Abstract
Signaling by the B cell receptor (BCR) promotes integrin-mediated adhesion and cytoskeletal reorganization. This results in B cell spreading, which enhances the ability of B cells to bind antigens and become activated. Proline-rich tyrosine kinase (Pyk2) and focal adhesion kinase (FAK) are related cytoplasmic tyrosine kinases that regulate cell adhesion, cell morphology, and cell migration. In this report we show that BCR signaling and integrin signaling collaborate to induce the phosphorylation of Pyk2 and FAK on key tyrosine residues, a modification that increases the kinase activity of Pyk2 and FAK. Activation of the Rap GTPases is critical for BCR-induced integrin activation as well as for BCR- and integrin-induced reorganization of the actin cytoskeleton. We now show that Rap activation is essential for BCR-induced phosphorylation of Pyk2 and for integrin-induced phosphorylation of Pyk2 and FAK. Moreover Rap-dependent phosphorylation of Pyk2 and FAK required an intact actin cytoskeleton as well as actin dynamics, suggesting that Rap regulates Pyk2 and FAK via its effects on the actin cytoskeleton. Importantly B cell spreading induced by BCR/integrin co-stimulation or by integrin engagement was inhibited by short hairpin RNA-mediated knockdown of either Pyk2 or FAK expression and by treatment with PF-431396, a chemical inhibitor that blocks the kinase activities of both Pyk2 and FAK. Thus Pyk2 and FAK are downstream targets of the Rap GTPases that play a key role in regulating B cell morphology.
Antibodies (Abs)2 made by B lymphocytes play a critical role in host defense against infection. Antigen-induced signaling by the B cell receptor (BCR) initiates an activation program that leads to B cell proliferation and subsequent differentiation into Ab-producing cells. BCR clustering by antigens or by anti-immunoglobulin (anti-Ig) Abs used as surrogate antigens initiates multiple signaling pathways that control gene expression, cell survival, and proliferation pathways (1–3).
BCR signaling also promotes integrin activation (4, 5), localized actin polymerization, reorganization of the actin cytoskeleton, and changes in B cell morphology (6, 7), all of which may facilitate B cell activation. Integrin activation and cell spreading is critical for the activation of B cells by membrane-bound antigens. Macrophages, dendritic cells, and follicular dendritic cells can present arrays of captured antigens to B cells (8, 9), and this may be one of the main ways in which B cells encounter antigens (10). BCR-induced integrin activation prolongs the interaction between the B cell and the antigen-presenting cell and also allows the B cell to spread on the surface of the antigen-presenting cell such that more BCRs can encounter and bind membrane-bound antigens (11). Subsequent contraction of the B cell membrane allows the B cells to gather the BCR-bound antigen into an immune synapse in which clustered antigen-engaged BCRs are surrounded by a ring of ligand-bound integrins. Formation of this immune synapse reduces the amount of antigen that is required for B cell activation (12, 13).
Recent work has shown that B cells in lymphoid organs may contact soluble antigens by extending membrane processes into a highly organized network of lymph-filled conduits (14). These conduits are created by fibroblastic reticular cells that partially ensheathe collagen fibrils. In addition to being rich in collagen, fibronectin, and other extracellular matrix (ECM) components, the fibroblastic reticular cells that form these conduits express high levels of intercellular adhesion molecule-1, the ligand for the αLβ2 integrin (lymphocyte function-associated antigen-1 (LFA-1)) on B cells (10). Thus B cells interacting with these conduits are likely to be in contact with integrin ligands, and integrin-dependent spreading may enhance the ability of B cells to extend membrane processes into the fibroblastic reticular cell conduit.
In addition to promoting cell spreading, integrins can act as co-stimulatory receptors that enhance signaling by many receptors including the T cell receptor and the BCR (15–17). Thus signaling proteins that regulate B cell spreading and that are also targets of BCR/integrin co-stimulation may play a key role in the activation of B cells by membrane-bound antigens as well as soluble antigens that are delivered to lymphoid organs by fibroblastic reticular cell conduits.
Proline-rich tyrosine kinase (Pyk2) and focal adhesion kinase (FAK) are related non-receptor protein-tyrosine kinases that integrate signals from multiple receptors and play an important role in regulating cell adhesion, cell morphology, and cell migration in many cell types (18–20). Integrins, receptor tyrosine kinases, antigen receptors, and G protein-coupled chemokine receptors all stimulate tyrosine phosphorylation of Pyk2 and FAK, a modification that increases the enzymatic activity of these kinases and allows them to bind SH2 domain-containing signaling proteins (21). FAK, which is expressed in almost all tissues (21), is a focal adhesion component that mediates integrin-dependent cell migration (22), cell spreading, and cell adhesion (18) in adherent cells as well as co-clustering of LFA-1 with the T cell receptor in lymphocytes (23). Pyk2 is expressed mainly in hematopoietic cells, osteoclasts, and the central nervous system (24) and is critical for chemokine-induced migration of B cells, macrophages, and natural killer cells (20, 25, 26) as well as the spreading of osteoclasts on vitronectin (27). FAK and Pyk2 are thought to mediate overlapping but distinct functions because Pyk2 expression only partially reverses the cell adhesion and migration defects in FAK-deficient fibroblasts (28).
In B cells, clustering of the BCR, β1 integrins, or β7 integrins induces tyrosine phosphorylation of both Pyk2 and FAK (29–33). FAK is involved in the chemokine-induced adhesion of B cell progenitors (34), and Pyk2 is required for chemokine-induced migration of mature B cells (25). However, the role of these kinases in BCR- and integrin-induced B cell spreading has not been investigated, and the signaling pathways that link the BCR and integrins to tyrosine phosphorylation of Pyk2 and FAK have not been elucidated.
We have shown previously that the ability of the BCR to induce integrin activation, B cell spreading, and immune synapse formation requires activation of the Rap GTPases (6, 17). In addition to binding effector proteins such as RapL and Rap1-interacting adaptor molecule (RIAM) that promote integrin activation (35–37), the active GTP-bound forms of Rap1 and Rap2 bind multiple proteins that control actin dynamics and cell morphology (38). Moreover we showed that BCR/integrin-induced phosphorylation of Pyk2 in B cells is dependent on Rap activation (17). However, this previous study did not address how Rap-GTP links the BCR and integrins to Pyk2 phosphorylation, whether Rap activation is important for FAK phosphorylation in B cells, or whether B cell spreading is regulated by Pyk2 or FAK. We now show that Pyk2 and FAK are differentially expressed and localized in B cells, that Pyk2 and FAK are important for B cell spreading, and that integrin engagement enhances BCR-induced phosphorylation of Pyk2 and FAK, a process that depends on both Rap activation and actin dynamics.
EXPERIMENTAL PROCEDURES
Antibodies and Inhibitors
Goat and donkey anti-mouse IgG and goat anti-mouse IgM were from Jackson ImmunoResearch Laboratories (West Grove, PA). Rat monoclonal anti-LFA-1 (anti-αL integrin) and anti-CD40 (1C10) were from eBioscience (San Diego, CA). A rat monoclonal Ab against very late antigen-4 (anti-VLA-4; anti-α4 integrin) was purified from culture supernatants of the PS/2 hybridoma (39) (from Dr. Bosco Chan, University of Western Ontario, London, Ontario, Canada). Rabbit anti-Erk, goat anti-Pyk2 (sc-1514), and goat anti-FAK (C-20) were from Santa Cruz Biotechnology (Santa Cruz, CA). The 4G10 monoclonal anti-phosphotyrosine (Tyr(P)) Ab was from Upstate (Charlottesville, VA). Abs to Tyr397-, Tyr576-, and Tyr577-phosphorylated FAK and Tyr579/Tyr580-phosphorylated Pyk2 were from BIOSOURCE International (Camarillo, CA). The rabbit polyclonal Ab against Tyr402-phosphorylated Pyk2, the murine monoclonal Ab against phosphorylated Erk, and the rabbit monoclonal Ab against Ser473-phosphorylated Akt were from Cell Signaling Technology (Danvers, MA). The monoclonal Ab to paxillin was from BD Biosciences. Horseradish peroxidase-conjugated donkey anti-goat IgG (Santa Cruz Biotechnology), goat anti-rabbit IgG (Bio-Rad), and goat anti-mouse IgG (GE Healthcare) were used for immunoblotting. Latrunculin A and jasplakinolide were from Calbiochem. PF-431396 has been described previously (40). The pCMV-δR8.91 and pCMV-VSV-G-M5 plasmids were a gift from Dorothee von Laer (Georg-Speyer Haus Chemotherapeutic Institute, Frankfurt, Germany).
Cells
B cells were isolated from the spleens of C57BL/6 mice using the magnetic-activated cell sorting B cell isolation kit (Miltenyi Biotec, Auburn, CA) to deplete non-B cells (41). The resulting cells were >98% B cells as determined by staining with anti-CD19-fluorescein isothiocyanate (BD Pharmingen). Activated B cells were obtained by culturing splenic B cells with 25 μg/ml lipopolysaccharide (LPS; Sigma-Aldrich) plus 5 ng/ml IL-4 (R&D Systems, Minneapolis, MN) for 2–3 days. A20 cells (ATCC, Manassas, VA) were maintained as described previously (17). Bulk populations of A20 cells stably transduced with the empty pMSCVpuro vector (BD Biosciences Clontech) or with pMSCVpuro/RapGAPII have been described previously (17).
