Abstract
In Archaea, an hexameric ATPase complex termed PAN promotes proteins unfolding and translocation into the 20 S proteasome. PAN is highly homologous to the six ATPases of the eukaryotic 19 S proteasome regulatory complex. Thus, insight into the mechanism of PAN function may reveal a general mode of action mutual to the eukaryotic 19 S proteasome regulatory complex. In this study we generated a three-dimensional model of PAN from tomographic reconstruction of negatively stained particles. Surprisingly, this reconstruction indicated that the hexameric complex assumes a two-ring structure enclosing a large cavity. Assessment of distinct three-dimensional functional states of PAN in the presence of adenosine 5′-O-(thiotriphosphate) and ADP and in the absence of nucleotides outlined a possible mechanism linking nucleotide binding and hydrolysis to substrate recognition, unfolding, and translocation. A novel feature of the ATPase complex revealed in this study is a gate controlling the “exit port” of the regulatory complex and, presumably, translocation into the 20 S proteasome. Based on our structural and biochemical findings, we propose a possible model in which substrate binding and unfolding are linked to structural transitions driven by nucleotide binding and hydrolysis, whereas translocation into the proteasome only depends upon the presence of an unfolded substrate and binding but not hydrolysis of nucleotide.
In eukaryotic cells most protein breakdown in the cytosol and nucleus is catalyzed by the 26 S proteasome. This ∼2.5-MDa (1) complex degrades ubiquitin-conjugated and certain non-ubiquitinated proteins in an ATP-dependent manner (2, 3). The 26 S complex is composed of one or two 19 S regulatory particles situated at the ends of the cylindrical 20 S proteasome. Within the 26 S complex, proteins are hydrolyzed in the 20 S proteasome. Tagged substrates, however, first bind to the 19 S regulatory particle, which catalyzes their unfolding and translocation into the 20 S subcomplex (4, 5). The 19 S regulatory particle consists of at least 17 different subunits (1, 6). Nine of these subunits form a “lid,” whereas the other eight subunits, including six ATPases, comprise the base of the 19 S particle. Electron microscopy (7–10) as well as cross-linking experiments (11, 12) have demonstrated that the six homologous ATPases are associated with the α rings of the 20 S particle.
Unlike eukaryotes, Archaea and certain eubacteria contain homologous 20 S particles but lack ubiquitin. Their proteasomes degrade proteins in association with a hexameric ATPase ring complex termed PAN (13). PAN appears to be the evolutionary precursor of the 19 S base, predating the coupling of ubiquitination and proteolysis in eukaryotes (14). In addition, PAN recognizes the bacterial targeting sequence ssrA (in analogy to the polyubiquitin conjugates in eukaryotes) and efficiently unfolds and translocates globular substrates, like green fluorescent protein, when tagged with ssrA (15). In both PAN and the 19 S proteasome regulatory complexes, ATP is essential for substrate unfolding and translocation and for opening of the gated channel in the α ring through which substrates enter the 20 S particle (15–17). Because this portal is quite narrow (18–20), only extended polypeptides can enter the 20 S proteasome. Consequently, a globular substrate must be unfolded by the associated ATPase complex to be translocated and digested within the 20 S particle.
PAN and the six ATPases found at the base of the 19 S particle are members of the AAA+ superfamily of multimeric ATPases which also includes the ATP-dependent proteases Lon and FtsH and the regulatory components of the bacterial ATP-dependent proteases ClpAP, ClpXP, and HslUV (8, 21). For mechanistic studies of the roles of ATP, the simpler archaeal PAN-20 S system offers many technical advantages over the much more complex 26 S proteasome. For example, prior studies of PAN (17, 22) demonstrated that unfolding of globular substrates (e.g. green fluorescent protein-ssrA) requires ATP hydrolysis. The same was also shown for the Escherichia coli ATP-dependent proteases ClpXP (23) and ClpAP (24). We have also shown that unfolding by PAN can take place on the surface of the ATPase ring in the absence of translocation (15). Thus, unfolding seems to proceed independently from protein translocation into the 20 S proteolytic particle. It is noteworthy that other studies suggest that proteins are unfolded by energy-dependent translocation through the ATPase ring (25, 26). These studies have suggested that the translocation of an unfolded polypeptide from the ATPase into the 20 S core is an active process that is coupled to ATP hydrolysis. A key to underline a detailed molecular mechanism for substrate binding, unfolding, and translocation by the proteasome regulatory ATPase complex is improved understanding of its architecture and the nucleotide-dependent structural transitions that afford these functions.
To date we and others have failed to generate micrographs suitable for three-dimensional reconstruction of PAN using single-particle EM analysis. Likewise, structural information regarding the three-dimensional architecture and subunit organization within the 19 S particle is very limited. In fact, high resolution three-dimensional information on the 19 S complex is not yet available. Most knowledge available is based on cross-linking experiments (11, 12) as well as EM structural analysis (7–10), which provided a three-dimensional model outline of the general architecture of the 26 S complex. Unlike the 19 S complex, the structure of the 20 S subcomplex was determined by x-ray crystallography (18, 19). In contrast to the highly homogenous structure of the 20 S complex, the structural heterogeneity and flexibility of the 19 S subcomplex is presumably reflected in multiple conformations, which in turn also contribute to the difficulty in generating a high resolution three-dimensional structural model of the 26 S proteasome. Accordingly, the initial goal of this study was to generate a three-dimensional model of PAN that will allow us to determine its general architecture and to correlate unique conformational transitions within this ATPase with the nucleotide state of the complex (i.e. in the presence of ATPγS, ADP, or in the absence of nucleotides).
Smith et al. (27) suggested a general architecture for the PAN-20 S complex based on two-dimensional averaging of a Thermoplasma acidophilum (TA)3 20 S proteasome and Methanococcus jannaschii (MJ) PAN hybrid complex in the presence of ATPγS. Based on side-view projections of that complex, these authors proposed that PAN assumes an overall structure similar to E. coli HslU (28–30).
We realized that although PAN appears heterogeneous in electron micrographs, it does not occupy all possible orientations when adsorbed to carbon-coated electron microscopy (EM) grids, a prerequisite for single particle analysis. This problem was overcome by applying electron tomography in conjunction with a three-dimensional averaging procedure that accounts for the missing wedge in the Fourier space of electron tomograms (31, 32). The three-dimensional model generated revealed an unexpected architecture leading to a possible molecular mechanism describing the function of PAN and presumably the 19 S ATPases.
EXPERIMENTAL PROCEDURES
Purification of PAN and the MJ 20 S Proteasome
PAN was purified according to Navon and Goldberg (15). The α and β subunits of the MJ 20 S proteasome were cloned into the pET22 plasmid downstream of the T7 promoter. The MJ 20 S complex was expressed in E. coli BL21 and purified by heating the resuspended bacterial pellet (50 mm Hepes, pH 7.5) and removing the aggregated E. coli proteins by centrifugation. The cleared lysate was loaded onto an anion exchange column (Mono Q), and PAN-containing fractions were pooled, concentrated, and dialyzed against 50 mm Hepes, pH 7.5.
