Abstract
OBJECTIVE: To examine the effect of the 7-valent pneumococcal conjugate vaccine in a well-characterized population in Olmsted County, Minnesota, with a combination of urban and rural residents likely to have a relatively low risk of invasive pneumococcal disease (IPD).
PATIENTS AND METHODS: This population-based study analyzed data from children younger than 5 years to determine the incidence of IPD from January 1, 1995, to December 31, 2007.
RESULTS: From 1995 through 2007, 29 cases of IPD were identified in the study population, but 2 patients denied research authorization; thus, 27 cases were available for review. From 1995-1999 to 2001-2003, the incidence of IPD decreased from 33.5 (95% confidence interval [CI], 16.6-50.5) to 10.8 (95% CI, 0.0-23.0) cases per 100,000 person-years (68% decrease; P=.046). The incidence subsequently increased to 15.2 (95% CI, 3.0-27.4) cases per 100,000 person-years from 2004 through 2007; however this change was not significant (P=.62). All cases of IPD with available serotype data from 2002 through 2007 (n=5) were due to non-7-valent conjugate vaccine serotypes.
CONCLUSION: Although the baseline incidence of IPD was much lower than that reported in other populations, the overall incidence of IPD decreased significantly in children younger than 5 years after introduction of a 7-valent conjugate vaccine.
From 1995-1999 to 2001-2003, the incidence of invasive pneumococcal disease in children younger than 5 years decreased from 33.5 to 10.8 cases per 100,000 person-years. The incidence increased to 15.2 cases per 100,000 person-years from 2004-2007; however, this change was not significant. The overall incidence of invasive pneumococcal disease decreased significantly after introduction of a 7-valent conjugate vaccine.
CDC = Centers for Disease Control and Prevention; CI = confidence interval; IPD = invasive pneumococcal disease; MIC = minimum inhibitory concentration; PCV-7 = 7-valent pneumococcal conjugate vaccine
After the introduction of a 7-valent pneumococcal conjugate vaccine (PCV-7) for children in early 2000 in the United States, the incidence of invasive pneumococcal disease (IPD) decreased substantially in several populations in the United States.1-7 These populations included metropolitan areas throughout the United States (Active Bacterial Core Surveillance),1 a health care system in Northern California (Kaiser Permanente),2 8 children's hospitals in the United States (US Pediatric Multicenter Pneumococcal Surveillance Group),3 the state of Alaska (Arctic Investigations Program),4 the metropolitan Atlanta area (Georgia Emerging Infections Program),5 and a White Mountain Apache tribe in northern Arizona.7 The baseline incidence of IPD among children younger than 5 years in these populations ranged from 62.5 cases per 100,000 in the Kaiser Permanente population,2 to 96.4 cases per 100,000 in the metropolitan areas of the Active Bacterial Core Surveillance population,1 to 139.7 cases per 100,000 in metropolitan Atlanta,5 to 473 cases per 100,000 in the White Mountain Apache tribe.7
To examine the effect of PCV-7 in a well-characterized population in the midwestern United States with a combination of urban and rural residents likely to have a relatively low risk of IPD,8 we conducted a population-based incidence study of IPD in Olmsted County, Minnesota, from 1995 through 2007.
PATIENTS AND METHODS
Population
Olmsted County (total population, 124,277; population <5 years, 8890; 2000 census data) is located in southeastern Minnesota and includes the central city of Rochester (total population, 85,806; population <5 years, 6401; 2000 census data) and the surrounding area. Most of the population is white (90.3% white; 2000 census data) and of Northern European ancestry. One unique feature of the Olmsted County population is that medical care is mainly self-contained within the community.8 This is due to the relative geographic isolation of Olmsted County from other urban centers; the closest competing medical centers are located in Minneapolis, MN (87 miles to the north), LaCrosse, WI (71 miles to the east), Iowa City, IA (198 miles to the south), and Sioux Falls, SD (235 miles to the west). Another unique feature is that essentially all original medical records from the providers in Olmsted County, including the Mayo Clinic and Olmsted Medical Center health care systems, are available for review through the Rochester Epidemiology Project, a medical records-linkage system that has received federal funding since 1966.8 These features allow for essentially complete ascertainment of all cases of a specific disease in residents of Olmsted County and thus comprise an ideal environment for population-based studies.
Case Ascertainment and Definitions
Invasive pneumococcal disease was defined as the isolation of Streptococcus pneumoniae from a normally sterile site,1 from an Olmsted County resident younger than 5 years. Cases of IPD were identified from January 1, 1995, through December 31, 2007, from the only 2 microbiology laboratories in Olmsted County: Mayo Clinic and Olmsted Medical Center. No distantly located reference laboratories were used by practitioners of Olmsted County. The institutional review boards at Mayo Clinic and Olmsted Medical Center approved the study and waived the requirement for informed consent.
