels). Total levels of β-catenin were not changed in SW480 cells (Fig. 1C, bottom panel). These data confirmed that PP2A is required for β-catenin dephosphorylation. To determine whether PP2A can directly dephosphorylate β-catenin, phosphorylated β-catenin was immunoprecipitated from HCT116 cells and incubated with purified PP1, PP5, and PP2A holoenzyme. PP2A efficiently dephosphorylated β-catenin within 30 min (Fig. 1D). PP2A can dephosphorylate in vitro phosphorylated GST-β-catenin as well (not shown).
PP2A Is Essential for Wingless Signaling by Regulating Armadillo Levels in Drosophila
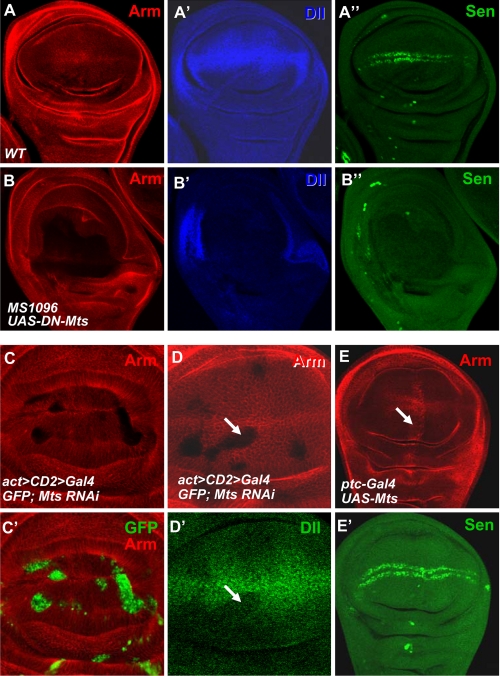
Our results and the results from a phosphatase reconstitution study (18) suggest that PP2A could dephosphorylate and stabilize β-catenin, thus playing positive roles in Wnt signaling. However, the in vivo functions of PP2A in Wnt signaling remain elusive likely due to multiple roles of PP2A in the Wnt signaling cascade. To define PP2A functions in vivo, we turned to the Drosophila wing development where Wingless (Wg), a member of the Wnt gene family, exerts a highly conserved biological influence through the canonical Wnt/β-catenin pathway. We examined the levels of β-catenin homolog Armadillo (Arm) and the expression of Wg target genes in Drosophila. We found that expressing dominant-negative PP2Ac, DN-Mts in Drosophila, by the wing-specific MS1096 gal4, caused significant reduction of Arm levels (Fig. 2B, compared with wild-type Arm staining in Fig. 2A) and severe suppression of Wg target genes such as distalless (dll) and senseless (sen) (compare Fig. 2, B′, B″ with A′, A″). Consistently, we found that mts RNAi blocked Arm accumulation (Fig. 2C), thus the attenuated Dll expression (Fig. 2D′) in Drosophila wing discs. We also performed a gain-of-function study by using the ptc-gal4 that drives gene expression in a stripe anteriorally to the anterior/posterior boundary of the wing disc (26). We found that expressing UAS-mts by ptc-gal4 elevated Arm levels (Fig. 2E).
FIGURE 2.
PP2A is essential for Drosophila Wg signaling. A, A′, and A″, a wild-type wing disc was stained with anti-Arm, Dll, and Sen. B, B′, and B″, UAS-DN-mts was expressed by MS1096 gal4 and immunostained for Arm, Dll, and Sen. C and C′, UAS-MtsRNAi was expressed by act>CD2>gal4 and larvae were grown at 20 °C, limiting the strength of mts RNAi that could cause cell lethality. Mts knockdown cells were marked by green fluorescent protein expression. D and D′, a wing disc expressing UAS-mtsRNAi was immunostained for Arm and Dll. Arrow in D indicates the destabilization of Arm and the arrow in D′ indicates the down-regulated Dll expression by mts RNAi. E and E′, a wing disc expressing UAS-mts by ptc-gal4 was immunostained with anti-Arm and Sen. Arrow in E indicates the elevation of Arm by the overexpression of Mts.
