Abstract
The Cre/loxP recombination system is a commonly used tool to alter the mouse genome in a conditional manner by deletion or inversion of loxP-flanked DNA segments. While Cre-mediated deletion is essentially unidirectional, inversion is reversible and therefore does not allow the stable alteration of gene function in cells that constitutively express Cre. Site-directed mutagenesis yielded a pair of asymmetric loxP sites (lox66 and lox71) that display a favorable forward reaction equilibrium. Here, we demonstrate that lox66/lox71 mediates efficient and predominantly unidirectional inversion of a switch substrate targeted to the mouse genome in combination with either inducible or cell type-specific cre-transgenes in vivo.
INTRODUCTION
Conditional mutagenesis has become a potent tool to analyze gene function in vivo. Site-specific recombinases such as bacteriophage P1-derived Cre are used extensively to modify the mammalian genome in a cell type- and/or time-specific manner (reviewed in 1,2). Cre is a 38 kDa protein that recognizes a 34 bp DNA target termed loxP (locus of X-over of P1), which consists of two 13 bp inverted repeats that serve as Cre monomer binding sites and an 8 bp spacer region. The spacer region is non-palindromic and provides the loxP site with an orientation; recombination can occur between two loxP sites of either identical or opposing orientation. The former results in excision of the intervening DNA, the latter in its inversion. Excision is accompanied by the deletion of one loxP site from the genome and is therefore essentially unidirectional. Inversion, on the other hand, yields two loxP sites that are indistinguishable from the original loxP pair due to the conservative nature of the recombination event (3), and recombination continues as long as Cre is present.
Site-directed mutagenesis of loxP sites has led to the discovery of mutant loxP pairs that display a favorable forward reaction equilibrium and might thus allow unidirectional Cre-mediated gene inversion (4). One such loxP pair consists of two asymmetric, mutant loxP sites that carry a 5 bp mutation in the distal end of either the left (lox66) or the right (lox71) inverted repeat. Cre-mediated recombination of a lox66/lox71 pair yields one loxP site with two mutated inverted repeats (lox72) and one wild-type (WT) loxP site (Fig. 1A). While lox66 and lox71 mediated recombination with an efficiency comparable to WT loxP sites in vivo in plants, recombination between lox72 and WT loxP was up to 8-fold reduced (4). More recently, two reports showed the successful application of lox66/lox71-mediated recombination in mouse embryonic stem (ES) cells (5,6). Although inversion appeared irreversible in ES cell colonies (6), a characterization of lox66/lox71-mediated recombination in cre-transgenic mouse strains in vivo is still missing.
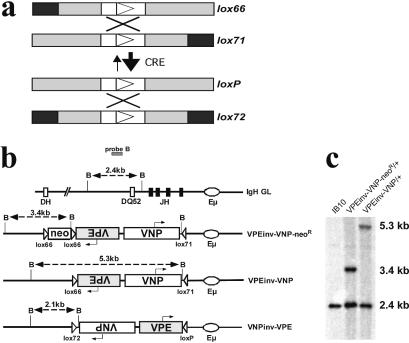
Figure 1.
Strategy to switch the IgH variable region exon employing lox66/lox71. (a) Schematic representing the Cre-mediated recombination of lox66 and lox71 resulting in lox72 and loxP. Inverted repeats are shown in grey, black boxes symbolize 5 bp mutations, the open box depicts the spacer region and the triangle represents its orientation. The difference in arrow sizes reflects the unbalanced equilibrium between the forward and reverse reactions. (b) Targeted integration of a VDJ switch cassette into the IgH locus. BamHI (B) restriction endonuclease map of the IgH germline JH region (IgH GL), the targeted VDJ switch cassette before neoR deletion (VPEinv-VNP-neoR) and before (VPEinv-VNP) and after (VNPinv-VPE) Cre-mediated inversion. Boxes depict D and J exons and the promotor-VDJ exons VPE and VNP as indicated, arrows symbolize the transcriptional orientation of VNP and VPE. The oval represents the Eµ enhancer and triangles depict (mutant) loxP sites. Double-headed arrows indicate BamHI fragments detected with probe B; fragment sizes are indicated. (c) Southern blot analysis of IB10 wild-type ES cells and the same targeted ES cell clone before (VPEinv-VNP-neoR) and after neoR deletion (VPEinv-VNP). Genomic DNA was digested with BamHI and hybridized to probe B.
