Abstract
The legless locomotion of snakes requires specific adaptations of their ventral scales to maintain friction force in different directions. The skin microornamentation of the snake Corallus hortulanus was studied by means of scanning electron microscopy and the friction properties of the skin were tested on substrates of different roughness. Skin samples from various parts of the body (dorsal, lateral, ventral) were compared. Dorsal and lateral scales showed similar, net-like microornamentation and similar friction coefficients. Average friction coefficients for dorsal and lateral scales on the epoxy resin surfaces were 0.331 and 0.323, respectively. In contrast, ventral scales possess ridges running parallel to the longitudinal body axis. They demonstrated a significantly lower friction coefficient compared to both dorsal and lateral scales (0.191 on average). In addition, ventral scales showed frictional anisotropy comparing longitudinal and perpendicular direction of the ridges. This study clearly demonstrates that different skin microstructure is responsible for different frictional properties in different body regions.
Keywords: Snake skin, Microornamentation, Friction, Biological materials, Biotribology
Introduction
Snakes lack legs and use the surface of the body itself to generate propulsion on the ground during locomotion. For this purpose, some frictional grip is mandatory, in order to get the force onto the ground. Depending on the snake species, type of movement, environment and preferred substrate, different parts of the body must have different functional requirements and therefore different frictional properties. Frictional properties of the contact pair depend on the stiffness of materials of the contact pair, their physico-chemical properties, and their surface profiles (Bowden and Tabor 1986; Scherge and Gorb 2001).
The snakes’ epidermis is made up of different layers with the innermost called the stratum germinativum. The outer layers, which are renewed during shedding, are, from the inside, α-, mesos-, β-layer, and Oberhäutchen. The Oberhäutchen, mainly consisting of β-keratin, is in direct contact with the environment. It is well known that the Oberhäutchen possesses a fine surface structure called microornamentation (Leydig 1873; Ruibal 1968), whose details were described by earlier authors with the use of electron microscopy (Hoge and Souza Santos 1953; Price 1983; Bea and Fontarnau 1986; Fontarnau and Bea 1987; Stille 1987; Chiasson et al. 1989; Chiasson and Lowe 1989; Price and Kelly 1989; Price 1990).
Functions of the lizard and snake microornamentation have been previously discussed in the literature. It was suggested that the microstructure functions as a kind of zip-fastener supporting the moulding process by holding old and new skin together until the old skin is entirely shed (Maderson 1966). Ruibal and Ernst (1965) proposed the function of surface strengthening by the surface microgrooves. Anti-contamination capability of the scales has been shown for uropeltid snakes (Gans and Baic 1977; Gower 2003). An anti-fouling effect for sea-snake scales was proposed by McCarthy (1987). In contrast to these functional interpretations of microornamentations, Price (1982) proposed that interspecific differences are independent of ecological or environmental factors.
A qualitative frictional analysis of the snake skin at the nanoscale has been performed previously by using atomic force microscopy in three species (Boa constrictor, Morelia spilotes, and Python regius) (Hazel et al. 1999). The authors found friction anisotropy by using the AFM-cantilever in anterior and posterior directions of the scale. They suggested that narrow, backward pointing “spikes” (referred to as microfibrils by the authors), as part of the microornamentation in ventral scales, were responsible for anisotropic properties. However, no comparison to scales from other regions of the body has been done so far. Since keratinised biological micro- and nano-structures may have specific interactions with various substrata as has been previously shown for gecko setae (Hiller 1968), we expect to reveal micro- and nanostructural adaptations to legless locomotion on the ventral and possibly lateral scale surface of the snake in a macroscopical tribological experiment.
The goal of the present study was to analyse relationships between the microstructure of scales and their frictional properties at different regions of the body in the Amazon tree boa Corallus hortulanus (Squamata, Boidae), which is a specialised arboreal snake. The high degree of specialisation was the reason for us to work on this particular species. Frictional properties of the skin were characterised on the smooth and rough substrata in different direction relative to the body axis of the snake.
Materials and methods
Animals and preparations
Two specimens of the Amazon tree boa C. hortulanus were used in this study. Specimens were killed by an overdose of isoflurane and stored at −70°C in polyethylene bags. Pieces of frozen snakes were cut off and thawed at room temperature. The skin was removed and carefully cleaned from remaining subdermal tissue. During the preparation, we avoided touching skin regions used in further experiments and microscopic analysis.
