Abstract
Mirk/Dyrk1B is a serine/threonine kinase widely expressed in colon cancers. Serum starvation induced HD6 colon carcinoma cells to enter a quiescent G0 state, characterized by a 2N DNA content and a lower RNA content than G1 cells. Compared with cycling cells, quiescent cells exhibited 16-fold higher levels of the retinoblastoma protein p130/Rb2, which sequesters E2F4 to block entry into G1, 10-fold elevated levels of the CDK inhibitor p27kip1, and 10-fold higher levels of Mirk. However, depletion of Mirk did not prevent entry into G0, but enabled quiescent HD6, SW480, and colo320 colon carcinoma cells to acquire some biochemical characteristics of G1 cells, including increased levels of cyclin D1 and cyclin D3 because of slower turnover, increased activity of their CDK4/cyclin D complexes, and increased phosphorylation and decreased E2F4 sequestering ability of the CDK4 target, p130/Rb2. As a result, depletion of Mirk allowed some cells to escape quiescence and enabled cells released from quiescence to traverse G1 more quickly. The kinase activity of Mirk was increased by the chemotherapeutic drug 5-fluorouracil (5-FU). Treatment of p53 mutant colon cancer cells with 5-FU led to an elongated G1 in a Mirk-dependent manner, as G1 was shortened by ectopic overexpression of cyclin D1 mutated at the Mirk phosphorylation site (T288A), but not by wild-type cyclin D1. Mirk, through regulating cyclin D turnover, and the CDK inhibitor p27, as shown by depletion studies, functioned independently and additively to regulate the exit of tumor cells from quiescence.
Solitary disseminated tumor cells that are negative for proliferation markers such as Ki67 are thought to be the source of tumor recurrence. These dormant tumor cells are quiescent, reversibly arrested in the G0 part of the cell cycle, and can re-enter the cell cycle under favorable clues from the microenvironment. Quiescence is not simply a long G1 period, but is characterized by specific changes in gene expression (1). Factors that allow the prolonged survival of quiescent tumor cells in vivo are of clinical relevance and include antioxidant proteins. A requirement for quiescence in normal hematopoietic stem cells is repression of the production of reactive oxygen species (ROS)2 (2), making it likely that cancer cells also require low ROS levels to maintain quiescence. The serine/threonine kinase Mirk was recently shown to reduce ROS levels in two pancreatic cancer cell lines by increasing transcription of a cohort of genes that detoxify superoxides and prevent the generation of hydroxyl radicals (3). The ROS-countering activity of Mirk was primarily exhibited in quiescent tumor cells because such cells had the highest levels of Mirk protein. Thus Mirk maintains the viability of quiescent pancreatic cancer cells through up-regulating antioxidant genes.
Mirk/Dyrk1B is a member of a conserved family of serine/threonine kinases that are activated by intramolecular tyrosine phosphorylation and which mediate maturation in different tissues: Mirk in skeletal muscle, Dyrk1A in the brain, etc. One role of Mirk in skeletal muscle differentiation after a stress signal of serum deprivation is to block cycling myoblasts in the G0 quiescent state (4) by phosphorylation of the cell cycle regulators cyclin D1 and p27kip1 (5, 6). Phosphorylation by Mirk at a conserved ubiquitination site initiated proteolysis of cyclin D1, while p27kip1 was stabilized following phosphorylation by Mirk at Ser-10. Recently the survival factors Bcl2 and BclXL were shown to mediate G0 quiescence in NIH3T3 cells and murine embryonic fibroblasts through increasing p27kip1 stability, which required phosphorylation of p27Ser-10 by Mirk (7). These observations are consistent with Mirk function as a G0 kinase in nontransformed cells.
Recently, observations have pointed to the importance of cancer cells arrested in a reversible quiescent state to undergo repair. Cell cycle restriction through the CDK inhibitor p21cip1 was shown to both limit DNA damage and maintain the self-renewal of leukemia stem cells (8). Expression of leukemia-associated oncogenes in mouse hematopoietic stem cells induced DNA damage and activated a p21cip1-dependent response that led to reversible cell cycle arrest in a quiescent state and DNA repair. The mechanisms that control this arrest include induction of p21cip1 in leukemia stem cells (8). Possibly Mirk retained the capacity to function as a G0 kinase to restrict cell cycling in cancer cells. This function of Mirk is the focus of the current study.
EXPERIMENTAL PROCEDURES
Materials
Antibodies CDK4, cyclins D1, D3, and E, and antibody to p130/Rb2 were from Santa Cruz Biotechnology. Rabbit polyclonal antibody to a unique sequence at the C terminus of Mirk was raised as described (9). 5-Fluorouracil (5-FU), nocodazole, and all other reagents were from Sigma or Calbiochem.
Cell Culture
All cell lines and the BJ human diploid fibroblast strain were obtained from ATCC and maintained in growth medium, Dulbecco's modified Eagle's medium containing 7% FBS (10). The HD6 subclone of the uncloned HT29 line was described previously (11, 12, 9), is mutant in p53, but exhibits wild-type ras genes (13). The wild-type cyclin D1 construct and cyclin D1-T288A had been generated in the same pCMV-tag2B constructs in earlier studies (5).
Cell Cycle Analysis
Cells were plated at ∼104/cm2 in growth medium. After attachment, cells were depleted of Mirk or mock-depleted, then cultured serum-free for 48 h to reach quiescence. Flow cytometry was performed as described after staining with either propidium iodide or for two-parameter analysis following staining with Hoechst 33258 and Pyronin Y (3).
RNA Interference
Synthetic RNAi duplexes targeting different regions of the Mirk/Dyrk1B mRNA sequence initiated at either base 649 (siA), base 840 (siC), or base 461 (siD) were obtained from Invitrogen and are unique to Mirk by Blast search. RNAi to p27kip1 was also obtained from Invitrogen. Controls were GC-matched scrambled sequences. Cells in 6-well plates were transfected per well with synthetic RNAi duplexes (5 μl) plus Lipofectamine 2000 (5 μl) in 250-μl serum-containing Opti-MEM for 48 h.
Immunoprecipitations
An aliquot of total cell lysate of 500 μg was immunoprecipitated with 1 μl of anti-Mirk rabbit polyclonal antibody overnight at 4 °C; the complexes were then collected by the addition of 20 μl of protein A-agarose, incubated for 1 h at 4 °C, washed three times with lysis buffer, and separated by SDS-PAGE. Identification of co-immunoprecipitated E2F4 and p130/Rb2 was performed as described (3).
In Vitro Kinase Assay
The kinase activity of Mirk was tested exactly as described (15) with the myelin basic protein (MBP) from Upstate Biotechnology, or with recombinant GST-HDAC5 (amino acids 1–283) prepared as described (16). CDK kinase activity was assayed on CDK4 bound to anti-CDK4-coupled agarose in an in vitro kinase assay using recombinant retinoblastoma protein (pRB, Santa Cruz Biotechnology).
Immunodetection
Immunodetection was performed as described (15).
RESULTS
HD6 Colon Carcinoma Cells Enter a Quiescent G0 State When Serum Starved
HT29/clone HD6 colon carcinoma cells were chosen for this study because, after culture to confluent density, they cease cell division and differentiate into the goblet cell lineage without the addition of chemical agents (17), suggesting that these cultured cells can enter a quiescent state. In earlier studies on Mirk effects on cell cycling, cell lines were used in which the Mirk gene was highly amplified, elevating Mirk protein levels 10–20-fold higher than in other cancer cell lines (3). In contrast, the Mirk gene is not amplified in HD6 cells, which may be more representative of the typical cancer cell in which Mirk is expressed. Stably overexpressed Mirk in HT29 subclones mediated cell survival, so these colon cancer cells were suitable for the study of Mirk function (9).
