Abstract
Proliferation inhibition of vascular smooth muscle cells (VSMCs) is governed by the activity of a transcription factor network. Krüppel-like factor 4 (Klf4), retinoic acid receptor (RARα), and platelet-derived growth factor receptor (PDGFR) are expressed in VSMCs and are components of such a network. However, the relationship among them in the regulation of VSMC proliferation remains unknown. Here, we investigated the mechanisms whereby Klf4 mediates the growth inhibitory effects in VSMCs through RARα and PDGFRβ. We demonstrated that Klf4 directly binds to the 5′ regulatory region of RARα, down-regulates RARα expression, and specifically inhibits RARα-mediated phosphatidylinositol 3-kinase (PI3K) and ERK signaling in cultured VSMCs induced by the synthetic retinoid Am80. Of particular interest, Klf4 inhibits RARα and PDGFRβ expression while blocking PI3K and ERK signaling induced by Am80 and PDGF-BB, respectively. The anti-proliferative effects of Klf4 on neointimal formation depend largely on PDGFR-mediated PI3K signaling without involvement of the RARα-activated signaling pathways. These findings provide a novel mechanism for signal suppression and growth inhibitory effects of Klf4 in VSMCs. Moreover, the results of this study suggest that Klf4 is one of the key mediators of retinoid actions in VSMCs.
The regulation of differentiation and proliferation of vascular smooth muscle cells (VSMCs)2 is known to be critical in blood vessel formation during embryogenesis and in pathological states such as atherogenesis, restenosis, and hypertension. There is considerable interest in identifying extracellular signals, signal transduction molecules, and transcription factors that are involved in the regulation of VSMC proliferation and differentiation (1, 2). Many different environmental cues are known to affect VSMC proliferation and differentiation, including endothelial cell-VSMC interactions, VSMC-matrix contacts, mechanical forces, and various cytokines such as platelet-derived growth factor BB (PDGF-BB), activin, and transforming growth factor-β1 (3, 4). The Krüppel-like factors (Klfs) are DNA-binding transcriptional regulators that regulate a diverse array of cellular processes, including development, differentiation, proliferation, and apoptosis. Members of this family bind similar CACCC elements on DNA (5). Klf4 was first identified as being highly expressed in epithelial cells, and gain/loss of function studies in vivo have confirmed a critical role for this factor in epithelial cell differentiation (6). Subsequent work has underlined the importance of Klf4 in a wide range of cellular processes, such as endothelial pro-inflammatory activation, macrophage gene expression, tumor cell development, and stem cell biology (7). Klf4 was also isolated as a Klf expressed in the vasculature and could be induced by shear stress (8). Klf4 has been shown to repress the transforming growth factor-β-dependent increase in VSMC differentiation markers, including those for α-smooth muscle actin and SM22α (1). A recent study showed that Klf4 represses myocardin-induced VSMC gene activation and myocardin expression itself (9). Although Klf4 is not normally expressed in differentiated SMCs, it is up-regulated in PDGF-BB-treated cultured SMCs and in response to vascular injury in vivo (10). Therefore, Klf4 may be a key effector of SMC phenotypic switching (11, 12).
The mitogen-activated protein kinase (MAPK) pathway is a key integration point in the signal transduction cascade that links diverse extracellular stimuli to proliferation, differentiation, and survival (13). Among the MAPK subfamilies, the extracellular signal-regulated kinase (ERK) MAPK is known to mediate terminal differentiation in many retinoid-induced cells (14, 15). There is also evidence for the existence of additional retinoid-activated signaling pathways. One such pathway involves the lipid kinase phosphatidylinositol 3-kinase (PI3K). An increasing body of experimental evidence has shown that PI3K regulates a variety of cellular functions, including proliferation, differentiation, and cell survival (16). It has been shown that cross-talk between the PI3K and ERK pathways may enhance or attenuate extracellular signals (17). In VSMCs, ligand-activated PDGF receptor (PDGFR) increases proliferation, whereas retinoic acid receptor (RARα) inhibits growth and promotes differentiation (18–21). PDGFR activation triggers a cascade of downstream signaling pathways, including PI3K and ERK, and enhances proliferation (22). In contrast, RARα activation triggers PI3K and MAPK pathways and stimulates actin remodeling (23).
Proliferation inhibition of VSMCs is governed by the activity of a transcription factor network. Klf4 is one component of such a network. To understand further the regulatory role of Klf4 in VSMC proliferation and differentiation, we characterized the signaling pathways activated by Klf4 and determined its ability to influence PDGFR and RARα downstream signaling. We demonstrated that Klf4 overexpression inhibits cell proliferation and PI3K and ERK pathways. The binding of Klf4 to the 5′ regulatory region of RARα down-regulates RARα expression and also leads to impaired RARα-mediated PI3K and ERK signaling. Although Klf4 was found to be itself regulated by the PI3K pathway activated by the synthetic retinoid Am80, the anti-proliferative effects of Klf4 are independent of RARα. We further identified that Klf4 plays important anti-proliferative roles through inhibition of the PDGFRβ-mediated PI3K pathway.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatment
Male Sprague-Dawley rats were anesthetized with sodium pentobarbital (Sigma); the aorta was removed, and VSMCs were isolated and cultured as previously described (24). VSMCs were maintained and passaged in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (HyClone), 100 units/ml of penicillin, and 100 μg/ml of streptomycin. In all experiments, only cell passages 3–5 were used. The experiments were initiated when the cells reached 70% confluence, unless stated otherwise. For studies on the effects of RARα, ERK, and PI3K/Akt inhibition, the cells were starved for 24 h and then left untreated or treated for 4 h with 10 μm of Am80, Am580, or PDGF-BB (10 ng/ml), DMSO, 25 μm of the PI3K inhibitor LY294002 (Promega) in DMSO, 50 μm of the ERK inhibitor PD98059 (Promega) in DMSO, or 20 μm of the RAR inhibitor Ro 41-5253 in DMSO (Biomol).
Adenovirus Expression Vector and Plasmids
The full-length rat Klf4 cDNA was cloned into the pAd/CMV/V5-DEST vector (Invitrogen) to create the Klf4 adenovirus pAd-Klf4 (25). The resulting constructs were packaged in A293 cells (ATCC) by transfection with Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Culture supernatants from individual A293 cells were used to infect VSMCs. The cells were passaged after 24 h and selected with 300 μg/ml G418 for 14 days. The Klf4 siRNA construct (PM-Klf4) was a generous gift from Dr. Gary K. Owens (9). To analyze the effects of RARα expression on cell signaling, we used pCMV-hRARα (kindly provided by Dr. Ronald M. Evans, Howard Hughes Medical Institute, San Diego, CA) to transfect VSMCs with Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Rat RARα siRNA was designed by custom Accell SMARTpool (Dharmacon) against the rat sequences for RARα (accession number NM_031528). The Accell nontargeting siRNA pool (Dharmacon) is composed of four siRNAs designed not to target any genes in human, mouse, or rat genomes and was microarray tested. The Accell siRNA delivery medium (Dharmacon) was used, according to the manufacturer's recommendations. After siRNA and adenovirus delivery, RARα, Klf4, and phosphorylated and total ERK1/2 levels were determined using Western blot analysis.
MTT Assay
The cell growth rates were evaluated by the tetrazolium dye-based MTT assay, as described previously (26). In brief, 1 × 104 cells/well were seeded in triplicate onto 96-well plates in Dulbecco's modified Eagle's medium. After 24 h, the cells were treated according to the experimental design. After each treatment, the medium was removed, and the cells were washed with phosphate-buffered saline. The MTT reagent was added at 2 mg/ml in Hank's buffer and incubated for 1 h until dark blue crystals could be seen in the cytoplasm under light microscopy. The crystals were dissolved in DMSO, and the absorbance was measured in a Thermo Fluroscan Ascent spectrometer at 570 nm with background subtraction at 650 nm. The results were expressed as the means of the absorbance relative to time 0 or the control ± S.E.
Reporter Gene Assays
A293 cells were transfected with a luciferase-harboring RARα promoter (gift from Dr. Giles E. Hardingham, University of Edinburgh, Edinburgh, Scotland, UK) and pcDNA3-Klf4 using Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. The cells were harvested after 48 h, and the activities of both firefly luciferase and Renilla luciferase were measured in the LB 955 Luminometer system using the dual luciferase reporter system (Promega), according to the manufacturer's recommendations. The activity of firefly luciferase was normalized to that of Renilla luciferase. A minimum of three independent transfections was performed for each experimental group (27).