EM Analysis and Image Processing
For electron tomography, an aliquot of PAN was diluted into buffer A (50 mm Tris-HCl, pH 7.5, and 1 mm MgCl2) to a final protein concentration of 25 μg/ml. When stated, the mixture was also complemented with 50 μm ATPγS, ADP, or no nucleotide. A 5-μl drop was applied onto 200 mesh carbon-coated copper grids. The specimens were transferred into a Phillips CM 200 FEG microscope equipped with a 2k TVIPS CCD camera. Data were collected in a fully automated manner using TVIPS tomography software. Tilt series were collected, typically covering an angular range from −60° to 60°, and sampled at 2° tilt increments with a 2-μm underfocus. The pixel size was 0.41 nm at the specimen level, which corresponds to a magnification of ×27,500.
Image Processing
Three-dimensional Reconstruction of the PAN Complex
All image processing operations were carried out with the EM package (33) and the TOM- and av3-toolbox (32, 34) for Matlab (MathWorks). A total number of 500 single particle-containing sub-tomograms of PAN-ATPγS volumes, 450 of PAN-ADP, 500 of PAN without nucleotide, and 250 of PAN Δ1–73 volumes were selected. The sub-tomograms containing particle volumes were extracted in silico and subjected to a three-dimensional averaging procedure (32). The resolutions of the different PAN structures were determined by Fourier shell correlation with 0.5 criteria. After a few iterations, the 6-fold symmetry of the structure became apparent. Therefore, imposed 6-fold symmetry was used in the refinement iterations. The PAN-ATPγS structure was resolved to 2.0-nm resolution, whereas the structures of PAN-ADP and PAN without nucleotide were resolved to 2.3 nm each. The PAN Δ1–73 structure was resolved to 2.5 nm. Three-dimensional surface-rendered visualizations were created with Amira 3.1 (TGS).
Two-dimensional Analysis of the MJ 20 S Proteasome
Electron micrographs were recorded digitally using a FEI Polara microscope equipped with a GIF energy filter (Gatan), a CCD camera at 1.5 μm underfocus, and a pixel size of 0.41 nm. A set of 1157 images of negatively stained proteasomes were aligned and averaged using the single particle procedures of the EM software package (33).
Single Particle Reconstruction of the MJ 20 S Proteasome
Electron micrographs were recorded as above. A set of 7000 images of negatively stained complexes were analyzed with the EMAN package (35). The images were low pass-filtered at 1.7 nm (below the first zero of the contrast transfer function). The structure was resolved to 2 nm of resolution, determined by Fourier shell correlation with 0.5 criteria.
PAN Fluorescence Activity Assays
The unfolding and degradation of YFP-ssrA was monitored by recording fluorescence over time. The reactions were performed in buffer B (50 mm Hepes, pH 7.5, 5 mm MgCl2, 1 mm ATP, and 30 mm KCl) at 45 °C. YFP-ssrA was excited at 485 nm, and fluorescence emission was recorded at 529 nm. Unless stated differently, the final concentration of YFP-ssrA in a typical reaction was 10 μm, and PAN-20 S complexes were present at a final concentration of 16 nm.
RESULTS
PAN Assumes a Two-ring Architecture
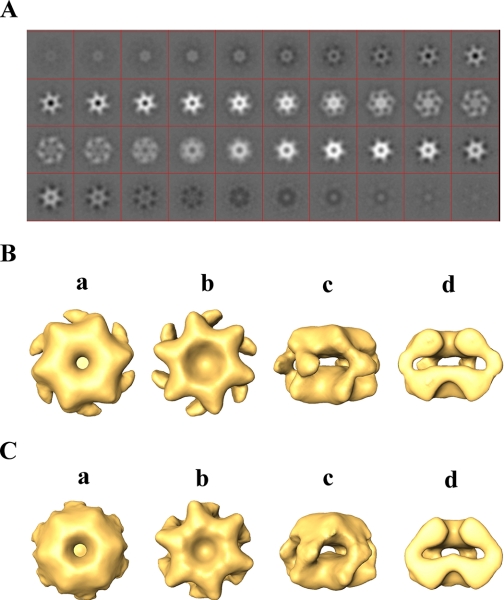
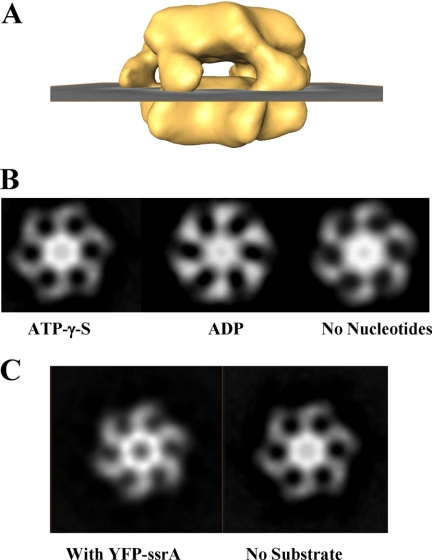
To gain insight into how substrates interact with PAN and how the conformation of PAN is affected by ATP binding and hydrolysis, we undertook an integrative approach combining biochemistry and structural analysis. Three-dimensional reconstruction of PAN (Fig. 1 and supplemental Fig. S1 and movie (PAN ATPγS.mpg)) indicated that the hexameric complex assumes a two-ring structure with a large cavity in its center. Fig. 1A depicts successive 0.4-nm-thick slices through the reconstructed volume of the PAN complex in the presence of ATPγS. The two rings have similar thicknesses of about 3 nm each, with connecting stems that converge in the mid-plane of the complex to form six symmetrical protruding “spikes” (Fig. 1Bc). Overall, the complex has a diameter of 14.5 nm and a height of 9.5 nm. It is noteworthy that two-dimensional projection of our three-dimensional model (see Fig. 7B) resembles the view seen by Smith et al. (27).
FIGURE 1.
Three-dimensional reconstruction of PAN in the presence of ATPγS. A, serial successive, 0.4-nm-thick slices through the three-dimensional-reconstructed volume of PAN (top ring to bottom ring) in the presence of ATPγS. B, orthogonal views of reconstructed PAN in the presence of ATPγS. a and b, top and bottom views, respectively; c, side view; d, cut-away side view. C, a surface rendering view of the three-dimensional reconstruction of PAN-ΔN (1–73)-ATPγS complex. a and b, top and bottom views, respectively; c, side view; d, cut-away side view.
FIGURE 7.
