Abstract
It is well established that CpG promotes pro-inflammatory cytokine and antibody production by B cells via the Toll-like receptor 9 (TLR9)-dependent pathway. However, scavenger receptors (SRs) are also capable of binding such pathogen-derived molecules, yet their contribution to CpG-induced signaling events has not yet been evaluated. Here we identified a novel TLR9-independent mechanism of CpG-induced signaling and immune function that is mediated by the scavenger B1 receptor (SR-B1). Specifically, we show that CpG/SR-B1 triggers calcium entry into primary B lymphocytes via phospholipase Cγ-1-mediated activation of TRPC3 channels and also B cell adhesion to vascular cell adhesion molecule-1. CpG-induced calcium signals and vascular cell adhesion molecule-1 adhesion are TLR9-independent and are mediated exclusively by SR-B1. Although pro-inflammatory cytokine and Ig production induced by CpG require TLR9 expression, we also found that SR-B1 negatively regulates TLR9-dependent production of interleukin-6, interleukin-10, and IgM. Thus, our results provide a novel perspective on the complexity of CpG signaling within B cells by demonstrating that SR-B1 is an alternative pathway for nucleic acid-induced signaling that provides feedback inhibition on specific TLR9-dependent responses of B cells. Consequently, these results have wide implications for understanding the mechanisms regulating immune tolerance to nucleic acids and pathogen-associated molecules.
Stimulus-induced dynamic changes in the concentration of cytoplasmic calcium are primary determinants of the activation, immunological function, and developmental fate of lymphocytes. Calcium signaling through the B cell antigen receptor (BCR)2 complex is initiated by the activation of proximal tyrosine kinases Lyn and Syk, which phosphorylate the adaptor BLNK to facilitate its association with and activation of PLCγ-2. PLCγ2 hydrolyzes phosphatidylinositol 4,5-bisphosphate into diacylglycerol and 1,4,5-inositol trisphosphate (IP3) (for review see Ref. 1), which activates IP3 receptor/channels that mediate Ca2+ release from endoplasmic reticulum into the cytosol (2) (for review see Refs. 3, 4). Ca2+ release from endoplasmic reticulum stores and the resulting depletion of Ca2+ (not an increase in cytoplasmic [Ca2+]) are the central and prerequisite events required to activate plasma membrane “store-operated” calcium release-activated calcium (CRAC) channels.
CRAC channels are responsible for antigen receptor-triggered calcium entry; however, a growing body of evidence suggests that CRAC channels do not underlie all the diverse calcium-regulated responses of lymphocytes, particularly those triggered by innate stimuli. For example, we previously identified several calcium-permeant non-selective cation channels (NSCCs) that are uniquely activated by distinct arachidonic acid-derived (eicosanoid) inflammatory mediators and by mechanical stimuli (5–7). Thus, multiple calcium-permeant channels with distinct activation mechanisms may underlie stimulus-specific calcium-dependent B cell functions in vivo. Surprisingly, a number of pathogen-associated Toll-like receptor agonists are known to be strong B cell mitogens, yet the potential for calcium-dependent signaling functions by these polyclonal B cell mitogens has not yet been fully evaluated.
Studies detailed in this report focus on the mechanism of calcium signaling elicited by unmethylated CpG DNA in primary B cells. Unmethylated CpG DNA is typically considered a pathogen-derived molecule that triggers polyclonal B cell activation, cytokine production, and immunoglobulin production via Toll-like receptor 9 (TLR9) engagement (8, 9). Because CpG induces a subset of the B cell responses normally elicited by cognate antigen binding to the BCR complex, we asked whether CpG stimulation mobilizes calcium. We found that while CpG stimulation and BCR engagement both elicit similar biphasic calcium signals, CpG-mediated calcium entry is regulated by TRPC3, a calcium-permeant NSCC of the canonical transient receptor potential (TRPC) channel family (10) and that, unlike the BCR, which couples to calcium entry via PLCγ-2, TRPC3 activation involves an adaptor like function of PLCγ-1.
We also report that CpG-mediated calcium signals are initiated by the scavenger receptor B1 (SR-B1) independently of TLR9. To our knowledge, this is the first demonstration of SR-B1 function in B lymphocytes; although scavenger receptors have been implicated in the responses of other immune cells. For example, bacterial pathogens and byproducts of apoptotic cells contribute to the pathogenesis of immune-mediated diseases, including lupus in part via MARCO and CD36 expressed by marginal zone macrophages (11). In naïve B cells, CD36 expression is largely restricted to marginal zone cells. Notably, CD36 cooperates with TLR2 to produce antibodies against phosphocholine, which is an endogenous antigen (13). Given our finding that CpG elicits calcium signals via SR-B1 on lymphocytes, we asked whether SR-B1 might also act cooperatively, in this case with TLR9, to trigger inflammatory responses of B cells. In fact, our results indicate that SR-B1 negatively regulates CpG/TLR9-mediated production of specific immunoglobulins (IgM) and pro-inflammatory cytokines (IL-6 and IL-10) by B cells. These findings have important implications for understanding how calcium is regulated in B cells, but also point to novel mechanisms by which pathogen-associated molecules regulate B cell activation.
EXPERIMENTAL PROCEDURES
Intracellular Calcium Measurements
B lymphocytes purified from spleens of C57Bl/6 mice by negative immunomagnetic separation (Stemcell Technologies) were loaded with fura-2 AM (3.0 μm, Invitrogen) and intracellular Ca2+ was measured by digital imaging microscopy as previously described (5). All results are expressed as the fura-2 fluorescence emission ratio at 510 nm. The BCR was engaged using soluble anti-mouse F(ab′)2 antibody (Jackson ImmunoResearch, West Grove, PA). Phosphorothioester derivatives of stimulatory (CpG, ODN1826: 5′-TCC ATG ACG TTC CTG ACG TT-3′) and a control variant of ODN1826 (GpC, 5′-TCC ATG AGC TTC CTG AGC TT-3′) were synthesized and high-performance liquid chromatography purified by Integrated DNA Technologies (Coralville, IA). CpG was used at a final concentration of 2.5 μm for calcium measurements unless stated otherwise.
