Abstract
2006 marked the centenary of the birth of Dr Elsie Widdowson, a pioneer of nutrition science. One of the hallmarks of Elsie Widdowson’s research was an integrative approach that recognised the importance of investigating the mechanisms underpinning a public health or clinical issue at all levels, looking into the physiology, comparative biology, intermediate metabolism and basic science. The theme of the present lecture, given in celebration of the work of Dr Widdowson, is mineral nutrition, with a particular focus on Ca, P and vitamin D. The contributions of Dr Widdowson to the early understanding of mineral nutrition are reviewed and the latest scientific findings in this rapidly-expanding field of research are presented.
Keywords: Calcium, Elsie Widdowson, Mineral metabolism, Phosphorus, Vitamin D
Dr Elsie Widdowson was a far-sighted pioneer of nutrition science who maintained enthusiasm for her subject throughout her long and distinguished career. One of the hallmarks of her research was an integrative approach to science, now paraphrased as ‘molecules to man’, which recognised the importance of investigating the mechanisms underpinning a public health or clinical issue at all levels, looking into the physiology, comparative biology, intermediate metabolism and basic science. The style of Elsie Widdowson’s research was to define a problem and then drill down, in the manner of a mine shaft, to the ‘heart of the matter’ in order to investigate the various underlying layers, consider the scientific evidence and offer solutions. This approach is the theme of the Human Nutrition Research Elsie Widdowson Lecture 2006, given to celebrate the centenary of Elsie Widdowson’s birth. The lecture comprises a journey down the ‘mine shaft of mineral nutrition’ and, on descending the shaft and visiting each stratum of the mine, there will be reflection on the contributions made by Elsie Widdowson and then the science will be brought up to date, focusing on Ca, P and vitamin D (Fig. 1).
Fig. 1.
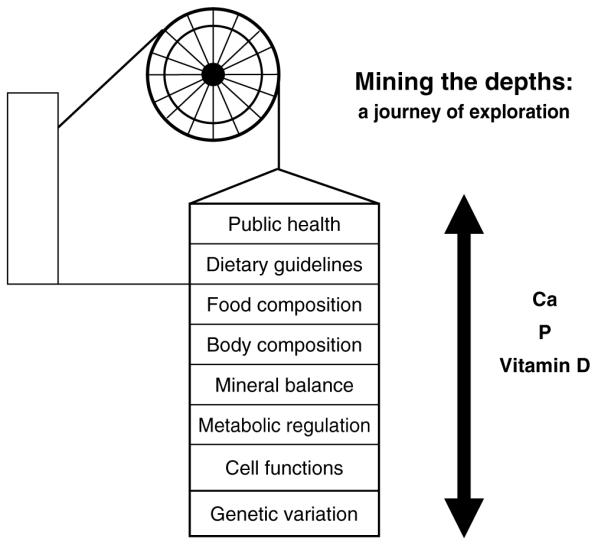
The mine shaft of mineral nutrition.
Stratum 1: public health
The journey starts where Elsie would have expected, i.e. in considering the public health problems that need addressing. Classically for Ca, P and vitamin D, the concern is about their importance for the growth and health of the skeleton. Although there are many diseases that can affect the skeleton, in the public health arena the focus of interest is on:
stunting, in which a child has an inappropriately low (>2 SD below the mean) height for their age;
rickets and osteomalacia, in which the growth plate and protein matrix of bone respectively do not mineralise correctly, resulting in bone deformities and pain. These conditions are caused predominantly, but not exclusively (see p. 516–17), by vitamin D deficiency;
fragility fractures associated with low bone mineral density, both in childhood, especially around the time of puberty, and in older adults with osteoporosis.
It is salutary to remember that, in the years before the Second World War when Elsie Widdowson and Robert McCance started their research into mineral nutrition, it was rickets that was the primary public health concern in the UK in relation to bone. At that time rickets was widespread among the general population of British children. Mercifully, now a thing of the past in the UK, at least among the general population, the prevalence of rickets remains scandalously high in many countries, especially in the Far East, Middle East and parts of Africa. For example, in approximately the last 10 years, prevalence rates as high as 66–70% have been reported in Mongolia and Tibet, 42% in Ethiopia and 9% in Nigeria. Even in Europe children with rickets are found among some minority groups with strict codes of dietary, religious or cultural practice that limit mineral and vitamin D supply. Stunting also remains a global public health issue, with as many as one-third of children in developing countries, approximately 182 million individuals, regarded as having an inappropriately-low height for their age.
It is, however, osteoporosis that is now of greatest concern in the Western world, and which exercises the minds of researchers and policy-makers. Osteoporosis refers to a condition in which mineral:protein in the skeleton is normal but in which there is overall loss of bone tissue and deterioration of the internal architecture, leading to an increased susceptibility to fracture. Osteoporosis is a major public health issue, affecting over three million of the UK population and causing >2 × 106 hip fractures worldwide each year. The incidence of hip fractures is predicted to rise by 3–4-fold between now and 2050 because of the increasing numbers of older adults living to an age when fractures are most common.
Further information on this area of discussion is available(1-3).
Stratum 2: dietary guidelines
It is clearly essential for health professionals and the general public to be provided with robust dietary guidelines in relation to Ca, P and vitamin D nutrition in order to optimise their skeletal health. Elsie Widdowson was well aware of the importance of this need and was at the vanguard of translating research evidence into government policy and practice. Together with Robert McCance, she was the architect of the war-time and post-war diet in the UK, and, in the years after the war, of the fortification of the UK diet with Ca and vitamin D. These fortification practices are still in place. It would probably frustrate Elsie to realise that there is currently little consensus between different advisory bodies about the dietary reference values for Ca and vitamin D and that defining optimal intakes for these nutrients remains a continuing area of controversy and debate. For example, the dietary reference value corresponding to the reference nutrient intake for Ca varies by 2–3-fold between different countries, and for vitamin D there are differences in philosophy about how to account for the contribution of sunlight exposure to vitamin D supply in the setting of dietary guidelines.
Further information on this area of discussion is available(3-11).
