Abstract
Maintenance of an intact mucosal barrier is critical to preventing damage to and infection of wet-surfaced epithelia. The mechanism of defense has been the subject of much investigation, and there is evidence now implicating O-glycosylated mucins on the epithelial cell surface. Here we investigate a new role for the carbohydrate-binding protein galectin-3 in stabilizing mucosal barriers through its interaction with mucins on the apical glycocalyx. Using the surface of the eye as a model system, we found that galectin-3 colocalized with two distinct membrane-associated mucins, MUC1 and MUC16, on the apical surface of epithelial cells and that both mucins bound to galectin-3 affinity columns in a galactose-dependent manner. Abrogation of the mucin-galectin interaction in four different mucosal epithelial cell types using competitive carbohydrate inhibitors of galectin binding, β-lactose and modified citrus pectin, resulted in decreased levels of galectin-3 on the cell surface with concomitant loss of barrier function, as indicated by increased permeability to rose bengal diagnostic dye. Similarly, down-regulation of mucin O-glycosylation using a stable tetracycline-inducible RNA interfering system to knockdown c1galt1 (T-synthase), a critical galactosyltransferase required for the synthesis of core 1 O-glycans, resulted in decreased cell surface O-glycosylation, reduced cell surface galectin-3, and increased epithelial permeability. Taken together, these results suggest that galectin-3 plays a key role in maintaining mucosal barrier function through carbohydrate-dependent interactions with cell surface mucins.
Mucosal surfaces comprise more than 400 m2 of the total surface area in humans (compared with 1.8 m2 for skin) and are, thus, by far the largest area of contact with the environment (1). Epithelial cells in mucosal surfaces are continuously faced with the critical function of forming a protective apical barrier that prevents cellular damage and infection while allowing the exchange of molecules with the extracellular milieu. Loss of barrier function is ascribed to numerous mucosal pathologies, such as dry eye (a disease affecting more than 5 million people in the United States), severe asthma, and inflammatory bowel disease (2–4). Integral to the apical surface of mucosal epithelia are cell surface-associated mucins, a group of high molecular weight glycoproteins defined by the presence of long amino-terminal, extracellular domains containing extensive sites for O-glycan attachment. O-Glycosylation is the most abundant post-translational modification of mucins and constitutes up to 80% of mucin's mass. It is thought that specific cell surface mucins and their O-glycans provide protection to the mucosal surface (5). Data from knock-out mice deficient in cell surface mucin MUC1 and core 3 β-1,3-N-acetylglucosaminyltransferase, an enzyme involved in the synthesis of mucin-type O-glycans in human colon, indicate the requirement for mucins and their O-glycans in maintaining barrier integrity in the gastrointestinal tract and the eye (6–9). However, the mechanism by which cell surface-associated mucins and their O-glycans contribute to forming the mucosal barrier on the epithelial glycocalyx remains poorly characterized.
Galectins are a family of animal β-galactoside-binding lectins, defined by their evolutionarily conserved carbohydrate recognition domain (10, 11). As many as 15 galectins have been identified in mammals, and they are widely distributed among different types of cells and tissues (12). Galectins have been implicated in numerous biological processes, including tumor cell adhesion and progression, immunity, inflammation, wound healing, and development (11, 13, 14). Galectin-3 is a 35-kDa protein originally identified as Mac-2, a cell surface antigen expressed on murine thioglycollate-elicited peritoneal macrophages (15). It is now established that galectin-3, like other galectins, can interact in a multivalent fashion and cross-link glycan ligands on cell surface receptors, such as with epidermal growth factor receptors and α5β1 integrin, to generate molecular lattices (16, 17). In this study we investigate whether galectin-3 participates in mucosal barrier function through its interaction with cell surface-associated mucins. We demonstrate here that two distinct cell surface mucins, MUC1 and MUC16, interact with galectin-3 on the apical surface of epithelial cells and that carbohydrate-mediated mucin-galectin-3 interactions play an important role in maintaining mucosal barrier function.
EXPERIMENTAL PROCEDURES
Cell Cultures
Telomerase-immortalized human corneal (HCLE)3 and conjunctival (HCjE) epithelial cells were kindly provided by Dr. Ilene Gipson (18). Tumor-derived human colorectal carcinoma (Caco-2), mammary gland carcinoma (BT-20), and endometrial (ECC-1) epithelial cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). Cells were plated at a seeding density of 5 × 104 cells/cm2 and maintained at 37 °C in 5% CO2. HCLE and HCjE cell cultures were grown in Dulbecco's modified Eagle's medium/F-12 supplemented with 10% calf serum and 10 ng/ml epidermal growth factor for 7 days to promote stratification and differentiation, as previously reported (18). Caco-2, BT-20, and ECC-1 cells were grown as recommended by ATCC (Caco-2: ATCC-formulated Eagle's minimum essential medium with 20% fetal bovine serum (FBS), BT-20, ATCC-formulated Eagle's minimum essential medium with 10% FBS, ECC-1, RPMI 1640 medium with 2 mm l-glutamine, 1.5 g/liter sodium bicarbonate, 4.5 g/liter glucose, 10 mm HEPES, 1.0 mm sodium pyruvate, 5% FBS) and analyzed 7 days after reaching confluence.
Colocalization of Galectin-3 and Cell Surface-associated Mucins
Galectin-3 was immunolocalized in frozen sections (6 μm) of human corneal donor rims and conjunctival biopsies and in cell cultures fixed in 4% paraformaldehyde using standard protocols. Briefly, sections and cultures were incubated with a rat monoclonal antibody against galectin-3 (1:10 diluted hybridoma fluid; antibody M3/38; ATCC) or a rabbit polyclonal, anti-human, galectin-3 antibody (1:50; H-160, Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C (15). The corresponding fluorescein isothiocyanate-conjugated secondary antibodies were applied (1:50) for 1 h at room temperature, and after a PBS wash specimens were cover-slipped using Vectashield mounting medium with propidium iodide (Vector Laboratories; Burlingame, CA). Incubation with primary antibodies was routinely omitted in control experiments. The sections and cultures were viewed by confocal microscopy on a TCS SP2 confocal laser scanning microscope (Leica Inc., Heidelberg, Switzerland). To determine whether galectin-3 colocalized with MUC1 and MUC16, double-labeling studies were performed. For this, a mixture of galectin-3 rabbit antibody (1:100) and OC125 mouse antibody against MUC16 (1:50; Dako Corp., Carpinteria, CA) or the 214D4 mouse antibody against MUC1 (1:100; Upstate Biotechnology Inc., Lake Placid, NY) was applied to tissue sections followed by a mixture of fluorescein isothiocyanate-conjugated anti-rabbit IgG and TRITC-conjugated anti-mouse IgG. The integrity of tight junctions in cell cultures was analyzed by immunostaining with the rabbit polyclonal anti-ZO-1 (Zymed Laboratories Inc., San Francisco, CA).