Scanning electron microscopy
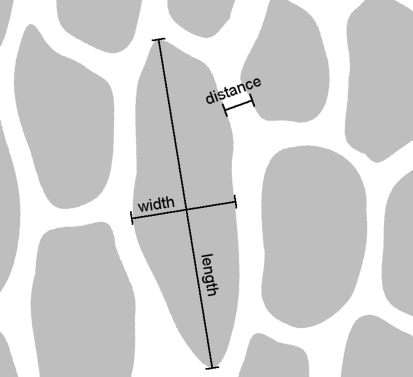
Small pieces of the skin were dehydrated for 15 min in absolute methanol and critical point dried using a critical point drying apparatus (E3000 Series, Quorum Technologies, UK). Dried samples were mounted on aluminum stubs, sputter coated with 6 nm of gold–paladium (SCD 500 Sputter Coater equipped with QSG 100 Quartz Film Thickness Monitor, BAL-TEC, Liechtenstein) and viewed using a Hitachi S-4800 (Hitachi, Japan) scanning electron microscope at an acceleration voltage of 3 kV. Surface structures were measured from SEM micrographs using the software SigmaScan Pro 5 (Systat Software Inc., USA). The length and the width of the grooves as well as distance between grooves were measured. The “length” is defined as the maximum dimension of a groove, which was always oriented in approximately rostro-caudal direction. The “width” of a groove is defined as the maximum dimension orthogonal to the length (see Fig. 1).
Fig. 1.
Schematic drawing of microornamentation to illustrate the method of measuring surface structures. Grooves are indicated in grey
Substrate preparation for friction measurements
Plates, made of the polymerised epoxy resin (61.3% NSA, 23.6% ERL 4221, 14.2% D.E.R. 736, 0.9% DMAE; for details see Spurr 1969) were used as substrate surfaces in measurements of the snake skin friction. The surfaces of the epoxy resin were positive replicas of glass and different polishing papers. In order to prepare the replicas, a two step molding technique was applied (Gorb 1999, 2007). The negative molds were made by coating the original surface attached to a plane wooden plate with a two-compound polymer polyvinylsiloxane (PVS) (President light body, Coltène, Switzerland). As original surfaces, smooth glass plates and polishing papers (Fibrmet Discs, Buehler, Germany) were used. Polishing papers used had grain sizes of 0.3, 1, 3, 9, and 12 μm. Additionally, the sandpapers of the grit size of P220, P100, and P60 were used. Grit sizes rougher than P60 resulted in a regular hooking of the scales edges. Thus the friction properties of the surface itself could not be measured. The PVS negatives were then used to obtain the positives by covering the negative surface with fluid epoxy resin and polymerizing it for 24 h at 70°C.
The final epoxy substrates had average surface roughnesses (Ra) of 0.08 (glass mold), 0.25, 0.42, 1.11, 2.25, 2.75, 12.67, 13.94 μm (the mold of P60 sandpaper was too rough to measure with the instruments available). These values are the means obtained from the measurements using three surface profiling instruments: surface perthometer Dektak 8 (Veeco Instruments Inc., USA), white light interferometer New View 5000 (Zygo Corporation, USA), and Surftest 301 (Mitutoyo Corporation, Japan). The scanned distances per sample were 3 mm for Dektak (all surfaces except P60) and three times 2.5 mm for Surftest (molds of 0.3 μm polishing paper to P220 sandpaper). For Niew View the examined areas were 0.07 × 0.05 mm (glass mold, 0.3, and 1 μm polishing paper), 0.14 × 0.11 mm (3 μm paper), and 0.36 × 0.20 mm (9 and 12 μm paper). Thus, different physical principles for evaluation of the surface roughness were used. The white light interferometer uses interferences caused by the roughness of the surface while the other two instruments scan the surface mechanically with a stylus.
Friction measurements
For friction measurements, the thawed fresh skin was wrapped around a polytetrafluoroethylen (PTFE) plate (10 × 10 × 3 mm) containing a hole of about 2 mm in diameter. The hole was situated in the centre of the plate and was not covered by the skin on one side. A few layers of cellulose were placed between the plate and skin, so that the skin could be kept moist by pouring water through the hole without wetting the surface of the scales. Otherwise, the capillary forces, caused by water, could have falsified the measurements.