Identification of PR55α as the Regulatory B Subunit That Controls β-Catenin Phosphorylation
PP2A holoenzyme comprises a catalytic C subunit, a scaffolding A subunit, and a regulatory B subunit, which controls substrate specificity (8–10). There are more than 20 regulatory B subunits in the human genome (9). We hypothesize that PP2A plays different roles and regulates multiple steps in the Wnt pathway through distinct regulatory B subunits. Our in vitro and in vivo studies suggest that PP2A regulates β-catenin phosphorylation. The regulatory B subunit that controls β-catenin dephosphorylation remains to be identified.
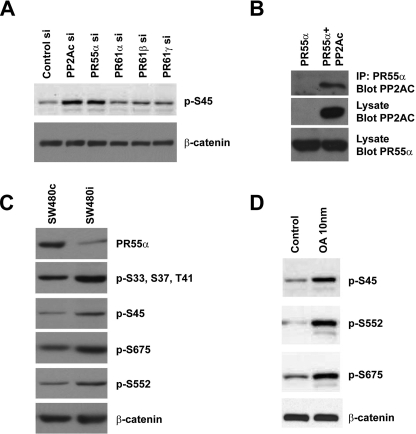
We performed another siRNA screening in SW480 cells using siRNAs targeting individual regulatory subunits of PP2A (supplemental Fig. S1, D–G). As a control, PP2Ac siRNA increased the levels of phosphorylated β-catenin (compare lane 2 with lane 1, Fig. 3A, top panel). In addition, we found that depletion of PR55α (also called B55α) significantly increased the levels of phosphorylated β-catenin (Fig. 3A). The levels of phosphorylated β-catenin were not increased by siRNAs targeting other PP2A regulatory subunits, such as PR61α (B56α), PR61δ (B56δ), and PR61ϵ (B56ϵ), although these subunits were implicated in Wnt signaling as well (15, 17, 20).
FIGURE 3.
Identification of PR55α as the regulatory B subunit that controls β-catenin phosphorylation. A, SW480 cells were transfected with siRNAs targeting several different regulatory subunits of PP2A. Negative control siRNA and PP2Ac siRNA were used as controls. Total β-catenin and phosphorylated β-catenin were analyzed by Western blot with anti-Ser(P)-45 and anti-β-catenin Abs. B, interaction between PR55α and PP2Ac. Myc-tagged PR55α was cotransfected with CS2 control plasmid or FLAG-tagged PP2Ac into HEK293T cells. PR55α protein was immunoprecipitated from cell lysates with an anti-Myc Ab. The presence of PP2Ac in the immunoprecipitated (IP) samples was analyzed by Western blot with an anti-FLAG Ab. The levels of PP2Ac and PR55α in the cell lysates were analyzed as control. C, SW480 cells were infected with lentiviruses that express control shRNA or PR55α shRNA. Stable cells were selected with puromycin. PR55α protein levels in these cells were analyzed by Western blot with an anti-PR55α Ab. Total β-catenin and phosphorylated β-catenin were analyzed with an anti-β-catenin Ab and phospho-specific Abs that recognize different phosphorylation sites of β-catenin. D, SW480 cells were treated with dimethyl sulfoxide or 10 nm OA. Total β-catenin and phosphorylated β-catenin were analyzed with an anti-β-catenin Ab and phospho-specific Abs against β-catenin.
PR55α comprises a seven-bladed β-propeller, or WD40 repeats (27). Each blade comprises four antiparallel β-strands. These domains are involved in protein-protein interactions. To analyze the binding between PR55α and PP2Ac, Myc-tagged PR55 and FLAG-tagged PP2Ac were co-transfected into HEK293T cells. As expected, PR55α forms a complex with PP2Ac (Fig. 3B). To confirm the siRNA results and further analyze the PR55α function, we generated stable SW480 cells that express PR55α shRNA (Fig. 3C and supplemental Fig. S3). PR55α was efficiently knocked down in these cells compared with the SW480 cells with control shRNA (Fig. 3C, top panel). Consistent with the siRNA result in Fig. 3A, the levels of both CKIα and GSK3-phosphorylated β-catenin were increased in SW480 cells expressing PR55α shRNA (Fig. 3C, second and third panels), with the total amount of β-catenin unchanged (Fig. 3C, bottom panel).