Electrophoresis and Western/Lectin Blotting
Assay for galectin-3 and c1galt1 in cell culture lysates was performed by Western blot analysis using a monoclonal rat antibody against galectin-3 (hybridoma M3/38) and a monoclonal mouse anti-human c1galt1 (Abnova Corp., Taipei, Taiwan), respectively. Proteins were extracted from serum-starved cell cultures with radioimmune precipitation assay buffer (150 μm NaCl, 50 μm Tris, pH 8.0, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS) plus Halt protease inhibitor mixture (Pierce). Protein concentration was determined using the bicinchoninic acid protein assay reagent kit (Pierce). Samples were diluted in Laemmli buffer, separated by SDS-PAGE, and transferred to nitrocellulose membranes (Bio-Rad). Protein blots were then incubated with anti-galectin-3 (undiluted hybridoma supernatant) or anti-c1galt1 (1:2,000) antibodies followed by peroxidase-conjugated anti-rat or anti-mouse IgG secondary antibodies, respectively. Housekeeping proteins were detected using antibodies to cytochrome c oxidase IV (1:2,000; Abcam Inc., Cambridge, MA) and glyceraldehyde-3-phosphate dehydrogenase (1:2,000; Santa Cruz Biotechnology). Positive binding was detected using the Pierce SuperSignal West Pico Chemiluminescent Substrate (Pierce). To determine the presence of galectin-3 in cell culture media, culture supernatants were dialyzed against distilled H20 at 4 °C using 12–14-kDa dialysis tubing (Spectra/Por®2; Spectrum Laboratories, Rancho Dominguez, CA), lyophilized, and analyzed by Western blot as described above. Mucin-type O-glycans on nitrocellulose membranes were detected by lectin blot analyses. For these, membranes were incubated with 1% polyvinylpyrrolidone in 0.1% Tween Tris-buffered saline, pH 7.5, for 1 h and then with biotin-labeled peanut agglutinin (25 μg/ml; Vector Laboratories) for 90 min at room temperature on a shaker. Membranes were developed using the Vectastain ABC kit (Vector Laboratories) followed by incubation with SuperSignal West Pico chemiluminescence substrate.
Galectin-3 Affinity Chromatography
Binding of galectin-3 to MUC1 and MUC16 was determined using a galectin-3 affinity matrix. The matrix was prepared by coupling 5 mg of recombinant human galectin-3 to cyanogen bromide-activated Sepharose 4B (Sigma Aldrich) according to the manufacturer's instructions. Cell culture lysates in radioimmune precipitation assay buffer were applied to a galectin-3 affinity column. The unbound components were removed by washing the column with PBS containing 0.1% Triton X-100 (PBST), and the bound proteins were eluted using PBST containing 0.1 m β-lactose. To confirm sugar binding specificity of the galectin-3 binding proteins, the column was eluted with a non-competing disaccharide, sucrose (0.1 m), in PBST before elution with β-lactose. Fractions eluted from the column with saccharides were dialyzed against water, lyophilized, and resolved by agarose gel electrophoresis (1%, w/v). Proteins were then transferred to nitrocellulose membranes by vacuum and probed with the 214D4 antibody (1:5,000) against MUC1 and the OC125 antibody (1:2,000) against MUC16 as described (18).
Biotinylation of Cell Surface Proteins
To determine the presence of galectin-3, MUC1, MUC16, and T-antigen on the epithelial cell surface glycocalyx, cell surface proteins on apical cell membranes of corneal epithelial cultures serum-starved for 2 h before analysis were biotinylated and isolated by chromatography on a neutravidin-agarose affinity column using the PinpointTM Cell Surface Protein Isolation kit (Pierce) according to the manufacturer's instructions. Membrane extracts of the labeled cells were analyzed for the presence of galectin-3, MUC1, MUC16, and T-antigen by Western/lectin blot analyses as described above. To determine whether biotinylation was restricted to the apical cell surface, the membrane extracts were analyzed for the presence of integrin α5 subunit (ITGA5), a glycoprotein primarily restricted to the basolateral cell membrane in corneal epithelium (19) using a rabbit polyclonal antibody (1:1,000) directed against the integrin α5 subunit (Chemicon, Temecula, CA).
C1galt1 Retroviral Production and Titer Assay
Retroviruses encoding hairpin siRNA were constructed using the pSUPER.retro.puro vector system containing a tetracycline-inducible H1 promoter (OligoEngine, Seattle, WA). Three double-stranded hairpin oligonucleotides designed to target the human c1galt1 gene (shRNA target sequences 1, (CAAACACGTCAAAGCTACT), 2 (TAAGCAAAGAAGCCTTGAA), and 3, TACAGATATCAACCTACCT) were cloned into the BglII/HindIII sites of pSUPER.retro.puro. The shRNA target sequences were found by Blast homology searching to have minimal similarity with other human genes. A scrambled sequence (GCGCGCTTTGTAGGATTCG) was used as a negative control. The viruses were transformed into chemically competent Escherichia coli (DH5α cells), and the correct orientation of the oligonucleotides was confirmed by DNA sequencing. pSUPER viruses were transfected in 293GPG packaging cells using Polyfect reagent (Qiagen, Valencia, CA). Viral supernatants were concentrated by ultracentrifugation at 50,000 × (4 °C, 90 min). Virions were resuspended in a minimal volume of 50 mm Tris-HCl, pH 7.8, 130 mm NaCl, 1 mm EDTA, separated into aliquots, and stored at −80 °C. Infectivity titers in NIH3T3 cells were determined 16 h after infection with serial dilutions of concentrated viral supernatants. Viral titers of at least 6 × 106 colony-forming units/ml, based on the percentage of crystal violet-positive cells in NIH3T3 cells, were used for viral infections.