Western Blot Analysis
The cells were lysed with 150 mm NaCl, 50 mm Tris-HCl (pH 7.5), 1% Nonidet P-40, 0.5% sodium deoxycholic acid, and Complete protease inhibitor mixture tablets (Roche Applied Science), and the protein was then isolated (28, 29). Total protein (70 μg) from each sample was separated by 8% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (Millipore). The membranes were blocked with 5% milk in TTBS for 2 h at room temperature and incubated overnight at 4 °C using the following primary antibodies: 1:500 rabbit anti-Klf4 (Santa Cruz), 1:500 mouse anti-ERK1/2 (Santa Cruz), 1:500 mouse antiphospho-ERK1/2 (Santa Cruz), 1:1000 rabbit anti-MEK1/2 (Cell Signaling), 1:1000 rabbit antiphospho-MEK1/2 (Cell Signaling), 1:1000 rabbit anti-p38 (Cell Signaling), 1:500 rabbit anti-PDGFR (Santa Cruz), 1:200 antiphospho-PDGFRβ (Santa Cruz), 1:500 anti-RARα (Santa Cruz), 1:500 anti-retinoic X receptor (RXRα) (Santa Cruz), and 1:1000 mouse anti-β-actin (Santa Cruz). The membranes were then incubated for 1 h at room temperature with a 1:5000 dilution of anti-rabbit/horseradish peroxidase or anti-mouse/horseradish peroxidase (Santa Cruz) and developed with the Chemiluminescence Plus Western blot analysis kit (Santa Cruz).
Balloon Injury Model and Drug Treatment
Animal housing and procedures were approved by the local Animal Care and Use Committee at Hebei Medical University. The animals were anesthetized with urethane (600 mg/kg) intraperitoneally. The thoracic-abdominal artery was de-endothelialized as described previously (29). In brief, the catheter was advanced from the left common carotid artery down to the level of the renal arteries three times with a 2F (60 cm) Fogarty catheter (Baxter, McGaw Park, IL). To attain a constant degree of vessel wall injury for each of the animals, we kept the diameter of the balloon and the resistance during withdrawal constant and the same for each of the animals. A single operator performed all of the procedures. After balloon injury, solutions (50 μl) of pAd-Klf4 (1010 plaque-forming unit/ml) or pAd (1010 plaque-forming unit/ml) were infused into the ligated segment of the common carotid artery for 15 min. The ligatures and catheter were then removed, the external carotid artery was ligated, and the incision was closed.
The drugs were administrated orally at a different dose beginning 1 day before balloon injury and continuing for 3 or 14 days thereafter. On days 3 and 14 after injury and administration of drugs orally, the animals were sacrificed with an overdose of pentobarbital (200 mg/kg), and the thoracic-abdominal artery was collected for Western blot or hematoxylin/eosin staining. The experiments were divided into 12 experimental groups: sham, injury, injury plus pAd, injury plus pAd-Klf4, injury plus pAd plus LY294002 (1.2 mg/kg/day), injury plus pAd-Klf4 plus LY294002, injury plus pAd plus PD98059 (0.5 mg/kg/day), injury plus pAd-Klf4 plus PD98059, injury plus pAd plus Am80 (1 mg/kg/day), injury plus pAd-Klf4 plus Am80, injury plus pAd plus Ro 41-5253 (0.5 mg/kg/day), and injury plus pAd-Klf4 plus Ro 41-5253.
Preparation of PDGF-BB-containing Pluronic Gel and Drug Treatment
Thirty percent (w/v) F-127 Pluronic gel solution was prepared and kept at 4 °C for 24 h. Five milligrams of PDGF-BB was dissolved in 1 ml of phosphate-buffered saline, and 1 ml of this solution was added to 9 ml of the previously cooled 30% Pluronic gel solution. Pluronic gel containing vehicle was formulated via the same method. Immediately following balloon injury, 100 μl of Pluronic gel containing PDGF-BB was applied to the exposed adventitial surface of an approximately 10-mm segment of the injured carotid artery, ∼5 mm proximal to the bifurcation of the internal and external carotid arteries using a 1-ml syringe with an 18-gauge blunted tip needle.
Morphology Analysis
At 14 days after balloon injury, six cross-sections from the middle of each abdominal artery were stained with hematoxylin/eosin. The neointimal and medial areas were calculated using the Image-Pro Plus Analyzer version 5.1 software (Media Cybernetics, Inc., Silver Spring, MD) in a blind manner. For each section, six random, noncontiguous microscopic fields were examined. An algorithm computed the mean thickness (in micrometers) of the intima (I) and media (M) in each field, from which the I/M thickness ratio was derived. To compute the mean thickness values (intima, media, and I/M) of an artery, all of the measurements performed on the three sections of the artery were averaged (29, 30). The data are expressed as the means ± S.D.
Immunohistochemistry
For antigen retrieval, deparaffinized formalin-fixed sections were boiled for 10 min in 10 mm sodium citrate (pH 6). Primary antibody (PCNA at 1:100, PDGFR at 1:100 dilution) was incubated overnight at 4 °C in 1% normal goat serum in phosphate-buffered saline.
Semi-quantitative PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription-PCR was performed as described previously (31). The 5′-ATTAATGAGGCAGCCACCTG-3′ and 5′-TCGTTGAACTCCTCGGTCT-3′, 5′-ACCCCTTCCTAGTGGTGGAC-3′ and 5′-GATACTGCGTCGGAAGAAGC-3′, and 5′-GGAACGAGAATGAGGTGGAG-3′ and 5′-GAGAAGGAGGCAATCAGCAG-3′ primer pairs were used for the Klf4, RARα, and RXRα genes, respectively. For the glyceraldehyde-3-phosphate dehydrogenase gene (used as the internal control), the 5′ACCACAGTCCATGCCATCAC-3′ and 5′TTCACCACCCTGTTGCTGTA-3′ oligonucleotides were used. The PCR conditions were 25 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s.
Chromatin Immunoprecipitation Assay
VSMCs at 80% confluence were cross-linked with 1% formaldehyde for 10 min, lysed as described above, and sonicated 5–10 times each for 10 s at 4 °C to reduce the average DNA length to 0.4–0.5 kb. The samples were diluted 10-fold and then precleared with protein A-agarose/salmon sperm DNA for 30 min at 4 °C followed by an overnight incubation at 4 °C with 1:500 anti-Klf4 or 1:500 anti-mouse IgG (as a negative control). The immune complexes were precipitated with protein A-agarose for 1 h. The samples were heated at 65 °C for 4 h and treated with proteinase K, and the DNA was extracted with phenol/chloroform. The primers for amplifying the RARα promoter region were designed as shown in Fig. 4D. The fidelity of the PCR products from each amplification cycle was verified by melting curve analyses and gel electrophoresis. All PCRs were performed in triplicate (32, 33).
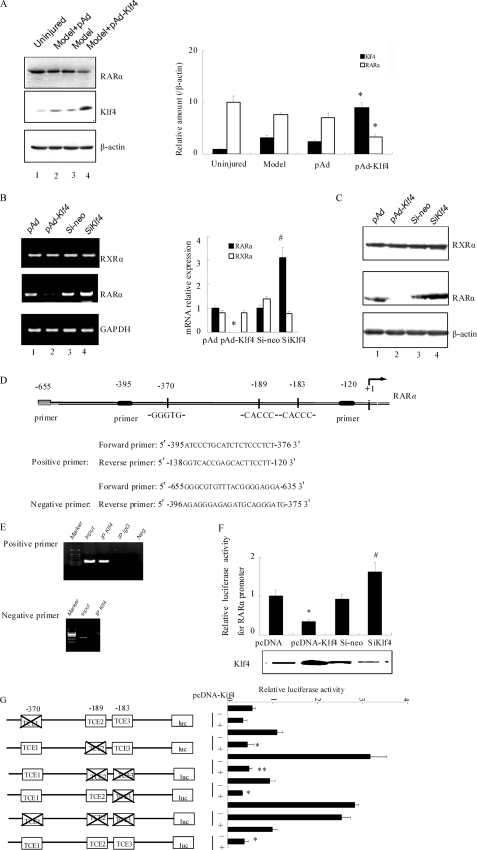
FIGURE 4.