Although CKIα and GSK3 phosphorylate the N terminus of β-catenin and control β-catenin degradation, other kinases can also phosphorylate β-catenin. It has been shown that AKT and PKA phosphorylated β-catenin at Ser-552 and Ser-675, which regulate the activation and nuclear translocation of β-catenin (28–30). When SW480 cells were treated with 10 nm OA, the levels of Ser-552 and Ser-675-phosphorylated β-catenin were also increased (Fig. 3D, second and third panels), suggesting that these sites are also regulated by protein phosphatase. Interestingly, we found that depletion of PR55α in SW480 cells increased the abundance of β-catenin with Ser-552 and Ser-675 phosphorylation (Fig. 3C, fourth and fifth panels), suggesting that PP2A/PR55α may affect the phosphorylation of Ser-552 and Ser-675 thus the activation and nuclear translocation of β-catenin.
PR55α Interacts with β-Catenin in Mammalian Cells
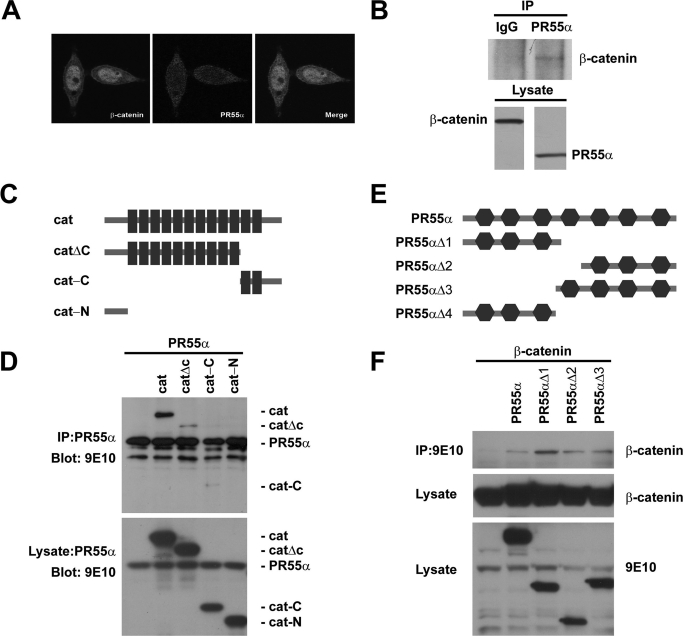
We analyzed the localization of PR55α in SW480 cells. PR55α was localized in both the cytoplasm and nucleus (Fig. 4A). As a control, β-catenin localized on the membrane and in the cytoplasm and significantly accumulated in the nucleus (Fig. 4A). To test if PR55α interacts with β-catenin, endogenous PR55α protein was immunoprecipitated from SW480 cells, and the presence of β-catenin was analyzed by Western blot. We found that β-catenin indeed binds PR55α (Fig. 4B). Although several phosphatases and their subunits are involved in Wnt signaling, PR55α is the first phosphatase subunit found to bind β-catenin. To analyze which domains of β-catenin bind PR55α, we generated several β-catenin deletions (Fig. 4C). Full-length β-catenin strongly bound PR55α (Fig. 4D). The C terminus containing armadillo repeats 11–12, or the N terminus containing armadillo repeats 1–10, bound PR55α weakly, whereas the N terminus with no armadillo repeat did not bind PR55α, suggesting that PR55α may bind multiple armadillo repeats of β-catenin (Fig. 4D).
FIGURE 4.
PR55α interacts with β-catenin. A, localization of PR55α in SW480 cells. SW480 cells were stained with Abs against β-catenin (green) and PR55α (red). B, endogenous PR55α binds endogenous β-catenin. PR55α was immunoprecipitated (IP) with an anti-PR55α Ab and β-catenin was analyzed by Western blot with an anti-β-catenin Ab. The endogenous PR55α and β-catenin in the cell lysates were analyzed as control. C, schematic diagram of β-catenin deletion constructs. The black boxes are armadillo repeats. D, armadillo domains of β-catenin bind PR55α. HEK293T cells were cotransfected with Myc-tagged PR55α and Myc-tagged β-catenin or its mutants. PR55α was immunoprecipitated with an anti-PR55α Ab. β-Catenin proteins were analyzed by Western blot with an anti-Myc Ab. The expression of these proteins was analyzed by Western blot with an anti-Myc Ab. E, schematic diagram of PR55α deletion constructs. The hexagons are WD40 repeats. F, β-catenin interacts with multiple domains of PR55α. HEK293T cells were cotransfected with FLAG-tagged β-catenin and Myc-tagged PR55α or its mutants. PR55α proteins were immunoprecipitated with 9E10-conjugated beads and β-catenin was analyzed by Western blot with an anti-FLAG Ab. The levels of PR55α and β-catenin proteins in the cell lysates were analyzed as control.