In the present study, we assessed whether a lox66/lox71-mediated genetic switch can be used to irreversibly alter the coding sequence of the B cell antigen receptor (BCR) variable domain in mature B cells in vitro. In addition, we analyzed the frequency of lox66/lox71-mediated forward and lox72/WT loxP-mediated reverse recombination in combination with two distinct cre transgenes in vivo.
MATERIALS AND METHODS
Generation of mice carrying a lox66/lox71-flanked VDJ switch cassette in the IgH locus
A targeting vector was designed to insert a lox66/lox71-flanked VDJ switch cassette (VNP and VPE in opposing orientations) into the mouse IgH locus. The VPE gene segment was PCR-amplified from hybridoma 80A3 (7) and linked to a 0.5 kb EcoRV–SalI fragment containing the VH promotor of the VH3-83 gene (8). A 2.1 kb BamHI–XhoI fragment containing the VNP gene segment and its VH promotor (9) and an inverted lox71 site 3′ of the VNP segment was ligated to the VH3-83-promotor-VPE fragment with the promotors facing away from each other. This cassette was subsequently fused to the 3′ end of a lox66-flanked TK-neoR gene (10) with the downstream lox66 site facing the inverted VPE segment. An 11 kb XhoI fragment containing 9 kb of genomic sequence upstream of DQ52 and the HSV-tk gene (11) served as the long arm of homology and a 0.8 kb EcoRI fragment containing part of the JH-Cµ intron (8) was used as the short arm of homology. The NotI-linearized targeting construct was transfected into IB10 ES cells (12) as described (13). G418 and Gancyclovir double-resistant ES cell colonies were screened for homologous recombination by Southern blotting using internal probe B and external probe RH (8). To delete the lox66-flanked neoR gene, correctly targeted clones were transfected with the circular Cre-encoding plasmid pIC-Cre (14) and G418-sensitive clones were analyzed for neoR deletion by Southern blot analysis. Two independent ES cell clones that carry the lox66/lox71-flanked VDJ switch cassette without the neoR gene were injected into CB.20 blastocysts. Resulting chimeric male mice were bred to CB.20 females for germline transmission.
Protein transduction of splenic B cells with TAT-NLS-Cre
Single cell suspensions of splenocytes were purified by MACS depletion using anti-CD43 beads as described by the manufacturer (Miltenyi, Bergisch Gladbach, Germany). The purity of B220+ cells was >99%. TAT-NLS-Cre transduction was essentially done as described (15). In brief, B cells were washed twice in serum-free medium (Hyclone, Logan, UT) and incubated at 5 × 106 cells/ml with the indicated dose of TAT-NLS-Cre in serum-free medium for 1 h at 37°C. Cells were then washed twice in complete medium (15) and cultured at a density of 2 × 106 cells/ml at 37°C and 10% CO2 for 5 days.
Induction of Mx-Cre transgene expression in vivo
VPEinv-VNP/+ Mx-cre mice or VNPinv-VPE/+ Mx-cre mice were given three doses of 400 µg poly(I)·poly(C) (Amersham Biosciences Corp., Piscataway, NJ) i.p. on days 0, 3 and 6 and analyzed either 3 days or 6–8 weeks thereafter.
Southern blot analysis of VPEinv-VNP inversion
Genomic DNA from 106 sorted cells or ∼10 µg of genomic DNA from indicated tissues was subjected to Southern blot analysis. To detect VPEinv-VNP inversion, DNA was digested with BamHI and hybridized to probe B (8), resulting in a 2.4 kb IgH germline fragment, a 5.3 kb fragment indicative of the VPEinv-VNP allele and a 2.1 kb fragment representing the inverted VNPinv-VPE allele. The signal intensities of each sample were quantified using a Storm 860 Molecular Dynamics scanner and ImageQuant software (Amersham Biosciences, Piscataway, NJ). The fraction of cells that had undergone lox66/lox71-mediated recombination was calculated as the ratio of VNPinv-VPE-intensity over the sum of VNPinv-VPE- and VPEinv-VNP-intensities or as the ratio of VNPinv-VPE-intensity over IgH GL intensity. The latter is, however, not applicable for B cells, since the IgH GL signal is lost upon IgH rearrangement. In all other tissues, both assays yielded comparable results. Based on the real time PCR results depicted in Figure 3C, the detection limit of recombination by Southern blot analysis was estimated to be between 5 and 10%.
