Abstract
Objective To test the efficacy of supplemental vitamin D and active forms of vitamin D with or without calcium in preventing falls among older individuals.
Data sources We searched Medline, the Cochrane central register of controlled trials, BIOSIS, and Embase up to August 2008 for relevant articles. Further studies were identified by consulting clinical experts, bibliographies, and abstracts. We contacted authors for additional data when necessary.
Review methods Only double blind randomised controlled trials of older individuals (mean age 65 years or older) receiving a defined oral dose of supplemental vitamin D (vitamin D3 (cholecalciferol) or vitamin D2 (ergocalciferol)) or an active form of vitamin D (1α-hydroxyvitamin D3 (1α-hydroxycalciferol) or 1,25-dihydroxyvitamin D3 (1,25-dihydroxycholecalciferol)) and with sufficiently specified fall assessment were considered for inclusion.
Results Eight randomised controlled trials (n=2426) of supplemental vitamin D met our inclusion criteria. Heterogeneity among trials was observed for dose of vitamin D (700-1000 IU/day v 200-600 IU/day; P=0.02) and achieved 25-hydroxyvitamin D3 concentration (25(OH)D concentration: <60 nmol/l v ≥60 nmol/l; P=0.005). High dose supplemental vitamin D reduced fall risk by 19% (pooled relative risk (RR) 0.81, 95% CI 0.71 to 0.92; n=1921 from seven trials), whereas achieved serum 25(OH)D concentrations of 60 nmol/l or more resulted in a 23% fall reduction (pooled RR 0.77, 95% CI 0.65 to 0.90). Falls were not notably reduced by low dose supplemental vitamin D (pooled RR 1.10, 95% CI 0.89 to 1.35; n=505 from two trials) or by achieved serum 25-hydroxyvitamin D concentrations of less than 60 nmol/l (pooled RR 1.35, 95% CI 0.98 to 1.84). Two randomised controlled trials (n=624) of active forms of vitamin D met our inclusion criteria. Active forms of vitamin D reduced fall risk by 22% (pooled RR 0.78, 95% CI 0.64 to 0.94).
Conclusions Supplemental vitamin D in a dose of 700-1000 IU a day reduced the risk of falling among older individuals by 19% and to a similar degree as active forms of vitamin D. Doses of supplemental vitamin D of less than 700 IU or serum 25-hydroxyvitamin D concentrations of less than 60 nmol/l may not reduce the risk of falling among older individuals.
Introduction
Each year one in three people aged 65 years or older experiences at least one fall,1 2 3 with 9% of falls leading to an emergency room visit and 5-6% resulting in a fracture.4 Fall prevention has, therefore, become a public health goal, especially as the older proportion of the population grows.
Vitamin D has direct effects on muscle strength modulated by specific vitamin D receptors present in human muscle tissue.5 6 Myopathy from severe vitamin D deficiency presents as muscle weakness and pain,7 but is reversible with vitamin D supplementation.6 In several trials of older individuals at risk for vitamin D deficiency, vitamin D supplementation improved strength, function, and balance in a dose-related pattern.4 8 9 Most importantly, these benefits translated into a reduction in falls.4 8 9
Overall, however, results have been mixed for fall prevention with vitamin D; for example, several trials of vitamin D have had non-significant results. This may be explained in part by the use of low doses of vitamin D, as suggested by a 2004 meta-analysis of limited data from three trials on supplemental vitamin D.10 Other potential explanations include the availability of vitamin D over the counter for the control group; the use of an open trial design, which biases trial results towards the null11; and incomplete assessment, inadequate definition, or incomplete ascertainment of falls during the entire observation period,12 again introducing a bias towards the null.
Several trials on vitamin D have been performed since 2004; thus, the importance of vitamin D dose for the prevention of falls should be reassessed. Specifically, we need to establish the optimum threshold of serum 25-hydroxyvitamin D3 (25(OH)D; calcidiol (25-hydroxycholecalciferol)) required to prevent falls in older individuals. Notably, two epidemiology studies among older individuals have found a dose-response relationship between lower extremity function and serum 25(OH)D concentrations,13 14 with one study identifying a threshold of 50 nmol/l for optimal function.13 The other larger study found that function continued to improve with increasing concentration, without any plateau.14
Both active forms of vitamin D and standard supplemental vitamin D have been suggested to prevent falls among older individuals, but no direct comparison of these two groups is available. Active forms of vitamin D do not need hydroxylation in the kidney, so their effect on falls should be influenced less by age related decline in kidney function than the effect of supplemental vitamin D. However, active forms of vitamin D cost more and are associated with a higher risk for hypercalcaemia than standard supplemental vitamin D.
The aim of this meta-analysis was to assess the efficacy of vitamin D supplementation, with and without calcium, for the prevention of falls among older persons by dose and serum concentration of 25(OH)D achieved. In addition, we assessed the efficacy of active forms of vitamin D compared with supplemental vitamin D in the prevention of falls.
Methods
Search strategy and data extraction
We conducted a systematic search for all English and non-English articles in Medline (Ovid, Pubmed) and the Cochrane central register of controlled trials from January 1960 to August 2008, in BIOSIS from January 1985 to July 2008, and in Embase from January 1991 to August 2008. Additional studies were identified by contacting experts, searching reference lists, and searching abstracts presented at the American Society for Bone and Mineral Research from 1995 to 2008. We used Medical Subject Headings (MeSH) as search terms, including terms related to trials (“randomised controlled trial,” “controlled clinical trial,” “random allocation,” “double-blind method,” and “single-blind method”), vitamin D (“cholecalciferol,” “hydroxycholecalciferol,” “calcifediol,” “ergocalciferol,” “calcidiol,” “vitamin D/blood/25-hydroxyvitamin D,” “1,25-dihydroxyvitamin D,” “1-α-hydroxyvitamin D,” “1-alpha-hydroxyvitamin D,” “calcitriol,” “alfacalcidol,” and “paricalcitol”), and falls (“falls,” “accidental falls,” “fall prevention,” and “balance”), and the terms “humans,” “elderly,” and “bone density.” Data extraction was conducted independently by two authors (HAB-F and JH). A consensus procedure was developed but was not necessary because of concordance.
Inclusion criteria
Randomised controlled trials of fall prevention with a defined oral dose of supplemental vitamin D (vitamin D3 (cholecalciferol) or vitamin D2 (ergocalciferol)) or oral active vitamin D (1α-hydroxyvitamin D3 (1α-hydroxycalciferol) or 1,25-dihydroxyvitamin D3 (1,25-dihydroxycholecalciferol)) in individuals aged 65 years or older with a minimum follow-up of three months were identified. To be included in the primary analysis, the trial design had to be double blind and the assessment of falls sufficiently specified according to the following criteria: (a) falls had to be a primary or secondary end point defined at the onset of the trial; (b) the study had to include a definition of falls and how they were assessed; and (c) falls had to be assessed for the entire trial period. Eligible studies that did not meet the criteria for the primary analysis were included in a sensitivity analysis, such as studies involving older patients in an unstable health state (that is, those recruited during acute inpatient care).
We excluded reviews, trials that were not randomised, trials that did not include a control group, observational studies, and animal studies. Given that health conditions that place patients at high risk for falls might mask the benefits of vitamin D on falls, we excluded studies that focused on patients with Parkinson’s disease, organ transplant recipients, or patients with stroke. We excluded trials that assessed intramuscular injection of vitamin D because this technique is invasive and has resulted in small but variable increases in 25(OH)D concentrations.15
Outcome measures
Our primary outcome measure was the relative risk of having at least one fall among persons receiving vitamin D with or without calcium compared with the risk among those individuals receiving placebo or calcium supplementation alone. We analysed separately the effect of supplemental vitamin D and active forms of vitamin D, and evaluated both dose and 25(OH)D concentrations achieved for supplemental vitamin D.