PR55α has 7 WD40 repeats. To test which WD40 repeats bind β-catenin, we generated a panel of PR55α deletions (Fig. 4E). Using the immunoprecipitation assay, we found that β-catenin binds multiple domains of PR55α (Fig. 4F). This is consistent with the result that multiple armadillo repeats of β-catenin bind PR55α (Fig. 4D).
PR55α Directly Binds Both β-Catenin and Axin
It has been reported that PP2Ac interacts with Axin (14). To test if PR55α binds Axin as well, Myc-tagged PR55α and FLAG-tagged Axin or β-catenin were cotransfected into HEK293T cells. Using an immunoprecipitation assay, we found that PR55α not only interacts with β-catenin, but also strongly interacts with Axin (Fig. 5A, lane 2, top panel). Our data indicate that PR55α falls into the Axin-β-catenin protein complex and further suggests that PR55α acts as the PP2A regulatory subunit in regulating β-catenin phosphorylation.
FIGURE 5.
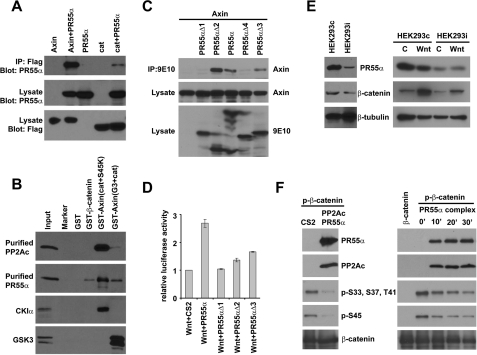
PR55α directly interacts with β-catenin and Axin and regulates PP2A-mediated β-catenin dephosphorylation. A, PR55α interacts with both Axin and β-catenin. HEK293T cells were cotransfected with Myc-tagged PR55α and FLAG-tagged Axin, or FLAG-tagged β-catenin. Axin and β-catenin were immunoprecipitated from the cell lysates using an anti-FLAG Ab. The presence of PR55α in the immunoprecipitated samples were analyzed with an anti-Myc Ab. The protein levels of Axin and β-catenin were analyzed with an anti-FLAG Ab. B, PR55α directly interacts with β-catenin and Axin. Purified histidine-tagged PP2Ac and PR55α were incubated with purified GST, GST-β-catenin, GST-Axin(Cat+S45K), and GST-Axin(G3+Cat), respectively. These GST-Axin fusion proteins have been described previously (4). GST fusion proteins were pulled down with glutathione-agarose beads. PP2Ac and PR55α were analyzed with anti-PP2Ac and anti-PR55α Abs. The binding between these GST proteins and CKIα and GSK3 were analyzed as controls. C, the C-terminal region of PR55α interacts with Axin. HEK293T cells were cotransfected with FLAG-tagged Axin and Myc-tagged PR55α or its mutants. PR55α proteins were immunoprecipitated with 9E10-conjugated beads and Axin was analyzed by Western blot with an anti-FLAG Ab. The levels of PR55α and Axin proteins in the cell lysates were analyzed as control. D, PR55α enhances Wnt signaling. Super8XTOPFlash was cotransfected with Wnt3A plus CS2 or Wnt3A plus PR55α constructs into HEK293T cells. Luciferase activity was analyzed and normalized. E, PR55α regulates β-catenin stability. Left panel, HEK293T cells were infected with lentiviruses that express control shRNA or PR55α shRNA. Stable cells were selected with puromycin. Cytoplasmic PR55α and β-catenin protein levels were analyzed by Western blot with anti-PR55α and anti-β-catenin Abs. Right panel, the HEK293T cell line contains PR55α shRNA (HEK293i) and the control HEK293T cell line were treated with control conditioned medium or Wnt3A-conditioned medium for 6 h. Cytoplasmic fractions were isolated from these cells. β-Catenin and PR55α levels were analyzed by Western blot using anti-β-catenin and anti-PR55α Ab. β-Tubulin was analyzed as a loading control. F, PP2A holoenzyme containing PR55α directly dephosphorylates β-catenin. GST-β-catenin was phosphorylated by CKI and GSK3 in vitro. Left panel, Myc-tagged PR55α and FLAG-tagged PP2Ac were cotransfected into HEK293T cells. HEK293T cells transfected with empty vector were used as control. The PR55α-PP2Ac complex was immunoprecipitated with 9E10-conjugated beads. The beads were resuspended in 1× phosphatase buffer and incubated with phosphorylated GST-β-catenin for 30 min. PR55α, PP2Ac, total β-catenin, and phosphorylated β-catenin were analyzed by Western blot. Right panel, PP2A holoenzyme containing FLAG-tagged PR55α was purified from HEK293T cells and incubated with phosphorylated β-catenin for 0, 10, 20, and 30 min. PR55α and the catalytic subunit of PP2A (PP2Ac) were analyzed by Western blot with anti-PR55α and anti-PP2Ac Abs. β-Catenin was analyzed by Western blot with Abs against phosphorylated β-catenin or total β-catenin.
