Abstract
In addition to its essential role in permitting mitochondrial import and oxidation of long chain fatty acids, carnitine also functions as an acyl group acceptor that facilitates mitochondrial export of excess carbons in the form of acylcarnitines. Recent evidence suggests carnitine requirements increase under conditions of sustained metabolic stress. Accordingly, we hypothesized that carnitine insufficiency might contribute to mitochondrial dysfunction and obesity-related impairments in glucose tolerance. Consistent with this prediction whole body carnitine dimunition was identified as a common feature of insulin-resistant states such as advanced age, genetic diabetes, and diet-induced obesity. In rodents fed a lifelong (12 month) high fat diet, compromised carnitine status corresponded with increased skeletal muscle accumulation of acylcarnitine esters and diminished hepatic expression of carnitine biosynthetic genes. Diminished carnitine reserves in muscle of obese rats was accompanied by marked perturbations in mitochondrial fuel metabolism, including low rates of complete fatty acid oxidation, elevated incomplete β-oxidation, and impaired substrate switching from fatty acid to pyruvate. These mitochondrial abnormalities were reversed by 8 weeks of oral carnitine supplementation, in concert with increased tissue efflux and urinary excretion of acetylcarnitine and improvement of whole body glucose tolerance. Acetylcarnitine is produced by the mitochondrial matrix enzyme, carnitine acetyltransferase (CrAT). A role for this enzyme in combating glucose intolerance was further supported by the finding that CrAT overexpression in primary human skeletal myocytes increased glucose uptake and attenuated lipid-induced suppression of glucose oxidation. These results implicate carnitine insufficiency and reduced CrAT activity as reversible components of the metabolic syndrome.
Disturbances in mitochondrial genesis, morphology, and function are increasingly recognized as components of insulin resistance and the metabolic syndrome (1–3). Still unclear is whether poor mitochondrial performance is a predisposing factor or a consequence of the disease process. The latter view is supported by recent animal studies linking diet-induced insulin resistance to a dysregulated mitochondrial phenotype in skeletal muscle, marked by excessive β-oxidation, impaired substrate switching during the fasted to fed transition, and coincident reduction of organic acid intermediates of the tricarboxylic acid cycle (4, 5). In these studies, both diet-induced and genetic forms of insulin resistance were specifically linked to high rates of incomplete fat oxidation and intramuscular accumulation of fatty acylcarnitines, byproducts of lipid catabolism that are produced under conditions of metabolic stress (5, 6). Most compelling, we showed that genetically engineered inhibition of fat oxidation lowered intramuscular acylcarnitine levels and preserved glucose tolerance in mice fed a high fat diet (5, 7). In aggregate, the findings established a strong connection between mitochondrial bioenergetics and insulin action while raising new questions regarding the roles of incomplete β-oxidation and acylcarnitines as potential biomarkers and/or mediators of metabolic disease.
In another recent investigation we found that oral carnitine supplementation improved insulin sensitivity in diabetic mice, in parallel with a marked rise in plasma acylcarnitines (8). This occurred in three distinct models of glucose intolerance; aging, genetic diabetes, and high fat feeding (8). The antidiabetic actions of carnitine were accompanied by an increase in whole body glucose oxidation, a surprising result given that carnitine is best known for its essential role in permitting mitochondrial translocation and oxidation of long chain acyl-CoAs. Carnitine palmitoyltransferase 1 (CPT1)2 executes the initial step in this process by catalyzing the reversible transesterification of long chain acyl-CoA with carnitine. The long chain acylcarnitine (LCAC) product of CPT1 traverses the inner membrane via carnitine/acylcarnitine translocase (CACT) and is then delivered to CPT2, which regenerates acyl-CoA on the matrix side of the membrane where β-oxidation occurs. Notably, however, in addition to its requisite role in fatty acid oxidation, carnitine also facilitates mitochondrial efflux of excess carbon fuels. Thus, in the event that rates of substrate catabolism exceed energy demand, accumulating acyl-CoA intermediates are converted back to acylcarnitines, which can then exit the organelle and the tissue. This aspect of carnitine function has remained relatively understudied.
The finding that carnitine supplementation improved glucose tolerance while increasing circulating acylcarnitines favors the interpretation that production and efflux of these metabolites is beneficial rather than detrimental (9, 10). Thus, at present, we view these metabolites as biomarkers rather than mediators of metabolic dysfunction. Acylcarnitine accumulation in insulin-resistant skeletal muscles might reflect a failed attempt to combat “mitochondrial stress” and/or an impediment in tissue export; either of which could arise should availability of free carnitine become limiting. Fitting with this scenario, we postulated that carnitine insufficiency might contribute to mitochondrial dysfunction and insulin resistance. To address this possibility carnitine homeostasis was examined in rodent models of obesity, diabetes, and aging. Our results show that chronic metabolic stress does indeed compromise whole body carnitine status. Low carnitine levels in severely obese rats were associated with aberrant mitochondrial fuel metabolism, whereas oral carnitine supplementation reversed these perturbations in concert with improved glucose tolerance and increased acylcarnitine efflux. Complementary studies in primary human myocytes suggest that the therapeutic actions of carnitine are mediated in part through carnitine acetyltransferase (CrAT), a mitochondrial matrix enzyme that promotes glucose disposal. These findings underscore the multifaceted roles of the carnitine shuttle system, not only in permitting β-oxidation but also for maintaining mitochondrial performance and glucose homeostasis in the face of energy surplus.
MATERIALS AND METHODS
Animals
Animal studies were approved by the Duke University Institutional Animal Care and Use Committee. Male Wistar rats (n = 36) were housed in a temperature-controlled environment with a 12:12-h light:dark cycle and allowed ad libitum access to food and water. Animals were randomly selected to receive either standard chow (7001, Harlan Teklad) or a 45% high fat (HF) diet (D12451; Research Diets) beginning at 3 months of age. After 10 months on HF diet, a subset of the animals (n = 6) were provided supplemental l-carnitine in their drinking water (300 mg/kg body weight/day), which was maintained until sacrifice at 15 months of age. Water consumption was monitored throughout the supplementation phase. On the day of sacrifice food was pulled 4 h prior to harvesting tissue, blood, and urine.
Glucose Tolerance Test
Overnight fasted blood was collected from the tail vein into tubes containing EDTA (1.7 mg/ml). Glucose (2 g/kg body weight) was injected intraperitoneally, blood was collected at 30, 60, 90, and 120 min post injection, and glucose was immediately analyzed in whole blood using a glucose oxidase method (BD Logic Analyzer, BD Medical, Franklin Lakes, NJ). Plasma was obtained for assessing insulin levels by enzyme-linked immunosorbent assay (Linco Research, Inc., St. Louis, MO).
Mitochondrial Isolation
Skeletal muscle mitochondria were prepared based on the procedure of Kerner et al. (11) with some modifications. Rat gastrocnemius muscles were removed under anesthesia and placed in ice cold KMEM buffer (100 mm KCl, 50 mm MOPS, 1 mm EGTA, 5 mm MgSO4, pH 7.4). The tissue was cleaned, blotted, weighed, finely minced, and suspended 10-fold diluted in KMEM plus 1 mm ATP. Twenty milliliters (2 g of tissue) of the homogenate was treated on ice with constant stirring for 10 min with protease (5 mg/g wet weight of tissue, Fluka 82518). The suspension was homogenized on ice using four passes with a Potter-Elvehjem homogenizer. The digestion was stopped by adding 20 ml of KMEM/ATP buffer supplemented with 0.2% BSA. The homogenate was centrifuged at 12,000 × g for 10 min at 4 °C, and the pellet was resuspended in 20 ml of KMEM/ATP/BSA buffer and centrifuged at 300 × g for 10 min at 4 °C, and the supernatant was filtered through two layers of surgical gauze. Mitochondria were pelleted by centrifugation at 7000 × g for 10 min at 4 °C, then washed once in 10 ml of KMEM/ATP/BSA buffer, centrifuged, transferred to a microcentrifuge tube using 2 × 0.75 ml volumes of KME buffer (100 mm KCl, 50 mm MOPS, 0.5 mm EGTA, pH 7.4), and centrifuged at 3500 × g for 10 min at 4 °C. The final pellet was resuspended in KME buffer and immediately used for oxidation assays.
Oxidation Studies
Substrate oxidation was assessed in isolated mitochondria using methods previously described (12). Fatty acid oxidation was measured as the capture of 14CO2 and 14C-acid-soluble metabolites (ASMs) from [1-14C]oleate (100 μm) in the presence and absence of cold pyruvate (5 mm). Pyruvate oxidation was assessed by measuring 14CO2 production from [2-14C]pyruvate (5 mm) in the presence and absence of cold oleate (100 μm). Pyruvate dehydrogenase (PDH) activity was measured in the presence and absence of l-carnitine (5 mm) by capturing 14CO2 from [1-14C]pyruvate (5 mm).
