Abstract
Ischemic stroke stimulates neurogenesis in the adult rodent brain. The molecules underlying stroke-induced neurogenesis have not been fully investigated. Using real-time reverse transcription-PCR, we found that stroke substantially up-regulated angiopoietin 2 (ANG2), a proangiogenic gene, expression in subventricular zone neural progenitor cells. Incubation of neural progenitor cells with recombinant human ANG2 significantly increased the number of β-III tubulin-positive cells, a marker of immature neurons, but did not alter the number of glial fibrillary acidic protein (GFAP)-positive cells, a marker of astrocytes, suggesting that ANG2 promotes neuronal differentiation. Blockage of the ANG2 receptor, Tie2, with small interference RNA (siRNA)-Tie2 attenuated recombinant human ANG2 (rhANG2)-increased β-III tubulin mRNA levels compared with levels in the progenitor cells transfected with control siRNA. Chromatin immunoprecipitation analysis revealed that CCAAT/enhancer-binding protein (C/EBPβ) up-regulated by rhANG2 bound to β-III tubulin, which is consistent with published data that there are several C/EBPβ binding sites in the promoter of β-III tubulin gene. In addition, rhANG2 enhanced migration of neural progenitor cells measured by single neurosphere assay. Blockage of Tie2 with siRNA-Tie2 and a Tie2-neutralizing antibody did not suppress ANG2-enhanced migration. However, inhibition of matrix metalloproteinases with GM6001 blocked ANG2-enhanced migration. Collectively, our data suggest that interaction of ANG2, a proangiogenic factor, with its receptor Tie2 promotes neural progenitor cell differentiation into neuronal lineage cells, whereas ANG2 regulates neural progenitor cell migration through matrix metalloproteinases, which do not require its receptor Tie2.
The mammalian brain contains neural stem and progenitor cells in the sub-granular zone of the dentate gyrus and the subventricular zone (SVZ)2 of the lateral ventricles to generate new neurons throughout lifetime (1–5). Neuroblasts generated in the SVZ migrate in chains rostrally toward the olfactory bulb where they differentiate into olfactory interneurons (6–8). Cerebral ischemia increases neurogenesis in the SVZ (9–12), and many SVZ neuroblasts migrate laterally toward the ischemic boundary zone (11, 13, 14). Upon arrival, some neuroblasts exhibit markers of striatal neurons (9, 10, 15). However, the molecules that mediate stroke-induced neurogenesis have not been fully investigated.
The angiopoietins, including angiopoietin 1 (ANG1) and angiopoietin 2 are a family of structurally related proteins that bind with similar specificity and affinity to a common endothelial cell-specific receptor-tyrosine kinase (Tie2) (16). ANG1 and ANG2/Tie2 signaling play important roles in the angiogenic process and hematopoiesis (17, 18). The function of ANG2 is context-dependent. When acting in the absence of angiogenic inducers (such as vascular endothelial growth factor), ANG2 induced endothelial cell apoptosis with consequent vascular regression (19). When acting in concert with vascular endothelial growth factor, ANG2 may stimulate endothelial cell migration and proliferation, thus serving as a permissive angiogenic signal (19, 20). ANG2 is also a critical effector of hypoxia-induced neovasculature and is involved in cerebral angiogenesis in the ischemic brain (21–24).
Emerging evidence indicates that angiogenesis is coupled with neurogenesis under physiological and pathophysiological conditions (25–32). Neuroblasts in the SVZ could use cerebral blood vessels as a scaffold to migrate to the ischemic striatum (25). Cerebral endothelial cells activated by stroke promote neural progenitor cell differentiation into neurons, whereas ischemic neural progenitor cells facilitate angiogenesis (29). Vascular endothelial growth factor mediates the coupling of angiogenesis and neurogenesis in ischemic brain (27, 29). In addition to vascular endothelial growth factor, stroke up-regulates ANG2 expression in SVZ neural progenitor cells (26). In the present study we investigated the effect of ANG2 on differentiation and migration of adult SVZ progenitor cells. There is no single marker to identify SVZ stem cells in the adult rodent brain. Therefore, in the present study the term “neural progenitor cells” was used to describe dividing cells with capacity for differentiation.
EXPERIMENTAL PROCEDURES
The Model of Middle Cerebral Artery Occlusion
Adult male mice (C57B/6J, 20–30 g) were employed in this study. Permanent right middle cerebral artery occlusion (MCAo) was induced by advancing a 6–0 surgical nylon suture (8.0–9.0 mm, determined by body weight) with an expanded tip from the external carotid artery into the lumen of the internal carotid artery to block the origin of the middle cerebral artery (33).
SVZ Cell Cultures
SVZ cells were dissociated from adult mice, as previously reported (1, 6, 34). The cells were plated at a density of 2 × 104 cells/ml in growth medium. Growth medium contains Dulbecco's modified Eagle's medium/F-12 medium (Invitrogen), 20 ng/ml epidermal growth factor (R&D Systems, Minneapolis, MN), and basic fibroblast growth factor (R&D Systems). Dulbecco's modified Eagle's medium/F-12 medium contains l-glutamine (2 mmol/liter), glucose (0.6%), putrescine (9.6 mg/ml), insulin (0.025 mg/ml), progesterone (6.3 ng/ml), apotransferrin (0.1 mg/ml), and sodium selenite (5.2 ng/ml). The generated neurospheres (primary spheres) were passaged by mechanical dissociation and reseeded as single cells at a density of 20 cells/μl. SVZ cells from ischemic brain were extracted 7 days after MCAo, a peak time of increase of neurogenesis (9, 13). SVZ cells used in all experiments were from passages 2–5.
Laser Capture Microdissection (LCM)
Briefly, frozen brain coronal sections stored at −80 °C were immediately immersed in acetone for 2 min of fixation and air-dried for 30 s. After a brief rinse with 0.1% diethylpyrocarbonate-treated phosphate-buffered saline, sections were stained with propidium iodide dye (1:3000 dilution, Sigma) for 5 min, rinsed with phosphate-buffered saline twice, and dehydrated in graded alcohols (75, 90, and 100% ethanol, 30 s each) and xylene for clearance for 5 min (26). All reaction steps were performed in RNase-free solutions. Sections were then air-dried under laminar flow for 10 min and immediately used for LCM. Dense SVZ cells on sections stained by propidium iodide were readily distinct from the ependymal cells that have cilia along the lateral wall of the lateral ventricle and from the adjacent striatal cells (35). In the non-ischemic mouse, the dorsal and ventral SVZ of the lateral wall was defined as a 20–30-μm-wide zone approximately of 2–3 cell bodies immediately adjacent to ependymal cells, whereas in the ischemic mouse, the SVZ was expanded to a 60–80-μm-wide zone. Propidium iodide-positive cells within the SVZ were captured onto a thermoplastic film mounted on optically transparent LCM caps using the PixCell II LCM System (Arcturus Bioscience Inc., Mountain View, CA). The following parameters were used during LCM: 7.5-mm laser spot size, 60 milliwatts of power, and 750-ms duration. The transfer film was examined under the microscope to ensure cell lysis. Caps with cells were immediately placed into Eppendorf tubes containing 350 μl of lysis buffer and stored at −80 °C before RNA isolation. Approximately 1000 cells were isolated in the SVZ from each animal.