Initial studies showed that most HD6 cells could be made to reversibly enter a quiescent state when the cells were cultured for 48 h in serum-free conditions in high glucose Dulbecco's modified Eagle's medium. A two-stage staining method used Hoechst dye to bind to all double-stranded DNA followed by Pyronin Y dye to bind RNA. The majority of serum-starved cells (74–88%) had a 2N DNA content by flow cytometric analysis after binding Hoechst 33258 (Fig. 1, C and D) or, in parallel experiments, propidium iodide (Fig. 2A, Table 1). Most serum-starved cells that had accumulated with a 2N DNA content also had a low RNA level (Fig. 1C), so were in G0 (Fig. 1D, gold cells). Cultures of the serum-starved cells were analyzed in parallel to cultures of serum-starved cells released by a change to growth medium containing the chemotherapeutic drug 5-FU to initiate S phase checkpoints, and the mitotic inhibitor nocodazole to block any remaining cycling cells in G2+M, so only one cell cycle was allowed in the 24-h release period. Analysis of serum-starved cultures by two-parameter flow cytometry demonstrated that 86% of cells were in G0, with few cells in other cell cycle phases (Fig. 1D), while after release from quiescence, most cells had entered the cell cycle, with only 22% remaining in G0 (Fig. 1D). Thus the majority of the quiescent HD6 cells remained capable of re-entering the cell cycle under appropriate conditions.
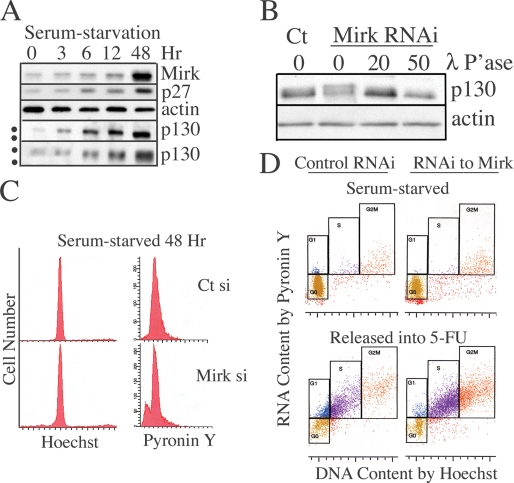
FIGURE 1.
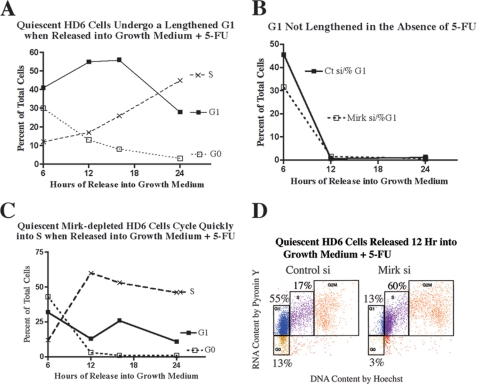
Characterization of quiescent HD6 colon carcinoma cells. A, HD6 colon carcinoma cells were cultured in serum-free medium for 0–48 h and examined for abundance of Mirk, p130/Rb2, p27kip1, and actin by Western blotting. The top four panels were scanned from a 10% acrylamide gel, while the lowest panel of p130/Rb2 was scanned from a 6% acrylamide gel. The dots mark the different positions of the more phosphorylated, slowly migrating p130 band, and the less phosphorylated, faster migrating p130 band. Similar data were obtained in a second experiment. B, E2F4 sequestering protein p130/Rb2HD6 is more phosphorylated in quiescent cells when Mirk is depleted. HD6 colon carcinoma cells were depleted of Mirk by transient transfection with RNAi duplex siA (Mirk si) or mock-depleted by transfection with GC-matched control RNAi (Ct si), cultured in serum-free medium for 48 h, then released into serum-containing growth medium. Lysates were treated with 20 or 50 μl of lambda phosphatase before Western analysis for p130/Rb2 and actin. Similar data obtained in two other experiments (not shown). C, HD6 colon carcinoma cells were depleted of Mirk or mock-depleted and then made quiescent as in B. 10,000 cells from each culture were analyzed for DNA content by staining with Hoechst 33258, then stained for RNA content (primarily polyribosomes) by Pyronin Y. Similar data showing accumulation of serum-starved cells with a 2N DNA content found in 3 two-parameter flow cytometric analyses using Hoechst/Pyronin Y staining and in 5 one-parameter flow cytometric analysis using propidium iodide to analyze DNA content (Table 1). D, serum-starved HD6 cells arrest predominantly in G0, but retain the capacity to enter cycle when released by addition of FBS. Data from C plotted together in the upper panel. In the lower panel, parallel cultures were released for 24 h by a change to growth medium containing 7% FBS, the toxic concentration of 1 μm 5-fluoruracil to damage DNA and block cycling cells in S phase by engagement of checkpoints, and 50 ng/ml nocodazole to block any remaining cycling cells in G2+M so that only one cell cycle was allowed. 10,000 cells from each culture were analyzed for DNA content by staining with Hoechst 33258, then stained for RNA content with Pyronin Y. The analyses of the serum-starved cultures and the released cultures were performed at the same time using the same gating. Similar data were seen in two additional experiments. The fraction of cells in each cell cycle position in the serum-starved culture control-depleted were 86% G0 (gold), 2% G1 (blue), 2% S (purple), and 5% G2+M (red) and Mirk-depleted were 71% G0, 1% G1, 1% S, and 7% G2+M. The serum-starved mock-depleted cultures released into 5-FU were 22% G0, 14% G1, 34% S, and 12% G2+M. The Mirk-depleted cultures released into 5-FU were 14% G0, 3% G1, 49% S, and 27% G2+M. The values do not equal 100% because of the presence of sub-G0 cells (not shown) and some cells with >2N DNA but lower RNA content than normal S phase cells.
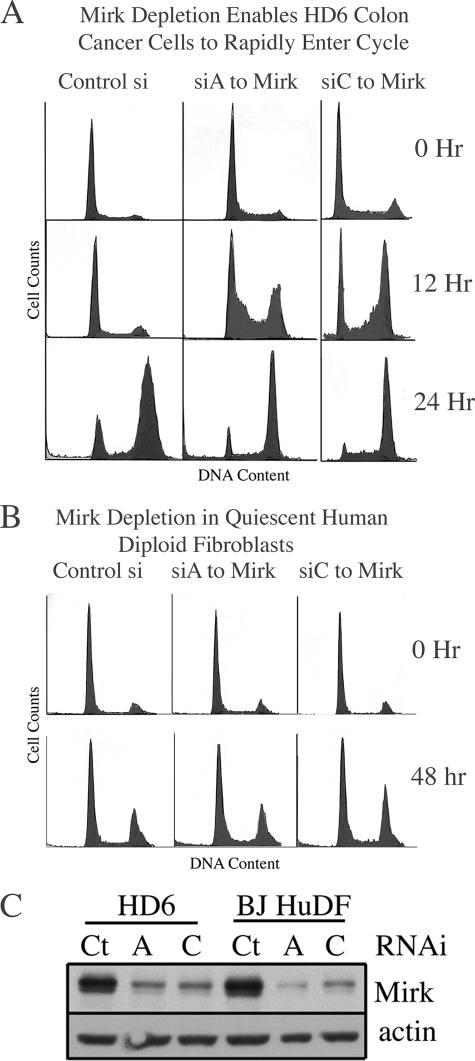
FIGURE 2.