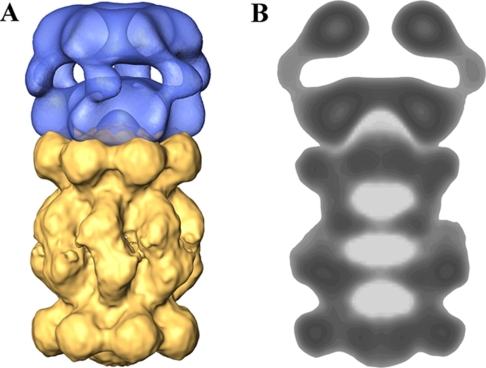
Three-dimensional model of the MJ PAN-20 S complex. A, a three-dimensional model of the PAN-20 S proteasome complex generated in silico based on the three-dimensional model of PAN and a reconstructed three-dimensional model of the MJ proteasome using negatively stained complexes. B, projection view through the PAN-20 S complex demonstrating spatial interactions between the MJ 20 S proteasome and PAN. The dimensions of the pseudo-ring and the cavity in the C-terminal ring of PAN allow direct interaction between the N-terminal region of the α-ring and the C-terminal residues of PAN.
To confirm that the structure depicted in Fig. 1B represents the architecture of a single hexameric PAN complex rather than that of a dodecamer (a dimer of hexamers), we determined the molecular weight of the complex by mass spectrometry. The detected mass of 294,570 ± 23 Da agrees with the predicted molecular mass of a single hexameric complex (36). In addition, to verify this result and localize the N and the C domains of the subunits in the complex, we engineered a mutant of PAN in which each of the subunits lacks the first 73 residues (PAN Δ1–73). We reconstructed the three-dimensional structure of this deletion mutant under similar conditions as employed with wild type PAN (supplemental movie PAN Δ1–73.mpg). As depicted in Fig. 1C, the bottom ring did not change, maintaining the same dimensions as in wild type PAN (compare Fig. 1, Bb to Cb). By contrast, the top ring was altered in response to the N-terminal deletions, with the “shoulders” supporting the connecting stems between the two rings being contracted and the protruding spikes becoming less pronounced. Thus, the sequence of PAN encodes for a protein, which upon oligomerization forms a structure comprising two rings. One ring, reminiscent in structure of the classical AAA domain (37), depicted in Fig. 1Bb, is presumably formed by the C-terminal domains of the six PAN monomers (residues 160–430, based on homology models). An additional ring (Fig. 1Ba) is assembled by the N-terminal domains of the monomers (residues 1–160, based on the predicated N-terminal coiled-coil sequence and N-terminal deletion mutants). In addition, the data also demonstrate that the coiled-coil region (or at least part of it) contributes to the formation of the spikes seen in the side view of the PAN complex (Fig. 1Bc) and appear to be trimmed in the ΔN mutant (Fig. 1Cc).
PAN Undergoes Conformational Transitions in Response to Nucleotide Hydrolysis and Release
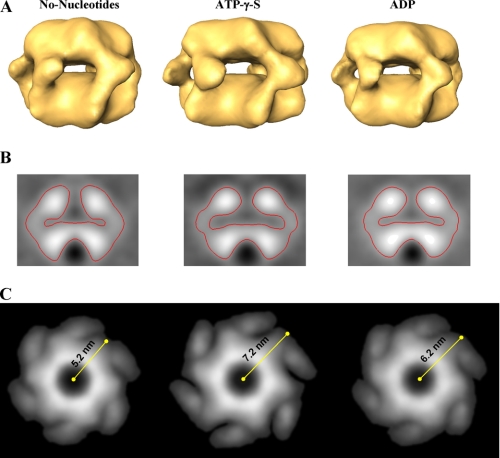
Despite the critical roles and conserved structural motifs shared by members of the AAA superfamily of ATPases, their mode of action is not well understood. In particular, the link between the ATPase catalytic cycle and the conformational changes responsible for the biological activity of these proteins has yet to be elucidated. Likewise, it is still unclear how nucleotide binding, hydrolysis, and release by PAN promote substrate binding, unfolding, and translocation into the 20 S proteasome. To address these outstanding questions, we generated a three-dimensional model of PAN in the presence of ADP and in the absence of nucleotides. In the presence of ATPγS, PAN is presumably locked in a conformation similar to that of the ATP-bound state. The ADP-bound form simulates the conformation that PAN assumes upon nucleotide hydrolysis. The structure realized in the absence of nucleotide represents the conformation of the complex upon nucleotide release, before reloading with ATP. As shown in Fig. 2, although the overall structural character of PAN did not change, the size of the internal cavity of the complex contracted upon nucleotide hydrolysis (as reflected in the structure of PAN preincubated with ADP) and became even smaller in the absence of nucleotides (Fig. 2B). In addition, the size and orientation of the spikes were also affected (Fig. 2C). As presented in Fig. 2C, the shoulders connecting the spikes to the top ring change their orientation and move inward upon ATP hydrolysis (i.e. the ADP state) and release (i.e. the no nucleotide state). We measured the dimensions of the upper ring by calculating the projection of these elements from averaged structures. The measured difference in radius between the ATPγS state and the “no nucleotide” state is about 2 nm (Fig. 2C), whereas in the presence of ADP the complex adopts an intermediate state. Together these data suggest that upon nucleotide binding (i.e. the ATPγS-bound state), PAN assumes a conformation in which the internal cavity is more accessible. Upon nucleotide hydrolysis, which probably occurs after or concomitantly with substrate entry into the internal chamber of the complex, the spikes change their conformation, presumably to exclude binding of additional substrate molecules. The expansion and contraction that are associated with nucleotide binding, hydrolysis, and release may promote the unfolding of the globular domain trapped (or partially trapped) within the internal chamber of PAN.
FIGURE 2.
PAN undergoes structural transitions in response to nucleotide binding, hydrolysis, and release. A, side view of PAN in the absence of nucleotides (left panel) in the presence of ATPγS (middle panel) and when incubated with ADP (right panel). B, the central slice of the three-dimensional structure of PAN in the absence of nucleotides (left panel), in the presence of ATPγS (middle panel), and when incubated with ADP (right panel). The volume of the internal chamber decreases upon nucleotide hydrolysis and release. C, the transition in the orientation of the spikes upon nucleotide hydrolysis and release is illustrated by top view projections of the different nucleotide states of the PAN complex.
