Abstract
p120 catenin is a major regulator of cadherin stability at cell-cell contacts and a modulator of Rho GTPase activities. In C2C12 myoblasts, N-cadherin is stabilized at cell contacts through its association with cholesterol-rich membrane domains or lipid rafts (LR) and acts as an adhesion-activated receptor that activates RhoA, an event required for myogenesis induction. Here, we report that association of p120 catenin with N-cadherin at cell contacts occurs specifically in LR. We demonstrate that interaction of p120 catenin with N-cadherin is required for N-cadherin association with LR and for its stabilization at cell contacts. LR disruption inhibits myogenesis induction and N-cadherin-dependent RhoA activation as does the perturbation of the N-cadherin-p120 catenin complex after p120 catenin knockdown. Finally, we observe an N-cadherin-dependent accumulation of RhoA at phosphatidylinositol 4,5-bisphosphate-enriched cell contacts which is lost after LR disruption. Thus, a functional N-cadherin-catenin complex occurs in cholesterol-rich membrane microdomains which allows the recruitment of RhoA and the regulation of its activity during myogenesis induction.
Skeletal myogenesis is a multistep process regulated by diffusible molecules and the interaction of muscle cell precursors with their neighbors and the extracellular matrix (1, 2). Particularly, N-cadherin-dependent intercellular adhesion has a major role in cell cycle exit and induction of skeletal muscle differentiation through activation of the Rho family GTPases. RhoA positively regulates MyoD expression and skeletal muscle differentiation because it is required for serum response factor-mediated activation of several muscle-specific gene promoters (3, 4).
Dynamic association of cadherin complexes at the plasma membrane (PM)4 is crucial for cadherin-mediated signaling. Their extracellular domain mediates homophilic cell-cell adhesion, whereas the intracellular domain associates with catenins that produce attachment sites for the F-actin cytoskeleton (5–7). The juxtamembrane domain of the cadherin cytoplasmic tail binds to p120 catenin, which regulates cadherin stability at cell contacts and modulates Rho GTPase activities (8–11). Cadherin stability is directly dependent on p120 catenin, and in its absence most cadherins are internalized and often degraded, suggesting that p120 catenin controls cadherin turnover at the cell surface (11, 12). Moreover, mutations in the E-cadherin region that bind to p120 catenin dissociate the E-cadherin-p120 catenin complex and disrupt strong cell adhesion, although interaction with other catenins remains intact (13). Cadherin stability at cell-cell contacts is also regulated by homophilic binding between extracellular domains and association with the F-actin cytoskeleton (14, 15). Association of N-cadherin with cholesterol-enriched microdomains, called lipid rafts (LR), at cell contacts, also stabilizes N-cadherin (16). Because p120 catenin interaction with cadherins and N-cadherin association with LR at cell contact sites are both involved in cadherin stability at cell contact sites, we asked whether p120 catenin association with N-cadherin required LR. We observed that their association occurred specifically in these cholesterol-rich domains. Moreover, using an N-cadherin mutant unable to bind to p120 catenin, we showed that the N-cadherin/p120 catenin interaction was required for N-cadherin association with LR and its stabilization at cell contacts. Because N-cadherin is implicated in the commitment to myogenesis through RhoA activation, we questioned whether its association with p120 catenin in LR was a prerequisite for RhoA activation. LR disruption inhibited myogenesis induction, association of p120 catenin with N-cadherin, and N-cadherin-dependent RhoA activation, as did the perturbation of the N-cadherin-p120 catenin complex after p120 catenin knockdown. Together, these data suggest a crucial role for the N-cadherin/p120 catenin association in LR in the regulation of RhoA activity during myogenesis induction.
EXPERIMENTAL PROCEDURES
Cell Lines
C2C12 myoblasts were cultured at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium/F-12 supplemented with 10% fetal calf serum; mouse L cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum.
p120 catenin knockdown C2C12 cell lines were obtained after infection with pRS human p120 catenin small interfering RNA or mouse p120 catenin small interfering RNA retroviruses (11). Different clones were isolated by limited dilution and grown in 3–5 μg/ml puromycin.
Drug Treatment, Transfection, and Immunocytochemistry
Cells were treated with amphotericin B (ampho B) (25 μg/ml for 4 h; Sigma), methyl-β-cyclodextrin (4 mm for 6 h; Sigma), or cholesterol oxidase (CO) (1 unit/ml for 2 h; Calbiochem) in culture medium containing 10 or 2% delipidized serum (Sigma-Aldrich). C2C12 myoblasts and L cells were transfected with N-cadherin-GFP, N-cadherin-RFP, N-cadherinAAA-YFP, PH-PLCδ-GFP, and RhoAWT-RFP by jetPEI (Qbiogen) according to the manufacturer's instructions. Ganglioside GM1 patching was performed as described (16).
Cells were fixed for 5 min in 4% paraformaldehyde (in phosphate-buffered saline) followed by a 2-min permeabilization in 0.1% Triton X-100 (in phosphate-buffered saline) and incubation in phosphate-buffered saline containing 1% bovine serum albumin. Primary antibodies were p120 catenin (1:100; BD Transduction Laboratories), N-cadherin (1:200; BD Transduction Laboratories), myogenin (1:30; Santa Cruz Biotechnology), and troponin T (1:100; Sigma). Secondary antibodies were Alexa Fluor 350-, Alexa Fluor 546-, or Alexa Fluor 488-conjugated goat anti-mouse antibody and Alexa Fluor 488-conjugated goat anti-rabbit antibody (Molecular Probes). Cells were stained for F-actin with tetramethylrhodamine B isothiocyanate or coumarin isothiocyanate-conjugated phalloidin (Sigma-Aldrich), and nuclei were stained with Hoechst (0.1 μg/ml; Sigma-Aldrich). Images were captured with a MicroMax 1300 CCD camera (RS-Princeton Instruments, Trenton, NJ) driven by MetaMorph software (version 7, Universal Imaging Corp., Westchester, PA). Images were processed using Adobe Photoshop and Adobe Illustrator.