Mirk depletion enables colon cancer cells to rapidly traverse G1 when released from quiescence, but does not detectably affect cycling of human diploid fibroblasts. A, HD6 colon carcinoma cells were depleted of Mirk by RNAi duplexes siA or siC or mock-depleted and then made quiescent as in Fig. 1. Cells were then released by change to medium containing 7% FBS and the mitotic inhibitor vinblastine at 2 nm so only one cell cycle would be analyzed by flow cytometry after propidium iodide staining. Data shown are representative of five replicate experiments, with the quantitation shown in Table 1. B, Mirk depletion does not detectably affect cell cycle progression of human diploid fibroblasts. Strain BJ cells were depleted of Mirk by transient transfection with RNAi duplexes siA and siC or mock-depleted with control RNAi by a 48-h transfection in growth medium with Lipofectamine 2000, then accumulated in G0/G1 by 24 h culture in serum-free medium. Cells were then released by a change to medium containing 7% FBS and 2 nm vinblastine to arrest cycling cells in G2+M and were analyzed for cell cycle distribution by flow cytometry 0 and 48 h following release. The number of cells analyzed per panel was 3680 ± 1218 (S.D.) (n = 6), with similar results in three similar experiments. C, parallel Western blots from the experiments shown in A and B, showing Mirk and actin levels in HD6 colon carcinoma cells and in BJ human diploid fibroblasts (BJ HuDF).
TABLE 1.
Mirk depletion increases the cycling of colon cancer cells, but not of normal diploid fibroblasts as analyzed by one-parameter flow cytometry in HD6 colon carcinoma cells
HD6 cells were depleted of Mirk by transient transfection with RNAi duplexes to Mirk or GC-matched controls, cultured in serum-free medium for 2 days, and then released by change to medium containing 7% FBS and the mitotic inhibitors vinblastine (2 nm) or nocodazole (50 ng/ml). Cells were stained for DNA content with propidium iodide before flow cytometry. The RNAi duplex siD to Mirk was used in the nocodazole release experiment. Mirk depletion in 2 other colon carcinoma cell lines decreased the fraction of serum-starved cells with a 2N DNA content 12%+/−3% (S.D.) in SW480 cultures and 7%+/−3% (S.D.) in SKCO1 cultures.
| % Cells in G0/G1 |
% Cells in S phase |
% Cells in G2+M |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Ct si | Mirk siA | Mirk siC/D | Ct si | Mirk siA | Mirk siC/D | Ct si | Mirk siA | Mirk siC/D | |
| 0 h | 74 (6)a | 63 (4) | 54 | 19 (3) | 29 (1) | 41 | 6 (3) | 8 (4) | 5 |
| + vinblastine | |||||||||
| 12 h | 71 | 31 | 15 | 16 | 45 | 50 | 13 | 23 | 35 |
| 24 h | 15 | 10 | 0 | 8 | 13 | 30 | 77 | 78 | 70 |
| + nocodazole | |||||||||
| 24 h | 35 | 5 | 19 | 27 | 14 | 26 | 37 | 81 | 54 |
a n = 5; mean (S.D.).
A time course analysis was used to characterize the quiescent cultures. During serum-starvation Mirk protein levels increased 10-fold, as did levels of the CDK inhibitor p27kip1, a well known marker of quiescent cells (Fig. 1A). The retinoblastoma protein family member p130/Rb2 sequesters the E2F4 transcription factor, preventing progression of mammalian cells into G1. The protein level of p130/Rb2 is elevated in quiescent cells and decreased in proliferating cells (18). Quiescent HD6 cells (48-h point, Fig. 1A) exhibited 16-fold higher levels of p130/Rb2 than cells in log phase growth (zero time point). When cells are stimulated to enter G1 by addition of mitogens, p130/Rb2 is phosphorylated by G1 cyclin/CDK complexes and migrates more slowly. When HD6 cells were in log phase growth, their p130/Rb2 exhibited slow mobility, while when cells entered a quiescent state after 48 h of serum starvation, their p130/Rb2 species exhibited a more rapid migration, consistent with less phosphorylation (Fig. 1A, compare upper and lower ●, left margin). The differentially phosphorylated p130/Rb2 forms were more readily seen in a lower percent gel (Fig. 1A, lowest panel). When cells are stimulated to enter G1 by addition of mitogens, p130 is phosphorylated by G1 cyclin/CDK complexes, migrates more slowly, and releases E2F4. Depletion of Mirk gave the same result as addition of FBS, conversion of p130/Rb2 by phosphorylation into slower-migrating species (Fig. 1B, compare lanes 1 and 2). Treatment of lysates with lambda phosphatase collapsed all of the p130/Rb2 bands into one high mobility form in a dose-dependent manner. Thus Mirk depletion in these quiescent colon carcinoma cells increased the activity of the CDK4/cyclin D complexes which inactivate the pRb2 protein by phosphorylation. Moreover, quiescent HD6 cells in G0 accumulate the G1 phase biochemical marker of inactivated p130/Rb2 when depleted of Mirk.
Depletion of Mirk Enabled Quiescent Colon Cancer Cells to Rapidly Re-enter Cycle When Serum Growth Factors Were Added
The role of Mirk in quiescent cells was evaluated by RNA interference using transient transfection of synthetic RNAi duplexes (Fig. 2C). Mirk-depleted cells still entered a G0 quiescent state, but fewer cells were found in G0 (71% versus 86%) and more in sub-G0 regions (Fig. 1C and data not shown). Depletion of Mirk in quiescent SU86.86 pancreatic cancer cells led to increased cell death because of a 2-fold higher intracellular level of ROS (3). A similar 2-fold increase in ROS levels was seen in Mirk-depleted colon cancer cells (data not shown), suggesting that the loss of cells from quiescent colon cancer cultures was a common feature when Mirk was depleted.
Surprisingly, when Mirk-depleted quiescent cells were released into growth medium, more cells entered cycle, with 76% in S and G2+M, versus 46% for the mock-depleted culture (Fig. 1D), suggesting that Mirk-depleted cells were more capable of exiting the quiescent state. To explore this further, Mirk was depleted by two RNAi duplexes, each targeted to a different region of the Mirk mRNA (Fig. 2C), and time course studies were done using flow cytometry after propidium iodide staining (Fig. 2A). By this analysis, more Mirk-depleted HD6, SKCO1, and SW480 colon cancer cells than control cells had slipped from quiescence during serum starvation and had a >2N DNA content (zero time points in Fig. 2A and Table 1). After 12 h of release from quiescence, the majority of HD6 cells depleted of Mirk by either RNAi duplex were either in S phase or G2/M, while the majority of the mock-depleted cells remained in G0/G1 (Fig. 2A). Similar results were found whether cells were released into medium containing either of two microtubule inhibitors, nocodazole or vinblastine (Table 1). Depletion of Mirk primed quiescent colon cancer cells so they could rapidly enter cycle when serum growth factors were restored.
Mirk Depletion Did Not Allow Quiescent Normal Diploid Fibroblasts to Re-enter Cycle
Because Mirk is widely expressed in normal tissues at low levels (9), depletion of Mirk might allow quiescent normal diploid cells to re-enter cycle. Culture of the human BJ strain of normal diploid fibroblasts in serum-free medium led cells to accumulate predominately with a 2N DNA content whether depleted of Mirk by either of two RNAi duplexes (Fig. 2C) or mock-depleted with GC-matched control RNAi, 70–74% (±2%) and 77 (±2)%, respectively (Fig. 2B, three replicate experiments, Table 1). A similar fraction of Mirk-depleted and mock-depleted cells were either in S phase or G2/M 24 and 48 h after release from serum-starvation (Fig. 2B, Table 2 and data not shown). Thus depletion of Mirk had no detectable effect on the cycling of normal diploid fibroblasts, possibly because these cells had a normal G1 checkpoint. Each of the three colon cancer cell lines studied in Fig. 2A and Tables 1 and 2 had mutant p53, so lacked the p53-induced p21 G1 checkpoint. Mirk protein was not detectable in colon cancer cell lines RKO and HCT116 with wild-type p53 (data not shown) so the effect of Mirk depletion could not be tested in these lines.
TABLE 2.
