Abstract
We present a novel voltage-sensitive hemicyanine dye ANNINE-6plus and describe its synthesis, its properties and its voltage-sensitivity in neurons. The dye ANNINE-6plus is a salt with a double positively charged chromophore and two bromide counterions. It is derived from the zwitterionic dye ANNINE-6. While ANNINE-6 is insoluble in water, ANNINE-6plus exhibits a high solubility of around 1 mM. Nonetheless, it displays a strong binding to lipid membranes. In contrast to ANNINE-6, the novel dye can be used to stain cells from aqueous solution without surfactants or organic solvents. In neuronal membranes, ANNINE-6plus exhibits the same molecular Stark effect as ANNINE-6. As a consequence, a high voltage-sensitivity is achieved with illumination and detection in the red end of the excitation and emission spectra, respectively. ANNINE-6plus will be particularly useful for sensitive optical recording of neuronal excitation when organic solvents and surfactants must be avoided as with intracellular or extracellular staining of brain tissue.
Keywords: Voltage-sensitive dye, Optical recording, Fluorescence, Membrane voltage, Nerve cells
Introduction
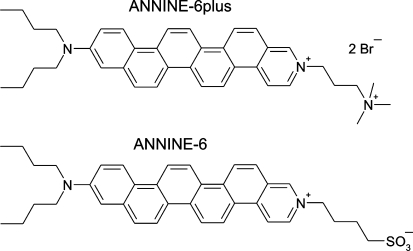
Optical recording with fast voltage-sensitive fluorescent dyes is a well-established method for observing the spatiotemporal electrical activity of nerve cells and of brain tissue (Cohen and Salzberg 1978; Loew 1982; Grinvald and Hildesheim 2004). A particularly useful class of dyes are the styryl-type hemicyanines such as RH-421 and Di-4-ANEPPS (Grinvald et al. 1982; Fluhler et al. 1985). In our previous work, hemicyanines without twistable single or double bonds were developed to eliminate the impact of complex intramolecular dynamics on the voltage-sensitive fluorescence (Ephardt and Fromherz 1989; Ephardt and Fromherz 1993; Röcker et al. 1996). The work resulted in the development of the zwitterionic dye ANNINE-6 (Fig. 1) containing six anellated benzene rings (Hübener et al. 2003). ANNINE-6 was found to exhibit a very high voltage-sensitivity that is exclusively based on a molecular Stark effect (Kuhn and Fromherz 2003). However, a serious problem with that dye is its extremely low solubility in water. Intracellular staining of brain tissue was impossible (Dombeck et al. 2005). Staining of cultured cells was achieved only with auxiliary agents like bile salt or polyol surfactant (Kuhn and Fromherz 2003; Kuhn et al. 2004).
Fig. 1.
Hemicyanine dyes with anellated benzene rings: dye salt ANNINE-6plus with double positively charged chromophore and two bromide counter ions and zwitterionic dye ANNINE-6
To overcome the problem of staining, we synthesized ANNINE-6plus (Fig. 1), a dye salt with a double positively charged chromophore and two bromide counterions. A similar approach was previously applied for styryl-type dyes (Grinvald et al. 1982). In the present note, we characterize ANNINE-6plus with regard to water solubility, membrane binding, cell staining and voltage sensitivity in neurons. We find that ANNINE-6plus exhibits both excellent solubility in water and a strong membrane binding. Cells are efficiently stained from aqueous solution, and a high voltage-sensitivity is observed in neurons.
Materials and methods
Synthesis
We synthesized ANNINE-6plus from a precursor of ANNINE-6 (Hübener et al. 2003). 11-Di-n-butylamino-3-aza-benzo[m] picene (0.2 g, 0.44 mM) and (3-bromopropyl)-trimethyl-ammonium bromide (Aldrich, 0.57 g, 2.2 mM) were dissolved in 3 ml DMF and heated for 9 h at 120°C. Upon cooling, Et2O (50 ml) was added to precipitate 11-Di-n-butylamino-3-(3-trimethyl-ammonium-propyl)-3-azonia-benzo[m] picene dibromide. After decanting the supernatant, the product (MW 717.6 Da) was purified by column chromatography (SiO2, 42–105 μm, YMC, with CHCl3:MeOH:H2O, 50:20:4) with a yield of 40 mg (12.6%, red solid, m.p. > 220°C). It was identified by 1H NMR and mass spectroscopy.