Mitochondrial Oxidative Phosphorylation and Integrity
Mitochondrial function was assessed based on the methods of Kerner et al. (11). Mitochondrial oxygen consumption was measured in duplicate in a 0.5-ml chamber of an OxyTherm oxygen electrode (Hansatech Instruments, Scotland) at 30 °C in respiration medium consisting of 80 mm KCl, 50 mm MOPS, 5 mm KH2PO4, 1 mm EGTA, and 0.1% fatty acid free BSA, pH 7.0. Mitochondrial integrity of all samples was tested each day in a separate polarographic assay consisting of sequential addition of 2 mm ADP, 2.5 mm NADH, 3 μm reduced cytochrome c, and 0.1% deoxycholate to elicit maximal respiration (13). Respiration of NADH and cytochrome c were 4.0 ± 1.6% and 10.5 ± 3.8%, respectively, of detergent-permeabilized maximal respiration through complex IV indicating good intactness of the prepared mitochondria.
Cell Culture
Primary human skeletal myocytes were grown and differentiated as previously described (14). Mature myotubes were lipid loaded with 1% BSA complexed with 1 mm 1:1 oleate:palmitate. After 24 h, cells were washed then maintained in medium containing l-carnitine (100–5000 μm) for an additional 24 h prior to collection of both cell lysates and medium for metabolic profiling. For gene overexpression studies, cytomegalovirus (CMV) promoter-driven recombinant adenovirus containing Myc-tagged rat carnitine acetyltransferase (AdCMV-CrAT) was constructed as previously described (15). A recombinant adenovirus encoding β-galactosidase (AdCMV-β-gal) was used as the control. Adenoviruses were amplified and purified as previously described (15). On day 3 following differentiation human myotubes were treated with 2 × 109 P/cm2 purified AdCMV-CrAT or AdCMV-β-gal. Three days later cells were harvested for protein and metabolic profiling or used to assess rates of glucose oxidation as previously described (5).
Subcellular localization of rCrAT was evaluated in C2C12 myoblasts (ATCC, Manassas, VA) as this analysis required a large number of cells. Myoblasts were raised in growth media (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, and 50 mg/ml gentamycin), treated with AdCMV-CrAT (2 × 109 P/cm2) upon reaching confluence, and harvested 72 h later by trypsinization. The cell suspension was transferred to a 15-ml Falcon tube and spun at 1000 × g for 10 min at 4 °C. The resulting pellet was resuspended in 1 ml of mitochondrial isolation buffer (0.3 m mannitol, 0.1% BSA, 0.2 mm EDTA, 10 mm HEPES, pH 7.4) and homogenized with a 2-ml Dounce homogenizer until 90% of cell membranes were disrupted. After centrifugation at 1,000 × g for 10 min at 4 °C to remove cellular debris, the supernatant was centrifuged at 14,000 × g for 15 min at 4 °C. The pellet was washed twice in mitochondrial isolation buffer, resuspended in CelLytic M buffer (Sigma-Aldrich) containing protease inhibitors (Sigma-Aldrich), and used as the purified mitochondrial fraction. The supernatant was centrifuged at 110,000 × g for 1 h at 4 °C, and the resultant supernatant was used as the cytosolic fraction.
Metabolic Profiling
Free carnitine and acylcarnitines in tissues, plasma, and urine were quantified using tandem mass spectrometry-based metabolic profiling as previously described (7, 16). Urinary carnitine/acylcarnitine levels were normalized to creatinine excretion.
Quantitative Reverse Transcription-PCR
High quality RNA was isolated, cDNA was synthesized, and mRNA was quantified as previously described (5). Sequence-specific primers and probes were designed using Primer Express software (Applied Biosystems, Foster City, CA) for rat/mouse carnitine acetyltransferase (CrAT). Sequences were as follows: forward, 5′-GCAGCTGGCATACTACAGGATCT-3′; reverse, 5′- AGGTGAAACATGCGCAGAGA-3′; and probe, 5′-TGGGCAGGCGTGTGCCACGTATGA-3′. All other genes were assessed using predesigned/prevalidated FAM-labeled Assays-on-Demand and normalized using values from a duplexed reaction with VIC-labeled 18 S endogenous control gene (Applied Biosystems).
Western Blot
Protein was isolated from rodent tissues and cell culture experiments using either radioimmune precipitation assay buffer (Sigma-Aldrich), 2× lysis buffer (20 mm Tris-HCl, 4% SDS, 10 mm NaF, 1 mm EDTA, and 20% glycerol, pH 6.8) or CelLytic (Sigma-Aldrich) supplemented with protease inhibitors (Sigma-Aldrich). Proteins from tissue (25 μg) and cell culture lysates (10 μg) were separated on 10% Criterion gels (Bio-Rad) and transferred to nitrocellulose prior to blocking with 5% milk. Blots were probed using antibodies against CrAT (generously provided by Dr. Fausto Hegardt), citrate synthase (Alpha Diagnostics International, San Antonio, TX), γ-tubulin, or glyceraldehyde-3-phosphate dehydrogenase (Sigma-Aldrich). Proteins were visualized using horseradish peroxidase-conjugated immunoglobulin G antibodies and ECL Plus chemiluminescence detection reagent with band intensity being quantified using ImageQuant software (Amersham Biosciences).
Statistics
JMP software version 7.0 (SAS Institute, Cary, NC) was used to perform multivariate correlation analyses of metabolites measured in Zucker diabetic fatty (ZDF) rats and lean controls. Unsupervised clustering of the Pearson correlation matrix of all pairwise comparisons among individual metabolites was then used to generate the heat map in Fig. 1a. Other statistical analyses were performed using SigmaStat (SysStat Software, Inc., Point Richmond, CA) or Microsoft Excel statistical package. Main effects of age and diet were detected by one-way or two-way analysis of variance using Tukey's post hoc analysis or Student's t test, as detailed in the figure legends. Within-group responses to experimental manipulations were evaluated using a paired t test, where appropriate. All data are presented as mean ± S.E., and the level of significance was established a priori at p ≤ 0.05.
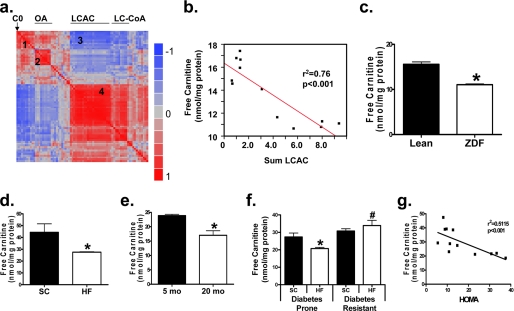
FIGURE 1.
Dimunition of free carnitine levels in insulin-resistant skeletal muscle. A heat map representation (a) of the pairwise correlation matrix was generated using metabolic profiles of gastrocnemius muscles from lean and obese Zucker diabetic fatty (ZDF) rats as described in a previous study (5). Each square represents the Pearson correlation coefficient between the metabolite of the column with that of the row, according to the color scale. Metabolite order was determined by unsupervised clustering of the correlations. Several organic acids (OA) and long chain acylcarnitines (LCAC) clustered together (clusters 2 and 4, respectively). Free carnitine (C0) correlated positively with several short chain acylcarnitines and amino acids (cluster 1) and negatively with several LCAC and long chain CoAs (LC-CoA (cluster 3)). The strong negative relationship between free carnitine and total LCAC is shown in panel b. Tandem MS/MS was used to measure free carnitine content of mixed gastrocnemius muscle specimens obtained from lean and ZDF rats (c), animals fed a standard chow (SC) or high fat (HF) diet for 3 months (d), young (5 month), and aged (20 month) rats fed an SC diet (e), and rats artificially selected for running capacity that exhibit disparate susceptibility to HF diet-induced insulin resistance (f and g). Tissues were harvested 4 h after food withdrawal (a–e), or after an overnight fast (f and g). Data represent means ± S.E. from 5–8 animals per group. Results were analyzed by one-way (c–e) or two-way (f) analysis of variance using Tukey's post hoc test to determine differences between groups. *, p < 0.05 versus control group; #, p < 0.05 diabetes prone versus diabetes resistant rats.
RESULTS
Carnitine Homeostasis Is Compromised by Chronic Metabolic Stress
Interactions between skeletal muscle carnitine content and metabolic dysregulation shown in Fig. 1a were initially identified by multivariate correlation analysis using a metabolomics data set acquired from gastrocnemius muscles of genetically obese ZDF rats and lean controls (5). Unsupervised cluster analysis of all pairwise correlations revealed a “hot spot” (cluster 1) reflecting strong positive correlations between free carnitine (C0), several short chain acylcarnitines and amino acids, and a “cold spot” (cluster 3) reflecting negative associations between free carnitine and several LCAC (Fig. 1b). In addition, the LCAC content of muscle correlated positively (r2 = 0.56, p < 0.005) with LCAC levels in plasma (not shown). These data suggested that excessive production and export of LCAC in the obese and/or diabetic state might lead to carnitine decline.