Mirk depletion increases the cycling of colon cancer cells but not of normal diploid fibroblasts as analyzed by one-parameter flow cytometry in BJ diploid human fibroblasts
Human diploid fibroblasts were depleted of Mirk for 24 h. Cells were stained for DNA content with propidium iodide before flow cytometry.
| +2 nm vinblastine | % Cells in G0/G1 |
% Cells in S phase |
% Cells in G2+M |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ct si | Mirk siA | Mirk siC | Ct si | Mirk siA | Mirk siC | Ct si | Mirk siA | Mirk siC | |
| 0 h | 77 (2)a | 70 (2) | 74 (2) | 8 (3) | 14 (4) | 8 (3) | 15 (5) | 16 (6) | 18 (4) |
| 24 h | 64 (6) | 62 (8) | 56 (13) | 19 (3) | 20 (8) | 28 (9) | 17 (6) | 17 (6) | 17 (6) |
| 48 h | 30 | 26 | 29 | 39 | 33 | 36 | 31 | 40 | 35 |
a n = 3; mean (S.D.).
Mirk Depletion Leads to a Rapid Transit of G1 in Colon Cancer Cells by Increasing Levels of G1 Cyclins
In microarray analysis, depletion of Mirk did not alter expression of cell cycle regulatory genes such as cyclins or CDK inhibitors, or any growth factor (not shown). However, in earlier studies with nontransformed Mv1Lu cells overexpression of Mirk was observed to initiate a reversible arrest in G0 because Mirk targeted the G1 cyclin, cyclin D1, for rapid turnover by phosphorylation at a ubiquitination site conserved in all cyclin D isoforms (5). Elevated levels of cyclin D1 are commonly seen in colon cancer (19) so all cells were expected to exhibit some level of this cyclin. Depletion of Mirk in SW480 and colo320 colon cancer cells by two RNAi duplexes targeted to different regions of the Mirk mRNA led to average 2-fold and 4-fold increases in cyclin D1 levels, respectively (Fig. 3A), because of an increase in cyclin D half-life (data not shown). Similarly, quiescent HD6 cells had an average of 3-fold more cyclin D3 and cyclin D1 if Mirk had been depleted because of less turnover (Fig. 3, B and E and data not shown). The Mirk phosphorylation site is conserved between cyclins D1 and D3, and Mirk has been shown to increase the turnover of both cyclins in pancreatic cancer cells (3). Depletion of Mirk in HD6 cells increased the half-life of cyclin D1 compared with control-depleted cells (Fig. 3E), accounting for the increased protein abundance. In earlier studies, stable Mirk transfectants of colon cancer cells were shown to exhibit a faster turnover of cyclin D1 (20). Thus, both Mirk depletion (this study) and Mirk overexpression studies showed that Mirk destabilized cyclin D isoforms in colon cancer cells.
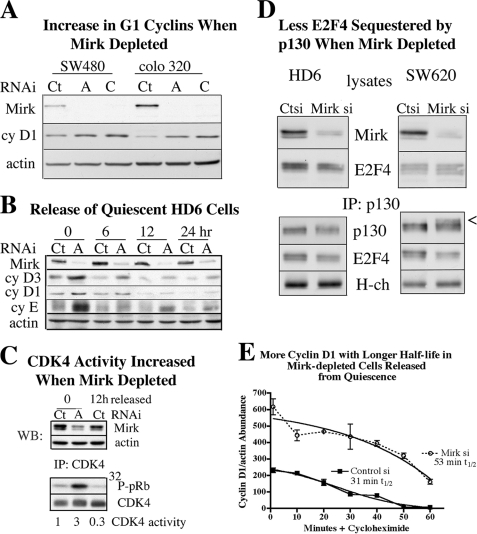
FIGURE 3.
Mirk destabilizes cyclin D in colon cancer cells. A, SW480 and Colo 320 colon carcinoma cells were depleted of Mirk or mock-depleted as in Fig. 1. Lysates were examined for levels of Mirk, cyclin D1, and actin. Mirk depletion reduced cyclin D1 levels 2-fold in SW480 cells and 4-fold in Colo 320 cells. B, Mirk was depleted in HD6 cells by siA and made quiescent as in Fig. 1, then released into growth medium containing 2 nm vinblastine to collect cycling cells in G2+M. Parallel cultures were examined at 0, 6, 12, and 24 h after release for Mirk, cyclin D1, cyclin D3, cyclin E, or actin by Western blotting. Data shown are representative of two experiments. C, Mirk was depleted in HD6 cells by siA and made quiescent as in Fig. 1, then released into growth medium containing 2 nm vinblastine to collect cycling cells in G2+M. Lysates were Western blotted for the abundance of Mirk and actin (upper) and immunoprecipitated with anti-CDK4 antibody conjugated to agarose. An in vitro kinase reaction was carried out on recombinant pRb (lower). The ratio of phospho-pRb to immunoprecipitated CDK4 is shown below the respective lanes and is representative of two experiments. D, Mirk was depleted in HD6 cells and in SW620 cells by siD and made quiescent for 3 days as in Fig. 1. The E2F4 sequestering protein p130/Rb2 was immunoprecipitated and the amounts of p130 and co-immunoprecipitated E2F4 were determined by Western blotting, with immunoglobulin heavy chain (H-ch) as control. Cell lysates were examined for total Mirk and E2F4 levels by Western blotting. E, Mirk was depleted in HD6 cells by siA and made quiescent as in Fig. 1, then released into growth medium containing 20 μg/ml cycloheximide for the times indicated before lysates were analyzed by Western blotting. Sigmoidal curve-fitting (r2 = 0.9782) yielded a half-life of 31 min for cyclin D1 in the control-depleted cells and 53 min for the Mirk-depleted cells (n = 2, mean ± S.D. shown).
Progression from G0 into G1 and through G1 is mediated by CDK4/cyclin complexes, which phosphorylate members of the retinoblastoma protein family, pRb and p130/Rb2. Depletion of Mirk led to more phosphorylation of p130/Rb2 (Fig. 1B), consistent with increased CDK4 activity. CDK4 activity was directly measured by immunoprecipitation of CDK4/cyclin complexes from mock-depleted and Mirk-depleted quiescent cultures of HD6 cells, and for another control, from mock-depleted cultures 12 h after release from quiescence (Fig. 3C) when levels of cyclins D3, D1, and E were at low levels as cells had entered cycle (Figs. 3B and 2A). Mirk-depleted quiescent cells exhibited about three times as much CDK4 activity as mock-depleted quiescent HD6 cells, consistent with their higher levels of cyclin D3 and D1 (Fig. 3C). When many HD6 cells were progressing through S and G2 12 h after release from quiescence (Fig. 2A), their CDK4 activity was even lower, reflecting the low levels of G1 cyclins (Fig. 3, B and C).
Depletion of Mirk led to inactivation of the G0 maintaining protein p130/Rb2, the retinoblastoma protein family member that blocks entry into G1. Mirk was significantly depleted in HD6 and in SW620 cells by a different duplex RNAi (siD) (Fig. 3D) than that used in Fig. 1B (siA); but in both experiments decreased mobility of p130 was seen when Mirk is depleted. Slower migrating p130/Rb2 bands consistent with more phosphorylation by cyclin D/CDK complexes were seen (arrowhead). The p130/Rb2 immunoprecipitates were analyzed by SDS-PAGE by Western blotting for E2F4 co-immunoprecipitated with p130, with loading controlled by the amount of antibody heavy chain (H-chain) in the immunoprecipitates. There was less E2F4 associated with p130/Rb2 in Mirk-depleted HD6 cells and in Mirk-depleted SW620 cells (Fig. 3D). Thus depletion of Mirk increased the percentage of p130 in more highly phosphorylated forms with lower mobility, that have less ability to sequester the transcription factor E2F4 which controls entry into G1. As a result, Mirk-depleted quiescent HD6 cells, although primarily in G0 (Fig. 1D), attained some biochemical markers of cells in G1: elevated levels of G1 cyclins, elevated CDK4 kinase activity on the retinoblastoma protein (Fig. 3), and phosphorylated p130/Rb2 (Figs. 1B and 3D).