Solubility
To evaluate the saturation concentration of ANNINE-6 and ANNINE-6plus an excess of dye powder in dioxane/water mixtures was stirred for 6 h at room temperature. After centrifugation, the dye concentration was determined by light absorption at a maximum around 440 nm (Cary 3E, Varian). An extinction coefficient of 25,000 M−1 cm−1 was determined from a defined concentration in ethanol.
Membrane binding
We studied the membrane binding of ANNINE-6plus by fluorescence titration with lipid vesicles (Bashford et al. 1979; Fromherz and Röcker 1994; Hinner et al. 2004). The vesicles were made from 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC, Lipoid KG, Ludwigshafen, Germany) by extrusion (MacDonald et al. 1991) through a polycarbonate filter with 100 nm pore size (Avestin Europe, Mannheim, Germany) in Tris–NaCl buffer (20 mM Tris, 100 mM NaCl, pH 8.1). The lipid concentration was determined by a chromogenic enzyme assay (Biomerieux, Marcy l´Etoile, France). Vesicle size was checked by quasielastic light scattering. Suspensions of vesicles were prepared with lipid concentrations from 40 nM to 2 mM. They were mixed with an equal volume of 1 μM ANNINE-6plus in Tris–NaCl in 2 ml plastic tubes (Eppendorf, Hamburg, Germany). After sonication for 30 min (Sonorex RK 510H, Bandelin, Berlin, Germany), the fluorescence was recorded at 600 nm with an excitation at 488 nm (SLM Amino 8100, Acton Research, Acton, MA) at 25°C). The increase of fluorescence intensity with increasing lipid concentration was evaluated in terms of a binding isotherm
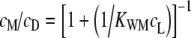 with the concentration cM of dye bound to the membrane, with a total dye concentration cD, with a lipid concentration cL and a binding constant KWM (Fromherz and Röcker 1994; Hinner et al. 2004). That relation has the form of a mass action law, but does not imply a 1:1 molecular association. It reflects a partitioning equilibrium of the dye between water and membrane (Fromherz and Röcker 1994). We assumed that only the outer monolayer of the vesicles was effective in dye binding.
with the concentration cM of dye bound to the membrane, with a total dye concentration cD, with a lipid concentration cL and a binding constant KWM (Fromherz and Röcker 1994; Hinner et al. 2004). That relation has the form of a mass action law, but does not imply a 1:1 molecular association. It reflects a partitioning equilibrium of the dye between water and membrane (Fromherz and Röcker 1994). We assumed that only the outer monolayer of the vesicles was effective in dye binding.
Staining
The staining solutions for giant lipid vesicles and for HEK293 cells with a dye concentration of 5 μg/ml were prepared from stock solutions of 0.5 mg/ml either with ANNINE-6plus in pure water (Millipore) or with ANNINE-6 in DMSO containing 20% Pluronic F127 (Molecular Probes). Giant vesicles were made by electroswelling from 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) in 300 mM aqueous saccharose (Dimitrov and Angelova 1988; Hinner et al. 2004), and transferred to polypropylene culture dishes (BD Biosciences) containing ANNINE-6 or ANNINE-6plus at a concentration of 5 μg/ml in 300 mM glucose. After sedimentation for around 10 min, fluorescence images were taken with a microscope (Axioskop, 63× water immersion objective, Zeiss) equipped with a CCD camera (Sony ICX 285, Theta Systems, Germany). Excitation was at 450 nm with a bandwidth of 50 nm and detection was beyond 515 nm. HEK293 cells (DSMZ, Braunschweig, Germany) were cultured at 37°C and 5% CO2 in Dulbecco’s modified eagle medium with 10% fetal calf serum and l-glutamine (Gibco). Before imaging, cells were washed with physiological buffer (5.4 mM KCl, 135 mM NaCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 5 mM HEPES, pH 7.3), incubated for 5 min in buffer containing the dye at a concentration of 5 μg/ml, washed twice in buffer and transferred to the microscope.