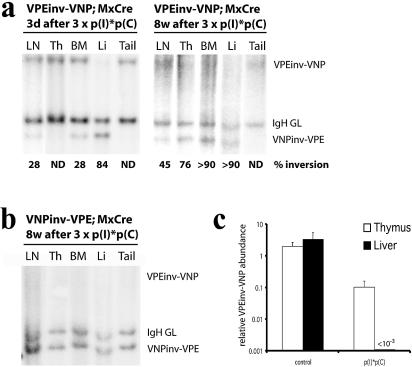
Figure 3.
lox66/lox71-mediated recombination efficiency in Mx-cre mice. (a) Adult VPEinv-VNP/+ Mx-cre mice were injected with poly(I)·poly(C) and analyzed 3 days or 8 weeks thereafter. Genomic DNA from lymph nodes (LN), thymus (Th), bone marrow (BM), liver (Li) and tail was digested with BamHI and hybridized to probe B. The percentage of cells that carry the VPEinv-VNP allele in its inverted configuration (VNPinv-VPE) is shown for each lane. These results are representative of at least two independent experiments. ND, not detected. (b) Adult VNPinv-VPE/+ Mx-cre mice were injected with poly(I)·poly(C) and analyzed 8 weeks thereafter as described in (a). (c) Quantitative PCR analysis to detect lox72/WT loxP-mediated reverse recombination in VNPinv-VPE/+ Mx-cre mice. Thymic and liver DNA was PCR amplified with primers detecting either the VPEinv-VNP allele or the TNFR-1 gene to normalize DNA content. The relative abundance of the VPEinv-VNP PCR product is shown for poly(I)·poly(C)-injected animals and VPEinv-VNP/+ control mice.
Real time PCR analysis to quantify lox72/WT loxP-mediated reverse recombination
Genomic DNA from liver and thymus of VPEinv-VNP/+ mice or VNPinv-VPE/+ Mx-cre mice that were injected three times with poly(I)·poly(C) and killed 8 weeks thereafter, was subjected to quantitative PCR analysis using the iCycler iQ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). The VPEinv-VNP allele was amplified using the 5′-primer LAH-53 (GGA CCT CCA TCT GCT CTT ATT T) located in the long arm of homology and the 3′-primer CDR3-PE (GGT CTA TTA CTG TGC AAG TTG G) located in the complementarity-determining region (CDR) 3 of VPE. TNFR-1 was amplified as described (16) to normalize the individual DNA samples. PCR was performed using SYBR Green PCR core reagents (Applied Biosystems, Foster City, CA) as described by the manufacturer. Two serial 1:5 dilutions were included for each sample and served to determine mean and standard deviation.
Flow cytometry and cell sorting
Single cell suspensions from bone marrow, spleen and thymus were stained with monoclonal antibodies (mAbs) conjugated to fluorescein isothyocyanate (FITC), phycoerythrin (PE) or allophycocyanin (APC). The following mAbs were used: anti-CD4 (GK1.5-4), anti-CD5 (53-7.3), anti-CD8 (53-6.7), anti-CD19 (1D3), FcBlock (2.4G2) (from BD PharMingen, San Diego, CA); anti-IgM (1B4B1) (from eBioscience, San Diego, CA); anti-NP (S43) (generated in our laboratory). Staining for NP was performed in two steps: cells were incubated with NP14-BSA (0.2 ng/ml), washed once with phosphate-buffered saline, 0.5% bovine serum albumin and subsequently stained with S43-APC. Stained cells were acquired on a FACSCalibur and data were analyzed with CellQuest software. Cell sorting was performed on a FACS Vantage (all Becton Dickinson, San Jose, CA). All analyses were restricted to live cells within the lymphocyte gate.
RESULTS
By choosing a genetic switch system that allows the inducible change of the BCR antigen specificity we were able to monitor Cre-mediated recombination both on the genomic level by Southern blot analysis and on the protein level using flow cytometry (FACS). The BCR consists of two identical immunoglobulin heavy (IgH) chains, two identical Ig light (IgL) chains and the signal transducing Igα/Igβ heterodimer. The paired variable domains of IgH and IgL chains (termed VDJ and VJ, respectively) form the antigen binding sites of the BCR (reviewed in 17) and a change in either the VDJ or the VJ domain generally results in a change of antigen specificity. We therefore engineered a lox66/lox71-flanked ‘VDJ switch cassette’, which consists of the two distinct VDJ gene segments VNP and VPE (7) in opposing transcriptional orientations, and inserted it into the mouse IgH locus by homologous recombination in ES cells. Depending on Cre-mediated recombination, B cells will express either the VNP or the VPE heavy chain. In combination with a λ1 light chain, the two heavy chains can be distinguished by surface staining: VNP/Igλ1 recognizes 4-hydroxy-3-nitrophenylacetyl (NP)-haptenated carrier proteins while VPE/Igλ1 binds PE. This allows the detection of lox66/lox71-mediated recombination in single λ1+ B cells (∼6% of all splenic B cells).