Generation of Stable HCLE Cell Lines for Inducible c1galt1 Knockdown
The pcDNA/6TR plasmid (Invitrogen) was used in accordance with the manufacturer's instructions to generate clones expressing the Tet repressor. Briefly, the linearized plasmid was transfected into HCLE cells with Polyfect (Qiagen), and cells were selected in 3 μg/ml Blasticidin (Invitrogen) for 10 days. The resulting clones were then screened for Tet repressor protein expression by Western blotting. The clone expressing the highest level of Tet repressor was chosen for subsequent viral infections with retroviruses carrying inducible-specific c1galt1 shRNA. For viral infections, Tet-inducible HCLE cells were plated at 4 × 104 cells per well in 6-well plates and incubated with 300 μl of concentrated virus-containing supernatant and 6 μg/ml Polybrene in a final volume of 1 ml for 16 h. This procedure was repeated 2 times, allowing cells to recover for 24 h between infections. Infected clones were obtained using antibiotic selection (puromycin) and further expanded to confluence to obtain stably transfected cells. For functional assays, doxycycline, a synthetic derivative of tetracycline, was administered to HCLE cells for 4 days after reaching confluency to induce shRNA expression. Expression of c1galt1 was determined by reverse transcription-PCR (forward primer, 5′-GCCAACATAAAGATGAGAACA-3′; reverse primer, 5′-TCTCCCCAGTGCTAAGTCTTC-3′) using previously published conditions (20).
Barrier Function Assay
Barrier function in cell cultures was assayed as previously described using differentiated epithelial cells that express robust amounts of cell surface mucins (21). Briefly, epithelial cell cultures grown on chamber slides (Lab-Tek, Naperville, IL) were serum-starved for 2 h, rinsed with PBS, and incubated for 5 min with 0.1% rose bengal (Aldrich) in PBS, Ca+2/Mg+2-free, pH 7.4. Rose bengal was then aspirated, and the cell layer was examined to assess the extent of dye penetrance using an inverted microscope (Nikon Eclipse TS100). Pictures were taken with a SPOT Insight Fire Wire Camera (Diagnostic Instruments, Inc.; Sterling Heights, MI). Images were processed further for dye penetrance quantification using ImageJ software (NIH, Bethesda, MD). In this assay rose bengal penetrance is dependent on the character of the cell surface glycocalyx; induction and abrogation of cell surface mucin biosynthesis result in reduced and increased rose bengal penetrance, respectively (21, 22). Therefore, protection from rose bengal penetration into epithelial cells indicated the presence of a fully functional mucosal barrier. Likewise, penetration and positive staining of the epithelia by rose bengal indicated the presence of a compromised mucosal barrier. To assess the role of galectin-3 in maintaining the integrity of the mucosal barrier, the barrier function assays were performed as described above, except that before incubation with rose bengal, the epithelial cells were preincubated with galectin inhibitors (β-lactose, modified citrus pectin (MCP)) and control saccharides (sucrose, maltose) in Dulbecco's modified Eagle's medium/F-12 at room temperature under gentle agitation for 1 h (the medium was changed every 15 min). For these experiments, five different field images were captured per well and two-to-four cultures were used per experimental condition. MCP was prepared by increasing the pH of citrus pectin (Sigma Aldrich; 0.5% in distilled H20) to pH 10.0 with 3 n NaOH for 30 min and then decreasing it to 3.0 with 3 n HCl for 10 h (23). The pH was then equilibrated to 6.3, and the solution was dialyzed against distilled H2O and lyophilized.
RESULTS
Transmembrane Mucins MUC1 and MUC16 Are Counter-receptors for Galectin-3
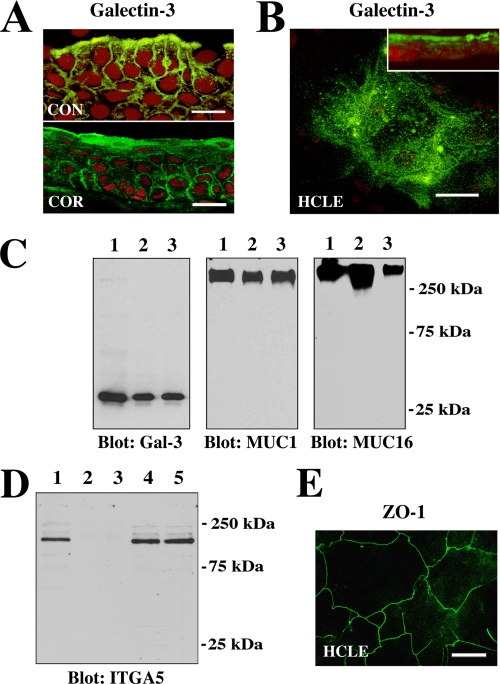
The presence of galectin-3 on apical membranes of human cornea and conjunctiva as well as in cultures of corneal epithelial cells was demonstrated by confocal immunofluorescence microscopy (Figs. 1, A and B). Galectin-3 was present throughout the entire epithelia in the tissue sections, being concentrated on the apical surface. In HCLE cells galectin-3 was observed primarily on apical membranes as seen in cell culture cross-sections (Fig. 1B, inset). To further confirm the apical location of galectin-3, cell surface proteins from HCLE cells were biotinylated and isolated by affinity chromatography on a neutravidin-agarose column. In these experiments galectin-3 was detected in the bound fraction eluted from the affinity column (Fig. 1C), confirming the apical localization of galectin-3. The biotinylation experiments also revealed the presence of the high molecular weight MUC1 and MUC16 mucins on apical cell membranes (Fig. 1C). The biotinylation was restricted to the apical surface proteins, as no labeling of ITGA5, a basolateral cell membrane glycoprotein in corneal epithelial cells, was detected in the bound fraction eluted from the affinity column (Fig. 1D, lanes 2 and 3). As shown in Fig. 1E, the HCLE cells express tight junction proteins in a pattern similar to that observed in normal corneal epithelium (24).
FIGURE 1.
Galectin-3 localizes at the apical surface of normal epithelia in vivo and in vitro. A, confocal immunofluorescence micrograph showing binding of the rat anti-galectin-3 antibody M3/38 (green) to apical membranes of apical epithelial cells in human conjunctival (CON) and corneal (COR) tissue cross-sections. Use of the rabbit anti-galectin-3 antibody H-160 resulted in a similar staining pattern (data not shown). Propidium iodide (red) was used to visualize the cell nuclei. Magnification bars = 20 μm. B, in HCLE cells, binding of the galectin-3 antibody was predominantly apical, as evidenced along the x-y axis (en face view) and in cell culture cross-sections (inset). Magnification bar = 20 μm. C, cell surface proteins on apical cell membranes of HCLE cells were biotinylated, purified through a neutravidin-agarose affinity column, and immunoblotted with anti-galectin-3, anti-MUC1, and anti-MUC16 antibodies. Control lanes were loaded with 15 μg of total protein from HCLE cell lysates (lane 1). Biotinylation experiments were performed in duplicate, and apical proteins were detected in the bound fraction eluted from the affinity column (lanes 2 and 3). D, to determine whether biotinylation was restricted to the apical cell surface, membrane extracts of labeled cells were analyzed for the presence of integrin α5 subunit (ITGA5), a basolateral cell membrane glycoprotein in corneal epithelium (19). In these experiments ITGA5 was not detected in the bound fraction eluted from the neutravidin affinity column (lanes 2 and 3) but was detected in the unfractionated extract (lane 1) as well as in the flow-through fractions (lanes 4 and 5). E, tight junctions in HCLE cells stained with the anti-ZO-1 antibody. The image was acquired along the x-y axis and corresponds to the distribution of tight junctions observed in normal corneal epithelial cells (24). Magnification bar = 50 μm.