Expression of Pyk2 and FAK
For immunoblotting with Abs to Pyk2 or FAK, cells were solubilized in radioimmune precipitation assay buffer (42). For quantitative RT-PCR, RNA was prepared using the RNeasy kit with QIAshredder columns (Qiagen, Valencia, CA) and converted into cDNA using the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). Equivalent amounts of cDNA were combined with TaqMan Fast Universal PCR Master Mix (Applied Biosystems) plus TaqMan Gene Expression Assay primers and probes (Applied Biosystems) specific for Pyk2 (Mm00552840_m1), FAK (Mm00433209_m1), or glyceraldehyde-3-phosphate dehydrogenase (Mm99999915_g1). PCRs and quantitation were performed using an Applied Biosystems 7500 Fast Real-Time PCR system. The amount of Pyk2 or FAK mRNA was normalized to the amount of glyceraldehyde-3-phosphate dehydrogenase mRNA for each sample.
RT-PCR Analysis of Pyk2 mRNA Splicing
PCR primers (5′-GTGGCCTCTCCTGAGTGTGT-3′ and 5′-GATCTTCTCTGCCTCCCAGA-3′) that flank the alternatively spliced exon of the mouse Pyk2 gene were used to amplify cDNA from resting and activated mouse B cells. These primers amplify a 738-bp fragment from cDNA generated from unspliced Pyk2 mRNA and a 612-bp fragment from cDNA from the hematopoietic cell-specific Pyk2 isoform in which a 126-bp exon is deleted. PCR products were separated on 2% agarose gels and visualized with CyberSafe DNA gel stain.
Immunofluorescence
Cells were fixed with 3% paraformaldehyde for 20 min and then permeabilized with phosphate-buffered saline plus 0.1% Tween 20 for 45 min. After blocking with phosphate-buffered saline containing 2% bovine serum albumin for 10 min, the cells were stained with goat Abs to Pyk2 or FAK for 45 min followed by Alexa Fluor 488-conjugated donkey anti-goat IgG (Molecular Probes-Invitrogen) for 30 min. Where indicated, cells were also stained with rat monoclonal Abs to LFA-1 or VLA-4 followed by Alexa Fluor 568-conjugated donkey anti-rat IgG (Molecular Probes-Invitrogen). The cells were washed and adhered to poly-l-lysine-coated coverslips, which were treated with Prolong Gold antifade reagent containing 4′,6-diamidino-2-phenylindole (Molecular Probes-Invitrogen) and mounted onto glass slides. Images were collected using an Olympus IX81/Fluoview1000 confocal microscope and processed using Olympus Fluoview 1.6 software.
Phosphorylation of Pyk2, FAK, Erk, Akt, and Paxillin
A20 cells or splenic B cells (1.5 × 107) in 1 ml of modified HEPES-buffered saline (41) were stimulated with anti-Ig Abs either while in suspension or 30 min after being added to wells of 6-well tissue culture plates coated with a collagen/fibronectin ECM (17, 43). This ECM was generated by sequentially coating the wells with a 2% gelatin solution and then fetal calf serum. To initiate integrin signaling, cells were added to wells that had been coated with Abs to LFA-1 or VLA-4 as described previously (6). Reactions were terminated by adding 0.25 ml of cold 5× lysis buffer (17). After 10 min on ice, insoluble material was removed by centrifugation. Where indicated, aliquots of cell lysate were removed to assess total protein tyrosine phosphorylation or the phosphorylation of Erk, Akt, and paxillin. Pyk2 and FAK were immunoprecipitated from cell lysates as described previously (34, 41).
Short Hairpin RNA (shRNA)-mediated Knockdown of Pyk2 and FAK Expression in A20 Cells
pGIPZ lentiviral vectors encoding GFP as well as microRNA-adapted shRNAs (shRNAmirs) specific for murine Pyk2 (catalogue number V2LMM_21947) or FAK (catalogue number V2LMM_37327) were purchased from Open Biosystems (Huntsville, AL). Lentiviruses were generated by transfecting 293T cells with the appropriate lentiviral vector (7.5 μg) together with 12.5 μg of pCMV-δR8.91 and 2 μg of pCMV-VSV-G-M5 (44, 45). Viral supernatants were collected 24 and 48 h after transfection and filtered through a 0.45-μm filter. A20 cells (6 × 105) were added to wells of a 6-well dish containing 3 ml of viral supernatant and then centrifuged at 2000 rpm for 1 h at 21 °C. Cells were cultured with 5 μg/ml puromycin to select for transduced cells.
Cell Spreading
Tissue culture plates were coated overnight at 4 °C with a rat anti-mouse LFA-1 monoclonal Ab (6) or with fibronectin (R&D Systems) and then blocked with phosphate-buffered saline containing 2% bovine serum albumin for 1 h. A20 cells (105 cells in 0.5 ml of RPMI 1640 medium with 2% fetal calf serum and 50 μm 2-mercaptoethanol) were pretreated with DMSO or PF-431396 for 45 min, added to the coated wells, and incubated at 37 °C. Cells scored as spread were phase dark and had an elongated or irregular shape with obvious membrane processes.
Rap Activation
Rap activation assays were performed as described previously (17). A GST-RalGDS fusion protein was used to selectively precipitate the active GTP-bound form of Rap, which was detected by immunoblotting with a Rap1 Ab (Santa Cruz Biotechnology).
RESULTS
Expression and Localization of Pyk2 and FAK in B Cells
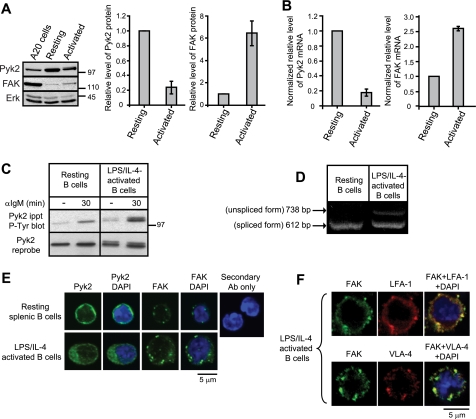
Because Pyk2 and FAK regulate cell morphology in many cell types, we asked whether both of these kinases were expressed in mature B cells from mouse spleen. Immunoblotting showed that resting B cells from mouse spleen expressed high levels of Pyk2 but only low levels of FAK (Fig. 1A). We also asked whether B cell activation altered the expression of Pyk2 or FAK because activated, but not resting, primary B cells undergo dramatic spreading when plated on integrin ligands or on immobilized Abs to CD44, CD23, or the BCR (46–48). Activating splenic B cells with LPS plus IL-4 for 2 days resulted in a 4–5-fold decrease in Pyk2 protein levels and a 6-fold increase in FAK levels (Fig. 1A). This likely reflects transcriptional regulation because a similar down-regulation of Pyk2 mRNA and up-regulation of FAK mRNA occurred upon B cell activation (Fig. 1B). A number of murine (WEHI-231, BAL17, and A20) and human B lymphoma cell lines (Ramos, Daudi, and Raji) expressed both Pyk2 and FAK (Fig. 1A and data not shown), consistent with these cells representing transformed versions of activated B cells. We also observed that Pyk2 from LPS/IL-4-activated B cells ran as a doublet on SDS-PAGE gels (Fig. 1, A and C). The higher molecular weight form of Pyk2 may be the unspliced form that has been reported to be highly expressed in brain but not in the spleen (49). Indeed RT-PCR showed that both the spliced and unspliced forms of Pyk2 mRNA were present in activated B cells, whereas only the spliced form was present in resting B cells (Fig. 1D). Both isoforms of the Pyk2 protein were tyrosine-phosphorylated upon BCR clustering in activated splenic B cells (Fig. 1C).
FIGURE 1.
Expression and localization of Pyk2 and FAK in B cells. A, Pyk2 and FAK protein levels in cell lysates (40 μg of protein) from A20 B lymphoma cells, resting splenic B cells, and splenic B cells that were activated with LPS plus IL-4 for 2 days were analyzed by sequential blotting with Abs to Pyk2, FAK, and Erk1/2 (loading control). Molecular mass markers (in kDa) are indicated to the right of each blot. The relative amount of Pyk2 or FAK protein in activated B cells compared with resting B cells (=1) was determined by quantifying band intensities with ImageJ and normalizing the values to the amount of Erk in the sample. The data represent the mean ± S.E. for three independent experiments. B, the relative amounts of Pyk2 and FAK mRNA in resting and activated splenic B cells were determined by quantitative RT-PCR. Values were normalized to the amount of glyceraldehyde-3-phosphate dehydrogenase mRNA in the same sample and are expressed as the amount of mRNA (average ± range for two independent experiments) relative to that in resting B cells (=1). C, resting and LPS/IL-4-activated splenic B cells were left unstimulated for 30 min (−) or were incubated with 10 μg/ml anti-IgM Abs for 30 min. Anti-Pyk2 immunoprecipitates (ippt) were analyzed by blotting with the 4G10 anti-Tyr(P) (P-Tyr) antibody (upper panel). The blots were then stripped and reprobed with an Ab to Pyk2 (lower panel). One of two experiments that yielded similar results is shown. D, RT-PCR analysis of alternatively spliced Pyk2 mRNA in resting splenic B cells and LPS/IL-4-activated B cells. PCR primers flanking the alternatively spliced exon distinguish the full-length mRNA from the spliced isoform, which is 126 bp shorter. Data are representative of three experiments with similar results. E, resting and LPS/IL-4-activated splenic B cells were permeabilized and stained with goat anti-Pyk2 or goat anti-FAK plus Alexa Fluor 488-conjugated secondary Ab. Nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI) (blue). No fluorescence was observed when the cells were stained with secondary Ab alone or with nonspecific goat IgG plus secondary Ab (supplemental Fig. 1). F, LPS/IL-4 activated splenic B cells were permeabilized and stained with Abs to FAK plus Abs to either LFA-1 or VLA-4. In E and F, each panel shows representative data from one of three experiments with similar results.