The targeting strategy for insertion of the VDJ switch cassette is depicted in Figure 1B. The VDJ switch allele is referred to as VPEinv-VNP before and VNPinv-VPE after inversion. In order to obtain a second mouse strain that carries the VNPinv-VPE allele in the germline, VPEinv-VNP/+ mouse mutants were crossed to deleter mice (18), which express Cre during early emryogenesis. Two out of six mice that carried the deleter allele also carried the VNPinv-VPE allele.
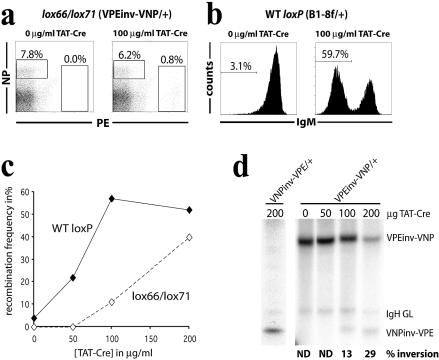
We first determined the efficiency of lox66/lox71-mediated recombination at different Cre concentrations in ex vivo isolated splenic B cells from VPEinv-VNP/+ mice. B cells that carry a WT loxP-flanked VNP gene segment (termed B1-8f) (19) in the same genomic position served as a control. In the B1-8f allele, the loxP sites are in identical orientation and the VNP segment is deleted in the presence of Cre resulting in the ablation of surface IgM. To prevent cell death of primary B cells in culture, we used B cells transgenic for a cDNA encoding the anti-apoptotic Bcl-2 protein (bcl-2tg) (20). Splenic B cells were incubated for 1 h with TAT-NLS-Cre at different concentrations. TAT-NLS-Cre is a fusion protein of a basic 11 amino acid peptide derived from HIV-TAT, a nuclear localization signal (NLS) and Cre (15). The hydrophobic TAT peptide has been reported to facilitate cellular uptake of recombinant proteins in vitro and in vivo (21,22). TAT-NLS-Cre-mediated inversion was analyzed 5 days after Cre transduction by surface staining for NP and PE (Fig. 2A) and by Southern blotting (Fig. 2D). Both assays yielded comparable inversion frequencies. To determine the efficiency of WT loxP-mediated deletion, we analyzed B1-8f/+ B cells for loss of surface IgM expression upon TAT-NLS-Cre transduction (Fig. 2B). The comparison of WT and mutant loxP sites shows a clear difference in recombination frequencies (Fig. 2C). While low doses of TAT-NLS-Cre (≤50 µg/ml) induce recombination in up to ∼25% of all B cells carrying WT loxP sites, no recombination is observed with lox66/lox71. To obtain recombination frequencies similar to those of WT loxP sites, mutant loxP sites have to be exposed to ∼3-fold higher concentrations of TAT-NLS-Cre. To address whether reverse recombination affects the overall recombination frequency in our system, we incubated B cells that carry the pre-inverted VNPinv-VPE allele with 200 µg/ml TAT-NLS-Cre (highest concentration used in the dose–response assay) and analyzed them 3 days after transduction. No reverse recombination could be detected by Southern blot analysis (Fig. 2C). Given the limited sensitivity of this approach, we conclude that <10% of cells have undergone reverse recombination (see Materials and Methods for details).
Figure 2.