Real-time Semiquantitative RT-PCR
Quantitative PCRs were performed using SYBR Green real-time PCR system. Total RNAs from cultured SVZ cells or LCM cells were extracted using Qiagen Mini kit or Qiagen Micro kit (Qiagen, Valencia, CA). cDNAs were prepared from total RNA using oligodT20, dNTP mix, first-strand buffer, dithiothreitol, RNaseOUT, and Superscript III (Invitrogen). Real-time RT-PCRs were performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA). Amplicon sizes were confirmed using RT-PCR, as previously described (36). Each sample was tested in triplicate, and relative gene expression was determined using the 2−ΔΔCT method (37). Primers to amplify the following transcripts are as follows: β-actin forward primer, 5′-CCATCATGAAGTGTGACGTTG, and reverse primer, 5′-CAATGATCTTGATCTTCATGGTG (150 bp); ANG1 forward primer, 5′-GATCTTACACGGTGCCGATT, and reverse primer, 5′-TTAGATTGGAAGGGCCACAG (118 bp); ANG2 forward primer, 5′-TCCAAGAGCTCGGTTGCTAT, and reverse primer, 5′-AGTTGGGGAAGGTCAGTGTG (114 bp); Tie2 forward primer, 5′-AAGCATGCCCATCTGGTTAC, and reverse primer, 5′-GCCTGCCTTCTTTCTCACAC (138 bp); bone morphogenetic protein 2 (Bmp2) forward primer, 5′- GTCGAAGCTCTCCCACTGAC, and reverse primer 5′-CAGGAAGCTTTGGGAAACAG (150 bp); Bmp4 forward primer, 5′-CGTTACCTCAAGGGAGTGGA, and reverse primer, 5′-ATGCTTGGGACTACGTTTGG (116 bp); β-III tubulin forward primer, 5′-TGAGGCCTCCTCTCACAAGT, and reverse primer, 5′-GGCCTGAATAGGTGTCCAAA (105 bp); Bmp type I receptor B (Bmpr1b), forward primer, 5′-AGCGCTATATGCCTCCAGAA, and reverse primer, 5′-CTCCTTGCAATCTCCCAGAG (114 bp); Smad5 forward primer, 5′-CTCCAGCTCCTCCATAGCAC, and reverse primer, 5′-ATTGTTGGGCTGGAAACAAG (109 bp).
Microarray Hybridization
The Oligo arrays (SABiosciences, Frederick, MD) were used, and hybridization procedures were performed as described by the manufacturer. The biotin dUTP-labeled cDNA probes were specifically generated in the presence of a designed set of gene-specific primers using total RNA and reverse transcriptase. The array filters were hybridized with biotin-labeled probes at 60 °C for 17 h. The filters were then washed twice with 2× saline sodium citrate buffer, 1% sodium dodecyl sulfate and twice with 0.1× saline sodium citrate, 1% sodium dodecyl sulfate at 60 °C for 15 min each. Chemiluminescent detection steps were performed by subsequent incubation of the filters with alkaline phosphatase-conjugated streptavidin and CDPStar substrate (SABiosciences).
For quantification, intensity of spots was measured by GEArray Expression Analysis Suite software, and then the average intensities derived from blank spots were subtracted. Four random pictures from the bottom of each insert were acquired. These relative intensities were used to compare gene expression levels between control and ANG2 treatment groups.
Chromatin Immunoprecipitation Assay (ChIP)
A ChIP assay was performed using the ChIP kit (Upstate, Charlottesville, VA). SVZ cells were cross-linked with 1% formaldehyde and sonicated to an average length of 200–500 bp. The chromatin solutions were precleared with the addition of Protein G beads for 2 h at 4 °C. The precleared chromatins were incubated with C/EBPβ antibody (2 μg, Santa Cruz Biotechnology, Inc. Santa Cruz, CA), normal IgG serum, or no antibody as a negative control overnight. The antibody/chromatin mixtures were precipitated with Protein G beads, and the beads were sequentially washed with ChIP wash buffer. Cross-linking was reversed by adding 4 μl of 5 m NaCl and incubating at 65 °C overnight. DNAs were purified by phenol/chloroform extraction and ethanol precipitation. The real-time PCR primers: forward primer, 5′-GCACCTGGGGTGAACTAAGA, and reverse primer, 5′-CCAAGGAGGAGGACAAAGAA (127 bp), were used to amplify the β-III tubulin promoter region flanking the C/EBPβ binding. Binding activities were calculated as the percentage of pre-immunoprecipitated input DNA.
In Situ Zymography
In situ gelatinolytic activity was performed on a single SVZ neurosphere using an 8-well chamber and DQ gelatin as a substrate (Molecular Probes, Eugene, OR) (38, 39). DQ-gelatin was dissolved in a concentration of 1 mg/ml in water and then 1:10 diluted in 1% (w/v) low gelling temperature-agarose (Sigma) in phosphate-buffered saline. The mixture was put on top of the cells and covered with a coverslip. After gelling of the agar at 4 °C, the incubation was performed for 1 h at room temperature.
Cleavage of DQ gelatin by MMPs resulted in a green fluorescent product (wavelengths: excitation, 495 nm; emission, 515 nm). Some SVZ neurospheres were incubated with GM601, a nonspecific inhibitor of MMP activity. Images were taken under a 63× objective of 2-photon laser confocal microscope (Carl Zeiss Inc.), and fluorescent density was compared in control and ANG2 treatment groups.
siRNA Electroporation in Vitro
siRNA against mouse Tie2 (sequences are provided in supplemental Data I), purchased from Dharmacon (Chicago, IL, catalog #M-045325-01) and siGLO (Dharmacon, catalog #D-001630-02), was used as the negative control. siRNAs were introduced into cells using a NucleofectorTM kit (Amaxa, Germany). Briefly, 200 pmol/well siRNAs were mixed with 100 μl of Nucleofector solution, and cell-DNA mixtures were transferred into a cuvette and electroporated using program A33. Total RNAs or proteins were extracted at 24 or 72 h after nucleofection for the following experiment.
Neurosphere Assay
To examine the effects of ANG2 on SVZ cell proliferation, single cells at a density of 10 cells/μl were incubated in the growth medium for 7 days, and bromodeoxyuridine (BrdUrd, 30 μg/ml, Sigma) was added 18 h before the termination of incubation (34). The percentage of BrdUrd-positive cells was measured.
To examine the effects of ANG2 on SVZ cell differentiation, neurospheres were plated directly onto laminin-coated glass coverslips in Dulbecco's modified Eagle's medium/F-12 medium containing 2% fetal bovine serum, which is referred to as differentiation medium, in the presence of various concentrations of recombinant human ANG2 (rhANG2). Every 4 days, half of the medium was replaced with fresh medium. Incubation was terminated 10 days after plating, and immunostaining for neuronal and astrocyte markers was performed for evaluation of cell differentiation.