The setup for the estimation of the frictional coefficient consisted of a flat motorized panel, which could be slowly inclined in a controllable way from the horizontal to the vertical position. The skin sample, prepared as mentioned above, was placed onto the epoxy resin substrates and the panel tilted at an angular velocity of 3.5° per second until the sample slid down. The sample orientation was randomly chosen but recorded in each experiment. Twelve individual samples from three different regions of the body (dorsal, ventral, lateral) were tested 14 times in four different orientations (rostro-caudad, caudo-rostrad, sinistro-dextrad, dextro-sinistrad for dorsal and ventral scales and rostro-caudad, caudo-rostrad, dorso-ventrad, ventro-dorsad for lateral scales, respectively). The data obtained for each surface for dorsal, lateral, and ventral scales were statistically compared (Kolmogorov–Smirnov test with software SPSS 16, SPSS Inc., USA) for each combination of longitudinal with transversal directions. Friction coefficients of opposite directions were pooled in the cases of sinistrad and dextrad in ventral and dorsal scales, as well as dorsad and ventrad in lateral scales.
Results
Electron microscopy
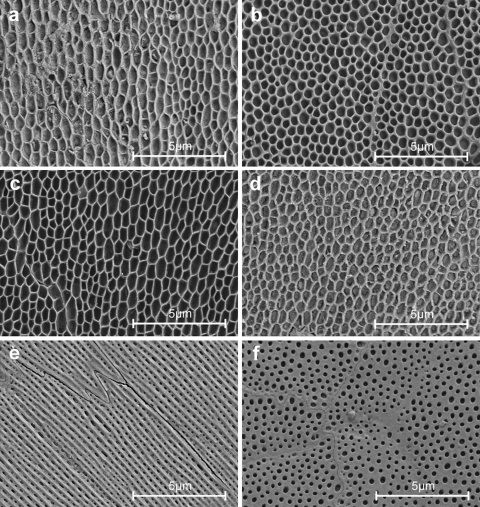
All body regions possess a regular fine microstructure (microornamentation) that is characterized by micropores and pits or indentions of various shape and diameter. The dimensions of these structures are summarized in Table 1. The microstructure of the ventral scales (Fig. 2e, f) clearly differs from dorsal (Fig. 2a, b) and lateral (Fig. 2c, d) scales in respect to pronounced longitudinal ridges at the scale areas with surface contact.
Table 1.
Dimensions of the microornamentation of Corallus hortulanus scales
| Location of the scale | Position on the scale | Mean (nm) | Standard deviation | Number of objects measured |
|---|---|---|---|---|
| Dorsal | Caudal–middle (Fig. 2a) | |||
| Length of the groove | 740 | 189 | 29 | |
| Width of the groove | 352 | 61 | 29 | |
| Distance between grooves | 107 | 31 | 62 | |
| Cranial (Fig. 2b) | ||||
| Length of the groove | 410 | 62 | 21 | |
| Width of the groove | 367 | 46 | 21 | |
| Distance between grooves | 133 | 12 | 19 | |
| Lateral | Middle (Fig. 2c) | |||
| Length of the groove | 773 | 214 | 31 | |
| Width of the groove | 356 | 68 | 31 | |
| Distance between grooves | 90 | 15 | 37 | |
| Ventral | Middle (Fig. 2e) | |||
| Length of the groove | 258 | 116 | 39 | |
| Width of the groove | 106 | 22 | 39 | |
| Distance between grooves | 285 | 46 | 66 | |
| Distance between ridges (crest to crest) | 327 | 45 | 51 | |
| Cranial (Fig. 2f) | ||||
| Diameter of the groove | 230 | 48 | 46 | |
| Distance between grooves | 236 | 45 | 89 | |
Fig. 2.
SEM micrographs of scale regions of Corallus hortulanus at a magnification of 10,000. a Dorsal scale, middle part, anterior is at the top. b Dorsal scale, anterior part, anterior is at the top. c Lateral scale, middle part, anterior is at the top. d Lateral scale, anterior part, anterior is at the top. e Ventral scale, anterior is in the upper left corner. f Ventral scale, most anterior part of the scale; this surface was covered by the adjacent rostral scale; anterior is at the top
Dorsal and lateral scales are characterized by a honeycomb or net like pattern with depressions about 750 nm long and about 350 nm wide on average at the middle of the scales. There is only slight structural anisotropy in the way that the depressions are elongated along the longitudinal axis of the snake (Fig. 2a, c). Towards the edges of the scales the depressions become shorter and therefore more circular (Fig. 2b, d).