Voltage sensitivity
The measurements of voltage sensitivity followed the procedure described by Kuhn and Fromherz (2003). In short, Retzius neurons were dissociated from the ganglia of Hirudo medicinalis and held in defined culture medium on the stage of a fluorescence microscope. After staining, defined intracellular voltages were applied with a whole-cell patch pipette. Both the wavelength of excitation and of emission was varied. The normalized fluorescence intensity F/FMAX at an intracellular voltage of −50 mV were measured as well as the relative change of fluorescence in response to a voltage step of 100 mV, i.e. the voltage sensitivity defined by SV = (ΔF/F)/100 mV. The data are plotted versus the wavenumber of excitation and emission that reflect the energy difference between excited state and ground state that is affected by the electrical field.
Results and discussion
Solubility and membrane binding
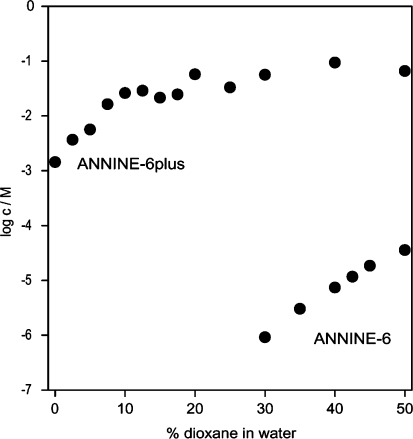
We found that ANNINE-6plus is well soluble in water whereas the solubility of ANNINE-6 in water was too low to be detected. To permit a quantitative comparison of the two dyes, we determined the saturation concentrations in a series of water/dioxane mixtures. The results are plotted in Fig. 2. The saturation concentration of ANNINE-6plus in pure water is about 1 mM. At 30% dioxane, we were able to determine a saturation concentration for ANNINE-6 of 1 μM. In the same solvent, the saturation concentration of ANNINE-6plus was enhanced to 0.1 M. There is a difference of solubility by five orders of magnitude.
Fig. 2.
Solubility of the dye salt ANNINE-6plus and of the zwitterionic dye ANNINE-6 as the logarithm of the saturation concentration in water/dioxane mixtures. At 30% (v/v) dioxane, the solubility of the two dyes differs by five orders of magnitude
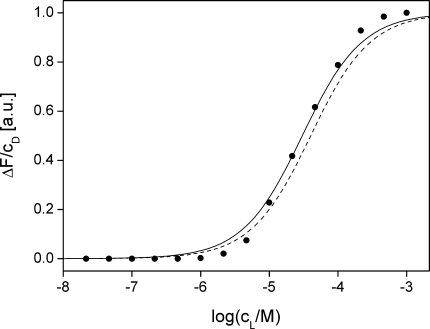
We studied the membrane binding of ANNINE-6plus by fluorescence titration with liposomes (Bashford et al. 1979; Fromherz and Röcker 1994; Hinner et al. 2004). An example is shown in Fig. 3. Fitting the normalized increase of fluorescence with a binding isotherm, we found a binding constant of KWM = 70,000 M−1 (standard deviation σ = 18,000 M−1 in four measurements) at a dye concentration of 0.5 μM. A similar titration of the zwitterionic ANNINE-6 was not possible due to the low solubility of the dye in water. For a comparison we may consider the well-known zwitterionic voltage-sensitive dye Di-4-ANEPPS, a homologous styryl-type hemicyanine, with a binding constant of KWM = 50,000 M−1 (Fluhler et al. 1985).