Confocal microscopy showed that Pyk2 and FAK had distinct subcellular localizations in B cells (Fig. 1E). In both resting and activated murine splenic B cells, Pyk2 was uniformly distributed in the cytoplasm with a diffuse pattern. In contrast, FAK was present in punctate structures in both resting and activated splenic B cells (Fig. 1E) as well as the A20 B cell line (data not shown). Activated splenic B cells had more FAK-containing puncta than resting splenic B cells and overall higher levels of FAK consistent with the immunoblotting data. These punctate FAK-containing structures also contained LFA-1 and to some extent VLA-4 (α4β1 integrin) (Fig. 1F), suggesting that FAK associates constitutively with these integrins in B cells.
Adhesion to ECM Enhances BCR-induced Tyrosine Phosphorylation of Pyk2 and FAK
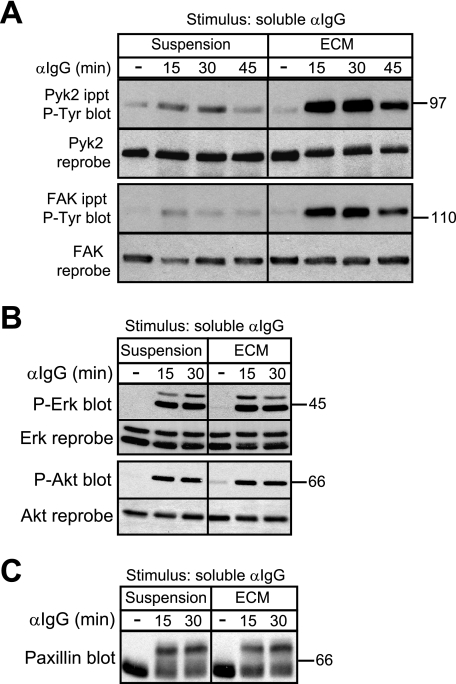
To examine the role of Pyk2 and FAK in BCR and integrin signaling in B cells, we used the A20 B lymphoma cell line, which expresses both Pyk2 and FAK. Consistent with the idea that integrins can act as co-stimulatory receptors that enhance BCR signaling, we showed previously that BCR-induced tyrosine phosphorylation of Pyk2 is substantially greater when A20 cells are plated on a collagen/fibronectin ECM that contains integrin ligands than when the cells are stimulated in suspension (17) (see also Fig. 2A). As was the case for Pyk2, BCR-induced tyrosine phosphorylation of FAK was also substantially increased when A20 cells were plated on ECM (Fig. 2A). The binding of integrins to ECM ligands did not cause an overall enhancement of BCR signaling but selectively augmented BCR-induced tyrosine phosphorylation of FAK and Pyk2. BCR-induced serine/threonine phosphorylation of Erk, Akt, and the cytoskeleton-associated adaptor protein paxillin was not enhanced by integrin engagement (Fig. 2, B and C). The selective targeting of Pyk2 and FAK by BCR/integrin co-stimulation suggests that these kinases may be important for integrin-dependent B cell responses.
FIGURE 2.
Adhesion of B cells to ECM selectively enhances BCR-induced tyrosine phosphorylation of Pyk2 and FAK. A20 cells were kept in suspension or plated on collagen/fibronectin ECM for 30 min before being stimulated with 20 μg/ml soluble anti-IgG for the indicated times. For unstimulated controls (−), A20 cells were kept in suspension or plated on collagen/fibronectin ECM for 30 min and then left unstimulated for an additional 45 min before being lysed. A, immunoprecipitated (ippt) Pyk2 and FAK were analyzed by immunoblotting with the 4G10 anti-Tyr(P) (P-Tyr) Ab. The blots were then reprobed with Abs to Pyk2 or FAK. A mock stimulation of cells with phosphate-buffered saline for 15 or 30 min did not increase phosphorylation of Pyk2 and FAK compared with cells left unstimulated for the entire duration of the experiment (supplemental Fig. 2). B, cell lysates were immunoblotted with Abs against the phosphorylated forms of Erk (P-Erk) or Akt (P-Akt) and then reprobed with Abs against total Erk or Akt. C, cell lysates were immunoblotted with a paxillin Ab. Serine/threonine phosphorylation of paxillin is indicated by a bandshift on SDS-PAGE gels and was dependent on the activity of the Erk and GSK-3 kinases (data not shown) as in T cells and macrophages (67, 68). For each panel, similar results were obtained in three experiments. αIgG, anti-IgG Ab.
BCR/Integrin-induced B Cell Spreading Involves Pyk2 and FAK
A20 cells spread dramatically when they are plated on fibronectin and then stimulated with anti-Ig Abs (6, 17). In this scenario BCR signaling activates β1 integrins (e.g. VLA-4), which bind to the fibronectin, and the combined BCR/integrin signaling leads to cell spreading. A20 cells also spread when plated on immobilized Abs that cluster the LFA-1 integrin, adopting a morphology similar to that of anti-Ig-activated A20 cells spreading on intercellular adhesion molecule-1, the physiological ligand for LFA-1 (6). This indicates that integrin signaling is sufficient to induce B cell spreading. Because integrin signaling selectively enhances the ability of the BCR to induce tyrosine phosphorylation of Pyk2 and FAK (Fig. 2) and can independently induce the phosphorylation of these kinases (see Fig. 5), we asked whether Pyk2 or FAK played a role in B cell spreading.
FIGURE 5.
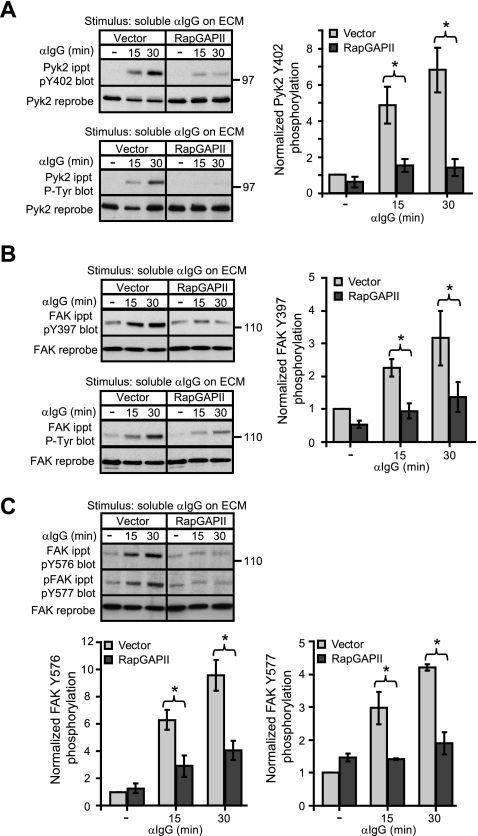
Rap activation is important for integrin-induced phosphorylation of Pyk2 and FAK. Vector control and RapGAPII-expressing A20 cells were plated in wells coated with 3.5 μg/cm2 anti-CD40 (control), anti-LFA-1, or anti-VLA-4 monoclonal Abs for 15 or 30 min (′). Anti-Pyk2 (A) and anti-FAK (B) immunoprecipitates (ippt) were analyzed by blotting with the 4G10 anti-Tyr(P) (P-Tyr) Ab. The blots were then stripped and reprobed with Abs against Pyk2 or FAK. For FAK phosphorylation, band intensities were normalized to the amount of total FAK for each sample and then expressed as the relative phosphorylation (mean ± S.E. for three experiments) compared with that for vector control cells plated on anti-CD40 (=1). *, p < 0.05 by Student's one-tailed paired t test.
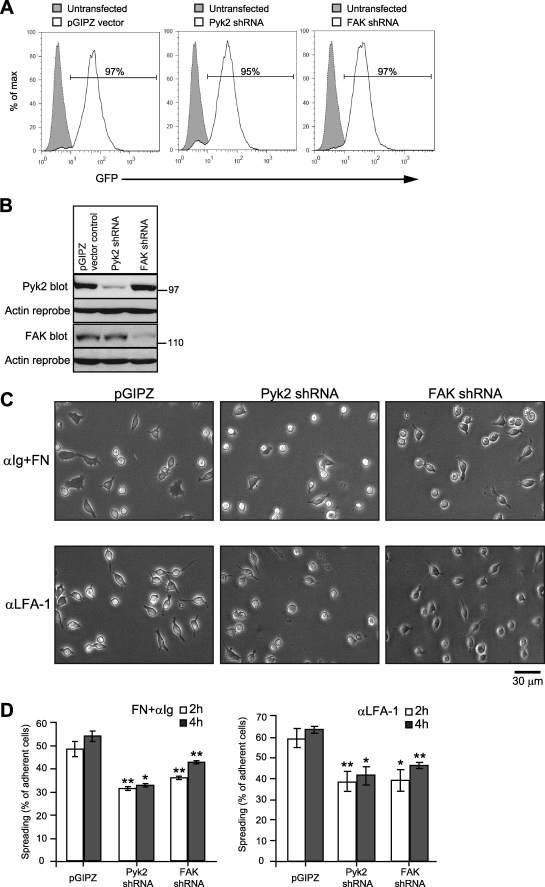
To test this, we used RNA interference to reduce the expression of Pyk2 or FAK in A20 cells. We established stable bulk populations of A20 cells containing the GFP-encoding pGIPZ lentiviral vector or derivatives of this vector that also encode shRNAs specific for either Pyk2 or FAK. The resulting cell populations were >95% GFP+ (Fig. 3A), and immunoblotting showed that the Pyk2 shRNA reduced the expression of Pyk2 by 83% without affecting FAK levels, whereas the FAK shRNA reduced the expression of FAK by 67% without affecting Pyk2 levels (Fig. 3B). Knocking down the expression of either Pyk2 or FAK caused a 30–40% reduction in the number of A20 cells that developed a spread, elongated morphology when plated on fibronectin and then stimulated with anti-Ig Abs (Fig. 3C). The same was true when A20 cells were plated on immobilized anti-LFA-1 Abs (Fig. 3C). Thus Pyk2 and FAK both contribute to BCR/integrin- and integrin-induced B cell spreading in A20 cells.