Stratum 3: food composition
There are many reasons why it is difficult to be confident about the optimal intakes of bone-forming minerals and vitamin D, but one of them is the uncertainty around what individuals actually eat, because of the variability in the nutrient intake of individuals and the nutritional content of foods. This area was one that Robert McCance and Elsie Widdowson sought to address by improving dietary assessment methodologies and by creating a series of food composition tables. Part of their legacy, the UK Food Tables (McCance and Widdowson’s The Composition of Foods) were first published in 1942 and have been updated regularly over the years. The tables have continued to be revised and updated to the present day, to take account of changes in food composition and to include the plethora of new foods available in the UK diet. This task is immensely challenging and has led to several ongoing initiatives to create accessible electronic databases both at the national and European level.
Such work has been instructive in indicating just how variable the composition of foods can be, and how this variability influences nutrient intake. This variability is particularly marked for Ca; customary Ca intake can vary by approximately 5-fold between individuals in the same population and between different populations, depending on the types of foods consumed. For example, the mean adult Ca intake in sub-Saharan Africa, where milk is rarely consumed, is approximately 200–300 mg/d whereas it averages 1000–1500 mg/d in Scandinavian countries, where milk is consumed regularly. A similar difference in intake can exist between those individuals with the lowest and highest consumption of Ca-rich foods within populations. Such variability is also seen in the Ca intake of exclusively breast-fed children. As the work of the author’s research group has shown, this variability results from the differences in the Ca content of breast milk between individual mothers and in the breast-milk intake of their infants, differences that are not related to the dietary Ca intake of the mother during lactation.
Further information on this area of discussion is available(2,12-16).
Stratum 4: body composition
How such differences in Ca supply translate into variations in the mineral content of the body leads to studies of body composition. Here, too, Elsie Widdowson led the way and made major contributions to knowledge. Together with her colleague John Dickerson, Elsie undertook the total chemical analysis of a small series of human cadavers. From this work they estimated that the Ca content of the newborn is approximately 25–30 g and of the adult human is approximately 1 kg, 99% of which is in the skeleton. From these data they calculated that it would require a Ca acquisition rate of 150–200 mg/d for a newborn to grow to adulthood, assuming maturity at 18 years, and that the rate might peak at approximately 400 mg/d at times of rapid growth, such as puberty.
Nowadays, there are many techniques that facilitate investigation of the skeleton in healthy living individuals. These techniques include instruments to measure bone mass and density, bone dimensions and angles and, at least in animal models, bone architecture. Dual-energy X-ray absorptiometry (DXA), in particular, has provided the opportunity to measure whole-body mineral content in much larger numbers of individuals than was possible for Elsie Widdowson and John Dickerson. The DXA data have supported their original estimates of whole-body Ca content and for childhood acquisition rates. In addition, the use of DXA has made it possible to define the pattern of change in bone mineral content in more detail. For example, longitudinal DXA studies have shown that, during puberty, the peak of bone mineral accrual occurs about 1 year after peak height velocity and that for a period of time the skeleton is relatively rarefied. This change may account to some extent for the increased incidence of distal forearm fractures at around this age. A second example, which comes from the work of the author’s group, is that reductions in bone mineral content of the axial skeleton (the spine and hip) occur in lactating women during the first months of breast-feeding. These changes are reversed during and after weaning and are not influenced by maternal Ca intake, indicating that they are part of the physiological response to lactation.
Bone is a living tissue and the skeleton contains three cell types: the osteoblast, the bone-forming cell, that makes and secretes the protein matrix of bone (collagen, osteoid); the osteocyte, which is a cell embedded within the miner-alised bone tissue and acts as a sensor of the internal skeletal environment, communicating via caniculi with cells on the bone surfaces; the osteoclast, the bone-resorbing cell, a large multinucleated cell with many pseudopodia, that dissolves away small areas of the bone surface, exposing the underlying tissue.
Osteoblasts, osteocytes and osteoclasts act in concert with one another to maintain and repair bone. There is an orchestrated progression of events, referred to as bone remodelling or bone turnover, in which the osteoclast first excavates a resorption pit, followed by recruitment of the osteoblast to the cavity in order to lay down fresh osteoid, which then becomes mineralised and forms new bone. The process of bone remodelling is mediated through cytokines produced by the bone cells. When the bone turnover rate is high there are many resorption cavities on skeletal surfaces being formed and refilled; when it is slower the number of pits formed is fewer. When the rate of turnover alters there is a change in the number of active resorption pits. This change can result in demonstrable changes in bone mineral density, as measured by instruments such as DXA that have very high precision. These effects are referred to as bone remodelling transients. However, such a change in bone mineral density, which is reversed when bone turnover returns to its original value, does not imply a long-term alteration in the total amount or strength of bone tissue in the skeleton.
Bone remodelling transients complicate the interpretation of intervention studies designed to look at the influence of diet on bone health. For example, Ca is a powerful anti-resorptive agent, i.e. an increase in Ca intake reduces the number of active resorption pits on bone surfaces. As a result, a measurable, but reversible, increase in bone mineral density can often be observed by DXA when Ca intake is increased, but this effect does not necessarily represent a change in the amount or strength of bone tissue in the skeleton. This finding emphasises the importance of understanding what influences mineral balance (the difference between intake and output) over a long period of time.
Further information on this area of discussion is available(1-3,14,17-21).
Stratum 5: mineral balance
The study of mineral balance is the field of research in which arguably Robert McCance and Elsie Widdowson made their greatest contributions to the understanding of mineral nutrition and in which they expended considerable effort. One of their most famous studies is ‘The Bread Experiment’ in which they investigated the different effects of brown and white bread on Ca balance by placing themselves, plus several of their colleagues, on a diet in which 40–50% energy was provided by bread. Over a period of 2 years they alternated the use of white and brown bread, taking careful measurements of all the Ca they consumed and excreted. This heroic study demonstrated that Ca absorption was lower on the brown bread diet than on the white bread diet. This disparity was matched by parallel changes in urinary Ca excretion, which thereby reduced the impact on Ca balance. The study also demonstrated that there is seasonal variation in Ca absorption and excretion, with higher values being recorded in the summer than the winter. These effects were shown to be specific to Ca; neither bread type nor season influenced the absorption and excretion of Mg. Overall, these results suggested that the human body has the ability to adapt efficiently to differences in the intake of Ca.