Quality assessment
We examined the following methodological features most relevant to the control of bias: randomisation; masking of treatment allocation; blinding; adherence; and withdrawals.16 17 Given that vitamin D is available over the counter, trials had to be double blind to be included in the primary analysis. Open design trials that met the general eligibility criteria were included in the sensitivity analysis.
Differences in vitamin D assays
Interlaboratory and interassay variation limit comparison between trials for achieved 25(OH)D concentrations,18 19 with competitive protein binding assays tending to yield higher concentrations than radioimmunoassays.20 To account for these variations, we adjusted the 25(OH)D values from the two studies where a competitive protein binding assay was used to radioimmunoassay equivalent values, according to the method described by Lips and colleagues.20
Statistical methods
Outcomes were analysed on an intention to treat basis by using random effect models.21 In addition, we calculated the difference in relative risks to determine the number needed to treat to prevent a person from falling.
Heterogeneity among studies was explored by predefined covariates using the Q statistic as a test (significant for P<0.10).22 The presence of heterogeneity suggests that the studies should not be pooled because of significant differences in results.23 In such cases, we explored heterogeneity by dose of vitamin D and 25(OH)D concentration achieved by using visual inspection and random effect meta-regression analysis. Additional subgroup analyses undertaken for supplemental vitamin D included type of vitamin D (D2 v D3), gender, age (<80 years v ≥80 years), treatment duration (<12 months v ≥12 months), level of independence (independent v institutionalised), and additional calcium supplementation. To evaluate publication bias, we used Begg’s test and Egger’s test with all eight trials from the primary analysis or all 15 trials from the sensitivity analysis.24 Statistical analysis was performed with STATA version 8.0 (StataCorp, College Station, TX, USA).
Results
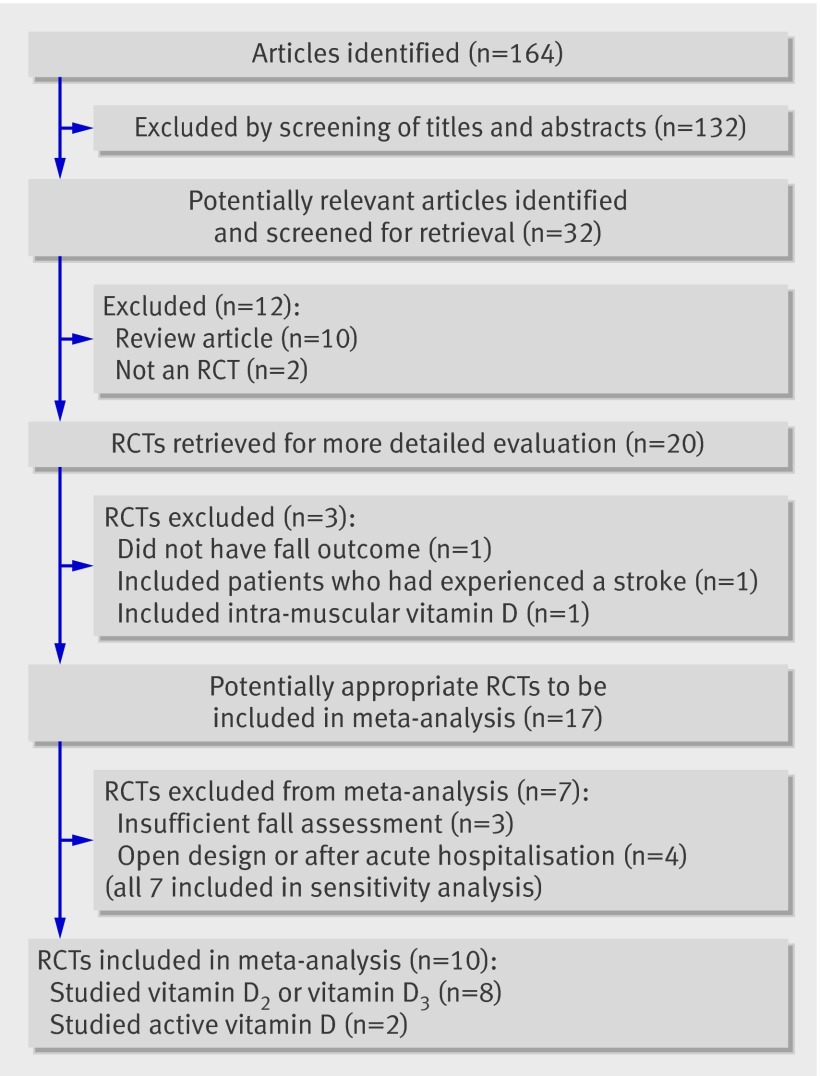
A total of 164 articles were found in our initial search, 132 of which could be excluded by screening the titles and abstracts (fig 1). A further 12 articles were excluded either because they did not detail randomised controlled trials or they were review articles. Ten more trials were excluded because they did not meet the inclusion criteria so that 10 randomised trials were included in the final analysis—eight that studied either vitamin D2 or vitamin D3 and two that assessed active forms of vitamin D.
Fig 1 Quorum flow chart. RCT=randomised controlled trial
Trials assessing supplemental vitamin D
We identified eight randomised controlled trials of supplemental vitamin D that met our inclusion criteria (box 1).w1-w8 All trials assessed vitamin D treatment for the prevention of falls as a primary or secondary outcome and were identified through our MeSH term search. One study consisted of a multiple dose trial in which the authors provided additional data for the four trial arms (200, 400, 600, and 800 international units (IU) D2 v placebo).w1
Box 1 Fall ascertainment in trials with supplemental vitamin D included in the primary analysis
Broe et al, 2007w1
Falls were defined as “a sudden, unintentional change in position causing a resident to fall on the ground.” Nursing staff filled out an incident report at the time of the event, the primary care physician verified it, and the information was entered into the incident report database.
Bischoff-Ferrari et al, 2006w2
Falls were defined as “unintentionally coming to rest on the ground, floor, or other lower level.” Participants were asked to send a postcard after every fall, which was then followed by a telephone call from a staff member to assess the circumstances of the fall. In addition, falls were ascertained at every follow-up visit (every six months).
Prince et al, 2008w3
Falls were defined as “unintentionally coming to rest on the ground, floor, or other lower level.” Patients were interviewed by study staff every six weeks via telephone or during clinic visits. The number of falls that had occurred in the previous six weeks and the associated features of the falls were recorded on a questionnaire.
Flicker et al, 2005w4
Falls were defined as “an event that results in a person coming to rest inadvertently on the ground or other lower level.” Residential care staff recorded falls continuously in diaries, and these were mailed monthly to the central study centre.
Pfeifer et al, 2008w5
A fall was defined as “falling on to the floor or ground, or hitting an object like a chair or stair.” Not included as falls were controlled or intentional movements towards a chair or bed or a near fall in which the participant caught oneself before falling on to the floor or ground. The number of falls was recorded in fall diaries—each day the participants had to make a cross in the diary depending on whether a fall had occurred or not. Every two months, the study participants were also asked via telephone interviews whether a fall had happened.
Bischoff et al, 2003w6
Falls were defined as “unintentionally coming to rest on the ground, floor, or other lower level.” Coming to rest against furniture or a wall was not counted as a fall. Falls were recorded by nurses on the inpatient units who had received training in the use of the fall protocol (which included recording date, time, circumstances, and injuries).