To assay the migration of ANG2 treated cells, we employed a Matrigel assay that had been used for measurements of neural progenitor cell motility (40). Briefly, a single neurosphere was seeded in the Matrigel of 96-well plates for 48 h. Migration of cells out of the neurosphere was measured at 0 and 48 h after seeding.
Immunohistochemistry and Quantification
Single, double, and triple immunofluorescent staining was performed on brain coronal sections and cultured cells, as previously described (9, 13, 26, 34, 40). The following primary antibodies were used in the present study: mouse anti-BrdUrd (1:100; Roche Applied Science), mouse anti-β-III tubulin (TuJ-1, 1:500; Covance the Development Services Co.), rabbit anti-glial fibrillary acidic protein (GFAP, 1:500; Dako Cytomation California Inc., Carpinteria, CA), rabbit anti-ANG2 (1:500, Abcam, Cambridge, MA), rabbit anti-ANG1 (1:500, Abcam), anti-mouse nestin (1:100, Pharmingen, San Jose, CA), goat anti-sox2 (1:100, Santa Cruz Biotechnology, Inc), goat anti-doublecortin (1:100, Santa Cruz Biotechnology, Inc). Cultured cells were fixed in 4% paraformaldehyde for 20 min at room temperature. Nonspecific binding sites were blocked with phosphate-buffered saline with 1% bovine serum albumin goat serum for 1 h at room temperature. The cells were then incubated with the primary antibodies listed above and with CY3 or fluorescein isothiocyanate-conjugated secondary antibodies. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (1:10,000, Vector Laboratories, Burlingame, CA).
Measurements of ANG2 immunoreactive cells were performed on five coronal sections per mouse subjected to 7 days after MCAo (34). ANG2-positive areas in the ipsilateral SVZ were digitized under a 20× objective (Olympus BX40) with use of a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research, St. Catharines, Ontario, Canada). ANG2 immunoreactive areas within the SVZ were determined by setting a threshold to distinguish signals from the background based on the original images. The data are presented as a percentage of positive immunoreactive area in the SVZ. For cultured cells, eight fields per well were randomly selected. The number of BrdUrd-, TuJ1-, and GFAP-positive cells as well as total 4′,6-diamidino-2-phenylindole nuclei was counted under a 40× objective (IX71; Olympus Optical, Tokyo, Japan), and the percentage of each cell type was determined.
Statistical Analysis
One-way analysis of variance or the Student-Newman-Keuls test was applied for multiple or two group comparisons, respectively. To analyze the effect of GM6001 on gelatinase activity in the absence or the presence of rhANG2, we designed a method. By assuming a treatment factor (T), rhANG2 (absence or presence), and a blocker factor (B), GM6001 (absence or presence) in which each has two levels (0, 1), response variable (changes of fluorescence, yij), and expected value of yij (μij), E(yij) = μij with indexes of rhANG2 treatment (i) and GM6001 (j). We used the complete 2 × 2 factorial design and two-way analysis of variance to analyze the effect of rhANG2 on MMP signals detected by in situ zymography. The analysis began with testing for T by B interaction using PROC MIXED in SAS with a contract statement, L = (μ11 − μ01) − (μ10 − μ00), followed by evaluating additive or sub-additive effects of the two factors of treatment and condition. The sub-additive effects would be identified if L<0 or (μ11 − μ01) − (μ10 − μ00), indicating that the rhANG2 treatment effect is greatly reduced in the presence of GM6001 compared with the rhANG2 treatment effect in the absence of GM6001. The data are presented as means ± S.D. A value of p < 0.05 was taken as significant.
RESULTS
Stroke Up-regulates ANG2 Expression in SVZ Neural Progenitor Cells
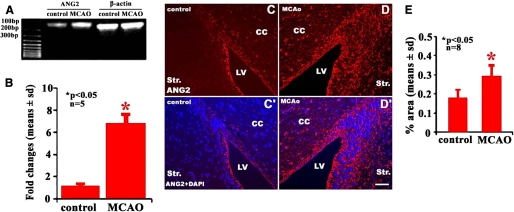
To analyze the effects of stroke on ANG expression in neural progenitor cells, SVZ cells from non-ischemic mice or mice subjected to 7 days after MCAo were isolated using LCM. Real-time RT-PCR analysis revealed that SVZ cells expressed ANG2 under non-ischemic conditions (Fig. 1A). However, mRNA levels of ANG2 significantly increased in SVZ cells isolated from mice 7 days after MCAo (Fig. 1, A and B). In parallel, immunostaining analysis revealed that in non-ischemic SVZ, ependymal cells, and SVZ cells were ANG2-positive (Fig. 1, C–E). However, MCAo (p < 0.05) significantly increased the number of ANG2-positive SVZ cells (Fig. C–E). Interestingly, MCAo did not significantly alter ANG1 mRNA expression in SVZ cells (1.2 ± 0.7 versus 1.1 ± 0.6 in non-ischemic SVZ cells, p > 0.05, n = 5). ANG1 immunoreactive cells were not detected in non-ischemic and ischemic SVZ cells (data not shown).
FIGURE 1.
Expression of ANG2 in SVZ neural progenitor cells. RT-PCR analysis revealed mRNA levels of ANG2 in non-ischemic and ischemic SVZ cells, and β-actin was used as an internal control (A and B). Panels C and D show ANG2-immunoreactive cells in non-ischemic and ischemic SVZ, respectively. Panel C′ and D′ show merged images of ANG2 immunoreactive and 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining cells. Panel E shows quantitative data of ANG2-positive SVZ cells. *, p < 0.05 versus the control group. Bar = 40 μm; LV, lateral ventricle; Str, striatum; CC, corpus callosum.
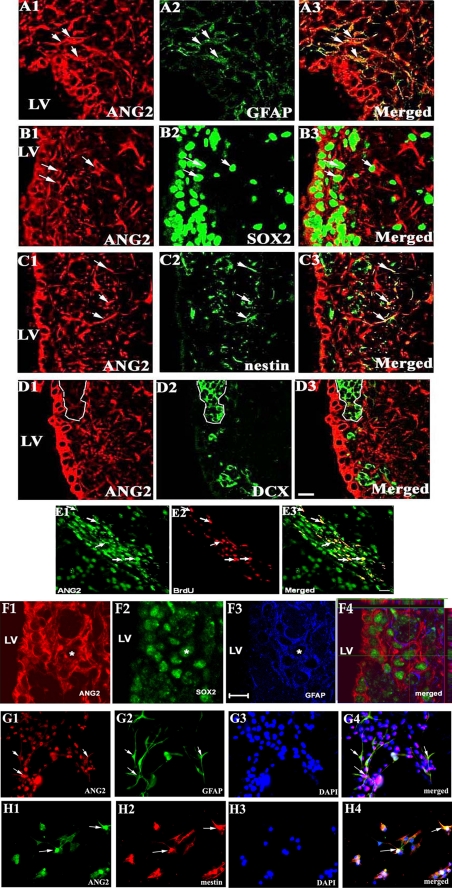
SVZ cells consist of a heterogeneous cell population (41). To identify cellular sources of ANG2, double and triple immunostaining was performed on the coronal and sagittal sections (Fig. 2). We found that ANG2-positive cells in the SVZ 7 days after MCAo were GFAP (Fig. 2, A1–A3)-, SOX2 (Fig. 2, B1–B3)-, or nestin (Fig. 2, C1–C3)-positive but doublecortin-negative (Fig. 2, D1–D3), suggesting that ANG2-expressing cells are neural progenitor cells but not neuroblasts. In addition, some ANG2-positive cells were BrdUrd-immunoreactive (Fig. 2, E1–E3). Triple immunofluorescent staining showed that a few of the ANG2-positive cells were GFAP- and SOX2-positive (Fig. 2, F1–F4). Morphologically, these ANG2-, SOX2-, and GFAP-positive astrocytes exhibited few processes (Fig. 2F), suggesting that these ANG2-positive astrocytes are stem cell-like astrocytes (42–45).