The Hydrophobic Character of PAN Alternates during the Nucleotide Cycle
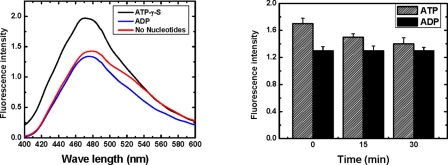
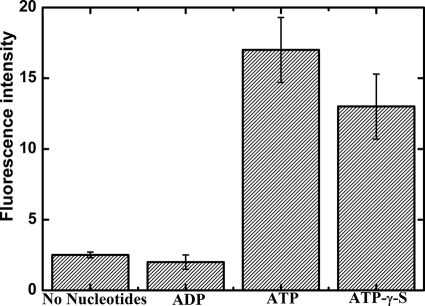
In the chaperonin GroEL, structural transitions are linked with direct nucleotide-dependent modulation of the hydrophobic character of the complex. To directly assess the hydrophobicity of the PAN complex in the various nucleotide-related states, we made use of 1-anilinonaphthalene-8-sulfonic acid (ANS). ANS is essentially non-fluorescent in water, only becoming appreciably fluorescent when bound to membranes (quantum yield ∼0.25) or proteins (quantum yield ∼0.7). These properties make ANS a sensitive indicator of conformational changes that modify the exposure of the probe to water (38, 39). Indeed, this probe was previously used to correlate the hydrophobicity of the GroEL complex with specific nucleotide-associated conformations (40–42). We, thus, incubated PAN (1 μm) in the presence of ATPγS or ADP or in the absence of nucleotides in standard reaction buffer complemented with ANS (5 μm). Fluorescence emission spectra (400–600 nm) were then recorded. Interestingly, the fluorescence intensity of a reaction mixture containing PAN and ATPγS was about 50% (±8%) higher than that of PAN incubated in the presence of ADP (Fig. 3, left panel). Although the no nucleotide conformation appeared reproducibly to be about 5–10% more fluorescent than was the ADP-associated conformation, these differences were not statistically significant. Our findings, thus, predict that incubating PAN with ATP and ANS should initially confer a conformation similar to that of PAN in the presence of ATPγS, reflected by high ANS fluorescence. Over time, as ATP is being hydrolyzed, the conformation would shift into the ADP-bound state, and ANS fluorescence intensity should drop accordingly. Indeed, as observed in Fig. 3 (right panel), the initial fluorescent intensity of ANS incubated with PAN and ATP was reduced over time, whereas the intensity of fluorescence of a similar sample incubated with ADP (or ATPγS) remained unchanged. Thus, the hydrophobic character of PAN increases as the internal cavity of PAN expands in response to nucleotide binding (i.e. the ATPγS state) and decreases upon nucleotide hydrolysis, with the associated reduction of PAN internal chamber volume (i.e. the ADP state).
FIGURE 3.
The hydrophobic character of PAN increases upon nucleotide binding and decreases after hydrolysis. Left panel, PAN (1 μm) was incubated in the presence of ATPγS, ADP, or in the absence of nucleotides. The reaction mixture was complemented with 5 μm ANS. The samples were excited at 370 nm, and fluorescence spectra (400–600 nm) were recorded. Right panel, PAN (1 μm) was incubated in the presence of ANS (5 μm), ATP, or ADP. Aliquots were removed at 15-min intervals, and their fluorescence spectra (400–600 nm) were recorded.
The C-terminal Ring of PAN Is Gated
Our structural analysis reveals that the opening in the C-terminal portion of the PAN complex is gated, as density is detected at the center of the bottom ring (Fig. 1Bb). However, we cannot exclude the existence of a low density region at the center of the bottom ring. Such an opening would have been revealed only through higher resolution structural determination. Initially, we monitored whether the nucleotide state of the complex (i.e. the presence of ATPγS or ADP or in the absence of nucleotides) affects the conformation of the gate. We compared a slice through the gated ring (Fig. 4A) from three-dimensional models of the different nucleotide states. As revealed in Fig. 4B, the gate remained closed, and its conformation was unaffected by the nucleotide state of the complex. However, when PAN was incubated with substrate (YFP-ssrA) in the presence of ATP, a loss of density was detected at the center of the bottom ring. This loss of density corresponds to an opening of the gated ring (Fig. 4C). It is noteworthy that gate opening was only induced when a substrate (YFP-ssrA) was incubated with ATP but not with ADP (data not shown).
FIGURE 4.
The state of the gated C-terminal ring of PAN is substrate-dependent. Density at the C-terminal ring of PAN was analyzed by projecting a 2 nm-thick section at the position, shown in A. B, slices of the gate region in the presence of ATPγS or ADP or in the absence of nucleotide. C, when PAN is incubated with substrate (YFP-SsrA) and ATP, the C-terminal ring opens.
To circumvent the need to unfold the substrate before its translocation through PAN, we used the readily unfolded β-casein as substrate (43). This allowed us to directly monitor whether gate opening is also nucleotide hydrolysis-dependent, as is the unfolding step, or whether binding of the nucleotide in the presence of an unfolded substrate is sufficient for gate opening. Interestingly, β-casein induced dilation of the gated ring in the presence of either ATP or ATPγS (data not shown). Thus, binding of ATP and not its hydrolysis in the presence of an unfolded substrate is sufficient to induce gate opening. As translocation through PAN feeds the proteasome, these findings imply that β-casein would be degraded by the PAN-20 S proteasome in the presence of ATPγS. That is provided that the association between the ATPase complex and the proteasome itself is supported when PAN is in the ATP-bound state (i.e. in the presence of ATPγS). As predicted, β-casein was degraded by the PAN-20 S complex in the presence of ATP or ATPγS but not when ADP was present or in the absence of nucleotides (Fig. 5).
FIGURE 5.
Nucleotide binding but not hydrolysis is required for casein translocation by PAN and degradation by the associated 20 S proteasome. The ability of ADP, ATP, and ATPγS to induce gate opening in the ATPase (PAN) and to promote translocation into the 20 S proteasome was estimated by monitoring the effect of these nucleotides on β-casein degradation. β-Casein was subjected to degradation by the archaeal PAN-20 S complex in the presence of ADP, ATP, ATPγS, or in the absence of nucleotides. The magnitude of β-casein degradation was estimated by the fluorescamine assay (43).
Both the PAN C termini and the N termini of the 20 S α-Ring Are Essential for PAN-20 S Functional Association
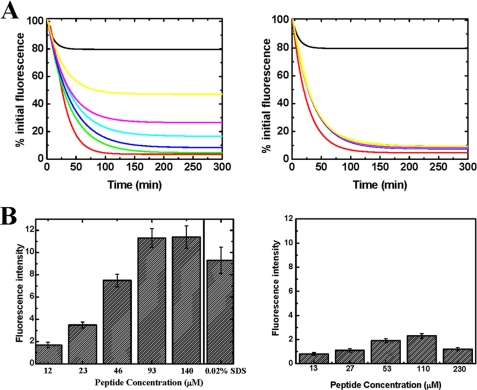
To further examine the importance of the C-terminal domain of PAN in the association with the 20 S proteasome, we chemically synthesized a peptide corresponding to the C-terminal 17 residues of PAN (amino acids 414–430) and monitored its ability to inhibit the degradation of YFP-ssrA by the PAN-20 S complex. Such inhibition is presumably mediated by competition for binding sites on the 20 S α-subunits, which are essential for the association between PAN and the 20 S proteasome (see “Discussion”). As portrayed in Fig. 6A, left panel, increasing the amount of the PAN C-terminal peptide resulted in significant inhibition of YFP-ssrA degradation. By contrast, when a mixture of nonspecific peptides was used at a similar concentration as was the C-terminal PAN peptide, inhibition of YFP-ssrA digestion was not observed (Fig. 6A, right panel). Thus, the C-terminal region of PAN plays a major role in the functional association of the PAN-20 S complex. This view is supported by the findings of Förster et al. (44), illustrating the critical role of the last residue of PAN (Lys-430) in the association of PAN with the 20 S proteasome and, by the findings of Rabl et al. (45), proposing that the C-terminal residues of PAN are responsible for docking the ATPase complex to the 20 S proteasome.