Isolation of Detergent-resistant Membrane Fractions and Immunoprecipitation
Cell lysates or PM-enriched fractions of C2C12 or L cells transfected or not with N-cadherin-GFP or N-cadherinAAA-YFP were fractionated through a 4-ml sucrose gradient as described (16). Fractions enriched in PM were prepared as described (16).
Pooled fractions 3–5 (LR fractions) and 8–10 (nonlipid raft (NLR) fractions) or fractions enriched in PM were analyzed by immunoblotting for the presence of N-cadherin, α-, β-, γ-, and p120 catenins, caveolin (antibodies from BD Transduction Laboratories), actin (Sigma-Aldrich), or transferrin receptor (Zymed Laboratories Inc.). Alternatively, LR and NLR fractions were also used for immunoprecipitation with anti-N-cadherin antibodies. In this case, fractions 3–5 were pooled and diluted 5-fold in 25 mm MOPS, pH 6.5, 150 mm NaCl, 1% Triton X-100 and then centrifuged at 4 °C at 100,000 × g for 18 h. Pellets were resuspended in 10 mm PIPES, pH 7, 100 mm NaCl, 300 mm sucrose, 3 mm MgCl2, 0,5% Nonidet P-40, 1 mm EDTA, 1 mm orthovanadate, 60 mm N-octylglucoside.
Protein concentration was determined with the BCA protein assay kit (Pierce). C2C12 myoblasts expressing N-cadherinAAA-YFP were lysed, and extracts were immunoprecipitated using an anti-GFP antibody (1:100; Roche Applied Science) and processed as described (17).
RhoA GTPase Activity Assay
Parental or p120 catenin shRNA C2C12 myoblasts were lysed and processed to measure the total and GTP-bound RhoA levels as described previously (18). Alternatively, C2C12 myoblasts plated on N-cadherin-Fc chimera-coated dishes obtained as described (18) were used.
Fluorescence Recovery after Photobleaching (FRAP)
Lateral diffusion coefficients and mobile fractions of N-cadherin-GFP and N-cadherinAAA-YFP expressed in mouse L cells were measured by FRAP using a Zeiss LSM Meta 510 confocal microscope as described (16).
RESULTS
p120 Catenin Association with N-cadherin Occurs in Cholesterol-rich Membrane Domains
To dissect the mechanisms of catenin association with N-cadherin, we questioned whether the localization in cholesterol-rich membrane microdomains influenced cadherin-catenin complex formation. Therefore, we co-immunoprecipitated similar amounts of proteins from either pooled fractions 3–5 (corresponding to LR fractions) or pooled fractions 8–10 (corresponding to NLR fractions) obtained from Triton X-100 lysates loaded onto sucrose density gradients (16). Although α-, β-, and γ-catenins associated with N-cadherin in both LR and NLR fractions, p120 catenin interacted with N-cadherin only in LR fractions (Fig. 1A). To confirm that the interaction of p120 catenin with N-cadherin was impossible in NLR fractions, we repeated the experiment but using a 10-fold higher amount of proteins from NLR fractions. Again, p120 catenin did not interact with N-cadherin, whereas α-, β-, and γ-catenins strongly associated with N-cadherin under these conditions (Fig. 1B, panel a). We confirmed that N-cadherin, β-catenin, and p120 catenin were present in both LR and NLR fractions (Fig. 1B, panel b). To confirm further the co-distribution of N-cadherin and p120 catenin in LR, we used a technique based on lateral cross-linking of the raft-associated ganglioside GM1 (19). Patching of GM1 with the cholera toxin B subunit and antibodies against cholera toxin B resulted in co-patching of both N-cadherin and p120 catenin (Fig. 1C). Moreover, LR disruption by cholesterol chelation through the addition of ampho B impaired p120 catenin association with N-cadherin monitored by immunoprecipitation without modifying their concentration (Fig. 1D, panels a and b). This result contrasts with previous data showing that CO treatment did not affect p120 catenin association with N-cadherin, although it perturbed N-cadherin accumulation and stabilization at cell-cell contacts (16). Thus, we carefully reinvestigated the effect of three drugs (i.e. ampho B, CO, and methyl-β-cyclodextrin) that affect cholesterol and confirmed that LR disruption by these compounds efficiently perturbed p120 catenin association with N-cadherin (data not shown). Nevertheless, data from cholesterol depletion experiments must be interpreted with caution because interactions between LR and F-actin cytoskeleton exist, and association of N-cadherin with LR requires F-actin cytoskeleton (16). These data suggest that p120 catenin associates with N-cadherin specifically in LR and that LR are essential for p120 catenin/N-cadherin association.