The theme of adaptation, which spawned the concept that for Ca ‘we need what we eat’, was taken further by Mark Hegsted in his seminal studies comparing populations with very different Ca intakes. By measuring the relationships between Ca intake and excretion, he demonstrated that the balance point, i.e. when excretion matches intake, occurs at approximately 700 mg/d in populations such as those in the USA and Europe with a mean intake of approximately this level, and at about 200 mg/d in Peru where Ca intake is much lower. The concept of adaptation was built on further by Elsie Widdowson, who showed in her studies of children in post-war Germany, that absorption increases when requirements are higher, for example during puberty. However, these studies also demonstrated that a net loss of Ca from the skeleton occurs during the period of accommodation to the new diet and suggested to Elsie, and her colleague Lois Thrussell, that adaptation to a low Ca intake might come at a cost. This possibility was also suggested by the studies of Ole Malm in the late 1950s. He measured Ca balance in Norwegian men over a prolonged period. He demonstrated that when their Ca intake was halved, from 1000 mg/d to 500 mg/d, adaptation to the lower Ca intake occurred but it took several months for urinary Ca excretion to decrease to match the reduced intake. During the transition period more Ca was lost from the body than was consumed, indicating a net loss of Ca from the skeleton during that time.
As a consequence, there have been long-standing concerns that, although able to survive on a low Ca intake, populations with a low Ca intake might not have optimal skeletal health and that they might benefit from a higher Ca intake. Over the years, the author’s group has been researching this question in a rural area of The Gambia, West Africa where Ca intake is very low, averaging approximately 200–400 mg/d. Randomised controlled intervention studies have been conducted that have focused on times of life when Ca requirements are high: during pregnancy; lactation; infancy; puberty. Taken overall, there does not appear to be much in the way of benefit for Gambians from an increase in Ca intake. The most recent study, published in 2006, looked at the consequences for the infant of a higher Ca intake by the mother during the second half of pregnancy. No significant difference was found in either the infant’s birth weight or growth (weight, length, bone mineral content) during the first year of life. It would appear that Gambians are, in general, well-adapted to their low Ca intake in terms of bone growth and mineralisation. However, clearly there must be limits to the adaptive process and also it is possible that there are long-term effects of a low Ca intake that are not related to skeletal health. It is important, therefore, to fully understand the way in which the metabolism of Ca, P and vitamin D is regulated and what happens when the supply of these nutrients is limited.
Further information on this area of discussion is available(22-31).
Stratum 6: metabolic regulation
The importance of elucidating the mechanisms underlying the metabolic regulation of mineral balance was recognised by Elsie Widdowson and Robert McCance. In 1939 they published a paper in the Biochemical Journal describing a series of human studies to investigate the excretion of Ca and other minerals following intravenous injection. They demonstrated that there was little evidence of excretion into the gut but rapid appearance of Ca in the urine. They concluded that the kidney ‘must be exceedingly sensitive to the levels of this metal in the serum’. In addition, they ended their paper with the prescient comment that ‘it would be most interesting and valuable to know in what way secretions of the thyroid and parathyroid affected the kidney’.
The pivotal role that the parathyroid gland plays in the regulation of Ca homeostasis is now recognised. It is essential for the normal functioning of the body for the concentration of ionised Ca in blood to be kept within very narrow limits. When serum ionised Ca concentration falls the parathyroid gland responds by increasing the secretion of parathyroid hormone (PTH). This hormone acts on the skeleton to release Ca from bone into the bloodstream and on the kidney to increase the reabsorption of Ca from the urinary filtrate back into the body. PTH also acts on the kidney to up-regulate the production of the renal enzyme (25-hydroxyvitamin D-1α-hydroxylase) that converts vitamin D, after it has been hydroxylated in the liver to 25-hydroxyvitamin D, into its active metabolite 1,25-dihydroxyvitamin D. This metabolite in turn acts on bone to further release Ca and on the intestine to increase the absorption of Ca (via the transient receptor potential cation channel, subfamily V, member 5). All these actions result in an increase in the supply of Ca into the bloodstream and the normalisation of serum ionised Ca concentration.
The mechanism by which the parathyroid gland recognises fluctuations in serum ionised Ca concentration has recently been identified; it is through the Ca2+-sensing receptor, a transmembrane G protein-coupled receptor. The molecule consists of a long extracellular domain with several Ca-binding sites, a membrane-spanning section and a short intracellular tail. When serum ionised Ca concentration falls, conformational changes occur in the extra-cellular domain because of a decrease in the number of occupied Ca-binding sites. These changes signal through to the intracellular domain, triggering a cascade of intracellular events that results in the release of PTH from internal stores, thus increasing PTH secretion.
In Western countries in which osteoporosis is common the elderly are often found to have a high serum PTH concentration, which is considered to be a risk factor for osteoporotic fracture. Paradoxically, some populations, such as those in The Gambia and northern China, have a high PTH concentration, either all year round or during the winter, throughout their adult lives, and yet the age-adjusted incidence of hip fracture in these countries is low compared with that of Western countries. It is possible that in these populations, because of their high PTH levels, they have developed an extent of skeletal resistance to the actions of PTH, and this factor protects them from excessive bone loss in old age. This possibility is continuing to be explored in ongoing research.
One of the side-effects of PTH is that it acts to raise the serum phosphate concentration (phosphate is the term given to the main inorganic forms of P in the body). This increase is a result of the release of phosphate from the skeleton along with Ca and the up-regulation by 1,25-dihydroxyvitamin D of phosphate absorption in the intestine (via the Na–phosphate cotransporter NPT2b) as well as Ca absorption. As P is relatively ubiquitous in most diets, an increase in PTH to rectify a fall in serum ionised Ca concentration would be likely to lead to a greater amount of phosphate entering the bloodstream. This effect, however, would be detrimental because a high serum phosphate concentration is a primary driver for calcification (formation of Ca deposits), not only in the skeleton but also ectopically in soft tissues, such as those of the vascular system and kidney. Furthermore, it would also lead to Ca being removed from the bloodstream, thus negating the efforts of the body to correct the fall in serum ionised Ca. Thus, it has been recognised for many years that the body must have ways of sensing and disposing of excess phosphate. The primary regulation is likely to be at the level of the kidney, because increases in serum phosphate load are rapidly cleared into the urine. However, the search for phosphate regulators, the so-called phosphatonins, has proved elusive until recently, when several factors have been identified that affect phosphate metabolism and may be part of a hormonal homeostatic system for phosphate. To date, the strongest candidate suggested as a physiological phosphatonin is fibroblast growth factor 23 (FGF23).