FIGURE 6.
The C-terminal peptide of PAN inhibits degradation of YFP-ssrA. A, left panel, the loss of YFP-ssrA fluorescence was measured as a function of time in the presence of the PAN C-terminal peptide. The green, blue, cyan, purple, and yellow curves represent reaction mixtures with increasing amounts of the peptide (20, 40, 60, 80, and 160 μm, respectively). Right panel, similar reactions as in A were carried out in the presence of the same concentrations of a nonspecific peptide. B, the C-terminal region of PAN induces gate opening in the α-ring of the 20 S proteasome. Left panel, β-casein degradation by the latent 20 S proteasome in the presence of increasing amounts of the PAN C-terminal peptide and in the presence of 0.02% SDS as a control to chemically induce gate opening. Right panel, β-casein degradation by the latent 20 S proteasome in the presence of increasing amounts of a mixture of nonspecific peptides. The degradation of β-casein was estimated by the fluorescamine assay. The contribution of the peptides themselves was determined in separate reaction and subtracted from the β-casein degradation reactions presented.
Next, we directly monitored whether the association of the C-terminal region of PAN with the 20 S proteasome induces dilation of the gated α-ring. We incubated latent TA 20 S proteasome with β-casein and monitored whether the addition of an increasing amount of peptide corresponding to the C terminus of PAN (see above) to reaction mixture led to activation of the proteasome (i.e. opening of the gated α-ring) and β-casein degradation. In a control experiment we assayed the digestion of β-casein under the same conditions while adding mixtures of nonspecific peptides at similar concentrations. We found that the presence of the C-terminal PAN peptide (Fig. 6B, left panel) but not of the control mixture resulted in β-casein degradation (Fig. 6B, right panel). Incubating the TA 20 S proteasome in the presence of 0.02% SDS (conditions believed to induce 20 S proteasome gate opening (46)) led to a comparable degree of β-casein degradation. Presumably, activation of the 20 S proteasome resulted from a conformational transition in the α-ring due to specific binding of the PAN C-terminal peptide. As shown in Fig. 6B, casein degradation by the PAN-20 S complex increases in response to greater concentration of the PAN C-terminal peptide, reaching saturation at about 100 μm, suggesting affinity in the μm range. These findings substantiate the notion that the C-terminal region of PAN plays a role in the association of this particle with the 20 S proteasome.
We generated a three-dimensional model of the MJ 20 S proteasome using single particle analysis and fused it in silico to our three-dimensional model of PAN (Fig. 7). In this synthetic complex, the gated C-terminal ring of PAN aligns very well with the gate region of the 20 S complex. The three-dimensional reconstruction of the proteasome indicates that the gate regions in the α ring form a pseudo-ring which surrounds the entry channel into the 20 S complex (Fig. 7). The dimensions of this pseudo-ring fit the internal cavity in the C-terminal ring of PAN (Fig. 7) and raise the possibility that the N-terminal tails of the α-ring subunits are also important for the association between PAN and the 20 S proteasome.
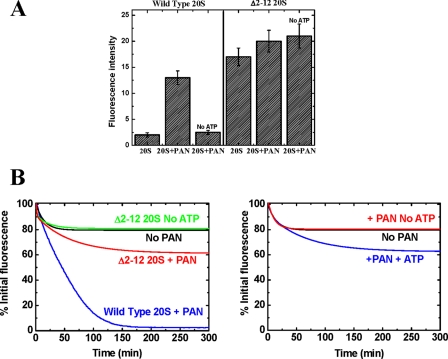
To characterize the contribution of the N-terminal residues of the 20 S α-subunits to the functional interaction between PAN and the 20 S proteasome, we monitored the ability of a version of the 20 S proteasome, in which the α-subunits lack their first 12 residues (17), to associate with PAN and degrade YFP-ssrA in the presence of ATP. As seen in Fig. 8A, right panel, this mutant 20 S proteasome (Δ2–12 20 S) degrades β-casein independent of PAN or ATP, presumably because the entrance to the α-ring is constitutively open, allowing loosely folded proteins, such as β-casein, to readily enter. By contrast, the Δ2–12 20 S complex failed to catalyze the degradation of YFP-ssrA in the presence of PAN and ATP (Fig. 8B, left panel). It is noteworthy that although YFP-ssrA was not degraded, a small ATP-dependent loss in YFP-ssrA fluorescence was detected (Fig. 8B, left panel). This loss of fluorescence is attributed to YFP-ssrA unfolding by PAN, as demonstrated in Fig. 8B, right panel, when YFP-ssrA was incubated together with similar amounts of PAN and ATP in the absence of the 20 S proteasome. In aggregate, these findings suggest that both the C-terminal regions of PAN as well as the N-terminal regions of the α-subunits of the proteasome are necessary for the formation of an active PAN-20 S complex.
FIGURE 8.
The N termini of the 20 S α-subunits are essential for degradation of YFP-ssrA by the PAN-20 S complex. A, ATP-dependent β-casein degradation by PAN and the TA 20 S proteasome or Δ2–12 TA 20 S proteasome was monitored using the fluorescamine assay. B, left panel, YFP-ssrA degradation reaction in the presence of wild type TA 20 S proteasome (blue) and Δ2–12 TA 20 S proteasome (red) in the presence and absence of ATP. Right panel, unfolding reaction of YFP-ssrA by PAN. In the presence of ATP some loss of YFP-ssrA fluorescence is detected (blue). The fluorescence loss is only partially due to the constant and contrasting unfolding-refolding reactions and represents the dynamic, steady state equilibrium.
DISCUSSION
The Three-dimensional Structure of PAN
Our three-dimensional reconstruction of PAN indicates the assembly of a two-ring structure with a large cavity between the two rings. In support of this model, three-dimensional cryo-EM analysis of the 26 S proteasome also revealed that the six ATPases found at the base of the 19 S particle form a dome-shaped structure (9). Likewise, other AAA+ ATPases associated with degradation, such as p97 and the bacterial complexes HslU, ClpX, ClpA, Lon, and others, all share such a two-ring architecture (47). The surprising finding with PAN was the fact that the second ring-forming domains (i.e. the N-terminal regions of the PAN monomers) are not AAA+ ATPase domains and were not predicted to form an additional ring. Moreover, VAT, an ATPase complex closely related to p97 and thought to regulate the TA proteasome in vivo (48), also shares this double-ring architecture. This is especially important because all previous mechanistic studies of PAN (derived from M. jannaschii) were performed using the TA 20 S proteasome (27). In addition, sequence alignment of PAN reveals two distinct homology domains. The N-terminal 160 residues aligned with the highest score to the N-terminal region of the ARC (AAA ATPase forming ring-shaped complexes) ATPase (49), which was also shown to form a ring structure. The remaining C-terminal 270 residues are highly homologous to the classical AAA+ domain found in p97 and 19 S ATPases known to form typical AAA+ hexameric rings. Taken together, our findings substantiate the notion that a two-ring structure enclosing a large cavity, which may alter its dimensions in response to nucleotide binding and hydrolysis, is essential for the unfolding and translocation.