FIGURE 2.
ANG2-positive SVZ cells in vivo and in vitro. Double immunofluorescent staining on coronal brain sections shows that ANG2-positive cells in the ischemic SVZ were GFAP (A1–A3, arrows)-, SOX2 (B1–B3, arrows)-, or nestin (C1–C3, arrows)-positive but not doublecortin (DCX)-positive (D1–D3, outlined by dashed lines). Some of ANG2-positive cells were BrdUrd-positive (E1–E3, arrows). Triple immunofluorescent staining showed that a few of ANG2-positive cells were GFAP- and SOX2-positive (F1–F4, a star). Panels G1 to H4 show that cultured SVZ cells were GFAP (G1–G4, arrows) or nestin positive (H1–H4, arrows). LV = lateral ventricle. Bar = 20 μm. DAPI, 4′,6-diamidino-2-phenylindole.
ANG2 Promotes Neuronal Differentiation of SVZ Neural Progenitor Cells
To examine the biological effects of ANG2 on neural progenitor cells, a neurosphere assay was employed in which SVZ cells were isolated from the SVZ of adult mice (40). When they were cultured in the medium containing growth factors, SVZ cells proliferated and formed spheres (1, 2, 4). Withdrawing growth factors, SVZ cells differentiated into Tuj1-positive immature neurons and GFAP-positive astrocytes (4, 46). Consistent with in vivo data, cultured SVZ cells were ANG2- and GFAP-positive (Fig. 2, G1–G4) or ANG2- and nestin-positive (Fig. 2, H1–H4).
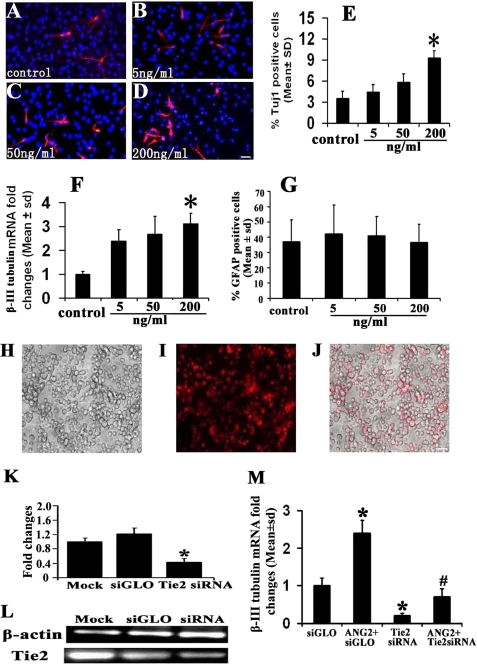
When SVZ cells were cultured in the growth medium in the presence of rhANG2 at concentrations of 50 and 200 ng/μl, rhANG2 did not affect the cell proliferation (81.1 ± 12.2 and 80.2 ± 7.8% versus 87.2 ± 5.3% in the control group, p > 0.05, n = 3). However, in the differentiation medium rhANG2 increased the number of Tuj1-positive cells (Fig. 3, A–E) and β-III tubulin transcript (Fig. 3F) in a dose-dependent manner with a significant increase at a dose of 200 ng/ml (p < 0.05, Fig. 3, E and F). Treatment with rhANG2 did not alter the number of GFAP-positive cells (56.5 ± 11.9%) compared with the control group (57 ± 14.4%, Fig. 3G). To examine whether ANG2 through the Tie2 receptor promotes neuronal differentiation, SVZ cells were electroporated with siRNA against mouse Tie2. Real-time RT-PCR analysis revealed that the siRNA suppressed ∼70% endogenous Tie2 mRNA in SVZ cells compared with the mRNA levels in SVZ cells transfected with control siRNA (siGLO), indicating high transfection efficiency in adult SVZ progenitor cells (Fig. 3, H–L). Attenuation of endogenous Tie2 expression in SVZ cells by siRNA against Tie2 significantly reduced β-III tubulin mRNA levels compared with the levels in SVZ cells transfected by siGLO (Fig. 3M). Moreover, siRNA against Tie2 abolished ANG2-increased β-III tubulin mRNA (Fig. 3M). To verify that down-regulation of β-III tubulin mRNA is specifically caused by blocking Tie2, SVZ cells were transfected by siRNA against lamin A/C (the sequence is provided in supplemental Data I, Dharmacon, catalog #D-001050-01-05), which does not target Tie2. We found the attenuation of endogenous lamin A/C by siRNA (44.7 ± 14.5 versus 100 ± 10.0% in control, n = 3, p < 0.05) did not affect β-III tubulin mRNA levels in the presence (100 ± 17.6 versus 93.0 ± 15.7% in control, n = 3, p > 0.05) or the absence of rhANG2 (94.7 ± 23.9 versus 100 ± 7.93% in control, n = 3, p > 0.05), indicating that the effect of endogenous Tie2 attenuated by siRNA on β-III tubulin expression is specific.
FIGURE 3.
The effect of ANG2 on neuronal differentiation. Immunofluorescent staining shows Tuj1-positive cells in various concentrations of rhANG2 (A–D). Panels E–G show quantitative data of the number of Tuj1 (E), β-III tubulin mRNA levels (F), and GFAP-positive cells (G). Incubation of SVZ neural progenitor cells with rhANG2 increased the number of Tuj1-positive cells in a dose-dependent manner (E). Panels H–J show control siRNA siGLO linked with CY3 (I and J, red color) was introduced into SVZ cells (H and J) with high electroporation efficiency (70%). Panel J is a merged image of panels H and I. Real-time RT-PCR analysis demonstrates that endogenous Tie2 mRNA in SVZ cells was significantly knocked down by siRNA against Tie2 compared with mock and siGLO control (K and L). Attenuation of endogenous Tie2 by siRNA against Tie2 suppressed β-III tubulin mRNA levels increased by rhANG2 (200 ng/ml, M). *, p < 0.05 versus the siGLO group; #, p < 0.05 versus the ANG2+siGLO group. The bar in D and J = 20 μm.