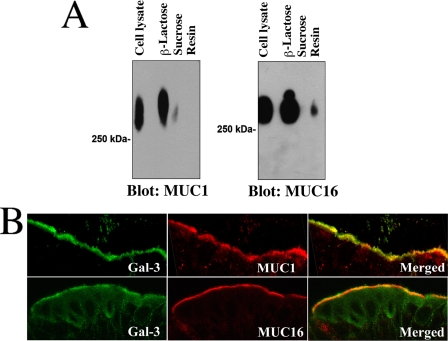
To assess whether cell surface-associated mucins directly associate with galectin-3, cell extracts were chromatographed on a galectin-3 affinity column. In these experiments both MUC1 and MUC16 bound to galectin-3 (Fig. 2A). The interaction between the cell surface mucins and galectin-3 was galactose-dependent, as MUC1 and MUC16 eluted from the galectin-3 affinity matrix with β-lactose but not sucrose. Also, in immunolocalization experiments, galectin-3 and the cell surface mucins MUC1 and MUC16 colocalized predominantly to the apical surface of human conjunctival epithelium (Fig. 2B).
FIGURE 2.
Cell surface mucins are counter-receptors for galectin-3 in polarized epithelial cells. A, HCLE cell culture lysates were applied to a galectin-3-agarose affinity column. After extensive washing, the column was eluted with 0.1 m β-lactose. To confirm sugar binding specificity of the galectin-3-binding proteins, the column was eluted with a non-competing disaccharide, 0.1 m sucrose, before elution with β-lactose. The presence of MUC1 (in the corresponding fractions) was detected by Western blot using the 214D4 antibody. β-Lactose effectively eluted MUC1 from the galectin-3 affinity column, indicating that MUC1-galectin-3 interaction was galactose-dependent. In contrast, little MUC1 was detected in the fraction eluted with sucrose. Similar results were obtained when protein blots were probed with OC125 antibody against MUC16, another cell surface-associated mucin expressed in HCLE cells. In these experiments some MUC16 was still detected in the galectin-3 affinity matrix (resin) after elution with β-lactose. B, galectin-3 colocalized with MUC1 and MUC16 in apical membranes of apical cells of the human conjunctival epithelium. Galectin-3 antibody binding was detected with a fluorescein isothiocyanate-conjugated secondary antibody (green), and the mucin antibody binding was detected using a TRITC-conjugated secondary antibody (red). The areas of colocalization of galectin-3 and MUC1 and MUC16 are shown in merged images.
Abrogation of Galectin Binding Impairs Mucosal Barrier Function
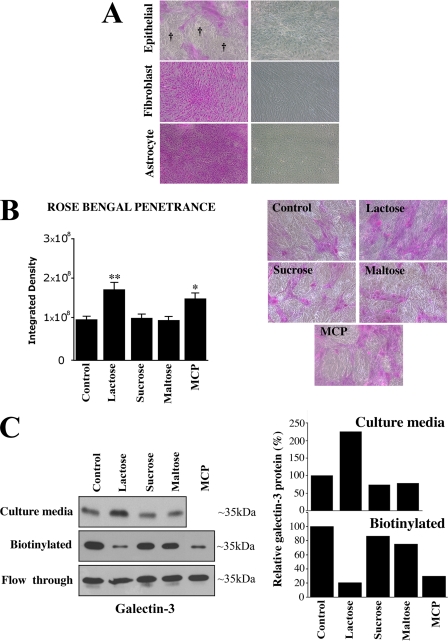
Pathological alteration of the mucosal barrier in human cornea and conjunctiva, such as keratoconjunctivitis sicca, allergy, and in patients with autoimmune disorders, is clinically evaluated by determining the extent of penetrance of the fluorescein-derivative rose bengal dye into the epithelium (25). As described earlier, in this assay, protection from rose bengal penetration into epithelial cells is indicative of the presence of a fully functional mucosal barrier, whereas penetration and positive staining of the epithelia by rose bengal is indicative of the presence of a compromised mucosal barrier. In in vitro assays, rose bengal penetrates undifferentiated epithelial cells that do not produce fully glycosylated cell surface mucins (21, 22). As shown in Fig. 3A, induction of epithelial cell differentiation, which is known to be associated with the robust expression of cell surface mucins (21), resulted in the appearance of islands of cells that excluded the rose bengal dye. These islands are not observed in non-mucin-producing cells such as corneal fibroblasts and brain astrocytes (Fig. 3A), which also express tight junction-specific proteins in vitro (26). To determine whether mucin-galectin interactions modulate epithelial barrier function, we evaluated dye penetrance in HCLE cells incubated with two well known competitive inhibitors of galectin binding, β-lactose and MCP. As shown in Fig. 3B, treatment of the epithelial cells with β-lactose and MCP resulted in an increase in rose bengal staining as compared with treatment with the non-inhibitory controls sucrose and maltose. In these experiments, increased rose bengal staining was associated with a marked decrease in levels of galectin-3 on the cell surface and an increase in lectin concentration in the culture media of HCLE cells treated with β-lactose (Fig. 3C), confirming that galectin-3 was released from the cell surface glycocalyx. Saccharide inhibitors used in the current study may also release other galectins. However, galectin-3 is the most abundant galectin present in the ocular surface epithelia (∼80% of the total galectin content (27)), and therefore, it is reasonable to expect that the effect of the inhibitors observed in the current study on rose bengal penetrance is likely to be in large part because of the release of galectin-3 itself.
FIGURE 3.