Structural Transitions in Response to Nucleotide Binding and Release
In this study we found three discrete conformations of PAN associated with three nucleotide states (i.e. in the presence of ATP, ADP, or no nucleotide) by assuming uniformity of all subunits in the ATPase complex at a given state. However, it is also possible that in the cellular environment different subunits might bind, hydrolyze, and release the nucleotide in a non-synchronous manner. In fact, such a model has been demonstrated (50). Yet, such non-coordinated action of the different subunits may also lead to a global transition. For example, global structural transitions may only occur once a certain number of subunits hydrolyze or release the nucleotide. Furthermore, even if nucleotide hydrolysis and release are not associated with globally coordinated contraction and expansion, the continuous conformational transition of individual subunits might be sufficient to promote unfolding of a globular domain trapped within the internal cavity of PAN, perhaps even more efficiently than would be realized by discrete “pulses” of contraction and expansion. In this respect our three-dimensional models of PAN are rather simplistic as the averaged structures are 6-fold-symmetrized, and thus, all of the subunits in a given nucleotide state appear to assume the same conformation. In fact, in pre-symmetrized structures, indications that only some of the subunits were bound to nucleotides were noted. However, we could not reliably underline such a distinction due to resolution limitation. If indeed only partial nucleotide occupancy existed under our experimental conditions, we would expect that differences between the fully loaded and completely vacant nucleotide states to be even more substantial. It is noteworthy that relative to the magnitude of the structural transitions and the net reduction and expansion of the GroEL internal cavity volume associated with the function of this chaperonin (51, 52), the conformational alterations detected in this study (Fig. 3) should be sufficient to promote unfolding in a related manner.
As mentioned above, it is appealing to consider a mechanism in which a globular domain is unfolded on internal surface of the PAN cavity (15). In fact, substrate trapping by ClpA (one of the E. coli ClpP protease regulating ATPase complexes) en route to degradation (or disassembly) was already demonstrated by Wickner and co-workers (53, 54). Supporting our findings, the substrate in those experiments was captured by the ClpA ATPase complex in the ATP-bound state.
Although our findings raise the possibility of a new mode of substrate entry into the ATPase complex through the side openings, we cannot exclude a possibility in which substrate enters through the top ring. In support of the latter possibility, substrates have already been visualized by EM adjacent to the top opening of the ClpX complex (55). Moreover, it is also possible that upon substrate binding on the lateral region of PAN by coiled-coil domains, a major conformational transition, moving the bound substrate toward the top opening, is induced. In fact, such motion was detected upon binding of substrate by the ClpA ATPase (56).
Complex Formation between PAN and the 20 S Proteasome
HslUV, one of the E. coli ATP-dependent degradation systems, is considered to be the closest bacterial homologue of the eukaryotic proteasome (57). This system is composed of two subcomplexes, namely HslV, serving as the protease (in analogy to the 20 S proteasome), and the regulatory hexameric ATPase complex, HslU, which is responsible for substrate recognition, unfolding, and translocation into HslV (in analogy to PAN and the 19 S particle). The crystal structure of HslU was determined on its own (29) and in association with HslV (58), allowing for a direct examination of the transition in the orientation of the HslU C-terminal residues (430–443) upon association with HslV (supplemental Fig. S2A). We propose that the high sequence homology of the C termini of PAN and HslU and their related roles in proteolysis make the function of the C termini of each monomer, within the HslU complex, in association with HslV a valid model for PAN and the 20 S proteasome.
Two major realizations emerge from the structural transition of the C termini of HslU upon its association with HslV (supplemental Fig. S2, A and B). First, the C termini of the monomers are located on the perimeter of the C-terminal ring of HslU and not in central channel leading to the protease. In addition, upon association with the protease (i.e. HslV), these C termini project of the ring-plane and protrude into the protease ring (supplemental Fig. S2B). These conclusions are also supported by localization of the C-terminal residues of the eukaryotic ATPase complex, p97/VCP (another complex homologous in structure and function to PAN) (59). In the corresponding three-dimensional structure, the C-terminal residues of p97 are located around the rim of cavity of the ATPase ring (supplemental Fig. S2C) and would presumably dip into the perimeter of the proteasome pseudo-ring (Fig. 7). Based on these model systems, the role of the C-terminal peptides of PAN in the association with the 20 S proteasome is presumably independent of opening the gated ATPase complex to allow release and translocation of unfolded protein into the proteasome (Fig. 4). In support, PAN promotes unfolding and release of YFP-ssrA even in the absence of docked 20 S proteasome (15).
In contrast to the C-terminal peptides of PAN, which by themselves are able to induce complete dilatation of the entry port to the 20 S proteasome (Fig. 6B), peptides corresponding to the 15 N-terminal residues of the α-subunits did not alter the ATPase activity of PAN nor its ability to unfold and release unfolded substrates (data not shown). Still, deletion of the first 12 residues of the TA α-subunits abolished the ability of the PAN-20 S complex to degrade YFP-ssrA (Fig. 8). Although not conclusive, these findings, taken together, suggest a mechanism in which the C termini of PAN initially dock into the 20 S proteasome, presumably at the periphery of the internal pseudo-ring (Fig. 7), as was also suggested previously (45). This interaction would invoke a conformational transition in the proteasome α-subunits, leading to dissociation of a mesh created by the seven α-subunit N-terminal residues gating the proteasome. Presumably, this transition would flip the N termini of the α-ring outward into the cavity of the ATPase ring, allowing substrate transit from the ATPase into the proteasome. Such a scenario is illustrated by comparing the crystal structure of the intact yeast 20 S proteasome (19) to that of the yeast 20 S proteasome in complex with PA-26 (60). As seen in supplemental Fig. S3, upon association of PA-26 C-terminal residues with boundaries of the 20 S internal pseudo-α ring, the inwardly collapsed meshed 20 S α-ring N-terminal residues flip and project outward into the C-terminal cavity of the PA-26. By analogy, we propose that such a scenario also occurs upon the association of PAN with the 20 S proteasome. Our findings suggest that the N-terminal residues of the proteasome α-ring assume a double role. When not in complex, these residues constitute a mesh gating entry to the 20 S proteasome. Upon association with the regulatory ATPase, the same residues contribute to stabilization of the complex, as both the C termini of PAN and the N termini of the 20 S α-ring are essential for active complex formation (Fig. 6). This model may partially circumvent the mis-symmetry between the regulatory complex (a hexamer) and the proteasome α-ring (a heptamer) realized upon interaction. The strong complementarity of the dimensions of the α-pseudo ring and the cavity of the associated PAN (Fig. 7) together with the notion that interactions between the N-terminal residues of the α-ring upon association with the regulatory complex might be highly dynamic and of lower specificity, may render such mis-symmetry less severe.