FGF23 promotes phosphate excretion into urine by the internalisation and down-regulation of the renal Na–phosphate cotransporters NPT2a and NPT2c, thereby decreasing the amount of phosphate that is reabsorbed from the urinary filtrate back into the bloodstream. FGF23 was originally identified in a number of rare phosphate-wasting diseases, such as tumour-induced osteomalacia, some inherited syndromes of hypophosphataemic rickets and fibrous dysplasia, in which it circulates in the blood at high concentrations. Recently, a physiological role has been identified for FGF23, in which it is produced by bone cells (osteoblasts and osteocytes) in response to raised serum phosphate and 1,25-dihydroxyvitamin D concentrations. In this model bone-derived FGF23 is secreted into the bloodstream where it is either inactivated, through cleavage by proteolytic enzymes, or travels to the kidney’s tubular epithelial cells where it locks onto the FGF receptor, thus setting off intracellular signals that result in fewer NPT2 co-transporters. This process reduces phosphate reabsorption from the urinary filtrate, increases urinary phosphate excretion and thereby lowers the amount of phosphate in the bloodstream. A physiological negative feedback loop regulates the production of FGF23 because FGF23 down-regulates the synthesis of renal 25-hydroxyvitamin D-1-α-hydroxylase and thus less 1,25-dihydroxyvitamin D is produced, resulting in a lower amount of FGF23 being secreted.
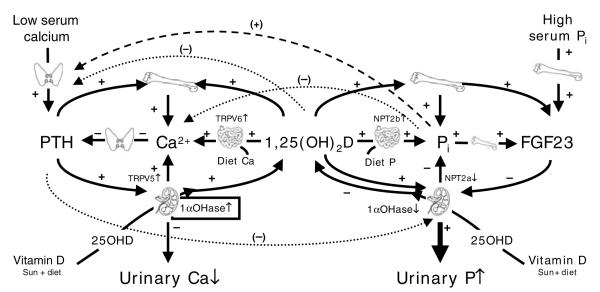
Thus, an integrated Ca–phosphate–vitamin D homeostatic system is beginning to be recognised that acts to maintain serum ionised Ca and phosphate concentrations within their physiological ranges during the every-day fluctuations in dietary supply, working to raise serum ionised Ca concentration when it falls and to decrease serum phosphate concentration when it rises (Fig. 2). Abnormal perturbations in any component of this system would be expected to lead to disease. A possible example of this system may have been identified in studies of Gambian children with ‘African Rickets’ because grossly elevated circulating FGF23 concentrations have recently been observed in children with rickets compared with reference children from the local community (A Prentice, M Ceesay, S Nigdikar, SJ Allen and JM Pettifor, unpublished results).
Fig. 2.
A proposed schematic for an integrated calcium–phosphorus–vitamin D homeostatic system. PTH, parathyroid hormone; 25OHD, 25-hydroxyvitamin D; TRPV5, TRPV6, transient receptor potential cation channel, subfamily V, members 5 and 6 respectively; 1αOHase, 25-hydroxyvitamin D-1α-hydroxylase; NTP2a, NTP2b, Na–phosphate cotransporters; FGF23, fibroblast growth factor 23; Pi, inorganic phosphorus; +, increased; −, decreased.
Cases of children with ‘African Rickets’ have been reported in several African countries including The Gambia, Nigeria and South Africa. Characteristically, such children have a serum 25-hydroxyvitamin D concentration within the range seen in unaffected children but have a low circulating phosphate concentration and either normal or raised PTH and 1,25-dihydroxyvitamin D concentrations; a biochemical profile that is not consistent with primary vitamin D deficiency. Also, these children come from populations whose intake of Ca is very low. These findings have led to the hypothesis that these children have rickets because of a chronically-inadequate dietary Ca supply that causes an elevation in PTH and hence in circulating 1,25-dihydroxyvitamin D concentration. These factors would result in an increase in the skeletal secretion of FGF23 into the bloodstream, a subsequent increase in urinary phosphate excretion and a decrease in serum phosphate concentration. It would be expected, in normal circumstances, that negative feedback would then occur to reduce the serum concentration of 1,25-dihydroxyvitamin D. However, it has been suggested that because of the chronically-low Ca intake of these children, the concentration of 1,25-dihydroxyvitamin D continues to be driven up by PTH and a perpetual positive feedback loop is set-up. These factors would result in urinary phosphate wasting, an abnormally-low serum phosphate concentration, poorly-mineralised bones and growth plates, and ultimately rickets and osteomalacia. Ongoing studies in The Gambia are designed to investigate whether this mechanism is involved in the aetiology of African rickets.
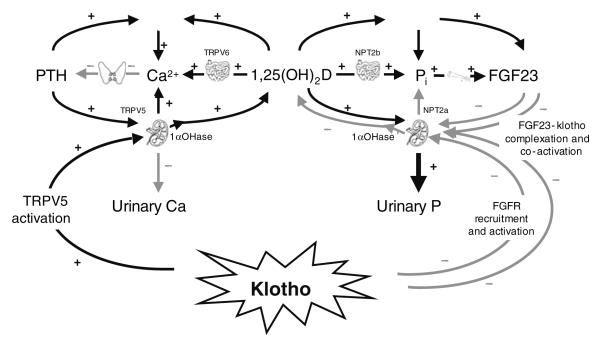
Although it is now possible to describe a simple integrated system to explain Ca and phosphate homeostasis, the picture is, in fact, more complicated. Several other factors are known to modulate phosphate metabolism, including frizzled-related protein-4 and matrix extra-cellular phosphoglycoprotein, which may play a role in an integrated system. The latest factor to be identified as a modulator of Ca and phosphate metabolism is klotho. In 2005 klotho was shown to activate the transient receptor potential cation channel V5 receptor in the intestine and thereby influence Ca absorption, and in 2006 it was shown to potentiate the action of FGF23 at the kidney by enabling it to signal through the canonical FGF receptor (FGF receptor 1(IIIc)) and thus increase urinary phosphate excretion (Fig. 3).