Competitive inhibition of galectin binding disrupts mucosal barrier. A, rose bengal was excluded from islands (†) of differentiated HCLE cells grown for 7 days in the presence of serum, conditions that induce biosynthesis of cell surface mucins and mucin-type O-glycans (21). On the other hand, the dye penetrated into cells that do not express cell surface mucins, such as corneal fibroblasts and brain astrocytes grown under the same conditions. Phase contrast images of cells before dye incubation are shown in the right panel. B, incubation of HCLE cells for 1 h with the galectin binding inhibitors β-lactose and MCP significantly enhanced the penetrance of rose bengal, whereas addition of sucrose, maltose, or media alone (control) did not (*, p < 0.05; **, p < 0.005). Representative micrographs are shown in the right panel. C, galectin-3 was analyzed in culture media and on the cell surface of HCLE cells after incubation for 1 h with galectin binding inhibitors and controls. To determine the presence of galectin-3 in cell culture media, culture supernatants were dialyzed and lyophilized, and non-biotinylated galectin-3 was analyzed by Western blot. To determine the presence of galectin-3 on the cell surface after harvesting the media, cell surface proteins were biotinylated, purified through a neutravidin-agarose affinity column, and immunoblotted with anti-galectin-3. Note the enrichment of non-biotinylated galectin-3 in the cell culture media and the reduction in biotinylated cell surface galectin-3 after incubation β-lactose and MCP. The amount of galectin-3 in the non-biotinylated/neutravidin flow-through did not differ under any of the experimental conditions used in this study. Experiments were performed in duplicate, and the amount of galectin-3 was quantified by densitometry (right) using ImageJ software.
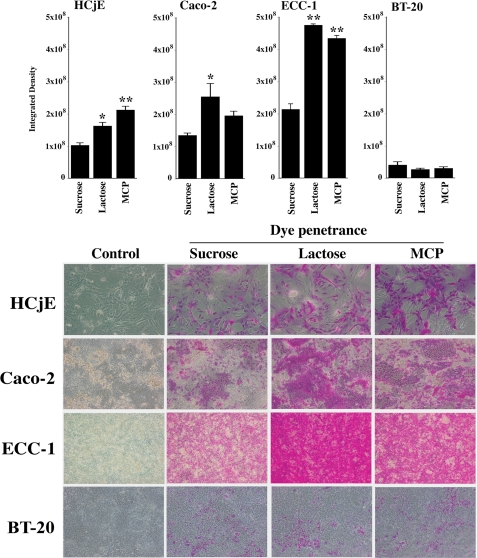
In addition to corneal epithelial cells, we evaluated the effect of β-lactose and MCP on rose bengal penetrance in other human mucin-producing epithelial cells, both normal and neoplastic. As shown in Fig. 4, dye penetrance increased in conjunctival (HCjE), colonic (Caco-2), and endometrial (ECC-1) epithelial cells (the latter two derived from adenocarcinomas) after treatment with both competitive inhibitors β-lactose and MCP but not with the control disaccharide sucrose. In each of the three cell types, there was an increase in the number of cells into which rose bengal penetrated after β-lactose and MCP treatment, and the staining appeared cytoplasmic.
FIGURE 4.
Rose bengal penetrance in normal (HCjE) and neoplastic (Caco-2, ECC-1, BT-20) epithelial cells. Different cell lines of epithelial origin were incubated for 1 h with the non-competitive inhibitor of galectin binding, sucrose, and with the competitive inhibitors, β-lactose and MCP, followed by rose bengal assay. Islands of cells excluding the rose bengal were observed in all epithelial cell lines grown for 7 days after confluence. Competitive inhibition of galactose-dependent surface interactions with β-lactose or MCP significantly increased rose bengal penetrance in HCjE, Caco-2, and ECC-1 cells but not in BT-20, suggesting that the galactose-dependent response of the epithelial cultures is cell type-specific (*, p < 0.01; **, p < 0.001). Representative micrographs are shown in the lower panel.
It is noteworthy that rose bengal penetrance in BT-20 mammary gland carcinoma epithelial cells did not significantly increase after the addition of galectin inhibitors as compared with control disaccharide, suggesting that the role of galactose-dependent interactions on barrier function is cell type-specific.
Silencing of c1galt1 Reduces Cell Surface O-Glycosylation with a Concomitant Loss of Cell Surface Galectin-3 and Epithelial Barrier Function
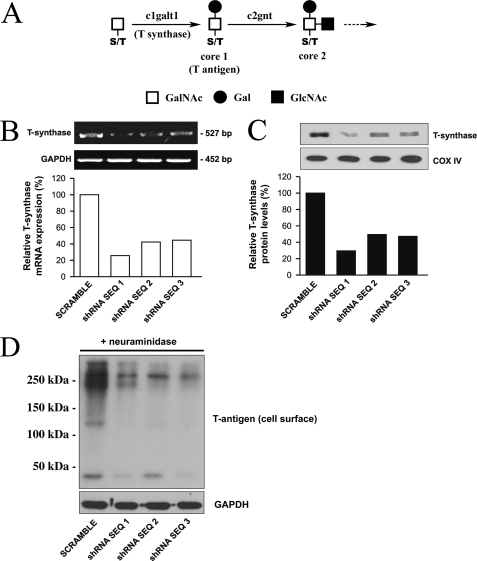
A critical step in mucin biosynthesis involves the synthesis of the core 1 disaccharide (Galβ1–3GalNAcα1-Ser/Thr), also known as the T-antigen or Thomsen-Friedenreich antigen. Core 1 is the most common core structure found on mucins, and it serves as a key precursor for all core 1 and core 2 mucin-type O-glycans (Fig. 5A). Unlike other carbohydrate structures that can be synthesized by several members of the same glycosyltransferase family, core 1 is synthesized by the action of a single glycosyltransferase, c1galt1 (also known as T-synthase or core 1 β3 galactosyltransferase), located on chromosome 7p14-p13 (28). This makes it possible to knock down the biosynthesis of T-antigen by inhibiting the expression of a single glycosyltransferase. T-antigen itself can serve as a natural ligand for galectin-3 in epithelial cells (29–32). In addition, it can serve as a substrate for the synthesis of extended glycan chains, which in turn could also function as ligands for galectin-3. To demonstrate that mucin O-glycans were involved in barrier formation and are responsible, at least in part, for cell surface localization of galectin-3, we generated a stable tetracycline-inducible RNA interfering cell line to knockdown c1galt1 as described under “Experimental Procedures.” Incubation of HCLE cells after stable expression of c1galt1 shRNA with doxycycline resulted in decreased levels of c1galt1 mRNA (% reduction: sequence 1 (74%), sequence 2 (58%), and sequence 3 (56%)) as compared with scramble control (Fig. 5B). Analyses of cell culture lysates from these cells also revealed that the amount of c1galt1 protein was reduced (% reduction: sequence 1 (71%), sequence 2 (51%), sequence 3 (53%)) as compared with scramble control (Fig. 5C). To confirm that down-regulation of c1galt1 mRNA and protein results in impaired mucin-type O-glycosylation, HCLE cells were biotinylated, and the cell membrane extracts, after elution from a neutravidin-agarose affinity column, were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with peanut agglutinin, a lectin that recognizes core 1 O-glycans bearing terminal galactose residues. The biotinylation assays on cell surface proteins from HCLE cells revealed that induction of c1galt1 shRNA resulted in substantial decrease of T-antigen at the cell surface (Fig. 5D), confirming the role of c1galt1 in modifying the O-glycan composition of the apical surface glycocalyx.