Dose–response characteristics of ex vivo isolated splenic bcl-2tg B cells from VPEinv-VNP/+ or B1-8f/+ mice to TAT-NLS-Cre. (a) FACS analysis of splenic B cells from VPEinv-VNP/+ bcl-2tg mice to determine lox66/lox71-mediated recombination frequency in vitro. CD43– MACS purified B cells were incubated with 0 and 100 µg/ml TAT-NLS-Cre and analyzed 5 days thereafter. Cells were analyzed for PE and NP binding and inversion frequency was calculated as the fraction of PE+ cells over the sum of PE+ and NP+ cells. (b) FACS analysis of splenic B cells from B1-8f/+ bcl-2tg mice to determine WT loxP-mediated recombination frequency in vitro. Cells were prepared and cultured as described in (a) and analyzed for IgM expression to determine the frequency of B1-8f deletion. (c) Dose–response curve of lox66/lox71 and WT loxP-mediated recombination to increasing amounts of TAT-NLS-Cre. Recombination frequencies for VPEinv-VNP inversion (lox66/lox71, hatched line) and B1-8f deletion (WT loxP, solid line) were calculated based on FACS analyses performed as in (a). For details see text. (d) Southern blot analysis of VPEinv-VNP/+ and VNPinv-VPE/+ bcl-2tg B cells 5 days after TAT-NLS-Cre transduction. Total B cell DNA was digested with BamHI and hybridized to probe B (see Fig. 1). Fragments representing VPEinv-VNP, VNPinv-VPE and IgH germline (GL) alleles are indicated. No lox72/WT loxP-mediated reverse recombination (VPEinv-VNP signal) could be detected in B cells from VNPinv-VPE/+ mice (left panel). In the case of VPEinv-VNP/+ bcl-2tg mice, the percentage of cells that carry the VNPinv-VPE allele as a result of lox66/lox71-mediated recombination is shown for each lane (right panel). ND, not detected.
We then analyzed the efficiency of lox66/lox71-mediated recombination in vivo in combination with the inducible Mx-cre transgene. In Mx-cre mice, Cre expression is under the control of the Type I interferon (IFN)-inducible Mx1 promotor (23). Injection of Type I IFN or poly(I)·poly(C), a double-stranded RNA homolog that induces an endogenous anti-viral Type I IFN response, leads to efficient Cre expression and subsequent Cre-mediated recombination (23). As potential (counter)selection of B cells after Cre-induced change of BCR specificity might obscure the actual recombination frequency, we focused our analysis on organs that harbor few or no BCR+ B cells, such as liver, thymus and bone marrow. We tested lox66/lox71-mediated recombination in adult VPEinv-VNP/+ Mx-cre mice both 3 days and 8 weeks after Cre induction by poly(I)·poly(C). To compare the recombination efficiencies of mutant and WT loxP sites, we also analyzed mice carrying WT loxP-flanked gene insertions in the same genomic position (7). Inversion efficiencies in lymphoid organs, liver and tail were analyzed by Southern blotting. In the liver, ∼80% of VPEinv-VNP/+ Mx-cre cells show lox66/lox71-mediated recombination both 3 days and 8 weeks after Cre induction, while less than 10% of tail tissue showed recombination at either of the two time points (Fig. 3A). These results closely resemble deletion efficiencies observed with WT loxP sites (7,23; data not shown). Unlike in liver and tail, recombination frequencies in thymus and bone marrow increased over time (Fig. 3A). Three days after induction, lox66/lox71-mediated recombination in thymus and bone marrow was between three and six times less efficient than WT loxP-mediated deletion in the same organs (7,23; data not shown). Eight weeks after induction, however, ∼75% of thymocytes and ∼90% of bone marrow cells carried the inverted allele. This indicates inefficient inversion in B and T lineage cells and bone marrow macrophages but efficient recombination in hematopoietic stem cells, which reconstitute bone marrow and thymus within 8 weeks after Cre induction. To determine the extent of lox72/WT loxP-mediated reverse recombination, we analyzed Mx-cre mice that carry the pre-inverted VNPinv-VPE allele. We were unable to detect reverse recombination 8 weeks after injection of poly(I)·poly(C) by Southern blotting in all tissues tested (Fig. 3B). Due to the limited sensitivity of this analysis, we also performed quantitative PCR on thymic and liver DNA of the same animals using light cycler analysis (Fig. 3C). No reverse recombination could be detected in the liver of poly(I)·poly(C)-injected VNPinv-VPE/+ Mx-cre animals, whereas up to 5% of thymocytes had undergone Cre-mediated reverse recombination. We conclude that lox66/lox71- mediated recombination can be efficiently induced in adult Mx-cre mice and reverse recombination appears to occur only in only a minor fraction of hematopoietic stem cells.