Fig. 3.
The potential involvement of klotho in the calcium–phosphorus–vitamin D homeostatic system. PTH, parathyroid hormone; TRPV5, TRPV6, transient receptor potential cation channel, subfamily V, members 5 and 6 respectively; 1αOHase, 25-hydroxyvitamin D-1α-hydroxylase; 1,25(OH)2D, 1,25-dihydroxyvitamin D; NTP2a, NTP2b, Na–phosphate cotransporters; Pi, inorganic phosphorus; FGF, fibroblast growth factor; FGFR, FGF receptor; +, increased; −, decreased.
Klotho is a protein that has received considerable interest since it was first identified, because of its connections with lifespan. In 1997 a mutant mouse was described that was depleted in klotho protein, referred to as the ‘klotho-mouse’. This mouse showed signs of premature ageing such as organ atrophy, hair loss, ectopic calcifications, arteriosclerosis, hyperphosphataemia, osteoporosis and impaired glucose metabolism. More recently, it has been shown that mice that over-express klotho protein have a longer lifespan than wild-type mice. These studies have led to the klotho gene being referred to widely as a longevity gene. Klotho is expressed in the kidney, parathyroid gland, brain, reproductive organs and pituitary gland and has the potential to act as a hormone and as a paracrine factor, influencing insulin signalling and Ca–phosphate metabolism. The wide-ranging activity of klotho raises the possibility that regulation of Ca and phosphate metabolism is an essential feature of many systems of the body, not just those connected with the skeleton.
Further information on this area of discussion is available(1,20,32-48).
Stratum 7: cell functions
In fact, it is now known that Ca, phosphate and vitamin D play important roles in the functions of many, if not all, cells in the body. There are many Ca-sensitive processes in cells that are invoked by signals generated by transient increases in intracellular ionised Ca concentration. Such increases can be the result of an increased influx of Ca into the cell from the extracellular fluid because of enhanced membrane transport or permeability, or due to the release of Ca from intracellular stores. There are too many examples to mention here but one example is where pulses of Ca coordinate the actions of the many millions of cells in the heart as it contracts and dilates. Ca and phosphate may also be important at the cellular level in other ways, e.g. in gut immunomodulation. It has been known for some time that small but persistent Ca losses occur in the mid-distal small bowel where there is luminal precipitation of Ca-rich microparticles. Jonathan Powell, a colleague at Human Nutrition Research Cambridge, has recently shown that these particles trap luminal material and are transported into phagocytes of the Peyer’s Patches, specialised regions of the intestine involved in immune surveillance and responsiveness. This ‘natural antigen transfection’, as Powell has termed it, may play a role in the development of immunotolerance in the gut, e.g. to different food antigens or symbiotic bacterial antigens, and this possibility is currently being followed up.
Another area in which Ca may be involved and that has engendered considerable interest in recent years is in the development of adiposity; often referred to as the ‘Zemel Hypothesis’. The possibility of a link between Ca and obesity has been suggested by inverse associations between Ca intake and BMI in some epidemiological studies. To date, however, the evidence is contradictory and not conclusive. It is perhaps noteworthy that those studies in which an association has been observed were largely conducted in the USA where dairy products are the major contributors to high Ca intakes, and it may therefore be some other diet or lifestyle factor that is responsible for the relationship. Nevertheless, several plausible mechanisms have been proposed for an effect of Ca on energy balance that are being actively explored by researchers at the present time. These proposals include: (1) an increase in Ca intake could increase satiety, thereby decreasing energy intake; (2) an increase in Ca intake could reduce the amount of fat available for absorption, because, as has been known for many years, Ca forms soaps with fatty acids in the intestine; (3) the decrease in PTH and, subsequently in 1,25-dihydroxyvitamin D, could lead to an increase in lipolysis and lipid oxidation by adipocytes, resulting in an increased thermic effect of food, thus reducing energy balance.
The jury is still out on the ‘Zemel Hypothesis’, but the third possible mechanism is an example of where mineral nutrition may influence cell functionality because of the action of 1,25-dihydroxyvitamin D as a regulator of gene transcription. This regulation occurs through its interaction with the vitamin D receptor (VDR). VDR are found in the cytoplasmic and nuclear compartments of many cells of the body, including those of bone, muscle, epithelium, adipose tissue, the central nervous system, the immune system and some cancers. The VDR, when activated by 1,25-dihydroxyvitamin D, forms a heterodimer with the retinoid X receptor. The resulting 1,25-dihydroxyvitamin D–VDR–retinoid X receptor complex locks on to specific VDR response elements in the promoter regions of specific genes and initiates transcription, modulated by several coactivators and corepressors, thereby regulating cell function. The intracellular concentration of 1,25-dihydroxyvitamin D is, therefore, an important element in the regulation of cellular processes and it is beginning to be recognised that, for many cells and tissues, this concentration is controlled by local extrarenal synthesis of 1,25-dihydroxyvitamin D (Fig. 4).
Fig. 4.
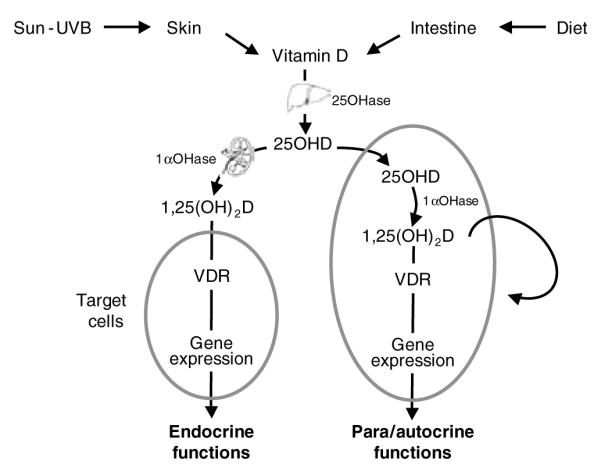
The renal and extrarenal synthesis of 1,25-dihydroxyvitamin D and its actions. 1aOHase, 25-hydroxyvitamin D-1α-hydroxylase; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D; VDR, vitamin D receptor; VDRE.