Because β-catenin directly binds Axin, PR55α could bind β-catenin indirectly through Axin. To test this possibility, we purified histidine-tagged PP2Ac and PR55α from Escherichia coli BL21(DE3). These proteins were incubated with purified GST, GST-β-catenin, GST-Axin(Cat+S45K), and GST-Axin(G3+Cat) (supplemental Fig. S4). GST-Axin(Cat+S45K) interacted with CKIα and GST-Axin(G3+Cat) interacted with GSK3 (Fig. 5B, bottom two panels), as we reported previously (4). PP2Ac bound GST-Axin(Cat+S45K) (Fig. 5B, top panel), as previously reported (14). In this study, we found that PR55α binds not only GST-Axin(Cat+S45K) but also GST-Axin(G3+Cat) and GST-β-catenin (Fig. 5B, second panel, lane 4). Neither PP2Ac nor PR55α binds GST protein. These results suggest that PP2A directly binds Axin, whereas PR55α directly binds both Axin and β-catenin. PP2A and PR55α may bind distinct domains of Axin. The binding between Axin(Cat) and PR55α was tested (supplemental Fig. S5). We found that the Axin(Cat) fragment is not sufficient for PR55α binding.
To test which WD40 repeats of PR55α bind Axin, we analyzed the binding between Axin and PR55α mutants (Fig. 4C). Using the immunoprecipitation assay, we found that unlike β-catenin, Axin only binds the PR55α fragments containing the last 3–4 WD40 repeats (Fig. 5C). To understand the function of PR55α in Wnt signaling, we performed a reporter assay in HEK293T cells using Super8xTOPFlash. PR55α alone has no significant effect on the reporter activity (not shown). However, when PR55α was cotransfected with Wnt3A, it synergized with Wnt3A and significantly increased the reporter activity (Fig. 5D). PR55α mutants PR55αΔ2 and PR55αΔ3 have less effect, whereas PR55αΔ1 has no effect on the reporter activity; this is consistent with their binding affinities to β-catenin and Axin. These results further suggest that PR55α plays a positive role in Wnt signaling.
PP2A/PR55α Regulates β-Catenin Degradation by Directly Dephosphorylating β-Catenin
To determine the function of endogenous PR55α on β-catenin degradation, we generated a stable HEK293T cell line that expresses PR55α shRNA. Cytoplasmic fractions were isolated from this cell line (HEK293i) and the control cell line (HEK293c). PR55α protein levels were significantly reduced by shRNA (Fig. 5E, left, top panel). PR55α depletion resulted in β-catenin degradation (Fig. 5E, left, middle panel). As a loading control, β-tubulin levels were not affected by PR55α shRNA (Fig. 5E, left, bottom panel). It is worth noting that PP2Ac depletion has similar results (14), suggesting that PR55α and PP2Ac work together in β-catenin regulation. These cells were treated with either control medium or Wnt3A-conditioned medium. Besides the above observations, we found that Wnt3A treatment increased the cytoplasmic levels of β-catenin more efficiently in HEK293c cells than that of the HEK293i cells (Fig. 5E, right, middle panel, lane 2, compared with lane 4), suggesting that PR55α enhances Wnt-regulated β-catenin stabilization by regulating β-catenin dephosphorylation.