5-Fluorouracil Increases Mirk Kinase Activity and Lengthens G1 in p53 Mutant Colon Cancer Cells
DNA damage activates p38MAPK in tumor cells with mutant p53 (21), such as HD6, SW480, and colo320 colon cancer cells. The kinase Mirk is activated by the same SAPK kinases MKK3 and MKK6 (10, 22, 15), which activate p38MAPK (23), so drugs that activate stress signaling would be expected to activate Mirk. In fact, the nucleoside analogue 5-FU induced a long-term 2–3-fold increase in Mirk kinase activity with activation seen at least for 24 h (Fig. 4A). Treatment with 5-FU led to lower cyclin D1 levels (Fig. 4B), possibly by initiating the rapid turnover of cyclin D1 through activation of Mirk, suggesting that 5-FU activation of Mirk would alter cell cycling.
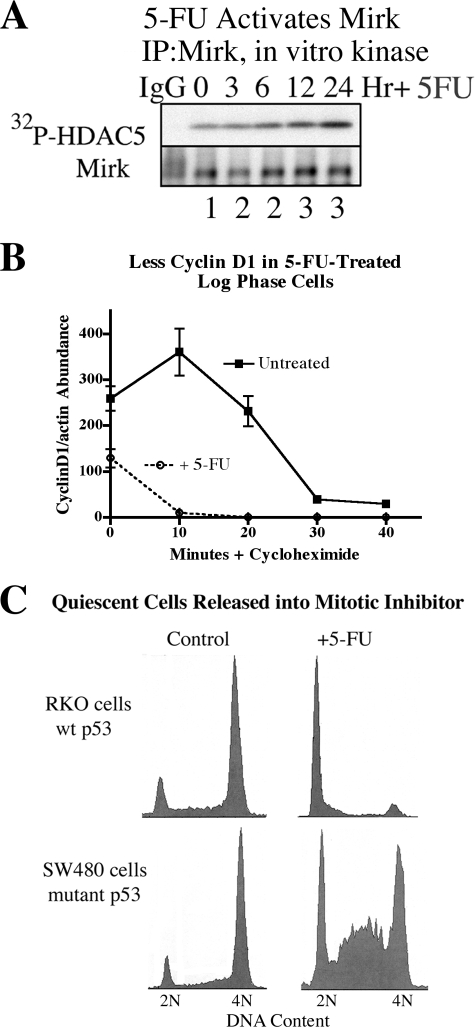
FIGURE 4.
Activation of Mirk kinase activity by the chemotherapeutic drug 5-FU correlates with a lengthened G1 phase in colon cancer cells with mutant p53. A, HD6 (p53 mutant) colon carcinoma cells were exposed to 10 μm 5-fluorouracil for 0–24 h. Mirk was immunoprecipitated and an immune complex kinase assay was performed using the 1–283 amino acid fragment of histone deacetylase 5 (HDAC5) as the substrate (Mirk site at Ser-279). The amount of Mirk in the immunoprecipitates was determined by Western blotting. The ratio of Mirk kinase activity to total Mirk protein is given below each lane. B, Mirk was depleted in HD6 cells by siA and made quiescent as in Fig. 1, then released into growth medium containing 1 μm 5-FU for 24 h. Cells were then treated with 20 μg/ml cycloheximide for the times indicated before lysates were analyzed by Western blotting (n = 2, mean ± S.D. shown). C, RKO (p53 wild-type) and SW480 (p53 mutant) colon carcinoma cells were made quiescent by culture in DMEM+0.2% FBS for 2 days, then released for 24 h into DMEM + 7% FBS plus the mitotic inhibitor nocodazole (50 ng/ml) to block cycling cells in G2+M, and either 1 μm 5-FU or no additives. Cell cycle position was determined by one-parameter flow cytometry for DNA content after propidium iodide staining.
Four colon cancer cell lines were made quiescent with at least 70% of cells in G0/G1, then released into growth medium containing 1 μm 5-FU and the mitotic inhibitor nocodazole to block any cycling cells in G2+M. RKO cells have wild-type p53 genes, so when released from quiescence, they were arrested by 5-FU at a G1 checkpoint through p53 induction of p21cip1 (Fig. 4C), while control cells not treated with 5-FU passed through G1 and S and were accumulated in G2+M by nocodazole. SW480, colo320, and HD6 cells are all deficient in p53 so they lack its G1 checkpoint. When released from quiescence into medium containing 5-FU for 24 h, some cells from each of these 3 lines were found accumulated in G1 (Figs. 4C and 1D, data not shown) as well as at the S and G2 checkpoints known to be mediated by the Chk1 and Chk2 checkpoint kinases (24, 25, 26). DNA damage initiated by 5-FU has been seen to activate a G1 checkpoint in some tumor cells by other investigators (27), so engagement of this checkpoint may cause the accumulation in G1 of HD6 (Fig. 1D), SW480 (Fig. 4D), and colo320 (data not shown) colon cancer cells.
Mirk-depleted Cells Rapidly Enter S Phase When Released from Quiescence, Even in the Presence of 5-FU
To more fully determine whether Mirk may mediate some aspects of this G1 checkpoint in p53 mutant colon cancer cells, time course experiments were performed following Mirk depletion. HD6 cultures of either Mirk-depleted or mock-depleted cells were made quiescent, then released for 0–24 h in the presence of 1 μm 5-FU, and cell cycle distribution was determined using two-parameter flow cytometry (Fig. 5). Cells were released in the presence of the mitotic inhibitor nocodazole so only one cell cycle was permitted. 12–24 h post-release most of the mock-depleted cells were in G1 (Fig. 5, A and D), while in sharp contrast, the Mirk-depleted cells accumulated primarily in S phase (Fig. 5, C and D), presumably at S phase checkpoints activated by 5-FU. As depicted by two-parameter flow cytometry after 12 h of release, 55% of the mock-depleted cells were in G1, while 60% of the Mirk-depleted cells had traversed G1 and were in S phase (Fig. 5D). Thus 5-FU could not induce any G1 checkpoint in the Mirk-depleted cells, which would prevent their rapid entry into S phase. 5-FU increased the length of G1 in HD6 cells because parallel studies showed that when 5-FU was not added, quiescent mock-depleted and Mirk-depleted rapidly left the G1 phase, leaving less than 2% in G1 after 12 h (Fig. 5B). Similar data were obtained by one-parameter flow cytometry with propidium iodide. 5-FU induced a longer G1 period in HD6 cells when Mirk was present, and the G1 peak was diminished when Mirk was depleted (n = 2, data not shown).
FIGURE 5.
When quiescent HD6 colon carcinoma cells were released into fresh growth medium containing 5-fluorouracil, they were maintained in G1 for several hours, while Mirk-depleted cells rapidly traversed G1 and entered S phase. HD6 cells were depleted of Mirk or mock-depleted made quiescent as in Fig. 1, then released by change to growth medium containing 1 μm 5-FU. 50 ng/ml of the mitotic inhibitor nocodazole was added to arrest any cycling cells in G2+M so that only one cell cycle was allowed, and cells were collected 6, 12, 16, and 24 h following release. A parallel experiment was performed with depletion of Mirk by RNAi duplex siD directed to another sequence in the Mirk mRNA, cells were collected 24 h after release, and data were averaged together with the data from the experiment utilizing siA. Similar results were seen in three experiments. A, time course showing cell cycle distribution of mock-depleted HD6 cells 6–24 h after release from quiescence in the presence of 1 μm 5-FU. B, time course showing percent of cells in G1 of either mock-depleted or Mirk-depleted HD6 cells 6–24 h after release from quiescence, no 5-FU added. C, identical time course to A, with Mirk-depleted HD6 cells. D, HD6 cells were depleted of Mirk or mock-depleted, made quiescent as in Fig. 1 and then released for 12 h into growth medium containing 1 μm 5-FU. 50 ng/ml of the mitotic inhibitor nocodazole was added to arrest any cycling cells in G2+M so that only one cell cycle was allowed. Cell cycle phases were color-coded: G0 (gold), G1 (blue), S (purple), G2+M (red). Note that at this time of release the majority of mock-depleted cells were in G1 while the majority of Mirk-depleted cells were in S.