Because skeletal muscle sequesters an estimated 95% of whole body carnitine reserves (17), we proceeded to survey this tissue as an indicator of systemic carnitine status. Consistent with our prediction, free carnitine levels in mixed gastrocnemius were diminished in multiple rat models of insulin resistance; including ZDF rats (Fig. 1c), diet-induced obesity (Fig. 1d), and aging (Fig. 1e). We also evaluated two contrasting inbred rat strains that were artificially selected based on intrinsic endurance exercise capacity (18). As compared with the low endurance capacity strain, high capacity rats are protected against diet-induced glucose intolerance (12). Fig. 1f shows that high capacity rats maintained skeletal muscle levels of free carnitine when fed an HF diet, whereas levels in the low capacity runners fell below normal. Moreover, linear regression analysis in high versus low capacity rats revealed a significant negative correlation (r2 = 0.5115, p < 0.01) between intramuscular free carnitine levels and insulin resistance, as assessed by the homeostatic assessment model (Fig. 1g). In aggregate, Fig. 1 shows that carnitine homeostasis is compromised in several rodent models of metabolic dysregulation.
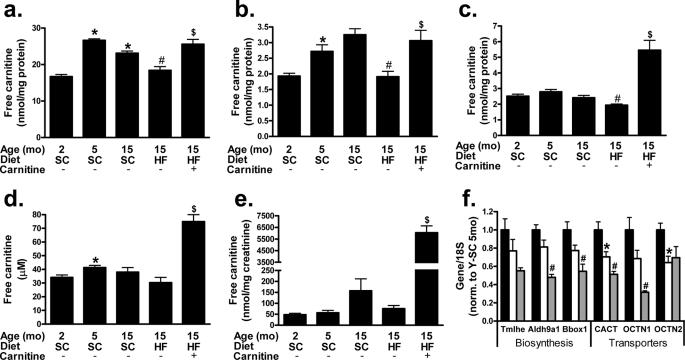
We next sought to gain a more comprehensive understanding of this phenotype by examining whole body carnitine regulation in aging rats fed either a standard chow (SC) or HF diet for 1 year. Our experimental paradigm focused on the interaction between aging and overnutrition and was designed to recapitulate middle-aged onset of metabolic syndrome in obese humans. During development from adolescence to adulthood (2–5 months of age) free carnitine levels increased in the skeletal muscle, liver, and plasma (Fig. 2, a, b, and d, respectively). Conversely, carnitine content in skeletal muscle (Fig. 2a) tended to decrease between adulthood and advanced age (5–15 months). The HF diet exacerbated age-related carnitine loss, as levels fell in each of the tissues analyzed (skeletal muscle, liver, and kidney; Fig. 2, a, b, and c, respectively), although concentrations in plasma were unchanged (Fig. 2d). Neither aging nor the HF diet affected urinary carnitine excretion (Fig. 2e), arguing against a potential impairment in renal reabsorption.
FIGURE 2.
Whole body carnitine status is compromised by lifelong high fat feeding and restored by carnitine supplementation. Tandem MS/MS was used to measure free carnitine levels in mixed gastrocnemius (a), liver (b), kidney (c), plasma (d), and urine (e) harvested 4 h after food withdrawal from young (Y, 5 month) or old (O, 15 month) animals fed a standard (SC) or high fat (HF) diet. Quantitative reverse transcription-PCR was used to measure the expression of hepatic genes involved in cellular (OCTN2) and mitochondrial (CACT and OCTN1) carnitine transport, as well as genes encoding the first (Trimethyllysine hydroxylase-ϵ (Tmlhe)), third (aldehyde dehydrogenase-9 family, member A1 (Aldh9a1)), and fourth/final (γ-butyrobetaine hydroxylase-1 (Bbox1)) reactions involved in carnitine biosynthesis; young (black), old (white), and old-HF (gray) (f). Data represent means ± S.E. from 5–8 animals per group. A one-way analysis of variance with Tukey's post hoc test was used to examine the effects of aging, whereas a Student's t test was used to determine differences due to HF diet and carnitine supplementation. *, p < 0.05 due to aging; #, p < 0.05 due to HFD; $, p < 0.05 due to carnitine supplementation.
To determine whether carnitine supplementation could restore whole body carnitine reserves and improve metabolic outcomes, a subset of the HF group received supplemental l-carnitine during the final 2 months of the diet. The intervention produced robust increases in whole body carnitine reserves (Fig. 2). Importantly, free carnitine content in skeletal muscle (Fig. 2a) and liver (Fig. 2b) was restored to levels found in the SC control animals, whereas that in kidney (Fig. 2c) and plasma (Fig. 2d) increased ∼2-fold. Excess carnitine was excreted in the urine (Fig. 2e).
Expression of Genes Involved in Carnitine Biosynthesis and Transport
l-Carnitine is a quaternary amine obtained from dietary sources or synthesized endogenously from trimethylated lysine residues derived from protein degradation (19). Because the liver is the principal site of carnitine biosynthesis in rats, we proceeded to examine hepatic expression of genes involved in carnitine homeostasis (Fig. 2f). Trimethyllysine hydroxylase-ϵ, aldehyde dehydrogenase-9 family, member A1, and γ-butyrobetaine hydroxylase-1 catalyze the initial, third, and final biosynthetic steps in de novo carnitine biosynthesis, respectively. Between 5 and 15 months of age hepatic expression of these genes tended to decrease, although the trends did not reach statistical significance. The HF diet resulted in a 30–40% reduction in mRNA levels of aldehyde dehydrogenase-9 family (member A1) and γ-butyrobetaine hydroxylase-1; a similar but non-significant trend was observed for trimethyllysine hydroxylase-ϵ. We then probed for genes involved in carnitine transport. Both aging and chronic HF feeding decreased expression of the mitochondrial carnitine transporters, CACT, and organic cation transporter 1 (OCTN1), whereas the plasmalemmal carnitine transporter OCTN2 was diminished in aged rats but did not vary in response to the HF diet. Collectively, these findings suggest that aging and overnutrition might compromise the capacity of the liver to synthesize and transport free carnitine.
Impact of Carnitine Supplementation on Whole Body Metabolism
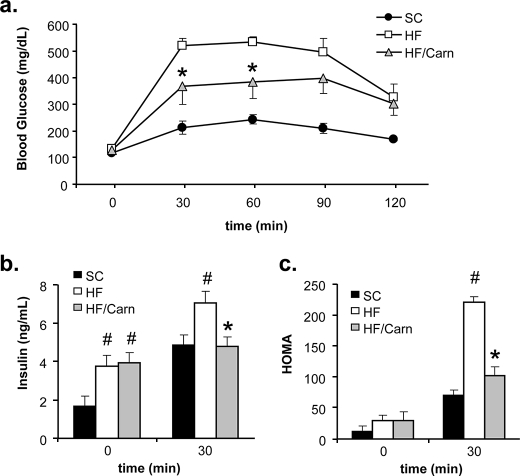
In previous reports we demonstrated that excessive β-oxidation contributes to mitochondrial dysregulation and muscle insulin resistance (5), whereas carnitine supplementation lowered whole body fat oxidation and improved glucose tolerance (8). The present study sought to examine the therapeutic utility of carnitine supplementation in a more severe model of lifelong overnutrition. Indeed, animals fed a HF diet for 12 months were grossly obese, because total body weight and fat pad (epididymal plus perirenal) weights were 28 and 160% higher, respectively, compared with SC controls (not shown). Predictably, animals fed the HF diet developed severe insulin resistance, evidenced by fasting hyperinsulinemia and impaired glucose tolerance (Fig. 3).
FIGURE 3.
Carnitine supplementation improved whole body glucose tolerance. Male Wistar rats were fed either a standard chow (SC) or high fat (HF) diet for 12 months, with or without oral carnitine therapy during the final 2 months (HF/Carn). Intraperitoneal glucose tolerance tests (a) were performed 5 weeks after initiation of carnitine therapy. Plasma insulin values obtained at basal and 30 min post glucose injection (b) were used to calculate the homeostatic assessment model index of insulin resistance (c). Data represent means ± S.E. from 5–8 animals per group. Results were analyzed by Student's t test for between group differences, and a paired t test was applied to detect the within group effect of carnitine. #, p < 0.05 difference versus SC controls; *, p < 0.05 due to carnitine supplementation.
During the final 2 months of the HF diet, half the cohort received carnitine supplementation. Glucose tolerance tests and mitochondrial assays were performed during the fifth and eighth weeks of supplementation, respectively. Although basal glucose and insulin levels were unchanged by the intervention (Fig. 3, a and b), results of the glucose tolerance test reflected a partial improvement in insulin sensitivity. Thus, compared with the unsupplemented HF diet group, both the glucose (Fig. 3a) and insulin (Fig. 3b) excursions during the glucose tolerance test were reduced in the carnitine-treated animals, resulting in a favorable change in the homeostatic assessment model index of insulin resistance (Fig. 3c).