Pfeifer et al, 2000w7
A fall was defined as “falling on to the floor or ground or hitting an object like a chair or stair.” Not included as falls were controlled or intentional movements toward a chair or bed or a near fall in which the participant caught herself before falling on to the floor or ground. The number of falls was recorded by questionnaires throughout the trial period.
Graafmans et al, 1996w8
A fall was defined as “unintentionally coming to rest at a lower level or on the ground.” Participants were asked to record in a diary any falls they had during a 28-week period. Every week, participants registered whether or not they had fallen, as well as the location, time, and circumstances of each fall.
Table 1 shows the characteristics of the eight double blind randomised controlled trials with sufficient fall assessment included in our study, including one randomised controlled trial with four study arms.w1 The eight trials involved 2426 individuals in total, 81% of whom were women, and participants had an approximate mean age of 80 years. All participants were in stable health and were living in the community or in nursing homes. Vitamin D3 was used in five studies and vitamin D2 in three studies. Vitamin D2 or D3 was given in a daily dose ranging from 200 IU to 1000 IU. Treatment duration varied from 2 months to 36 months. Calcium supplementation was used in both treatment and placebo groups in five randomised controlled trials, and the dose varied between 500 mg/day and 1200 mg/day. In one study, calcium was provided only in the treatment group, and vitamin D alone was compared with placebo in two trials. Adherence varied between 68% and 100%, with seven out of eight trials reporting adherence of 80-100%.
Table 1.
Randomised controlled trials of supplemental vitamin D included in the primary analyses
| Number of participants | Age (years; mean (SD)) | Sample population | Treatment (daily dose) | Adherence | Study duration (months) | 25(OH)D serum concentration (mmol/l; mean (SD)) | |||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Control group | Assay used | |||||||
| High dose trials | |||||||||
| Broe et al, 2007w1 | 48 (36 women, 12 men) | 89 (5) | Nursing home patients | 800 IU vitamin D2 or placebo | 98% | 5 | 54 (23) to 75 (15) at 5 months (n=17). DiaSorin equivalent mean values: 43 to 60 at 5 months | 53 (29) to 55-60 at 5 months (n=20). DiaSorin equivalent mean values: 42 to 48 | Competitive protein binding assay |
| Bischoff-Ferrari et al, 2006 w2 | 445 (246 women, 199 men) | 71 (5) | Ambulatory individuals | 700 IU vitamin D3 + 500 mg calcium (citrate malate) or placebo | 93% | 36 | 76 (35) to 107 (38) at 36 months (n=182). DiaSorin equivalent mean values: 67 to 95 | 73 (32) to 72 (30) at 36 months (n=195). DiaSorin equivalent mean values: 65 to 64 | Competitive protein binding assay |
| Prince et al, 2008w3 | 302 women | 77 (5) | Patients with history of falling recruited from emergency departments, the electoral roll, and receiving home nursing services. | 1000 IU vitamin D2 + 500 mg calcium (calcium carbonate) or 500 mg calcium + placebo | 86% | 12 | 45 to 60 at 12 months | 44.3 to 49 at 12 months | Radioimmunoassay |
| Flicker et al, 2005w4 | 625 (593 women, 32 men) | 83 (8) | Residental care patients | Initially 10 000 IU D2 weekly, then 1000 IU D2 + 600 mg calcium or placebo + 600 mg calcium (calcium carbonate) | 68% | 24 | 25-60 at baseline: (89% of participants). No follow-up | 25-60 at baseline (89% of participants). No follow-up | Radioimmunoassay |
| Pfeifer et al, 2008w5 | 242 (121 women, 121 men) | 77 (4) | Ambulatory individuals | 800 IU vitamin D3 + 1000 mg calcium or 1000 mg calcium + placebo | 80% | 20 | 55.4 (18.5) to 84.5 (18.0) at 12 months (n=122) | 53.8 (18.4) to 56.6 (20) at 12 months (n=120) | Radioimmunoassay |
| Bischoff et al, 2003w6 | 122 women | 85 (7) | Institutionalised patients | 800 IU vitamin D3 + 1200 mg calcium or 1200 mg calcium + placebo | 100% | 3 | 30.8 (23-55) to 65.5 (49.8-82.8) at 3 months (n=45) | 29 (23-55) to 28.5 (24.5-41.5) at 3 months (n=44) | Radioimmunoassay |
| Pfeifer et al, 2000w7 | 137 women | 74 (1) | Community dwelling individuals | 800 IU vitamin D3 + 1200 mg calcium or 1200 mg calcium + placebo | 96% | 2 with treatment plus 10 without treatment | 25.7 (13.6) to 66.1 (33.1) at 2 months (n=70) | 24.6 (12.1) to 42.9 (33.1) at 2 months (n=67) | Radioimmunoassay |
| Low dose trials | |||||||||
| Broe et al, 2007w1 | 51 (39 women, 12 men) | 92 (6) | Nursing home patients | 200 IU vitamin D2 or placebo | 98% | 5 | 45 (23) to 60 (20) at 5 months follow-up (n=24). Diasorin equivalent mean values: 36 to 48 | 50 (23) to 61 (34) at 5 months (n=23). Diasorin equivalent mean values: 40 to 49 | Competitive protein binding assay |
| Broe et al, 2007w1 | 50 (38 women, 12 men) | 88 (5) | Nursing home patients | 400 IU vitamin D2 or placebo | 98% | 5 | 53 (28) to 55 (22) at 5 months (n=24). Diasorin equivalent mean values: 42 to 44 | 50 (23) to 61 (34) at 5 months (n=23). Diasorin equivalent mean values: 40 to 49 | Competitive protein binding assay |
| Broe et al, 2007w1 | 50 (37 women, 13 men) | 89 (6) | Nursing home patients | 600 IU vitamin D2 or placebo | 98% | 5 | 40 (19) to 60 (20) at 5 months (n=23). Diasorin equivalent mean values: 32 to 48 | 50 (23) to 61 (34) at 5 months (n=23). Diasorin equivalent mean values: 40 to 49 | Competitive protein binding assay |
| Graafmans et al, 1996w8 | 354 (302 women, 52 men) | 83 (6) | Ambulatory patients in homes and apartments for older individuals | 400 IU vitamin D3 + estimated calcium intake from dairy products (800-1000 mg/d) | 85% | 7 | Not defined | Not defined | Not defined |
Fall prevention with oral supplemental vitamin D
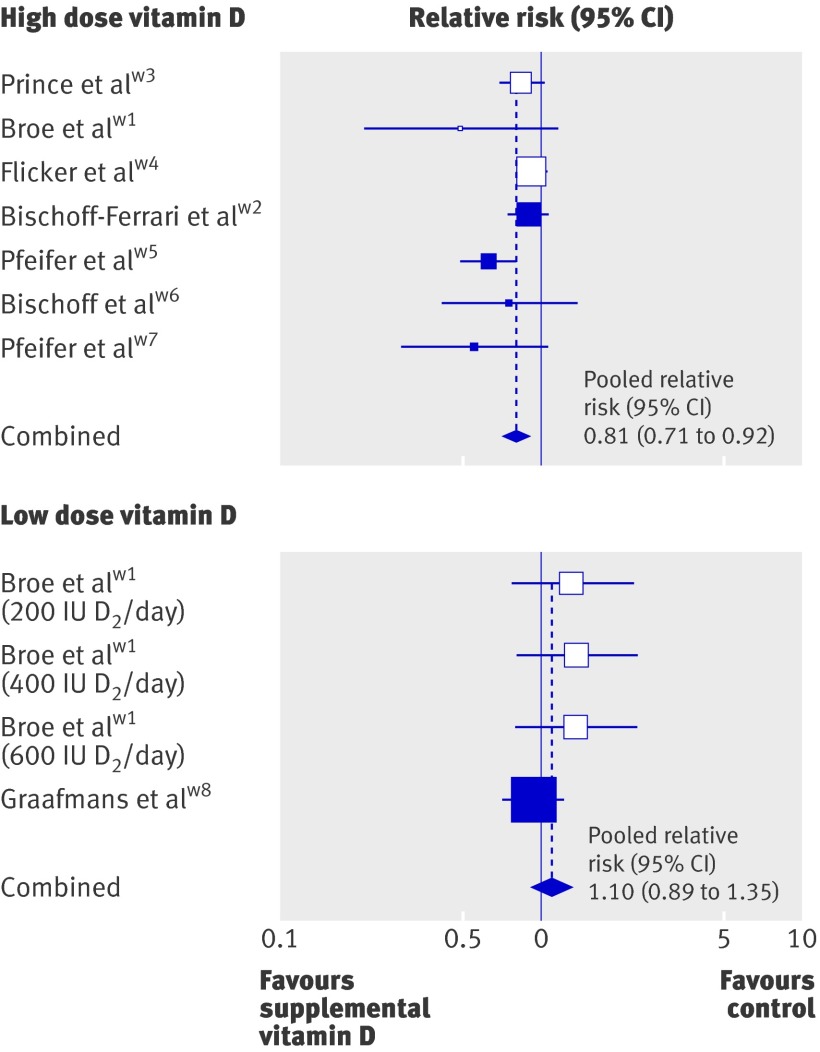
In the eight randomised controlled trials, the pooled relative risk for any dose of vitamin D preventing a fall was 0.87 (95% CI 0.77 to 0.99). However, heterogeneity in results was seen among studies (Q test: P=0.05), although this was resolved after stratifying trials by daily dose (200-600 IU v 700-1000 IU).
