Abstract
OBJECTIVE: To examine the rate and correlates of completion of the quadrivalent human papillomavirus vaccine (HPV4) 3-dose regimen because nonadherence to the regimen may adversely affect vaccine efficacy.
PARTICIPANTS AND METHODS: Female members of Kaiser Permanente Southern California who were 9 to 26 years old, received the first dose of HPV4 between October 2006 and March 2007, and maintained health plan membership 12 months afterward were identified and followed up for regimen completion. We examined the following: (1) demographics/socioeconomic status, (2) primary care physician characteristics, (3) historical health service utilization, (4) women's health-related conditions, and (5) selected immune-related conditions for their association with completion in 2 age groups: 9 to 17 years and 18 to 26 years. Multivariable log-binomial regression was used to directly estimate relative risk (RR).
RESULTS: Of the 34,193 females who initiated HPV4, the completion rate was 41.9% in the 9- to 17-year-old group and 47.1% in the 18- to 26-year-old group. Black race (RR, 0.70; 95% confidence interval [CI], 0.64-0.77) and lower neighborhood education level were associated with lower regimen completion. However, those in the 9- to 17-year-old group who were covered by the state-subsidized program Medi-Cal were more likely to complete the regimen (RR, 1.14; 95% CI, 1.07-1.22). Historical hospitalizations and emergency department visits (RR, 0.92; 95% CI, 0.87-0.96; and RR, 0.96; 95% CI, 0.94-0.98 per visit, respectively) and having a pediatrician were also predictors of noncompletion. A history of sexually transmitted diseases, abnormal Papanicolaou test results, and immune-related conditions (eg, asthma/infections) were not associated with regimen completion.
CONCLUSION: These findings suggest that factors such as race or socioeconomic status should be considered when human papillomavirus vaccination programs are being designed and evaluated.
Of 34,193 females who initiated the quadrivalent human papillomavirus vaccine, the completion rate was 42% in the 9- to 17-year-old group and 47% in the 18- to 26-year-old group. Black race and lower neighborhood educational level were associated with lower regimen completion. Thus, factors such as race and socioeconomic status should be considered when human papillomavirus vaccination programs are being designed and evaluated.
CI = confidence interval; HPV = human papillomavirus; HPV4 = quadrivalent human papillomavirus vaccine; ICD-9 = International Classification of Diseases, Ninth Revision; KITS = Kaiser Immunization Tracking System; KPSC = Kaiser Permanente Southern California; PCP = primary care physician; RR = relative risk; SES = socioeconomic status; STD = sexually transmitted disease
The quadrivalent human papillomavirus vaccine (HPV4) (Gardasil, Merck & Co, Inc), given in 3 injections, has been shown to be efficacious in preventing cervical cancer and other conditions caused by human papillomavirus (HPV) types 6, 11, 16, and 18.1,2 However, the 3-dose regimen of the vaccine may impose difficulty for successful HPV immunization in eligible females (ie, 9-26 years old) because of the lack of regular or prespecified health care encounters in these age groups. This may be particularly true in resource-poor settings. In the United States, HPV vaccination is in general not required by state law. As a result, the 3-dose regimen may not always be completed by those who initiate the vaccine. The Advisory Committee on Immunization Practice recommends that the second and third doses be administered 2 and 6 months after the first dose.3 The vaccine label suggests that the 3 doses be completed within 12 months. On the basis of a survey conducted by the Centers for Disease Control and Prevention, approximately 25% of female adolescents between 13 and 17 years of age initiated the vaccine in 20074; however, only one-fourth of these individuals had completed the 3-dose series, potentially because of insufficient follow-up at the time of the survey.4 The number of those who initiated the vaccine and actually completed the 3-dose regimen is unclear. Furthermore, whether socioeconomic status (SES) or other health-related factors are associated with regimen completion is unclear. These issues are of public health importance because incomplete vaccination may result in suboptimal disease protection and may jeopardize the cost-effectiveness of the vaccination program. Thus, research addressing these issues is needed to inform clinical practice and future development of HPV immunization programs.
To this end, we investigated the rate and correlates for HPV4 regimen completion among those who initiated the vaccine in a managed care population of Kaiser Permanente Southern California (KPSC). A previous study suggested that education level and medical history were predictors for hepatitis B vaccine regimen adherence.5 We further explored physician and utilization-related characteristics and examined whether the following factors were correlates for completion of the 3-dose regimen among those who initiated HPV4: (1) demographics and SES, (2) primary care physician (PCP) characteristics, (3) historical health service utilization, (4) women's health-related medical history, and (5) several immune-related medical conditions (including rheumatoid arthritis, asthma, allergy, and infections).