Klf4 down-regulates the expression and activity of RARα. A, immediately following balloon injury, vehicle, pAd, or pAd-Klf4 was infused into the segment of the common carotid artery as described under “Experimental Procedures.” Three days later, the arteries were harvested, and Western blot analysis was performed with antibodies against RARα, Klf4, and β-actin as a loading control. Blots from a representative experiment are shown on the left. Right panel, densitometry analysis was carried out and normalized to β-actin. The bars represent the means ± S.E. of three independent experiments. *, p < 0.05 versus pAd group. B, VSMCs were infected with pAd, pAd-Klf4, control siRNA (si-neo), or siKlf4 for 2 days before lysis and processing for semi-quantitative reverse transcription-PCR. Left panel, result from a representative experiment. Right panel, densitometry analysis for RXRα (open bars) and RARα (filled bars) mRNA was carried out and normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA levels. The bars represent the means ± S.E. of three independent experiments. *, p < 0.05 versus pAd group; #, p < 0.05 versus si-neo group. C, VSMCs were infected as indicated in Fig. 4B and harvested. Western blot analysis was performed with antibodies against RXRα, RARα, and β-actin as a loading control. D, primers used for amplification of the rat RARα promoter for chromatin immunoprecipitation assays. E, chromatin immunoprecipitation assays using anti-Klf4 immunoprecipitates were performed with untreated VSMCs. Nonimmune IgG was used as negative control for immunoprecipitation, DNA was replaced with water as the PCR negative control, and Input corresponds to extracted DNA from the sample prior to immunoprecipitation. The results are from representative experiments that have been repeated three times with similar results. F, A293 cells were transiently cotransfected with the 5′ regulatory region of RARα (−395 to −120 bp) fused in-frame in the pGL3-Basic luciferase reporter vector plasmid together with either pcDNA empty vector, pcDNA-Klf4, control si-neo, or siKlf4. Forty-eight hours later, the cells were harvested, and the activity of firefly luciferase was measured and normalized to that of Renilla luciferase. The bars represent the means ± S.E. from three independent experiments. *, p < 0.05 versus pcDNA group; #, p < 0.05 versus si-neo group. G, A293 cells were cotransfected with the RARα promoter-luciferase constructs indicated on the left of the figure with either pcDNA or pcDNA-Klf4. Twenty-four hours later, luciferase activity was measured as indicated above. The values represent the means ± S.E. of three independent experiments. *, p < 0.05 versus pcDNA group; **, p < 0.01 versus pcDNA group.
Site-directed Mutagenesis of the TCE Element of RARα Promoter
Site-directed mutation of the TCE element of RARα promoter was carried by PCR using oligonucleotide primers that contain a base substitution at the TCE element. The reactions were carried out using a QuikChange site-directed mutagenesis kit (Stratagene). The introduced mutation was verified by DNA sequence analysis. PCR primers used in site-directed mutagenesis of the Klf4-binding sites of RARα promoter were: ΔTCE1 (−370, caccc → cgccc), ΔTCE2 (−189, caccc → cgccc), ΔTCE3 (−183, caccc → gggcg), ΔTCE2/3 (−183 and −189, caccc → cgccc), and ΔTCE1/2/3 (−183, −189, and −370, caccc → cgccc).
Statistical Analysis
All of the data are expressed as the means ± S.E. Differences between two groups were assessed using analysis of variance followed by a Student's t test.
RESULTS
Overexpression of Klf4 Inhibits Neointimal Hyperplasia
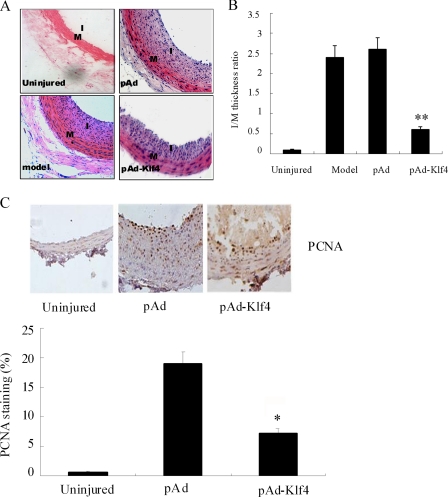
At 14 days after balloon injury, the model and pAd-infected animals showed abundant neointimal hyperplasia (Fig. 1A), which was significantly lower in pAd-Klf4-infected animals, and reduction of the I/M ratio (2.30 ± 0.40 versus 0.52 ± 0.15 in pAd versus pAd-Klf4-infected animals, respectively, p < 0.01). The uninjured arteries revealed no significant neointimal hyperplasia (Fig. 1B). Cell proliferation was examined by immunohistochemical staining of the tissue with a monoclonal antibody to PCNA. Within the intimal lesions of the model animals, 18.2% of cells were PCNA-positive. By contrast, only 7.13% of cells were PCNA-positive in the media of the pAd-Klf4-treated animals, indicating that Klf4 overexpression markedly inhibits proliferation of medial SMCs (Fig. 1C).
FIGURE 1.
Klf4 overexpression reduces neointimal hyperplasia. A, neointima hyperplasia 14 days after balloon injury. Representative sections of hemotoxylin and eosin-stained arterial section from uninjured, model, or pAd- or pAd-Klf4-infected rats. Magnification, ×200. B, morphometric data. **, p < 0.01 versus model group (n = 6 in each group). C, evaluation of VSMC proliferative index by immunohistochemical staining using anti-PCNA. The PCNA index was calculated as follows: PCNA-positive cells/total cells × 100. The bars represent the means ± S.D. of six independent experiments. *, p < 0.05 versus pAd group.
Klf4 Is Important for VSMC Growth Arrest in Vitro
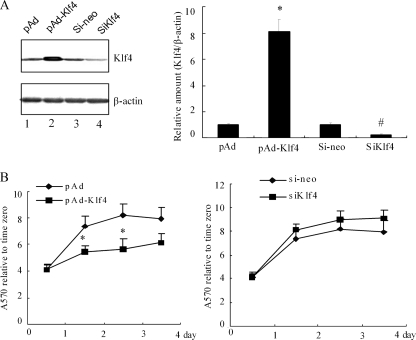
Klf4 has previously been shown to induce growth arrest and suppress cell cycle progression in NIH3T3 cells by inhibiting both G1/S transition and entry into mitosis (34). To investigate the role of Klf4 in VSMC growth arrest, VSMCs were either infected with pAd-Klf4 or treated with siRNA directed against Klf4 (siKlf4). The extent of the Klf4 overexpression or knockdown is shown by Western blotting of the total cell lysate (Fig. 2A). Using MTT assays, we observed that the Klf4-overexpressing cells have a significantly lower proliferation rate as compared with the controls, whereas knockdown of endogenous Klf4 slightly increased cell growth (Fig. 2B). These results further confirm a physiological role for Klf4 in VSMC growth arrest.
FIGURE 2.
Klf4 reduces VSMC cell proliferation. A, VSMCs were infected with pAd, pAd-Klf4, control siRNA (si-neo), or siKlf4 for 2 days before harvesting. The expression of Klf4 was assessed by Western blot analysis. The blots were reprobed for β-actin as a loading control. Left panel, blots from a representative experiment are shown. Right panel, densitometry analysis was carried out and normalized to β-actin. The Klf4 signal in pAd- and si-neo-infected cells were given the value of 1.0. The bars represent the means ± S.E. of three independent experiments. *, p < 0.05 versus control pAd group; #, p < 0.05 versus si-neo group. B, effect of Klf4 overexpression (left panel) or siRNA-mediated Klf4 knockdown (right panel) on VSMC cell proliferation by MTT assay. The values represent fold increase relative to time 0. The experiments were performed in triplicate, and each value is the mean ± S.E. of three independent experiments. *, p < 0.05 versus control plasmid pAd using Student's t test.