FIGURE 3.
A20 cell spreading involves both Pyk2 and FAK. Lentiviral transduction was used to establish stable bulk populations of A20 cells expressing the GFP-encoding pGIPZ vector or derivates encoding a Pyk2-specific shRNA or a FAK-specific shRNA. A, representative fluorescence-activated cell sorting plots showing GFP expression by the transduced A20 cells (solid lines) compared with untransduced parental A20 cells (shaded curves). The percentage of transduced cells that were GFP+ is indicated. B, immunoblot analysis of Pyk2 and FAK protein levels in cell lysates (40 μg of protein/lane). The Pyk2 and FAK blots were reprobed with Abs to actin (loading control). C, A20 cells transduced with the pGIPZ vector, Pyk2 shRNA, or FAK shRNA were plated on wells coated with 2.63 μg/cm2 fibronectin and then stimulated with 10 μg/ml soluble anti-IgG for 4 h (upper panels) or were plated on wells coated with 2.63 μg/cm2 anti-LFA-1 Abs for 4 h (bottom panels). Representative images are shown. D, the percentage of adherent A20 cells that had spread after 2 or 4 h as indicated by being phase dark with an elongated or irregular shape was determined. The data are presented as the average ± S.E. for >300 cells counted in each of three experiments. *, p < 0.05; **, p < 0.01 by Student's one-tailed paired t test compared with vector control cells. αIgG, anti-IgG Abs; αLFA-1, anti-LFA-1 Ab; FN, fibronectin.
BCR/Integrin-induced Tyrosine Phosphorylation of Pyk2 and FAK Depends on Activation of the Rap GTPases
Because activation of the Rap GTPases is critical for BCR- and integrin-induced B cell spreading (6, 17) and Pyk2 and FAK contribute to this process, we hypothesized that Rap activation would be important for BCR-induced tyrosine phosphorylation of Pyk2 and FAK. The phosphorylation of Pyk2 and FAK on conserved tyrosine residues increases their kinase activity (21, 50). The initial event in receptor-induced activation of these kinases is phosphorylation of Pyk2 on Tyr402 or FAK on Tyr397. This is thought to occur via dimerization and transphosphorylation (51). Src family kinases can then bind via their SH2 domain to the phosphorylated Pyk2 Tyr402 or FAK Tyr397 and phosphorylate Pyk2 at Tyr579/Tyr580 or FAK at Tyr576/Tyr577. Phosphorylation of Pyk2 and FAK on these activation loop residues is required for maximal activity of these kinases toward substrates (21). We showed previously that Rap activation is required for BCR/integrin-induced phosphorylation of Pyk2 on Tyr579/Tyr580 (17). However, it was not known whether this reflected a role for Rap activation in Tyr402 phosphorylation or the Src family kinase-mediated phosphorylation of Tyr579/Tyr580. Moreover the role of Rap activation in BCR/integrin-induced FAK phosphorylation had not been assessed.
To address these questions, we blocked Rap activation in A20 cells by expressing the Rap-specific GTPase-activating protein, RapGAPII (52). RapGAPII converts the Rap1 and Rap2 GTPases to their inactive GDP-bound state, and RapGAPII expression has been widely used to assess the role of Rap activation (53, 54). We have shown that RapGAPII expression completely blocks anti-Ig-, chemokine-, and phorbol ester-induced Rap activation in A20 cells without inhibiting other signaling reactions such as phosphorylation of mitogen-activated protein kinases or Akt (17, 42).
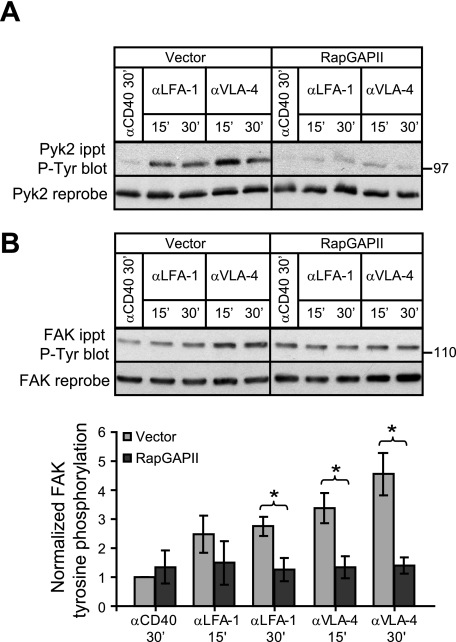
Preventing Rap activation via RapGAPII expression significantly inhibited tyrosine phosphorylation of Pyk2 on Tyr402 when A20 cells were plated on ECM and stimulated with soluble anti-Ig antibodies (Fig. 4A). This corresponded with inhibition of total Pyk2 tyrosine phosphorylation as assessed using anti-Tyr(P) Abs (Fig. 4A). Thus BCR/integrin-induced phosphorylation of Pyk2 on Tyr402, the first step in Pyk2 activation, is dependent on Rap activation. The same was true for FAK. The use of phosphorylation site-specific Abs showed that blocking Rap activation significantly inhibited BCR/integrin-induced phosphorylation of FAK on Tyr397 (Fig. 4B) as well as the subsequent phosphorylation of FAK on Tyr576/Tyr577 (Fig. 4C). Consistent with this, the total tyrosine phosphorylation of FAK as detected using the 4G10 anti-Tyr(P) Ab was also inhibited when Rap activation was blocked (Fig. 4B). Thus during BCR/integrin co-stimulation, Rap activation is critical for the initial step in the activation of Pyk2 and FAK, phosphorylation of Pyk2 on Tyr402 and FAK on Tyr397. As a consequence Rap activation is also required for the subsequent Src family kinase-mediated phosphorylation of the activation loop tyrosine residues of Pyk2 and FAK.
FIGURE 4.
BCR/integrin-induced tyrosine phosphorylation of Pyk2 and FAK depends on activation of the Rap GTPases. Vector control and RapGAPII-expressing A20 cells were cultured for 30 min in wells coated with collagen/fibronectin ECM before being stimulated with 20 μg/ml anti-IgG for the indicated times. For unstimulated controls (−), A20 cells were plated on collagen/fibronectin ECM for 30 min and then left unstimulated for another 30 min before being lysed. A, anti-Pyk2 immunoprecipitates (ippt) were probed with an Ab against Pyk2 that is phosphorylated on Tyr402 (pY402) or with the 4G10 anti-Tyr(P) (P-Tyr) Ab before being reprobed with an anti-Pyk2 Ab. B, anti-FAK immunoprecipitates were probed with an Ab that recognizes FAK that is phosphorylated on Tyr397 (pY397) or with the 4G10 anti-Tyr(P) Ab before being reprobed with an anti-FAK Ab. C, anti-FAK immunoprecipitates were probed sequentially with Abs that recognize FAK that is phosphorylated on either Tyr576 or Tyr577 before being reprobed with an anti-FAK Ab. The relative levels of Pyk2 and FAK phosphorylation were determined by quantifying band intensities using ImageJ, normalizing the values to the total amount of Pyk2 or FAK in the same lane, and expressing the values (mean ± S.E. for three experiments) relative to the Pyk2 or FAK phosphorylation levels in unstimulated vector control cells (=1). *, p < 0.05 by Student's one-tailed paired t test.
The requirement for Rap activation in BCR/integrin co-stimulation-induced phosphorylation of Pyk2 and FAK could reflect a role for Rap activation in one or more of the following processes: coupling BCR signaling pathways to the phosphorylation of Pyk2 and FAK, activating integrins such that ligand binding initiates outside-in integrin signaling, or coupling integrin signaling pathways to the phosphorylation of Pyk2 and FAK. We have shown previously that Rap activation is essential for the BCR to stimulate integrin activation (17). Therefore we now investigated whether Rap activation was also an essential component of the signaling pathways that link the BCR and integrins to the phosphorylation of Pyk2 and FAK. Because integrin engagement greatly enhances BCR-induced phosphorylation of Pyk2 and FAK, we first tested the hypothesis that integrin signaling induces Pyk2 and FAK phosphorylation in a Rap-dependent manner.
Rap Activation Is Important for Integrin-induced Phosphorylation of Pyk2 and FAK
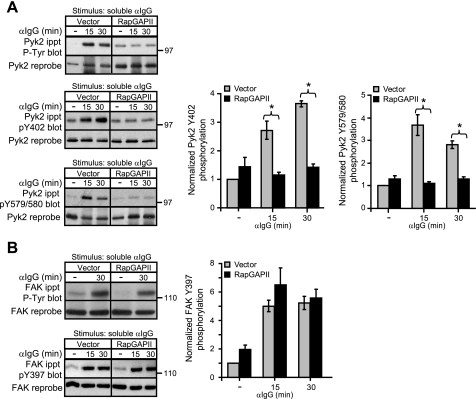
To initiate integrin signaling without stimulating the cells through the BCR, we plated A20 cells on wells coated with Abs against the LFA-1 or VLA-4 integrins. We have shown previously that the ability of A20 cells to spread on immobilized anti-integrin Abs or on immobilized intercellular adhesion molecule-1 is dependent on Rap activation (6). Moreover Ab-induced clustering of LFA-1 activates Rap1 in A20 cells (6). Fig. 5A shows that plating A20 cells on wells coated with Abs to LFA-1 or VLA-4 resulted in increased Pyk2 phosphorylation compared with cells plated on wells coated with an isotype-matched control monoclonal Ab against CD40. Both LFA-1- and VLA-4-induced Pyk2 phosphorylation was substantially reduced in the RapGAPII-expressing A20 cells in which Rap activation was blocked (Fig. 5A). Similarly FAK phosphorylation, which was increased 3–4-fold by clustering VLA-4 and to a lesser extent by clustering LFA-1, was significantly reduced when Rap activation was blocked (Fig. 5B). Thus Rap activation is required for integrin signaling to induce tyrosine phosphorylation of Pyk2 and FAK.