The pooled relative risk for the seven studies with 700-1000 IU supplemental vitamin D a day (1921 individuals) was 0.81 (95% CI 0.71 to 0.92) suggesting that a high dose of vitamin D a day reduced the risk of a person falling by 19% (table 2, fig 2). The pooled risk difference for the high dose was 9.4% (95% CI 5.1% to 13.7%; P<0.0001), so the number needed to treat was 11 (95% CI 7 to 20) for a treatment duration of 2-36 months.
Table 2 .
Primary pooled analysis and primary subgroup analyses for high doses of supplemental vitamin D (700-1000 IU) and the prevention of falls
| Study (daily dose of vitamin D) | Number of participants | Number of fallers/total treated | Number of fallers/total control | Effect relative risk | Lower 95% CI | Upper 95% CI | Q test P value | I2 | Fall reduction | P value: difference between subgroups |
|---|---|---|---|---|---|---|---|---|---|---|
| Broe et al, 2007w1 (800 IU D2) | 48 | 5/23 | 11/25 | 0.49 | 0.21 | 1.16 | — | — | — | — |
| Bischoff-Ferrari et al, 2006w2 (700 IU D3) | 445 | 107/219 | 124/226 | 0.89 | 0.74 | 1.07 | — | — | — | — |
| Prince et al, 2008w3 (1000 IU D2) | 302 | 80/151 | 95/151 | 0.84 | 0.69 | 1.02 | — | — | — | — |
| Flicker et al, 2005w4 (1000 IU D2) | 625 | 170/313 | 185/312 | 0.92 | 0.80 | 1.05 | — | — | — | |
| Pfeifer et al, 2008w5 (800 IU D3) | 242 | 48/121 | 76/121 | 0.63 | 0.49 | 0.81 | — | — | — | — |
| Bischoff et al, 2003w6 (800 IU D3) | 122 | 14/62 | 18/60 | 0.75 | 0.41 | 1.37 | — | — | — | — |
| Pfeifer et al, 2000w7 (800 IU D3) | 137 | 11/70 | 19/67 | 0.55 | 0.29 | 1.06 | — | — | — | — |
| Primary pooled analysis | 1921 | 435/959 | 528/962 | 0.81 | 0.71 | 0.92 | 0.12 | 41% | -19% | — |
| Primary subgroup analyses | ||||||||||
| D3 only (w2,w6,w5,w7) | 946 | 180/472 | 237/474 | 0.74 | 0.58 | 0.93 | 0.12 | 49% | -26% | 0.28 |
| D2 only (w1(800),w3,w4) | 975 | 255/487 | 291/488 | 0.88 | 0.77 | 1.00 | 0.32 | 12% | -12% | |
| Men only (w1(800),w2) | 211 | 51/105 | 52/106 | 0.99 | 0.75 | 1.31 | 0.58 | 0% | -1% | 0.34 |
| Women only* | 1468 | 336/733 | 400/735 | 0.85 | 0.76 | 0.95 | 0.36 | 9% | -15% | |
| D3 women (w2,w6,w7) | 505 | 84/253 | 112/252 | 0.78 | 0.63 | 0.95 | 0.55 | 0% | -22% | 0.27 |
| D2 women (w1(800),w3,w4) | 963 | 252/480 | 288/483 | 0.87 | 0.74 | 1.03 | 0.21 | 35% | -13% | |
| Age 65-79 and/or independent (w2,w3,w5,w7) | 1126 | 246/561 | 314/565 | 0.77 | 0.65 | 0.93 | 0.10 | 52% | -23% | 0.46 |
| Age 80+ and/or institutionalised (w1(800),w4,w6) | 795 | 189/398 | 214/397 | 0.86 | 0.70 | 1.07 | 0.32 | 13% | -14% | |
| Less than 12 months’ treatment duration (w1(800),w6,w7) | 307 | 30/155 | 48/152 | 0.62 | 0.42 | 0.91 | 0.67 | 0% | -38% | 0.07 |
| At least 12 months’ treatment duration (w2,w3,w4,w5) | 1614 | 305/804 | 480/810 | 0.83 | 0.72 | 0.96 | 0.09 | 56% | -17% | |
| Main effect vitamin D (w1(800),w3,w4,w5,w6,w7) | 1476 | 327/740 | 404/736 | 0.77 | 0.65 | 0.92 | 0.09 | 48% | -23% | — |
Subgroups with at least two available trials are presented.
Q test: P<0.100 indicates heterogeneity.
I2 estimates above 25% are considered to represent modest heterogeneity, and values above 50% represent large heterogeneity beyond chance.
*Given that the Flicker et al trial included only 5% men, we added the total study population finding of Flicker to the female subgroup.
Fig 2 Fall prevention with high dose (700-1000 IU a day) and low dose (200-600 IU a day) of supplemental vitamin D. Boxes represent relative risks, and the size of the boxes is proportional to the size of the high dose supplemental vitamin D trials included in the primary analysis. Error bars represent 95% confidence intervals. Shaded boxes indicate trials with vitamin D3, and white boxes indicate those with vitamin D2
The pooled relative risk for the two trials with a dose of less than 700 IU (200-600 IU) vitamin D a day was 1.10 (95% CI 0.89 to 1.35; Q test: P=0.42) indicating that less than 700 IU vitamin D a day did not reduce fall risk (table 3, fig 2).
Table 3 .