Klf4 Inhibits PI3K/Akt and ERK Activation
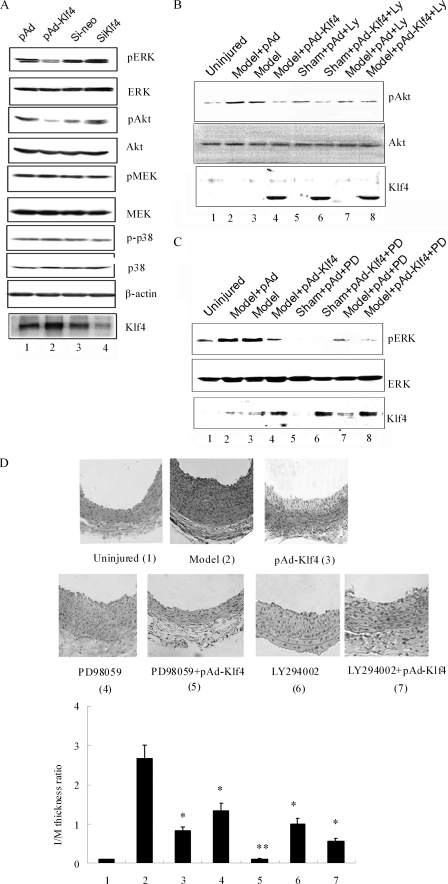
Activation of PI3K and MAPK signaling is crucial for many fundamental cellular processes, including proliferation. In the following experiments, we wanted to determine the signaling pathways that are involved in the anti-proliferative actions of Klf4 in VSMCs. Total cell lysates were examined by Western blotting for the phosphorylation levels of Akt, MEK1/2, ERK1/2, and p38 (Fig. 3A). Klf4 overexpression decreased the basal levels of phospho-Akt and phospho-ERK1/2, whereas the levels of total ERK and Akt remained unchanged (Fig. 3A). On the other hand, knockdown of Klf4 slightly increased Akt and ERK1/2 phosphorylation levels. The signal associated with phosphoactive p38 and phospho-MEK was similar irrespective of the level of Klf4 expression. The data support the hypothesis that Klf4 is a specific regulator of PI3K/Akt and ERK signaling.
FIGURE 3.
Klf4 inhibits constitutive activation of PI3K/Akt and ERK and blocks neointimal hyperplasia after balloon injury. A, VSMCs are infected with pAd, pAd-Klf4, si-neo, or siKlf4 as indicated in the legend of Fig. 2 prior to harvesting. The cell lysates were subjected to Western blot analysis with the antibodies indicated on the right. Note that Klf4 overexpression is associated with lower levels of phosphoactive ERK (pERK, first panel) and Akt (pAkt, third panel). Conversely, Klf4 knockdown increased constitutive Akt and ERK phosphorylation, indicating that Klf4 exerts an inhibitory tone on PI3K/Akt and ERK activation. B and C, an inhibitor of PI3K (LY294002, Ly, B) or ERK (PD98059, PD, C) was given by mouth to rats for 24 h, followed by balloon injury and infusion of pAd or pAd-Klf4 as indicated under “Experimental Procedures.” Three days later, the arteries were harvested, and Western blot analysis was performed with antibodies against phosphoactive and total Akt (B) or phosphoactive and total ERK (C). Representative blots of three independent experiments are shown. D, neointimal hyperplasia was assessed 14 days after balloon injury in the presence or absence of ERK and PI3K inhibitors. Neointimal formation was calculated as the I/M ratio as indicated in Fig. 1A. *, p < 0.05 versus model group (n = 6 in each group). **, p < 0.01 versus model group (n = 6 in each group).
To test whether Klf4 could inhibit PI3K/Akt and ERK signaling in vivo, total cell extracts from injured arteries, harvested 3 days after balloon injury, were prepared and examined by Western blotting. There was an increase in the levels of phospho-Akt and phospho-ERK1/2 upon injury, and pAd-Klf4 infection decreased both of these levels without affecting total Akt and ERK protein levels (Fig. 3, B and C). We also evaluated the effect of selective pharmacological inhibitors of PI3K and ERK in vivo and examined their ability to modulate Klf4-dependent actions toward VSMC proliferation and neointimal formation in vivo. Oral administration of the PI3K antagonist LY294002 (1.2 mg/kg/day) and the ERK-specific antagonist PD98059 (0.5 mg/kg/day) abrogated the activation of PI3K and ERK after arterial injury in rats (Fig. 3, B and C, compare lane 7 versus lane 2). In preliminary experiments, we found that the concentrations of these inhibitors elicited maximal inhibition of PI3K and ERK activities without being toxic to the animals (data not shown). As shown in Fig. 3 (B and C), combinations of Klf4 infection and antagonist treatment further attenuated ERK phosphorylation compared with antagonist treatment alone, indicating the synergistic inhibitory effect of Klf4 and ERK antagonist (Fig. 3C, lane 4 versus lane 8).
We then examined whether the pharmacological inhibition of ERK and PI3K/Akt pathways antagonizes neointimal formation after balloon injury in pAd and pAd-Klf4-infected animals (Fig. 3D). Whereas the I/M ratio increased significantly 14 days after injury (Fig. 3D, Model), oral administration of PD98059 and LY294002 led to a 50–60% decrease in the I/M ratio (p < 0.01) and seemed to be able to exert stronger protection against neointimal formation in pAd-Klf4-infected rats when compared with pAd-infected animals (p < 0.05).
Klf4 Binds to and Regulates RARα
PDGFR and RARα are expressed in VSMCs and play a key role in the regulation of cell proliferation and differentiation. Moreover, both of these receptors can activate PI3K and ERK pathways (18–21). Because Klf4 could inhibit proliferation (25), we speculated that the anti-proliferative effects of Klf4 are mediated via inhibition of PDGFR signaling and/or increase in RARα function. To examine whether Klf4 regulates RARα, pAd-Klf4 was delivered to the vessel wall after balloon injury, and expression of endogenous RARα was assessed in the injured arteries by Western blotting. Unexpectedly, it was observed that overexpression of Klf4 down-regulated RARα expression (Fig. 4A, p < 0.05). To further verify the effect of Klf4 on RARα expression, VSMCs were either infected with pAd-Klf4 or subjected to siRNA-mediated knockdown of endogenous Klf4. Semi-quantitative reverse transcription-PCR analysis showed a marked decrease in RARα mRNA levels in Klf4-overexpressed cells and a 3-fold increase in RARα mRNA expression upon Klf4 knockdown (Fig. 4B). The profile of RARα protein levels was consistent with the mRNA data (Fig. 4C). Importantly, no parallel change in RXRα mRNA and protein levels was detectable under these experimental conditions (Fig. 4, B and C).
To determine whether Klf4 could directly regulate RARα gene transcription, we used the TESS computational program and examined the promoter region of RARα for putative Klf4-binding sites. Two typical Klf4-binding sites (CACCC) and its reverse orientation sequence (GTGGG) were identified within the rat RARα promoter from positions −395 to −120 (Fig. 4D). Chromatin immunoprecipitation assays were then used to get insight into the in vivo interaction between Klf4 and the RARα promoter. The results showed that upon using an anti-Klf4 antibody to precipitate the DNA-protein complex, Klf4 was recruited onto the promoter of RARα but failed to bind when chromatin samples were immunoprecipitated with a control mouse IgG or amplified by negative primers (Fig. 4E). To analyze the functional roles of Klf4 on RARα gene expression, we cloned the −1500 to +65 region of the RARα promoter into the pGL3-Basic luciferase reporter vector and transfected the construct into A293 cells along with pAd-Klf4 or siRNA-mediated Klf4 knockdown. The promoter activity level was decreased by 65% upon Klf4 overexpression, whereas a 40% increase in reporter activity was observed in Klf4-depleted cells (Fig. 4F). These data support the notion that Klf4 is a negative regulator of RARα expression. As shown in Fig. 4D, there are three TCE elements on the RARα promoter. We were interested to know which response elements contributed to the Klf4 responsiveness toward RARα promoter activity by cotransfecting A293 cells with Klf4-expressing plasmid and reporter constructs harboring various TCE deletion mutants. The results showed that inactivation of a single TCE2 or TCE3 element had minimal effect, if any, on basal and Klf4-responsive promoter activity levels as compared with wild-type promoter (Fig. 4G). Although simultaneous inactivation of TCE2 and TCE3 increased the basal reporter activity by 3-fold, it did not interfere with the inhibitory effects of Klf4. Inactivation of TCE1 repressed promoter activity. However, when the three TCE elements were inactivated, the repressive action of Klf4 was lost (Fig. 4G), indicating that the TCE1 element plays a critical role in the Klf4-mediated transcriptional repression of RARα, whereas TCE2 and TCE3 exert a tonic inhibition on basal RARα promoter activity.