Rap Activation Is Important for BCR-induced Phosphorylation of Pyk2 but Not for BCR-induced Phosphorylation of FAK
When A20 cells were stimulated with anti-Ig Abs while in suspension, BCR clustering induced tyrosine phosphorylation of both Pyk2 and FAK although to a much lesser extent than when the cells were plated on ECM (Fig. 2). Because integrin engagement is likely to be minimal when the cells are in suspension, this may reflect integrin-independent BCR signaling events. Therefore we asked whether Rap activation was important for integrin-independent phosphorylation of Pyk2 and FAK by the BCR. When we kept vector control and RapGAPII-expressing A20 cells in suspension and stimulated them with soluble anti-Ig Abs, blocking Rap activation completely abrogated BCR-induced phosphorylation of Pyk2 at Tyr402 and Tyr579/Tyr580 (Fig. 6A). In contrast, blocking Rap activation did not impair the ability of the BCR to increase tyrosine phosphorylation of FAK, as judged using the 4G10 anti-Tyr(P) Ab, or more specifically phosphorylation of FAK at Tyr397 (Fig. 6B). Thus both BCR-induced (Fig. 6A) and integrin-induced (Fig. 5A) Pyk2 phosphorylation required Rap activation, whereas integrin-induced FAK phosphorylation was dependent on Rap activation (Fig. 5B), but BCR-induced FAK phosphorylation was Rap-independent (Fig. 6B).
FIGURE 6.
Rap activation is important for BCR-induced tyrosine phosphorylation of Pyk2 but not FAK. Vector control and RapGAPII-expressing A20 cells were stimulated in suspension with 20 μg/ml anti-IgG for the indicated times. For unstimulated controls (−), A20 cells were left in suspension for 30 min without being stimulated. A, anti-Pyk2 immunoprecipitates (ippt) were probed with either the 4G10 anti-Tyr(P) (P-Tyr) Ab, an Ab against Pyk2 that is phosphorylated on Tyr402, or an Ab against Pyk2 that is phosphorylated on Tyr579/Tyr580. The blots were then stripped and reprobed with a Pyk2 Ab. B, anti-FAK immunoprecipitates were probed with either the 4G10 anti-Tyr(P) Ab or an Ab against FAK that is phosphorylated on Tyr397. The blots were then stripped and reprobed with a FAK Ab. Band intensities were normalized to the amount of total Pyk2 or FAK for each sample and then expressed as the relative phosphorylation (mean ± S.E. for three experiments) compared with that for unstimulated vector control cells (=1). *, p < 0.05 by Student's one-tailed paired t test. The values for FAK phosphorylation in vector and RapGAPII-expressing cells were not significantly different by this test.
The Role of Rap Activation in the Phosphorylation of Pyk2 and FAK Corresponds to a Requirement for Actin Dynamics
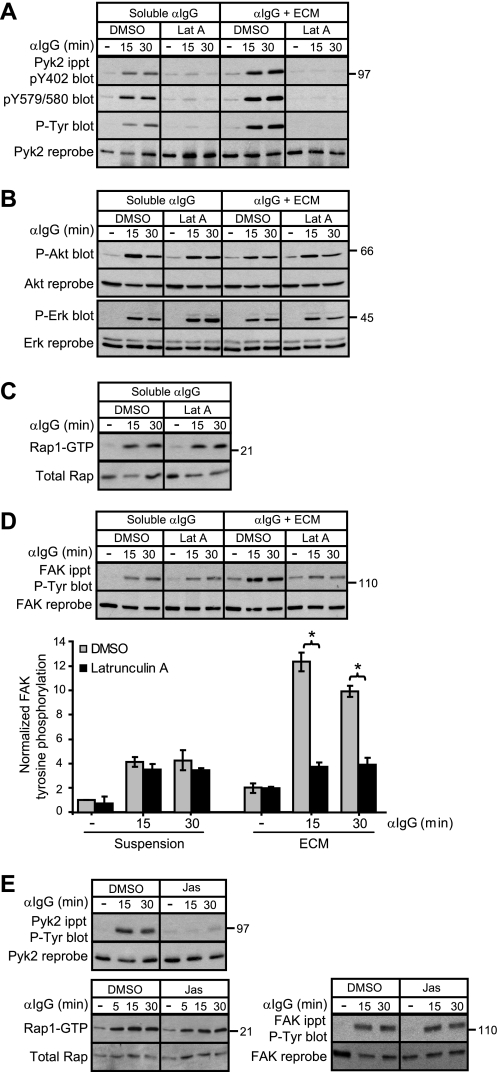
Because Rap activation is required for maximal BCR-induced increases in polymerized F-actin in A20 cells (17), we hypothesized that Rap might regulate the phosphorylation of Pyk2 and FAK via its ability to promote actin polymerization or stabilize actin filaments. To test this, we pretreated A20 cells with latrunculin A, a drug that prevents the addition of actin monomers to existing actin filaments, thereby leading to a loss of F-actin. Confocal microscopy showed that a 30-min treatment with latrunculin A led to a nearly complete loss of F-actin in A20 cells (data not shown). In the presence of latrunculin A, anti-Ig-induced phosphorylation of Pyk2 at Tyr402 and Tyr579/Tyr580 was almost completely blocked both when the cells were stimulated in suspension and when they were stimulated while on ECM (Fig. 7A). Similar results were obtained using cytochalasin D (data not shown), another drug that leads to the loss of F-actin. An intact actin cytoskeleton was not required for other BCR signaling events such as phosphorylation of Erk or Akt (Fig. 7B). Importantly latrunculin A did not block BCR-induced Rap1 activation (Fig. 7C), consistent with the idea that F-actin acts downstream of Rap activation to promote Pyk2 phosphorylation.
FIGURE 7.
Rap-dependent phosphorylation of Pyk2 and FAK requires actin dynamics. A20 cells in suspension or plated on ECM were pretreated with 10 μm latrunculin A or an equivalent volume of DMSO for 30 min before being stimulated with 20 μg/ml soluble anti-IgG for the indicated times. For unstimulated controls (−), A20 cells were kept in suspension or plated on collagen/fibronectin ECM for 30 min and then left unstimulated for another 30 min before being lysed. A, anti-Pyk2 immunoprecipitates (ippt) were sequentially probed with an Ab that recognizes Pyk2 that is phosphorylated at Tyr402, an Ab that recognizes Pyk2 that is phosphorylated at Tyr579/Tyr580, the 4G10 anti-Tyr(P) (P-Tyr) Ab, and an anti-Pyk2 Ab. B, cell lysates were assayed for phosphorylation of Akt (P-Akt) and Erk (P-Erk) as in Fig. 2B. C, a GST-RalGDS fusion protein was used to selectively precipitate the active GTP-bound form of Rap1, which was detected by immunoblotting with a Rap1 Ab (upper panel). The lower panel shows the amount of Rap1 in the cell lysates. D, tyrosine phosphorylation of FAK was assessed by blotting anti-FAK immunoprecipitates with the 4G10 anti-Tyr(P) Ab and then reprobing with an anti-FAK Ab. FAK phosphorylation (mean ± S.E. for three experiments) relative to that in unstimulated DMSO-treated cells kept in suspension (=1) is graphed. *, p < 0.05 by Student's one-tailed paired t test. E, A20 cells in suspension were pretreated with 1 μm jasplakinolide or an equivalent volume of DMSO for 30 min before being stimulated with 20 μg/ml anti-IgG for the indicated times or being left unstimulated for 30 min (−). Pyk2 and FAK tyrosine phosphorylation as well as Rap1 activation was assessed. For each panel, similar results were obtained in three experiments. Lat A, latrunculin A; Jas, jasplakinolide.
For FAK phosphorylation, the requirement for an intact actin cytoskeleton paralleled the requirement for Rap activation. When A20 cells were stimulated with anti-Ig while in suspension, BCR-induced FAK phosphorylation was unaffected by blocking Rap activation (Fig. 6B) or by disrupting the actin cytoskeleton with latrunculin A (Fig. 7D). In contrast, when the cells were stimulated while on ECM, BCR/integrin-induced FAK phosphorylation was significantly reduced by disrupting the actin cytoskeleton with latrunculin A (Fig. 7D) and by blocking Rap activation (Fig. 4B). Thus a Rap- and F-actin-dependent pathway links integrins, but not the BCR, to FAK phosphorylation.
The ability of latrunculin A and cytochalasin D to block BCR-induced Pyk2 phosphorylation could reflect a requirement for actin filaments, which may act as signaling platforms, or a requirement for the dynamic assembly and disassembly of actin filaments. To distinguish these possibilities, we used jasplakinolide, a drug that prevents actin filament disassembly (55). When A20 cells were stimulated in suspension, jasplakinolide treatment completely inhibited BCR-induced phosphorylation of Pyk2 while having no effect on BCR-induced Rap1 activation (Fig. 7E). Thus both disrupting actin filaments and stabilizing actin filaments inhibited the Rap-dependent phosphorylation of Pyk2 by the BCR. In contrast, BCR-induced phosphorylation of FAK in cells that were kept in suspension did not require Rap activation and was unaffected by either latrunculin A (Fig. 7D) or jasplakinolide (Fig. 7E).