RNA and Protein Analysis
Total RNA was extracted from purified splenic B cells or bone narrow derived macrophages with an RNeasy kit (Qiagen, CA), and cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Invitrogen, CA), and random hexamer primers. cDNA was subject to 40 amplification cycles with SR primers (300 nm) of the following sequences: SRA sense, 5′-AGA ATT TCA GCA TGG CAA CTG; SRA antisense, 5′-ACG GAC TCT GAC ATG CAG TG; MARCO sense, 5′-GCA CTG CTG CTG ATT CAA GTT C-3′; MARCO antisense, 5′-AGT TGC TCC TGG CTG GTA TG-3′; SRB1 sense, 5′-GAC GGG CGT CCA GAA TTT C-3′; SR-B1 antisense, 5′-AGC GAG GAT TCG GGT GTC AT-3′; SRB2 sense, 5′-AAAAGGCATGCATCCCAACA-3′; SRB2 antisense, 5′-GTC CTG ATG TCT CCC GTT TCA-3′; CD36 sense, 5′-GTG ACG TGG CAA AGA ACA G-3′; CD36 antisense, 5′-AAA GGA GGC TGC GTC TGT G-3′; Macrosialin sense, 5′-TCC AAG ATC CTC CAC TGT TG-3′; Macrosialin antisense, 5′-CAT TGT ATT CCA CCG CCA TG-3′; LOX1 sense, 5′-ATG AAT TTG GAA ATG GCT TTT G-3′; LOX1 antisense, 5′-TCA CTG AGT TAG CAA TAA ATT TG-3′; SRECI sense, 5′-CTG GGC CGT CAT GGT AAG AA-3′; SRECI antisense, 5′-GGG CCA TAG GGA CCA TCT CT-3′; SRECII sense, 5′-CAA CGT TTT TGT GGA AGC TTC AG-3′; and SRECII antisense, 5′-GCA GTT GAG TGT GTT GTC TAG GTC AT-3′. For Western analysis, B cells lysates with ∼30 μg of protein were separated by electrophoresis. Proteins transferred to nitrocellulose membrane were detected by enhanced chemiluminescence (ECL).
RNA Inhibition
siRNA transfection of primary cells was performed by a modification of the procedure previously described (14). Purified splenic B cells were first activated with lipopolysaccharide (0.5 μg/ml) for 24 h, then incubated (5 million B cells) in 500 μl of culture medium (without 2-mercaptoethanol or antibiotics) containing 1000 pmol of TRPC3 or SR-B1 SMARTpool siRNA (Dharmacon) for 10 min at room temperature in an electroporation cuvette (BTX, 4-mm gap). Transfection was performed using a single 10-ms pulse at 295 V with a BTX ECM 830 electroporator. Transfected cells were washed and cultured for an additional 48 h in complete medium, and then used in functional experiments. The efficiency of RNA interference-mediated protein suppression was assessed by immunoblot analysis using antibodies against targeted proteins and typically exceeded 90% suppression. Antibodies against TRPC3 were obtained from Alomone Laboratories (Jerusalem, Israel), SR-B1 were obtained from Novus Biologicals (Littleton, CO), and phosphotyrosine (4G11) from Millipore (Billerica, MA).
Functional Assays
Single cell suspensions were prepared from dissociated spleens. Purified B cells (2 × 105) were stimulated with CpG or other agonists for 48–72 h in RPMI medium containing 10% fetal bovine serum, 2 mm glutamine, 100 units/ml penicillin G, 100 μg/ml streptomycin sulfate, non-essential amino acids, OPI media supplement (Sigma), and 50 μm 2-mercaptoethanol. Ig levels in culture supernatants at 48 h were measured using a kit (Southern Biotech). Supernatants collected at 72 h were analyzed for IL-6 and IL-10 by enzyme-linked immunosorbent assay (eBiosciences). Samples were processed in duplicate and recombinant cytokines used to establish standard curves (Peprotech, Rocky Hill, NJ) ranging from 8 to 4000 pg/ml. Cell integrin avidity changes were assessed using a VCAM-1 adhesion assay as previously described (5).
Electrophysiology
Patch clamp recordings were performed on primary B lymphocytes identified visually by negative immunofluorescence (CD3 negative) in the microscope recording chamber. Patch pipettes were fabricated with a 4- to 6-MΩ (for whole cell recording) or 5- to 10-MΩ (for single channel recording) tip resistance (Sutter Instrument, Novato, CA) from borosilicate glass and were back-filled with appropriate internal solution. Liquid junction potentials were calculated and were corrected manually with the patch clamp amplifier or post analysis. Capacitance and access resistance were monitored continuously. Between 50 and 70% of the series resistance was electronically compensated to minimize voltage errors. Command potentials were generated using an EPC-9 patch clamp amplifier and currents acquired, stored, and analyzed using PulseFit software (Heka Elektronic, Germany). Single channel amplitude frequency analysis was performed using QuB software (15).
Whole cell currents were recorded using the standard whole cell mode of the patch clamp technique. Cells were first held at 0 mV, then stepped to −80 mV and subjected to a linear voltage ramp (100 ms) to 80 mV with a sampling interval of 0.2 ms. This ramp protocol was repeated every 2 s, and the time course of the whole cell currents was obtained by plotting the mean current amplitude at −80 mV for each cycle. Cell membrane capacitance values were used to estimate the cell surface area, and results are expressed as the current amplitude per unit membrane capacitance (pA/pF). All ramp currents were leak-corrected by subtracting ramp currents obtained after establishing a stable whole cell recording from the ramp current obtained after agonist-induced current activation. Measurements of the relative Ca2+ versus Na+ permeability (PCa/PNa) were calculated using the measured reversal potential of CpG-induced currents recorded in a bath solution containing 30 mm Ca2+ and a pipette solution containing 10 mm Na+ using the equation, Erev = RT/2F ln{4PCa2+[Ca2+]o/PNa+[Na+]i}. Single channel recordings were also obtained using the cell-attached configuration of the patch clamp as previously described (7). Membrane-permeant drugs were typically applied by direct addition to the bath. For some whole cell, patch clamp recording measurements, membrane-impermeant monoclonal antibodies were dialyzed from the recording microelectrode into the cytoplasm of single cells to neutralize the function of PLCγ isoforms as previously demonstrated by us and others (5, 16). Direct visualization of cytoplasmic filling with fluorescein isothiocyanate-conjugated antibodies demonstrated that the time required for complete cytoplasmic dialysis using this approach is <2 min.