FIGURE 1.
p120 catenin interacts with N-cadherin exclusively in LR. A, C2C12 myoblasts were lysed in 1% Triton X-100 and fractionated on a sucrose gradient. Fractions 3–5 (LR) and fractions 8–10 (NLR) were pooled. 30 μg of each fraction was immunoprecipitated (IP) using an anti-N-cadherin antibody and probed for α-, β-, γ-, and p120 catenin. WB, Western blotting. B, panel a, NLR fractions (300 μg) were immunoprecipitated using an anti-N-cadherin antibody and tested for α-, β-, γ-, and p120 catenin. Panel b, cell lysates of C2C12 myoblasts (10 μg), LR (2 μg), and NLR fractions (10 μg) were probed for N-cadherin (top panel) and p120 catenin expression (bottom panel). Results are representative of three independent experiments. C, GM1 was labeled with rhodamine-conjugated cholera toxin B subunit and subsequently patched by the addition of a secondary antibody (panel a). Distributions of N-cadherin-GFP (panel b), p120 catenin (panel c), or merged image with the patched GM1 are shown (panel d). Arrows indicate some GM1 patches in which N-cadherin and p120 catenin are found. Scale bar, 5 μm. D, panel a, cell lysates of C2C12 myoblasts treated with ampho B (Amph. B), or not, were immunoprecipitated using an anti-N-cadherin antibody and probed for the presence of p120 catenin. Panel b, 20 μg of cell lysates of C2C12 myoblasts treated with ampho B, or not, were probed for p120 catenin and N-cadherin.
Disruption of p120 Catenin Binding to N-cadherin Impairs N-cadherin Association with LR and Destabilizes N-cadherin at Cell Junctions
To analyze whether p120 catenin binding to N-cadherin was involved in the recruitment of N-cadherin in LR, we used an N-cadherin mutant with a triple alanine mutation in the juxtamembrane domain (N-cadherinAAA) that abolishes binding to p120 catenin (20). Although N-cadherinAAA did not bind to p120 catenin, it surprisingly co-localized with p120 catenin in C2C12 myoblasts (data not shown). This unexpected observation was actually explained by the formation of heterodimers between N-cadherinAAA and endogenous wild-type (WT) N-cadherin that we detected in immunoprecipitation experiments (data not shown). Thus, to examine the p120 catenin contribution to the recruitment of N-cadherin into LRs, we decided to pursue our experiments using the N-cadherinAAA mutant in mouse L cells that do not express endogenous cadherins. Indeed, in these cells, the problem of heterodimerization between WT N-cadherin and N-cadherinAAA was excluded.
In L cells, N-cadherin-GFP accumulated at cell-cell contacts (Fig. 2A, panel a) as it does in C2C12 myoblasts (Fig. 2C, panel b). Conversely, N-cadherinAAA-YFP was rarely at cell contacts in L cells (Fig. 2A, panel c). This difference in N-cadherin-GFP and N-cadherinAAA-YFP localization in L cells was not because of a variation in p120 catenin expression level (Fig. 2A, right panel). We analyzed the recruitment of N-cadherin-GFP and N-cadherinAAA-YFP in LR by isolating LR from enriched PM preparations on sucrose gradient. We observed a similar amount of N-cadherin-GFP and N-cadherinAAA-YFP at the PM by Western blotting (Fig. 2B, panel a) and by monitoring the level of PM-associated N-cadherin-GFP and N-cadherinAAA-YFP by cell surface biotinylation (data not shown). Conversely, in pooled fractions 3–5 (corresponding to LR fractions) we detected N-cadherin-GFP but not N-cadherinAAA-YFP (Fig. 2B, panel b). Finally, we analyzed the distribution of N-cadherin-GFP and the LR marker ganglioside GM1 in parental and p120 catenin shRNA C2C12 myoblasts (see Fig. 6, A and B, for a description of these cells). In parental cells, both N-cadherin and p120 catenin co-localized at cell-cell contacts with GM1, in agreement with their common distribution and association in biochemically isolated LR (6) (see also Fig. 1) (Fig. 2C). In p120 catenin shRNA myoblasts, N-cadherin neither accumulated nor co-localized with GM1 at cell-cell contacts. These data indicate that recruitment of N-cadherin in LR requires interaction with p120 catenin.
FIGURE 2.
Disruption of p120 catenin binding to N-cadherin impairs its association with LR. A, mouse L cells transfected with plasmids encoding N-cadherin-GFP (Ncad/GFP) or N-cadherinAAA-YFP (NcadAAA/YFP) were fixed 20 h after transfection and monitored for fluorescent protein and p120 catenin distribution. N-cadherinAAA and p120 catenin do not accumulate at cell contacts (arrowheads in panels c and d), whereas N-cadherin and p120 catenin do (arrows in panels a and b). Scale bar, 10 μm. The graph shows the percentage of cells with N-cadherin localization at cell contacts. Results are shown as mean ± S.E. of three independent experiments (50 cells were counted for each condition). Cell lysates (20 μg) of L cells expressing N-cadherin-GFP or N-cadherinAAA-YFP were probed for p120 catenin and α-tubulin expression. B, panel a, 30-μl fractions enriched in PM obtained from L cells expressing N-cadherin-GFP or N-cadherinAAA-YFP were probed for GFP and transferrin receptor (TfR) expression. Panel b, PM fractions from L cells expressing N-cadherin-GFP or N-cadherinAAA-YFP were fractionated through sucrose gradients. Fractions 3–5 (i.e. LR) were pooled and probed for N-cadherin-GFP and N-cadherinAAA-YFP expression with an anti-GFP antibody. Actin and Tfr are loading controls. Results are representative of three independent experiments. C, analysis of the distribution of the LR marker ganglioside GM1 by using rhodamine-conjugated cholera toxin B (panels a and e) and of p120 catenin (panels c and g) in control and p120 catenin shRNA C2C12 myoblasts expressing N-cadherin-GFP (panels b and f). Panels d and f, merge of N-cadherin-GFP and GM1. Arrows indicate cell-cell contacts displaying GM1 and N-cadherin co-localization. Scale bar, 10 μm.
FIGURE 6.