Expression of Cyclin D1 Mutated at the Mirk Phosphorylation Site Enabled HD6 Cells to Move More Rapidly into Cycle
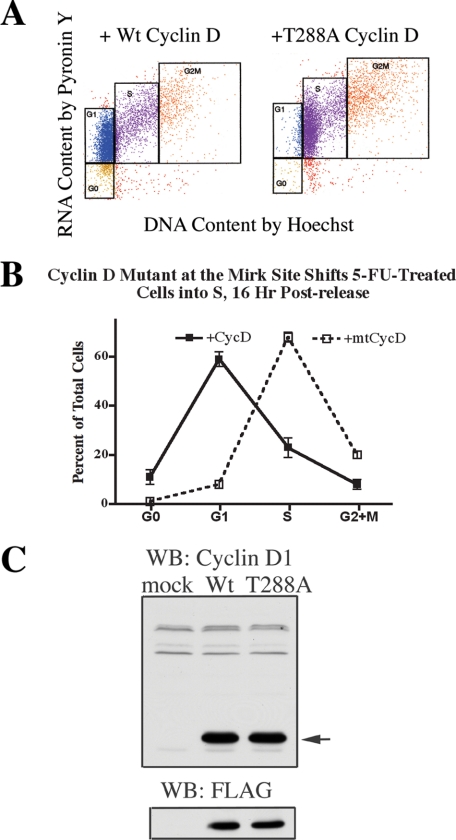
Ectopic expression of G1 cyclins was enough to initiate DNA synthesis in quiescent human fibroblasts (28) while enforced overexpression of D-type cyclins shortened the G1 interval (29), so cyclin D constructs should alter cycling of HD6 cells. If the phosphorylation of cyclin D isoforms was the major contribution of Mirk in slowing transit through G1, overexpression of a cyclin D1 construct mutated at the Mirk phosphorylation site would block Mirk function and allow cells to rapidly cycle into S, where they would be blocked by 5-FU-initiated checkpoints. Both wild-type cyclin D1 and the mutant cyclin D1-T288A had been generated in the same pCMV-tag2B constructs in earlier studies (5), and constructs were expressed at a similar level as shown by immunoblotting with either antibody to cyclin D1 or an antibody to their FLAG-epitope tag (Fig. 6C). The exogenous cyclin D1 constructs were about 24-fold as abundant as the endogenous cyclin D1 (see tiny faster migrating band, Fig. 6C), so were likely to displace the endogenous forms in complexes with G1 CDKs. HD6 cells were transiently transfected with either cyclin D1-T288A or wild-type cyclin D1, made quiescent, then released for 16 h into growth medium containing 1 μm 5-FU and a mitotic inhibitor so only one cell cycle was allowed. In the culture with ectopic wild-type cyclin D1 expression, 58% of the cells were found in G1, while in cultures expressing the Mirk site mutant cyclin D1-T288A, cells rapidly transited G1 and 5-FU treatment activated the expected S phase checkpoints, with 68% of cells arrested throughout S phase (Fig. 6, A and B). This result is a phenocopy of the effect of Mirk depletion on cell cycle progression in Fig. 5D, where 55% of the mock-depleted cells were found in G1, and 60% of the Mirk-depleted cells were found in S. Thus, the major cell cycle function of Mirk in these colon cancer cells is destabilizing the cyclin D isoforms that control transit through G1.
FIGURE 6.
Expression of cyclin D1 mutated at the Mirk phosphorylation site conserved in all cyclin D isoforms gives a phenocopy of Mirk-depletion: cells can move into cycle even when challenged with 5-FU. HD6 cells were transfected with expression plasmids (5 μg) for either wild-type FLAG-cyclin D1 or FLAG-cyclin D1-T288A, or an empty vector, for 48 h in growth medium together with siA to deplete Mirk (data not shown) or with GC-matched control RNAi duplexes to mock deplete Mirk, made quiescent as in Fig. 1, then released for 16 h into growth medium containing 1 μm 5-FU. The cells which remained cycling under these conditions were arrested in G2+M by 50 ng/ml nocodazole. 95% of cells either depleted of Mirk or mock-depleted were collected at G2+M by nocodazole in control cultures not transfected with cyclin D constructs (not shown). Cells were analyzed by two-parameter flow cytometry after staining with Hoechst 33258 for DNA followed by Pyronin Y for RNA. 6341 ± 649 cells were analyzed per sample. A, two-parameter flow cytometry showing the effect of overexpression of wild-type cyclin D1 or mutant cyclin D1-T288A on cell cycle distribution in mock-depleted cells released from quiescence in the presence of 1 μm 5-FU for 16 h. B, plot of the data from A. C, lysates from HD6 cells transfected with either wild-type FLAG-cyclin D1, FLAG-cyclin D1-T288A, mutated at the Mirk phosphorylation site, or mock-transfected for 48 h in growth medium. Lysates analyzed for expression of plasmids by Western blotting for cyclin D1 (arrow) or for the FLAG tag. The upper bands in the cyclin D1 blot are cross-reacting bands showing equal loading. The endogenous cyclin D1 is seen as a low abundant band migrating just before the exogenous cyclin D constructs.
In control experiments, overexpression of mutant cyclin D1-T288A together with Mirk depletion led to even more movement through the cell cycle with the majority of cells in G2+M (data not shown). In the absence of 5-FU, 21% of cells transfected with wild-type cyclin D1 construct and 34% of cells transfected with the mutant cyclin D1 were found in S phase and less than 2% in G1, under the experimental conditions. In contrast, in cultures not transfected with cyclin D1 constructs, 95–96% of control or of Mirk-depleted cells had progressed to G2+M and were arrested by nocodazole (data not shown). Thus neither the wild-type nor the mutant T288A cyclin D constructs blocked cells in G1 under experimental conditions.
Depletion of the CDK Inhibitor p27 Enhances Exit Out of G0 Initiated by Mirk Depletion
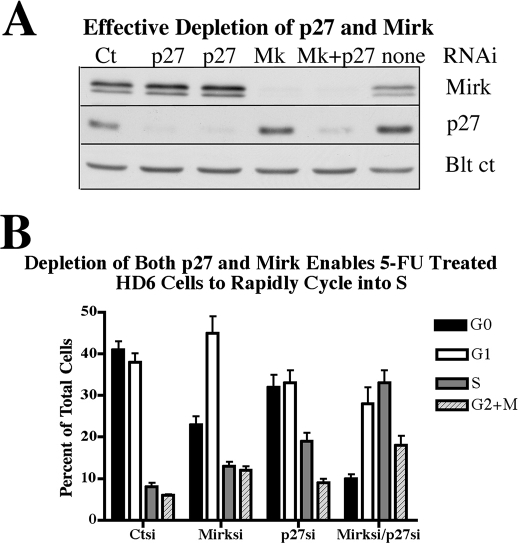
Mirk blocks cycling myoblasts in the G0 quiescent state (4), in part by stabilization of the p27kip1 protein by phosphorylation at Ser-10 (6). More recently, the survival factors Bcl2 and BclXL were shown to mediate G0 quiescence in NIH3T3 cells and murine embryonic fibroblasts through increasing p27kip1 stability, in a manner which required phosphorylation of p27Ser10 by Mirk (7). These studies suggested that Mirk maintained elevated p27 levels to keep nontransformed cells in a quiescent state. Possibly, Mirk prevented the exit of HD6 colon carcinoma cells from G0 by stabilizing p27 as well as destabilizing cyclin D, or perhaps Mirk and p27 worked independently to maintain quiescence. To test these hypotheses, HD6 cells were depleted of Mirk, p27 or both Mirk and p27 (Fig. 7A). Mirk depletion did not decrease p27 levels in this and other experiments (Fig. 7A, also data not shown) suggesting that Mirk did not function to stabilize p27 in these cancer cells. Cells were then made quiescent and then released by a switch to growth medium containing 5-FU and nocodazole to prevent the released cells from undergoing more than one cell cycle. The experimental conditions were chosen so that about 40% of the released control cells were still in G0 and 40% were in G1 (Fig. 7B). Depletion of Mirk led cells to move more quickly from G0 into G1 with a few (13%) reaching S phase. Depletion of p27 enabled cells to leave both G0 and G1, allowing 18% of cells to enter S phase. While depletion of Mirk and p27 independently reduced the fraction of cells in G0 to 18 and 9%, respectively, simultaneous depletion of Mirk and p27 had an additive effect, reducing the fraction of G0 cells from 41 to 10%, and enabling most of the cells to progress into S (33%) and G2+M (18%). Thus, p27 and Mirk blocked the exit of HD6 colon carcinoma cells from G0 by working in an additive fashion.