As described above (Fig. 5, A and B), both PR55α and PP2Ac bind Axin. Because Axin regulates β-catenin phosphorylation, it is also possible that PR55α indirectly regulates β-catenin phosphorylation by regulating Axin. We have shown that β-catenin can be dephosphorylated by a commercial PP2A holoenzyme (Fig. 1D), but it is not clear which regulatory B subunit is presented. To test whether PR55α directly regulates β-catenin dephosphorylation, Myc-tagged PR55α and FLAG-tagged PP2Ac were cotransfected into HEK293T cells. The PR55α-PP2A complex was immunoprecipitated with FLAG-conjugated beads and incubated with phosphorylated β-catenin (Fig. 5F, left panel). Both CKIα and GSK3 phosphorylations of β-catenin were decreased upon incubation with PR55α/PP2A, whereas total β-catenin levels remain unchanged (Fig. 5F, left panel), suggesting that the PR55α-PP2Ac complex can dephosphorylate β-catenin. To test if PR55α can recruit endogenous PP2A complex for β-catenin dephosphorylation, we purified PP2A holoenzyme containing PR55α with FLAG-conjugated beads, and the holoenzyme was eluted from the beads with FLAG peptides. We demonstrated that the purified PP2A holoenzyme contains both PR55α and endogenous PP2Ac, and can directly dephosphorylate β-catenin in vitro (Fig. 5F, right panel).
DISCUSSION
β-Catenin phosphorylation by protein kinases has been well studied (31). How phosphatase regulates β-catenin is less understood. PP2A has been shown to dephosphorylate β-catenin in vitro (18). However, PP2A has been suggested to play both positive and negative roles in Wnt signaling. Our study demonstrated that PP2A regulates β-catenin phosphorylation both in vitro and in vivo. The specificity of PP2A in Wnt signaling is regulated by the regulatory B subunit. We demonstrated that PR55α directly interacts with β-catenin and regulates PP2A-mediated β-catenin dephosphorylation.
PP2A Regulates β-Catenin Dephosphorylation
The PP2A inhibitor, OA, increased the levels of phosphorylated β-catenin in HEK293T cells (Fig. 1A). However, the levels of β-catenin are elevated rather than attenuated by OA treatment (Fig. 1A, bottom panel). This is probably because OA regulates multiple phosphatases that play different roles in β-catenin phosphorylation and degradation. For example, OA also inhibits PP1 and PP5 when used at higher concentrations. Another possibility is that PP2A may regulate the degradation of phosphorylated β-catenin (32).
Because β-catenin degradation but not phosphorylation is inhibited in colon cancer cell line SW480, the SW480 cell line provides a better model to study β-catenin phosphorylation. The siRNA experiments in SW480 cells suggests that PP2A is essential for β-catenin dephosphorylation. The in vitro phosphatase assay further demonstrated that PP2A is both necessary and sufficient for β-catenin dephosphorylation. Within 30 min, only PP2A dephosphorylated β-catenin (Fig. 1D). However, after 2 h of incubation, PP1 can also dephosphorylate β-catenin, and PP5 weakly dephosphorylated β-catenin (supplemental Fig. S2), suggesting that PP1 and PP5 are active, but not as specific as PP2A in β-catenin dephosphorylation. In addition, siRNA results suggest that PP1 and PP5 are not required for β-catenin dephosphorylation.
PP2A Function in Wingless Signaling in Drosophila
To test PP2A function in vivo, we analyzed Arm and its target genes in Drosophila. Loss-of-function of PP2A resulted in decreased levels of Arm (Fig. 2, B and C). Overexpression of PP2A resulted in increased levels of Arm (Fig. 2E). However, under such conditions, we did not observe changes in Wg target genes expression (Fig. 2E′; not shown). It could be possible that Arm dephosphorylation by PP2A was not sufficient to activate Arm, or, PP2A has additional role(s) in regulating Arm signaling activity. Our finding that PP2A plays a positive role in Wnt signaling is consistent with a previous study (11). However, PP2A also plays negative roles in other studies probably by regulating different substrates. It is important to further determine how PP2A specificity is regulated in Wnt signaling.
PP2A Regulatory Subunit, PR55α, Regulates β-Catenin Dephosphorylation
We performed siRNA screening for the regulatory B subunits of PP2A. Our result demonstrates that PR55α is required for β-catenin dephosphorylation and further suggests that PP2A regulates different steps of Wnt signaling through distinct regulatory subunits (Fig. 3A). We found that PR55α also regulates the phosphorylation of β-catenin Ser-552 and Ser-675 (Fig. 3, C and D). This may be one of the reasons why the elevation of Arm caused by overexpressing Mts did not ectopically activate Wg target genes (Fig. 2E′, data not shown). The Ser-675 site is highly conserved among species although the function of this phosphorylation has not yet been demonstrated in Drosophila or other in vivo models.