Ventral scales possess a fine structure consisting of ridges oriented along the longitudinal body axis. These ridges are separated by 300 nm wide grooves running more or less parallel to each other. The ridges build up a system of rails that presumably represent the principal contact zone with the substrate. Depressions on the ventral scales are about one-third the diameter of those found on dorsal and lateral scales. The cell borders are usually oriented along the ridges. Such geometry results in a zigzag line at the anterior and posterior ends of the cells and straight lines at their sides. The anterior part of each ventral scale, covered by the next overlapping anterior scale, does not possess any ridges. This part is almost flat with circular depressions, which are on average 230 nm in diameter (Fig. 2f).
Friction measurements
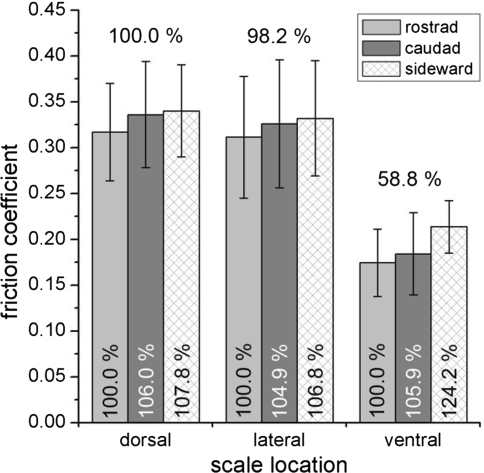
Altogether 5,346 friction coefficient (FC) values were recorded. They ranged from 0.15 to 0.47 (Table 2). The ventral scales with a FC mean of 0.191 represent the lowest value. That is about 40% less than mean FCs of dorsal (0.331) and lateral (0.323) scales (Fig. 3). The intermediate range of roughness within the nine different roughnesses always represented the lowest friction coefficients regardless of the origin of scales (ventral, dorsal, lateral). For each sliding direction (rostrad, caudad and sideward) and each roughness, ventral scale friction differed significantly from dorsal and lateral scales (P < 0.01) whereas dorsal and lateral scales appeared to be similar (Fig. 4d–f).
Table 2.
Descriptive statistics of the friction measurements
| Orientation | Roughness Ra (in μm) | N | Dorsal scales (mean ± SD) | Lateral scales (mean ± SD) | Ventral scales (mean ± SD) |
|---|---|---|---|---|---|
| Rostrad | 0.08 | 52 | 0.454 ± 0.069 | 0.412 ± 0.093 | 0.207 ± 0.038 |
| Rostrad | 0.25 | 52 | 0.367 ± 0.058 | 0.353 ± 0.074 | 0.218 ± 0.042 |
| Rostrad | 0.42 | 52 | 0.325 ± 0.052 | 0.311 ± 0.053 | 0.203 ± 0.066 |
| Rostrad | 1.11 | 52 | 0.300 ± 0.043 | 0.268 ± 0.064 | 0.170 ± 0.037 |
| Rostrad | 2.26 | 52 | 0.293 ± 0.044 | 0.280 ± 0.075 | 0.153 ± 0.032 |
| Rostrad | 2.75 | 52 | 0.272 ± 0.040 | 0.281 ± 0.062 | 0.161 ± 0.022 |
| Rostrad | 12.67 | 52 | 0.261 ± 0.050 | 0.250 ± 0.035 | 0.144 ± 0.019 |
| Rostrad | 13.94 | 52 | 0.283 ± 0.056 | 0.301 ± 0.063 | 0.151 ± 0.027 |
| Rostrad | * | 52 | 0.299 ± 0.067 | 0.346 ± 0.079 | 0.163 ± 0.038 |
| Caudad | 0.08 | 52 | 0.472 ± 0.067 | 0.416 ± 0.090 | 0.227 ± 0.055 |
| Caudad | 0.25 | 52 | 0.384 ± 0.076 | 0.349 ± 0.075 | 0.222 ± 0.043 |
| Caudad | 0.42 | 52 | 0.344 ± 0.058 | 0.320 ± 0.066 | 0.204 ± 0.057 |
| Caudad | 1.11 | 52 | 0.308 ± 0.048 | 0.283 ± 0.072 | 0.165 ± 0.032 |
| Caudad | 2.26 | 52 | 0.301 ± 0.048 | 0.289 ± 0.081 | 0.153 ± 0.025 |
| Caudad | 2.75 | 52 | 0.271 ± 0.040 | 0.283 ± 0.060 | 0.162 ± 0.029 |
| Caudad | 12.67 | 52 | 0.266 ± 0.037 | 0.279 ± 0.049 | 0.157 ± 0.043 |
| Caudad | 13.94 | 52 | 0.303 ± 0.059 | 0.307 ± 0.043 | 0.181 ± 0.063 |
| Caudad | * | 52 | 0.375 ± 0.090 | 0.408 ± 0.092 | 0.188 ± 0.055 |
| Sideward | 0.08 | 94 | 0.455 ± 0.061 | 0.408 ± 0.086 | 0.233 ± 0.037 |