Primary pooled analysis for low doses of supplemental vitamin D (<700 IU) and the prevention of falls
| Study (daily dose of vitamin D) | Number of participants | Number of fallers/total treated | Number of fallers/total control | Effect relative risk | Lower 95% CI | Upper 95% CI | Q test P value | I2 | Fall reduction |
|---|---|---|---|---|---|---|---|---|---|
| Broe et al, 2007w1(200) (200 IU D2) | 51 | 15/26 | 11/25 | 1.31 | 0.76 | 2.27 | — | — | — |
| Broe et al, 2007w1(400) (400 IU D2) | 50 | 15/25 | 11/25 | 1.36 | 0.79 | 2.35 | — | — | — |
| Broe et al, 2007w1(600) (600 IU D2) | 50 | 15/25 | 11/25 | 1.36 | 0.79 | 2.35 | — | — | — |
| Graafmans et al, 1996w8 (400 IU D3) | 354 | 62/177 | 66/177 | 0.94 | 0.71 | 1.24 | — | — | — |
| Primary pooled analysis (w1(200,400,600),w8) | 505 | 107/253 | 99/252 | 1.10 | 0.89 | 1.35 | 0.42 | 0% | +10% (ns) |
Q test: P<0.100 indicates heterogeneity.
I2 estimates above 25% are considered to represent modest heterogeneity, and values above 50% represent large heterogeneity beyond chance.
ns=not significant.
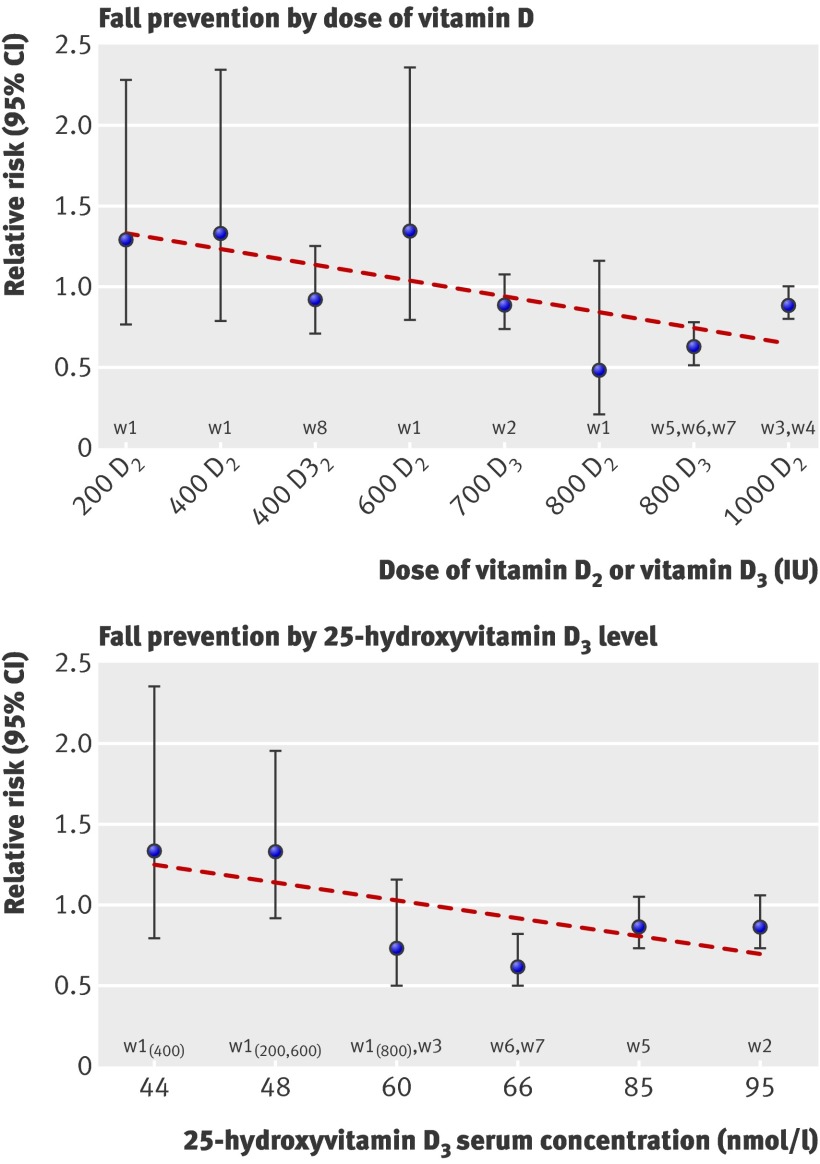
Achieved serum 25(OH)D concentrations of 60 nmol/l or more resulted in a 23% fall reduction (pooled relative risk 0.77, 95% CI 0.65 to 0.90), whereas concentrations of less than 60 nmol/l had no effect on number of falls (pooled relative risk 1.35, 95% CI 0.98 to 1.84).
Figure 3 shows the relationship between vitamin D and falls, and suggests that fall prevention begins with a daily dose of 700 IU supplemental vitamin D. This threshold was confirmed in a meta-regression of 2426 individuals, in which a significant inverse relationship was found between dose and the risk of sustaining at least one fall (beta estimate for dose: ≥700 IU v <700 IU=−0.337; P=0.02). Figure 3 also suggests that a 25(OH)D concentration of 60 nmol/l is required for fall prevention. This possibility was likewise confirmed by a meta-regression of 1447 individuals (two trials did not provide 25(OH)D dataw4 w8), which indicated a significant inverse relationship between 25(OH)D serum concentration and the risk of sustaining at least one fall (beta estimate for 25(OH)D concentration: ≥60 nmol/l v <60 nmol/l=−0.586; P=0.005).
Fig 3 Fall prevention by dose and achieved 25(OH)D concentrations. Circles represent relative risks and error bars represent 95% confidence intervals. Trendline is based on series of effect sizes (circles). There were three trials with 800 IU D3,w5 w6 w7 so the effect size for 800 IU D3 is the pooled result from these three trials. Likewise, the effect size for 1000 IU D2 is the pooled result from the two trials with 1000 IU D2.w3 w4 We have listed the same dose D2 and D3 separately in the graph to account for their potential different impact on fall reduction. As there were two data points from the Broe et al trial that reached 48 nmol/l,w1 two trials that reached 60 nmol/l,w1 w3 and two trials that reached 66 nmol/l,w6 w7 we pooled each of the sets. On the basis of visual inspection of figure 3, the benefits of vitamin D for fall risk started at a dose of 700 IU a day
Primary subgroup analyses with trials assessing high doses of supplemental vitamin D
In subgroup analyses of the trials that assessed a high dose of supplemental vitamin D (700-1000 IU), the pooled relative risk reduction was 12% in trials that used vitamin D2 compared with 26% for trials that used vitamin D3 (P=0.28; table 2). The combined effect of calcium plus vitamin D compared with placebo in one study showed a relative risk reduction of 19%.w2 The main effect of vitamin D, either vitamin D compared with placebo or vitamin D plus calcium compared with calcium only, was tested in six studies. The pooled relative risk reduction for these six studies was 23% (relative risk 0.77, 95% CI 0.65 to 0.92); thus, the main effect of vitamin D may not depend on additional calcium supplementation. The effect of vitamin D in women was tested in six studies (n=1468), which had a pooled relative risk reduction of 15% compared with 19% in men and women combined. Data on men from two trials (n=211) were limited.w1 w2 Treatment duration did not modulate the effect of vitamin D significantly: fall reduction was 38% with a treatment duration of less than 12 months in three small trials (relative risk 0.62, 95% CI 0.42 to 0.91) compared with 17% with a treatment duration of 12 months or more in four larger trials (relative risk 0.83, 95% CI 0.72 to 0.96; P=0.07). The benefits of 700-1000 IU vitamin D a day on risk of falls were present in both ambulatory and institutionalised older individuals, as well as in trials with a mean age 65-79 years or higher.