RESULTS
CpG Elicits a Large Calcium Elevation in B Cells
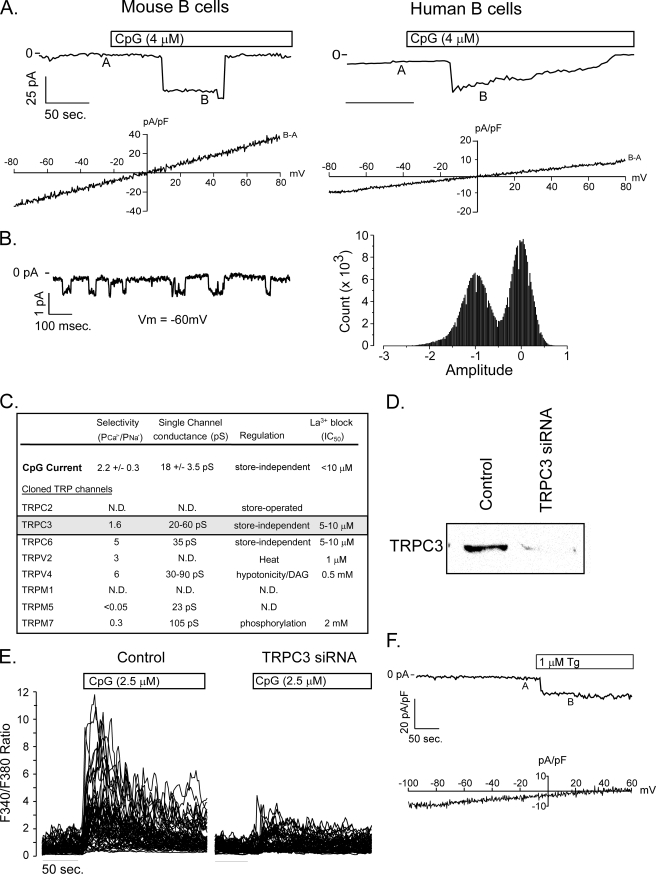
Given that unmethylated bacterial CpG DNA is a powerful polyclonal B cell mitogen and that calcium regulates lymphocyte activation, proliferation, and effector functions, studies were initiated to examine whether CpG mobilizes calcium in primary B lymphocytes. We found that CpG elicited a large [Ca2+]i increase comparable to that triggered by BCR engagement (Fig. 1A). Notably, this response, which was observed in both murine and human primary B cells (Fig. 1B), did not require TLR9 (Fig. 1C) or MyD88 expression (supplemental Fig. S1), suggesting the presence of a previously unrecognized CpG-induced TLR9-independent pathway. Although CpG-induced calcium mobilization was not observed in a previous studies (17, 18), low concentrations (∼100 nm) utilized also did not produce a response in our hands (Fig. 1D, left panel). These findings suggested that different thresholds may exist for unique CpG-induced functional effects. Importantly, the calcium signals we observe are not selective for CpG, as a control oligonucleotide that differed from CpG 1826 only by the inversion of two CG motifs (GpC) elicited a signal with the identical dose sensitivity as CpG 1826 (Fig. 1D, right panel). Thus, together with the data demonstrating CpG-induced calcium mobilization in TLR9-deficient B cells, these data indicate that DNA (both CpG and GpC) elicits calcium signals through a novel TLR9-independent pathway that is less restrictive in its nucleotide recognition.
FIGURE 1.
CpG DNA elicits TLR9 independent Ca2+ signals in mouse and human primary B lymphocytes. A, BCR engagement (left panel) and CpG (right panel) produce a similar biphasic elevation in cytoplasmic [Ca2+] in purified mouse B cells. B, CpG elicits Ca2+release from intracellular stores in Ca2+-free medium in both mouse and human primary B cells. Subsequent superfusion with Ca2+ containing solution produces a secondary increase in cytoplasmic Ca2+ due to extracellular Ca2+ influx. C, CpG induced Ca2+ release from stores and Ca2+ entry into primary B cells occurs does not require TLR9 expression. CpG produced a similar transient elevation of cytosolic Ca2+ in TLR9+/+ (left panels) and TLR9−/− B cells bathed in Ca2+-free extracellular Ca2+ that decayed to baseline levels within 2 min. Reintroduction of extracellular Ca2+ in the continued presence of CpG, produced a sustained secondary increase in free cytosolic Ca2+ concentration indicative of entry through activated plasma membrane channels. D, the threshold for CpG-induced Ca2+ mobilization is between 250 and 500 nm CpG. GpC mobilizes Ca2+ with a similar dose threshold. In all experiments, intracellular Ca2+ was measured in single cells using fura-2 fluorescence emission ratio imaging as described under “Experimental Procedures.”
CpG-induced Calcium Entry Is Mediated by TRPC3 NSCCs
Because the calcium signal triggered by CpG superficially resembles that initiated by BCR engagement, we next asked whether it occurs by a similar mechanism. Antigen receptor-induced calcium entry occurs through CRAC channels, which are activated by intracellular calcium store depletion. In calcium-free medium, we found that CpG produced a transient elevation in cytoplasmic calcium due to release from intracellular stores and subsequent perfusion with calcium replete medium produced a secondary sustained [Ca2+] elevation in primary mouse and human B cells (see Fig. 1, B and C) consistent with entry via plasma membrane calcium-permeant channels. We used the patch clamp to determine whether CpG, like the BCR, activates CRAC channels or if distinct store-operated or store-independent channels are responsible for CpG-induced calcium entry. CpG treatment elicited relatively large amplitude inward currents (−37.4 ± 10.0 picoamps/picofarad, Vm = −80 mV, n = 26) that exhibited a linear current-voltage relationship and a reversal potential near 0 mV in primary mouse (−4.2 ± 1.6 mV; Fig. 2A, left panels) and human B cells (Fig. 2A, right panels). The single channel conductance (Fig. 2B, 18.8 ± 0.2 pS, n = 5) and the relative Ca2+ versus Na+ permeability (2.2 ± 0.3, n = 4) suggest that calcium-permeant NSCCs and not CRAC channels are responsible for CpG-induced calcium entry. We previously identified the expression of eight TRP family NSCCs in murine B cells (5), and, of these, TRPC3 has biophysical properties that most closely resemble currents elicited by CpG (Fig. 2C). To determine if TRPC3 contributed functionally to the CpG-induced calcium elevations that we observe, primary B cells were transfected with siRNA, to suppress TRPC3 expression (Fig. 2D). Notably reduction of TRPC3 expression substantially attenuated CpG-induced calcium signals (Fig. 2E) but had no effect on BCR-induced responses. Neither CpG- nor BCR-induced Ca2+ signals were affected by suppression of the SR MARCO (supplemental Fig. S2). Together, these results indicate that CpG-induced calcium signaling is regulated by activation of TRPC3.
FIGURE 2.