PARTICIPANTS AND METHODS
As the largest managed care organization in southern California, KPSC serves more than 3 million members. Members of KPSC are broadly representative of the diverse racial/ethnic and socioeconomic backgrounds of the source population in southern California. By nature of the prepaid managed care system, members of KPSC have relatively equal health care coverage. In particular, HPV4 is offered to eligible female members without additional out-of-pocket cost (variations in office visit copay exist but are small). Female members between age 9 and 26 years on October 1, 2006, who received the first dose of HPV4 between October 2006 and March 2007 and maintained KPSC membership for the following 12 months were included in this study. October 2006 was chosen as the start of the investigation because HPV4 was approved for the KPSC vaccine formulary in September 2006, and its administration started in October 2006. This study protocol was approved by the KPSC Institutional Review Board.
HPV4 Regimen Completion
Information on HPV4 vaccination was obtained using the Kaiser Immunization Tracking System (KITS). This system tracks all immunization and skin tests given at KPSC and is the Kaiser Permanente legal record for immunization. Records of HPV4 vaccination in KITS were linked to female members through the unique medical record number. Date and dose of vaccination were available from KITS. Receipt of the 3 doses of HPV4 was assessed during the 12-month period (as recommended by the vaccine label) for each individual.
Characteristics of Participants
Demographic variables such as age, race/ethnicity, and length of health plan membership were available from the KPSC membership files. Health plan members' addresses were mapped to US census block data,6 which provided information on neighborhood education and income status. Medi-Cal status was used as an indicator for individual level SES. Medi-Cal is California's Medicaid health care program that covers children and adults with limited income and resources.
The characteristics of participants' PCP, such as specialty, age, sex, and affiliated medical center, were obtained from the KPSC provider files. Members' medical history and health service utilization are constantly recorded in the KPSC information infrastructure, which includes electronic health records and multiple specialized databases (such as pharmacy and laboratory testing). Historical health service utilization in a 2-year interval between October 2004 and September 2006 was characterized by the number of (1) all outpatient visits, (2) primary care visits, (3) inpatient hospitalizations (excluding pregnancy-related hospitalizations), (4) emergency department visits, and (5) out-of-plan visits.
For women's health-related medical history, obstetric history, history of sexually transmitted diseases (STDs, including genital/anal warts, chlamydia, gonorrhea, genital herpes, trichomoniasis, and syphilis), Papanicolaou test screening, abnormal results of Papanicolaou tests, and use of oral contraceptives or birth control patches were assessed. Specific medical histories were ascertained by computer search of corresponding International Classification of Diseases, Ninth Revision, (ICD-9) codes specific to each condition. For assessing the obstetric history, we used a positive urine/serum pregnancy test result, a record in the KPSC perinatal service system, an abortion record, or 2 or more codes for diagnosis indicative of pregnancy. Information from inpatient, outpatient, and emergency department visits and claims were searched for obstetric history. A history of Papanicolaou testing and abnormal results of testing were assessed through KPSC electronic records of laboratory procedures. Clinical guidelines require that females 18 years and older undergo at least 1 Papanicolaou test every 3 years.7 Therefore, Papanicolaou testing within the 3-year interval before study baseline (October 2003-September 2006) was assessed. Data on use of oral contraceptives and contraceptive patches were collected from the KPSC pharmacy dispensing system.
For immune-related medical history, several prespecified conditions were assessed in the 2 years before baseline (October 2004-September 2006): (1) rheumatoid conditions (juvenile rheumatoid arthritis and rheumatoid arthritis); (2) asthma; (3) general allergy (including hay fever, eczema, contact dermatitis, urticaria, food allergies, and unspecified allergy); (4) drug allergy; (5) number of infections (including urinary tract infections, respiratory tract infections, intestinal infections, and skin infections); and (6) oral antibiotic use (number of prescriptions) for bacterial infections. Notations of at least 2 ICD-9 codes specific to the conditions were required to define the history of the condition (to reduce false-positive results and to capture the more severe cases). Drug allergy was assessed either by ICD-9 codes or through documentation of drug allergic/adverse reactions in electronic health records. Data on use of antibiotics were collected from the KPSC pharmacy dispensing system. Antibiotic use included only oral antibiotic use and excluded prescriptions of 21 days or more for tetracycline, minocycline, doxycycline, erythromycin, and clindamycin to reduce the effect of antibiotic used for acne treatment.