Sensitivity analysis of supplemental vitamin D
In a sensitivity analysis, we examined the effect size for supplemental vitamin D when including studies meeting less stringent quality criteria. Seven studies were identified for the sensitivity analysis,w9-w15 six through our MeSH term search and one in the abstracts of the American Society for Bone and Mineral Research (table 4).w12 Three of these trials were excluded from the primary analysis for their insufficient assessment of falls,w9-w11 with incomplete fall assessment for the entire trial period in two studies.w9 w10 Three trials had an open study design and were not blinded,w12 w13 w14 and one trial enrolled patients in unstable health states.w15
Table 4.
Trials of supplemental vitamin D excluded from the primary analyses but included in sensitivity analyses
| Source | Reason for exclusion | Number of participants | Age (years; mean (SD)) | Sample population | Treatment (daily dose) | Adherence | Study duration (months) | 25(OH)D serum concentration (mmol/l; mean (SD)) | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Control group | ||||||||
| Grant et al, 2005w9 | Insufficient fall assessment | 5292 (4481 women, 811 men) | 77 (6) | Individuals who were mobile before developing a low trauma fracture | 800 IU vitamin D3 with or without 1000 mg calcium (calcium carbonate) | 47% | 24 | 38 (16) to 62 (19.5) at 12 months | 38 (16) to 45.8 (18) at 12 months |
| Trivedi et al, 2003w10 | Insufficient fall assessment | 2686 (2037 men, 649 women) | 75 (5) | Community dwelling individuals | 800 IU vitamin D3 (100 000 IU every 4 months) or placebo | 80% | 60 | 74.3 (20.7) at 48 months | 53.4 (21.1) at 48 months |
| Chapuy et al, 2002w11 | Insufficient fall assessment | 583 women | 85 (7) | Ambulatory individuals living in apartment houses for elderly persons | 800 IU vitamin D3 + 1200 mg calcium (tri-calcium phosphate) or placebo | 95% | 24 | 21.3 (13.3) to 77.5 at 24 months | 22.8 (17.3) to 15 from bar graph at 24 months |
| Kärkkäinen et al, 2007w12 | Open study design | 3432 women | 67 | Community dwelling individuals | 800 IU vitamin D3 + 1 g calcium or control group (no placebo) | Adherence not stated | 36 | Not done | Not done |
| Law 2 et al, 006w13 | Open study design | 3717 (2788 women, 929 men) | 85 | Patients living in residential accommodation | 1100 IU vitamin D2 (100 000 IU ergocalciferol every 3 months) or no treatment (no placebo) | Adherence not stated | 10 | 47 (35-102) to 74 (52-110) at 3 months | Not done |
| Harwood et al, 2004w14 | Open study design | 76 women | 82 (67-92) | Patients in rehabilitation wards, previously community dwelling | 800 IU vitamin D3 + 1 g calcium or control group (no placebo) | Adherence not stated | 12 | 30 (6-75), to 50 at 12 months | 30 (12-64), to 27 at 12 months |
| Latham et al, 2003w15 | Patients in unstable health | 243 (129 women, 114 men) | 80 (77-81) | Patients in acute care for any reason | 1600 IU vitamin D2 (300 000 IU as single dose) or placebo | 100% | 6 | 37.5 (35-45) to 60 at 3 months | 47.5 (40-52.5) to 47.5 at 3 months |
The pooled effect of all 15 eligible trials including 17 786 individuals of any dose and any quality fall assessment suggested a non-significant 7% fall reduction with vitamin D (relative risk 0.93, 95% CI 0.87 to 1.01). However, variation among the 15 trials was larger than expected (Q test: P=0.009).
After adding the seven randomised controlled trials that did not meet our criteria for the primary analysis to the seven trials originally included the primary analysis, the pooled relative risk for the high dose of supplemental vitamin D (700-1000 IU vitamin D a day) was 0.92 (95% CI 0.85 to 0.99; total 17 281 individuals; table 5). However, variation among the 14 trials was larger than expected (Q-test: P=0.006), suggesting that adding the lower quality trials to the analysis introduces heterogeneity.
Table 5.
Sensitivity analysis of the seven high dose trials from the primary analysis and the seven eligible high dose trials that did not meet the criteria for the primary analysis
| Study (daily dose of vitamin D) | Number of participants | Number of fallers/total treated | Number of fallers/total control | Effect relative risk | Lower 95% CI | Upper 95% CI | Q test P value | I2 | Fall reduction |
|---|---|---|---|---|---|---|---|---|---|
| Pooled primary analysis of the seven high dose trials (w1(800),w2,w3,w4,w5,w6,w7) | 1921 | 435/959 | 528/962 | 0.81 | 0.71 | 0.92 | 0.12 | 41% | -19% |
| Sensitivity analysis including the seven high dose trials that did not meet criteria for primary analysis | |||||||||
| Grant et al, 2005w9 (800 IU D3) | 5292 | 380/2649 | 381/2643 | 1.00 | 0.87 | 1.14 | — | — | — |
| Trivedi et al, 2003w10 (800 IU D3) | 2038 | 254/1027 | 261/1011 | 0.96 | 0.83 | 1.11 | — | — | — |
| Chapuy et al, 2002w11 (800 D3) | 583 | 251/393 | 118/190 | 1.03 | 0.90 | 1.17 | — | — | — |
| Kärkkäinen et al, 2007w12 (800 IU D3) | 3432 | 180/1718 | 209/1714 | 0.86 | 0.71 | 1.04 | — | — | — |
| Law et al, 2006 w13 (1100 IU D2) | 3717 | 770/1762 | 833/1955 | 1.03 | 0.95 | 1.10 | — | — | — |
| Harwood et al, 2004w14 (800 IU D3) | 76 | 7/39 | 13/37 | 0.51 | 0.23 | 1.11 | — | — | — |
| Latham et al, 2003w15 (1600 IU D2) | 222 | 64/108 | 60/114 | 1.13 | 0.89 | 1.42 | — | — | — |
| Pooled sensitivity analysis (w1(800),w2,w3,w4,w5,w6,w7,w9,w10,w11,w12,w13,w14,w16) | 17 281 | 2341/8655 | 2403/8626 | 0.92 | 0.85 | 0.99 | 0.006 | 56% | -8% |
Q test: P<0.100 indicates heterogeneity.
I2 estimates above 25% are considered to represent modest heterogeneity, and values above 50% represent large heterogeneity beyond chance.
Trials of oral active forms of vitamin D
We found two randomised controlled trials of active forms of vitamin D that met our inclusion criteria (table 6).w16 w17 Falls were addressed as a primary outcome measure in both studies. No additional trials were identified for a sensitivity analysis. The two trials included 624 individuals with a mean age of 73 years, 70% of whom were women. In both studies, patients in the treatment group were more likely to experience hypercalcaemia (up to 3 mmol/l) than were those in the control group. The incidence of hypercalcaemia was twice as frequent in the treatment group as in the placebo group in one trial (12% v 6%),25 without details in the other.26 The pooled relative risk for fall prevention for active forms of vitamin D was 0.78 (95% CI 0.64 to 0.94), and active forms of vitamin D reduced the risk of falls by 22% (table 7).
Table 6.
Randomised controlled trials of active forms of vitamin D included in the primary analyses
| Source | Number of participants | Age (years; mean (SD)) | Sample population | Treatment (daily dose) | Adherence | Study duration (months) | 25(OH)D serum concentration (mmol/l; mean (SD)) | |
|---|---|---|---|---|---|---|---|---|
| Treatment group | Control group | |||||||
| Dukas et al, 2004w16 | 278 (191 women, 187 men) | 75 (5) | Community dwelling individuals | 1 μg 1α-hydroxyvitamin D3 or placebo | 84% among women, 87% among men | 9 | 78.0 (21.6) to 60.7 (19.7) at 9 months | 70.8 (26.8) at baseline at 9 months |
| Gallagher et al, 2001w17 | 246 women | 71 (4) | Community dwelling individuals | 0.5 μg 1,25-dihydroxyvitamin D3 or placebo | 70% | 36 | 74.8 (29.0) to 55.5 (20.5) at 36 months | 80.5 (27.4) to 63.2 (19.7) at 36 months |
Table 7.