CpG induced calcium entry is mediated by TRPC3. A, patch clamp recording of steady-state current elicited by CpG in primary mouse (left) and human (right) B cells at −70 mV holding potential. Voltage ramps (−80 mV to +80 mV) recorded at A were subtracted from ramps applied at B, and the I-V relationship was plotted beneath each macroscopic current. The current reversal potential of ∼0 mV in both cell types indicates that CpG activates a non-selective cation channel in primary B cells. These currents are representative of measurements obtained from more than 25 mouse and 3 of 8 human primary B cells. B, single channel activity of CpG-induced channel at a holding voltage of −60 mV (left) and current amplitude frequency histogram (right). C, the calculated single channel conductance, relative Ca2+ versus Na+ permeability, and store dependence of activation for CpG currents in B lymphocytes and published values for TRP family members (53, 54) expressed by primary murine B cells (5) indicate that CpG-induced currents most closely resemble TRPC3. D, Western analysis demonstrates almost complete suppression of TRPC3 protein expression in primary murine B lymphocytes. Lysates were obtained from 5 million primary murine B lymphocytes 48 h after transfection with TRPC3 siRNA. E, TRPC3 siRNA transfection did not affect BCR-induced Ca2+ signals, but almost completely suppressed CpG-induced responses. E, thapsigargin elicits an inward current in primary B cells (upper panel). The background-subtracted I-V current (B − A, bottom panel) exhibits inward rectification consistent with CRAC and not non-selective cation channel activation by thapsigargin.
Native TRPC3 is not store-operated; however, some studies have reported that ectopically expressed TRPC3 channels can operate in a store-dependent manner (19). Given that CpG triggers calcium release from stores (see Fig. 1, B and C), we examined whether calcium store depletion activates endogenous TRPC3-like currents in primary B cells. Thapsigargin, which blocks sarco-endoplasmic reticulum ATPase that maintain stores in a filled state, elicited CRAC currents in B cells (Fig. 2F) but did not elicit non-selective cation currents. Together, these data suggest that CpG-induced calcium signals are mediated by TRPC3 activation via a store-independent mechanism.
Mechanism of CpG-induced Calcium Mobilization
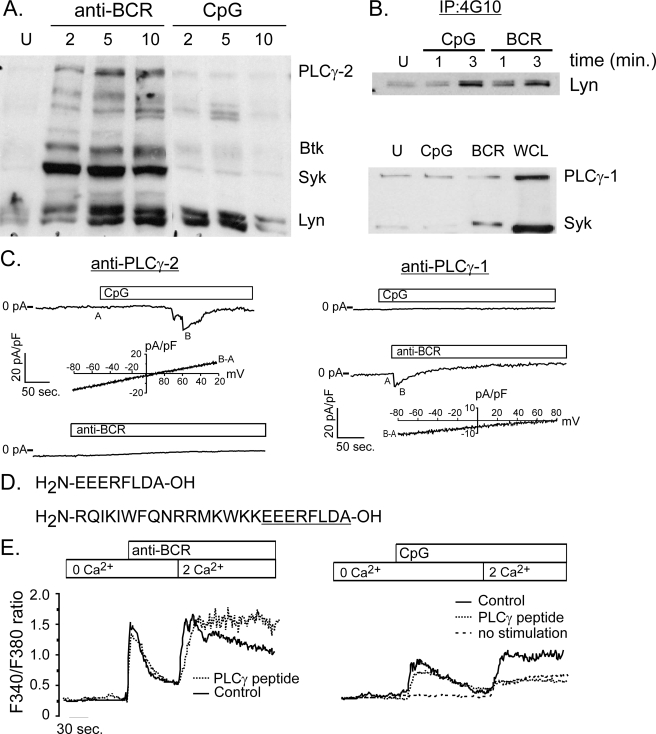
Given that CpG, like BCR engagement, induces calcium release from stores, we next examined whether CpG activates the proximal effector proteins involved in BCR-mediated calcium mobilization. Although CpG increases phosphorylation of dual protein bands with the mobility of Lyn kinase, it does not induce phosphorylation of other critical molecules which link the BCR to calcium release from stores, including Syk, Btk, BLNK, or PLCγ-2 (Fig. 3A). We confirmed that Lyn was indeed phosphorylated by CpG by performing a Lyn immunoblot analysis of phosphotyrosine immunoprecipitates (Fig. 3B, top panel). Given that PLCg-2 is not phosphorylated by CpG, we next examined whether CpG activates PLCγ-1. PLCγ-1 is not considered a major isoform in B cell; however, it was detectable in primary B cells (Fig. 3B, bottom panel, right lane). Because neither CpG nor BCR engagement induced a measurable change in PLCγ-2 or PLCγ-1 phosphorylation (Fig. 3B, bottom panel), we used a direct approach to determine whether TRPC3 activation by CpG requires either PLCγ-1 or PLCγ-2. To do this we neutralized these distinct isoforms in situ by dialyzing isoform-specific antibodies into the cytoplasm of single B cells. Antibodies used in this approach are capable of recognizing two-dimensional forms of these proteins and were identical to those we used previously to define the mechanism of mechanical signaling in B cells (see “Experimental Procedures” and Ref. 5). As expected, we found that PLCγ-2 neutralization blocked BCR-induced CRAC channel activation and did not affect CpG-induced TRPC3 activation. Surprisingly the converse was observed for CpG responses. PLCγ-1 neutralization in situ efficiently blocked CpG activation of TRPC3 channels, yet it did not disrupt activation of CRAC channels by BCR engagement (Fig. 3C). Together, these results demonstrate that neutralization of these proteins in situ is specific, as the BCR response served as both a positive and negative control for these antibodies, and that although CpG-mediated activation of TRPC3 is store independent, it is regulated by PLCγ-1.
FIGURE 3.
CpG-induced calcium signals are mediated by PLCγ-1. A, phosphotyrosine (4G10) Western analysis of protein lysates from BCR- and CpG-activated primary murine B cells. BCR engagement induces phosphorylation of key enzymes, including Lyn, Syk, Btk, and PLCγ-2. By contrast, CpG stimulation does not activate Syk, Btk, or PLCγ-2, but does trigger a transient increase in tyrosine phosphorylation of a protein with mobility identical to Lyn kinase. B, Western blot analysis of phosphotyrosine immunoprecipitates confirms Lyn phosphorylation by CpG at levels comparable to that induced by BCR stimulation (upper panel). Although PLCγ-1 is expressed in primary B cells, neither CpG nor BCR engagement triggers a detectable increase in its phosphorylation (bottom panel). An increase in phospho-Syk levels following BCR engagement indicates that cells are activated by this stimulus. C, intracellular dialysis of anti-PLCγ-1 antibody (right) but not PLCγ-2 (left) blocks CpG activation of TRPC3 currents in primary B cells. By contrast, anti-PLCγ-2, but not anti-PLCγ-1 blocks CRAC current activation by anti-BCR antibody. I-V plots below CpG and CRAC macroscopic currents are shown to demonstrate identity of respective currents. D, sequence is shown for peptide that represents the domain within PLCγ-1 that interacts with TRPC3 channels. Also shown (bottom) is the same PLCγ-1 peptide constructed with an N-terminal antennapedia sequence (underlined) to render the peptide membrane permeant (55). E, the membrane-permeant PLCγ-1/TRPC3 peptide was used to examine the role of TRPC3 in BCR- and CpG-induced Ca2+ entry in primary B cells. The peptide did not inhibit BCR-induced Ca2+ release from stores or subsequent Ca2+entry into cells (left). By contrast, this PLCγ-1/TRPC3 interacting domain peptide completely blocked CpG-induced Ca2+ entry (right). The residual Ca2+ entry observed in the presence of peptide was also seen in unstimulated cells following switch from Ca2+ free to Ca2+ containing external solution (dotted line).