To determine lox66/lox71-mediated recombination in mice that express Cre in a cell type-specific manner, we analyzed VPEinv-VNP/+ mice that carry the CD4-cre transgene (24). CD4-cre mice show essentially complete deletion of WT loxP-flanked gene segments in CD4+/CD8+ double-positive (DP) thymocytes and mature T cells (24,25). Moreover, the VPEinv-VNP allele is not expressed in T cells and thus not subject to potential (counter)selection upon its inversion. DP thymocytes and splenic T cells (CD5+/CD19–) from VPEinv-VNP/+ CD4-cre mice were sorted using flow cytometry and inversion efficiencies were determined by Southern blotting. CD19+ splenic B cells served as a negative control. Recombination was observed in 40% of DP thymocytes and ∼95% of splenic T cells (Fig. 4). The higher recombination efficiency in splenic T cells may be due to the fact that, on average, mature T cells have been exposed to Cre longer than DP thymocytes, in which CD4-Cre expression is initiated. The observation that 5% of splenic T cells carry the original VPEinv-VNP allele could reflect either incomplete inversion or the occurrence of reverse recombination in maximally 5% of mature T cells.
Figure 4.
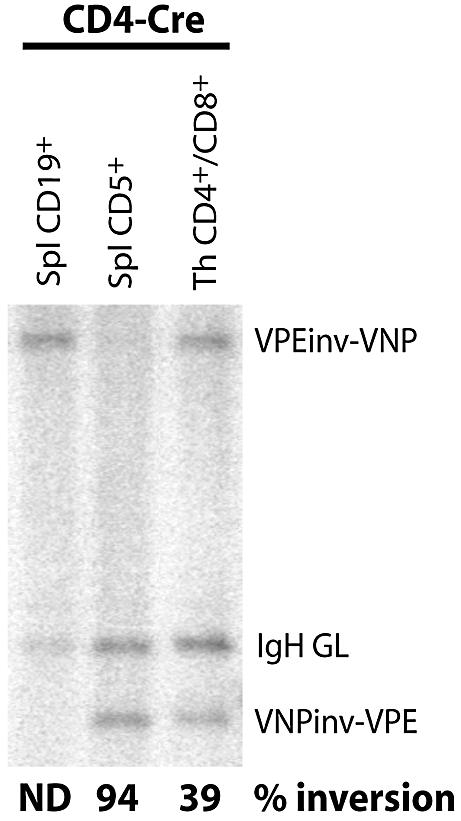
VPEinv-VNP-inversion in 5-week-old VPEinv-VNP/+ CD4-cre mice. Genomic DNA from FACS-sorted splenic B cells (CD19+), T cells (CD5+) and sorted CD4+/CD8+ thymocytes was digested with BamHI and hybridized to probe B. Inversion frequencies are indicated for each sample. ND, not detected.
DISCUSSION
Cre/loxP-mediated inversion of genomic DNA segments represents a useful tool to alter the mouse genome in a cell type- and/or tissue-specific manner. However, its applicability is hampered by the fact that this reaction is fully reversible. Here, we report successful application of the mutant loxP pair lox66/lox71 to mediate efficient and essentially unidirectional inversion of a VDJ switch cassette both in vitro and in two different cre-transgenic mouse models in vivo.
In an in vitro dose–response analysis we show that the lox66/lox71 pair has a higher threshold for the initiation of Cre-mediated recombination than WT loxP sites and requires 2- to 3-fold higher Cre concentrations to recombine with an efficiency comparable to WT loxP sites (Fig. 2). We were unable to detect reverse recombination in vitro by Southern blotting. Given that the forward reaction occurred in less than 40% of cells at the maximal dose of TAT-NLS-Cre and is known to be considerably more efficient than the reverse reaction (4), this is not a surprising result. Sensitivity of Southern blot analysis is, however, limited and we cannot rule out low levels of reverse recombination. Toxicity of the TAT-NLS-Cre fusion protein at doses >200 µg/ml did not allow us to further increase the TAT-NLS-Cre concentration.
Since Cre/loxP-mediated recombination is predominantly used for conditional mutagenesis in vivo and different cre-transgenic mouse strains are likely to display varying levels of Cre expression, it was important to address the efficiencies of lox66/lox71-mediated forward and lox72/WT loxP-mediated reverse recombination in vivo.
We show that in mice which carry Cre under the control of either a cell-type specific or an inducible promotor, lox66/lox71-mediated inversion is highly favored over the lox72/WT loxP-mediated reverse reaction. In both cases, the overall recombination efficiency of mutant loxP sites is slightly reduced when compared to WT loxP sites. Nevertheless, lox66/lox71-mediated inversion is almost complete in liver and hematopoietic stem cells of poly(I)·poly(C)-treated Mx-cre animals (Fig. 3A) and in mature T cells from CD4-cre mice (Fig. 4). Up to 5% of Mx-cre-transgenic hematopoietic stem cells undergo lox72/WT loxP-mediated reverse recombination upon poly(I)·poly(C) treatment (Fig. 3C), and a similar reversion frequency might account for the incomplete inversion observed in mature CD4-cre T cells (Fig. 4). We conclude that reverse recombination can occur at low levels and, based on previous work in plants (4), appears to depend on the concentration of Cre.