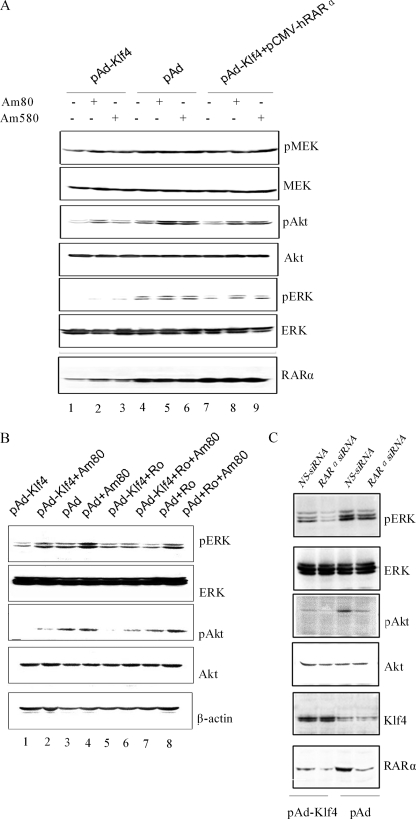
Klf4 Inhibits RARα-mediated Activation of the PI3K/Akt and ERK Pathways
RARα activates the MEK/ERK and PI3K pathways (35). We next investigated whether Klf4-mediated inhibition of PI3K and ERK stems from impaired RARα signaling in VSMCs. pAd- and pAd-Klf4-infected cells were treated with the specific RARα agonists Am80 and Am580 for 4 h, and then total cell lysates were analyzed by Western blotting. Stimulation of endogenous RARα increased the levels of phosphoactive Akt in pAd-infected cells and much less efficiently in Klf4-overexpressing cells (Fig. 5A). On the other hand, phosphoactive ERK levels were not significantly higher in pAd-infected cells after Am80 or Am580 stimulation as compared with basal; however, overexpression of Klf4 repressed both basal and retinoid-stimulated pERK levels in the absence of ERK degradation. We then attempted to evaluate the effects of ectopic expression of RARα on the biological actions of Klf4 in pAd-Klf4-infected VSMCs. The results show that RARα overexpression restored basal and retinoid-dependent responses in pAd-Klf4-infected cells to levels approaching those of pAd-infected cells (Fig. 5A). It follows, therefore, that inhibition of endogenous RARα activity with the specific antagonist, Ro 41-5253, would mimic Klf4 action. Whereas treatment of the cells with Am80 partially restored levels of phosphoactive ERK and Akt in pAd-Kfl4-infected VSMCs, the incubation with Ro 41-5253 blocked ERK activation but not that of Akt in response to Am80 (Fig. 5B, first and third panels, lanes 1 and 2 versus lanes 5 and 6). The role of Klf4 in endogenous RARα signaling was further examined by transfection of pAd- and pAd-Klf4-infected VSMCs with RARα siRNA. Depletion of RARα blocked the levels of phosphoactive ERK and Akt both in control and Klf4-overexpressing cells; however, knockdown of RARα was accompanied by an increased ability of Klf4 to inhibit ERK activation (Fig. 5C, first and third panels).
FIGURE 5.
Effects of Klf4 on RARα-mediated increase in phosphoactive Akt and ERK levels. A, VSMCs were infected with pAd, pAd-Klf4, or pAd-Klf4 plus pCMV-hRARα for 48 h. Serum-starved cells were incubated with vehicle (DMSO) or the RARα agonists Am80 (10 μm) and Am580 (10 μm) for 4 h before harvesting. The cell lysates were immunoblotted with the indicated antibodies. B, pAd- and pAd-Klf4-infected VSMCs were serum-starved for 24 h and then treated with either Am80, the RARα antagonist Ro 41-5253 (20 μm), or the combination Am80 + Ro 41-5253 for 4 h before harvesting. C, pAd- and pAd-Klf4-infected VSMCs were transfected with control (NS) siRNA or rat RARα siRNA and harvested 48 h later. In all experiments, the cells were lysed, and Western blot analysis was carried out with the indicated antibodies. The blots are from representative experiments that have been repeated three times with similar results.
Inhibitory Effect of Klf4 on Neointimal Formation Does Not Require RARα Signaling
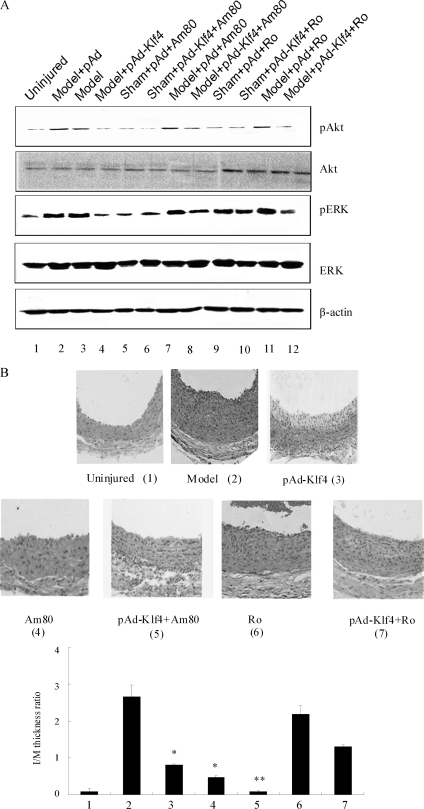
We then tested whether oral administration of Am80 (1 mg/kg/day) or Ro 41-5253 (1 mg/kg/day) would affect neointima hyperplasia after carotid balloon injury. As demonstrated earlier, the levels of phosphoactive Akt and ERK were markedly elevated in the arteries of pAd-infected animals 3 days post injury (Fig. 6A, first and third panels, lane 2 versus lane 1). Administration of Am80 to this group of animals failed to increase the phosphorylation signal further (compare lane 2 versus lane 7). Whereas the levels of Akt and ERK phosphorylation in pAd-Klf4-infected animals were low after injury, the combined treatment with Am80 partially restored these levels (compare lane 4 versus lane 8), although to intensities lower than in the pAd + Am80 group (compare lane 8 versus lane 7). When pAd-infected animals were treated with the RARα antagonist Ro 41-5253, there was increased phosphorylation of Akt and ERK in the arteries after injury (Fig. 6A, compare lane 11 versus lane 9), similar to the levels of the injured pAd-infected group (compare lane 11 versus lane 2), which indicates that RARα activation has little effect on the phosphorylation of Akt and ERK induced by balloon injury. Finally, treatment of pAd-Klf4-infected animals with either vehicle or Ro 41-5253 gave rise to a similar phosphorylation pattern for Akt and ERK after balloon injury (compare lane 4 versus lane 12).
FIGURE 6.
Klf4-induced growth arrest is not dependent on RARα. A, Am80 or Ro 41-5253 was given orally to pAd- and pAd-Klf4-infected animals that were subjected or not to vascular injury. Three days later, the injured arteries were harvested and analyzed by Western blotting using the indicated antibodies. β-Actin was used as a loading control. The blots are representative of three independent animals. B, Am80 or Ro 41-5253 was given orally to pAd- and pAd-Klf4-infected animals that were subjected to balloon injury. Fourteen days later, arterial sections were subjected to histological analysis. Neointimal formation was evaluated by calculating the I/M ratio as indicated in Fig. 1A. *, p < 0.05 versus model group (n = 6 in each group).
To examine neointima formation in response to RARα modulators, we harvested balloon-injured arteries and performed histological analysis. Unexpectedly, we observed that the injury-induced neointima hyperplasia was diminished with Am80 treatment (Fig. 6B). Although Klf4 overexpression also led to reduced neointima formation, the treatment of pAd-Klf4-infected animals with Am80 nearly suppressed hyperplasia. Conversely, RARα inhibition with Ro 41-5253 did not confer protection after balloon injury (Fig. 6B, compare group 6 versus group 2). These results establish that the protective effect of Am80 against injury-mediated neointima formation is independent of PI3K/Akt and ERK pathway activation induced by this synthetic retinoid. These data support the hypothesis that the anti-proliferative responses of pAd-Klf4 do not occur through impaired RARα expression and/or signaling.