| Sideward | 0.25 | 94 | 0.402 ± 0.061 | 0.348 ± 0.075 | 0.242 ± 0.036 |
| Sideward | 0.42 | 94 | 0.333 ± 0.049 | 0.310 ± 0.046 | 0.220 ± 0.040 |
| Sideward | 1.11 | 94 | 0.310 ± 0.038 | 0.273 ± 0.051 | 0.191 ± 0.025 |
| Sideward | 2.26 | 94 | 0.297 ± 0.046 | 0.283 ± 0.065 | 0.185 ± 0.020 |
| Sideward | 2.75 | 94 | 0.287 ± 0.040 | 0.284 ± 0.046 | 0.194 ± 0.021 |
| Sideward | 12.67 | 94 | 0.297 ± 0.042 | 0.289 ± 0.039 | 0.212 ± 0.028 |
| Sideward | 13.94 | 94 | 0.322 ± 0.057 | 0.362 ± 0.061 | 0.221 ± 0.031 |
| Sideward | * | 94 | 0.360 ± 0.068 | 0.437 ± 0.106 | 0.234 ± 0.045 |
Shown are the mean friction coefficients for all roughnesses for dorsal, lateral and ventral scales. An asterisk in the roughness column stands for the roughness of the P60 sandpaper which was not measurable
Fig. 3.
Friction coefficients of scales. Columns show mean values of corresponding friction measurements for different roughnesses and directions. Error bars indicate standard deviations. The percentages indicated over column are set to 100% for dorsal scales and the percentages within the columns are set to 100% for rostrad direction within one scale location
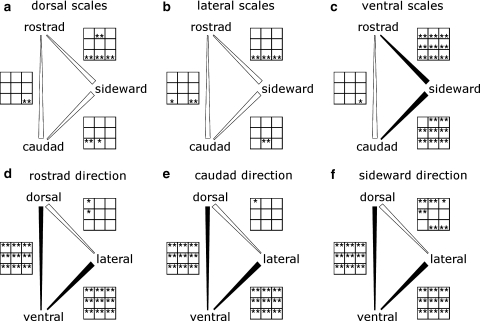
Fig. 4.
Comparison of friction coefficients estimated in different sliding directions (a–c) and of scales from different body regions (d–f). Broad end of the connecting line indicates where significantly higher coefficients of friction were found more often on the nine different roughnesses. Filled connections are used when one direction (or body region) has significantly higher friction coefficients on the majority of the nine roughnesses (94 and 100% in these cases). Open connections indicate that half or less of the roughnesses have significantly higher friction coefficients. The asterisks in the panels next to the connecting lines show the levels of significance for the nine roughnesses. (first row 0.08, 0.25, and 0.42 μm; second row 1.11, 2.26, and 2.75 μm; third row 12.67 and 13.94 μm, and replication of P60 sandpaper; no asteriskP ≥ 0.05, *0.01 < P < 0.05, **P < 0.01). Asterisks at the top of box indicate that the friction coefficient of the direction (or body region) at the upper tip of the comparison bar is significantly higher at the corresponding roughness
The comparison of FCs measured in rostro-caudal versus medio-lateral direction revealed most pronounced frictional anisotropy in ventral scales. Ventral scales showed an increase of 20.6% compared to only 4.8% (dorsal scales) and 4.3% (lateral scales) in medio-lateral direction (Fig. 3).
This anisotropy was observed in all nine roughnesses tested on ventral scales (Fig. 4c). The results of testing of rostrad against sideward direction are highly significant on all roughnesses. For caudad against sideward direction, eight of nine roughnesses showed highly significant differences. Rostrad against caudad for ventral scales and all directional combinations for dorsal and lateral scales do not show such a consistency throughout the different roughnesses (Fig. 4a–c).
However, lateral and dorsal scales show some significant results scattered among the three coarser roughnesses (Fig. 4a, b).