ANG2 Changes Gene Profiles of SVZ Neural Progenitor Cells
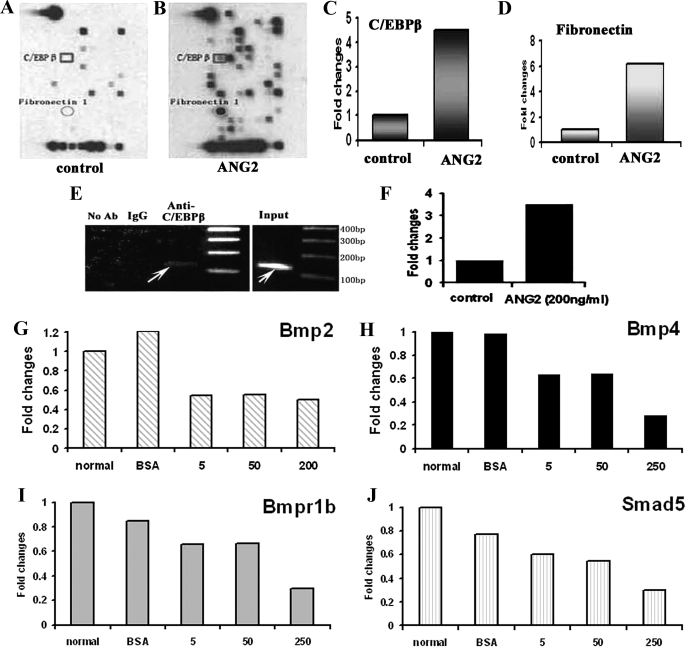
To examine signaling changes in SVZ cells treated with exogenous ANG2, a signal finder microarray containing 113 genes was performed. Treatment of SVZ cells with rhANG2 up-regulated (above the ratio 1.5) 23 genes and down-regulated (below the ratio 0.5) 38 genes (Fig. 4, A and B, and supplemental Data II). Among the up-regulated genes, C/EBPβ and fibronectin1 transcripts were increased by 4.0- and 6.9-fold, respectively, compared with the levels in the control group (Fig. 4, A to D). Using PromoSer software and Transcription Element Search Software, we found that there are several C/EBPβ binding sites in the promoter of the β-III tubulin gene. To examine whether C/EBPβ binds to the β-III tubulin gene promoter in SVZ progenitor cells, ChIP was performed. A binding band was detected on ChIP (Fig. 4E, arrow), and rhANG2 treatment enhanced the binding of C/EBPβ to the promoter of β-III tubulin gene (Fig. 4F), suggesting that C/EBPβ is involved in ANG2-promoted neuronal differentiation.
FIGURE 4.
The effect of ANG2 on gene expression. Signaling transduction microarray analysis shows a profile in SVZ cells non-treated (A) and treated with rhANG2 (200 ng/ml, B) for 24 h. Representative microarrays show up-regulated C/EBPβ (square, A–C) and fibronectin 1 (circle, A, B, and D) genes in SVZ cells treated with rhANG2 compared with SVZ cells only. The ChIP assay reveals binding of C/EBPβ to the β-III tubulin gene promoter sequence (E, arrows), and ANG2 promoted the binding of C/EBPβ (E, arrows, F). Real-time RT-PCR analysis (G–J) confirmed that rhANG2 treatment reduced mRNA levels of Bmp2 (G), Bmp4 (H), Bmp type I receptor B (I), and BMP signaling effectors, SMAD1 (J). Ab, antibody; BSA, bovine serum albumin.
The Bmp/Smad signaling pathway promotes the generation of astrocytes and antagonizes neurogenesis by the inhibition of pro-neurogenic activity of basic helix-loop-helix factors (47–49). Among down-regulated genes, two members of Bmp family, Bmp2 and Bmp4, were decreased by 0.35 and 0.5 times, respectively, after rhANG2 treatment (supplemental Data II). Real-time RT-PCR analysis confirmed that rhANG2 treatment reduced mRNA levels of Bmp2 and Bmp4 (Fig. 4, G and H). In addition, rhANG2 decreased Bmp type I receptor B (Bmpr1b) and BMP signaling effectors, SMAD5 (Fig. 4, I and J). Thus, down-regulation of the Bmp/Smad signaling pathway may also contribute to exogenous ANG2-promoted neuronal differentiation.
ANG2 Enhances SVZ Neural Progenitor Cell Migration
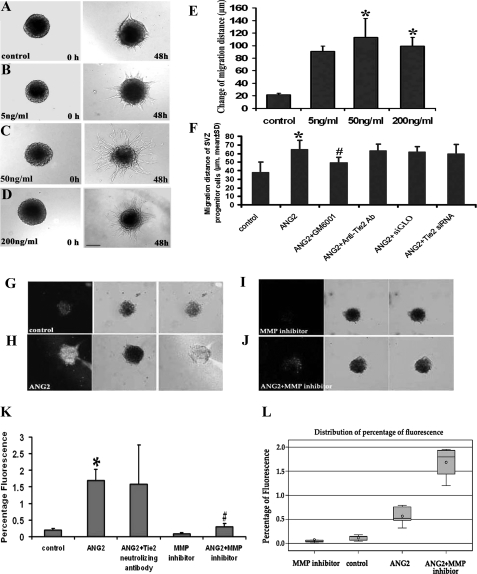
To examine if ANG2 affects SVZ cell migration, a single neurosphere migration assay was employed (12, 40, 50). Incubation of neurospheres with rhANG2 at 50 and 200 ng/ml for 48 h significantly increased the migration distance of cells out of the neurosphere seeded in a Matrigel with the growth medium compared with the distance in the control group (Fig. 5, A–E). Blockage of the ANG2 receptor, Tie2, with a neutralizing antibody or attenuation of endogenous Tie2 mRNA with siRNA against Tie2 did not inhibit the ANG2-increased migration distance (Fig. 5F). In contrast, a general MMP inhibitor, GM6001, blocked rhANG2-increased cell migration (48.7 ± 7.1 μm versus 64.5 ± 10.6 μm in rhANG2, n = 12, p < 0.05, Fig. 5F). In situ zymography analysis revealed that MMP signals were present in neurospheres in the absence of rhANG2- and GM6001-reduced MMP signals (Fig. 5G). Incubation of neurospheres with rhANG2 (200 ng/ml) significantly increased MMP signals in SVZ cells (Fig. 5, H–K), which was substantially blocked by GM6001 (Fig. 5, J and K). Statistical analysis revealed that there was an interaction between rhANG2 alone and GM6001 alone groups with estimated L = −1.07 and p < 0.01. GM6001 blocked MMP signals by 3-fold (p < 0.01, Fig. 5). These data suggest that MMPs mediate ANG2-enhanced cell migration.
FIGURE 5.