Statistical Analyses
We first calculated the percentage of females who completed the 3 doses (as well as those who received 2 doses) among those who initiated the vaccine in the 12-month assessment period by age. The distribution of demographics, physician characteristics, health service utilization, and selected medical history was compared between those who completed the 3 doses (regimen completers) and those who did not (noncompleters), stratified by 2 age groups: 9 to 17 years (defined as adolescent girls in this study) and 18 to 26 years (defined as young women in this study). Stratification was used because factors associated with regimen completion may be different for adolescent girls and young women. The age cutoff was chosen because parental consent is required at Kaiser Permanente for vaccination for children younger than 18 years. Age was modeled as a categorical variable for adolescent girls and as a continuous variable for young women on the basis of the crude assessment for the age trend of regimen completion.
The crude and adjusted associations with HPV4 regimen completion were estimated using log-binomial regression. Because the overall proportion of regimen completion was much greater than 10%, the log-binomial model was used to directly estimate relative risk (RR). When the log-binomial model failed to converge properly, the log-normal model was used as an approximation. Multivariable models that simultaneously considered measures from different categories were then constructed. Because of potential collinearity within a category, a subset of measures from each category was used in the multivariable analyses. To this end, Pearson correlation coefficients were calculated for the association between measures within the same category to identify those that appeared to measure similar constructs. On the basis of the RR estimates and the associated P values for each variable (mutually adjusted for other measures in the same category), 2 or 3 measures from each category were selected for the multivariable analysis. The selection criteria were based on significant associations in both P<.05 and magnitude of the point estimate of the RR (eg, ≥1.20). Furthermore, subjective knowledge was used to select measures likely to represent different underlying constructs. All demographic and SES variables, length of membership, and physician-affiliated medical center were always included in the multivariable analysis. Women's health-related medical history was considered only for young women (18-26 years old) because these conditions are rare in adolescent girls.
Using the aforementioned method, the following measures were selected for the final multivariable model. All demographic variables (age, race/ethnicity, neighborhood income and education level, Medi-Cal status); historical PCP visits, hospitalizations, and emergency department visits; and history of allergy and antibiotic use were selected. In addition, for young women, PCP specialty and sex, obstetric history, and use of oral contraceptives or patches were included. Multivariable analyses were performed stratified by the age group. Because the 11- and 12-year-old age group is currently recommended for routine HPV vaccination, we also conducted a secondary analysis restricted to that age group. All analyses were conducted using SAS statistical software version 9 (SAS Institute, Cary, NC).
RESULTS
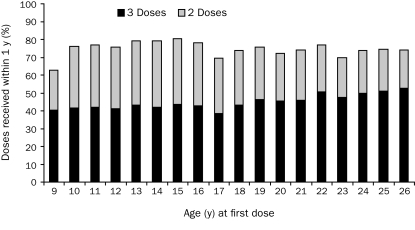
A total of 34,193 females aged 9 through 26 years received the first dose of HPV4 between October 2006 and March 2007 (ie, 9.5% of all female members in this age range who maintained health plan membership during that period), and 29,598 maintained their health plan membership for the following 12 months. These 29,598 females (24,676 in the 9- to 17-year-old age group and 4922 in the 18- to 26-year-old age group) were included in the study. Among them, 12,663 (42.8%) completed the 3-dose regimen within the 12-month period. The completion rate was 41.9% in adolescent girls (9-17 years) and 47.1% in young women (18-26 years). Rate of regimen completion by yearly age is shown in the Figure. Another 33.6% (age 9-26 years) received 2 doses during the assessment period (Figure). The distribution of demographics and other measures of interests by HPV4 regimen completion status, stratified by age groups, are listed in Table 1.
FIGURE.
Percentage of participants who completed the 3-dose regimen of quadrivalent human papillomavirus vaccine and percentage who received 2 doses within 12 months, stratified by age.