Discussion
Reptile scale microornamentation has been previously investigated in the context of phylogeny and ecological adaptations (Picado 1931; Hoge and Souza Santos 1953; Ruibal and Ernst 1965; Porter 1967; Ruibal 1968; Stewart and Daniel 1972, 1973, 1975; Gans and Baic 1977; Price 1982, 1983; Peterson 1984a, b; Peterson and Bezy 1985; Renous et al. 1985; Stille 1987; Irish et al. 1988; Price and Kelly 1989; Chiasson and Lowe 1989; Chiasson et al. 1989; Price 1990; Harvey 1993; Harvey and Gutberlet 1995; Maderson et al. 1998; Arnold 2002; Gower 2003). Microornamentation characters, such as micropits, cells size and shape, and raised posterior cell edges seem to be evolved several times independently among taxa, which is interesting from phylogenetical and ecological points of view (Arnold 2002). In phylogenetic reconstructions, microornamentation has been used as an argument to support relationships of species (in snakes: Picado 1931; Price 1983; Stille 1987; Chiasson and Lowe 1989; Chiasson et al. 1989; Price and Kelly 1989; Gower 2003; in lizards: Stewart and Daniel 1975; Peterson and Bezy 1985; Harvey 1993; Harvey and Gutberlet 1995; Arnold 2002).
Previous authors claimed that structural differences in microornamentations in different species do not represent any adaptations to different environments (Price 1982; Peterson 1984a, b; Peterson and Bezy 1985), because similar microstructures have been found in taxa living in different habitats and a variety of microstructures can be found in different species occupying similar ecological niches.
However, it seems to be unlikely that microornamentation is a character independent of selective pressures. Especially in snakes, the contact between the body surface and the environment demands a functional adaptation of the surface. Since there might be multiple ways to achieve similar functional tasks at the ultrastructural level of the scales, the comparison of FCs of different body regions within one species aids in understanding of functional significance of the microornamentation and provides a good basis for further interspecies comparison.
Possible functional significance of microstructure of ventral scales in different boid snakes (B. constrictor, M. spilotes, P. regius) has been shortly discussed in the work by Hazel et al. (1999), who demonstrated frictional anisotropy at the nanoscale in caudo-rostral direction versus rostro-caudal direction in contact with an AFM cantilever tip. How applicable are these results to real situation remains unknown. The friction anisotropy in caudo-rostral direction versus rostro-caudal direction is not supported by the present study, where friction has been estimated at various substrata in a more realistic situation. We could not find significant anisotropy in caudo-rostral versus rostro-caudal direction on the majority of roughnesses tested. However, this discrepancy between Hazel et al. (1999) and our results might be due to the different species investigated. In contrast to the species used by Hazel et al. (1999), in C. hortulanus it is difficult recognize to point the rostral or caudal orientation based on the appearance of the ultrastructure (Fig. 1e). Furthermore, representatives of the genera Morelia, Boa and Python are capable of the non-undulating rectilinear movement, whereas Corallus species are not (personal observations). A caudo-rostral anisotropy seems to be advantageous in rectilinear locomotion.
Snake locomotion by means of lateral undulation involves continuous movement of the ventral scales over the ground and load generation against resisting points at the side of the animal (Mosauer 1932a, b; Gray 1946; Gray and Lissmann 1950). When climbing upwards C. hortulanus bends its body ventrally against higher resisting points, comparable to the lateral bending during lateral undulation (personal observation). As friction impedes locomotion by lateral undulation, a reduction of friction in the longitudinal direction is beneficial for the propulsion of the snake. At the same time, high perpendicular friction or a fixed object as a counter bearing is needed to avoid slipping when forces are applied (Mosauer 1932a, b; Gray 1946). This principle has been used in constructing snake robots where wheels provide such kind of a frictional anisotropy (Hirose and Morishime 1990).
Our results show for the first time, that there is a significant anisotropy of friction coefficient for the ventral scales with higher coefficients for sideward directions. This anisotropy is constant over the broad range of different roughnesses tested. We would expect selective pressures on functional adaptations to cope with a variety of substrates and roughnesses during locomotion. Thus, we interpret our results clearly as functional adaptation of snake scale microornamentation. In contrast the dorsal and lateral scales do not show the consistency in anisotropy over the range of different roughnesses. This fact additionally supports the specialization of ventral scales.
The dorsal and lateral scales do show higher friction coefficients compared to the ventral ones (Fig. 3). Since the dorsal and lateral scales are hardly involved in surface contact, their high friction coefficients are not expected to be of functional relevance in locomotion. However, friction is not the only factor that may cause evolutionary change and may explain structural differences between dorsal and ventral scales. Dirt shedding capability and anti-reflection property are other characters of scales which depend on the microstructure (for lizards see Arnold 2002). Optimization of surface for one of these functions (dirt shedding, anti-reflection, friction) is most likely in conflict with the optimization for one another. A trade-off is to be expected depending on the selective pressure.