FIGURE 7.
Depletion of both the CDK inhibitor p27 and Mirk enables 5-FU treated HD6 cells to rapidly cycle into S phase. HD6 cells were depleted of p27, Mirk or both by transient transfection with RNAi duplexes for 6 h, not the 48 h used in all prior experiments, made quiescent as in Fig. 1, and then released for 16 h into growth medium containing 1 μm 5-FU. The cells which remained cycling under these conditions were arrested in G2+M by 50 ng/ml nocodazole. A, Western blots of parallel cultures showing depletion of Mirk (Mk) and p27 with RNAi duplexes. Blt ct, blot control of cross-reacting band. B, cells were analyzed by two-parameter flow cytometry after staining with Hoechst 33258 for DNA followed by Pyronin Y for RNA. 7014 ± 2526 cells were analyzed per sample. Mean ± S.D. is shown. Values for untreated proliferating cells not in graph were 19% in G0, 29% in G1, 22% in S, and 29% in G2+M.
DISCUSSION
Quiescent cells degrade their ribosomes, allowing G0 cells to be identified by their 2N DNA content and their low RNA content using two-parameter flow cytometry and by the presence of biochemical markers, including a multiprotein complex containing p130/Rb2 binding to and sequestering the transcription factor E2F4 and elevated levels of the CDK inhibitor p27kip1. In cells arrested in G0 by serum deprivation, many common promoter sites are bound by a multiprotein DREAM complex containing p130/E2F4 (30). In normal fibroblasts, arrest in G0 is an active process maintained by a set of proteins differing from those active in other cell cycle stages. The transcription factor ATF6α is a survival factor for quiescent but not proliferative squamous carcinoma cells and activates mTOR signaling (31). Recently mTOR was shown to maintain quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and thus the generation of reactive oxygen species (2). The quiescence of these adult stem cells is essential for their long term function and is thought to require the hypoxic conditions found in their bone marrow niche. Quiescent cells have reduced energy metabolism and less mitochondrial contents. Mitochondrial oxidation can generate ROS, which is known to be harmful to hematopoietic stem cells. Deletion of mTOR in a mouse model led to more mitochondria, more ROS, and the transition of quiescent stem cells into rapid cycling (2).
Quiescent cells are also proficient in transcription-coupled repair (32), and as a result, are relatively resistant to apoptosis-induced by DNA damage, nutrient starvation, or oxidative stress. Normal diploid fibroblasts carrying the bacterial lacI gene in a lambda shuttle vector have been used to quantitate DNA damage and repair in quiescent and in proliferating cells in primary culture. N-Ethyl-N-nitrosourea treatment of such quiescent normal diploid fibroblasts led to less premutagenic damage in the transgene and one-fifth as many mutations, compared with proliferating cells, and quiescent cells were proficient in transcription-coupled repair (33, 32). The capacity to enter quiescence is one of the defining characteristics of stem cells in somatic tissues (34, 35, 36). In chronic myeloid leukemia, quiescent stem cells have been identified that are exceptionally refractory to cytotoxic treatments (37), possibly because of their increased capacity for repair. Leukemia stem cells required a transient arrest in a quiescent state to repair DNA damage induced by introduction of oncogenes (8). This requirement for quiescence is not limited to leukemia cells. When colon carcinomas entered a G0 quiescence state they were more resistant to oxidative stress damage (38, 39).
In the current study, the kinase Mirk has been shown to prevent colon carcinoma cells from exiting a quiescent G0 state, at least in part, through destabilization of G1 cyclins. Mirk was recently shown to reduce ROS levels in two pancreatic cancer cell lines by increasing transcription of a cohort of genes which detoxify superoxides and which prevent the generation of hydroxyl radicals (3). The ROS-countering activity of Mirk was primarily exhibited in quiescent tumor cells, because such cells had the highest levels of Mirk protein. In colon cancer cells Mirk was at its highest abundance in G0 (Fig. 1); it also reduced ROS levels (data not shown) and caused cell cycle restriction. Three colon cancer cell lines, HD6, SW480, and colo320 depleted of Mirk by either of two synthetic RNAi duplexes were able to accumulate G1 cyclins, including cyclin D1, even though they had been made quiescent by serum starvation. Elevated levels of G1 cyclins activated the CDK4 kinase which in turn increased its phosphorylation of retinoblastoma proteins. These included the p130/Rb2 protein that blocks entry into G1 by sequestering the transcription factor E2F4. As a result, depletion of Mirk led to increased phosphorylation of p130/Rb2, which inactivated its sequestering capacity for E2F4. Thus quiescent colon cancer cells depleted of Mirk accumulated some of the properties of cells in G1. Release of quiescence by addition of serum growth factors led to a more rapid transit of G1 than that seen in control cells, as if Mirk-depleted cells were “primed” to leak out of a quiescent state. Mirk-depleted cells rapidly exited G0 when released from quiescence and accumulated in G1, while depletion of both Mirk and the CDK inhibitor p27 enabled most cells to exit G0 and enter cycle (Fig. 7B). In addition, a few Mirk-depleted colon cancer cells “leaked' out of G0, traversed G1, and were seen in S phase in the absence of added serum growth factors (Fig. 2A and Table 1).
Because depletion of Mirk altered the cycling of colon cancer cells, the effects of activation of Mirk were studied. 5-FU activated Mirk as a kinase and furthermore 5-FU treatment allowed HD6 cells to undergo a prolonged G1 arrest in a Mirk-dependent manner. One of the Mirk substrates in a variety of cell types is the cyclin D group of G1 cyclins, which Mirk phosphorylates at a conserved ubiquitination site, T288A in cyclin D1 (5), (40). The role of phosphorylation of cyclin D isoforms was supported by the effects on cell cycling of cyclin D1-T288A mutated at the Mirk phosphorylation site. Overexpression of this mutant cyclin D prevented 5-FU-activated Mirk from inducing a lengthened G1 phase by rapid turnover of cyclin D. The 5-FU-induced G1 checkpoint was maintained if ectopic wild-type cyclin D1 was expressed instead, showing that Mirk phosphorylation of cyclin D1 was an essential part of Mirk function in the checkpoint.
GSK3β is widely known for its destabilization of cyclin D1 by phosphorylation at Thr-286 (41). Mirk phosphorylates cyclin D1 at the adjacent site of Thr-288 in a series of cancer cells and nontransformed cells (5, 40, 3), with both amino acids known to be ubiquitinated following phosphorylation (42). In earlier studies Mirk was found to phosphorylate cyclin D1 bound to GSK3β, while purified recombinant Mirk and GSK3β phosphorylated cyclin D1 in additive fashion (5). The Mirk-related kinase Dyrk1A has been hypothesized to act as a primer kinase for GSK3β in phosphorylation of eIF2Bϵ (43), and in some cells Mirk/dyrk1B may act as a primer for GSK3β destabilization of cyclin D isoforms by phosphorylation. The related protein Dyrk2 acts as a scaffold to facilitate assembly of an E3 ligase (14), suggesting that this family of related kinases functions in controlling protein turnover.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 CA67405 from the NCI (to E. F.). This work was also supported by the Jones/Rohner Foundation.