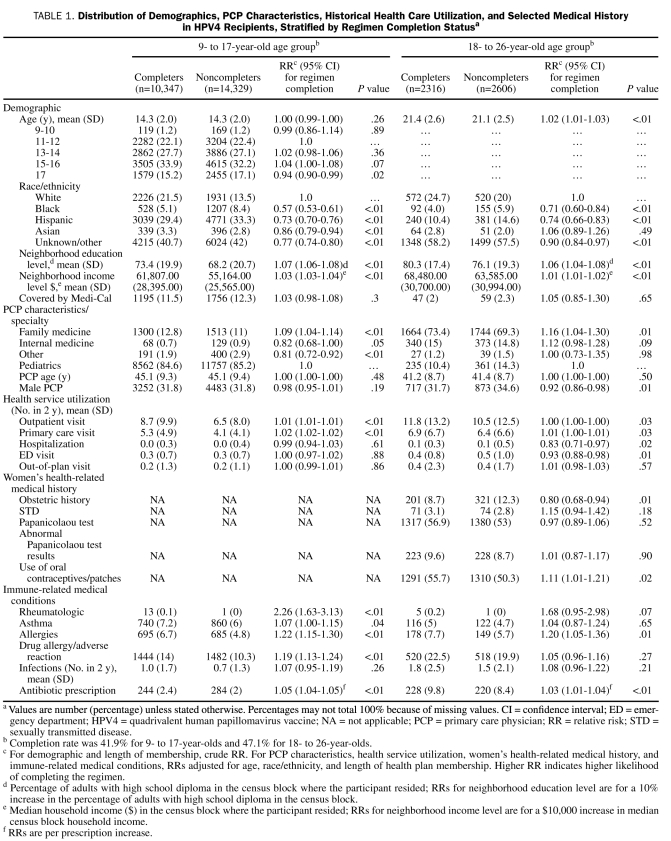
TABLE 1.
Distribution of Demographics, PCP Characteristics, Historical Health Care Utilization, and Selected Medical History in HPV4 Recipients, Stratified by Regimen Completion Statusa
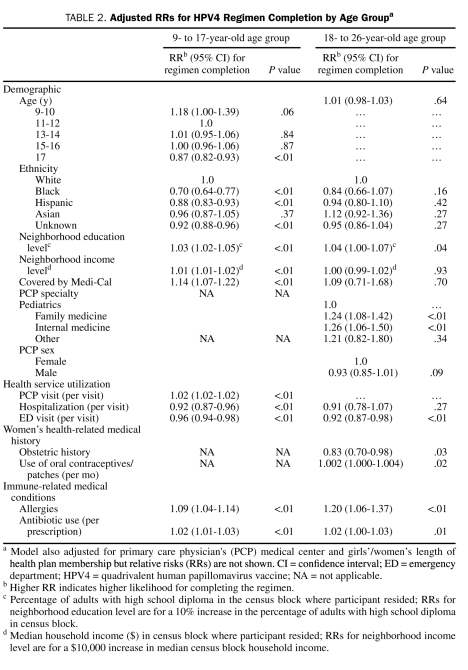
Results from multivariable models are shown in Table 2. Older age was slightly associated with lower likelihood of HPV4 regimen completion in adolescent girls (RR for age 17 years, 0.87; 95% CI [confidence interval], 0.82-0.93) compared with 11- to 12-year-olds). Compared with white adolescent girls, black and Hispanic adolescent girls had lower likelihood of completing the regimen (RR, 0.70; 95% CI, 0.64-0.77; and RR, 0.88; 95% CI, 0.83-0.93; respectively). Higher neighborhood education level was positively associated with regimen completion in both age groups. However, adolescent girls who were covered by Medi-Cal were also more likely to complete the regimen (RR, 1.14; 95% CI, 1.07-1.22). Although the number of historical PCP visits was positively associated with regimen completion, the number of historical hospitalizations and emergency department visits was inversely related to this outcome. In general, history of allergy or of antibiotic use was a predictor of higher likelihood of regimen completion in both age groups, independent of number of PCP visits. In addition, for young women, PCP specialty was a predictor for regimen completion: those with a pediatrician PCP were less likely to complete the regimen. Young women with a male PCP were also slightly less likely to complete the 3-dose regimen (RR, 0.93; 95% CI, 0.85-1.01). The number of oral contraceptive or patch prescriptions was not associated with regimen completion in a meaningful magnitude in the final model. Obstetric history was inversely associated with regimen completion (RR, 0.83; 95% CI, 0.70-0.98) (Table 2).
TABLE 2.
Adjusted RRs for HPV4 Regimen Completion by Age Groupa
In the secondary analyses that were restricted to the 11- to 12-year age group, results were similar as in the analyses for all adolescent girls (RRs in the same direction and magnitude, although some were not statistically significant), except antibiotic use, which was not a predictor for HPV regimen completion (RRper prescription, 0.99; 95% CI, 0.96-1.02) in this age group.