Direct interactions between PLCγ-1 and TRPC3 have been identified in cell lines, and it appears that this physical interaction regulates TRPC3 activity (20, 21). To determine if PLCγ-1 serves a similar adaptor-like role for TRPC3 activation in B cells, we generated a membrane permeant peptide, corresponding to the domain of PLCγ-1 that interacts with TRPC3, to serve as a competitive inhibitor of this interaction (Fig. 3D, 10987). Notably, peptide 10987 completely blocked CpG (Fig. 3E, left panel)-, but not BCR-mediated calcium mobilization (Fig. 3E, right panel). These conditions had no effect on CpG-induced calcium release from stores but strongly suppressed calcium entry. The small degree of residual calcium entry in the presence of the PLCγ-1 peptide was similar to that observed upon solution change and did not reflect cell activation by CpG. Consequently, our inability to elicit CRAC currents in B cells with CpG and the fact that CpG-induced calcium entry is fully suppressed when TRPC3 activation is blocked indicates that TPC3 alone underlies this response. Together, these data suggest that TRPC3 activation is regulated by a physical interaction with PLCγ-1.
SR-B1 Mediates CpG-induced TRPC3 Activation
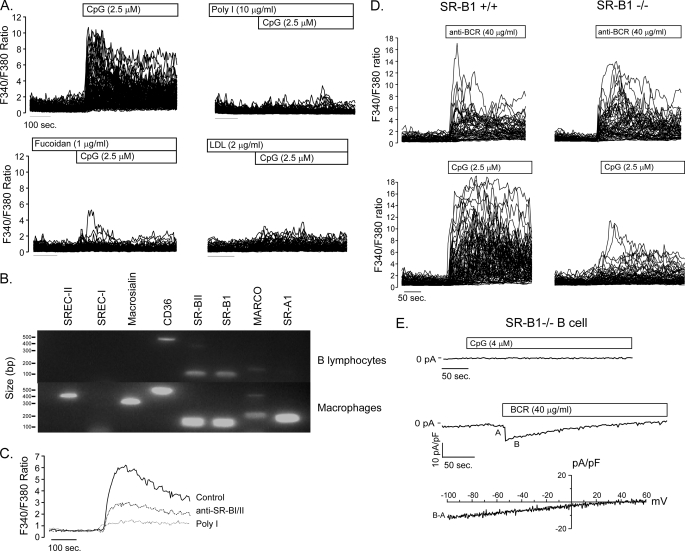
Given that TLR9 and MyD88 are not required for CpG-induced calcium mobilization, we next sought to identify the responsible receptor. We focused on the scavenger receptors (SRs) as candidates; because, like TLRs, they bind pathogen-associated molecules and have been shown to regulate macrophage and dendritic cell responses to TLR ligands such as lipopolysaccharide and CpG (22, 23). To determine if SRs were capable of mediating CpG-induced calcium signals in B cells, we first evaluated the effect of several broadly specific SR antagonists (poly-inosine, fucoidan, and low density lipoprotein). Notably each of the SR antagonists tested blocked CpG-induced calcium responses in primary B cells (Fig. 4A), implicating SRs in this response to CpG. Reverse transcription-PCR analysis of SR expression identified MARCO, CD36, SR-B1, and its splice variant SR-B2 in primary mouse B cells (Fig. 4B). Given that B cells express multiple SR and the broad specificity of antagonists tested, we next evaluated the effect of individual anti-SR antibodies on CpG-induced calcium signaling. Although neither anti-MARCO nor -CD36 antibodies affected CpG-induced calcium signals (supplemental Fig. S2), the anti-SR-B1/2 antibody was clearly inhibitory (Fig. 4C). To directly verify the requirement for SR-B1 in CpG-induced calcium signaling, we examined the response of SR-B1-deficient B cells (24) to this ligand. In the absence of SR-B1 expression, CpG failed to mobilize calcium (Fig. 4D) or elicit a cation current (Fig. 4E) in primary B cells. This failure of CpG-induced signaling does not reflect a general calcium defect; however, because BCR-induced calcium elevations and CRAC channel activation were unaffected by SR-B1 deficiency (Fig. 4E). Taken together, these data indicate that SR-B1 mediates CpG-induced calcium mobilization in B lymphocytes.
FIGURE 4.
Role of scavenger receptor B1 in CpG-mediated calcium signaling. A, CpG-induced Ca2+ signals are blocked by the non-selective scavenger receptor ligands poly-inosine (Poly I), fucoidan, and low density lipoprotein (LDL). B, reverse transcription-PCR analysis demonstrates expression of CD36, MARCO, and splice variants SR-B1 and SR-B2 in primary B lymphocytes. RNA isolated from macrophages served as a positive control for SREC-I, SREC-II, macrosialin, and SR-A1 primers. C, anti-SR-B1 antibody partially blocks CpG-induced Ca2+ signals. D, SR-B1 expression is required for CpG- but not BCR-induced Ca2+ signaling. BCR engagement produces similar Ca2+ signals in SR-B1−/− (upper right) B cells as in SR-B1+/+ controls (upper left); however, SR-B1−/− B lymphocytes are nearly unresponsive to CpG stimulation (lower right). E, CpG fails to elicit a NSCC current in SR-B1−/− lymphocytes, while anti-BCR antibody activates normal CRAC currents in SR-B1−/− B cells, consistent with a requirement for SR-B1 in CpG-mediated TRPC3-dependent Ca2+ entry in primary B cells.