Fig. 3.
Titration of the dye salt ANNINE-6plus with lipid vesicles of palmitoyl-oleoyl-phosphatidyl-choline (POPC). The normalized increase of fluorescence intensity ΔF divided by the dye concentration cD at each data point is plotted versus the logarithm of the lipid concentration cL. It reflects the fraction of lipid-bound dye. The data are fitted with a binding constant KWM = 66,300 M−1 for the partitioning equilibrium between water and membrane. For comparison, a computed titration curve is drawn (dashed line) for the zwitterionic dye Di-4-ANEPPS with KWM = 50,000 M−1 because a titration of ANNINE-6 is not possible due to its low solubility
ANNINE-6plus strongly binds to lipid membranes despite its good water solubility. There is a tremendously different impact of the changed head group on solubility and on membrane binding. This can be explained by the different influence of the bromide counterions on the two processes. The dissolution of ANNINE-6 with its unpolar chromophore is energetically unfavourable because the strong dispersion interactions in the crystal are not compensated by solvation with water. While that unfavourable solvation of the chromophore also applies to ANNINE-6plus, the dissolution of the whole dye salt is promoted by the stoichiometrically coupled release of two bromide counterions that are well soluble. However, in the binding process of ANNINE-6plus to a lipid membrane the bromide counterions play no role. The binding is governed by the interaction of the unpolar chromophore with the hydrophobic membrane interior as with a zwitterionic dye. The two bromide ions are not involved because they remain in the aqueous phase.
Staining
The binding constant KWM for the interaction of dye and lipid describes a partitioning equilibrium between the two phases of water and of lipid membrane (Fromherz and Röcker 1994). The density of dye molecules in the membrane is given by KWMcW/aL with a dye concentration cW in water at an area per lipid molecule aL. For example, at a concentration cW = 10 μM of ANNINE-6plus with KWM = 70,000 M−1 and aL = 0.7 nm2, we expect a strong staining of the membrane with a density of 1 molecule/nm2.
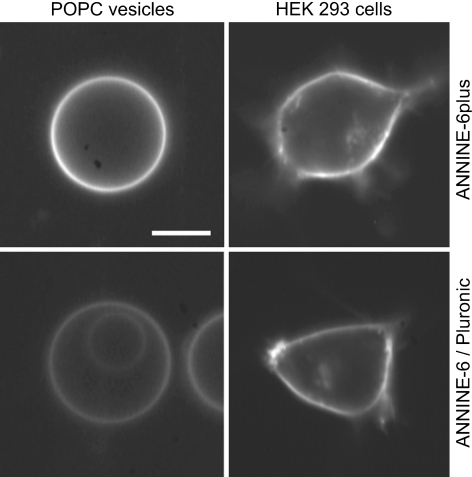
We qualitatively checked the staining efficiency for giant vesicles and for HEK293 cells. We used ANNINE-6plus that was added from a stock solution in pure water, and for comparison ANNINE-6 that was added from a stock solution in DMSO with 20% of the polyol surfactant Pluronic F127 (Kuhn et al. 2004). In both cases the final concentration was 5 μg/ml. Fluorescence pictures of giant vesicles and HEK293 cells are shown in Fig. 4. Both the giant vesicles and the cells are well stained by ANNINE-6plus. The staining is at least as strong as by ANNINE-6 solubilized with DMSO/Pluronic F127. With HEK293 cells, the internalization of the dye was slow with a dominant staining of plasma membrane after 20 h at room temperature.
Fig. 4.