Effect of p120 catenin silencing on myogenic differentiation and RhoA activation. A, the expression of p120 catenin, N-cadherin, and α-tubulin is shown in one representative clone and parental C2C12 myoblasts. B, p120 catenin (panels b and d) and N-cadherin (panels a and c) expression in parental myoblasts (panels a and b) and in one representative p120 catenin shRNA clone (panels c and d). C, cell lysates (20 μg/well) of parental and p120 catenin shRNA myoblasts were cultured in DM for 4 days and probed for myogenin, troponin T, and α-tubulin expression. D, phase-contrast images of parental (panel a) and p120 catenin shRNA myoblasts (panel b) after 4 days in DM. E, F-actin expression in parental (panel a) and p120 catenin shRNA myoblasts (panel b). Scale bar, 10 μm. F, GTP-bound RhoA was measured using GST-Rho-binding domain of the RhoA effector Rhotekin in lysates from parental (left panels) and p120 catenin shRNA (right panels) C2C12 cells grown in growth medium (GM) or DM for 6 h. RhoA was detected by immunoblotting. Data are representative of three independent experiments. G, parental, p120 catenin shRNA and p120 catenin shRNA myoblasts expressing RFP-tagged RhoAWT were cultured in DM for 2 days and probed for myogenin expression by immunohistochemistry. The histogram represents the percentage of myogenin-positive cells and summarizes the data from three independent sets of experiments; 60–70 cells were analyzed in each experiment.
We showed previously that N-cadherin association with LR allows its stabilization at cell-cell contacts, enabling the formation of a functional adhesive complex (16). To analyze the role of p120 catenin binding in N-cadherin stabilization at the cell contacts, we compared the assembly dynamics of N-cadherin-GFP and N-cadherinAAA-YFP using fluorescent recovery after FRAP experiments. We measured the diffusion coefficients and mobile fractions of N-cadherin-GFP and N-cadherinAAA-YFP at cell-cell contacts of living L cells. We found a 2-fold increase in the lateral diffusion of N-cadherinAAA-YFP compared with N-cadherin-GFP (Fig. 3, A and B). Moreover, the N-cadherinAAA-YFP mobile fraction was bigger than that of N-cadherin-GFP, indicating that N-cadherinAAA-YFP was free to diffuse in the PM for the entire duration of the experiment. Typical images of FRAP experiments from N-cadherin-GFP- and N-cadherinAAA-YFP-expressing cells are shown in Fig. 3C. Altogether, these data suggest that association of p120 catenin with N-cadherin participates in the recruitment of this cadherin to LR and in its immobilization during contact establishment.
FIGURE 3.
Disruption of p120 catenin binding to N-cadherin increases its mobile pool and lateral mobility at cell-cell contact sites. A, shown are the first 95 s of photobleach recovery curves of N-cadherin-GFP (Ncad/GFP) and N-cadherinAAA-YFP (NcadAAA/YFP). The solid lines represent the best fit to the lateral diffusion equation as described in Ref. 16. B, diffusion coefficients and mobile fractions of N-cadherin-GFP and N-cadherinAAA-YFP in membranes involved in cell contacts. Significance (by paired Student's t test) is shown on the graph. C, shown are typical images of a FRAP experiment. Fluorescence of selected area (circles) of N-cadherin or N-cadherinAAA-dependent cell-cell contacts (visualized in the left panels) was photobleached (the first image recorded after bleaching is marked by an asterisk), and fluorescence recovery was measured with time.
LR Disruption Prevents Myogenesis Induction and RhoA Activation
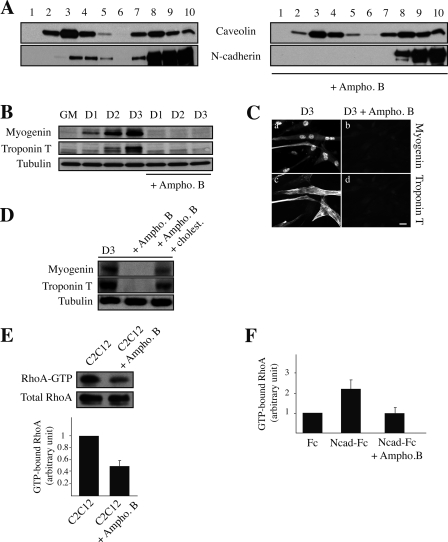
We showed previously that LR disruption leads to inhibition of cell-cell adhesion and disorganization of N-cadherin- dependent cell-cell contacts (16). We now analyzed the effect of LR disruption on myogenesis. Treatment of C2C12 myoblasts with ampho B impaired N-cadherin association with LR (Fig. 4A) as observed after the addition of CO or methyl-β-cyclodextrin (16). We next examined whether LR disruption could affect the expression of muscle-specific proteins by Western blot analysis and immunocytochemistry. Although parental C2C12 myoblasts expressed myogenin and troponin T after the addition of differentiation medium (DM), C2C12 myoblasts treated with ampho B (Fig. 4, B and C, respectively) or with CO or methyl-β-cyclodextrin (data not shown) did not. We were also unable to detect myotube formation (data not shown). The addition of culture medium with cholesterol restored myogenesis (Fig. 4D).
FIGURE 4.