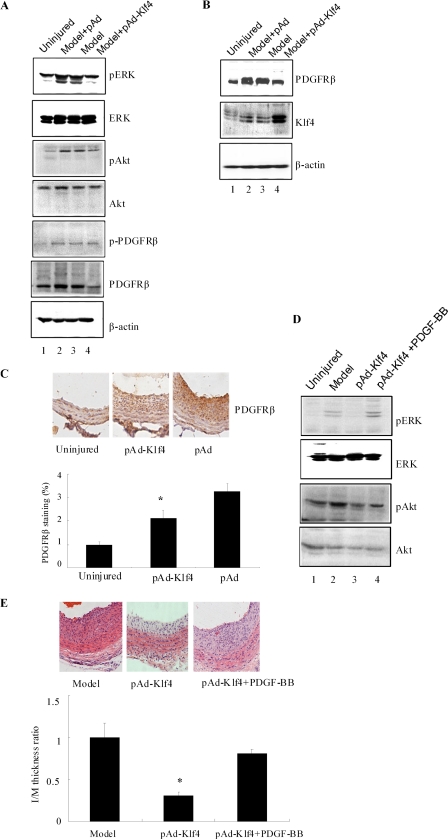
Inhibitory Effects of Klf4 on Neointimal Formation Require Alteration in PDGFR Signaling
Because the anti-proliferative effects of Klf4 are not mediated through RARα inhibition, we examined whether alteration in PDGFR expression and/or signaling could explain growth inhibition by Klf4. Three days after balloon injury, total lysates from the injured arteries were prepared and subjected to Western blotting. There was a clear reduction in PDGFRβ expression in pAd-Klf4-infected animals (Fig. 7A, sixth panel). Probing the membrane with an antibody against the tyrosine-phosphorylated form of PDGFR did not reveal major alteration (Fig. 7A, fifth panel). In agreement with the results in Fig. 3 (B and C), Klf4 overexpression sharply reduced the levels of phosphoactive ERK and Akt that were induced by balloon injury (Fig. 7A, first and third panels). We also evaluated PDGFRβ expression in arteries that were harvested 14 days after balloon injury. Using Western blotting (Fig. 7B) and immunohistochemical analyses (Fig. 7C), it was observed that PDGFRβ staining was markedly weaker in pAd-Klf4-infected animals and coincided with reduced neointima hyperplasia. Lastly, the effect of local delivery of PDGF-BB on neointima formation after carotid balloon injury was examined. As shown in Fig. 7D, PDGF-BB reversed the inhibitory effects of pAd-Klf4 on Akt and ERK activation. Fourteen days after balloon injury, the injured arteries were subjected to histological analysis. Treatment of pAd-Klf4-infected animals with PDGF-BB led to an increase in neointima formation approaching that of the model animals, thus suggesting that PDGF-BB reversed the anti-proliferative effects of pAd-Klf4 (Fig. 7E).
FIGURE 7.
Inhibition of PDGFβ receptor expression and signaling contributes to Klf4-mediated reduction in injury-induced neointimal hyperplasia. A and B, 3 (A) and 14 (B) days after pAd- and pAd-Klf4-infected animals were subjected to vascular injury, the injured arteries were harvested and analyzed by Western blotting using the indicated antibodies. Note the decreased expression of PDGFβ receptor in pAd-Klf4-infected rats following injury. The blots are representative of three independent animals. C, 14 days after vascular injury, the arterial sections were subjected to immunohistochemical staining using anti-PDGFR. PDGFR staining was evaluated as indicated in Fig. 1C. *, p < 0.05 versus control pAd group. D and E, PDGF-BB was given locally to pAd- and pAd-Klf4-infected animals that were subjected to vascular injury. D, 3 days later, the injured arteries were harvested and analyzed by Western blotting using the indicated antibodies. The blots are representative of three independent animals. E, 14 days later, the arterial sections were subjected to histological analysis. Neointimal formation was evaluated by calculating the I/M ratio as indicated in Fig. 1A. *, p < 0.05 versus model group (n = 6 in each group).
Klf4 Inhibits PDGFRβ-mediated Activation of PI3K/Akt Pathway
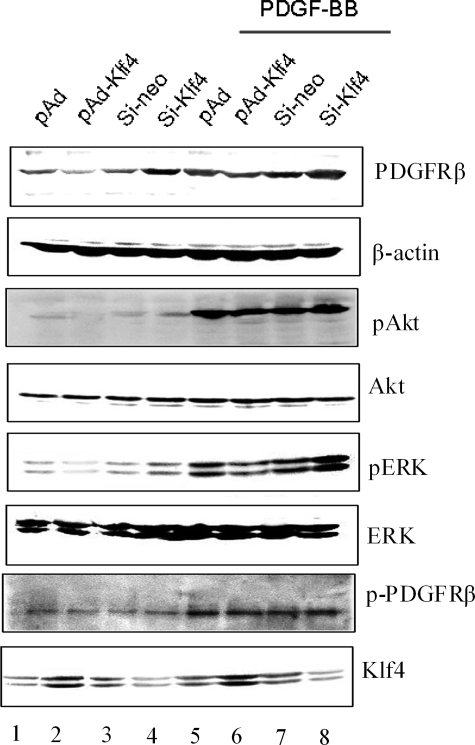
It is well known that liganded PDGFRβ activates the MEK/ERK and PI3K/Akt pathways (19, 22). To investigate whether the regulation of PDGFR signaling by Klf4 occurs via alteration in ERK and Akt phosphorylation, we tested the potency of PDGF-BB in VSMCs when Klf4 is ectopically expressed or the endogenous Klf4 is depleted. It was observed that PDGF-BB-induced phosphorylation of Akt was diminished with Klf4 overexpression but higher upon Klf4 silencing (Fig. 8, third panel). In contrast, the levels of phosphoactive ERK in cells treated with PDGF-BB for 1 h were not significantly different from the basal levels (Fig. 8, fifth panel). The extent of ligand-induced tyrosine phosphorylation of PDGFR was unaffected by the relative expression of Klf4 (Fig. 8, compare seventh and eighth panels, lanes 5–8). These results demonstrate that Klf4 diminishes PDGFRβ-mediated activation of the PI3K/Akt pathway.
FIGURE 8.
Klf4 inhibits PDGFβ receptor expression and signaling in cultured VSMCs. VSMCs were infected with pAd, pAd-Klf4, control siRNA (si-neo), and Klf4 siRNA (si-Klf4) for 2 days followed by incubation in the absence or presence of PDGF-BB (10 ng/ml) for 1 h. The cells were harvested, and Western blot analysis was carried out with the indicated antibodies. The blots are representative of three independent experiments.
DISCUSSION
Proliferation and differentiation of VSMCs are tightly regulated processes (36), whose abnormalities lead to vascular proliferative diseases. Recent studies indicate that Klf4 regulates VSMC proliferation and differentiation by multiple mechanisms, including enhancement of P53 expression and regulation of VSMC marker gene expression (37). In this study, we first determined that overexpression of Klf4 inhibited neointimal hyperplasia caused by balloon injury (Fig. 1). To assess the role of Klf4 in the control of VSMC proliferation, we examined the effect of overexpressing and knocking down Klf4. In cultured VSMCs, Klf4 overexpression inhibited VSMC proliferation stimulated by serum, and Klf4 knockdown promoted cell growth (Fig. 2). These observations suggest that Klf4 is an inhibitor of VSMC proliferation. Indeed, Klf4 was reported to mediate cell growth inhibition by activating numerous genes that encode negative regulators of the cell cycle and suppressing the expression of genes that promote cell cycle progression (38, 39). We have found that overexpression of Klf4 could up-regulate differentiation marker genes SM22α and α-smooth muscle actin and down-regulate the dedifferentiation marker gene SMemb. In addition, transcriptional profiling has further revealed a global inhibitory function for Klf4 in regulating the expression of genes involved in macromolecular synthesis, including transcription, protein, and cholesterol biosynthesis (39, 40). However, it remains unclear whether Klf4 regulates RAR and PDGFR, which comprise a transcriptional factor network regulating proliferation and differentiation of VSMCs.