In the case of C. hortulanus the ventral scales have lower friction than dorsal and lateral scales, but are shiny, whereas the two last-mentioned scales are matt. Generally, a matt surface is less easily spotted by moving predators or prey which should be beneficial to an ambush hunter.
The basal, i.e. most anterior, regions of ventral scales revealed no anisotropic microstructures and are covered by the caudal edge of an adjacent rostral scale. This can be regarded as a further argument, supporting functional significance of the anisotropic microstructure of ventral scales in the non overlapping areas which are in contact with the substrate.
The results of friction measurements and SEM analysis revealed a correlation between microstructural pattern of the ventral scales and frictional anisotropy. Longitudinal ridges are involved in friction reduction in the sliding direction. Scales from other regions of the snakes’ body show higher friction on respective surfaces (168%). Further analysis has to reveal whether the lower friction in ventral scales is to support propulsion of the snake or to avoid abrasion of the contacting scales.
Acknowledgments
Cornelia Miksch (Max Planck Institute for Metals Research, Stuttgart) assisted with the microscopy techniques. This work, as part of the European Science Foundation EUROCORES Programme FANAS was supported by the German Science Foundation DFG (contract no. GO 995/4-1) and the EC Sixt Framework Programme (contract no. ERAS-CT-2003-980409) to S.G. The experiments comply with the “Principles of animal care”, publication no. 86–23, revised 1985 of the National Institute of Health, and also with the current laws of Germany.
Contributor Information
R. A. Berthé, Phone: +49-228-735488, FAX: +49-228-735458, Email: berthe@uni-bonn.de
G. Westhoff, Email: gwesthoff@uni-bonn.de
S. N. Gorb, Email: sgorb@zoologie.uni-kiel.de
References
- Arnold EN (2002) History and function of scale microornamentation in lacertid lizards. J Morphol 252:145–169 [DOI] [PubMed]
- Bea A, Fontarnau R (1986) The study of the sloughing cycle in snakes by means of scanning electron microscopy. In: Roček Z (ed) Studies in herpetology. Charles University, Prague, pp 373–376
- Bowden FP, Tabor D (1986) The friction and lubrication of solids. Clarendon Press, Oxford
- Chiasson RB, Lowe CH (1989) Ultrastructural scale patterns in Nerodia and Thamnophis. J Herpetol 23:109–118 [DOI]
- Chiasson RB, Bentley DL, Lowe CH (1989) Scale morphology in Agkistrodon and closely related crotaline genera. Herpetologica 45:430–438
- Fontarnau R, Bea A (1987) A quick, simple method of replicating for scanning electron microscopy applied to the oberhautchen micro-ornamentation study. J Herpetol 21:366–369 [DOI]
- Gans C, Baic D (1977) Regional specialisation of reptilian scale surface: relation of texture and biological role. Science 195:1348–1350 [DOI] [PubMed]
- Gorb SN (1999) Ultrastructure of the thoracic dorso-medial field (TDM) in the elytra-to-body arresting mechanism in tenebrionid beetles (Coleoptera: Tenebrionidae). J Morphol 240:101–113 [DOI] [PubMed]
- Gorb SN (2007) Visualisation of native surfaces by two-step molding. Micros today 15:44–46
- Gower DJ (2003) Scale microornamentation of uropeltid snakes. J Morphol 258:249–268 [DOI] [PubMed]
- Gray J (1946) The mechanism of locomotion in snakes. J Exp Biol 23:101–120 [DOI] [PubMed]
- Gray J, Lissmann HW (1950) The kinetics of locomotion of the grass-snake. J Exp Biol 26:354–367
- Harvey MB (1993) Microstructure, ontology, and evolution of scale surface in xenosaurid lizards. J Morphol 216:161–177 [DOI] [PubMed]
- Harvey MB, Gutberlet RL (1995) Microstructure, evolution, and ontogeny of scale surface in cordylid and gerrhosaurid lizards. J Morphol 226:121–139 [DOI] [PMC free article] [PubMed]
- Hazel J, Stone M, Grace MS, Tsukruk VV (1999) Nanoscale design of snake skin for reptation locomotions via friction anisotropy. J Biomech 32:477–484 [DOI] [PubMed]