FIGURE 5.
Abrogation of c1galt1 impairs cell surface O-glycosylation. A, c1galt1 (T-synthase) adds galactose to N-acetylgalactosamine (GalNAc) on serine or threonine (S/T) residues to form core 1 O-glycans in the Golgi complex. Core 1 structures can be further processed by other glycosyltransferases, such as c2gnt that forms the core 2 structure. B, HCLE cells transfected with three shRNA sequences targeting the c1galt1 gene and a scramble control were treated with doxycycline (1 μg/ml). Total RNA (5.0 μg) was analyzed by reverse transcription-PCR. The c1galt1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (loading control) amplifications were performed for 25 and 30 cycles, respectively. Five microliters of PCR mixture were electrophoresed on 1.5% agarose gel and stained by ethidium bromide. Cells transfected with c1galt1 shRNA sequences, had reduced levels of c1galt1 mRNA as compared with cells transfected with scramble shRNA control. C, cell culture lysates collected after treatment with doxycycline (1 μg/ml) were resolved by SDS-PAGE. Ten micrograms of total lysates were subjected to Western blotting using antibodies against T-synthase and cytochrome c oxidase IV (COX IV), the latter serving as loading control. D, to detect the expression level of the T-antigen on cell surface glycoproteins in HCLE cells transfected with c1galt1 shRNA sequences, cell surface proteins were biotinylated and purified through a neutravidin-agarose affinity column, and the eluted material was probed with peanut agglutinin. Before the lectin blot, membranes were pretreated with neuraminidase from Arthrobacter ureafaciens (10 milliunits/ml) in sodium acetate 50 mm, pH 5.0, for 2 h at 37 °C to expose the T-antigen. Cells transfected with c1galt1 shRNA had reduced levels of the T-antigen as compared with scramble control. Glyceraldehyde-3-phosphate dehydrogenase served as a loading control.
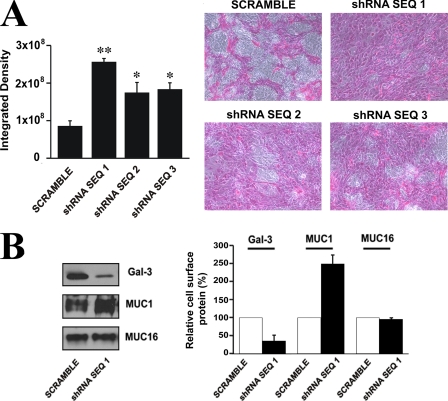
We next tested whether down-regulation of c1galt1 impairs barrier permeability in corneal epithelial cells. As shown in Fig. 6A, rose bengal uptake was significantly higher in cultures transfected with the three shRNA sequences to c1galt1 as compared with scramble control, confirming the role of mucin-type O glycans in epithelial barrier formation. Cells transfected with shRNA sequence 1, the most efficient in down-regulating c1galt1, were further analyzed for the expression level of cell surface galectin-3. Biotinylation assays revealed that the levels of galectin-3 protein on the surface of cells transfected with shRNA sequence 1 decreased as compared with scramble control (Fig. 6B), consistent with the role of the mucin-galectin-3 interactions in maintaining the cell surface barrier. As expected, abrogation of c1galt1 did not result in the reduction of MUC1 or MUC16 protein levels at the cell surface (Fig. 6B). In fact, increased levels of MUC1 protein at the cell surface was detected in the c1galt1 knockdown cells, suggesting that aberrant O-glycosylation alters the presentation of MUC1 on the cell surface and/or enhances recognition of the 214D4 antibody toward its antigen. Taken together, these results indicate that abrogation of c1galt1 alters the carbohydrate composition of the epithelial cell surface mucins, resulting in reduced cell surface galectin-3 and impaired mucosal barrier function.
FIGURE 6.
C1galt1 contributes substantially to epithelial barrier function. A, treatment of stable HCLE cell lines containing three c1galt1 shRNA sequences with doxycycline (1 μg/ml) for 4 days after reaching confluence resulted in increased permeability to the rose bengal diagnostic dye as compared with the scramble control (*, p < 0.05; **, p < 0.005). Representative micrographs are shown in the right panel. B, C1galt1 knockdown cells contain reduced levels of cell surface galectin-3. Cell surface proteins of HCLE cells transfected with c1galt1 shRNA sequence 1 were biotinylated, purified through a neutravidin-agarose affinity column, and analyzed by Western blot. A decrease in biotinylated galectin-3 was observed in cells transfected with c1galt1 shRNA as compared with the scramble control. In contrast, as expected, there was no reduction in the expression of biotinylated MUC1 and MUC16 cell surface proteins in the c1galt1 knockdown cells. Densitometric values (right) were normalized to scramble control. Experiments were performed in duplicate.
DISCUSSION
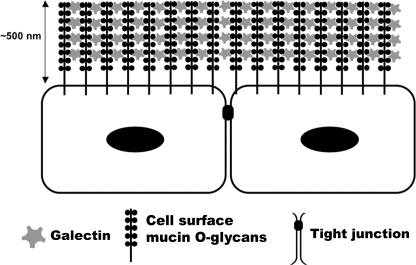
Cell surface mucins constitute major components of individual cell membranes on apical epithelial cells, where they can extend up to 500 nm above the plasma membrane; that is, far above all other membrane-associated proteins (33). Recent studies have provided direct evidence of the role of mucins in maintaining mucosal integrity. For instance, bacteria promptly crossed the gastrointestinal and ocular surface barriers in Muc1−/− mice, colonizing and inducing more epithelial damage than in wild-type animals (6, 8). Silencing MUC16 expression by siRNA in corneal epithelial cells also resulted in increased adherence of bacterial pathogens and increased permeability to rose bengal diagnostic dye (22). In addition, abrogation of mucin O-glycans has highlighted the role of specific glycan chains of mucins in maintaining mucosal barrier function. Mice lacking C3GnT, an enzyme involved in the biosynthesis of core-3-derived O-glycans (a major mucin O-glycan core in colon) showed increased permeability to low molecular weight fluorescein isothiocyanate-dextran and were more susceptible to experimental colitis (7). However, the mechanism by which mucin promotes barrier function is not well understood. Because of their high degree of glycosylation (up to 80% of the mucin mass is carbohydrate), mucins constitute potential binding partners for animal lectins. Our data provide a new model by which cell surface-associated mucins interact in a carbohydrate-dependent manner with galectin-3 on apical membranes to promote the formation of the intrinsic mucosal barrier (Fig. 7).