Effect of LR disruption on myogenesis and RhoA activation. A, C2C12 myoblasts treated with ampho B (Ampho. B, right panel) or mock-treated (left panel) were fractionated on sucrose gradients. 30 μl of each fraction was analyzed for N-cadherin and caveolin distribution by immunoblotting. B, cell lysates (20 μg/well) of C2C12 myoblasts treated with ampho B, or not, cultured in growth medium (GM) or DM as indicated (D = day) were assessed by Western blot analysis for myogenin, troponin T, and α-tubulin expression. C, C2C12 myoblasts treated with ampho B, or not, cultured in DM for 3 days were analyzed by immunocytochemistry for myogenin and troponin T. Scale bar, 10 μm. D, C2C12 myoblasts cultured in DM containing ampho B alone or ampho B and cholesterol (cholest.) for 3 days were analyzed by Western blotting for myogenin, troponin T, and α-tubulin expression. E, GTP-bound RhoA level was measured using glutathione S-transferase fused to the Rho-binding domain of the RhoA effector Rhotekin (GST-RBD) in lysates obtained from C2C12 myoblasts treated with ampho B or not. RhoA was detected by immunoblotting. The histogram shows a quantification of the results of three independent experiments. F, GTP-bound RhoA was measured in lysates obtained from C2C12 myoblasts plated on surfaces coated with either anti-Fc antibody or N-cadherin-Fc (Ncad-Fc) and treated with ampho B or not. The histogram represents GTP-bound RhoA after normalization to the amount of total RhoA protein. Results are the mean of three independent experiments.
Treatment with ampho B (Fig. 4E) or CO (data not shown) also led to a marked decrease in RhoA activity, known to be essential for myogenesis (18, 21). We thus asked whether LR disruption specifically impaired the increase in RhoA activity mediated by N-cadherin adhesion. We measured RhoA activity by pulldown assays in C2C12 myoblasts plated onto dishes coated with either anti-Fc antibody or N-cadherin-Fc ligand, which allowed us to mimic N-cadherin-mediated adhesion (18). LR disruption by incubation with ampho B again decreased RhoA activity (Fig. 4F), which was restored by the addition of culture medium with cholesterol (data not shown). These experiments clearly show that LR are involved in the activation of RhoA downstream of N-cadherin.
Phosphatidylinositol 4,5-Bisphosphate (PI(4,5)P2) and RhoA Are Found at Sites of N-cadherin Adhesion in a p120 Catenin-dependent Manner
We next wanted to analyze at the cell-cell contacts the content in lipid second messengers known to play a major role in protein recruitment and activation. Thus, we investigated whether PI(4,5)P2, a phosphoinositide known to be accumulated in LR (22, 23), was present at the N-cadherin-mediated cell-cell contacts. C2C12 myoblasts were transfected with a construct expressing the PH domain of PLCδ fused to GFP (PH-PLCδ-GFP) that specifically binds to PI(4,5)P2 (24). At cell contacts in C2C12 myoblasts, PI(4,5)P2 accumulated and co-localized with N-cadherin (Fig. 5A, panels a and b). This co-localization was lost after Ca2+ chelation with EGTA (panels c and d) and after LR disruption (data not shown). In L cells, PI(4,5)P2 accumulated at cell contacts only when N-cadherin was expressed (Fig. 5C, compare panel a with d). In the absence of N-cadherin expression, PI(4,5)P2 was found in F-actin-rich polymerization areas (panels a and b). LR disruption impaired the recruitment of PI(4,5)P2 at N-cadherin-dependent cell-cell contacts (panels f–h). We also investigated whether PI(4,5)P2 localization at cell-cell contacts correlated with RhoA accumulation in the same place. For this purpose, C2C12 myoblasts were co-transfected with PH-PLCδ-GFP and RhoAWT-RFP. In isolated C2C12 myoblasts, we observed no co-localization between RhoA and PI(4,5)P2 (Fig. 5B, panels a–d), whereas in contacting cells in which N-cadherin was engaged in cell-cell contacts, RhoA and PI(4,5)P2 were co-localized (panels e–h). Treatment with ampho B impaired co-localization of RhoA and PI(4,5)P2 in contacting C2C12 myoblasts (data not shown). In L cells, RhoA was not accumulated at cell-cell contacts (Fig. 5D, panel a), whereas it did so after N-cadherin expression (panels b–d). Again, LR disruption impaired the recruitment of RhoA at N-cadherin-dependent cell-cell contacts (panels e–g). Conversely, N-cadherinAAA did not recruit RhoA at cell contacts (Fig. 5E). Altogether, these data show that N-cadherin engagement and its subsequent recruitment in LR allow PI(4,5)P2 localization at cell-cell contacts, enabling RhoA accumulation at this place.
FIGURE 5.
PI(4,5)P2 and RhoA are found at sites of N-cadherin adhesion. A, C2C12 myoblasts transfected with PH-PLCδ-GFP (panels b and d) were treated with EGTA (panels c and d). Cells were stained for N-cadherin (panels a and c). Scale bar, 10 μm. B, C2C12 myoblasts transfected with PH-PLCδ-GFP and RhoAWT-RFP. In confluent conditions (panels e–h) PH-PLCδ-GFP and RhoAWT-RFP co-localize at cell contact sites (arrows in panel g), whereas they do not in isolated myoblasts (panels a–d). Scale bar, 10 μm. C, L cells transfected with PH-PLCδ-GFP alone (panel a) or together with N-cadherin-RFP (panels c and d) were stained for F-actin (panels b and e). PH-PLCδ-GFP is located in F-actin-rich structures (lamellipodia) in L cells (panel a) and is recruited at the cell contacts when N-cadherin-RFP is expressed (panel d, arrows). Recruitment at cell contacts is lost after LR disruption (panels f–h). Scale bar, 10 μm. D, L cells were transfected with RhoAWT-RFP alone (panel a) or together with N-cadherin-GFP (panels b and c). RhoAWT-RFP is recruited at cell contacts when N-cadherin-GFP is expressed, and they co-localize (panel d). This is lost after LR disruption (panels e–g). Scale bar, 10 μm. E, L cells were co-transfected with N-cadherinAAA-YFP (panel a) and RhoAWT-RFP (panel b). Scale bar, 10 μm.