Staining of giant lipid vesicles and of HEK293 cells by ANNINE-6plus (top) and by ANNINE-6/DMSO/Pluronic F127 (bottom) at a total dye concentration of 5 μg/ml. Scale bar 10 μm. The conditions of fluorescence microscopy were identical in all cases. The lipid vesicles were allowed to sediment for 5 min in 300 mM glucose before the pictures were taken. The cells were stained for 5 min in physiological buffer (5.4 mM KCl, 35 mM NaCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 5 mM HEPES, pH 7.3) and washed twice with buffer
Voltage sensitivity
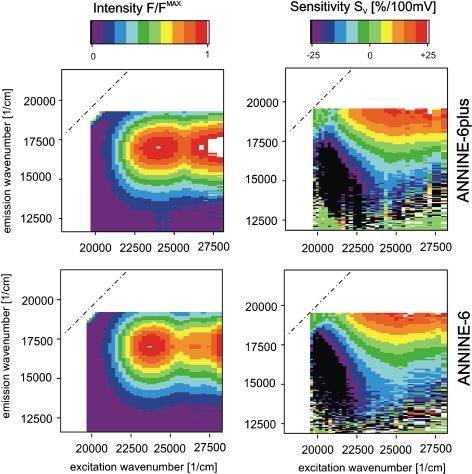
We determined the voltage-sensitivity of ANNINE-6plus in neurons using Retzius cells from the leech. Steps of membrane voltage were applied with a whole-cell patch-pipette. As a reference, the normalized fluorescence intensity F/FMAX is shown in Fig. 5 (left) at an intracellular voltage of −50 mV as a function of the wavenumbers of excitation and emission. The relative change of the fluorescence intensity to a voltage step of 100 mV—the voltage sensitivity SV = (ΔF/F)/100 mV—is plotted in Fig. 5 (right). For comparison, the data for ANNINE-6 are also plotted (Kuhn and Fromherz 2003). Apparently the fluorescence spectra are very similar for both dyes with maxima of excitation and emission at 23,930 and 17,250 cm−1 for ANNINE-6plus and 23,673 and 17,456 cm−1 for ANNINE-6. Also the sensitivity spectra are very similar. For both the dyes, a positive change of the membrane voltage leads to a decrease of the fluorescence intensity in the red and an increase in the blue for the excitation as well as for the emission. We fitted the data of ANNINE-6plus by lognormal functions for excitation and emission, and evaluated spectral blue shifts of 160 and 200 cm−1 for excitation and emission by a depolarization of 100 mV, as compared to 163 and 170 cm−1 for ANNINE-6 (Kuhn and Fromherz 2003). These shifts are equal within the uncertainties of measurement and fit.
Fig. 5.
Voltage-sensitive fluorescence of ANNINE-6plus (top) and ANNINE-6 (bottom) in a neuron (Retzius cell from Hirudo medicinalis). Left Normalized fluorescence intensity F/FMAX as a function of the wavenumber of excitation and emission. The peak reflects the S0–S1 transition. The S0–S2 transition is excited at high wavenumbers. Right Voltage sensitivity SV = (ΔF/F)/100 mV—the relative change of fluorescence intensity by a 100 mV change of membrane voltage. The fluorescence is lowered in the red and enhanced in the blue of the excitation and of the emission spectrum. Dashed lines mark the diagonals of the two dimensional spectra
The similar spectra of excitation and emission for ANNINE-6plus and ANNINE-6 indicate a similar interaction of the chromophores with the plasma membrane. The similar voltage-sensitivities indicate a similar interaction of the chromophores with the electrical field in the membrane. Thus we can attribute the voltage-sensitivity of ANNINE-6plus to a molecular Stark effect as described for ANNINE-6 with a chromophore that is located at the outer surface of the plasma membrane with a preferential orientation along the membrane normal. The conclusions about optical recording with ANNINE-6 (Kuhn and Fromherz 2003) remain fully valid for ANNINE-6plus: a high voltage-sensitivity is attained at a high signal-to-noise ratio by an intense narrow band illumination in the red of the excitation spectrum and a sensitive broad band detection in the red of the emission spectrum.