FIGURE 7.
Proposed model of galectin-mucin barrier formation on epithelial surfaces. Our data indicate that two membrane-associated mucins, MUC1 and MUC16, interact with galectin-3 on the epithelial glycocalyx of individual cell membranes to provide a barrier under physiological conditions. Soluble galectin-3 can form multivalent complexes that help to organize mucin assembly on the surface of the cell. The galectin-mucin barrier may regulate the transcellular flux of extracellular components into epithelial cells.
We demonstrated that (i) galectin-3 is expressed in robust amounts on apical membranes of corneal and conjunctival epithelia, (ii) galectin-3 on apical membranes colocalizes with MUC1 and MUC16, and (iii) both MUC1 and MUC16 bind to a galectin-3 affinity column in a carbohydrate-dependent manner. These findings lead us to conclude that MUC1 and MUC16 are counter-receptors of galectin-3 in ocular mucosal epithelia. Mucin-galectin-3 interactions have been shown to play a role in cancer progression by promoting cancer cell endothelial adhesion (29); however, the significance of the mucin-lectin interaction has thus far not been evaluated in the context of epithelial barrier function in normal and disease states. The most significant finding of the current study is that mucin-galectin-3 interactions contribute substantially to the integrity of mucosal epithelial barrier function. As assessed by rose bengal penetrance, the barrier function was disrupted after treatment with inhibitors of galectin-3 binding (β-lactose and MCP) but not with non-competitive saccharides in four of the five epithelial cell lines tested in this study. These data in conjunction with our findings that the disruption of the mucosal barrier by treatment with β-lactose is associated with reduced concentration of galectin-3 on the cell surface with a concomitant increase in galectin-3 concentration in media suggest that galectin-3 directly contributes to the integrity of the mucosal barrier. That galectin-3 interactions with mucins contribute to the integrity of the mucosal barrier is further suggested by our findings that targeted disruption of the c1galt1 gene, which plays a key role in the biosynthesis of core 1 O-glycans on mucosal surfaces, also resulted in disruption of the mucosal barrier with a concomitant reduction of galectin-3 on the apical cell surface.
Regarding the mechanism by which the mucin-galectin-3 interaction contributes to the integrity of the mucosal barrier, we propose that mucins, which are defined by the presence of amino acid tandem repeat domains with multiple O-glycan chains, form strong complexes with multivalent galectin-3 at the epithelial cell surface, as shown in the model in Fig. 7. This model is supported by (i) our findings showing galectin-3 binds to endogenous MUC1 and MUC16 under physiological conditions, (ii) published findings showing that galectin-3 promptly precipitates as a pentamer in the presence of multivalent N-acetyllactosamine ligands (a process mediated through the proline- and glycine-rich amino-terminal domain (34)), and (iii) studies showing that multivalent galectin-3 cross-links its counter-receptors (16, 17, 35, 36) and that the resulting galectin-ligand lattices on the cell surface are robust and resistant to lateral movement of membrane components (37).
Based on our data showing that treatment with saccharide inhibitors of galectin-3 binding disrupted the epithelial barrier in HCLE, HCjE, Caco-2, and ECC-1 cells, but not in BT-20 cells, it would appear that galectin-3 carbohydrate-mediated interactions modulate the mucosal barrier function in many, but not all, cell types.
Overall, the results of this study provide a new model in which association of epithelial cell surface mucins and galectins contributes to maintaining the intrinsic mucosal barrier. This model has potential applications to the understanding of barrier dysfunction in epithelial diseases. Alternatively, transient disruption of the mucin-galectin interaction could prove beneficial to the enhancement of drug delivery into mucosal surfaces for therapeutic purposes.
Acknowledgments
We thank Andrew Moore and Ashley Woodward for technical assistance and Drs. Zieske and Gerhardinger of the Schepens Eye Research Institute for providing human corneal fibroblasts and mouse brain astrocytes, respectively. We also appreciate the aid of Dr. Ilene Gipson and Ann Tisdale in the immunolocalization studies.
This work was supported, in whole or in part, by National Institutes of Health Grants R01EY014847 (to P. A.) and R01EY07088 (to N. P.) (NEI), and a challenge grant from Research to Prevent Blindness (to Tufts medical center).
- HCLE
- human corneal epithelial cells
- HCjE
- human conjunctival epithelial cells
- Caco-2
- colorectal carcinoma
- BT-20
- mammary gland carcinoma
- ECC-1
- endometrial epithelial cells
- MCP
- modified citrus pectin
- TRITC
- tetramethylrhodamine isothiocyanate
- PBS
- phosphate-buffered saline
- shRNA
- short hairpin RNA.