Impact of Carnitine Supplementation on Mitochondrial Metabolism
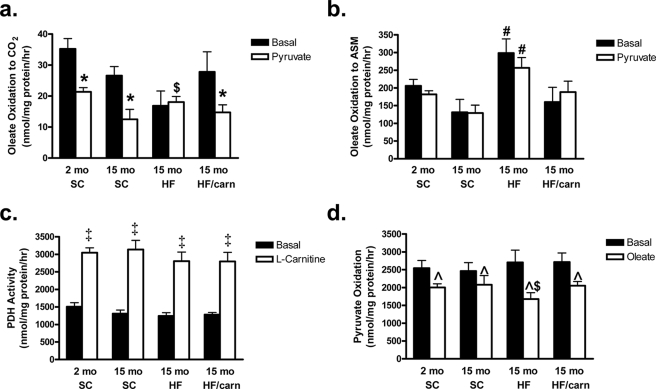
We next sought to test our hypothesis that carnitine supplementation might reverse the dysregulated mitochondrial phenotype that occurs in response to overfeeding (4, 5). Aging from 2 to 15 months resulted in a modest, generalized decrease in mitochondrial fatty acid utilization, as both complete (CO2 production (Fig. 4a)) and incomplete (ASM production (Fig. 4b)) oleate oxidation trended downward. Pyruvate-mediated suppression of [1-14C]oleate oxidation to 14CO2 was similar in mitochondria harvested from the 2- and 15-month animals, consistent with appropriate substrate switching. By contrast, mitochondria from the HF group exhibited a striking imbalance in the ratio of incomplete to complete fat oxidation, such that complete oleate oxidation to CO2 declined while incomplete oxidation to ASM increased markedly. In addition, pyruvate-mediated inhibition of fat oxidation was completely abolished by chronic HF feeding. Remarkably, however, the carnitine intervention not only restored the ratio of incomplete:complete fat oxidation to that in the SC controls but also rescued pyruvate-induced substrate switching.
FIGURE 4.
Carnitine supplementation reverses diet-induced mitochondrial dysregulation. Mitochondria were isolated from mixed gastrocnemius muscles of young (2 month) or old (15 month) male Wistar rats fed either standard chow (SC) or high fat (HF) diet for 12 months, without or with oral carnitine supplementation administered during the final 2 months (HF/Carn). Oxidation of [1-14C]oleate (100 μm) to 14CO2 (a) or 14C-labeled acid soluble metabolites (ASM; panel b) was measured ± pyruvate (5 mm) as an index of substrate switching. PDH activity (c) and pyruvate oxidation (d) were assessed as the liberation of 14CO2 from [1-14C]pyruvate (5 mm) ± l-carnitine (5 mm) or [2-14C]pyruvate (5 mm) ± oleate (100 μm), respectively. Data represent means ± S.E. from 5–8 animals per group. A Student's t test was used to evaluate between group differences, and paired t tests were applied to detect within-group responses to pyruvate, carnitine, and oleate. *, p < 0.05 pyruvate-induced inhibition of oleate oxidation; $, p < 0.05 difference in substrate switching between SC versus HF diet groups; #, p < 0.05 difference between SC and HF diet groups; ‡, p < 0.05 effect of l-carnitine on PDH activity; ^, p < 0.05 oleate-induced inhibition of pyruvate oxidation.
Based on earlier reports (8, 20) we reasoned that carnitine-mediated improvements in mitochondrial metabolism (Fig. 4, a and b) might be at least partly mediated via increased PDH activity. However, results in Fig. 4c argue against this possibility as mitochondrial PDH activity assessed in vitro (using [1-14C]pyruvate) was unaffected by carnitine supplementation administered in vivo. As shown previously, addition of carnitine in vitro to isolated mitochondria increased PDH activity 2-fold, but this effect was similar among groups. We also examined oxidation of [2-14C]pyruvate as an index of tricarboxylic acid cycle function. In the absence of fatty acid, pyruvate oxidation was similar among groups. Addition of oleate inhibited pyruvate oxidation in all groups, but this effect was most pronounced in mitochondria from the HF/un-supplemented group (Fig. 4d). Considering the lack of pyruvate-induced switching in Fig. 4a, we suspect that much of the 14CO2 produced in the pyruvate oxidation assay might occur via anaplerotic entry into the tricarboxylic acid cycle through the pyruvate carboxylase reaction. Taken together, the results suggest that muscle mitochondria derived from animals fed a lifelong HF diet invariably select fatty acids as a preferred fuel, and moreover, that carnitine supplementation reverses this preference and promotes pyruvate utilization. Interestingly, these changes in mitochondrial substrate metabolism were not accompanied by alterations in respiratory function, as assessed by classic oximetry (supplemental Fig. S1).
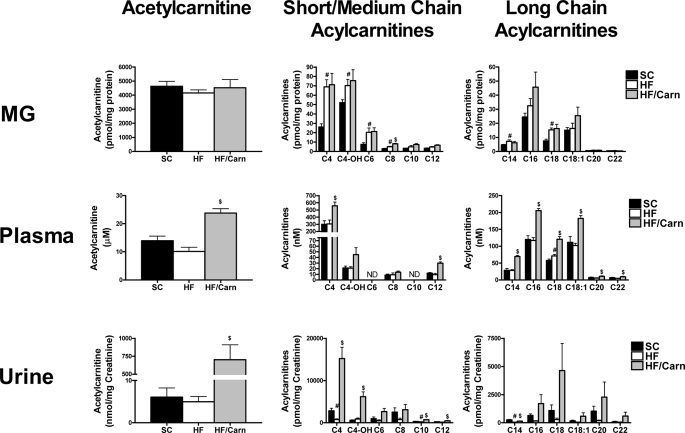
Carnitine Supplementation Promotes Acylcarnitine Efflux
Mass spectrometry was employed to evaluate nutrient-induced changes in production and compartmentalization of acylcarnitine metabolites (Fig. 5). In agreement with our previous findings (4, 5), HF feeding increased accumulation of several short, medium, and LCAC in skeletal muscle. By contrast, acylcarnitine levels in the liver and kidney decreased in the HF as compared with the SC group (supplemental Fig. S2), whereas plasma concentrations were relatively unchanged (Fig. 5). Urinary acylcarnitine excretion (Fig. 5) trended lower in animals fed the HF diet, but rose dramatically after 8 weeks of oral carnitine supplementation. Most notable was a 140-fold increase in urinary levels of acetylcarnitine (C2), typically the most abundant species in all compartments. The rise in acylcarnitine excretion in the supplemented group corresponded with an approximate 2-fold increase in plasma levels of most species, including acetylcarnitine, whereas the tissue acylcarnitine content was generally unchanged in skeletal muscle (Fig. 5), normalized in liver and doubled in kidney (supplemental Fig. S1). Thus, carnitine supplementation did not result in appreciable accumulation of acylcarnitines within tissues, but rather, appeared to facilitate efflux and excretion of these metabolites. Based on these results, we postulated that carnitine therapy might lower tissue content of long chain acyl-CoAs. On the contrary, whereas obesity was associated with robust elevations in several long chain acyl-CoA species, the carnitine treatment was without affect on these metabolites (supplemental Fig. S3).
FIGURE 5.
Effects of carnitine supplementation on acylcarnitine profiles. Tandem MS/MS was used to measure acylcarnitine levels in mixed gastrocnemius (MG), plasma, and urine harvested from male Wistar rats at 15 months of age fed either standard chow (SC) or high fat (HF) diet for 12 months, without or with oral carnitine supplementation administered during the final 2 months (HF/Carn). Data represent means ± S.E. from 5–8 animals per group. Results were analyzed by Student's t test. #, p < 0.05 compared with SC diet; $, p < 0.05 compared with HF group without carnitine.