SR-B1 Negatively Regulates TLR9-dependent B Cell Activation
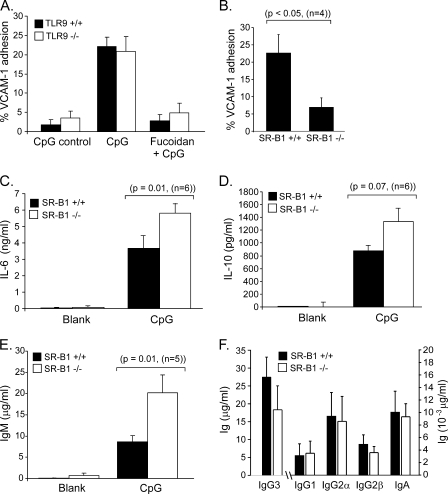
Although little is known about the role of SRs in B cells, it is noteworthy that Toll-like receptors and scavenger receptors expressed by B cells bind a similar range of cellular and pathogen-associated molecules (23, 25–27). Moreover, TLR2 co-engagement with the scavenger receptor CD36 positively regulates B cell activation (13). Given our findings that CpG activates the CD36-related class B family member SR-B1, we asked whether SR-B1 serves a similar co-stimulatory role in TLR9-mediated activation of B cells. We found that neither SR-B1 nor TRPC3 activation was required for NFκB activation (IκBα degradation and p65 activation) or B cell proliferation (supplemental Fig. S3). However, in addition to calcium mobilization we found that VLA-4 integrin activation (VCAM-1 adhesion) by CpG was independent of TLR9 (Fig. 5A) and fully dependent upon SR-B1 expression (Fig. 5B). CpG did not increase B cell adhesion to the LFA-1 ligand ICAM-1 (supplemental Fig. S3), suggesting a selective role for SR-B1 and TRPC3 in B cell adhesion and trafficking.
FIGURE 5.
Regulation of IgM, IL-6, and IL-10 production and VCAM-1 adhesion by SR-B1 signaling in B lymphocytes. A, CpG triggers an increase in VCAM-1 adhesion of primary B cells (filled bars). The increase was observed 30 min after CpG stimulation and was completely blocked by the scavenger receptor blocking ligand fucoidan. The ability of CpG to trigger an increase in VCAM-1 adhesion, and inhibition by fucoidan, is independent of TLR9 expression as these responses are similar in TRL9+/+ (filled bars) and TLR9−/− (open bars) B lymphocytes. B, CpG-induced VCAM-1 adhesion is significantly attenuated in B cells lacking SR-B1 expression. C, SR-B1 negatively regulates CpG-induced production of immunoregulatory cytokines IL-6 and IL-10, and IgM by B cells. SR-B1+/+ and SR-B1−/− primary B lymphocytes were stimulated in vitro, culture supernatants were harvested 48 h after stimulation and assayed by enzyme-linked immunosorbent assay. In response to CpG, SR-B1-deficient B cells elaborated roughly 2-fold more IL-6 (C), IL-10 (D), and IgM (E) than did wild-type cells. F, CpG elicited the production of significant amounts of IgG3 and relatively small amounts of IgG1, IgG2α, IgG2β, and IgA. However, unlike the effect on IgM production, SR-B1 deficiency did not regulate production of these other Ig isotypes.
Another important immunoregulatory function of lymphocytes is the production of cytokines and immunoglobulin. We examined the elaboration by B cells of several cytokines previously shown to be elicited by CpG stimulation (28, 29) and found that IL-6, IL-10, as well as IgM production are absolutely dependent upon TLR9 expression. However, B cells lacking SR-B1 consistently produce more IL-6 (Fig. 5C), IL-10 (Fig. 5D), and IgM (Fig. 5E) than SR-B1+/+ counterparts, indicating that SR-B1 negatively regulates TLR9-dependent production of these factors. CpG also elicited production of other antibody isotypes, most notably IgG3; however, SR-B1 expression did not regulate the production of IgA, IgG3, IgG2α, IgG2β, or IgG1 (Fig. 5F). Thus, in contrast to the cooperative role reported between TLR2 and CD36 during B cell activation (13), SR-B1 is clearly not co-stimulatory and appears to play a negative immunomodulatory role by exerting feedback inhibition on CpG/TLR9-dependent production of IgM, IL-6, and IL-10.
DISCUSSION
In this report, we demonstrate that CpG elicits a robust calcium signal in primary B lymphocytes that is superficially similar to that triggered by BCR engagement but mechanistically and functionally distinct. CpG-induced Ca2+ entry is mediated by PLCγ-1-dependent activation of TRPC3, which is a calcium-permeant store-independent non-selective cation channel (10, 30). By contrast, BCR-mediated calcium entry occurs via PLCγ-2-dependent activation of CRAC channels. Interestingly, although TRPC3 activation is PLCγ-1-dependent, CpG did not induce phosphorylation of PLCγ-1 or PLCγ-2. Rather our data indicate that PLCγ-1 operates as an adaptor that interacts with and activates TRPC3.
Another surprising finding of these studies was that CpG triggers calcium release from intracellular stores, yet does not activate CRAC channels. The may reflect partial calcium store depletion, which is insufficient to trigger STIM1 oligomerization and relocalization to junctional endoplasmic reticulum domains as required for activating CRAC channels (31, 32). Indeed, recent studies suggest a Kd for Ca2+ regulation of STIM1 activation in the 200–600 μm range (33). Although we found that BCR engagement and CpG induce Ca2+ release from stores, CpG-induced transients were sometimes smaller and may reflect less depletion (see Fig. 3E). However, at best, our measurements indirectly assess the extent of calcium release from stores; because, changes in the steady-state cytosolic [Ca2+] do not account for concurrent changes in sarco-endoplasmic reticulum calcium ATPase and plasma membrane calcium ATPase activity or cellular buffering that would mask differences in Ca2+ store depletion/release by CpG versus BCR. An alternative possibility is that IP3 produced by CpG, possibly via activation of PLCγ-1, is spatially restricted to IP3 receptors or functionally distinct calcium stores that do not support CRAC channel activation.
Finally, it is possible that CRAC channel activation (i.e. functional interactions between STIM1 and Orai1) requires a critical regulatory partner, which is activated by the BCR but not by CpG via SR-B1. Indeed, we favor this possibility because two B cell tyrosine kinases (Lyn and Syk) and the adaptor SLP-65 (BLNK) appear to regulate CRAC channel activation by mechanisms that are distinct from their proximal signaling role in PLCγ-2 activation and IP3 generation (34–37). This general idea is also supported by our previous observation that the cytoskeletal associated protein WAVE2 regulates CRAC channel activation in T cells at a step distal to IP3-mediated calcium depletion of stores (38). Given the requirement proposed for Syk in post store regulation of calcium signaling and CRAC channel activation; our results suggest that the inability of SR-B1 to activate CRAC channels in primary B cells may stem from its inability to activate Syk.