- ROS
- reactive oxygen species
- RNAi
- RNA interference
- 5-FU
- 5-fluorouracil
- CDK
- cyclin-dependent kinase
- FBS
- fetal bovine serum.
REFERENCES
- 1.Coller H., Sang L., Roberts J. M. (2006) PLOS Biol. 4,329–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C., Liu Y., Liu R., Ikenoue T., Guan K. L., Liu Y., Zheng P. (2008) J. Exp. Med. 205,2397–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng X., Ewton D. Z., Friedman E. (2009) Cancer Research 69,3317–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng X., Ewton D. Z., Pawlikowski B., Maimone M., Friedman E. (2003) J. Biol. Chem. 278,41347–41354 [DOI] [PubMed] [Google Scholar]
- 5.Zou Y., Ewton D. Z., Deng X., Mercer S. E., Friedman E. (2004) J. Biol. Chem. 279,27790–27798 [DOI] [PubMed] [Google Scholar]
- 6.Deng X., Mercer S. E., Shah S., Ewton D. Z., Friedman E. (2004) J. Biol. Chem. 279,22498–22504 [DOI] [PubMed] [Google Scholar]
- 7.Janumyan Y., Cui Q., Yan L., Sansam C. G., Valentin M., Yang E. (2008) J. Biol. Chem. 283,34108–34120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viale A., De Franco F., Orleth A., Cambiaghi V., Giuliani V., Bossi D., Ronchini C., Ronzoni S., Muradore I., Monestiroli S., Gobbi A., Alcalay M., Minucci S., Pelicci P. G. (2009) Nature 457,51–56 [DOI] [PubMed] [Google Scholar]
- 9.Lee K., Deng X., Friedman E. (2000) Cancer Res. 60,3631–3637 [PubMed] [Google Scholar]
- 10.Lim S., Jin K., Friedman E. (2002) J. Biol. Chem. 277,25040–25046 [DOI] [PubMed] [Google Scholar]
- 11.Yan Z., Chen M., Perucho M., Friedman E. (1997) J. Biol. Chem. 272,30928–30936 [DOI] [PubMed] [Google Scholar]
- 12.Yan Z., Deng X., Chen M., Xu Y., Ahram M., Sloane B. F., Friedman E. (1997) J. Biol. Chem. 272,27902–27907 [DOI] [PubMed] [Google Scholar]
- 13.Huang F., Hsu S., Yan Z., Winawer S., Friedman E. (1994) Oncogene 9,3701–3706 [PubMed] [Google Scholar]
- 14.Maddika S., Chen J. (2009) Nat. Cell Biol. 11,409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin K., Lim S., Mercer S. E., Friedman E. (2005) J. Biol. Chem. 280,42097–42105 [DOI] [PubMed] [Google Scholar]
- 16.Deng X., Ewton D. Z., Mercer S. E., Friedman E. (2005) J. Biol. Chem. 280,4894–4905 [DOI] [PubMed] [Google Scholar]
- 17.Deng X., Bellis S., Yan Z., Friedman E. (1999) Cell Growth Diff. 10,11–18 [PubMed] [Google Scholar]
- 18.Smith E. J., Leone G., Nevins J. R. (1998) Cell Growth Diff. 9,297–303 [PubMed] [Google Scholar]
- 19.Wang H. L., Wang J., Xiao S. Y., Haydon R., Stoiber D., He T. C., Bissonnette M., Hart J. (2002) Int. J. Cancer 101,301–310 [DOI] [PubMed] [Google Scholar]
- 20.Ewton D. Z., Lee K., Deng X., Lim S., Friedman E. (2003) Int. J. Cancer 103,21–28 [DOI] [PubMed] [Google Scholar]
- 21.Reinhardt H. C., Aslanian A. S., Lees J. A., Yaffe M. B. (2007) Cancer Cell 11,175–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim S., Zou Y., Friedman E. (2002) J. Biol. Chem. 277,49438–49445 [DOI] [PubMed] [Google Scholar]
- 23.Raingeaud J., Whitmarsh A. J., Barrett T., Dérijard B., Davis R. J. (1996) Mol. Cell. Biol. 16,1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zachos G., Rainey M. D., Gillespie D. A. (2003) EMBO J. 22,713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson H. M., Jones R., Walker M., Zachos G., Brown R., Cassidy J., Gillespie D. A. (2006) Oncogene 25,5359–5369 [DOI] [PubMed] [Google Scholar]
- 26.Xiao Z., Xue J., Sowin T. J., Zhang H. (2006) Mol. Cancer Ther. 5,1935–1943 [DOI] [PubMed] [Google Scholar]
- 27.Yoshikawa R., Kusunoki M., Yanagi H., Noda M., Furuyama J. I., Yamamura T., Hashimoto-Tamaoki T. (2001) Cancer Res. 61,1029–1037 [PubMed] [Google Scholar]
- 28.Connell-Crowley L., Elledge S. J., Harper J. W. (1998) Curr. Biol. 8,65–68 [DOI] [PubMed] [Google Scholar]
- 29.Sherr C. J., Roberts J. M. (2004) Genes Dev. 18,2699–2711 [DOI] [PubMed] [Google Scholar]
- 30.Litovchick L., Sadasivam S., Florens L., Zhu X., Swanson S. K., Velmurugan S., Chen R., Washburn M. P., Liu X. S., DeCaprio J. A. (2007) Mol. Cell 26,539–551 [DOI] [PubMed] [Google Scholar]
- 31.Schewe D. M., Aguirre-Ghiso J. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105,10519–10524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bielas J. H., Heddle J. A. (2004) DNA Repair 3,711–717 [DOI] [PubMed] [Google Scholar]
- 33.Bielas J. H., Heddle J. A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97,11391–11396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sell S. (2004) Crit. Rev. Oncol. Hematol. 51,1–28 [DOI] [PubMed] [Google Scholar]
- 35.Cheng T., Rodrigues N., Shen H., Yang Y., Dombkowski D., Sykes M., Scadden D. T. (2000) Science 287,1804–1808 [DOI] [PubMed] [Google Scholar]
- 36.Kippin T. E., Martens D. J., van der Kooy D. (2005) Genes Dev. 19,756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes D. J., Melo J. V. (2006) Cell Cycle 5,2862–2866 [DOI] [PubMed] [Google Scholar]
- 38.Kops G. J., Dansen T. B., Polderman P. E., Saarloos I., Wirtz K. W., Coffer P. J., Huang T. T., Bos J. L., Medema R. H., Burgering B. M. (2002) Nature 419,316–321 [DOI] [PubMed] [Google Scholar]
- 39.Kops G. J., Medema R. H., Glassford J., Essers M. A., Dijkers P. F., Coffer P. J., Lam E. W., Burgering B. M. (2002) Mol. Cell. Biol. 22,2025–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi-Yanaga F., Mori J., Matsuzaki E., Watanabe Y., Hirata M., Miwa Y., Morimoto S., Sasaguri T. (2006) J. Biol. Chem. 281,38489–38497 [DOI] [PubMed] [Google Scholar]
- 41.Diehl J. A. (2002) Cancer Biol. Ther. 1,226–231 [DOI] [PubMed] [Google Scholar]
- 42.Germain D., Russell A., Thompson A., Hendley J. (2000) J. Biol. Chem. 275,12074–12079 [DOI] [PubMed] [Google Scholar]
- 43.Woods Y. L., Cohen P., Becker W., Jakes R., Goedert M., Wang X., Proud C. G. (2001) Biochem. J. 355,609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]