The effect of rhANG2 on SVZ cell migration. Micrographs (A–D) show cell migration out of neurospheres seeded in Matrigel with various concentrations of rhANG2 during a 48-h period. Panel E shows quantitative data of migration distance 48 h after rhANG2 treatment. Blockage of Tie2 with a neutralized antibody against Tie2 or siRNA-Tie2 did not suppress ANG2-enhanced cell migration (F), whereas suppression of MMP with an MMP inhibitor GM6001 significantly reduced ANG2-enhanced migration (F). In situ gelatinase zymography of SVZ neurospheres (G–K) shows that incubation of SVZ neurospheres with rhANG2 significantly increased MMP activity (white in H and J) compared with the control group (white in G). However, incubation of neurospheres with rhANG2 in the presence of an MMP inhibitor GM6001 abolished ANG2-increased MMP activity (white in I and J). *, p < 0.05 versus control group; #, p < 0.05 versus ANG2 group. n = 12 per group. Bar = 50 μm. A box plot (L) displays summary statistics for the distribution of values for control (control), control + GM6001 (MMP inhibitor), rhANG2 (ANG2), and rhANG2 + GM6001 (ANG2+MMP inhibitor) groups. The outer bounds of the box represent the first and third quartiles. The line or circle inside the box represents the median or mean, respectively. The markers outside the box, referred to as outliers, represent data points that are outside of the 25th and 75th percentiles.
DISCUSSION
Stroke increases neurogenesis and induces migration of neuroblasts in the SVZ to the ischemic boundary region in the adult rodent brain (9–11, 13, 14). The present study shows that MCAo up-regulates ANG2, a proangiogenic factor, in SVZ neural progenitor cells. In vitro assays indicate that ANG2 promotes SVZ neural progenitor cell differentiation into neuronal lineage cells through its receptor Tie2 and that ANG2 enhances neural progenitor cell motility independent on Tie2 and possibly via activation of MMPs. Together, these data suggest that in addition to angiogenesis, ANG2 mediates neurogenesis which provides additional mechanisms underlying stroke-induced neurogenesis.
ANG2 deactivates Tie2 and antagonizes the Tie 2 agonist ANG1 to destabilize established vasculature, allowing new vessel formation to occur in the endothelium (51, 52). However, the role of ANG2 in the neurogenesis remains unknown. Our data show that rhANG2 promoted adult SVZ neural progenitor cells to differentiate into neuronal lineage cells through its receptor-Tie2, which was associated with up-regulation of transcriptional factor C/EBPβ. The C/EBP family is composed of basic leucine zipper DNA-binding proteins (α, β, γ, δ, ϵ, and ζ) that recognize a common DNA binding sequence and regulate signal transduction to cellular differentiation in numerous developing neural tissues (53, 54). C/EBPβ is involved in neurogenesis by the binding neurogenic basic helix-loop-helix transcription factor; that is, NeuroD members (55, 56). Our ChIP results show that incubation of SVZ cells with rhANG2 dramatically increased binding of C/EBPβ to the promoter of the β-III tubulin gene, which is consistent with the observation that there are several C/EBPβ binding sites in a promoter of the β-III tubulin gene using the Transcription Element Search System (TESS). Thus, our data suggest that interaction of ANG2 with the Tie2 receptor in SVZ neural progenitor cells increases C/EBPβ binding to the promoter of β-III tubulin gene, which may lead to increased neuronal differentiation. In addition, the present study showed that ANG2-promoted neuronal differentiation was associated with down-regulation of BMP family gene expression. BMPs are a family of cytokines belonging to the transforming growth factor-β superfamily (57). BMP2 and BMP4 promote gliogenesis and antagonize neurogenesis to maintain the stem cell phenotype (4, 58). Thus, in addition to C/EBPβ, down-regulation of BMP-SMAD genes could contribute to ANG2-enhanced neuronal differentiation. The present study also indicates that the endogenous ANG2/Tie2 pathway mediates neuronal differentiation because attenuation of endogenous Tie2 by siRNA reduced β-III tubulin expression.
After stroke, neuroblasts in the SVZ migrate toward the ischemic boundary regions of the striatum. Chemokines, such as stromal-derived factor 1α and its receptor CXCR4, regulate neuroblast motility after stroke (50, 59, 60). Our data demonstrate that rhANG2 enhanced cultured neural progenitor cell migration, and knockdown of Tie2 with siRNA in the neural progenitor cells did not block ANG2-enhanced migration. These data suggest that ANG2 mediates motility of neural progenitor cells, which is independent of its receptor Tie2. Although ANG2 regulates angiogenesis through interaction with Tie2 receptor, emerging studies on cancer cells and fibroblasts show that the highly conserved COOH-terminal fibrinogen-like receptor binding domain of ANG2 has a functional association with the integrin receptors, and ANG2 activates integrin signaling and stimulates cell adhesion in Tie2 knock-out cells (18, 61–64). ANG2 induced glioma cell invasion by stimulating MMP2 expression through the αVβ1integrin, but not the Tie2 receptor (17). We and others have shown that activation of MMP2 and MMP9 in SVZ cells mediates neuroblast migration under physiological and ischemic conditions and that stroke up-regulates integrin αV expression in SVZ cells (20, 26, 65). The present study shows that rhANG2 up-regulated fibronectin, a ligand of integrins, and activated MMPs in SVZ cells, whereas blockage of MMPs suppressed the effect of ANG2 on the cell migration. Collectively, these data suggest that ANG2 may interact with integrins to activate MMPs that mediate neural progenitor cell motility.
SVZ cells are heterogeneous and contain type A to type C cells (41). In adult rodents, a subset of SVZ GFAP-positive astrocytes (type B cells) are considered as the origin of neural stem cells that are derived from radial glia (7, 66). Type B cells generate transit-amplifying (type C) cells that give rise to neuroblasts (type A cells) and oligodendrocytes (7, 66). Neuroblasts born in the SVZ niche migrate tangentially through the rostral migratory stream to the olfactory bulb (2–4). Bipolar and unipolar GFAP-expressing cells are neural stem cells in the SVZ of adult rodent, and adult neural stem cells express SOX2 (42, 45). Our triple immunostaining demonstrated that a few of the ANG2-positive cells in the SVZ were GFAP- and SOX2-positive, and these cells had few processes. In addition, many ANG2-positive cells were nestin (one of markers for type C cells), but not doublecortin (a marker of type A cells)-positive. Collectively, these data suggest that ANG2 was primarily derived from types B and C cells but not from type A neuroblasts. ANG2 is a secreted protein (67, 68). Therefore, both autocrine and paracrine ANG2 could play important roles in mediating neuronal differentiation and neuroblast migration. Intriguingly, ANG2 was also expressed in ependymal cells, and MCAo further increased ANG2 expression. Under physiological conditions, there is debate about whether ependymal cells are neural stem cells in the adult SVZ (35, 69). After stroke, we and others have demonstrated that ependymal cells in the lateral ventricles exhibit neural stem cell characteristics properties (70, 71). Thus, it is warranted to investigate a role of ANG2 in ependymal cells under normal and ischemic conditions.
Supplementary Material
Acknowledgment
We are grateful to Cindi Roberts who provided the technical support for immunohistochemistry.
This work was supported, in whole or in part, by National Institutes of Health Grants PO1 NS23393, PO1 NS42345, and RO1 HL064766.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Data I and II.
- SVZ
- subventricular zone
- ANG2
- angiopoietin 2
- rhANG2
- recombinant human ANG2
- GFAP
- glial fibrillary acidic protein
- siRNA
- small interference RNA
- ChIP
- chromatin immunoprecipitation
- C/EBPβ
- CCAAT/enhancer-binding protein
- MMP
- matrix metalloproteinase
- MCAo
- middle cerebral artery occlusion
- LCM
- laser capture microdissection
- Bmp2
- bone morphogenetic protein 2
- BrdUrd
- bromodeoxyuridine
- RT
- reverse transcription
- BrdUrd
- bromodeoxyuridine.