PR55α Directly Interacts with β-Catenin and Axin
PR55α but not PP2Ac directly binds β-catenin. PR55α also binds Axin. The armadillo domains of β-catenin interact with PR55α. The WD40 repeats of PR55α may have a redundant function in β-catenin binding (Fig. 4F). In contrast, only the last 3–4 WD40 repeats of PR55α bind Axin (Fig. 5C). Besides the WD40 repeats, PR55α also contains two α helices, which are localized after the fourth and fifth SW40 repeats (27). These α helices may be involved in Axin binding.
PR55α Containing PP2A Holoenzyme Dephosphorylated β-Catenin
We purified PP2A holoenzyme with FLAG-tagged PR55α. The in vitro dephosphorylation results strongly suggest that PR55α directly regulates PP2A-mediated β-catenin dephosphorylation (Fig. 5F). It will be interesting to determine whether PR55α also regulates Axin phosphorylation and whether Axin regulates PR55α/PR2A activity in future experiments.
Our mechanistic studies of β-catenin dephosphorylation by PP2A/PR55α are fully consistent with the genetic results of the PR55α mutant in Drosophila (11). The Drosophila PR55α homolog is encoded by twins (tws). In the tws− wing disc, both Arm and its target genes were down-regulated, suggesting that Twins is required for Arm stabilization (11). We also found that overexpression of Twins increased Arm in the wing disc.5
In summary, we provide first and direct evidence that PR55α regulates the specificity of PP2A-mediated β-catenin dephosphorylation. Our work further suggests that PP2A may perform different roles in Wnt signaling by taking advantage of different regulatory B subunits. These findings are not only important for cell signaling and developmental biology, but also have important implications for cancer biology. It has been reported that PR61γ (B56γ) but not PR55α suppressed human cell transformation (33), suggesting that PP2A regulatory B subunits play distinct roles in tumorigenesis. This may be partially due to their distinct roles in Wnt signaling.
Supplementary Material
Acknowledgments
We thank Tianyan Gao, Paul Evans, Timothy Hofstra, Xi He, and Mark Evers for suggestions and helpful discussions. We are grateful to Drs. Randall Moon, Xinhua Feng, Sean Carroll, Hugo Bellen, and Amita Sehgal for valuable reagents; Vienna Drosophila RNAi Center for RNAi flies; Developmental Studies Hybridoma Bank for Arm antibody; and Hongge Jia for assistance with Mts overexpression experiment.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
H. Jia, Y. Liu, and J. Jia, unpublished results.
- CKI
- casein kinase 1
- GSK
- glycogen synthase kinase
- PP2Ac
- catalytic subunit of PP2A
- siRNA
- small interfering RNA
- shRNA
- short hairpin RNA
- HEK
- human embryonic kidney
- Ab
- antibody
- GST
- glutathione S-transferase
- OA
- okadaic acid
- Arm
- armadillo
- DN
- dominant-negative.