Perturbation of N-cadherin-Catenin Complexes Inhibits Myogenesis Induction and RhoA Activation
To investigate further the role of N-cadherin-p120 catenin complexes in RhoA activation and myogenesis induction, we generated stable C2C12 cell lines in which the expression of p120 catenin was inactivated by RNA interference (p120 catenin shRNA) (Fig. 6, A and B). Parental and C2C12 myoblasts expressing human p120 catenin shRNA (hp120 catenin shRNA) were used as controls (data not shown). As reported previously (11, 25), we observed that p120 catenin silencing led to a decrease in N-cadherin expression (Fig. 6, A and B). We next examined whether p120 catenin silencing affected the expression of myogenin and troponin T (Fig. 6C). After 4 days in DM, parental C2C12 myoblasts expressed myogenin and troponin T, whereas p120 catenin shRNA myoblasts did not. In addition, we observed many myotubes in parental (Fig. 6D, panel a) and control hp120 catenin shRNA cells (data not shown) but only few in the p120 catenin shRNA myoblasts (Fig. 6D, panel b).
Because N-cadherin-mediated adhesion activates RhoA, which allows myogenesis induction (18, 21), we analyzed the effect of p120 catenin silencing on RhoA activity in parental or p120 catenin shRNA C2C12 myoblasts. We first used the organization of the F-actin cytoskeleton as a functional read-out. No modification was observed in either parental or p120 catenin shRNA C2C12 myoblasts (Fig. 6E). We then analyzed RhoA activity using pulldown assays. We observed an increase in the RhoA GTP level after DM addition in parental but not in p120 catenin shRNA C2C12 myoblasts (Fig. 6F). To confirm the involvement of RhoA in myogenesis inhibition after p120 catenin knockdown, we transfected p120 catenin shRNA C2C12 myoblasts with an RFP-tagged construct expressing RhoA WT. Cells were cultured in DM, fixed, and analyzed for expression of myogenin (Fig. 6G). RhoA expression partially rescued the inhibition of myogenesis induction caused by p120 catenin silencing. This shows that perturbation of the N-cadherin-p120 catenin complex inhibits RhoA activation and subsequently myogenesis induction.
DISCUSSION
Skeletal myogenesis is a multistep process regulated by various molecules (1, 2). N-cadherin-dependent adhesion controls induction of myogenesis through activation of RhoA which positively regulates MyoD expression and activation of several muscle-specific gene promoters (3, 4).
Dynamic association of cadherin complexes at the PM is crucial for cadherin-mediated signaling. The juxtamembrane domain of the cadherin cytoplasmic tail binds p120 catenin, which is a major regulator of cadherin stability at cell-cell contacts and a modulator of Rho GTPase activities (8–11). Mutations in the region of E-cadherin that binds to p120 uncoupled the E-cadherin-p120 catenin complex and disrupted strong adhesion, although the interaction with other catenins remains intact (13). Moreover, association of N-cadherin with cholesterol-enriched microdomains called LR at the cell-cell contacts also stabilizes N-cadherin (16). Because p120 catenin association with cadherins and N-cadherin association with LR at the cell-cell contact sites are both involved in cadherin stability, we have analyzed whether p120 catenin association with N-cadherin requires LR. We observed that the association of p120 catenin with N-cadherin occurs specifically in these microdomains and reciprocally that LR are essential for p120 catenin/N-cadherin association. It has been suggested that p120 catenin is a relatively minor component of the cadherin complex because only a fraction of p120 catenin co-precipitates with cadherins in the presence of detergents (26, 27). This fraction of p120 catenin might be the one associated with N-cadherin in cholesterol-rich membrane domains.
Although it is known that binding to p120 catenin promotes cadherin stabilization at cell-cell contacts, the underlying mechanisms are largely unknown (9, 11, 13, 28). We demonstrate that p120 catenin associates with N-cadherin specifically and exclusively in LR and that, when this interaction is abolished, N-cadherin is not accumulated in LR and not stabilized at cell-cell contacts. This finding raises questions about how p120 catenin is recruited in LR and which mechanisms are involved in cadherin stabilization. Besides the function of the Arm domain of p120 catenin which binds to the N-cadherin juxtamembrane domain, p120 catenin could also sense the lipid environment or interact with a protein located in these microdomains. Recently, the p120 catenin C-terminal region was proposed to be involved in the recruitment of cytoplasmic E-cadherin to the plasma membrane (29). p120 catenin has been also involved in membrane stabilization of Desmoglein 3 through its binding to a juxtamembrane domain of this cadherin. This observation reinforces the potential contribution of the lipid environment in p120 catenin binding to this cadherin (30). Structural analysis of the p120 catenin C-terminal region revealed the presence of potential cholesterol recognition/interaction amino acid consensus motifs5 that favor the interaction with cholesterol (31). In summary, binding to p120 catenin might stabilize N-cadherin through interaction with cholesterol-rich microdomains. This might contribute to N-cadherin clustering, which enhances its adhesive strength. Moreover, binding to p120 catenin also has an indirect stabilizing function because it masks a conserved dileucine motif in N-cadherin juxtamembrane domain that is necessary for endocytosis (32). Indeed, unstabilized cadherin caused by deficient binding to p120 catenin is endocytosed (11).