Conclusion
We have shown that the anellated hemicyanine dye ANNINE-6plus exhibits both good solubility in water and a strong membrane binding. Cells are efficiently stained from aqueous solution. ANNINE-6plus exhibits the same high voltage-sensitivity in neurons as described for ANNINE-6. Thus ANNINE-6plus has all properties that are required for sensitive optical recording of neuronal excitation in situations when organic solvents and surfactants must be avoided as with intracellular or extracellular staining of brain tissue.
Acknowledgments
We thank Sonja Golla, Michaela Morawetz, and Stephanie Stumhofer for their excellent technical assistance with dye synthesis, measurements of solubility and binding constants, and Armin Lambacher and David Ng for critical reading of the manuscript.
References
- Bashford CL, Chance B, Smith JC, Yoshida T (1979) The behaviour of oxonol dyes in phospholipid dispersions. Biophys J 25:63–85 [DOI] [PMC free article] [PubMed]
- Cohen LB, Salzberg BM (1978) Optical measurements of membrane potential. Rev Physiol Biochem Pharmacol 83:35–88 [DOI] [PubMed]
- Dimitrov DS, Angelova MI (1988) Lipid swelling and liposome formation mediated by electric fields. Bioelectrochem Bioenerg 19:323–336 [DOI]
- Dombeck DA, Sacconi L, Blanchard-Desce M, Webb WW (2005) Optical recording of fast neuronal membrane potential transients in acute mammalian brain slices by second harmonic generation microscopy. J Neurophysiol 94:3628–2626 [DOI] [PubMed]
- Ephardt H, Fromherz P (1989) Fluorescence and photoisomerization of an amphiphilic aminostilbazolium dye as controlled by the sensitivity of radiationless deactivation to polarity and viscosity. J Phys Chem B 93:7717–7725 [DOI]
- Ephardt H, Fromherz P (1993) Fluorescence of amphiphilic hemicyanine dyes without free double-bonds. J Phys Chem B 97:4540–4547 [DOI]
- Fluhler E, Burnham VG, Loew LM (1985) Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry 24:5749–5755 [DOI] [PubMed]
- Fromherz P, Röcker C (1994) Staining of biomembranes by amphiphilic hemicyanine dyes. Ber Bunsenges Phys Chem 98:128–131
- Grinvald A, Hildesheim R (2004) VSDI: A new era in functional imaging of cortical dynamics. Nat Rev Neurosci 5:874–885 [DOI] [PubMed]
- Grinvald A, Hildesheim R, Farber IC, Anglister L (1982) Improved fluorescent probes for the measurement of rapid changes in membrane potential. Biophys J 39:301–308 [DOI] [PMC free article] [PubMed]
- Hinner MJ, Hübener G, Fromherz P (2004) Enzyme induced staining of biomembranes with voltage-sensitive fluorescent dyes. J Phys Chem B 108:2445–2453 [DOI] [PubMed]
- Hübener G, Lambacher A, Fromherz P (2003) Anellated hemicyanine dyes with large symmetrical solvatochromism of absorption and fluorescence. J Phys Chem B 107:7896–7902 [DOI]
- Kuhn B, Fromherz P (2003) Anellated hemicyanine dyes in a neuron membrane: molecular Stark effect and optical voltage recording. J Phys Chem B 107:7903–7912 [DOI]
- Kuhn B, Fromherz P, Denk W (2004) High sensitivity of Stark-shift voltage-sensing dyes by one- or two-photon excitation near the red spectral edge. Biophys J 87:631–639 [DOI] [PMC free article] [PubMed]
- Loew LM (1982) Design and characterization of electrochromic membrane probes. J Biochem Biophys Methods 6:243–260 [DOI] [PubMed]
- MacDonald RC, MacDonald RI, Menco BPM, Takeshita K, Subbarao NK, Hu LR (1991) Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta 1061:297–303 [DOI] [PubMed]
- Röcker C, Heilemann A, Fromherz P (1996) Time-resolved fluorescence of hemicyanine dye: dynamics of rotamerism and resolvation. J Phys Chem B 100:12172–12177 [DOI]