A number of potential signaling pathways have been implicated in the regulation of growth arrest and differentiation by Klf4. Klf4 has been demonstrated to be either upstream or downstream of the PI3K and MAPK pathways, which may be regulated by the RAR or PDGFR, in different cell types (12). Here, we showed that the levels of phospho-Akt and phospho-ERK 1/2 were reduced in Klf4-overexpressing cells (Fig. 3), indicating that Klf4 is a specific regulator of both signaling pathways. Multiple mechanisms may be involved in the regulation of PI3K and MAPK signaling by Klf4 in VSMCs. These include changes at the level of transcription for proliferation- and differentiation-associated receptors or at the level of interaction with cofactors. Indeed, cross-talk between the PI3K/Akt and ERK pathways has been demonstrated in a diverse set of experimental conditions and in different cell types (41). Functional interactions between these two pathways could play a major role in regulating cell proliferation under particular conditions (42). Our data suggest that both PI3K and ERK antagonists could abrogate the activation of PI3K and ERK by balloon injury, consistent with overexpression of Klf4. PDGFR and RARα are expressed in VSMCs and play a key role in the regulation of cell proliferation and differentiation (18–21). Binding of the specific ligands to these receptors leads to the activation of the PI3K and ERK pathways (22, 35). Because Klf4 exerts anti-proliferative effects through inhibition of PI3K and ERK pathways, we speculated that these actions of Klf4 could be mediated via either PDGFR inhibition or increase in RARα function.
Retinoids modulate cell growth, apoptosis, and differentiation (40, 43). The biological effects of retinoids are mainly mediated by their receptors, i.e. RARα and RXR. RARα interacts with both all-trans-retinoic acid and 9-cis-retinoic acid and has three isoforms (α, β, and γ) (44). The selective activation of these receptors results in distinct biological effects on different cell types (45, 46). It has been suggested that retinoids regulate the expression of distinct target genes by activating different signaling pathways and/or exert opposing effects on the same target gene (47). In fact, most studies on the biological functions of RARα have utilized fibroblasts or cancer cells; these have limited insight into the impact of RARα expression on VSMCs. Recently, the synthetic retinoid Am80, a RARα-specific agonist, was demonstrated to inhibit neointima formation through RARα-induced apoptosis and suppress smooth muscle phenotypic modulation by inhibiting Klf5 (44, 46). Based on the observation that Klf5 and Klf4 have similar structures but opposite effects on VSMC proliferation (48, 49), we hypothesized that the interaction between Klf4 and RARα may also play a key role in regulating VSMC proliferation and differentiation. Most interestingly, we found that RARα inhibition by Klf4 overexpression occurs at the transcriptional level (Fig. 4). Further studies suggest that Klf4 binds to the RARα promoter region in the vicinity of a typical Klf4-binding site and can decrease the transcriptional activity of RARα by 65%, indicating that Klf4 is a negative regulator of RARα expression (Fig. 4). Mutation analysis of the potential Klf4-binding sites within the RARα promoter region indicated that TCE1 element is required for the Klf4-mediated transcriptional repression of RARα, whereas TCE2 and TCE3 play a tonic inhibition on basal RARα promoter activity (Fig. 4), similar to the negative regulation of the transforming growth factor-β1/Smad3 function by Klf4 (1). Ligand-activated RARα up-regulates PI3K and ERK signaling. We speculate that Klf4-mediated inhibition of PI3K and ERK is caused by impaired RARα signaling. Indeed, when Klf4-overexpressing VSMCs were treated with RARα agonists Am80 and Am580, the levels of phosphoactive Akt and ERK were restored (Fig. 5). Inhibition of RARα activity would mimic Klf4 action (Fig. 5), indicating that Klf4 could inhibit RARα expression while blocking PI3K and ERK signaling induced by Am80. We then detected whether oral administration of Am80 or Ro 41-5253 would affect neointima hyperplasia after carotid balloon injury. Administration of Am80 to animals failed to increase the PI3K and ERK signaling, and unexpectedly, the injury-induced neointima hyperplasia was diminished with Am80 treatment (Fig. 6), consistent with the observation of Nagai and co-workers (44). They proved that Am80 suppressed neointima formation by inhibiting KLF5 (44). These data support the hypothesis that the anti-proliferative responses of pAd-Klf4 do not occur in the intact animal through impairing RARα expression and/or signaling. But the expression of the differentiation-related gene RARα is sharply reduced by Klf4, which provides a novel mechanism for the regulation of cell proliferation and differentiation in VSMCs. Klf4 creates a feedback loop of the differentiation effects of the RAR by blocking RARα signaling through inhibition of RARα expression. Thus, we believe that this differentiation-attenuating effect of Klf4 on VSMCs is a by-product, which is to maintain a balance between proliferation and differentiation. Because we have identified that growth arrest effect of Klf4 is not dependent on RARα-PI3K-ERK pathway, this prompted us to further examine whether the growth arrest effects of Klf4 is related to the PDGFR. There are two PDGF receptors, PDGFRα and PDGFRβ. Sano et al. (50) found that blockade of PDGFRα alone had no effect on neointimal formation. PDGFRβ in SMCs plays an important role in SMCs migration and proliferation (50). So, we detected the effect of PDGFRβ directly. We found that Klf4 overexpression significantly down-regulated PDGFRβ expression and phosphorylation of Akt and ERK (Fig. 7). Locally delivered PDGF-BB after carotid balloon injury reversed the inhibitory effect of pAd-Klf4, indicating that the inhibitory effect of Klf4 on neointimal formation is dependent on PDGFRβ-mediated PI3K/Akt and ERK signaling. From these results, we speculated and provided experimental evidence that the anti-proliferative actions of Klf4 stem from its ability to block PDGFRβ expression without altering its phosphorylation, which in turn leads to subsequent attenuation of PI3K and ERK signaling.
In conclusion, we show that the zinc finger transcription factor Klf4 is a central player in a regulatory loop that controls proliferation and differentiation in VSMCs. In addition, we demonstrate for the first time that Klf4 is an inhibitory factor of the RARα gene in VSMCs. So, we conclude that Klf4 plays a dual role: 1) Klf4-induced growth arrest is not dependent on RARα, but Klf4 can mediate the feedback loop of the RARα by blocking the RARα-sustained signaling through inhibition of RARα expression; and 2) Klf4-induced growth arrest is dependent on the PDGFRβ-mediated PI3K and ERK pathways. The results of the present study also provide a novel insight into the actions of RARs in VSMCs and a new mechanism for signal amplification or suppression and for the control of growth inhibitory effects in VSMCs.
This work was supported, in whole or in part, by the Intramural Research Program of the NIA, National Institutes of Health. This work was also supported by Program for Major State Basic Research Development Program of China Grant 2008CB517402, National Natural Science Foundation of People's Republic of China Grants 30770787, 30670845, and 30871272, Key Project of Chinese Ministry of Education Grant 206016, and Hebei Natural Science Foundation of People's Republic of China Grants C2007000831 and C2009001541.
- VSMC
- vascular smooth muscle cell
- Klf
- Krüppel-like factor
- ERK
- extracellular signal-regulated kinase
- PI3K
- phosphatidylinositol 3-kinase
- RAR
- retinoic acid receptor
- MAPK
- mitogen-activated protein kinase
- DMSO
- dimethyl sulfoxide
- siRNA
- small interfering RNA
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MEK
- MAPK/ERK kinase
- RXR
- retinoic X receptor
- I/M
- intima-to-media
- PCNA
- proliferating cell nuclear antigen
- TCE
- transforming growth factor-β control element
- siKlf4
- siRNA directed against Klf4.