Classically, 1,25-dihydroxyvitamin D is thought of as being synthesised in the kidney by the renal 1-α-hydroxylase from 25-hydroxyvitamin D. 25-hydroxyvitamin D is the long-lived metabolite of vitamin D that is produced in the liver from vitamin D synthesised in the skin through the action of summer sunlight or supplied from the diet. After conversion in the kidney renal 1,25-dihydroxyvitamin D is secreted into the circulation and travels to its target cells, where it is taken up, interacts with VDR and alters gene expression via VDR response elements in the genome. In this way, 1,25-hydroxyvitamin D behaves as an endocrine hormone, acting primarily on cells involved in the Ca–phosphate homeostatic system, such as those of the intestine, bone and parathyroid gland. More recently, it has been recognised that in some situations circulating 25-hydroxyvitamin D is taken up by extrarenal target cells and acts as a substrate for intracellular 1-α-hydroxylase. This action enables the local production of 1,25-hydroxyvitamin D for the activation of VDR and modulation of gene expression via VDR response elements. In this scenario 1,25-dihydroxyvitamin D acts as an autocrine or paracrine factor.
This process is illustrated in a recent paper by Liu and colleagues, which describes a possible role for 1,25-dihydroxyvitamin D in the body’s fight against tuberculosis. In an elegant series of experiments, the authors have demonstrated that macrophages and monocytes contain intracellular 1-α-hydroxylase, that this enzyme can be activated by Mycobacterium tuberculosis through interaction with the cell’s toll-like receptors (toll-like receptor 2/1), that this interaction increases the local synthesis of 1,25-dihydroxyvitamin D that in turn up regulates the production of cathelicidin, an antimicrobial protein, which reduces bacterial viability. Liu et al. speculate that this mechanism may explain why patients with tuberculosis are often found to have a very low serum concentration of 25-hydroxyvitamin D while having a highly elevated concentration of 1,25-dihydroxyvitamin D, presumably as a result of spill over from the cells of the immune system fighting the disease. It may also provide an explanation for the perceived utility, in earlier days, of two common treatments for tuberculosis, i.e. to take cod-liver oil and to visit Mediterranean countries, both of which would have increased the supply of vitamin D and helped to maintain serum 25-hydroxyvitamin D concentration.
Further information on this area of discussion is available(20,33,49-53).
Stratum 8: genetic variability
The discussion of the role of Ca and vitamin D in regulating cellular function leads to the last stratum in the mine shaft of mineral nutrition, i.e. genetic variation. The interaction of the Ca–phosphate–vitamin D system with the genome suggests the likelihood that differences in genetic makeup may influence the body’s responses to these nutrients. Elsie Widdowson was fully aware of this possibility, which she termed ‘nutritional individuality’, and made it the subject of an address to the Nutrition Society in 1962, which was subsequently published in the Proceedings of the Nutrition Society. In a later paper on the topic, she reminded her readers of the words of Claude Bernard some 100 years earlier: ‘Physiologists and physicians must never forget that the living being is an organism with its own individuality’. She went on to write that ‘We are now beginning to realize how very widely normal healthy people differ from each other in all sorts of ways, and . . . . . . . . . . . . . . which influence our nutritional requirements’.
A large number of studies have identified polymorphisms, both exonic and intronic, in genes associated with the Ca–phosphate–vitamin D axis. However, after years of research, no simple genetic predictor of bone health or fracture risk has emerged, although there are many candidates that are being explored. Of these possibilities, the VDR gene has probably received the greatest attention. Several polymorphisms have been identified, such as the one recognised by the Taq1 restriction enzyme. In some studies, but not all, the VDR genotype tt has been associated with a lower bone mineral density, and hence a higher fracture risk. However, the same genotype has also been associated with a decreased risk of tuberculosis, a reminder that an evolutionary advantage in one situation may be disadvantageous in another, and that this factor should be borne in mind when considering dietary strategies to address public health problems.
Further information on this area of discussion is available(5,54-60).
Return to stratum 1: public health
Having completed the journey, consideration returns to the importance of Ca, P and vitamin D nutrition for public health in 2006. It is now clear that many other aspects of physiology and metabolism must be taken into consideration in addition to the traditional ones of growth and skeletal health (Fig. 5). These inter-relationships are only just beginning to be unravelled, and from being one of the least fashionable areas of nutrition research mineral and vitamin D nutrition is rapidly becoming one of the hottest topics in the field. However, it is hoped that the present paper is a reminder that the scientific foundations of this work were laid many years ago. So, on leaving the mine shaft of mineral nutrition and looking out on the new horizons of mineral and vitamin D research that lie ahead, it is important to remember those giants on whose shoulders we climb, and especially, in her centenary year, Elsie Widdowson.
Fig. 5.
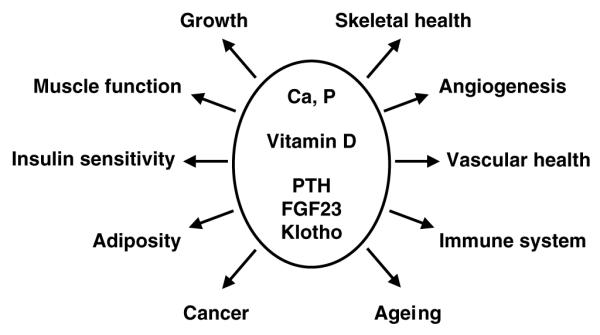
The health interactions of calcium, phosphorus and vitamin D. PTH, parathyroid hormone; FGF23, fibroblast growth factor 23.
Postscript
2006 was the centenary of the birth of Dr Elsie Widdowson. To mark this event and to celebrate her achievements the annual Elsie Widdowson Lecture, established by MRC Human Nutrition Research, was held at Churchill College, Cambridge. This year’s speaker was Dr Ann Prentice, Director of MRC Human Nutrition Research and current President of the Nutrition Society. The lecture is usually given to an invited audience and is not published. This year, however, the lecture was held in association with the Winter Meeting of the Nutrition Society jointly with the Neonatal Society on ‘New horizons for a new century’ held to honour Elsie Widdowson (Fig. 6), and Dr Prentice agreed to provide a written version of the presentation, based on the transcript, with selected references and illustrations for the Proceedings of the Nutrition Society. Although not in the usual house style, it is anticipated that the paper will be of interest to readers.
Fig. 6.
Elsie Widdowson 1906–2000.