Pneumococcal susceptibility testing was performed by agar dilution (1995-2007) at Mayo Clinic and by agar dilution (1995-1998) and Microstrep plus panel (Dade Behring, Brooksfield, CT; 1999-2007) at Olmsted Medical Center. Invasive pneumococcal disease isolates were defined as penicillin susceptible or nonsusceptible (ie, of intermediate susceptibility or resistant) on the basis of Clinical and Laboratory Standards Institute guidelines according to the current breakpoint for oral penicillin dosing and the previous breakpoint for parenteral penicillin dosing.
Pneumococcal serotypes were defined as follows: (1) PCV-7 serotype (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F); (2) PCV-7 related serotype (serotypes 6A, 9A, 9L, 9N, 18A, 18B, 18F, 19A, 19B, 19C, 23A, and 23B); (3) 23-valent pneumococcal polysaccharide vaccine (PPV-23) serotype (serotypes 1, 2, 3, 5, 7F, 8, 10A, 11A, 12F, 15B, 20, 22F, and 33F); and (4) nonvaccine serotype (all other serotypes).1
Pneumococcal serotyping was performed at the Minnesota Department of Health with type-specific antisera (Quellung reaction). Pneumococcal clinical syndromes have been previously defined.9
Vaccination with PCV-7 began in Olmsted County in July 2000. Because pneumococcal vaccination rates were not available for Olmsted County, survey data from the Centers for Disease Control and Prevention (CDC) were used to estimate vaccination rates. For PCV-7 vaccination coverage among children 19 to 35 months of age in Minnesota, the National Immunization Survey data of the CDC were used.10
Statistical Analyses
For calculating the incidence of IPD, the entire Olmsted County population younger than 5 years was considered at risk of infection. The denominator age- and sex-specific person-years were derived from decennial census figures. For the years after the last decennial census in 2000, a population growth rate of 1.9% was used to project these numbers. Incidence rates were age- and sex-adjusted to the US white population in 2000. Only initial episodes of IPD were included as incident cases. Poisson regression was used to examine the temporal and sex effects on the incidence of IPD. The time covariate was categorized into pre-PCV-7 (January 1, 1995, to December, 31, 1999), immediate post-PCV-7 (December 1, 2001, to December 31, 2003), and distant post-PCV-7 (December 1, 2004, to December 31, 2007) periods.4 A transition period was defined as December 1, 2000, to December 31, 2000, to allow for PCV-7 uptake, from which data were excluded in the analysis. If there were no incident IPD cases in males or females within a subgroup in at least 1 of the 3 periods, a correction factor of 0.5 was applied to each stratum count so that regression estimates could be calculated. The level of significance for statistical testing was defined as P<.05 (2-sided). All analyses were performed using SAS version 8 software (SAS Institute, Cary, NC).
RESULTS
Twenty-nine cases of IPD were identified in children younger than 5 years. For 2 children with IPD, research authorization was refused, and their records were not used for research purposes in accordance with the Minnesota Research Authorization Statute. Therefore, 27 (93%) of the 29 IPD cases were available for review.
Demographic Characteristics
Of the 27 patients, 20 (74%) were white, 3 (11%) were African American, 2 (7%) were Asian, and 2 (7%) were of unknown race; 15 (56%) were male. The median age of the study patients was 1 year (range, 3 months to 4 years).
Incidence
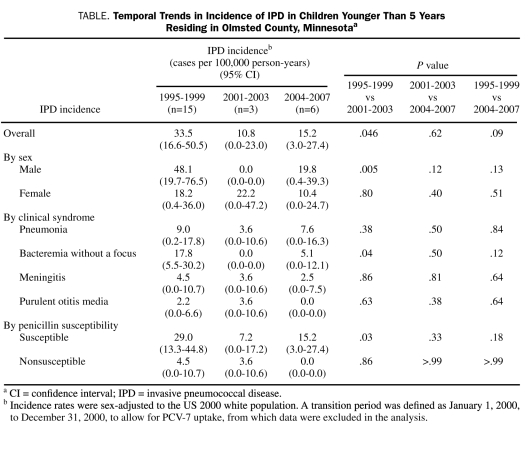
From 1995-1999 (pre PCV-7 period) to 2001-2003 (immediate post PCV-7 period), the incidence of IPD decreased 68%, from 33.5 (95% confidence interval [CI], 16.6-50.5) to 10.8 (95% CI, 0.0-23.0) cases per 100,000 person-years (P=.046) (Table). This was mainly a result of a decrease in the incidence of IPD in males (from 48.1 [95% CI, 19.7-76.5] cases per 100,000 person-years in 1995-1999 to 0.0 cases in 2001-2003; P=.005). During the same periods, the incidence of IPD did not change significantly in females (from 18.2 [95% CI, 0.4-36.0] cases per 100,000 person-years in 1995-1999 to 22.2 [95% CI, 0.0-47.2] cases in 2001-2003; P=.80). The incidence of IPD increased 29%, from 10.8 (95% CI, 0.0-23.0) cases per 100,000 person-years in 2001 through 2003 to 15.2 (95% CI, 3.0-27.4) cases per 100,000 person-years in 2004 through 2007 (distant post PCV-7 period), but this change was not significant (P=.62) (Table).