DISCUSSION
In the current study, the rate of completion of the 3-dose regimen of HPV4 in a 12-month period was less than 50% among those who initiated the vaccination. Higher neighborhood education levels, Medi-Cal status for adolescent girls, and history of allergy were positive predictors for regimen completion. In contrast, black and Hispanic adolescent girls, young women who had a pediatrician PCP, and a history of hospitalizations/emergency department visits were correlates for nonadherence to the 3-dose regimen. The rate of regimen completion for HPV4 has not been extensively reported. Because there is no regular health care system encounter for females in these age groups, some may complete the 3-dose series beyond the recommended 12-month period. However, we explored this possibility by analyzing regimen completion through September 2008. With an average of 2 years of follow-up for our study participants, the 3-dose completion rate increased to 50%, a limited increase in completion rate beyond a 12-month period. Hence, those who did not complete the regimen in 12 months seemed unlikely to complete the regimen. The relevance of regimen nonadherence depends on the immune protection offered by incomplete dosing. However, disease protection from 1 or 2 HPV4 injections or delayed dosing is still unclear. Therefore, it is important to understand current obstacles for regimen adherence and to design and implement appropriate interventions to enhance adherence.
We found that blacks in particular were at greater risk of failing to complete the HPV4 regimen. Irrespective of race/ethnicity, lower neighborhood education level was also a significant predictor for nonadherence. However, being covered by the state-subsidized program Medi-Cal was positively associated with regimen completion by adolescent girls. Although Medi-Cal status may reflect the individual level SES better than census track information, these results still suggest a concern of SES disparity for completion of the regimen. Our observation on neighborhood education level is consistent with a previous study that reported that education is a strong predictor for hepatitis B vaccine regimen adherence.5 However, that same study also reported that those with low education level were highly responsive to the intervention of study for regimen adherence (telephone reminders).5 Given that cervical cancer in the United States predominantly affects racial/ethnic minorities and/or those who are socioeconomically disadvantaged,8,9 appropriate intervention should be designed to enhance the regimen adherence in these subgroups.
None of the PCP characteristics we examined were important predictors for regimen completion by adolescent girls. However, young women with a pediatrician PCP were less likely to complete the regimen. The reason for this observation is unclear, although it could be due to lower health care need for those young women who still see a pediatrician. We also found that young women who had a male PCP had a somewhat lower likelihood of completing the regimen than those with a female PCP. This is consistent with our previous observation that those with a male PCP were less likely to initiate HPV vaccination. A previous national survey found that female physicians were more likely to recommend the HPV vaccine than male physicians.10 The underlying determinants of this finding (eg, comfort level of communication with female patients) could potentially affect both the likelihood of vaccine initiation and regimen completion by their patients. These results suggest that the effect of physician's attitudes on successful HPV vaccination deserves further study.
Physician office visits were associated with completion of the regimen by adolescent girls. This is not surprising since encounters with the health care system provide opportunity for vaccination for this age group that lacks regular and prespecified health care visits. We also observed an inverse relationship between the number of historical hospitalization/emergency department visits and HPV4 regimen completion. This observation may be due to ill patients being overwhelmed by their existing conditions and less attentive to other preventive care needs. This phenomenon has been documented in other studies of persons with chronic disease.11
The HPV4 is not indicated during pregnancy, and therefore it is somewhat reassuring that young women with obstetric history were less likely to receive the 3 doses.3 Interestingly, histories of STDs, abnormal Papanicolaou test results, pap screening, or use of oral contraceptive/patches were not important predictors of regimen completion by young women. We identified several immune-related medical conditions associated with regimen completion, including allergy and use of antibiotics (for young women who had 5 antibiotic prescriptions in the 2-year period before study baseline [RR, 1.10] compared with those who had no antibiotic prescription). These results suggest that females who may have more frequent encounters with the health care system are more likely to complete the vaccination series. Because immune-related conditions are outcomes of interest in current observational vaccine safety studies, these results provide some reassurance that females with immune-related conditions were unlikely to be underrepresented in these studies.
Several potential limitations need to be considered in interpreting results of our study. Most importantly, KPSC members may have had access to the vaccine at other health care settings, such as university health care centers. Our tracking system does track vaccines received outside the KPSC system if this is known to the physicians or nurses. However, misclassification of the study outcome (ie, regimen completion) could result if HPV4 received outside KPSC was not reported by the member. Although the magnitude of this misclassification is unclear and needs to be further studied, we suspect most members would choose to receive the vaccine at Kaiser Permanente because there is no out-of-pocket charge. Therefore, we do not expect the impact of this outcome misclassification to be major.