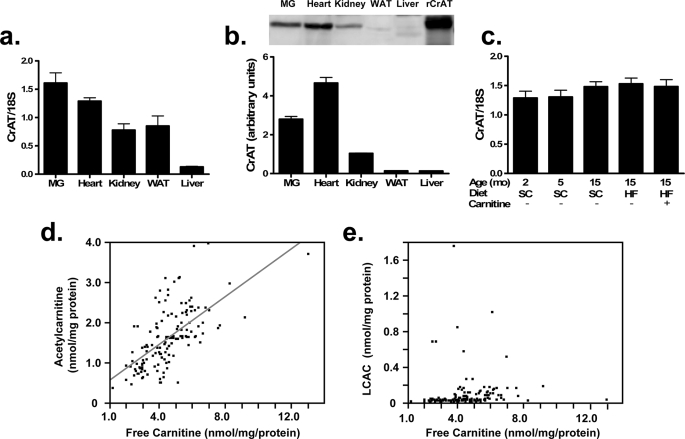
Acetylcarnitine is generated by CrAT, an enzyme predominantly localized to the mitochondrial matrix but also present within peroxisomes and the endoplasmic reticulum. CrAT mRNA (Fig. 6a) and protein (Fig. 6b) expression is most abundant in skeletal muscle and heart, consistent with the finding that acetylcarnitine levels are likewise highest in these tissues (Fig. 5). Lower levels of CrAT were also detected in kidney, liver, and adipose tissue. CrAT mRNA expression in skeletal muscle was unaffected by aging and diet (Fig. 6c), suggesting that fluctuations in acetylcarnitine production were attributable to changes in carnitine availability and/or enzyme activity. We then examined the relationship between muscle content of free carnitine, acetylcarnitine, and LCAC in skeletal muscles comprised of various fiber types harvested from young and older animals fed either a standard or HF diet as described in Fig. 2. This analysis revealed a strong positive correlation between acetylcarnitine and free carnitine (r2 = 0.401, p < 0.001) (Fig. 6d), whereas free carnitine was unrelated to LCAC (Fig. 6e). Thus, modest physiological fluctuations in free carnitine levels appear to influence the activity of CrAT but not CPT1.
FIGURE 6.
Tissue distribution of CrAT. CrAT mRNA (a) and protein (b) expression were determined in mixed gastrocnemius (MG), heart, kidney, liver, and white adipose tissue (WAT). Recombinant rat CrAT (rCrAT) was expressed in human skeletal myotubes and served as a positive control. CrAT mRNA levels were unaffected by experimental treatments (c). Relationships between free carnitine and acetylcarnitine (d) or total long chain acylcarnitines (LCAC) (e) were evaluated using metabolites measured in soleus, red and white quadriceps, and extensor digitorum longus muscles.
Acylcarnitine Efflux from Cultured Human Myocytes
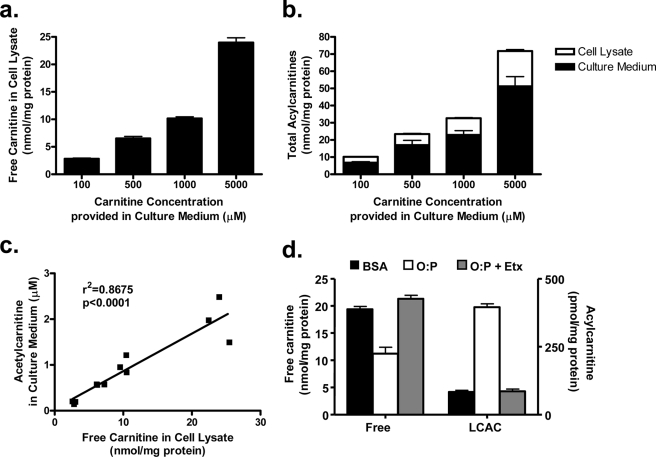
Considering the tissue distribution of CrAT and the high carnitine content of skeletal muscle, this tissue likely produces a large fraction of acetylcarnitine measured in plasma and urine. Fitting with this prediction, muscle concentrations of free carnitine correlated positively (R = 0.39, p < 0.02) with plasma levels of acetylcarnitine (not shown). Thus, carnitine supplementation in obese animals might alleviate mitochondrial stress (Fig. 4) and promote glucose oxidation (Fig. 3) (8) by facilitating export of excess acyl species. This concept was tested by measuring acylcarnitine production and export in primary human myotubes grown in culture. To mimic the lipid-loaded conditions of obese rats, myotubes were incubated overnight with 1 mm fatty acid and then exposed to chase medium with increasing doses of carnitine. Intracellular carnitine content increased dose-dependently as a function of the extracellular concentration (Fig. 7a). The acylcarnitine profiles measured in the cell system closely resembled those obtained from rat muscle and plasma, because acetylcarnitine was the most abundant species (not shown). Roughly 70% of the total acylcarnitine pool was detected in the medium regardless of the incubation conditions, confirming that skeletal myocytes harbor a robust capacity for acylcarnitine export (Fig. 7b). Increasing the carnitine concentration of the medium from 100 μm to 5 mm produced a dose-dependent increase in acetylcarnitine export into the medium (not shown), which correlated strongly with free carnitine content of the cell lysates (Fig. 7c). These results are consistent with in vitro kinetic assays showing that CrAT activity is principally regulated by substrate availability (21, 22). Similar to the acylcarnitine profile of ZDF rats (Fig. 1a), lipid exposure lowered myocyte content of free carnitine in association with LCAC accumulation and export. These effects were completely prevented by addition of the CPT1-inhibitor, etomoxir (Fig. 7d), indicating that cellular carnitine depletion was caused by excessive production of LCAC.
FIGURE 7.
Acylcarnitine production and export by primary human skeletal myotubes. Primary human skeletal myotubes were pulsed with a 1:1 mixture of oleate:palmitate (1 mm) for 24 h in the absence of carnitine, followed by 24-h exposure to chase medium containing 100–5000 μm carnitine, but lacking fatty acid. Mass spectrometry was used to assess free carnitine content within skeletal myotubes (a), total acylcarnitine content (medium plus cell lysates) (b), and acetylcarnitine exported into the medium. Intracellular free carnitine content correlated positively with the amount of acetylcarnitine exported (c). The effect of 24-h lipid exposure (500 μm oleate:palmitate plus 500 μm carnitine) on free carnitine and LCAC content of cells was measured in the absence or presence of 100 μm etomoxir (Etx) (d). Data represent the mean ± S.E. from at least two separate experiments.
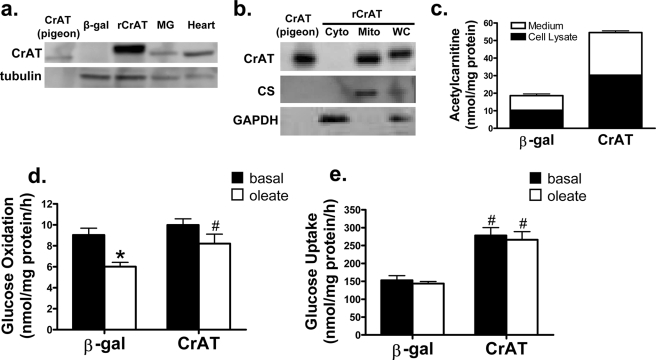
Lastly, to gain more direct evidence that CrAT serves as an important effector of carnitine supplementation, we used recombinant adenovirus encoding Myc-tagged rat CrAT to specifically increase enzyme activity in human myotubes. Treatment with this virus resulted in robust overexpression of rat CrAT as compared with the rAdCMV-β-gal-treated control cells (Fig. 8a). As determined by subcellular fractionation analyses, recombinant CrAT was present in the mitochondrial but not the cytosolic fraction, thus confirming proper localization. Treatment of cells with rAdCMV-CrAT resulted in a 3-fold increase in acetylcarnitine production and export (Fig. 8c). The impact of increased CrAT activity on glucose metabolism was examined in the presence and absence of fatty acids to mimic the metabolic milieu of obesity. In control cells, addition of fatty acids resulted in a robust decrease in glucose oxidation (Fig. 8d), as expected. By contrast, myotubes treated with rAdCMV-CrAT maintained high rates of glucose oxidation regardless of lipid availability. Maintenance of glucose oxidation in myotubes expressing Myc-CrAT was accompanied by a 2-fold increase in uptake of the non-metabolizable glucose analog, 2-deoxyglucose (Fig. 8e). Although the impact of CrAT overexpression on glucose oxidation was modest compared with the 2-fold increase in 2-deoxyglucose uptake, it is important to consider that a large fraction of the acetyl-CoA derived from the PDH reaction was probably exported into the medium as acetylcarnitine (Fig. 8c). In sum, heightened production and efflux of acetylcarnitine in cultured myocytes attenuated lipid-induced suppression of glucose oxidation and promoted glucose disposal, consistent with the effects of carnitine supplementation in vivo.
FIGURE 8.
Overexpression of CrAT in primary human skeletal myotubes promotes glucose uptake and oxidation. Skeletal myocytes were pretreated with recombinant adenoviruses encoding β-galactoside (β-gal) or Myc-tagged rat CrAT. Protein expression of rCrAT in primary human myotubes was detected using an antibody that recognizes rodent but not human CrAT (a). CrAT localization was evaluated using mitochondrial (Mito) and cytosolic (Cyto) fractions and whole cell lysates (WC) prepared from C2C12 myoblasts exposed to rAdCMV-CrAT, followed by SDS-PAGE and Western blot analysis using the aforementioned antibody (b). Metabolic experiments were performed 72 h after virus treatment. Acetylcarnitine levels in cell lysates and medium (c) was assessed 24 h after addition of carnitine. Myotube oxidation of [U-14C]glucose (d) and cellular uptake of [2-3H]deoxyglucose (e) were assessed during a 2-h exposure to radiolabel in the presence or absence of 100 μm oleate. Data represent the mean ± S.E. from 3–4 separate experiments. Results were analyzed by two-way analysis of variance. *, p < 0.05, effect of oleate compared with basal. #, p < 0.05, effect of CrAT compared with β-galactosidase.