TABLE.
Temporal Trends in Incidence of IPD in Children Younger Than 5 Years Residing in Olmsted County, Minnesotaa
Vaccination Data
On the basis of the National Immunization Survey of the CDC,10 the estimated coverage with 3 or more doses of PCV-7 among children 19 to 35 months of age in the state of Minnesota was (yearly point estimate [%] ± 95% CI): 48.2±6.8 (2002), 72.9±6.7 (2003), 77.3±6.5 (2004), 86.6±5.3 (2005), 92.5±3.6 (2006), and 95.7±2.8 (2007).
Of 10 children younger than 5 years who developed IPD after July 2000, 7 received at least 3 doses of PCV-7 before the diagnosis of IPD, 2 received no doses of PCV-7 (cases occurred in 2000 and 2001), and 1 had no available vaccination data.
Serotype Data
Pneumococcal serotype data were available for 5 (63%) of the 8 IPD cases from 2002 through 2007; serotype data before 2002 were not available. The serotypes of the 3 cases of IPD from 2002 through 2004 in children younger than 5 years were as follows (serotypes are listed in parentheses): nonvaccine serotype, 1 case (35F); unknown serotype, 2 cases. The serotypes of the 5 cases of IPD from 2005 through 2007 in children were as follows: PCV-7 related serotype, 1 case (19A); PPV-23 serotype, 2 cases (15B, 22F); nonvaccine serotype, 1 case (34); and unknown serotype, 1 case. For the period 2002 through 2007, no cases of IPD were due to PCV-7 serotypes. Serotype data were available for 5 of the 7 children who received at least 3 doses of PCV-7 before the diagnosis of IPD, and none were due to PCV-7 serotypes.
Clinical Syndromes and Penicillin Susceptibility
Of 27 IPD cases from 1995 through 2007, 11 (41%) presented as bacteremia without a focus, 9 (33%) as pneumonia, 5 (19%) as meningitis, and 2 (7%) as purulent otitis media with pneumococcal bacteremia. Of 27 IPD isolates from 1995 through 2007, 24 (89%) were penicillin susceptible, and 3 (11%) were penicillin nonsusceptible. Of 19 IPD isolates before 2002, 16 had a penicillin minimum inhibitory concentration (MIC) of less than or equal to 0.06 μg/mL (susceptible), 2 had a penicillin MIC of 2 μg/mL (nonsusceptible), and 1 had a penicillin MIC of 1 μg/mL (nonsusceptible). Of 8 IPD isolates from 2002 through 2007, all 8 isolates had penicillin MIC values less than or equal to 0.06 μg/mL (susceptible). Trends in IPD incidence stratified by clinical syndrome and penicillin susceptibility are listed in the Table.
DISCUSSION
In the population of Olmsted County, where the pre-PCV-7 incidence of IPD in children younger than 5 years (33.5 cases per 100,000 person-years) was much lower than the pre-PCV-7 incidence of IPD in other populations (ranging from 62.5 to 473 cases per 100,000 person-years),1,2,5,7 we observed a similar substantial decrease (68%) in the incidence of IPD after introduction of PCV-7. The decrease in IPD incidence in children in Olmsted County was likely related to the introduction of PCV-7 in July 2000; however, we cannot definitively make this claim given our descriptive study design and inability to causally link changes in IPD incidence with the introduction of PCV-7. The increasing PCV-7 vaccine coverage in the state of Minnesota over time and the relatively high coverage (1 of 7 states to have ≥95% coverage in 2007)10 provide some support for this hypothesis.
The substantial decrease in the incidence of IPD in male children younger than 5 years from 1995-1999 to 2001-2003 but not in female children younger than 5 years is unusual. Temporal trends in sex-specific incidence of IPD in children have not been reported in previously published studies1-7; thus, we cannot compare our findings with other populations. The reason for this finding is unclear but could be related at least in part to the relatively low number of cases in 2001 through 2003, which may have contributed to instability in the incidence figures for that period.