A Model for PAN Function
One model we favor involves a mechanism whereby two types of sites on the ATPase interact with a tagged substrate. The first site provides high affinity interactions with the specific tag (i.e. the ssrA binding site). The second class of sites represents a lower specificity, hydrophobic patch located on the internal surface of the central cavity of PAN. Initially, the high affinity tag (ssrA) binds to the ATPase complex and a globular domain is introduced into the internal cavity of PAN. Next, thermal perturbations of the tethered protein together with nucleotide-dependent expansion and contraction of the ATPase complex as well as alteration of the hydrophobic character of the internal surface of PAN would result in greater and greater interaction of the globular domain with the hydrophobic surfaces, dissolving the substrate step by step on the internal surface of the complex. One can consider this model as being analogous to the manner by which a fly would interact with a flypaper, namely, where each movement of the fly results in greater entanglement on the adhesive.
The role of nucleotide binding and hydrolysis could be to induce conformational changes that promote unfolding and regulate exposure of the hydrophobic patches (Fig. 3). This model is appealing because substrate binding is highly specific (through a universal tag), yet unfolding proceeds regardless of the structure of the specific substrate, and hence, any tagged protein can be unfolded. Once a globular protein (or domain) is unfolded, the gated C-terminal ring of the ATPase opens (Fig. 4) and, upon interaction with the 20 S α-ring, induces opening of the entry port leading into the proteasome. A functional complex between PAN and the proteasome is, thus, formed. Complex formation depends on the integrity of both the C termini of PAN as well as the N termini of the proteasome α-subunits. Although the unfolding of a globular domain is ATP hydrolysis-dependent, gate opening and the translocation into the proteasome is nucleotide binding but not hydrolysis-dependent (Fig. 5).
Supplementary Material
This work was supported in part by the Israeli Science Foundation Grants ISF 802/04 (to A. N.) and ISF 794/02 (to O. M.), German Minerva Foundation Grant 780040 (to A. N.), German-Israeli Foundation for Scientific Research and Development Grant GIF I-845-210.9/2004), and a special donation from Rolando Uziel (to A. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Movies PAN ATPγS.mpg and PAN Δ1–73.mpg.
- TA
- T. acidophilum
- ATPγS
- adenosine 5′-O-(thiotriphosphate)
- EM
- electron microscopy
- MJ
- M. jannaschii
- ANS
- 1-anilinonaphthalene-8-sulfonic acid
- YFP
- yellow fluorescent protein.
REFERENCES
- 1.Hölzl H., Kapelari B., Kellermann J., Seemüller E., Sümegi M., Udvardy A., Medalia O., Sperling J., Müller S. A., Engel A., Baumeister W. (2000) J. Cell Biol. 150,119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coux O., Tanaka K., Goldberg A. L. (1996) Annu. Rev. Biochem. 65,801–847 [DOI] [PubMed] [Google Scholar]
- 3.Voges D., Zwickl P., Baumeister W. (1999) Annu. Rev. Biochem. 68,1015–1068 [DOI] [PubMed] [Google Scholar]
- 4.Braun B. C., Glickman M., Kraft R., Dahlmann B., Kloetzel P. M., Finley D., Schmidt M. (1999) Nat. Cell Biol. 1,221–226 [DOI] [PubMed] [Google Scholar]
- 5.Strickland E., Hakala K., Thomas P. J., DeMartino G. N. (2000) J. Biol. Chem. 275,5565–5572 [DOI] [PubMed] [Google Scholar]
- 6.Glickman M. H., Rubin D. M., Fried V. A., Finley D. (1998) Mol. Cell. Biol. 18,3149–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walz J., Erdmann A., Kania M., Typke D., Koster A. J., Baumeister W. (1998) J. Struct. Biol. 121,19–29 [DOI] [PubMed] [Google Scholar]
- 8.Baumeister W., Walz J., Zühl F., Seemüller E. (1998) Cell 92,367–380 [DOI] [PubMed] [Google Scholar]
- 9.Nickell S., Mihalache O., Beck F., Hegerl R., Korinek A., Baumeister W. (2007) Biochem. Biophys. Res. Commun. 353,115–120 [DOI] [PubMed] [Google Scholar]
- 10.da Fonseca P. C., Morris E. P. (2008) J. Biol. Chem. 283,23305–23314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendil K. B., Hartmann-Petersen R., Tanaka K. (2002) J. Mol. Biol. 315,627–636 [DOI] [PubMed] [Google Scholar]
- 12.Hartmann-Petersen R., Tanaka K., Hendil K. B. (2001) Arch. Biochem. Biophys. 386,89–94 [DOI] [PubMed] [Google Scholar]
- 13.Zwickl P., Ng D., Woo K. M., Klenk H. P., Goldberg A. L. (1999) J. Biol. Chem. 274,26008–26014 [DOI] [PubMed] [Google Scholar]
- 14.Zwickl P., Seemüller E., Kapelari B., Baumeister W. (2001) Adv. Protein Chem. 59,187–222 [DOI] [PubMed] [Google Scholar]
- 15.Navon A., Goldberg A. L. (2001) Mol. Cell 8,1339–1349 [DOI] [PubMed] [Google Scholar]
- 16.Köhler A., Cascio P., Leggett D. S., Woo K. M., Goldberg A. L., Finley D. (2001) Mol. Cell 7,1143–1152 [DOI] [PubMed] [Google Scholar]
- 17.Benaroudj N., Zwickl P., Seemüller E., Baumeister W., Goldberg A. L. (2003) Mol. Cell 11,69–78 [DOI] [PubMed] [Google Scholar]
- 18.Löwe J., Stock D., Jap B., Zwickl P., Baumeister W., Huber R. (1995) Science 268,533–539 [DOI] [PubMed] [Google Scholar]
- 19.Groll M., Ditzel L., Löwe J., Stock D., Bochtler M., Bartunik H. D., Huber R. (1997) Nature 386,463–471 [DOI] [PubMed] [Google Scholar]
- 20.Groll M., Bajorek M., Köhler A., Moroder L., Rubin D. M., Huber R., Glickman M. H., Finley D. (2000) Nat. Struct. Biol. 7,1062–1067 [DOI] [PubMed] [Google Scholar]