DISCUSSION
In the present investigation carnitine decline emerged as a common trait of insulin resistant states, including advanced age, genetic diabetes, and diet-induced obesity. Chronic high fat feeding exacerbated age-related decrements in carnitine homeostasis, coincident with reduced hepatic expression of carnitine regulatory genes and increased sequestration of carnitine in the skeletal muscle acylcarnitine pool. Low carnitine levels in obese rats were associated with impaired fuel metabolism in isolated mitochondria, evidenced by a striking imbalance between complete and incomplete fat oxidation and abnormal substrate selection. Most remarkable, these perturbations were reversed by short term oral carnitine supplementation.
Whole body carnitine homeostasis is regulated at multiple levels, including dietary intake, intestinal absorption, de novo biosynthesis, and renal reabsorption. In omnivorous humans, animal products provide an estimated 75% of whole body carnitine reserves (23). Conversely, vegetarians meet most of their carnitine needs via endogenous production (23). Importantly however, the typical vegetarian diet, which is high in fruits and vegetables and low in fat and calories, naturally lessens carnitine requirements. By contrast, more customary Western diets are comprised of a wide variety of highly processed convenience foods that are low in carnitine content but rich in fat and sugar, similar to the experimental diet used in this study. Our findings suggest that heavy consumption of these foods can provoke an imbalance between carnitine supply and demand.
Early studies suggested that carnitine biosynthesis is limited by availability of trimethyllysine, a byproduct of lysosomal protein degradation (24, 25). However, more current evidence suggests that carnitine reserves can be compromised by depletion of essential micronutrients and/or metabolites, such as iron, vitamin C, and α-ketoglutarate (26–31), all of which are required cofactors for carnitine biosynthetic enzymes. Other clinical factors that disrupt carnitine status include renal dysfunction and use of drugs that form carnitine conjugates (32, 33). Conversely, compounds belonging to the fibrate class of hypolipidemic drugs raise systemic carnitine levels, in parallel with increased hepatic expression of the carnitine biosynthetic machinery (34, 35). These compounds activate PPARα, a lipid-sensing nuclear receptor that functions as a master regulator of fatty acid metabolism. Recent studies in PPARα null mice confirmed an essential role for this nuclear receptor in regulating carnitine homeostasis, because these animals have a profound systemic carnitine deficiency associated with reduced hepatic expression of OCTN2 and γ-butyrobetaine hydroxylase-1 (35, 36). Consistent with these reports, short term high fat feeding (4 days), which activates the PPAR network, increases systemic carnitine levels (34, 37). By contrast, we found that chronic high fat feeding diminished expression of genes involved in carnitine biosynthesis, in line with emerging evidence that overnutrition disrupts hepatic PPARα function (5, 38). In sum, diet-induced obesity appears to set the stage for carnitine decline at multiple levels; increased mitochondrial load, poor nutrient quality, and perturbations in hepatic gene regulation.
Recent attention on the role of mitochondrial dysfunction in age-related metabolic disorders has sparked renewed interest in the therapeutic potential of mitoprotective nutrients. Supplemental l-carnitine is one such therapy that is Food and Drug Administration-approved for treating inborn errors in metabolism, including genetic mutations in CACT and CPT2, as well as various mitochondrial dehydrogenase deficiencies (39). Patients afflicted with these disorders present with nonketotic hypoglycemia, muscle weakness, pronounced elevations in LCAC, and systemic carnitine depletion. Numerous case reports have found that carnitine therapy alleviates these symptoms (reviewed in Ref. 40). More direct evidence linking carnitine imbalance to mitochondrial dysfunction comes from the juvenile visceral steatosis (jvs) mice, a genetic model of primary carnitine deficiency. These animals harbor a spontaneous point mutation in OCTN2, resulting in severely depleted tissue carnitine content and marked derangements in mitochondrial genesis and morphology, as well as whole body lipid and energy metabolism (41, 42).
Relatively few studies have evaluated deficiency and/or repletion of carnitine in the context of overnutrition and diabetes. We previously reported that carnitine supplementation effectively restored insulin responsiveness in multiple mouse models of glucose intolerance (8). In the present study, carnitine therapy improved whole body glucose tolerance in grossly obese rats that had been feeding on a HF diet for a full year. Although the rescue was only partial, any improvement could be considered remarkable given the severity of the model. Taken together, the two investigations provide compelling evidence that oral carnitine supplements administered to obese rodents not only restores tissue carnitine content, but also enhances glucose disposal and mitochondrial performance. A limited number of small scale clinical studies have likewise reported positive outcomes of l-carnitine therapy in humans (43–45). In these studies, acute carnitine infusion during a hyperinsulinemic-euglycemic clamp was found to increase glucose disposal and whole body carbohydrate oxidation in insulin-resistant subjects (43, 44). Despite these promising results, studies to evaluate the potential antidiabetic efficacy of long term carnitine therapy are surprisingly sparse. We found only a single report in which investigators reported that 12 weeks of carnitine supplementation decreased fasting blood glucose levels in a cohort of 35 subjects (45).
The precise mechanisms underlying the therapeutic utility of carnitine in rodents remain unclear, although mitochondrial energy metabolism is a probable target. In addition to its prominent role in permitting mitochondrial import and oxidation of long chain fatty acids, carnitine also enables mitochondrial export of excess carbon fuel, thereby buffering intramitochondrial imbalances between acyl-CoA load and tricarboxylic acid cycle activity. This latter function may be critically important in the settings of obesity and diabetes, because these disorders impose a heavy lipid load on muscle mitochondria (5, 46, 47). Herein, we found that lifelong high fat feeding resulted in a more severe disconnect between complete and incomplete fatty acid oxidation as compared with that observed with shorter duration diets. For example, 3 months of high fat feeding did not compromise mitochondrial capacity to fully oxidize lipid substrate (5), whereas in the present study prolonged overnutrition lowered rates of complete fat oxidation in concert with a marked rise in incomplete oxidation. These results are reminiscent of those reported in moderately obese compared with severely obese humans (1, 48, 49). Interestingly, herein and in previous reports (50, 51), alterations in mitochondrial energetics occurred absent overt perturbations in respiratory control or capacity. Thus, in both humans and animals, mitochondrial dysregulation appears to occur as a progressive manifestation of chronic obesity, but does not necessarily encompass defects in respiratory function.
A key finding of this study was that short term carnitine supplementation reversed the perturbations in mitochondrial fuel metabolism caused by 12 months of HF feeding. The salutary effects of the intervention were associated with striking increases in plasma and urinary acetylcarnitine. Previous work suggested that tissue export of acetylcarnitine lowers local concentrations of acetyl-CoA, which in turn favors increased pyruvate dehydrogenase activity and higher rates of glucose oxidation (8, 20). Likewise, we showed that addition of carnitine to isolated mitochondria increased PDH activity. This mechanism presumably contributes to enhanced glucose oxidation in vivo. Importantly, however, PDH activity was similar between the supplemented and control groups when measured at a fixed carnitine concentration in isolated mitochondria, the same assay conditions that revealed an improvement in the balance between complete and incomplete fat oxidation. These results imply that chronic carnitine depletion/repletion in vivo leads to mitochondrial adaptations beyond changes in PDH. In addition to regulating the activities of CPT1 and PDH, the carnitine shuttle system is thought to maintain mitochondrial function by lowering intramitochondrial acyl-CoA content and/or recycling free CoA (Fig. 9). Thus, carnitine might combat mitochondrial stress by preserving free CoA levels in the face of excess substrate. This, in turn, could impact a number of mitochondrial enzymes and/or acyl-CoA-dependent signaling mechanisms, including those involving acylation, succinylation, and acetylation of targeted proteins (52).
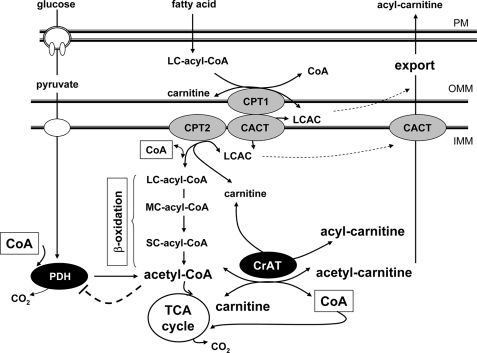
FIGURE 9.