Common limitations are associated with the use of electronic records. For example, we had no information on sexual behaviors (eg, whether individuals have been sexually active) that would help to further interpret the results of sexually related medical history. We could not capture free services rendered outside of KPSC facilities, such as free STD clinics and Planned Parenthood clinics. Teenagers who want oral contraceptives or STD treatment may visit free service clinics rather than their PCP. As a result, we expect some degree of misclassification of these variables. Furthermore individual race/ethnicity data were lacking for approximately 40% (in adolescent girls) to 60% (in young women) of the participants. Those with missing race/ethnicity data are less likely to have been hospitalized in the past or enrolled in KPSC more recently (hospitalizations and length of membership were adjusted in the multivariable model). Therefore, our findings on race/ethnicity apply only to those whose race/ethnicity information was available.
Our study has several important strengths, including a population-based design that minimized participation bias, use of electronic medical records that avoided recall bias, and a large sample size.
CONCLUSION
Black and Hispanic adolescent girls, females who reside in neighborhoods with lower average education levels, young women who have a pediatrician PCP, and those with histories of several hospitalizations/emergency department visits were less likely to complete the 3-dose regimen of HPV4. These findings provide useful insight for developing public health programs to enhance proper HPV vaccination, targeting populations susceptible to nonadherence. Of note, our findings represent the early postlicensure experience of HPV vaccination in a managed care setting. At KPSC, the HPV vaccine is listed in the clinical practice guidelines, and physicians and nurses receive member-specific automated vaccination reminders when a member seeks health care. Currently, patient incentives or outreach has not been offered in our setting for HPV4 regimen completion. Regimen completion rate may vary between settings, may change over time, and thus should be monitored continuously.
Footnotes
This work was supported by a research grant from Merck & Co, Inc [EPI08014.036.02]. Drs Chao, Slezak, and Jacobsen received research funding from Merck & Co, Inc, to work on this study and a related study, and Dr Jacobsen is an unpaid consultant for Merck & Co, Inc. Dr Velicer is an employee of Merck & Co, Inc.
REFERENCES
- 1.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271-278 [DOI] [PubMed] [Google Scholar]
- 2.Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006December4;95(11):1459-1466 Epub 2006 Nov 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1-24 [PubMed] [Google Scholar]
- 4.Department of Health and Human Services. Centers for Disease Control and Prevention Vaccination coverage among adolescents aged 13-17 years - United States, 2007 [published correction appears in MMWR Morb Mortal Wkly Rep. 2009;58(1):10] MMWR Morb Mortal Wkly Rep. 2008;57(40):1100-1103 [PubMed] [Google Scholar]
- 5.Sellors J, Pickard L, Mahony JB, et al. Understanding and enhancing compliance with the second dose of hepatitis B vaccine: a cohort analysis and a randomized controlled trial. CMAJ 1997;157(2):143-148 [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Petitti DB, Enger S. Limitations and potential uses of census-based data on ethnicity in a diverse community. Ann Epidemiol. 2004;14(5):339-345 [DOI] [PubMed] [Google Scholar]
- 7.U.S. Preventive Services Task Force Screening for cervical cancer: recommendations and rationale. Am Fam Physician 2003;67(8):1759-1766 [PubMed] [Google Scholar]
- 8.Liu T, Wang X, Waterbor JW, Weiss HL, Soong SJ. Relationships between socioeconomic status and race-specific cervical cancer incidence in the United States, 1973-1992. J Health Care Poor Underserved 1998;9(4):420-432 [DOI] [PubMed] [Google Scholar]
- 9.McDougall JA, Madeleine MM, Daling JR, Li CI. Racial and ethnic disparities in cervical cancer incidence rates in the United States, 1992-2003. Cancer Causes Control. 2007December;18(10):1175-1186 Epub 2007 Sep 6 [DOI] [PubMed] [Google Scholar]
- 10.Riedesel JM, Rosenthal SL, Zimet GD, et al. Attitudes about human papillomavirus vaccine among family physicians. J Pediatr Adolesc Gynecol. 2005;18(6):391-398 [DOI] [PubMed] [Google Scholar]
- 11.Kiefe CI, Funkhouser E, Fouad MN, May DS. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med. 1998;13(6):357-365 [DOI] [PMC free article] [PubMed] [Google Scholar]