Primary pooled analysis for active forms of vitamin D and the prevention of falls
| Study (daily dose of vitamin D) | Number of participants | Number of fallers/total treated | Number of fallers/total control | Effect relative risk | Lower 95% CI | Upper 95% CI | Q test P value | I2 | Fall reduction |
|---|---|---|---|---|---|---|---|---|---|
| Dukas et al, 2004w16 (1α-hydroxyvitamin D3) | 378 | 40/192 | 46/186 | 0.84 | 0.58 | 1.22 | — | — | — |
| Gallagher et al, 2001w17 (1,25-dihydroxyvitamin D3) | 246 | 59/123 | 78/123 | 0.76 | 0.60 | 0.95 | — | — | — |
| Pooled primary analysis (w16,w17) | 624 | 99/315 | 124/309 | 0.78 | 0.64 | 0.94 | 0.63 | 0% | -22% |
Q test: P<0.100 indicates heterogeneity.
I2 estimates above 25% are considered to represent modest heterogeneity, and values above 50% represent large heterogeneity beyond chance.
Fall prevention with active forms of vitamin D compared with high dose supplemental vitamin D
The pooled relative risk for fall prevention of 0.78 for active forms of vitamin D was similar to the pooled relative risk of 0.81 for high dose of supplemental vitamin D. The ratio of the two effect sizes (pooled relative risk supplemental vitamin D/pooled relative risk active forms of vitamin D) was 1.04 (95% CI 0.84 to 1.31), suggesting that active forms and standard forms of vitamin D have statistically indistinguishable effects on fall prevention.
Test for publication bias
We found no evidence for publication bias in the eight supplemental vitamin D trials, according to both Begg’s test and Egger’s test.24 Although the Begg’s test funnel plot indicated a potential absence of negative studies with small sample sizes, a trim and fill analysis did not confirm this possibility.27 28
Discussion
In this meta-analysis of eight double blind randomised controlled trials, the efficacy of supplemental vitamin D for fall prevention depended on dose and achieved 25(OH)D concentrations among individuals aged 65 years and older. No fall reduction was observed for a daily dose of less than 700 IU vitamin D or achieved serum 25(OH)D concentrations below 60 nmol/l. Daily vitamin D doses in the range of 700 IU to 1000 IU or achieved serum concentrations between 60 nmol/l and 95 nmol/l reduced the risk of falling by 19%. Given the absence of data beyond these beneficial ranges, our analyses don’t preclude the possibility that higher doses of vitamin D or higher achieved 25(OH)D concentrations would have been even more efficient in reducing falls. At the high dose range of 700 IU to 1000 IU a day, the benefit of vitamin D was not significantly affected by type of supplemental vitamin D, gender, age, or level of independence. Notably, fall prevention with a high dose might not depend on additional calcium supplementation and was attained with treatment for less than 12 months (2-5 months). The benefit was sustained for 12-36 months.
An important risk factor for falls is muscle weakness, which is a prominent feature of the clinical syndrome of vitamin D deficiency and could plausibly mediate fracture risk through increasing susceptibility to falls.29 Binding of vitamin D to its nuclear receptor in muscle tissue may lead to de novo protein synthesis,29 30 a benefit that appears to precede the effect of vitamin D on bone.2
Comparison with other studies
Our findings confirm those in an earlier meta-analysis on falls from 2004,10 which showed that any vitamin D reduced falls in older individuals by 22%. Of three trials with supplemental vitamin D included in this earlier meta-analysis, one trial of 400 IU vitamin D a day showed a neutral effect,w8 whereas two trials with 800 IU a day suggested a beneficial effect on risk of falls (odds ratio 0.65, 95% CI 0.40 to 1.00).w6 w7 Since then, five double blind trials with sufficient quality fall assessment have been performed.w1 w2 w3 w4 w5 Our meta-analysis including these trials confirmed a benefit of 700 IU to 1000 IU vitamin D a day. Lending further support to our findings, several double blind randomised controlled trials have documented fracture prevention with 700-800 IU vitamin D a day,12 31 32 but not with 400 IU a day.33 34 35
We found a 38% reduction in the risk of falling for the high dose range of vitamin D with treatment for 2-5 months, and a sustained 17% fall reduction with treatment for 12-36 months. Indirect support for a rapid and sustained effect of vitamin D on falls comes from the large fracture trial by Chapuy and colleagues.36 Although falls were not assessed in this trial, supplementation most likely reduced the number of falls, leading to the reduction in fracture risk apparent after six months.
Fall reduction was assessed as one of the end points in a 2007 evidence report on vitamin D commissioned by the United States Department of Health and Human Services.37 The authors combined nine blinded and two open design trials (n=13 888) of any oral dose of vitamin D (D2 and D3) with or without calcium compared with calcium or placebo. Their pooled result suggested a non-significant 8% reduction in falls with vitamin D (odds ratio 0.92, 95% CI 0.85 to 1.00). Heterogeneity by dose was not detected. The result of the evidence report is consistent with our sensitivity analysis of all 15 eligible trials regardless of vitamin D dose and quality of fall assessment: we found a non-significant 7% fall reduction with vitamin D in 17 786 individuals. Variation among the 15 trials was larger than expected (Q test: P=0.009), however, even for the high dose sensitivity analysis (Q test: P=0.006).
A 2007 meta-analysis that focused on vitamin D3 found a 12% reduction in falls by pooling four trials irrespective of their vitamin D dose or quality of fall assessment.38 Our analysis of five trials with sufficient quality fall assessment showed that high doses of vitamin D3 reduced falls by 26%.
We addressed the additional importance of calcium in our primary analysis of high dose trials and documented a 23% fall reduction on the basis of results from six high quality trials of vitamin D alone compared with placebo or vitamin D plus calcium compared with calcium.w1 w3-w7 Vitamin D in combination with calcium compared with placebo reduced falls by 19% according to limited data from one trial.w2 Thus, the effect of 700-1000 IU vitamin D a day on falls may not depend on additional calcium supplementation, which could be explained by the calcium sparing effect of vitamin D.39 40
Active forms of vitamin D reduced falls by 22% and the high dose supplemental vitamin D reduced falls by 19%, suggesting no difference in efficacy between these alternatives in unselected older persons. However, the efficacy data for active forms of vitamin D was drawn from relatively few studies. In addition, active forms cost more and have a higher risk profile, so we believe adequate dosing of supplemental vitamin D should be preferred. Importantly, the efficacy of active forms of vitamin D adds to the evidence that improved vitamin D status reduces the risk of falling in older individuals.
Limitations of study
As with all meta-analyses, this review has the potential for publication bias. However, we found no evidence for publication bias using the Begg’s test and the Egger’s test with all eight trials.24 Although the Begg’s test funnel plot suggested a possible absence of negative studies with small sample sizes, the trim and fill analysis did not confirm this suggestion.27 28 With respect to trial quality, our primary analysis was restricted to trials with a double blind design and sufficient quality fall assessment to address the efficacy of vitamin D for fall prevention. In our sensitivity analysis that included additional trials with an open study design or insufficient fall assessment, study variation was larger than expected for the pooled result from all 15 trials. Even within the 14 high dose trials, variation between trials was larger than expected, supporting our pre-defined strategy of focusing on fall efficacy from double blind trials with sufficient fall assessment.