REFERENCES
- 1.Childers N. K., Bruce M. G., McGhee J. R. (1989) Annu. Rev. Microbiol. 43,503–536 [DOI] [PubMed] [Google Scholar]
- 2.Gipson I. K., Argueso P., Beuerman R., Bonini S., Butovich I., Dana R., Dartt D., Gamache D., Ham B., Jumblatt M., Korb D., Kruse F., Ogawa Y., Paulsen F., Stern M., Sweeney D. F., Tiffany J., Ubels J., Willcox M. (2007) Ocul. Surf. 5,179–19317508121 [Google Scholar]
- 3.Davies D. E. (2001) Curr. Allergy Asthma Rep. 1,127–133 [DOI] [PubMed] [Google Scholar]
- 4.Einerhand A. W., Renes I. B., Makkink M. K., van der Sluis M., Büller H. A., Dekker J. (2002) Eur. J. Gastroenterol. Hepatol. 14,757–765 [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth M. A., Swanson B. J. (2004) Nat. Rev. Cancer 4,45–60 [DOI] [PubMed] [Google Scholar]
- 6.Kardon R., Price R. E., Julian J., Lagow E., Tseng S. C., Gendler S. J., Carson D. D. (1999) Invest. Ophthalmol. Vis. Sci. 40,1328–1335 [PubMed] [Google Scholar]
- 7.An G., Wei B., Xia B., McDaniel J. M., Ju T., Cummings R. D., Braun J., Xia L. (2007) J. Exp. Med. 204,1417–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAuley J. L., Linden S. K., Png C. W., King R. M., Pennington H. L., Gendler S. J., Florin T. H., Hill G. R., Korolik V., McGuckin M. A. (2007) J. Clin. Invest. 117,2313–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuckin M. A., Every A. L., Skene C. D., Linden S. K., Chionh Y. T., Swierczak A., McAuley J., Harbour S., Kaparakis M., Ferrero R., Sutton P. (2007) Gastroenterology 133,1210–1218 [DOI] [PubMed] [Google Scholar]
- 10.Barondes S. H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K. (1994) Cell 76,597–598 [DOI] [PubMed] [Google Scholar]
- 11.Dumic J., Dabelic S., Flögel M. (2006) Biochim. Biophys. Acta 1760,616–635 [DOI] [PubMed] [Google Scholar]
- 12.Rabinovich G. A., Toscano M. A., Jackson S. S., Vasta G. R. (2007) Curr. Opin. Struct. Biol. 17,513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Z., Said N., Amin S., Wu H. K., Bruce A., Garate M., Hsu D. K., Kuwabara I., Liu F. T., Panjwani N. (2002) J. Biol. Chem. 277,42299–42305 [DOI] [PubMed] [Google Scholar]
- 14.Leffler H., Carlsson S., Hedlund M., Qian Y., Poirier F. (2004) Glycoconj. J. 19,433–440 [DOI] [PubMed] [Google Scholar]
- 15.Ho M. K., Springer T. A. (1982) J. Immunol. 128,1221–1228 [PubMed] [Google Scholar]
- 16.Lagana A., Goetz J. G., Cheung P., Raz A., Dennis J. W., Nabi I. R. (2006) Mol. Cell. Biol. 26,3181–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Partridge E. A., Le Roy C., Di Guglielmo G. M., Pawling J., Cheung P., Granovsky M., Nabi I. R., Wrana J. L., Dennis J. W. (2004) Science 306,120–124 [DOI] [PubMed] [Google Scholar]
- 18.Gipson I. K., Spurr-Michaud S. J., Argueso P., Tisdale A., Ng T. F., Russo C. L. (2003) Invest. Ophthalmol. Vis. Sci. 44,2496–2506 [DOI] [PubMed] [Google Scholar]
- 19.Stepp M. A., Spurr-Michaud S., Gipson I. K. (1993) Invest. Ophthalmol. Vis. Sci. 34,1829–1844 [PubMed] [Google Scholar]
- 20.Qin W., Zhou Q., Yang L. C., Li Z., Su B. H., Luo H., Fan J. M. (2005) J. Intern. Med. 258,467–477 [DOI] [PubMed] [Google Scholar]
- 21.Argüeso P., Tisdale A., Spurr-Michaud S., Sumiyoshi M., Gipson I. K. (2006) Invest. Ophthalmol. Vis. Sci. 47,113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blalock T. D., Spurr-Michaud S. J., Tisdale A. S., Heimer S. R., Gilmore M. S., Ramesh V., Gipson I. K. (2007) Invest. Ophthalmol. Vis. Sci. 48,4509–4518 [DOI] [PubMed] [Google Scholar]
- 23.Albersheim P., Neukom H. (1960) Arch. Biochem. Biophys. 90,46–51 [DOI] [PubMed] [Google Scholar]
- 24.Ryeom S. W., Paul D., Goodenough D. A. (2000) Mol. Biol. Cell 11,1687–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feenstra R. P., Tseng S. C. (1992) Ophthalmology 99,605–617 [DOI] [PubMed] [Google Scholar]
- 26.Bauer H., Stelzhammer W., Fuchs R., Weiger T. M., Danninger C., Probst G., Krizbai I. A. (1999) Exp. Cell Res. 250,434–438 [DOI] [PubMed] [Google Scholar]
- 27.Mantelli F., Schaffer L., Dana R., Head S. R., Argüeso P. (2009) Invest. Ophthalmol. Vis. Sci. 50,2666–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju T., Brewer K., D'Souza A., Cummings R. D., Canfield W. M. (2002) J. Biol. Chem. 277,178–186 [DOI] [PubMed] [Google Scholar]
- 29.Yu L. G., Andrews N., Zhao Q., McKean D., Williams J. F., Connor L. J., Gerasimenko O. V., Hilkens J., Hirabayashi J., Kasai K., Rhodes J. M. (2007) J. Biol. Chem. 282,773–781 [DOI] [PubMed] [Google Scholar]
- 30.Glinsky V. V., Glinsky G. V., Rittenhouse-Olson K., Huflejt M. E., Glinskii O. V., Deutscher S. L., Quinn T. P. (2001) Cancer Res. 61,4851–4857 [PubMed] [Google Scholar]
- 31.Bresalier R. S., Byrd J. C., Wang L., Raz A. (1996) Cancer Res. 56,4354–4357 [PubMed] [Google Scholar]
- 32.Khaldoyanidi S. K., Glinsky V. V., Sikora L., Glinskii A. B., Mossine V. V., Quinn T. P., Glinsky G. V., Sriramarao P. (2003) J. Biol. Chem. 278,4127–4134 [DOI] [PubMed] [Google Scholar]
- 33.Hilkens J., Ligtenberg M. J., Vos H. L., Litvinov S. V. (1992) Trends Biochem. Sci. 17,359–363 [DOI] [PubMed] [Google Scholar]
- 34.Ahmad N., Gabius H. J., André S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C. F. (2004) J. Biol. Chem. 279,10841–10847 [DOI] [PubMed] [Google Scholar]
- 35.Inohara H., Raz A. (1995) Cancer Res. 55,3267–3271 [PubMed] [Google Scholar]
- 36.Delacour D., Greb C., Koch A., Salomonsson E., Leffler H., Le Bivic A., Jacob R. (2007) Traffic 8,379–388 [DOI] [PubMed] [Google Scholar]
- 37.Nieminen J., Kuno A., Hirabayashi J., Sato S. (2007) J. Biol. Chem. 282,1374–1383 [DOI] [PubMed] [Google Scholar]