Abbreviations
- DXA
dual-energy X-ray absorptiometry
- FGF23
fibroblast growth factor 23
- PTH
parathyroid hormone
- VDR
vitamin D receptor
References
- 1.Pettifor JM, Prentice A, Cleaton-Jones P. The skeletal system. In: Gibney M, Macdonald I, Roche H, editors. Textbook of Nutrition and Metabolism. Blackwell Publishing; London: 2003. pp. 247–283. [Google Scholar]
- 2.Prentice A. Diet, nutrition and prevention of osteoporosis. Public Health Nutr. 2004;7:237–254. doi: 10.1079/phn2003590. [DOI] [PubMed] [Google Scholar]
- 3.Prentice A, Schoenmakers I, Laskey MA, de Bono S, Ginty F, Goldberg GR. Nutrition and bone growth and development. Proc Nutr Soc. 2006;65:348–360. doi: 10.1017/s0029665106005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prentice A, Branca F, Decsi T, Michaelsen KF, Fletcher RJ, Guesry P, Manz F, Vidailhet M, Pannemans D, Samartín S. Energy and nutrient dietary reference values for children in Europe: methodological approaches and current nutritional recommendations. Br J Nutr. 2004;92(Suppl. 2):S83–S146. doi: 10.1079/bjn20041159. [DOI] [PubMed] [Google Scholar]
- 5.Prentice A. What are the dietary requirements for calcium and vitamin D? Bone. 2001;28(Suppl. 5):253. doi: 10.1007/s00223-001-0035-0. [DOI] [PubMed] [Google Scholar]
- 6.Department of Health . Nutrition and Bone Health: With Particular Reference to Calcium and Vitamin D. Report of the Subgroup on Bone Health (Working Group on the Nutritional Status of the Population) of the Committee on Medical Aspects of Food and Nutrition Policy. The Stationery Office; London: 1998. [PubMed] [Google Scholar]
- 7.Institute of Medicine Food and Nutrition Board . Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; Washington, DC: 1997. [PubMed] [Google Scholar]
- 8.Department of Health and Social Security . Recommended Intakes of Nutrients for the United Kingdom. H. M. Stationery Office; London: 1969. [Google Scholar]
- 9.Department of Health and Social Security . Rickets and Osteomalacia. H. M. Stationery Office; London: 1980. [Google Scholar]
- 10.McCance RA, Widdowson EM. An Experimental Study of Rationing. H. M. Stationery Office; London: 1946. [Google Scholar]
- 11.Committee of the Privy Council for Medical Research . Medical Research in War. Report of the Medical Research Council for the Years 1939–45. H. M. Stationery Office; London: 1948. [Google Scholar]
- 12.McCance RA, Widdowson EM. Chemical Composition of Foods. H. M. Stationery Office; London: 1942. [Google Scholar]
- 13.Paul AA, Southgate DAT. McCance and Widdowson’s The Composition of Foods. 4th ed H. M. Stationery Office; London: 1978. [Google Scholar]
- 14.Prentice A, Laskey MA, Jarjou LMA. Lactation and bone development: implications for the calcium requirements of infants and lactating mothers. In: Bonjour J-P, Tsang RC, editors. Nutrition and Bone Development. Vestey/Lippincott-Raven Publishers; Philadelphia, PA: 1999. pp. 127–145. [Google Scholar]
- 15.McCance RA, Widdowson EM. Food requirements and food intakes. Br Med J. 1937;ii:311–315. doi: 10.1136/bmj.2.3997.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food Standards Agency . McCance and Widdowson’s The Composition of Foods. Sixth Summary Edition Royal Society of Chemistry; Cambridge: 2002. [Google Scholar]
- 17.Prentice A, Bates CJ. An appraisal of the adequacy of dietary mineral intakes in developing countries for bone growth and development in children. Nutr Res Rev. 1993;6:51–69. doi: 10.1079/NRR19930006. [DOI] [PubMed] [Google Scholar]
- 18.Widdowson EM, Dickerson JWT. Chemical composition of the body. In: Comar CL, Bronner F, editors. Mineral Metabolism. Academic Press; New York: 1964. pp. 1–247. [Google Scholar]
- 19.Prentice A, Bonjour J-P, Branca F, Cooper C, Flynn A, Garabedian M, Muller D, Pannemans D, Weber P. PASSCLAIM – Bone health and osteoporosis. Eur J Nutr. 2003;42(Suppl. 1):128–149. doi: 10.1007/s00394-003-1103-1. [DOI] [PubMed] [Google Scholar]
- 20.American Society for Bone and Mineral Research . Primer of the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6th ed The American Society for Bone and Mineral Research; Washington, DC: 2006. [Google Scholar]
- 21.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan Bone Mineral Accrual Study. J Bone Miner Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 22.McCance RA, Widdowson EM. Mineral metabolism of healthy adults on white and brown bread. J Physiol. 1942;101:44–85. doi: 10.1113/jphysiol.1942.sp003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCance RA, Widdowson EM. Seasonal and annual changes in the calcium metabolism of man. J Physiol. 1943;102:42–49. doi: 10.1113/jphysiol.1943.sp004012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malm OJ. Calcium requirement and adaptation in adult man. Scand J Clin Lab Invest. 1958;10(Suppl. 36):1–289. [PubMed] [Google Scholar]
- 25.Kanis JA, Passmore R. Calcium supplementation of the diet. I. Br Med J. 1989;298:137–140. doi: 10.1136/bmj.298.6667.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegsted DM. From chick nutrition to nutrition policy. Annu Rev Nutr. 2000;20:1–19. doi: 10.1146/annurev.nutr.20.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Widdowson EM, Thrusell LA. Studies of under-nutrition, Wuppertal 1946–9. XXVI. The absorption and excretion of nitrogen, calcium, magnesium and phosphorus. Spec Rep Ser Med Rest Counc (G B) 1951;275:296–312. [PubMed] [Google Scholar]
- 28.Hegsted DM. Calcium and osteoporosis. J Nutr. 1986;116:2316–2319. doi: 10.1093/jn/116.11.2316. [DOI] [PubMed] [Google Scholar]
- 29.Jarjou L, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg G, Cole TJ. Randomized, placebo-controlled calcium supplementation study of pregnant Gambian women: effects on breast-milk calcium concentration and infant birth weight, growth and bone mineral accretion in the first year of life. Am J Clin Nutr. 2006;83:657–666. doi: 10.1093/ajcn.83.3.657. [DOI] [PubMed] [Google Scholar]