Although the incidence of IPD increased 29% from 2001-2003 to 2004-2007, our study was underpowered to detect a statistically significant difference. Although the numbers were small and the increase was not statistically significant, the increase in IPD incidence in children younger than 5 years correlates with a statistically significant increase in the incidence of IPD in adults aged 50 years and older from 2001-2003 to 2004-2007.11
Potential explanations for the possible increase in IPD incidence from 2001-2003 to 2004-2007 in children younger than 5 years include the following: (1) natural fluctuations in IPD incidence related to factors such as seasonal respiratory virus activity, antibiotic use, and clonal strains moving in and out of the community; (2) “replacement disease” resulting from nasopharyngeal colonization with non-PCV-7 type pneumococci among both vaccinees (a direct effect of PCV-7) and among unvaccinated children (an indirect effect of PCV-7)4; or (3) changes in demographics of the Olmsted County population, leading to an influx of persons with a higher risk of IPD. Although data regarding PCV-7 coverage among children younger than 5 years in Olmsted County were not available, statewide data indicate that the vaccine coverage of PCV-7 is high. Consequently, a decrease in vaccine coverage is an unlikely explanation for this observation.
Our study has several key strengths. First, we report IPD data from a well-characterized population with a pre-PCV-7 incidence of IPD that is much lower than in other populations. This allows for extrapolation of our findings to other similar populations. Second, our study was performed in a geographically isolated population, allowing for essentially complete ascertainment of all IPD cases and accurate incidence calculations. Third, our study was performed during a 13-year period and used consistent data abstraction methods.
Our study has some limitations. First, the relatively small population in Olmsted County limited the number of IPD cases, especially in the post-PCV-7 periods and restricted calculation of serotype specific incidence. Second, pneumococcal serotype data were available only from 2002-2007, which did not allow us to make conclusions about changes in serotype distributions before the introduction of PCV-7 in 2000. Third, the Olmsted County population differs from metropolitan populations, and our findings should be generalized to similar populations.
CONCLUSION
A significant decrease was noted in the incidence of IPD in children younger than 5 years in Olmsted County, Minnesota, after the introduction of PCV-7 in 2000. Further surveillance is needed in the upcoming years to monitor for increasing IPD incidence in young children.
Acknowledgments
We thank Mary Ann Butler at the Olmsted Medical Center Hospital laboratory for assistance in obtaining microbiology data and Barbara Yawn, MD, at the Olmsted Medical Center for her assistance with vaccination data. Neither received compensation for her work on this study.
Footnotes
This work was made possible by grant R01 AR30582 (Rochester Epidemiology Project) from the National Institutes of Health (NIH) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, by grant UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the NIH, and the NIH Roadmap for Medical Research, and in part by intramural research funding by the Small Grants Program and the Baddour Family Fund Research Grants Program from Mayo Clinic's site in Rochester, MN.
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
REFERENCES
- 1.Whitney CG, Farley MM, Hadler J, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737-1746 [DOI] [PubMed] [Google Scholar]
- 2.Black S, France EK, Isaacman D, et al. Surveillance for invasive pneumococcal disease during 2000-2005 in a population of children who received 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26(9):771-777 [DOI] [PubMed] [Google Scholar]
- 3.Kaplan SL, Mason EO, Jr, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 2004;113(3, pt 1):443-449 [DOI] [PubMed] [Google Scholar]
- 4.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2007;297(16):1784-1792 [DOI] [PubMed] [Google Scholar]
- 5.Albrich WC, Baughman W, Schmotzer B, Farley MM. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007June15;44(12):1569-1576 Epub 2007 May 8 [DOI] [PubMed] [Google Scholar]
- 6.Hicks LA, Harrison LH, Flannery B, et al. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007November1;196(9):1346-1354 Epub 2007 Oct 4 [DOI] [PubMed] [Google Scholar]
- 7.Lacapa R, Bliss SJ, Larzelere-Hinton F, et al. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis. 2008;47(4):476-484 [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266-274 [DOI] [PubMed] [Google Scholar]
- 9.Lexau CA, Lynfield R, Danila R, et al. Active Bacterial Core Surveillance Team Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005;294(16):2043-2051 [DOI] [PubMed] [Google Scholar]
- 10.Statistics and surveillance: immunization coverage in the U.S. Children only. Centers for Disease Control and Prevention Web site http://www.cdc.gov/vaccines/stats-surv/imz-coverage.htm#nis Accessed June 10, 2009
- 11.Tsigrelis C, Tleyjeh IM, Lahr BD, Nyre LM, Virk A, Baddour LM. Trends in invasive pneumococcal disease among older adults in Olmsted County, Minnesota. J Infect. doi: 10.1016/j.jinf.2009.07.004. [published online ahead of print July 14, 2009] doi:10.1016/j.jinf.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]