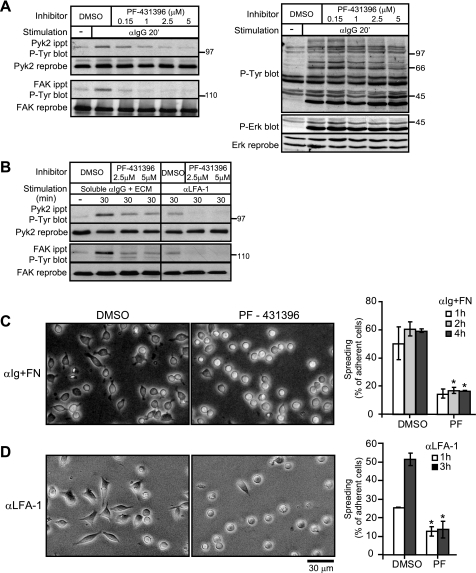
B Cell Spreading Requires Pyk2/FAK Kinase Activity
We have shown that Rap activation is important for BCR/integrin-induced tyrosine phosphorylation of Pyk2 and FAK (Figs. 4 and 5) and for BCR- and integrin-induced B cell spreading (6, 17). This suggests that activated Rap may promote B cell spreading at least in part by facilitating the phosphorylation-dependent activation of Pyk2 and FAK. Indeed knocking down the expression of either Pyk2 or FAK reduced B cell spreading (Fig. 3C). To specifically address the role of Pyk2 and FAK kinase activity in BCR/integrin-induced B cell spreading, we used PF-431396, a potent and highly selective pyrimidine-based inhibitor of both Pyk2 and FAK (40). Consistent with the idea that the tyrosine phosphorylation of Pyk2 and FAK involves an initial autophosphorylation or transphosphorylation step, treating A20 cells with PF-431396 blocked anti-Ig-induced tyrosine phosphorylation of Pyk2 and FAK when the cells were stimulated in suspension (Fig. 8A) and when they were stimulated on ECM (Fig. 8B). The phosphorylation of Pyk2 and FAK induced by clustering LFA-1 with plate-bound Abs was also inhibited by PF-431396 (Fig. 8B). PF-431396 treatment was not cytotoxic as judged by 7-amino-actinomycin D staining (data not shown) and did not reduce the ability of the BCR to stimulate Erk phosphorylation or overall protein tyrosine phosphorylation (Fig. 8A), which is dependent on the activation of both Src family kinases and the Syk tyrosine kinase. Thus, PF-431396 appeared to selectively inhibit BCR-induced tyrosine phosphorylation of Pyk2 and FAK. Importantly this correlated with a significant inhibition of A20 cell spreading. PF-431496 treatment significantly reduced the number of A20 cells that developed an elongated, spread morphology after being stimulated with anti-Ig Abs while on fibronectin (Fig. 8C). The spreading of A20 cells plated on immobilized anti-LFA-1 Abs was also significantly reduced by PF-431396 treatment (Fig. 8D). Thus the kinase activity of Pyk2 and/or FAK is required for both BCR/integrin- and integrin-induced B cell spreading.
FIGURE 8.
An inhibitor of Pyk2/FAK activity blocks B cell spreading. A, A20 cells in suspension were treated with the indicated concentrations of PF-431396 or with DMSO for 45 min before being stimulated with 20 μg/ml anti-IgG for 20 min. For unstimulated controls (−), A20 cells were kept in suspension for 45 min and then left unstimulated for another 20 min before being lysed. Pyk2 and FAK immunoprecipitates (ippt) were analyzed by blotting with the 4G10 anti-Tyr(P) (P-Tyr) Ab (left panel) before being reprobed with Abs against Pyk2 or FAK. The same cell lysates were analyzed for total tyrosine phosphorylation using the 4G10 anti-Tyr(P) Ab and for Erk phosphorylation (P-Erk) (right panel). B, A20 cells were treated with PF-431396 or DMSO for 45 min. The cells were then added to fibronectin/collagen ECM-coated wells and stimulated for 30 min with soluble anti-Ig. Alternatively the cells were added to wells coated with 2.63 μg/cm2 anti-LFA-1 Abs for 30 min. Pyk2 and FAK immunoprecipitates were analyzed by blotting with the 4G10 anti-Tyr(P) Ab. C, A20 cells were plated on wells coated with 2.63 μg/cm2 fibronectin (FN) and stimulated with 10 μg/ml anti-IgG in the presence of DMSO or 2.5 μm PF-431396 (PF) for 1, 2, or 4 h. Representative images of the 4-h time point are shown. D, A20 cells were plated on wells coated with 2.63 μg/cm2 anti-LFA-1 Ab in the presence of DMSO or 2.5 μm PF-431396 for 1 or 3 h. Representative images of the 3-h time point are shown. Cell surface expression of LFA-1 was not affected by PF-431396 treatment (data not shown). The percentage of adherent A20 cells that had spread (mean ± S.E. for >150 cells counted in each of three experiments) as indicated by being phase dark with an elongated or irregular shape was determined for each time point. *, p < 0.05 by Student's one-tailed paired t test compared with DMSO-treated cells.
DISCUSSION
The binding of antigens by B cells often occurs in the context of integrin engagement. Integrin-dependent cell spreading enhances the ability of B cells to contact antigens, and integrin signaling may synergize with BCR signaling to promote both B cell spreading and activation. The Pyk2 and FAK kinases are key regulators of cell morphology, and in this report we showed that the kinase activities of Pyk2 and FAK are important for BCR/integrin-induced B cell spreading. Moreover we showed that integrins enhance the ability of the BCR to phosphorylate Pyk2 and FAK on their auto/transphosphorylation sites, the initial step in the activation of these kinases. Finally we showed that both Rap activation and actin dynamics were critical for BCR/integrin-induced phosphorylation of Pyk2 and FAK.
We had shown previously that integrin engagement enhances BCR-induced Pyk2 phosphorylation (17), and we have now shown that the same is true for FAK phosphorylation. Moreover by clustering integrins with Abs, we showed that integrin signaling was sufficient to induce tyrosine phosphorylation of Pyk2 and FAK in B cells. Thus signaling by antigen-clustered BCR complexes and ligand-bound integrins can have additive effects on the phosphorylation of Pyk2 and FAK. This highlights the ability of integrins to act as co-stimulatory receptors that collaborate with lymphocyte antigen receptors. Pyk2 and FAK appeared to be selective targets of the BCR/integrin collaboration as integrin engagement did not enhance BCR-induced phosphorylation of other signaling proteins such as Erk, Akt, and paxillin.
We also showed that activation of the Rap GTPases was critical for BCR/integrin signaling to induce the phosphorylation of Pyk2 and FAK on their auto/transphosphorylation sites as well as tyrosine residues in their activation loops. Although Rap-GTP likely contributes to Pyk2 and FAK phosphorylation by activating integrins on B cells (17), we found that activated Rap also acts downstream of the BCR to promote Pyk2 phosphorylation and downstream of integrins to promote the phosphorylation of Pyk2 and FAK. The active GTP-bound form of Rap binds multiple effector proteins that promote actin polymerization and the stabilization of F-actin polymers (38). Many of the downstream consequences of Rap activation may therefore reflect its role in reorganization of the actin cytoskeleton. Indeed we found that the Rap-dependent steps in Pyk2 and FAK phosphorylation were also blocked by actin-disrupting drugs. This suggests that Rap-GTP promotes Pyk2 and FAK phosphorylation via its ability to remodel the actin cytoskeleton. Rap1 activation was not dependent on actin dynamics, suggesting that the requirement for actin remodeling lies downstream of Rap activation.
Although Pyk2 phosphorylation has been shown to require an intact actin cytoskeleton in a number of cell types (21), how this contributes to Pyk2 phosphorylation is not clear. Phosphorylation of Pyk2 on Tyr402 may involve Pyk2 dimerization and subsequent transphosphorylation (51). Rap-dependent actin polymerization could create a cytoskeletal platform that promotes Pyk2 dimerization. However, we found that treating B cells with the actin-stabilizing agent jasplakinolide also prevented tyrosine phosphorylation of Pyk2, indicating that polymerized F-actin is not sufficient to support receptor-induced Pyk2 phosphorylation. Dynamic remodeling of the actin cytoskeleton may be required for efficient Pyk2 dimerization. Alternatively Pyk2-dependent phosphorylation in vivo may require cycles of actin polymerization and depolymerization that regulate either the kinase activity of Pyk2 or the accessibility of its catalytic site.
In contrast to Pyk2, Rap activation and actin dynamics were required for integrin-induced FAK phosphorylation but not for BCR-induced FAK phosphorylation in A20 B lymphoma cells. For integrin-induced FAK phosphorylation, Rap activation was required for the initial step in FAK activation, phosphorylation of Tyr397, an event that is initiated by transphosphorylation and that can be amplified by Src family kinase (56). How Rap activation and F-actin contribute to integrin-induced FAK Tyr397 phosphorylation is not clear. Our microscopy data suggest that FAK constitutively co-localizes with integrins in B cells. Rap activation and actin polymerization could therefore contribute to the recruitment and/or stabilization of other proteins that regulate FAK Tyr397 phosphorylation. In adherent cells that form focal adhesions, integrin activation results in the recruitment of talin to the integrin α and β chain cytoplasmic domains (57). This allows FAK to interact with paxillin and undergo autophosphorylation. At the same time, activation of Src family kinases by protein-tyrosine phosphatase α increases the phosphorylation of FAK at Tyr397. Further work is required to determine whether Rap and F-actin promote integrin-dependent FAK phosphorylation by regulating these steps in B cells. Interestingly Rap activation and F-actin were not required for BCR-induced phosphorylation of FAK when the cells were in suspension, a situation in which there is minimal integrin engagement. FAK has been reported to associate constitutively with the Src family kinase Lyn and with the BCR in WEHI-231 B lymphoma cells (33). FAK phosphorylation could therefore be a proximal Rap-independent BCR signaling event that is initiated by BCR.