Proposed role of carnitine and CrAT in regulating mitochondrial energetics. TheLCAC product of CPT1 traverses the inner mitochondrial membrane (imm) via CACT and is then delivered to CPT2, which regenerates acyl-CoA on the matrix side of the membrane. The enzymes of β-oxidation degrade long chain acyl-CoAs to shorter species through a recurrent, multistep process that yields one two-carbon molecule of acetyl-CoA in each successive cycle. When acyl-CoA production exceeds consumption, these intermediates can be converted back to their acylcarnitine counterparts and exported from the mitochondria into the general circulation. CrAT, a mitochondrial matrix enzyme that prefers short-chain acyl esters, regulates mitochondrial metabolism by lowering the acetyl-CoA/free CoA ratio and regenerating free CoA, which is used by PDH and the tricarboxylic acid (TCA) cycle enzyme, α-ketoglutarate dehydrogenase. Futile production and export of LCAC might compromise the intramitochondrial pool of carnitine, thus limiting CrAT activity.
Our results strongly suggest that the therapeutic actions of carnitine supplementation involve CrAT, because overexpression of this enzyme in primary human myotubes promoted glucose uptake and mitigated lipid-induced suppression of glucose oxidation. Additional studies are needed to gain a more complete, mechanistic understanding of how CrAT regulates mitochondrial energetics. Nevertheless, this is the first direct evidence showing that enhanced CrAT activity opposes the “Randle effect” (e.g. lipid-induced lowering of glucose oxidation) (53). In the context of normal physiology, this regulatory mechanism might take effect during the transition between fasting and feeding.
CrAT is localized primarily within the mitochondrial matrix, prefers short-chain acyl-CoAs and catalyzes a reversible reaction with an equilibrium constant of 1.5–1.8 (54, 55). Thus, enzyme activity and direction is thought to be predominantly regulated by substrate/product concentrations within the mitochondrial matrix. Regulatory factors controlling the relative distribution of carnitine between intra- and extramitochondrial compartments are poorly understood. Because CPT1 resides on the outer mitochondrial membrane, this enzyme might gain first access to limited amounts of free carnitine, whereas the intramitochondrial carnitine pool could be more susceptible to depletion. As such, we reason that mild carnitine deficiency might permit β-oxidation while selectively compromising CrAT activity. This possibility is supported by our finding that skeletal muscle levels of free carnitine correlated strongly with tissue content of acetylcarnitine but was unrelated or negatively related to levels of LCAC.
Although our study highlighted skeletal muscle as a key beneficiary of carnitine supplementation, the intervention probably influenced metabolic regulation in multiple tissues. We emphasize, however, that free carnitine content and CrAT expression are most abundant in skeletal and cardiac muscles; thus these tissues are likely to account for a large fraction of the serum acylcarnitine pool. Our results support this notion as we found that 70–90% of acylcarnitines generated by human skeletal myocytes were exported into the culture medium. This observation prompts intriguing questions regarding the biological role(s) of exported acylcarnitines. In carnitine-supplemented animals, urinary excretion of total acylcarnitines increased 130-fold. Still, a large proportion of the circulating acylcarnitines are retained by renal reabsorption (56), implying that these molecules might be destined for other metabolic fates (57–59). Interestingly, acetylcarnitine supplements have proven therapeutically effective in models of neuronal dysfunction. Positive outcomes include increased ATP levels in the brain, faster neural conduction, improved cognition, diminished oxidative stress, and enhanced mitochondrial function (60–63). These reports tempt speculation that the CrAT system evolved as a mechanism to redirect excess carbons from highly oxidative skeletal muscle and heart to neurons and other cell types that are less competent consumers of long chain fatty acids. An inter-organ shuttle of this kind could play a vital role during episodes of energy stress, including starvation and sustained exercise.
In summary, this study shows that carnitine status is compromised in multiple rodent models of obesity and glucose intolerance. We are not suggesting that carnitine decline is a root cause of metabolic failure, but rather that it is a consequence of chronic lipid surplus that exacerbates energy dysregulation. In line with this premise, we provide compelling evidence that carnitine repletion improved metabolic control in obese rats. The importance of the carnitine antiport system in combating metabolic disease and regulating mitochondrial function warrants further investigation and larger scale human trials.
Supplementary Material
Acknowledgments
We thank Chris Newgard for his thoughtful evaluation of this manuscript. We thank the dedicated staff of the Metabolomics and Biomarker Core of the Sarah W. Stedman Nutrition and Metabolism Center.
This work was supported, in whole or in part, by National Institutes of Health Grants P30-AG028716 and R01-AG028930 (to D. M. M.) and F32-DK080609 (to R. N.). This work was also supported by the American Diabetes Association (to D. M. M.) and a John A. Hartford Duke Center for Excellence Award (to T. R. K).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- CPT1
- -2,carnitine palmitoyltransferases 1 and 2
- PPAR
- peroxisome proliferator-activated receptor
- LCAC
- long-chain acylcarnitine
- CACT
- carnitine/acylcarnitine translocase
- CrAT
- carnitine acetyltransferase
- HF
- high fat
- MOPS
- 4-morpholinepropanesulfonic acid
- BSA
- bovine serum albumin
- ASM
- acid-soluble metabolite
- PDH
- pyruvate dehydrogenase
- CMV
- cytomegalovirus
- Ad
- adenovirus
- ZDF
- Zucker diabetic fatty
- SC
- standard chow
- OCTN1
- -2,organic cation transporters 1 and 2
- MS
- mass spectrometry
- MS/MS
- tandem MS.
REFERENCES
- 1.Kelley D. E., Goodpaster B., Wing R. R., Simoneau J. A. (1999) Am. J. Physiol. 277,E1130–E1141 [DOI] [PubMed] [Google Scholar]
- 2.Morino K., Petersen K. F., Shulman G. I. (2006) Diabetes 55, Suppl. 2, S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrauwen P., Hesselink M. K. (2004) Diabetes 53,1412–1417 [DOI] [PubMed] [Google Scholar]
- 4.Koves T. R., Li P., An J., Akimoto T., Slentz D., Ilkayeva O., Dohm G. L., Yan Z., Newgard C. B., Muoio D. M. (2005) J. Biol. Chem. 280,33588–33598 [DOI] [PubMed] [Google Scholar]
- 5.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., Lopaschuk G. D., Muoio D. M. (2008) Cell Metab. 7,45–56 [DOI] [PubMed] [Google Scholar]
- 6.Wood J. C., Magera M. J., Rinaldo P., Seashore M. R., Strauss A. W., Friedman A. (2001) Pediatrics 108,E19. [DOI] [PubMed] [Google Scholar]
- 7.An J., Muoio D. M., Shiota M., Fujimoto Y., Cline G. W., Shulman G. I., Koves T. R., Stevens R., Millington D., Newgard C. B. (2004) Nat. Med. 10,268–274 [DOI] [PubMed] [Google Scholar]
- 8.Power R. A., Hulver M. W., Zhang J. Y., Dubois J., Marchand R. M., Ilkayeva O., Muoio D. M., Mynatt R. L. (2007) Diabetologia 50,824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roe C. R., Hoppel C. L., Stacey T. E., Chalmers R. A., Tracey B. M., Millington D. S. (1983) Arch. Dis. Child 58,916–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrigoni-Martelli E., Caso V. (2001) Drugs Exp. Clin. Res. 27,27–49 [PubMed] [Google Scholar]