REFERENCES
- 1.Liu Y., Sinha S., Owens G. (2003) J. Biol. Chem. 278,48004–48011 [DOI] [PubMed] [Google Scholar]
- 2.Papanicolaou K. N., Izumiya Y., Walsh K. (2008) Circ. Res. 102,16–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan R., Agrotis A., Bobik A. (2007) Cardiovasc. Res. 74,223–234 [DOI] [PubMed] [Google Scholar]
- 4.King K. E., Iyemere V. P., Weissberg P. L., Shanahan C. M. (2003) J. Biol. Chem. 278,11661–11669 [DOI] [PubMed] [Google Scholar]
- 5.Haldar S. M., Ibrahim O. A., Jain M. K. (2007) J. Mol. Cell Cardiol. 43,1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki T., Aizawa K., Matsumura T., Nagai R. (2005) Arterioscler. Thromb. Vasc. Biol. 25,1135–1141 [DOI] [PubMed] [Google Scholar]
- 7.Feinberg M. W., Cao Z., Wara A. K., Lebedeva M. A., Senbanerjee S., Jain M. K. (2005) J. Biol. Chem. 280,38247–38258 [DOI] [PubMed] [Google Scholar]
- 8.Feinberg M. W., Lin Z., Fisch S., Jain M. K. (2004) Trends Cardiovasc. Med. 14,241–246 [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Sinha S., McDonald O. G., Shang Y., Hoofnagle M. H., Owens G. K. (2005) J. Biol. Chem. 280,9719–9727 [DOI] [PubMed] [Google Scholar]
- 10.McDonald O. G., Wamhoff B. R., Hoofnagle M. H., Owens G. K. (2006) J. Clin. Invest. 116,36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pidkovka N. A., Cherepanova O. A., Yoshida T., Alexander M. R., Deaton R. A., Thomas J. A., Leitinger N., Owens G. K. (2007) Circ. Res. 101,792–801 [DOI] [PubMed] [Google Scholar]
- 12.Kawai-Kowase K., Owens G. K. (2007) Am. J. Physiol. Cell Physiol. 292,C59–C69 [DOI] [PubMed] [Google Scholar]
- 13.Milella M., Kornblau S. M., Estrov Z., Carter B. Z., Lapillonne H., Harris D., Konopleva M., Zhao S., Estey E., Andreeff M. (2001) J. Clin. Invest. 108,851–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yen A., Roberson M. S., Varvayanis S., Lee A. T. (1998) Cancer Res. 58,3163–3172 [PubMed] [Google Scholar]
- 15.Pettersson F., Couture M. C., Hanna N., Miller W. H. (2004) Oncogene 23,7053–7066 [DOI] [PubMed] [Google Scholar]
- 16.Rosner D., Stoneman V., Littlewood T., McCarthy N., Figg N., Wang Y., Tellides G., Bennett M. (2006) Am. J. Pathol. 168,2054–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell M., Allen W. E., Sawyer C., Vanhaesebroeck B., Trimble E. R. (2004) Circ. Res. 95,380–388 [DOI] [PubMed] [Google Scholar]
- 18.Mellgren A. M., Smith C. L., Olsen G. S., Eskiocak B., Zhou B., Kazi M. N., Ruiz F. R., Pu W. T., Tallquist M. D. (2008) Circ. Res. 103,1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi H., Igarashi M., Hirata A., Sugae N., Tsuchiya H., Jimbu Y., Tominaga M., Kato T. (2004) Arterioscler. Thromb. Vasc. Biol. 24,2095–2101 [DOI] [PubMed] [Google Scholar]
- 20.Uruno A., Sugawara A., Kanatsuka H., Kagechika H., Saito A., Sato K., Kudo M., Takeuchi K., Ito S. (2005) Circulation 112,727–736 [DOI] [PubMed] [Google Scholar]
- 21.Lü L., Yao T., Zhu Y. Z., Huang G. Y., Cao Y. X., Zhu Y. C. (2003) Am. J. Physiol. Heart Circ. Physiol. 285,H1370–H1377 [DOI] [PubMed] [Google Scholar]
- 22.Mehrhof F. B., Schmidt-Ullrich R., Dietz R., Scheidereit C. (2005) Circ. Res. 96,958–964 [DOI] [PubMed] [Google Scholar]
- 23.Day R. M., Lee Y. H., Park A. M., Suzuki Y. J. (2006) Am. J. Respir. Cell Mol. Biol. 34,695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han M., Wen J. K., Zheng B., Cheng Y., Zhang C. (2006) Am. J. Physiol. Cell Physiol. 291,C50–C58 [DOI] [PubMed] [Google Scholar]
- 25.Wang C., Han M., Zhao X. M., Wen J. K. (2008) J. Biochem. 144,313–321 [DOI] [PubMed] [Google Scholar]
- 26.Yang Y., Goldstein B. G., Nakagawa H., Katz J. P. (2007) FASEB J. 21,543–550 [DOI] [PubMed] [Google Scholar]
- 27.Zheng B., Wen J. K., Han M., Zhou A. R. (2004) Biochim. Biophys. Acta 1690,1–10 [DOI] [PubMed] [Google Scholar]
- 28.Zheng B., Wen J. K., Han M. (2008) FEBS J. 275,1568–1578 [DOI] [PubMed] [Google Scholar]
- 29.Liu B., Han M., Wen J. K. (2008) J. Pharmacol. Exp. Ther. 324,292–298 [DOI] [PubMed] [Google Scholar]
- 30.Zhang X. H., Zheng B., Han M., Miao S. B., Wen J. K. (2009) FEBS Lett. 583,1231–1236 [DOI] [PubMed] [Google Scholar]
- 31.Zheng B., Han M., Wen J. K. (2008) Biochemistry 73,353–357 [DOI] [PubMed] [Google Scholar]
- 32.Zheng B., Han M., Wen J. K., Zhang R. (2008) Biochem. J. 409,683–690 [DOI] [PubMed] [Google Scholar]
- 33.Li A. Y., Han M., Zheng B., Wen J. K. (2008) FEBS Lett. 582,243–248 [DOI] [PubMed] [Google Scholar]
- 34.McConnell B. B., Ghaleb A. M., Nandan M. O., Yang V. W. (2007) Bioessays 29,549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonyak M. A., Boehm J. E., Cerione R. A. (2002) J. Biol. Chem. 277,14712–14716 [DOI] [PubMed] [Google Scholar]
- 36.McDonald O. G., Owens G. K. (2007) Circ. Res. 100,1428–1441 [DOI] [PubMed] [Google Scholar]
- 37.Autieri M. V. (2008) Circ. Res. 102,1455–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowland B. D., Bernards R., Peeper D. S. (2005) Nat. Cell Biol. 7,1074–1082 [DOI] [PubMed] [Google Scholar]
- 39.Wei D., Kanai M., Huang S., Xie K. (2006) Carcinogenesis 27,23–31 [DOI] [PubMed] [Google Scholar]
- 40.Haxsen V., Adam-Stitah S., Ritz E., Wagner J. (2001) Circ. Res. 88,637–644 [DOI] [PubMed] [Google Scholar]
- 41.Venkatachalam K., Mummidi S., Cortez D. M., Prabhu S. D., Valente A. J., Chandrasekar B. (2008) Am. J. Physiol. Heart Circ. Physiol. 294,H2078–H2087 [DOI] [PubMed] [Google Scholar]
- 42.Gervais M., Dugourd C., Muller L., Ardidie C., Canton B., Loviconi L., Corvol P., Chneiweiss H., Monnot C. (2006) Mol. Biol. Cell 17,3940–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kada N., Suzuki T., Aizawa K., Matsumura T., Ishibashi N., Suzuki N., Takeda N., Munemasa Y., Sawaki D., Ishikawa T., Nagai R. (2007) Arterioscler. Thromb. Vasc. Biol. 27,1535–1541 [DOI] [PubMed] [Google Scholar]
- 44.Fujiu K., Manabe I., Ishihara A., Oishi Y., Iwata H., Nishimura G., Shindo T., Maemura K., Kagechika H., Shudo K., Nagai R. (2005) Circ. Res. 97,1132–1141 [DOI] [PubMed] [Google Scholar]
- 45.Miano J. M., Berk B. C. (2001) Arterioscler. Thromb. Vasc. Biol. 21,724–726 [DOI] [PubMed] [Google Scholar]
- 46.Ou H., Haendeler J., Aebly M. R., Kelly L. A., Cholewa B. C., Koike G., Kwitek-Black A., Jacob H. J., Berk B. C., Miano J. M. (2000) Circ. Res. 87,881–887 [DOI] [PubMed] [Google Scholar]
- 47.Chen Q., Ma Y., Ross A. C. (2002) Immunology 107,199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y., Goldstein B. G., Chao H. H., Katz J. P. (2005) Cancer Biol. Ther. 4,1216–1221 [DOI] [PubMed] [Google Scholar]
- 49.Ghaleb A. M., Nandan M. O., Chanchevalap S., Dalton W. B., Hisamuddin I. M., Yang V. W. (2005) Cell Res. 15,92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sano H., Sudo T., Yokode M., Murayama T., Kataoka H., Takakura N., Nishikawa S., Nishikawa S. I., Kita T. (2001) Circulation 103,2955–2960 [DOI] [PubMed] [Google Scholar]