- 21.Larsen C. N., Finley D. (1997) Cell 91,431–434 [DOI] [PubMed] [Google Scholar]
- 22.Benaroudj N., Goldberg A. L. (2000) Nat. Cell Biol. 2,833–839 [DOI] [PubMed] [Google Scholar]
- 23.Kenniston J. A., Baker T. A., Fernandez J. M., Sauer R. T. (2003) Cell 114,511–520 [DOI] [PubMed] [Google Scholar]
- 24.Weber-Ban E. U., Reid B. G., Miranker A. D., Horwich A. L. (1999) Nature 401,90–93 [DOI] [PubMed] [Google Scholar]
- 25.Lee C., Schwartz M. P., Prakash S., Iwakura M., Matouschek A. (2001) Mol. Cell 7,627–637 [DOI] [PubMed] [Google Scholar]
- 26.Matouschek A. (2003) Curr. Opin. Struct. Biol. 13,98–109 [DOI] [PubMed] [Google Scholar]
- 27.Smith D. M., Kafri G., Cheng Y., Ng D., Walz T., Goldberg A. L. (2005) Mol. Cell 20,687–698 [DOI] [PubMed] [Google Scholar]
- 28.Rohrwild M., Pfeifer G., Santarius U., Müller S. A., Huang H. C., Engel A., Baumeister W., Goldberg A. L. (1997) Nat. Struct. Biol. 4,133–139 [DOI] [PubMed] [Google Scholar]
- 29.Bochtler M., Hartmann C., Song H. K., Bourenkov G. P., Bartunik H. D., Huber R. (2000) Nature 403,800–805 [DOI] [PubMed] [Google Scholar]
- 30.Song H. K., Hartmann C., Ramachandran R., Bochtler M., Behrendt R., Moroder L., Huber R. (2000) Proc. Natl. Acad. Sci. U. S. A. 97,14103–14108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck M., Förster F., Ecke M., Plitzko J. M., Melchior F., Gerisch G., Baumeister W., Medalia O. (2004) Science 306,1387–1390 [DOI] [PubMed] [Google Scholar]
- 32.Förster F., Medalia O., Zauberman N., Baumeister W., Fass D. (2005) Proc. Natl. Acad. Sci. U. S. A. 102,4729–4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harauz G., Cicicopol C., Hegerl R., Cejka Z., Goldie K., Santarius U., Engel A., Baumeister W. (1996) J. Struct. Biol. 116,290–301 [DOI] [PubMed] [Google Scholar]
- 34.Nickell S., Förster F., Linaroudis A., Net W. D., Beck F., Hegerl R., Baumeister W., Plitzko J. M. (2005) J. Struct. Biol. 149,227–234 [DOI] [PubMed] [Google Scholar]
- 35.Tang G., Peng L., Baldwin P. R., Mann D. S., Jiang W., Rees I., Ludtke S. J. (2007) J. Struct. Biol. 157,38–46 [DOI] [PubMed] [Google Scholar]
- 36.Medalia N., Sharon M., Martinez-Arias R., Mihalache O., Robinson C. V., Medalia O., Zwickl P. (2006) J. Struct. Biol. 156,84–92 [DOI] [PubMed] [Google Scholar]
- 37.Dreveny I., Kondo H., Uchiyama K., Shaw A., Zhang X., Freemont P. S. (2004) EMBO J. 23,1030–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arighi C. N., Rossi J. P., Delfino J. M. (1998) Biochemistry 37,16802–16814 [DOI] [PubMed] [Google Scholar]
- 39.Raha T., Chattopadhyay D., Chattopadhyay D., Roy S. (1999) Biochemistry 38,2110–2116 [DOI] [PubMed] [Google Scholar]
- 40.Smoot A. L., Panda M., Brazil B. T., Buckle A. M., Fersht A. R., Horowitz P. M. (2001) Biochemistry 40,4484–4492 [DOI] [PubMed] [Google Scholar]
- 41.Kusmierczyk A. R., Martin J. (2000) J. Biol. Chem. 275,33504–33511 [DOI] [PubMed] [Google Scholar]
- 42.Golbik R., Zahn R., Harding S. E., Fersht A. R. (1998) J. Mol. Biol. 276,505–515 [DOI] [PubMed] [Google Scholar]
- 43.Kisselev A. F., Akopian T. N., Goldberg A. L. (1998) J. Biol. Chem. 273,1982–1989 [DOI] [PubMed] [Google Scholar]
- 44.Förster A., Masters E. I., Whitby F. G., Robinson H., Hill C. P. (2005) Mol. Cell 18,589–599 [DOI] [PubMed] [Google Scholar]
- 45.Rabl J., Smith D. M., Yu Y., Chang S. C., Goldberg A. L., Cheng Y. (2008) Mol. Cell 30,360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akopian T. N., Kisselev A. F., Goldberg A. L. (1997) J. Biol. Chem. 272,1791–1798 [DOI] [PubMed] [Google Scholar]
- 47.White S. R., Lauring B. (2007) Traffic 8,1657–1667 [DOI] [PubMed] [Google Scholar]
- 48.Gerega A., Rockel B., Peters J., Tamura T., Baumeister W., Zwickl P. (2005) J. Biol. Chem. 280,42856–42862 [DOI] [PubMed] [Google Scholar]
- 49.Wolf S., Nagy I., Lupas A., Pfeifer G., Cejka Z., Müller S. A., Engel A., De Mot R., Baumeister W. (1998) J. Mol. Biol. 277,13–25 [DOI] [PubMed] [Google Scholar]
- 50.Martin A., Baker T. A., Sauer R. T. (2005) Nature 437,1115–1120 [DOI] [PubMed] [Google Scholar]
- 51.Ranson N. A., Farr G. W., Roseman A. M., Gowen B., Fenton W. A., Horwich A. L., Saibil H. R. (2001) Cell 107,869–879 [DOI] [PubMed] [Google Scholar]
- 52.Ranson N. A., Clare D. K., Farr G. W., Houldershaw D., Horwich A. L., Saibil H. R. (2006) Nat. Struct. Mol. Biol. 13,147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gottesman S., Maurizi M. R., Wickner S. (1997) Cell 91,435–438 [DOI] [PubMed] [Google Scholar]
- 54.Pak M., Wickner S. (1997) Proc. Natl. Acad. Sci. U. S. A. 94,4901–4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ortega J., Singh S. K., Ishikawa T., Maurizi M. R., Steven A. C. (2000) Mol. Cell 6,1515–1521 [DOI] [PubMed] [Google Scholar]
- 56.Ishikawa T., Maurizi M. R., Steven A. C. (2004) J. Struct. Biol. 146,180–188 [DOI] [PubMed] [Google Scholar]
- 57.Rohrwild M., Coux O., Huang H. C., Moerschell R. P., Yoo S. J., Seol J. H., Chung C. H., Goldberg A. L. (1996) Proc. Natl. Acad. Sci. U. S. A. 93,5808–5813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sousa M. C., Trame C. B., Tsuruta H., Wilbanks S. M., Reddy V. S., McKay D. B. (2000) Cell 103,633–643 [DOI] [PubMed] [Google Scholar]
- 59.DeLaBarre B., Brunger A. T. (2005) J. Mol. Biol. 347,437–452 [DOI] [PubMed] [Google Scholar]
- 60.Whitby F. G., Masters E. I., Kramer L., Knowlton J. R., Yao Y., Wang C. C., Hill C. P. (2000) Nature 408,115–120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.