The difference in WT loxP- and lox66/lox71-mediated recombination frequencies observed in vitro and in vivo is likely to reflect intrinsic differences between WT and mutant loxP sites. In the case of the latter, Cre/loxP complex formation might be impaired due to inefficient recruitment of the Cre monomer to mutated inverted repeats. Potential differences in accessibility of loxP-flanked alleles can be ruled out since both WT and mutant loxP sites analyzed in this study were targeted to the same genomic positions.
Taken together, lox66/lox71-mediated recombination represents an easily applicable tool for the inducible and essentially unidirectional alteration of the mouse genome by Cre-mediated gene inversion in vitro and in vivo. Recently, Schnuetgen et al. (26) described a different approach to achieve unidirectional Cre-mediated gene inversion employing a combination of WT and mutant loxP sites (lox511), which efficiently recombine with themselves but very inefficiently with each other (27). This system has been reported to yield a high inversion frequency in vivo with undetectable levels of reverse recombination. However, low levels of loxP/lox511-mediated reverse recombination are still possible as the detection limit of the PCR approach used to quantify this event was not determined.
A potential limitation to the use of inverted loxP sites in vivo is Cre-mediated chromosome loss in early embryos due to dicentric chromosome formation. This event has been detected previously using a Y chromosome that carries multiple loxP sites in opposing orientations (28). However, in our system, inversion essentially blocks dicentric chromosome formation since lox72 recombines only very inefficiently with WT loxP; and the fact that we readily obtained mice harboring the inverted VNPinv-VPE allele in combination with the deleter allele suggests that Cre-mediated dicentric chromosome formation during early embryogenesis does not represent a major problem in lox66/lox71-mediated gene inversion.
Acknowledgments
ACKNOWLEDGEMENTS
We thank N. Barteneva for cell sorting, A. Egert, A. Roth, V. Dreier, D. Ghitza, B. Hampel and C. Uthoff-Hachenberg for technical help and M. Alimzhanov for providing lox66 and lox71 containing plasmids. This work was supported by grants from the Volkswagen Stiftung, the National Cancer Institute (POI CA92625-0) and the National Institutes of Health (R37 AI054636).
REFERENCES
- 1.Tronche F., Casanova,E., Turiault,M., Sahly,I. and Kellendonk,C. (2002) When reverse genetics meets physiology: the use of site-specific recombinases in mice. FEBS Lett., 529, 116–121. [DOI] [PubMed] [Google Scholar]
- 2.Rajewsky K., Gu,H., Kuhn,R., Betz,U.A., Muller,W., Roes,J. and Schwenk,F. (1996) Conditional gene targeting. J. Clin. Invest., 98, 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo F., Gopaul,D.N. and van Duyne,G.D. (1997) Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse. Nature, 389, 40–46. [DOI] [PubMed] [Google Scholar]
- 4.Albert H., Dale,E.C., Lee,E. and Ow,D.W. (1995) Site-specific integration of DNA into wild-type and mutant lox sites placed in the plant genome. Plant J., 7, 649–659. [DOI] [PubMed] [Google Scholar]
- 5.Araki K., Araki,M. and Yamamura,K. (1997) Targeted integration of DNA using mutant lox sites in embryonic stem cells. Nucleic Acids Res., 25, 868–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z. and Lutz,B. (2002) Cre recombinase-mediated inversion using lox66 and lox71: method to introduce conditional point mutations into the CREB-binding protein. Nucleic Acids Res., 30, e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruyama M., Lam,K.P. and Rajewsky,K. (2000) Memory B-cell persistence is independent of persisting immunizing antigen. Nature, 407, 636–642. [DOI] [PubMed] [Google Scholar]
- 8.Pelanda R., Schwers,S., Sonoda,E., Torres,R.M., Nemazee,D. and Rajewsky,K. (1997) Receptor editing in a transgenic mouse model: site, efficiency and role in B cell tolerance and antibody diversification. Immunity, 7, 765–775. [DOI] [PubMed] [Google Scholar]