- 30.Prentice A, Jarjou LMA, Cole TJ, Stirling DM, Dibba B, Fairweather-Tait S. Calcium requirements of lactating Gambian mothers: effects of a calcium supplement on breastmilk calcium concentration, maternal bone mineral content, and urinary calcium excretion. Am J Clin Nutr. 1995;62:58–67. doi: 10.1093/ajcn/62.1.58. [DOI] [PubMed] [Google Scholar]
- 31.Prentice A. Studies of Gambian and UK children and adolescents: insights into calcium requirements and adaptation to a low calcium intake. In: Burckhard P, Dawson-Hughes B, Heaney RP, editors. Proceedings of the 6th International Symposium on Nutritional Aspects of Osteoporosis; Amsterdam: Elsevier; 2007. pp. 15–24. [Google Scholar]
- 32.McCance RA, Widdowson EM. LXIV. The fate of calcium and magnesium after intravenous administration to normal persons. Biochem J. 1939;33:523–529. doi: 10.1042/bj0330523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleet JC. Molecular regulation of calcium homeostasis. In: Weaver CM, Heaney RP, Raisz LG, editors. Calcium in Human Health. Humana Press; Totowa, NJ: 2006. pp. 163–190. [Google Scholar]
- 34.Brown EM. The extracellular Ca2 + -sensing receptor: central mediator of systemic calcium homeostasis. Annu Rev Nutr. 2000;20:507–533. doi: 10.1146/annurev.nutr.20.1.507. [DOI] [PubMed] [Google Scholar]
- 35.Aspray TJ, Yan L, Prentice A. Parathyroid hormone and rates of bone formation are raised in perimenopausal rural Gambian women. Bone. 2005;36:710–720. doi: 10.1016/j.bone.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Yan L, Zhou B, Wang X, D’Ath S, Laidlaw A, Laskey MA, Prentice A. Older people in China and UK differ in the relationships between parathyroid hormone, vitamin D and bone mineral status. Bone. 2003;33:620–627. doi: 10.1016/s8756-3282(03)00216-3. [DOI] [PubMed] [Google Scholar]
- 37.Pettifor JM. Nutritional rickets. In: Glorieux F, Jueppner H, Pettifor JM, editors. Pediatric Bone – Biology and Diseases. Elsevier Science; San Diego, CA: 2003. pp. 541–566. [Google Scholar]
- 38.White KE, Larsson TM, Econs MJ. The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr Rev. 2006;27:221–241. doi: 10.1210/er.2005-0019. [DOI] [PubMed] [Google Scholar]
- 39.Berndt T, Kumar R. The phosphatonins and the regulation of phosphorus homeostasis. BoneKEy-Osteovision. 2005;2:5–16. doi: 10.1152/ajprenal.00072.2005. [DOI] [PubMed] [Google Scholar]
- 40.Saito H, Maeda A, Ohmoto S, et al. Circulating FGF-23 is regulated by 1a,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 41.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishnan FK. 1alpha,25-dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 42.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 44.Urakawa I, Yamazuki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Kukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 45.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 46.Negri AL. The klotho gene: a gene predominantly expressed in the kidney is a fundamental regulator of aging and calcium/phosphorus metabolism. J Nephrol. 2005;18:654–658. [PubMed] [Google Scholar]
- 47.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kettler M, Floege J. Calcification and the usual suspect phosphate: still guilty but there are other guys behind the scenes. Nephrol Dial Transplant. 2006;21:33–35. doi: 10.1093/ndt/gfi270. [DOI] [PubMed] [Google Scholar]
- 49.Awumey EM, Bukoski R. Cellular functions and fluxes of calcium. In: Weaver CM, Heaney RP, Raisz LG, editors. Calcium in Human Health. Humana Press; Totowa, NJ: 2006. pp. 13–35. [Google Scholar]
- 50.Tallini YN, Ohkura M, Choi BR, et al. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2 + indicator GCaMP2. Proc Natl Acad Sci USA. 2006;103:4753–4758. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Morilla I, Thoree V, Powell JJ, Kirkby KJ, Grime GW. Identification and quantitative analysis of calcium phosphate microparticles in intestinal tissue by nuclear microscopy. Nucl Instrum Methods Phys Res B. 2006;249:665–669. [Google Scholar]
- 52.Pele L, Powell JJ. Microparticles: a link between modern life and inflammatory bowel disease. In: Vucelic B, Colombel J-F, Gasche C, Scholmerich J, editors. Inflammatory Bowel Disease: Translation from Basic Research to Clinical Practice. Falk Symposium. vol. 140. Springer; Dubrovnik, Croatia: 2005. pp. 123–137. [Google Scholar]
- 53.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 54.Widdowson EM. Nutritional individuality. J Coll Gen Pract. 1963;19(Suppl.2):10–16. [PMC free article] [PubMed] [Google Scholar]
- 55.Widdowson EM. Nutritional individuality. Proc Nutr Soc. 1962;21:121–128. doi: 10.1079/pns19620023. [DOI] [PubMed] [Google Scholar]
- 56.Ferrari S. Single gene mutations and variations affecting bone turnover and strength: a selective 2006 update. BoneKEy-Osteovision. 2006;3:11–29. [Google Scholar]
- 57.Prentice A. The relative contribution of diet and genotype to bone development. Proc Nutr Soc. 2001;60:1–8. doi: 10.1079/pns200072. [DOI] [PubMed] [Google Scholar]
- 58.Spector TD, Keen RW, Arden NK, et al. Influence of vitamin D receptor genotype on bone mineral density in postmenopausal women: a twin study in Britain. Br Med J. 1995;310:1357–1360. doi: 10.1136/bmj.310.6991.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellamy R, Ruwende C, Corrah T, McAdam KPWJ, Thursz M, Whittle HC, Hill AVS. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:723–724. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 60.Teegarden D. Dietary calcium and obesity. In: Weaver CM, Heaney RP, Raisz LG, editors. Calcium in Human Health. Humana Press; Totowa, NJ: 2006. pp. 327–340. [Google Scholar]