- Hiller U (1968) Untersuchungen zum Feinbau und zur Funktion der Haftborsten von Reptilien. Z Morphol Tiere 62:307–362 [DOI]
- Hirose S, Morishime A (1990) Design and control of a mobile robot with an articulated body. Int J Robot Res 9:99–114 [DOI]
- Hoge AR, Souza Santos P (1953) Submicroscopic structure of “stratum corneum” of snakes. Science 118:410–411 [DOI] [PubMed]
- Irish FJ, Williams EE, Seling E (1988) Scanning electron microscopy of changes in epidermal structure occurring during the shedding cycle in squamate reptiles. J Morphol 197:105–126 [DOI] [PubMed]
- Leydig F (1873) Über die äusseren Bedeckungen der Reptilien und Amphibien. Archiv für mikroskopische Anatomie 9:753–794 [DOI]
- Maderson PFA (1966) Histological changes in the epidermis of the Tokay (Gekko gecko) during the sloughing cycle. J Morphol 116:39–50 [DOI]
- Maderson PFA, Rabinowitz T, Tandler B, Alibardi L (1998) Ultrastructural contributions to an understanding of the cellular mechanisms involved in lizard skin shedding with comments on the function and evolution of a unique lepidosauran phenomenon. J Morphol 236:1–24 [DOI] [PubMed]
- McCarthy CJ (1987) Sea snake puzzles. In: van Gelder JJ, Strijbosch H, Bergers PJM (eds) In: Proceedings of the fourth ordinary general meeting of the Scocietas Europaea Herpetologica, Nijmegen, pp 279–284
- Mosauer W (1932a) On the locomotion of snakes. Science 76:583–585 [DOI] [PubMed]
- Mosauer W (1932b) Über die Ortsbewegung der Schlangen. Zoologisches Jahrbuch (Abteilung für allgemeine Zoologie und Physiologie der Tiere) 52:191–215
- Peterson JA (1984a) The scale microarchitecture of Sphenodon punctatus. J Herpetol 18:40–47 [DOI]
- Peterson JA (1984b) The microstructure of the scale surface in iguanid lizards. J Herpetol 18:437–467 [DOI]
- Peterson JA, Bezy RL (1985) The microstructure and evolution of scale surfaces in xantusiid lizards. Herpetologica 41:298–324
- Picado C (1931) Epidermal microornaments of the crotalinae. Bull Antivenin Inst Am 4:104–105
- Porter WP (1967) Solar radiation through the living body walls of vertebrates with emphasis on desert reptiles. Ecol Monogr 37:273–296 [DOI]
- Price RM (1982) Dorsal snake scale microdermatoglyphics: ecological indicator or taxonimical tool? J Herpetol 16:294–306 [DOI]
- Price RM (1983) Microdermatoglyphics: the Liodytes-Regina problem. J Herpetol 17:292–294 [DOI]
- Price RM (1990) Microdermatoglyphics: an appeal for standardization of methodology and terminology with comments on recent studies of North American natricines. J Herpetol 24:324–325 [DOI]
- Price RM, Kelly P (1989) Microdermatoglyphics: basal patterns and transition zones. J Herpetol 23:244–261 [DOI]
- Renous S, Gasc J, Diop A (1985) Microstructure of the tegumentary surface of the Squamata (Reptilia) in relation of their spatial position and their locomotion. Forts Zool 30:478–479
- Ruibal R (1968) The ultrastructure of the surface of lizard scales. Copeia 1968(4):698–703 [DOI]
- Ruibal R, Ernst V (1965) The structure of the digital setae of lizards. J Morphol 117:271–294 [DOI] [PubMed]
- Scherge M, Gorb SN (2001) Biological micro- and nanotribology. Springer, Berlin
- Spurr RA (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultra Mol Struct R 26:31–43 [DOI] [PubMed]
- Stewart GR, Daniel RS (1972) Scales of the lizard Gekko gecko: surface structure examined with the scanning electron microscope. Copeia 1972:252–257 [DOI]
- Stewart GR, Daniel RS (1973) Scanning electron microscopy of scales from different body regions of three lizard species. J Morphol 139:377–388 [DOI] [PubMed]
- Stewart GR, Daniel RS (1975) Microornamentation of lizard scales: some variation and taxonomic correlation. Herpetologica 31:117–130
- Stille B (1987) Dorsal scale microdermatoglyphics and rattlesnake (Crotallus and Sisturus) phylogeny (Reptilia: Viperidae: Crotalinae). Herpetologica 43:89–104