REFERENCES
- 1.Reynolds B. A., Weiss S. (1992) Science 255, 1707–1710 [DOI] [PubMed] [Google Scholar]
- 2.Johansson C. B., Momma S., Clarke D. L., Risling M., Lendahl U., Frisén J. (1999) Cell 96, 25–34 [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla A., Seri B., Doetsch F. (2002) Brain Res. Bull 57, 751–758 [DOI] [PubMed] [Google Scholar]
- 4.Doetsch F., Caillé I., Lim D. A., García-Verdugo J. M., Alvarez-Buylla A. (1999) Cell 97, 703–716 [DOI] [PubMed] [Google Scholar]
- 5.Palmer T.D., Takahashi J., Gage F. H. (1997) Mol. Cell Neurosci. 8, 389–404 [DOI] [PubMed] [Google Scholar]
- 6.Morshead C. M., Reynolds B. A., Craig C. G., McBurney M. W., Staines W. A., Morassutti D., Weiss S., van der Kooy D. (1994) Neuron 13, 1071–1082 [DOI] [PubMed] [Google Scholar]
- 7.García-Verdugo J. M., Doetsch F., Wichterle H., Lim D. A., Alvarez-Buylla A. (1998) J. Neurobiol. 36, 234–248 [DOI] [PubMed] [Google Scholar]
- 8.Luskin M. B. (1998) J. Neurobiol. 36, 221–233 [PubMed] [Google Scholar]
- 9.Zhang R. L., Zhang Z. G., Zhang L., Chopp M. (2001) Neuroscience 105, 33–41 [DOI] [PubMed] [Google Scholar]
- 10.Jin K., Minami M., Lan J. Q., Mao X. O., Batteur S., Simon R. P., Greenberg D. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 4710–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parent J. M., Vexler Z. S., Gong C., Derugin N., Ferriero D. M. (2002) Ann. Neurol. 52, 802–813 [DOI] [PubMed] [Google Scholar]
- 12.Jin K., Wang X., Xie L., Mao X. O., Zhu W., Wang Y., Shen J., Mao Y., Banwait S., Greenberg D. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13198–13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R., Zhang Z., Wang L., Wang Y., Gousev A., Zhang L., Ho K. L., Morshead C., Chopp M. (2004) J. Cereb. Blood Flow Metab. 24, 441–448 [DOI] [PubMed] [Google Scholar]
- 14.Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. (2002) Nat. Med. 8, 963–970 [DOI] [PubMed] [Google Scholar]
- 15.Yamashita T., Ninomiya M., Hernández A. P., García-Verdugo J. M., Sunabori T., Sakaguchi M., Adachi K., Kojima T., Hirota Y., Kawase T., Araki N, Abe K., Okano H., Sawamoto K. (2006) J. Neurosci. 26, 6627–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis S., Aldrich T. H., Jones P. F., Acheson A., Compton D. L., Jain V., Ryan T. E., Bruno J., Radziejewski C., Maisonpierre P. C., Yancopoulos G. D. (1996) Cell 87, 1161–1169 [DOI] [PubMed] [Google Scholar]
- 17.Hu B., Jarzynka M. J., Guo P., Imanishi Y., Schlaepfer D. D., Cheng S. Y. (2006) Cancer Res. 66, 775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imanishi Y., Hu B., Jarzynka M. J., Guo P., Elishaev E., Bar-Joseph I., Cheng S. Y. (2007) Cancer Res. 67, 4254–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobov I. B., Brooks P. C., Lang R. A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11205–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y., Lee C., Shen F., Du R., Young W. L., Yang G. Y. (2005) Stroke 36, 1533–1537 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z. G., Zhang L., Tsang W., Soltanian-Zadeh H., Morris D., Zhang R., Goussev A., Powers C., Yeich T., Chopp M. (2002) J. Cereb. Blood Flow Metab. 22, 379–392 [DOI] [PubMed] [Google Scholar]
- 22.Lin T. N., Wang C. K., Cheung W. M., Hsu C. Y. (2000) J. Cereb. Blood Flow Metab. 20, 387–395 [DOI] [PubMed] [Google Scholar]
- 23.Beck H., Acker T., Wiessner C., Allegrini P. R., Plate K. H. (2000) Am J. Pathol. 157, 1473–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R. G., Zhu X. Z. (2002) Acta Pharmacol. Sin. 23, 405–411 [PubMed] [Google Scholar]
- 25.Ohab J. J., Fleming S., Blesch A., Carmichael S. T. (2006) J. Neurosci. 26, 13007–13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X. S., Zhang Z. G., Zhang R. L., Gregg S., Morris D. C., Wang Y., Chopp M. (2007) J. Cereb. Blood Flow Metab. 27, 564–574 [DOI] [PubMed] [Google Scholar]
- 27.Sun Y., Jin K., Xie L., Childs J., Mao X. O., Logvinova A., Greenberg D. A. (2003) J. Clin. Invest. 111, 1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thored P., Wood J., Arvidsson A., Cammenga J., Kokaia Z., Lindvall O. (2007) Stroke 38, 3032–3039 [DOI] [PubMed] [Google Scholar]
- 29.Teng H., Zhang Z. G., Wang L., Zhang R. L., Zhang L., Morris D., Gregg S. R., Wu Z., Jiang A., Lu M., Zlokovic B. V., Chopp M. (2008) J. Cereb. Blood Flow Metab. 28, 764–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopp M., Zhang Z. G., Jiang Q. (2007) Stroke 38, Suppl. 2, 827–831 [DOI] [PubMed] [Google Scholar]
- 31.Tavazoie M., Van der Veken L., Silva-Vargas V., Louissaint M., Colonna L., Zaidi B., Garcia-Verdugo J. M., Doetsch F. (2008) Cell Stem Cell. 3, 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen Q., Wang Y., Kokovay E., Lin G., Chuang S. M., Goderie S. K., Roysam B., Temple S. (2008) Cell Stem Cell. 3, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao Y., Yang G. Y., Zhou L. F., Stern J. D., Betz A. L. (1999) Brain Res. Mol. Brain Res. 63, 366–370 [DOI] [PubMed] [Google Scholar]
- 34.Wang L., Zhang Z. G., Zhang R. L., Jiao Z. X., Wang Y., Pourabdollah-Nejad D. S., LeTourneau Y., Gregg S. R., Chopp M. (2006) J. Cereb. Blood Flow Metab. 26, 556–564 [DOI] [PubMed] [Google Scholar]
- 35.Chiasson B. J., Tropepe V., Morshead C. M., van der Kooy D. (1999) J. Neurosci. 19, 4462–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katakowski M., Zhang Z., deCarvalho A. C., Chopp M. (2005) Neurosci. Lett. 385, 204–209 [DOI] [PubMed] [Google Scholar]