- 11.Kerner J., Turkaly P. J., Minkler P. E., Hoppel C. L. (2001) Am. J. Physiol. Endocrinol. Metab. 281,E1054–E1062 [DOI] [PubMed] [Google Scholar]
- 12.Noland R. C., Thyfault J. P., Henes S. T., Whitfield B. R., Woodlief T. L., Evans J. R., Lust J. A., Britton S. L., Koch L. G., Dudek R. W., Dohm G. L., Cortright R. N., Lust R. M. (2007) Am. J. Physiol. Endocrinol. Metab. 293,E31–E41 [DOI] [PubMed] [Google Scholar]
- 13.Puchowicz M. A., Varnes M. E., Cohen B. H., Friedman N. R., Kerr D. S., Hoppel C. L. (2004) Mitochondrion. 4,377–385 [DOI] [PubMed] [Google Scholar]
- 14.Muoio D. M., Way J. M., Tanner C. J., Winegar D. A., Kliewer S. A., Houmard J. A., Kraus W. E., Dohm G. L. (2002) Diabetes 51,901–909 [DOI] [PubMed] [Google Scholar]
- 15.Becker T. C., BeltrandelRio H., Noel R. J., Johnson J. H., Newgard C. B. (1994) J. Biol. Chem. 269,21234–21238 [PubMed] [Google Scholar]
- 16.Haqq A. M., Lien L. F., Boan J., Arlotto M., Slentz C. A., Muehlbauer M. J., Rochon J., Gallup D., McMahon R. L., Bain J. R., Stevens R., Millington D., Butler M. D., Newgard C. B., Svetkey L. P. (2005) Contemp. Clin. Trials 26,616–625 [DOI] [PubMed] [Google Scholar]
- 17.Brass E. P. (1995) Clin. Ther. 17,176–185; discussion 175 [DOI] [PubMed] [Google Scholar]
- 18.Koch L. G., Britton S. L. (2001) Physiol. Genomics 5,45–52 [DOI] [PubMed] [Google Scholar]
- 19.Vaz F. M., Wanders R. J. (2002) Biochem. J. 361,417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uziel G., Garavaglia B., Di Donato S. (1988) Muscle Nerve 11,720–724 [DOI] [PubMed] [Google Scholar]
- 21.Bhuiyan A. K., Bartlett K., Sherratt H. S., Agius L. (1988) Biochem. J. 253,337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chase J. F. (1967) Biochem. J. 104,510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiber A., Kerner J., Hoppel C. L. (2004) Mol. Aspects Med. 25,455–473 [DOI] [PubMed] [Google Scholar]
- 24.Davis A. T., Hoppel C. L. (1986) J. Nutr. 116,760–767 [DOI] [PubMed] [Google Scholar]
- 25.Rebouche C. J., Lehman L. J., Olson L. (1986) J. Nutr. 116,751–759 [DOI] [PubMed] [Google Scholar]
- 26.Citak E. C., Citak F. E., Kurekci A. E. (2006) Pediatr. Hematol. Oncol. 23,381–385 [DOI] [PubMed] [Google Scholar]
- 27.Mikhail M. M., Mansour M. M. (1976) Clin. Chim. Acta 71,207–214 [DOI] [PubMed] [Google Scholar]
- 28.Okamoto M., Ueno Y. (2006) J. Clin. Forensic Med. 13,26–29 [DOI] [PubMed] [Google Scholar]
- 29.Otsuka M., Matsuzawa M., Ha T. Y., Arakawa N. (1999) J. Nutr. Sci. Vitaminol. (Tokyo) 45,163–171 [DOI] [PubMed] [Google Scholar]
- 30.Fischer G. M., Nemeti B., Farkas V., Debreceni B., Laszlo A., Schaffer Z., Somogyi C., Sandor A. (2000) Biochim. Biophys. Acta 1501,200–210 [DOI] [PubMed] [Google Scholar]
- 31.Farkas V., Bock I., Cseko J., Sandor A. (1996) Biochem. Pharmacol. 52,1429–1433 [DOI] [PubMed] [Google Scholar]
- 32.Okudaira N., Fujigaki M., Nakayoshi T., Komiya I., Sugiyama Y. (2001) Pharm. Res. 18,439–445 [DOI] [PubMed] [Google Scholar]
- 33.Brass E. P., Mayer M. D., Mulford D. J., Stickler T. K., Hoppel C. L. (2003) Clin. Pharmacol. Ther. 73,338–347 [DOI] [PubMed] [Google Scholar]
- 34.Koch A., Konig B., Stangl G. I., Eder K. (2008) Exp. Biol. Med. (Maywood.) 233,356–365 [DOI] [PubMed] [Google Scholar]
- 35.van Vlies N., Ferdinandusse S., Turkenburg M., Wanders R. J., Vaz F. M. (2007) Biochim. Biophys. Acta 1767,1134–1142 [DOI] [PubMed] [Google Scholar]
- 36.Makowski L., Noland R. C., Koves T. R., Xing W., Ilkayeva O. R., Muehlbauer M. J., Stevens R. D., Muoio D. M. (2009) FASEB J. 23,586–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cederblad G. (1987) Am. J. Clin. Nutr. 45,725–729 [DOI] [PubMed] [Google Scholar]
- 38.Li X., Monks B., Ge Q., Birnbaum M. J. (2007) Nature 447,1012–1016 [DOI] [PubMed] [Google Scholar]
- 39.Longo N., Amat di San Filippo C., Pasquali M. (2006) Am. J. Med. Genet. C. Semin. Med. Genet. 142C,77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winter S. C. (2003) J. Inherit. Metab. Dis. 26,171–180 [DOI] [PubMed] [Google Scholar]
- 41.Kaido M., Fujimura H., Ono A., Toyooka K., Yoshikawa H., Nishimura T., Ozaki K., Narama I., Kuwajima M. (1997) Eur. Neurol. 38,302–309 [DOI] [PubMed] [Google Scholar]
- 42.Tein I. (2003) J. Inherit. Metab. Dis. 26,147–169 [DOI] [PubMed] [Google Scholar]
- 43.Capaldo B., Napoli R., Di Bonito P., Albano G., Saccà L. (1991) Diabetes Res. Clin. Pract. 14,191–195 [DOI] [PubMed] [Google Scholar]
- 44.Mingrone G., Greco A. V., Capristo E., Benedetti G., Giancaterini A., De Gaetano A., Gasbarrini G. (1999) J. Am. Coll. Nutr. 18,77–82 [DOI] [PubMed] [Google Scholar]
- 45.Rahbar A. R., Shakerhosseini R., Saadat N., Taleban F., Pordal A., Gollestan B. (2005) Eur. J. Clin. Nutr. 59,592–596 [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Roves P., Huss J. M., Han D. H., Hancock C. R., Iglesias-Gutierrez E., Chen M., Holloszy J. O. (2007) Proc. Natl. Acad. Sci. U.S.A. 104,10709–10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hancock C. R., Han D. H., Chen M., Terada S., Yasuda T., Wright D. C., Holloszy J. O. (2008) Proc. Natl. Acad. Sci. U.S.A. 105,7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hulver M. W., Berggren J. R., Cortright R. N., Dudek R. W., Thompson R. P., Pories W. J., MacDonald K. G., Cline G. W., Shulman G. I., Dohm G. L., Houmard J. A. (2003) Am. J. Physiol. Endocrinol. Metab. 284,E741–E747 [DOI] [PubMed] [Google Scholar]
- 49.Thyfault J. P., Kraus R. M., Hickner R. C., Howell A. W., Wolfe R. R., Dohm G. L. (2004) Am. J. Physiol. Endocrinol. Metab. 287,E1076–E1081 [DOI] [PubMed] [Google Scholar]
- 50.Anderson E. J., Lustig M. E., Boyle K. E., Woodlief T. L., Kane D. A., Lin C. T., Price J. W., 3rd, Kang L., Rabinovitch P. S., Szeto H. H., Houmard J. A., Cortright R. N., Wasserman D. H., Neufer P. D. (2009) J. Clin. Invest. 119,573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacLellan J. D., Gerrits M. F., Gowing A., Smith P. J., Wheeler M. B., Harper M. E. (2005) Diabetes 54,2343–2350 [DOI] [PubMed] [Google Scholar]
- 52.Kim S. C., Sprung R., Chen Y., Xu Y., Ball H., Pei J., Cheng T., Kho Y., Xiao H., Xiao L., Grishin N. V., White M., Yang X. J., Zhao Y. (2006) Mol. Cell 23,607–618 [DOI] [PubMed] [Google Scholar]
- 53.Randle P. J., Garland P. B., Hales C. N., Newsholme E. A. (1963) Lancet 1,785–789 [DOI] [PubMed] [Google Scholar]
- 54.Farrell S. O., Fiol C. J., Reddy J. K., Bieber L. L. (1984) J. Biol. Chem. 259,13089–13095 [PubMed] [Google Scholar]
- 55.Pieklik J. R., Guynn R. W. (1975) J. Biol. Chem. 250,4445–4450 [PubMed] [Google Scholar]
- 56.Huang W., Shaikh S. N., Ganapathy M. E., Hopfer U., Leibach F. H., Carter A. L., Ganapathy V. (1999) Biochem. Pharmacol. 58,1361–1370 [DOI] [PubMed] [Google Scholar]
- 57.Aureli T., Puccetti C., Di Cocco M. E., Arduini A., Ricciolini R., Scalibastri M., Manetti C., Conti F. (1999) Eur. J. Biochem. 263,287–293 [DOI] [PubMed] [Google Scholar]
- 58.Farrell S., Vogel J., Bieber L. L. (1986) Biochim. Biophys. Acta 876,175–177 [DOI] [PubMed] [Google Scholar]
- 59.Lligona-Trulla L., Arduini A., Aldaghlas T. A., Calvani M., Kelleher J. K. (1997) J. Lipid Res. 38,1454–1462 [PubMed] [Google Scholar]
- 60.Ames B. N., Liu J. (2004) Ann. N.Y. Acad. Sci. 1033,108–116 [DOI] [PubMed] [Google Scholar]
- 61.Dhitavat S., Ortiz D., Shea T. B., Rivera E. R. (2002) Neurochem. Res. 27,501–505 [DOI] [PubMed] [Google Scholar]
- 62.Nalecz K. A., Miecz D., Berezowski V., Cecchelli R. (2004) Mol. Aspects Med. 25,551–567 [DOI] [PubMed] [Google Scholar]
- 63.Ricciolini R., Scalibastri M., Kelleher J. K., Carminati P., Calvani M., Arduini A. (1998) J. Neurochem. 71,2510–2517 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.