A key finding was that Pyk2 and FAK are important for B cell spreading that is initiated by BCR/integrin co-stimulation or by integrin clustering. This is consistent with Pyk2 and FAK being downstream targets of Rap because blocking Rap activation also prevents B cell spreading (6, 17). Knocking down the expression of either Pyk2 or FAK in A20 B lymphoma cells reduced the ability of these cells to undergo cell spreading, whereas PF-431396, a dual specificity inhibitor of the kinase activities of both Pyk2 and FAK, substantially inhibited A20 cell spreading. This suggests that both Pyk2 and FAK contribute to the ability of A20 B lymphoma cells to undergo cell spreading. Moreover the use of PF-431396 showed that the kinase activities of Pyk2 and FAK were critical for B cell spreading.
Although it is not known how Pyk2 and FAK promote B cell spreading, these kinases may coordinate the activation of Rac, Cdc42, and RhoA, GTPases that control cytoskeletal organization. In T lymphocytes, Pyk2 binds Vav (50), an exchange factor that activates Rac. Both Pyk2 and FAK can interact with the RhoA activator p190RhoGEF (58), and in fibroblasts Pyk2 associates with Wrch1, a Cdc42-like GTPase that promotes the formation of filopodia (59). Pyk2 and FAK can also bind the p85 subunit of phosphoinositide 3-kinase following integrin ligation (60, 61). Phosphatidylinositol 3,4,5-trisphosphate produced by phosphoinositide 3-kinase activates Vav and promotes Rac-dependent actin polymerization and cytoskeletal rearrangement. Pyk2 and FAK can also bind and phosphorylate the scaffolding proteins p130Cas and paxillin, which can then recruit the Rac activators DOCK180 and PAK-interacting exchange factor (PIX), leading to Rac-dependent membrane ruffling (61).
An interesting observation was that when B cells were activated with LPS plus IL-4 Pyk2 levels decreased, but FAK levels increased significantly. B cells activated in this manner resemble antigen-activated germinal center (GC) B cells, which proliferate within lymphoid organ follicles and undergo somatic hypermutation of their Ig genes. These GC B cells then compete for limiting amounts of antigen that are displayed on the surface of follicular dendritic cells, which provide the B cells with survival signals. GC B cells interacting with follicular dendritic cells in vivo exhibit a spread morphology with multiple membrane processes (62, 63). This presumably increases their ability to detect antigens on the surface of the follicular dendritic cell. The activation-induced increase in FAK expression may reflect a switch from the motile phenotype of a circulating B cell to the more adhesive phenotype of an activated GC B cell. FAK expression and activation are associated with sustained adhesion at least in B cell progenitors (34). A number of adhesion molecules including α6 integrin are up-regulated in activated GC B cells (64, 65), and gene expression profiling has shown that FAK mRNA levels are elevated in GC B cells (66). Thus, the increased expression of FAK after B cell activation may be part of a proadhesion gene expression program in which FAK promotes integrin-dependent adhesion and cell spreading, which facilitate BCR-antigen interactions that provide survival signals for GC B cells. Similarly the change in Pyk2 mRNA splicing in activated B cells may allow Pyk2 to interact with additional proteins that control cell adhesion or cytoskeletal reorganization. In summary, we have shown that Pyk2 and FAK are downstream targets of the Rap GTPases that play an important role in B cell spreading, a process that contributes to B cell activation.
Supplementary Material
Acknowledgments
We thank Sonja Christian (University of British Columbia) for technical assistance, Dr. Dorothee von Laer (Georg-Speyer Haus, Frankfurt, Germany) for providing the pCMV-δR8.91 and pCMV-VSV-G-M5 plasmids, and Dr. Hanne Ostergaard (University of Alberta) and Dr. Michael Schaller (University of North Carolina) for advice and reagents.
This work was supported by Canadian Institutes of Health Research Operating Grant 68865 (to M. R. G.) and by graduate student fellowships (to K. W. K. T.) from the Michael Smith Foundation for Health Research and the Natural Sciences and Engineering Research Council of Canada.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- Ab
- antibody
- BCR
- B cell receptor
- Ig
- immunoglobulin
- ECM
- extracellular matrix
- LFA-1
- lymphocyte function-associated antigen-1
- Pyk2
- proline-rich tyrosine kinase
- FAK
- focal adhesion kinase
- VLA-4
- very late antigen-4
- Tyr(P)
- phosphotyrosine
- LPS
- lipopolysaccharide
- shRNA
- short hairpin RNA
- GC
- germinal center
- SH2
- Src homology 2
- Erk
- extracellular signal-regulated kinase
- RT
- reverse transcription
- GFP
- green fluorescent protein
- IL-4
- interleukin-4
- GAP
- GTPase-activating protein.
REFERENCES
- 1.Dal Porto J. M., Gauld S. B., Merrell K. T., Mills D., Pugh-Bernard A. E., Cambier J. (2004) Mol. Immunol. 41,599–613 [DOI] [PubMed] [Google Scholar]
- 2.Kurosaki T. (2002) Nat. Rev. Immunol. 2,354–363 [DOI] [PubMed] [Google Scholar]
- 3.Niiro H., Clark E. A. (2002) Nat. Rev. Immunol. 2,945–956 [DOI] [PubMed] [Google Scholar]
- 4.Spaargaren M., Beuling E. A., Rurup M. L., Meijer H. P., Klok M. D., Middendorp S., Hendriks R. W., Pals S. T. (2003) J. Exp. Med. 198,1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris E. S., McIntyre T. M., Prescott S. M., Zimmerman G. A. (2000) J. Biol. Chem. 275,23409–23412 [DOI] [PubMed] [Google Scholar]
- 6.Lin K. B., Freeman S. A., Zabetian S., Brugger H., Weber M., Lei V., Dang-Lawson M., Tse K. W., Santamaria R., Batista F. D., Gold M. R. (2008) Immunity 28,75–87 [DOI] [PubMed] [Google Scholar]
- 7.Westerberg L., Greicius G., Snapper S. B., Aspenström P., Severinson E. (2001) Blood 98,1086–1094 [DOI] [PubMed] [Google Scholar]
- 8.Bergtold A., Desai D. D., Gavhane A., Clynes R. (2005) Immunity 23,503–514 [DOI] [PubMed] [Google Scholar]
- 9.Qi H., Egen J. G., Huang A. Y., Germain R. N. (2006) Science 312,1672–1676 [DOI] [PubMed] [Google Scholar]
- 10.Bajénoff M., Egen J. G., Koo L. Y., Laugier J. P., Brau F., Glaichenhaus N., Germain R. N. (2006) Immunity 25,989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleire S. J., Goldman J. P., Carrasco Y. R., Weber M., Bray D., Batista F. D. (2006) Science 312,738–741 [DOI] [PubMed] [Google Scholar]
- 12.Carrasco Y. R., Fleire S. J., Cameron T., Dustin M. L., Batista F. D. (2004) Immunity 20,589–599 [DOI] [PubMed] [Google Scholar]
- 13.Carrasco Y. R., Batista F. D. (2006) EMBO J. 25,889–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roozendaal R., Mempel T. R., Pitcher L. A., Gonzalez S. F., Verschoor A., Mebius R. E., von Andrian U. H., Carroll M. C. (2009) Immunity 30,264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann M. F., McKall-Faienza K., Schmits R., Bouchard D., Beach J., Speiser D. E., Mak T. W., Ohashi P. S. (1997) Immunity 7,549–557 [DOI] [PubMed] [Google Scholar]
- 16.Schwartz M. A., Ginsberg M. H. (2002) Nat. Cell Biol. 4,E65–68 [DOI] [PubMed] [Google Scholar]
- 17.McLeod S. J., Shum A. J., Lee R. L., Takei F., Gold M. R. (2004) J. Biol. Chem. 279,12009–12019 [DOI] [PubMed] [Google Scholar]
- 18.Rovida E., Lugli B., Barbetti V., Giuntoli S., Olivotto M., Dello Sbarba P. (2005) Biol. Chem. 386,919–929 [DOI] [PubMed] [Google Scholar]
- 19.van Buul J. D., Anthony E. C., Fernandez-Borja M., Burridge K., Hordijk P. L. (2005) J. Biol. Chem. 280,21129–21136 [DOI] [PubMed] [Google Scholar]
- 20.Okigaki M., Davis C., Falasca M., Harroch S., Felsenfeld D. P., Sheetz M. P., Schlessinger J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100,10740–10745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avraham H., Park S. Y., Schinkmann K., Avraham S. (2000) Cell. Signal. 12,123–133 [DOI] [PubMed] [Google Scholar]