Another important and novel aspect of this study is that both CpG-induced calcium signaling and VCAM-1 adhesion are independent of TLR9 and instead are mediated by SR-B1. Although scavenger receptors are analogous to Toll-like receptors in their capacity to recognize a broad range of pathogen-associated molecules, relatively little is known about the biological consequences of pathogen signaling through them. In addition to pathogens, SR's also recognize apoptotic cell products, including DNA, lipoproteins, and phospholipids, each of which has the capacity to act as an autoantigen and trigger inflammatory disease. In fact, MARCO- and SR-A-deficient mice have elevated anti-DNA antibody titers compared with wild-type mice following inoculation with syngeneic apoptotic cells (11), suggesting that these scavenger receptors regulate the clearance of antigens that can trigger autoimmunity. Consistent with a role for these SRs in the clearance of cellular debris, mice with a propensity to develop spontaneous autoimmune disease and patients with systemic lupus erythematosis both have elevated antibody titers against MARCO and SR-A (11).
In our studies, we initially focused on the response of B cells to the pathogen-derived TLR9 ligand unmethylated CpG DNA because of its ability to elicit a large calcium signal and induce polyclonal B cell activation. However, because stimulatory TLR9 motifs present in lower abundance in mammalian DNA may also be physiological ligands for SR, our findings have much broader implications for the regulation of tolerance to self antigens. Recent work suggests that these endogenous CpG motifs may be enriched in DNA released from apoptotic cells (18, 39, 40). Thus, CpG islands in host DNA may also induce dual activation of TLR9 and SR-B1 (41) and negative regulatory responses like the one we have identified for SR-B1 could play a general role in controlling the balance between normal and pathological activation by pathogen and/or self-derived nucleic acids.
In addition to maintaining tolerance to self-DNA, SRs seem to play a general role in regulating the intensity of inflammatory responses to antigen. For example, it has been shown that scavenger receptor A family members negatively regulate immune activity triggered by tumors, fungal pathogens, and apoptotic cells (11, 42, 43). In our studies, we have shown that dual engagement of SR-B1 and TLR9 by CpG negatively regulates the production of pro-inflammatory mediators such as IL-6 and IL-10. Although the role of these cytokines in promoting autoimmune disease is complex and not fully resolved, IL-6 and IL-10 are biomarkers of systemic lupus erythematosis (44) and have been implicated in its pathogenesis (45–49). IL-6 regulates B cell differentiation and Ig production, but this action seems more critical to isotype switching and not IgM production (50). Therefore, elevated IL-6 following CpG stimulation of SR-B1-deficient B cells is unlikely to underlie their increased IgM production. Although we did not observe regulation of other antibody isotypes by SR-B1, this does not preclude an effect on production of DNA and chromatin-specific pathogenic IgGs (51). In fact, anti-IL-6 (and IL-10) clinical trials conducted on patients with systemic lupus erythematosis have shown promising initial results (52). Given the pleiotropic and pro-inflammatory effects of IL-6 and IL-10, SR-B1-mediated suppression may be an important mechanism for limiting the production of these and other TLR9-induced pro-inflammatory mediators and pathogenic antibodies.
In conclusion, our results provide a novel perspective on mechanisms of Ca2+-regulated cell activation and differentiation by cognate antigen, pathogen-derived molecules, and inflammatory mediators that induce functionally limited polyclonal responses in B cells. Together with our previously published data (5–7), we have now identified four distinct TRP family calcium permeant channels in B lymphocytes, each of which is activated by distinct receptors or pro-inflammatory stimuli. Thus, TRP channels integrate an array of innate signals, including those generated by pathogens, inflammatory mediators (eicosanoids), and osmotic or shear stress, with adaptive stimuli generated by antigen engagement of the BCR. These distinct calcium signaling pathways provide a means for B cells to integrate complex environmental cues into context specific calcium-regulated biological responses.
Supplementary Material
Acknowledgments
We thank Dan Rader and Jeff Billheimer for SR-B1-deficient mice and Thamara Hewavitharana for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant AI 39678 (to B. D. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- BCR
- B cell receptor
- NSCC
- non-selective cation channel
- CRAC
- calcium release-activated calcium
- TRP
- transient receptor potential
- SR-B1
- scavenger receptor B1
- TLR
- Toll-like receptor
- PLC
- phospholipase C
- VCAM-1
- vascular cell adhesion molecule-1
- IP3
- 1,4,5-inositol trisphosphate
- IL
- interleukin
- siRNA
- small interference RNA.
REFERENCES
- 1.Berridge M. J. (1993) Nature 361,315–325 [DOI] [PubMed] [Google Scholar]
- 2.Sugawara H., Kurosaki M., Takata M., Kurosaki T. (1997) EMBO J. 16,3078–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyakawa T., Maeda A., Yamazawa T., Hirose K., Kurosaki T., Iino M. (1999) EMBO J. 18,1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagar R. E., Burgstahler A. D., Nathanson M. H., Ehrlich B. E. (1998) Nature 396,81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q. H., Liu X., Wen Z., Hondowicz B., King L., Monroe J., Freedman B. D. (2005) J. Immunol. 174,68–79 [DOI] [PubMed] [Google Scholar]
- 6.Zhu P., Liu X., Labelle E. F., Freedman B. D. (2005) J. Immunol. 175,4981–4989 [DOI] [PubMed] [Google Scholar]
- 7.Liu X., Zhu P., Freedman B. D. (2006) Am. J. Physiol. Cell Physiol. 290,C873–C882 [DOI] [PubMed] [Google Scholar]
- 8.Bauer S., Kirschning C. J., Häcker H., Redecke V., Hausmann S., Akira S., Wagner H., Lipford G. B. (2001) Proc. Natl. Acad. Sci. U.S.A. 98,9237–9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. (2000) Nature 408,740–745 [DOI] [PubMed] [Google Scholar]