REFERENCES
- 1.Clevers H. (2006) Cell 127,469–480 [DOI] [PubMed] [Google Scholar]
- 2.Logan C. Y., Nusse R. (2004) Annu. Rev. Cell. Dev. Biol. 20,781–810 [DOI] [PubMed] [Google Scholar]
- 3.Moon R. T. (2005) Sci. STKE. 2005,cm1. [DOI] [PubMed] [Google Scholar]
- 4.Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. (2002) Cell 108,837–847 [DOI] [PubMed] [Google Scholar]
- 5.Liu C., Kato Y., Zhang Z., Do V. M., Yankner B. A., He X. (1999) Proc. Natl. Acad. Sci. U.S.A. 96,6273–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strovel E. T., Wu D., Sussman D. J. (2000) J. Biol. Chem. 275,2399–2403 [DOI] [PubMed] [Google Scholar]
- 7.Luo W., Peterson A., Garcia B. A., Coombs G., Kofahl B., Heinrich R., Shabanowitz J., Hunt D. F., Yost H. J., Virshup D. M. (2007) EMBO J. 26,1511–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho U. S., Xu W. (2007) Nature 445,53–57 [DOI] [PubMed] [Google Scholar]
- 9.Janssens V., Longin S., Goris J. (2008) Trends Biochem. Sci. 33,113–121 [DOI] [PubMed] [Google Scholar]
- 10.Virshup D. M. (2000) Curr. Opin. Cell. Biol. 12,180–185 [DOI] [PubMed] [Google Scholar]
- 11.Bajpai R., Makhijani K., Rao P. R., Shashidhara L. S. (2004) Development 131,1007–1016 [DOI] [PubMed] [Google Scholar]
- 12.Creyghton M. P., Roël G., Eichhorn P. J., Hijmans E. M., Maurer I., Destrée O., Bernards R. (2005) Genes Dev. 19,376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creyghton M. P., Roël G., Eichhorn P. J., Vredeveld L. C., Destrée O., Bernards R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103,5397–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu W., Zeng L., Costantini F. (1999) J. Biol. Chem. 274,3439–3445 [DOI] [PubMed] [Google Scholar]
- 15.Li X., Yost H. J., Virshup D. M., Seeling J. M. (2001) EMBO J. 20,4122–4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratcliffe M. J., Itoh K., Sokol S. Y. (2000) J. Biol. Chem. 275,35680–35683 [DOI] [PubMed] [Google Scholar]
- 17.Seeling J. M., Miller J. R., Gil R., Moon R. T., White R., Virshup D. M. (1999) Science 283,2089–2091 [DOI] [PubMed] [Google Scholar]
- 18.Su Y., Fu C., Ishikawa S., Stella A., Kojima M., Shitoh K., Schreiber E. M., Day B. W., Liu B. (2008) Mol. Cell 32,652–661 [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto H., Hinoi T., Michiue T., Fukui A., Usui H., Janssens V., Van Hoof C., Goris J., Asashima M., Kikuchi A. (2001) J. Biol. Chem. 276,26875–26882 [DOI] [PubMed] [Google Scholar]
- 20.Yang J., Wu J., Tan C., Klein P. S. (2003) Development 130,5569–5578 [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama N., Yin D., Malbon C. C. (2007) J. Mol. Signal. 2,11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J., Zhang W., Evans P. M., Chen X., He X., Liu C. (2006) J. Biol. Chem. 281,17751–17757 [DOI] [PubMed] [Google Scholar]
- 23.Zhang W., Chen X., Kato Y., Evans P. M., Yuan S., Yang J., Rychahou P. G., Yang V. W., He X., Evers B. M., Liu C. (2006) Mol. Cell. Biol. 26,2055–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams D. G., Wadzinski B. E. (2007) Methods Mol. Biol. 365,101–111 [DOI] [PubMed] [Google Scholar]
- 25.Jia H., Liu Y., Yan W., Jia J. (2009) Development 136,307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson R. L., Grenier J. K., Scott M. P. (1995) Development 121,4161–4170 [DOI] [PubMed] [Google Scholar]
- 27.Xu Y., Chen Y., Zhang P., Jeffrey P. D., Shi Y. (2008) Mol. Cell 31,873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang D., Hawke D., Zheng Y., Xia Y., Meisenhelder J., Nika H., Mills G. B., Kobayashi R., Hunter T., Lu Z. (2007) J. Biol. Chem. 282,11221–11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He X. C., Yin T., Grindley J. C., Tian Q., Sato T., Tao W. A., Dirisina R., Porter-Westpfahl K. S., Hembree M., Johnson T., Wiedemann L. M., Barrett T. A., Hood L., Wu H., Li L. (2007) Nat. Genet. 39,189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taurin S., Sandbo N., Qin Y., Browning D., Dulin N. O. (2006) J. Biol. Chem. 281,9971–9976 [DOI] [PubMed] [Google Scholar]
- 31.Huang H., He X. (2008) Curr. Opin. Cell. Biol. 20,119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing Y., Clements W. K., Le Trong I., Hinds T. R., Stenkamp R., Kimelman D., Xu W. (2004) Mol. Cell 15,523–533 [DOI] [PubMed] [Google Scholar]
- 33.Chen W., Possemato R., Campbell K. T., Plattner C. A., Pallas D. C., Hahn W. C. (2004) Cancer Cell. 5,127–136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.