- 37.Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 38.Mook O. R., Van Overbeek C., Ackema E. G., Van Maldegem F., Frederiks W. M. (2003) J. Histochem. Cytochem. 51, 821–829 [DOI] [PubMed] [Google Scholar]
- 39.Snoek-van Beurden P.A., Von den Hoff J. W. (2005) Biotechniques 38, 73–83 [DOI] [PubMed] [Google Scholar]
- 40.Liu X. S., Zhang Z. G., Zhang R. L., Gregg S. R., Wang L., Yier T., Chopp M. (2007) J. Neurosci Res. 85, 2120–2125 [DOI] [PubMed] [Google Scholar]
- 41.Reznikov K., Acklin S. E., van der Kooy D. (1997) Dev. Dyn. 210, 328–343 [DOI] [PubMed] [Google Scholar]
- 42.Bylund M., Andersson E, Novitch B. G., Muhr J. (2003) Nat. Neurosci. 6, 1162–1168 [DOI] [PubMed] [Google Scholar]
- 43.Graham V., Khudyakov J., Ellis P., Pevny L. (2003) Neuron 39, 749–765 [DOI] [PubMed] [Google Scholar]
- 44.Komitova M, Eriksson P. S. (2004) Neurosci Lett. 369, 24–27 [DOI] [PubMed] [Google Scholar]
- 45.Garcia A. D., Doan N. B., Imura T., Bush T. G., Sofroniew M. V. (2004) Nat. Neurosci. 7, 1233–1241 [DOI] [PubMed] [Google Scholar]
- 46.Consiglio A., Gritti A., Dolcetta D., Follenzi A., Bordignon C., Gage F. H., Vescovi A. L., Naldini L. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 14835–14840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viñals F., Reiriz J., Ambrosio S., Bartrons R., Rosa J. L., Ventura F. (2004) EMBO J. 23, 3527–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Y., Nadal-Vicens M., Misono S., Lin M. Z., Zubiaga A., Hua X., Fan G., Greenberg M. E. (2001) Cell 104, 365–376 [DOI] [PubMed] [Google Scholar]
- 49.Shou J., Rim P. C., Calof A. L. (1999) Nat. Neurosci. 2, 339–345 [DOI] [PubMed] [Google Scholar]
- 50.Robin A. M., Zhang Z. G., Wang L., Zhang R. L., Katakowski M., Zhang L., Wang Y., Zhang C., Chopp M. (2006) J. Cereb. Blood Flow Metab. 26, 125–134 [DOI] [PubMed] [Google Scholar]
- 51.Maisonpierre P. C., Suri C., Jones P. F., Bartunkova S., Wiegand S. J., Radziejewski C., Compton D., McClain J., Aldrich T. H., Papadopoulos N., Daly T. J., Davis S., Sato T. N., Yancopoulos G. D. (1997) Science 277, 55–60 [DOI] [PubMed] [Google Scholar]
- 52.Yuan H. T., Khankin E. V., Karumanchi S. A., Parikh S. M. (2009) Mol. Cell. Biol. 29, 2011–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lekstrom-Himes J., Xanthopoulos K. G. (1998) J. Biol. Chem. 273, 28545–28548 [DOI] [PubMed] [Google Scholar]
- 54.Sterneck E., Johnson P. F. (1998) J. Neurochem. 70, 2424–2433 [DOI] [PubMed] [Google Scholar]
- 55.Calella A. M., Nerlov C., Lopez R. G., Sciarretta C., von Bohlen und Halbach O., Bereshchenko O., Minichiello L. (2007) Neural Dev. 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uittenbogaard M., Martinka D. L., Johnson P. F., Vinson C., Chiaramello A. (2007) J. Neurosci. Res. 85, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. (1988) Science 242, 1528–1534 [DOI] [PubMed] [Google Scholar]
- 58.Gross R. E., Mehler M. F., Mabie P. C., Zang Z., Santschi L., Kessler J. A. (1996) Neuron 17, 595–606 [DOI] [PubMed] [Google Scholar]
- 59.Thored P., Arvidsson A., Cacci E., Ahlenius H., Kallur T., Darsalia V., Ekdahl C. T., Kokaia Z., Lindvall O. (2006) Stem Cells 24, 739–747 [DOI] [PubMed] [Google Scholar]
- 60.Imitola J., Raddassi K., Park K. I., Mueller F. J., Nieto M., Teng Y. D., Frenkel D., Li J., Sidman R. L., Walsh C. A., Snyder E. Y., Khoury S. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 18117–18122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis S., Papadopoulos N., Aldrich T. H., Maisonpierre P. C., Huang T., Kovac L., Xu A., Leidich R., Radziejewska E., Rafique A., Goldberg J., Jain V., Bailey K., Karow M., Fandl J., Samuelsson S. J, Ioffe E., Rudge J. S., Daly T. J., Radziejewski C., Yancopoulos G. D. (2003) Nat. Struct. Biol. 10, 38–44 [DOI] [PubMed] [Google Scholar]
- 62.Cascone I., Napione L., Maniero F., Serini G., Bussolino F. (2005) J. Cell Biol. 170, 993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carlson T. R., Feng Y., Maisonpierre P. C., Mrksich M., Morla A. O. (2001) J. Biol. Chem. 276, 26516–26525 [DOI] [PubMed] [Google Scholar]
- 64.Weber C. C., Cai H., Ehrbar M., Kubota H., Martiny-Baron G., Weber W., Djonov V., Weber E., Mallik A. S., Fussenegger M., Frei K., Hubbell J. A., Zisch A. H. (2005) J. Biol. Chem. 280, 22445–22453 [DOI] [PubMed] [Google Scholar]
- 65.Wang L., Zhang Z. G., Zhang R. L., Gregg S. R., Hozeska-Solgot A., LeTourneau Y., Wang Y., Chopp M. (2006) J. Neurosci. 26, 5996–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doetsch F., García-Verdugo J. M., Alvarez-Buylla A. (1997) J. Neurosci. 17, 5046–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pichiule P., Chavez J. C., LaManna J. C. (2004) J. Biol. Chem. 279, 12171–12180 [DOI] [PubMed] [Google Scholar]
- 68.Fiedler U., Scharpfenecker M., Koidl S., Hegen A., Grunow V., Schmidt J. M., Kriz W., Thurston G., Augustin H. G. (2004) Blood 103, 4150–4156 [DOI] [PubMed] [Google Scholar]
- 69.Chojnacki A. K., Mak G. K., Weiss S. (2009) Nat. Rev. Neurosci. 10, 153–163 [DOI] [PubMed] [Google Scholar]
- 70.Zhang R. L., Zhang Z. G., Wang Y., LeTourneau Y., Liu X. S., Zhang X., Gregg S. R., Wang L., Chopp M. (2007) J. Cereb. Blood Flow Metab. 27, 1201–1212 [DOI] [PubMed] [Google Scholar]
- 71.Carlén M., Meletis K., Göritz C., Darsalia V., Evergren E., Tanigaki K., Amendola M., Barnabé-Heider F., Yeung M. S., Naldini L., Honjo T., Kokaia Z., Shupliakov O., Cassidy R. M., Lindvall O., Frisén J. (2009) Nat. Neurosci. 12, 259–267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.