Moreover, we show that the association of p120 catenin with N-cadherin in LR is involved in N-cadherin-mediated RhoA activation. This suggests that at cell contacts, the N-cadherin/p120 catenin association with LR allows the stabilization and formation of a functional adhesive complex able to organize signal transduction pathways in this specific area of the membrane (16). Because RhoA is recruited at cell contacts after N-cadherin engagement (18) and its activity is required for N-cadherin stabilization at cell contacts in C2C12 myoblasts (33), we conclude that p120 catenin is required for N-cadherin-dependent RhoA activation which in turn allows N-cadherin stabilization at cell contacts. Interestingly mammalian RhoA and its Drosophila homolog, Rho1, interact directly with p120 catenin (34, 35), and we have also detected a fraction of RhoA in LR (data not shown). Moreover, at cell contacts in C2C12 myoblasts, N-cadherin, RhoA, and PI(4,5)P2, a phosphoinositide known to be accumulated in LR (22, 23) and to play a critical role in the dynamic organization of the actin cytoskeleton (36, 37), were co-localized (Fig. 5, A and B). This co-localization was lost after LR disruption. In L cells, PI(4,5)P2 and RhoA accumulated at cell contacts only when N-cadherin was expressed. Again, LR disruption impaired the recruitment of PI(4,5)P2 and RhoA at N-cadherin-dependent cell-cell contacts. Conversely, N-cadherinAAA did not recruit RhoA at N-cadherin-mediated cell contacts. Recruitment and activation of phosphatidylinositol phosphate 5-kinase γ at sites of N-cadherin ligation resulting in PI(4,5)P2 production have been reported (38). Segregation of PI(4,5)P2 might allow the recruitment of PH domain-containing proteins, such as a guanine exchange factor for RhoA that still remains to be identified. Altogether, these data suggest that p120 catenin might be involved in recruiting RhoA in LR at cell contacts to allow its activation by a guanine exchange factor and consequently its downstream function.
Finally, our results show that LR are required for the formation of the N-cadherin complex, downstream RhoA activation, and subsequent myogenesis induction. Inhibition of myogenesis was already reported after perturbation of the N-cadherin-p120 catenin complex upon silencing of p120 catenin (39). In contrast to data obtained in fibroblasts (8), p120 knock down in myoblasts does not increase RhoA activity as measured by Rhotekin pulldown assay and analysis of actin stress fibers, but the loss of p120 catenin impairs RhoA activation induced by specific N-cadherin engagement. This was confirmed by the rescue of myogenesis inhibition in p120 catenin shRNA myoblasts after RhoA expression. These data obtained in myoblasts agree with the role of RhoA during myogenesis induction (18, 21). Indeed, RhoA has been reported to regulate MyoD expression and skeletal muscle cell differentiation positively because it has been demonstrated to be required for serum response factor-mediated activation of several muscle-specific gene promoters (3, 4). Roles for p120 catenin in controlling Rho GTPases activity emerged underlying the existence of cell type-specific mechanisms. Indeed, in NIH3T3 fibroblasts, p120 catenin can target suppression of Rho to cadherin complexes via transient recruitment of p190RhoGAP (8), and in CHO cells, p120 appears to be essential for cadherin-mediated activation of Rac1 (40).
p120 catenin is known to regulate cell-cell adhesion through its interaction with the cytoplasmic juxtamembrane domain of cadherins. Here, we identified a novel mechanism by which p120 catenin acts as a direct stabilizator of N-cadherin at the cell contacts through its association with cholesterol-rich membrane microdomains. How p120 catenin senses this peculiar lipid membrane environment remains to be determined. LR serve as plateforms for protein segregation and signaling and are often modified in cancer cells. Such modifications might thus participate in the pertubation of p120 catenin activity in tumor cells.
Acknowledgments
We thank the imaging facility at CRBM and C. Benistant for discussions. We are grateful to Al Reynolds for shp120 catenin and to K. Green for N-cadherinAAA-YFP constructs.
This work was supported in part by the Ligue Nationale contre le Cancer (Equipe labellisée), Association Française contre les Myopathies, Agence Nationale de la Recherche, and Association Française pour la Recherche contre le Cancer.
N. Taulet, F. Comunale, C. Fayard, S. Charrasse, S. Bodin, and C. Gauthier-Rouvière, unpublished observations.
- PM
- plasma membrane
- ampho B
- amphotericin B
- CO
- cholesterol oxidase
- DM
- differentiation medium
- FRAP
- fluorescence recovery after photobleaching
- GFP
- green fluorescent protein
- GM1
- monosialotetrahexosylganglioside
- LR
- lipid raft(s)
- MOPS
- 4-morpholinepropanesulfonic acid
- NLR
- nonlipid raft
- PH
- pleckstrin homology
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PIPES
- 1,4-piperazinediethanesulfonic acid
- PLC
- phospholipase C
- RFP
- red fluorescent protein
- shRNA
- short hairpin RNA
- WT
- wild type
- YFP
- yellow fluorescent protein.