Implications for future research
We found a greater fall reduction in studies with a maximum vitamin D daily dose of 1000 IU a day than in studies with lower doses; therefore, higher doses may be even more effective. Such doses should be explored in future research to optimise the fall prevention benefit with vitamin D.
Conclusions
Doses of 700 IU to 1000 IU supplemental vitamin D a day could reduce falls by 19% or by up to 26% with vitamin D3. This benefit may not depend on additional calcium supplementation, was significant within 2-5 months of treatment, and extended beyond 12 months of treatment. Conversely, our results do not support the clinical use of vitamin D doses below 700 IU a day for the prevention of falls among older individuals. A 25(OH)D concentration of at least 60 nmol/l is required for fall prevention; therefore, a daily intake of at least 700 IU supplemental vitamin D is warranted in all individuals age 65 and older. Notably, good adherence is essential as the effect of vitamin D on falls will not be proportional below 700 IU a day. Furthermore, it is possible that greater benefits may be achieved with the use of vitamin D3 instead of vitamin D2. Finally, active forms of vitamin D do not appear to be more effective than 700-1000 IU of supplemental vitamin D for fall prevention in older persons.
What is already known on this topic
Recent systematic reviews suggest a non-significant reduction in falls among individuals receiving supplemental vitamin D
Vitamin D has a direct beneficial effect on muscle, and improved strength and balance in several trials in older persons
What this study adds
A dose of 700-1000 IU supplemental vitamin D a day reduced falls by 19%, and by up to 26% with vitamin D3, within 2-5 months of treatment initiation
Vitamin D may not reduce falls at doses of less than 700 IU a day
Active forms of vitamin D do not appear to be more effective for fall prevention than 700-1000 IU of supplemental vitamin D
Contributors: All authors contributed to the conception and design of the study and interpretation of data. The analysis was performed by HAB-F and JH, with methodological support by JEO, DPK, and JBW. HAB-F drafted the manuscript with input from all authors. The approval of the final version of the manuscript was given by all co-authors. HAB-F acts as guarantor.
Funding: This project was funded by a Swiss National Foundations Professorship Grant (PP00B-114864), the Velux Foundation, the Baugarten Foundation, the Vontobel Foundation, and a fellowship from the Robert Bosch Foundation. DPK was funded by the National Institute on Aging (grant P01 AG004390).
Sponsors: No sponsors participated in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Competing interests: None declared.
In order to ensure compliance with the National Institutes of Health policy, all US authors retain the rights to: provide a copy of the authors’ final manuscript, including all modifications from the publishing and peer review process, to the National Library of Medicine’s PubMed Central database at the time the manuscript is accepted for publication; authorise the NIH to make such copy of the manuscript available in digital form for public access in PubMed Central no later than 12 months after publication; prepare derivative works from the manuscript; authorise others to make any use of the manuscript provided that it is not sold for a profit and that the author receives credit as author and the journal in which the manuscript has been published is cited as the source of first publication; and distribute copies of the manuscript in connection with teaching and research by the author and by the author’s employer.
Cite this as: BMJ 2009;339:b3692
References
- 1.Blake AJ, Morgan K, Bendall MJ, Dallosso H, Ebrahim SB, Arie TH, et al. Falls by elderly people at home: prevalence and associated factors. Age Ageing 1988;17:365-72. [DOI] [PubMed] [Google Scholar]
- 2.Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol 1996;143:1129-36. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319:1701-7. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res 2003;18:343-51. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res 2004;19:265-9. [DOI] [PubMed] [Google Scholar]
- 6.Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int 2000;66:419-24. [DOI] [PubMed] [Google Scholar]
- 7.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet 1976;1:626-9. [DOI] [PubMed] [Google Scholar]
- 8.Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int 2009;20:315-22. [DOI] [PubMed] [Google Scholar]
- 9.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res 2000;15:1113-8. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff-Ferrari HA, Dawson-Hughes B, Willett CW, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of vitamin D on falls: a meta-analysis. JAMA 2004;291:1999-2006. [DOI] [PubMed] [Google Scholar]
- 11.Law M, Withers H, Morris J, Anderson F. Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in elderly people in residential accommodation. Age Ageing 2006;35:482-6. [DOI] [PubMed] [Google Scholar]
- 12.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab 2007; 92: 2058-65. [DOI] [PubMed]
- 14.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged >=60 y. Am J Clin Nutr 2004;80:752-8. [DOI] [PubMed] [Google Scholar]
- 15.Heikinheimo RJ, Haavisto MV, Harju EJ, Inkovaara JA, Kaarela RH, Kolho LA, et al. Serum vitamin D level after an annual intramuscular injection of ergocalciferol. Calcif Tissue Int 1991;49:S87. [DOI] [PubMed] [Google Scholar]
- 16.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
- 17.Cook DJ, Sackett DL, Spitzer WO. Methodologic guidelines for systematic reviews of randomized control trials in health care from the Potsdam Consultation on Meta-Analysis. J Clin Epidemiol 1995;48:167-71. [DOI] [PubMed] [Google Scholar]
- 18.Jongen MJ, Van Ginkel FC, van der Vijgh WJ, Kuiper S, Netelenbos JC, Lips P. An international comparison of vitamin D metabolite measurements. Clin Chem 1984;30:399-403. [PubMed] [Google Scholar]
- 19.Jongen MJ, van der Vijgh WJ, van Beresteyn EC, van den Berg H, Bosch R, Hoogenboezem T, et al. Interlaboratory variation of vitamin D1 metabolite measurements. J Clin Chem Clin Biochem 1982;20:753-6. [DOI] [PubMed] [Google Scholar]
- 20.Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int 1999;9:394-7. [DOI] [PubMed] [Google Scholar]
- 21.Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med 1995;14:395-411. [DOI] [PubMed] [Google Scholar]
- 22.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559-73. [DOI] [PubMed] [Google Scholar]
- 23.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693-708. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Smith GD, Altman DG. Systemic reviews in health care: meta-analysis in context. BMJ Publishing Group, 2001.
- 25.Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab 2001;86:3618-28. [DOI] [PubMed] [Google Scholar]
- 26.Dukas L, Bischoff HA, Lindpaintner LS, Schacht E, Birkner-Binder D, Damm TN, et al. Alfacalcidol reduces the number of fallers in a community-dwelling elderly population with a minimum calcium intake of more than 500 mg daily. J Am Geriatr Soc 2004;52:230-6. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [DOI] [PubMed] [Google Scholar]
- 29.Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev 1986;7:434-447. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen OH, Lund B, Saltin B, Andersen RB, Hjorth L, Melsen F, et al. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Colch) 1979;56:157-61. [DOI] [PubMed] [Google Scholar]
- 31.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670-6. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck A, Staehelin HB, Orav JE, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 2009;169:551-61. [DOI] [PubMed] [Google Scholar]
- 33.Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med 1996;124:400-6. [DOI] [PubMed] [Google Scholar]
- 34.Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res 2002;17:709-15. [DOI] [PubMed] [Google Scholar]
- 35.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669-83. [DOI] [PubMed] [Google Scholar]
- 36.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992;327:1637-42. [DOI] [PubMed] [Google Scholar]
- 37.Cranny A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess 2007;158:1-235. [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson C, Gaugris S, Sen SS, Hosking D. The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: a meta-analysis. QJM 2007;100:185-92. [DOI] [PubMed] [Google Scholar]
- 39.Heaney RP, Barger-Lux MJ, Dowell MS, Chen TC, Holick MF. Calcium absorptive effects of vitamin D and its major metabolites. J Clin Endocrinol Metab 1997;82:4111-6. [DOI] [PubMed] [Google Scholar]
- 40.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 2003;22:142-6. [DOI] [PubMed] [Google Scholar]