- 22.van Seventer G. A., Salmen H. J., Law S. F., O'Neill G. M., Mullen M. M., Franz A. M., Kanner S. B., Golemis E. A., van Seventer J. M. (2001) Eur. J. Immunol. 31,1417–1427 [DOI] [PubMed] [Google Scholar]
- 23.Giannoni E., Chiarugi P., Cozzi G., Magnelli L., Taddei M. L., Fiaschi T., Buricchi F., Raugei G., Ramponi G. (2003) J. Biol. Chem. 278,36763–36776 [DOI] [PubMed] [Google Scholar]
- 24.Andreev J., Simon J. P., Sabatini D. D., Kam J., Plowman G., Randazzo P. A., Schlessinger J. (1999) Mol. Cell. Biol. 19,2338–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guinamard R., Okigaki M., Schlessinger J., Ravetch J. V. (2000) Nat. Immunol. 1,31–36 [DOI] [PubMed] [Google Scholar]
- 26.Gismondi A., Jacobelli J., Strippoli R., Mainiero F., Soriani A., Cifaldi L., Piccoli M., Frati L., Santoni A. (2003) J. Immunol. 170,3065–3073 [DOI] [PubMed] [Google Scholar]
- 27.Lakkakorpi P. T., Bett A. J., Lipfert L., Rodan G. A., Duong le T. (2003) J. Biol. Chem. 278,11502–11512 [DOI] [PubMed] [Google Scholar]
- 28.Sieg D. J., Iliæ D., Jones K. C., Damsky C. H., Hunter T., Schlaepfer D. D. (1998) EMBO J. 17,5933–5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Astier A., Avraham H., Manie S. N., Groopman J., Canty T., Avraham S., Freedman A. S. (1997) J. Biol. Chem. 272,228–232 [DOI] [PubMed] [Google Scholar]
- 30.Freedman A. S., Rhynhart K., Nojima Y., Svahn J., Eliseo L., Benjamin C. D., Morimoto C., Vivier E. (1993) J. Immunol. 150,1645–1652 [PubMed] [Google Scholar]
- 31.Manie S. N., Astier A., Wang D., Phifer J. S., Chen J., Lazarovits A. I., Morimoto C., Freedman A. S. (1996) Blood 87,1855–1861 [PubMed] [Google Scholar]
- 32.Glodek A. M., Honczarenko M., Le Y., Campbell J. J., Silberstein L. E. (2003) J. Exp. Med. 197,461–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mlinaric-Rascan I., Yamamoto T. (2001) FEBS Lett. 498,26–31 [DOI] [PubMed] [Google Scholar]
- 34.Glodek A. M., Le Y., Dykxhoorn D. M., Park S. Y., Mostoslavsky G., Mulligan R., Lieberman J., Beggs H. E., Honczarenko M., Silberstein L. E. (2007) Leukemia 21,1723–1732 [DOI] [PubMed] [Google Scholar]
- 35.Katagiri K., Maeda A., Shimonaka M., Kinashi T. (2003) Nat. Immunol. 4,741–748 [DOI] [PubMed] [Google Scholar]
- 36.Lafuente E. M., van Puijenbroek A. A., Krause M., Carman C. V., Freeman G. J., Berezovskaya A., Constantine E., Springer T. A., Gertler F. B., Boussiotis V. A. (2004) Dev. Cell 7,585–595 [DOI] [PubMed] [Google Scholar]
- 37.Han J., Lim C. J., Watanabe N., Soriani A., Ratnikov B., Calderwood D. A., Puzon-McLaughlin W., Lafuente E. M., Boussiotis V. A., Shattil S. J., Ginsberg M. H. (2006) Curr. Biol. 16,1796–1806 [DOI] [PubMed] [Google Scholar]
- 38.Bos J. L. (2005) Curr. Opin. Cell Biol. 17,123–128 [DOI] [PubMed] [Google Scholar]
- 39.Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. (1991) J. Exp. Med. 173,599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckbinder L., Crawford D. T., Qi H., Ke H. Z., Olson L. M., Long K. R., Bonnette P. C., Baumann A. P., Hambor J. E., Grasser W. A., 3rd, Pan L. C., Owen T. A., Luzzio M. J., Hulford C. A., Gebhard D. F., Paralkar V. M., Simmons H. A., Kath J. C., Roberts W. G., Smock S. L., Guzman-Perez A., Brown T. A., Li M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104,10619–10624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durand C. A., Westendorf J., Tse K. W., Gold M. R. (2006) Eur. J. Immunol. 36,2235–2249 [DOI] [PubMed] [Google Scholar]
- 42.McLeod S. J., Li A. H., Lee R. L., Burgess A. E., Gold M. R. (2002) J. Immunol. 169,1365–1371 [DOI] [PubMed] [Google Scholar]
- 43.Freundlich B., Avdalovic N. (1983) J. Immunol. Methods 62,31–37 [DOI] [PubMed] [Google Scholar]
- 44.Yee J. K., Friedmann T., Burns J. C. (1994) Methods Cell Biol. 43,99–112 [DOI] [PubMed] [Google Scholar]
- 45.Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. (1996) Science 272,263–267 [DOI] [PubMed] [Google Scholar]
- 46.Santos-Argumedo L., Kincade P. W., Partida-Sánchez S., Parkhouse R. M. (1997) Immunology 90,147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elenström C., Severinson E. (1989) Growth Factors 2,73–82 [DOI] [PubMed] [Google Scholar]
- 48.Davey E. J., Thyberg J., Conrad D. H., Severinson E. (1998) J. Immunol. 160,5366–5373 [PubMed] [Google Scholar]
- 49.Xiong W. C., Macklem M., Parsons J. T. (1998) J. Cell Sci. 111,1981–1991 [DOI] [PubMed] [Google Scholar]
- 50.Ostergaard H. L., Lysechko T. L. (2005) Immunol. Res. 31,267–282 [DOI] [PubMed] [Google Scholar]
- 51.Park S. Y., Avraham H. K., Avraham S. (2004) J. Biol. Chem. 279,33315–33322 [DOI] [PubMed] [Google Scholar]
- 52.Christian S. L., Lee R. L., McLeod S. J., Burgess A. E., Li A. H., Dang-Lawson M., Lin K. B., Gold M. R. (2003) J. Biol. Chem. 278,41756–41767 [DOI] [PubMed] [Google Scholar]
- 53.Mochizuki N., Ohba Y., Kiyokawa E., Kurata T., Murakami T., Ozaki T., Kitabatake A., Nagashima K., Matsuda M. (1999) Nature 400,891–894 [DOI] [PubMed] [Google Scholar]
- 54.Ohba Y., Mochizuki N., Matsuo K., Yamashita S., Nakaya M., Hashimoto Y., Hamaguchi M., Kurata T., Nagashima K., Matsuda M. (2000) Mol. Cell. Biol. 20,6074–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bubb M. R., Senderowicz A. M., Sausville E. A., Duncan K. L., Korn E. D. (1994) J. Biol. Chem. 269,14869–14871 [PubMed] [Google Scholar]
- 56.Zeng L., Si X., Yu W. P., Le H. T., Ng K. P., Teng R. M., Ryan K., Wang D. Z., Ponniah S., Pallen C. J. (2003) J. Cell Biol. 160,137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlaepfer D. D., Mitra S. K., Ilic D. (2004) Biochim. Biophys. Acta 1692,77–102 [DOI] [PubMed] [Google Scholar]
- 58.Lim Y., Lim S. T., Tomar A., Gardel M., Bernard-Trifilo J. A., Chen X. L., Uryu S. A., Canete-Soler R., Zhai J., Lin H., Schlaepfer W. W., Nalbant P., Bokoch G., Ilic D., Waterman-Storer C., Schlaepfer D. D. (2008) J. Cell Biol. 180,187–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruusala A., Aspenström P. (2008) Mol. Cell. Biol. 28,1802–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarkar S., Svoboda M., de Beaumont R., Freedman A. S. (2002) Leuk. Lymphoma 43,1663–1671 [DOI] [PubMed] [Google Scholar]
- 61.Mitra S. K., Hanson D. A., Schlaepfer D. D. (2005) Nat. Rev. Mol. Cell Biol. 6,56–68 [DOI] [PubMed] [Google Scholar]
- 62.Allen C. D., Okada T., Tang H. L., Cyster J. G. (2007) Science 315,528–531 [DOI] [PubMed] [Google Scholar]
- 63.Hauser A. E., Junt T., Mempel T. R., Sneddon M. W., Kleinstein S. H., Henrickson S. E., von Andrian U. H., Shlomchik M. J., Haberman A. M. (2007) Immunity 26,655–667 [DOI] [PubMed] [Google Scholar]
- 64.Ambrose H. E., Wagner S. D. (2004) Immunology 111,400–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klein U., Tu Y., Stolovitzky G. A., Keller J. L., Haddad J., Jr., Miljkovic V., Cattoretti G., Califano A., Dalla-Favera R. (2003) Proc. Natl. Acad. Sci. U.S.A. 100,2639–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alizadeh A. A., Eisen M. B., Davis R. E., Ma C., Lossos I. S., Rosenwald A., Boldrick J. C., Sabet H., Tran T., Yu X., Powell J. I., Yang L., Marti G. E., Moore T., Hudson J., Jr., Lu L., Lewis D. B., Tibshirani R., Sherlock G., Chan W. C., Greiner T. C., Weisenburger D. D., Armitage J. O., Warnke R., Levy R., Wilson W., Grever M. R., Byrd J. C., Botstein D., Brown P. O., Staudt L. M. (2000) Nature 403,503–511 [DOI] [PubMed] [Google Scholar]
- 67.Robertson L. K., Mireau L. R., Ostergaard H. L. (2005) J. Immunol. 175,8138–8145 [DOI] [PubMed] [Google Scholar]
- 68.Cai X., Li M., Vrana J., Schaller M. D. (2006) Mol. Cell. Biol. 26,2857–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.