- 9.Sonoda E., Pewzner-Jung,Y., Schwers,S., Taki,S., Jung,S., Eilat,D. and Rajewsky,K. (1997) B cell development under the condition of allelic inclusion. Immunity, 6, 225–233. [DOI] [PubMed] [Google Scholar]
- 10.Ferradini L., Gu,H., De Smet,A., Rajewsky,K., Reynaud,C.A. and Weill,J.C. (1996) Rearrangement-enhancing element upstream of the mouse immunoglobulin kappa chain J cluster. Science, 271, 1416–1420. [DOI] [PubMed] [Google Scholar]
- 11.Taki S., Meiering,M. and Rajewsky,K. (1993) Targeted insertion of a variable region gene into the immunoglobulin heavy chain locus. Science, 262, 1268–1271. [DOI] [PubMed] [Google Scholar]
- 12.Torres R.M. and Kuhn,R. (1997) Laboratory Protocols for Conditional Gene Targeting. Oxford University Press, Oxford, UK. [Google Scholar]
- 13.Pasparakis M. and Kollias,G. (1995) Production of cytokine transgenic and knockout mice. In Balkwill,F.R. (ed.), Cytokines: A Practical Approach. Oxford University Press, Oxford, UK, pp. 297–324. [Google Scholar]
- 14.Kuhn R., Lohler,J., Rennick,D., Rajewsky,K. and Muller,W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell, 75, 263–274. [DOI] [PubMed] [Google Scholar]
- 15.Peitz M., Pfannkuche,K., Rajewsky,K. and Edenhofer,F. (2002) Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc. Natl Acad. Sci. USA, 99, 4489–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeffer K., Matsuyama,T., Kundig,T.M., Wakeham,A., Kishihara,K., Shahinian,A., Wiegmann,K., Ohashi,P.S., Kronke,M. and Mak,T.W. (1993) Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell, 73, 457–467. [DOI] [PubMed] [Google Scholar]
- 17.Bassing C.H., Swat,W. and Alt,F.W. (2002) The mechanism and regulation of chromosomal V(D)J recombination. Cell, 109 (suppl.), S45–S55. [DOI] [PubMed] [Google Scholar]
- 18.Schwenk F., Baron,U. and Rajewsky,K. (1995) A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res., 23, 5080–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam K.P., Kuhn,R. and Rajewsky,K. (1997) In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell, 90, 1073–1083. [DOI] [PubMed] [Google Scholar]
- 20.Strasser A., Whittingham,S., Vaux,D.L., Bath,M.L., Adams,J.M., Cory,S. and Harris,A.W. (1991) Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl Acad. Sci. USA, 88, 8661–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarze S.R., Ho,A., Vocero-Akbani,A. and Dowdy,S.F. (1999) In vivo protein transduction: delivery of a biologically active protein into the mouse. Science, 285, 1569–1572. [DOI] [PubMed] [Google Scholar]
- 22.Schwarze S.R., Hruska,K.A. and Dowdy,S.F. (2000) Protein transduction: unrestricted delivery into all cells? Trends Cell Biol., 10, 290–295. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn R., Schwenk,F., Aguet,M. and Rajewsky,K. (1995) Inducible gene targeting in mice. Science, 269, 1427–1429. [DOI] [PubMed] [Google Scholar]
- 24.Wolfer A., Bakker,T., Wilson,A., Nicolas,M., Ioannidis,V., Littman,D.R., Lee,P.P., Wilson,C.B., Held,W., MacDonald,H.R. et al. (2001) Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nature Immunol., 2, 235–241. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Supprian M., Courtois,G., Tian,J., Coyle,A.J., Israel,A., Rajewsky,K. and Pasparakis,M. (2003) Mature T cells depend on signaling through the IKK complex. Immunity, 19, 377–389. [DOI] [PubMed] [Google Scholar]
- 26.Schnutgen F., Doerflinger,N., Calleja,C., Wendling,O., Chambon,P. and Ghyselinck,N.B. (2003) A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat. Biotechnol., 21, 562–565. [DOI] [PubMed] [Google Scholar]
- 27.Hoess R.H., Wierzbicki,A. and Abremski,K. (1986) The role of the loxP spacer region in P1 site-specific recombination. Nucleic Acids Res., 14, 2287–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewandoski M. and Martin,G.R. (1997) Cre-mediated chromosome loss in mice. Nature Genet., 17, 223–225. [DOI] [PubMed] [Google Scholar]