- 10.Hofmann T., Obukhov A. G., Schaefer M., Harteneck C., Gudermann T., Schultz G. (1999) Nature 397,259–263 [DOI] [PubMed] [Google Scholar]
- 11.Wermeling F., Chen Y., Pikkarainen T., Scheynius A., Winqvist O., Izui S., Ravetch J. V., Tryggvason K., Karlsson M. C. (2007) J. Exp. Med. 204,2259–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deleted in proof
- 13.Won W. J., Bachmann M. F., Kearney J. F. (2008) J. Immunol. 180,230–237 [DOI] [PubMed] [Google Scholar]
- 14.Gomez T. S., Kumar K., Medeiros R. B., Shimizu Y., Leibson P. J., Billadeau D. D. (2007) Immunity 26,177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin F., Auerbach A., Sachs F. (1996) Biophys. J. 70,264–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y. X., Kotlikoff M. I. (2000) J. Physiol. 523,131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busconi L., Bauer J. W., Tumang J. R., Laws A., Perkins-Mesires K., Tabor A. S., Lau C., Corley R. B., Rothstein T. L., Lund F. E., Behrens T. W., Marshak-Rothstein A. (2007) J. Immunol. 179,7397–7405 [DOI] [PubMed] [Google Scholar]
- 18.Uccellini M. B., Busconi L., Green N. M., Busto P., Christensen S. R., Shlomchik M. J., Marshak-Rothstein A., Viglianti G. A. (2008) J. Immunol. 181,5875–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Löf C., Blom T., Törnquist K. (2008) J. Cell Physiol. 216,245–252 [DOI] [PubMed] [Google Scholar]
- 20.van Rossum D. B., Patterson R. L., Sharma S., Barrow R. K., Kornberg M., Gill D. L., Snyder S. H. (2005) Nature 434,99–104 [DOI] [PubMed] [Google Scholar]
- 21.Patterson R. L., van Rossum D. B., Ford D. L., Hurt K. J., Bae S. S., Suh P. G., Kurosaki T., Snyder S. H., Gill D. L. (2002) Cell 111,529–541 [DOI] [PubMed] [Google Scholar]
- 22.Józefowski S., Sulahian T. H., Arredouani M., Kobzik L. (2006) J. Leukoc. Biol. 80,870–879 [DOI] [PubMed] [Google Scholar]
- 23.Gordon S. (2002) Cell 111,927–930 [DOI] [PubMed] [Google Scholar]
- 24.Rigotti A., Trigatti B. L., Penman M., Rayburn H., Herz J., Krieger M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94,12610–12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gough P. J., Gordon S. (2000) Microbes Infect. 2,305–311 [DOI] [PubMed] [Google Scholar]
- 26.Pearson A. M. (1996) Curr. Opin. Immunol. 8,20–28 [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay S., Gordon S. (2004) Immunobiology 209,39–49 [DOI] [PubMed] [Google Scholar]
- 28.Krieg A. M. (2003) Nat. Med. 9,831–835 [DOI] [PubMed] [Google Scholar]
- 29.Krieg A. M. (2002) Annu. Rev. Immunol. 20,709–760 [DOI] [PubMed] [Google Scholar]
- 30.Hurst R. S., Zhu X., Boulay G., Birnbaumer L., Stefani E. (1998) FEBS Lett. 422,333–338 [DOI] [PubMed] [Google Scholar]
- 31.Luik R. M., Wang B., Prakriya M., Wu M. M., Lewis R. S. (2008) Nature 454,538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luik R. M., Wu M. M., Buchanan J., Lewis R. S. (2006) J. Cell Biol. 174,815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stathopulos P. B., Li G. Y., Plevin M. J., Ames J. B., Ikura M. (2006) J. Biol. Chem. 281,35855–35862 [DOI] [PubMed] [Google Scholar]
- 34.Takata M., Sabe H., Hata A., Inazu T., Homma Y., Nukada T., Yamamura H., Kurosaki T. (1994) EMBO J. 13,1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung S. C., Limnander A., Kurosaki T., Weiss A., Korenbrot J. I. (2007) J. Cell Biol. 177,317–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abudula A., Grabbe A., Brechmann M., Polaschegg C., Herrmann N., Goldbeck I., Dittmann K., Wienands J. (2007) J. Biol. Chem. 282,29059–29066 [DOI] [PubMed] [Google Scholar]
- 37.Kulathu Y., Hobeika E., Turchinovich G., Reth M. (2008) EMBO J. 27,1333–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolz J. C., Gomez T. S., Zhu P. M., Li S., Medeiros R. B., Shimizu Y., Burkhardt J. K., Freedman B. D., Billadeau D. D. (2006) Curr. Biol. 16,24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viglianti G. A., Lau C. M., Hanley T. M., Miko B. A., Shlomchik M. J., Rothstein A. (2003) Immunity 19,837–847 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y. Q., Krieg A. M. (2003) Int. Immunol. 15,223–231 [DOI] [PubMed] [Google Scholar]
- 41.Leadbetter E. A., Rifkin I. R., Hohlbaum A. M., Beaudette B. C., Shlomchik M. J., Marshak-Rothstein A. (2002) Nature 416,603–607 [DOI] [PubMed] [Google Scholar]
- 42.Hollifield M., Bou Ghanem E., de Villiers W. J., Garvy B. A. (2007) Infect. Immun. 75,3999–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X. Y., Facciponte J., Chen X., Subjeck J. R., Repasky E. A. (2007) Cancer Res. 67,4996–5002 [DOI] [PubMed] [Google Scholar]
- 44.Chun H. Y., Chung J. W., Kim H. A., Yun J. M., Jeon J. Y., Ye Y. M., Kim S. H., Park H. S., Suh C. H. (2007) J. Clin. Immunol. 27,461–466 [DOI] [PubMed] [Google Scholar]
- 45.Yin Z., Bahtiyar G., Zhang N., Liu L., Zhu P., Robert M. E., McNiff J., Madaio M. P., Craft J. (2002) J. Immunol. 169,2148–2155 [DOI] [PubMed] [Google Scholar]
- 46.Ishida H., Muchamuel T., Sakaguchi S., Andrade S., Menon S., Howard M. (1994) J. Exp. Med. 179,305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyrrell-Price J., Lydyard P. M., Isenberg D. A. (2001) Clin. Exp. Immunol. 124,118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lenert P., Brummel R., Field E. H., Ashman R. F. (2005) J. Clin. Immunol. 25,29–40 [DOI] [PubMed] [Google Scholar]
- 49.Llorente L., Richaud-Patin Y. (2003) J. Autoimmun. 20,287–289 [DOI] [PubMed] [Google Scholar]
- 50.Kopf M., Herren S., Wiles M. V., Pepys M. B., Kosco-Vilbois M. H. (1998) J. Exp. Med. 188,1895–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richards H. B., Satoh M., Shaw M., Libert C., Poli V., Reeves W. H. (1998) J. Exp. Med. 188,985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anolik J. H., Aringer M. (2005) Best Pract. Res. Clin. Rheumatol. 19,859–878 [DOI] [PubMed] [Google Scholar]
- 53.Ramsey I. S., Delling M., Clapham D. E. (2006) Annu. Rev. Physiol. 68,619–647 [DOI] [PubMed] [Google Scholar]
- 54.Owsianik G., Talavera K., Voets T., Nilius B. (2006) Annu. Rev. Physiol. 68,685–717 [DOI] [PubMed] [Google Scholar]
- 55.Derossi D., Joliot A. H., Chassaing G., Prochiantz A. (1994) J. Biol. Chem. 269,10444–10450 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.