REFERENCES
- 1.Buckingham M. (2001) Curr. Opin. Genet. Dev. 11,440–448 [DOI] [PubMed] [Google Scholar]
- 2.Knudsen K. A. (1990) Curr. Opin. Cell Biol. 2,902–906 [DOI] [PubMed] [Google Scholar]
- 3.Carnac G., Primig M., Kitzmann M., Chafey P., Tuil D., Lamb N., Fernandez A. (1998) Mol. Biol. Cell 9,1891–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei L., Zhou W., Croissant J. D., Johansen F. E., Prywes R., Balasubramanyam A., Schwartz R. J. (1998) J. Biol. Chem. 273,30287–30294 [DOI] [PubMed] [Google Scholar]
- 5.Yap A. S., Brieher W. M., Gumbiner B. M. (1997) Annu. Rev. Cell Dev. Biol. 13,119–146 [DOI] [PubMed] [Google Scholar]
- 6.Drees F., Pokutta S., Yamada S., Nelson W. J., Weis W. I. (2005) Cell 123,903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada S., Pokutta S., Drees F., Weis W. I., Nelson W. J. (2005) Cell 123,889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wildenberg G. A., Dohn M. R., Carnahan R. H., Davis M. A., Lobdell N. A., Settleman J., Reynolds A. B. (2006) Cell 127,1027–1039 [DOI] [PubMed] [Google Scholar]
- 9.Reynolds A. B. (2007) Biochim. Biophys. Acta 1773,2–717175391 [Google Scholar]
- 10.Anastasiadis P. Z., Reynolds A. B. (2001) Curr. Opin. Cell Biol. 13,604–610 [DOI] [PubMed] [Google Scholar]
- 11.Davis M. A., Ireton R. C., Reynolds A. B. (2003) J. Cell Biol. 163,525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao K., Allison D. F., Buckley K. M., Kottke M. D., Vincent P. A., Faundez V., Kowalczyk A. P. (2003) J. Cell Biol. 163,535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thoreson M. A., Anastasiadis P. Z., Daniel J. M., Ireton R. C., Wheelock M. J., Johnson K. R., Hummingbird D. K., Reynolds A. B. (2000) J. Cell Biol. 148,189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams C. L., Nelson W. J., Smith S. J. (1996) J. Cell Biol. 135,1899–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert M., Choquet D., Mège R. M. (2002) J. Cell Biol. 157,469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Causeret M., Taulet N., Comunale F., Favard C., Gauthier- Rouvière C. (2005) Mol. Biol. Cell 16,2168–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mary S., Charrasse S., Meriane M., Comunale F., Travo P., Blangy A., Gauthier-Rouvière C. (2002) Mol. Biol. Cell 13,285–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charrasse S., Meriane M., Comunale F., Blangy A., Gauthier-Rouvière C. (2002) J. Cell Biol. 158,953–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harder T., Scheiffele P., Verkade P., Simons K. (1998) J. Cell Biol. 141,929–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Kojima S., Borisy G. G., Green K. J. (2003) J. Cell Biol. 163,547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovett F. A., Gonzalez I., Salih D. A., Cobb L. J., Tripathi G., Cosgrove R. A., Murrell A., Kilshaw P. J., Pell J. M. (2006) J. Cell Sci. 119,4828–4840 [DOI] [PubMed] [Google Scholar]
- 22.Bodin S., Giuriato S., Ragab J., Humbel B. M., Viala C., Vieu C., Chap H., Payrastre B. (2001) Biochemistry 40,15290–15299 [DOI] [PubMed] [Google Scholar]
- 23.Pike L. J., Casey L. (1996) J. Biol. Chem. 271,26453–26456 [DOI] [PubMed] [Google Scholar]
- 24.Holz R. W., Hlubek M. D., Sorensen S. D., Fisher S. K., Balla T., Ozaki S., Prestwich G. D., Stuenkel E. L., Bittner M. A. (2000) J. Biol. Chem. 275,17878–17885 [DOI] [PubMed] [Google Scholar]
- 25.Ireton R. C., Davis M. A., van Hengel J., Mariner D. J., Barnes K., Thoreson M. A., Anastasiadis P. Z., Matrisian L., Bundy L. M., Sealy L., Gilbert B., van Roy F., Reynolds A. B. (2002) J. Cell Biol. 159,465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staddon J. M., Smales C., Schulze C., Esch F. S., Rubin L. L. (1995) J. Cell Biol. 130,369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozawa M., Kemler R. (1998) J. Biol. Chem. 273,6166–6170 [DOI] [PubMed] [Google Scholar]
- 28.Anastasiadis P. Z. (2007) Biochim. Biophys. Acta 1773,34–46 [DOI] [PubMed] [Google Scholar]
- 29.Liu H., Komiya S., Shimizu M., Fukunaga Y., Nagafuchi A. (2007) Cell Struct. Funct. 32,127–137 [DOI] [PubMed] [Google Scholar]
- 30.Kanno M., Isa Y., Aoyama Y., Yamamoto Y., Nagai M., Ozawa M., Kitajima Y. (2008) Exp. Cell Res. 314,1683–1692 [DOI] [PubMed] [Google Scholar]
- 31.Epand R. M. (2008) Biochim. Biophys. Acta 1778,1576–1582 [DOI] [PubMed] [Google Scholar]
- 32.Miyashita Y., Ozawa M. (2007) J. Biol. Chem. 282,11540–11548 [DOI] [PubMed] [Google Scholar]
- 33.Comunale F., Causeret M., Favard C., Cau J., Taulet N., Charrasse S., Gauthier-Rouvière C. (2007) Biol. Cell 99,503–517 [DOI] [PubMed] [Google Scholar]
- 34.Magie C. R., Pinto-Santini D., Parkhurst S. M. (2002) Development 129,3771–3782 [DOI] [PubMed] [Google Scholar]
- 35.Castaño J., Solanas G., Casagolda D., Raurell I., Villagrasa P., Bustelo X. R., García de Herreros A., Duñach M. (2007) Mol. Cell. Biol. 27,1745–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin H. L., Janmey P. A. (2003) Annu. Rev. Physiol. 65,761–789 [DOI] [PubMed] [Google Scholar]
- 37.Janmey P. A., Lindberg U. (2004) Nat. Rev. Mol. Cell Biol. 5,658–666 [DOI] [PubMed] [Google Scholar]
- 38.El Sayegh T. Y., Arora P. D., Ling K., Laschinger C., Janmey P. A., Anderson R. A., McCulloch C. A. (2007) Mol. Biol. Cell 18,3026–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gavard J., Marthiens V., Monnet C., Lambert M., Mège R. M. (2004) J. Biol. Chem. 279,36795–36802 [DOI] [PubMed] [Google Scholar]
- 40.Goodwin M., Kovacs E. M., Thoreson M. A., Reynolds A. B., Yap A. S. (2003) J. Biol. Chem. 278,20533–20539 [DOI] [PubMed] [Google Scholar]