Abstract
The term acute coronary syndrome (ACS) refers to any group of clinical symptoms compatible with acute myocardial ischemia and includes unstable angina (UA), non—ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI). These high-risk manifestations of coronary atherosclerosis are important causes of the use of emergency medical care and hospitalization in the United States. A quick but thorough assessment of the patient's history and findings on physical examination, electrocardiography, radiologic studies, and cardiac biomarker tests permit accurate diagnosis and aid in early risk stratification, which is essential for guiding treatment. High-risk patients with UA/NSTEMI are often treated with an early invasive strategy involving cardiac catheterization and prompt revascularization of viable myocardium at risk. Clinical outcomes can be optimized by revascularization coupled with aggressive medical therapy that includes anti-ischemic, antiplatelet, anticoagulant, and lipid-lowering drugs. Evidence-based guidelines provide recommendations for the management of ACS; however, therapeutic approaches to the management of ACS continue to evolve at a rapid pace driven by a multitude of large-scale randomized controlled trials. Thus, clinicians are frequently faced with the problem of determining which drug or therapeutic strategy will achieve the best results. This article summarizes the evidence and provides the clinician with the latest information about the pathophysiology, clinical presentation, and risk stratification of ACS and the management of UA/NSTEMI.
ACC = American College of Cardiology; ACE = angiotensin-converting enzyme; ACS = acute coronary syndrome; ADP = adenosine diphosphate; AHA = American Heart Association; BNP = B-type natriuretic peptide; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CHF = congestive heart failure; CI = confidence interval; CK-MB = muscle and brain fraction of creatine kinase; CRP = C-reactive protein; CURE = Clopidogrel in Unstable Angina to Prevent Recurrent Events; ECG = electrocardiography; ED = emergency department; GP = glycoprotein; HR = hazard ratio; IV = intravenous; LDL = low-density lipoprotein; LMWH = low—molecular-weight heparin; LV = left ventricular; MI = myocardial infarction; NSTEMI = non—ST-segment elevation MI; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation MI; TIMI = Thrombolysis in Myocardial Infarction; UA = unstable angina; UFH = unfractionated heparin
The term acute coronary syndrome (ACS) refers to any group of clinical symptoms compatible with acute myocardial ischemia and covers the spectrum of clinical conditions ranging from unstable angina (UA) to non—ST-segment elevation myocardial infarction (NSTEMI) to ST-segment elevation myocardial infarction (STEMI). Unstable angina and NSTEMI are closely related conditions: their pathophysiologic origins and clinical presentations are similar, but they differ in severity. A diagnosis of NSTEMI can be made when the ischemia is sufficiently severe to cause myocardial damage that results in the release of a biomarker of myocardial necrosis into the circulation (cardiac-specific troponins T or I, or muscle and brain fraction of creatine kinase [CK-MB]). In contrast, the patient is considered to have experienced UA if no such biomarker can be detected in the bloodstream hours after the initial onset of ischemic chest pain. Unstable angina exhibits 1 or more of 3 principal presentations: (1) rest angina (usually lasting >20 minutes), (2) new-onset (<2 months previously) severe angina, and (3) a crescendo pattern of occurrence (increasing in intensity, duration, frequency, or any combination of these factors). Each year in the United States, approximately 1.36 million hospitalizations are required for ACS (listed either as a primary or a secondary discharge diagnosis), of which 0.81 million are for myocardial infarction (MI) and the remainder are for UA. Roughly two-thirds of patients with MI have NSTEMI; the rest have STEMI.1
PATHOPHYSIOLOGY OF ACS
Initiation of Atherosclerosis: Role of the Endothelium
Atherosclerosis is the ongoing process of plaque formation that involves primarily the intima of large- and medium-sized arteries; the condition progresses relentlessly throughout a person's lifetime, before finally manifesting itself as an acute ischemic event. Several coronary risk factors influence this process, including hypercholesterolemia, hypertension, diabetes, and smoking.2-4 These risk factors damage the endothelium of the blood vessel and result in endothelial dysfunction, which plays a pivotal role in initiating the atherosclerotic process. A dysfunctional endothelium is characterized by reduced bioavailability of nitric oxide and by excessive production of endothelin 1, which impairs vascular hemostasis; increased expression of adhesion molecules (eg, selectins, vascular cell adhesion molecules, and intercellular adhesion molecules); and increased thrombogenicity of blood through the secretion of several locally active substances.5,6
Progression of Atherosclerotic Plaque: Role of Inflammation
Once the endothelium has been damaged, the inflammatory cells, especially monocytes, migrate into the subendothelium by binding to endothelial adhesion molecules; once in the subendothelium, they undergo differentiation, becoming macrophages. Macrophages digest oxidized low-density lipoprotein (LDL) that has also penetrated the arterial wall, transforming into foam cells and causing the formation of fatty streaks. The activated macrophages release chemoattractants and cytokines (eg, monocyte chemoattractant protein 1, tumor necrosis factor α, and interleukins) that perpetuate the process by recruiting additional macrophages and vascular smooth muscle cells (which synthesize extracellular matrix components) at the site of the plaque. Macrophages also elaborate matrix metalloproteinases, enzymes that digest the extracellular matrix and lead to plaque disruption.3 The ratio between smooth muscle cells and macrophages plays an important role in plaque vulnerability and the propensity for rupture. Although plaque rupture may result in ACS, more often, in fact in 99% of cases, it is clinically silent.7 The rate of progression of atherosclerotic lesions is variable, nonlinear, and unpredictable.8
Stability of Plaques and Tendency for Rupture
The stability of atherosclerotic plaques varies. Characteristics of so-called high-risk or vulnerable plaques include a large lipid core, thin fibrous caps, a high density of macrophages and T lymphocytes,9,10 a relative paucity of smooth muscle cells,11 locally increased expression of matrix metalloproteinases that degrade collagen,12,13 eccentric outward remodeling,14,15 and increases in plaque neovascularity and intraplaque hemorrhage.16 The composition of human atherosclerotic plaques is strikingly heterogeneous, even within the same person.17 Inflammation, a particularly important determinant of the “vulnerability” of plaques,9,18 is related to an increase in the activity of macrophages at the site of plaque; this increased activity leads to an enlargement of the lipid core and a thinning of the plaque cap, characteristics that render the plaque more vulnerable to rupture. Elevated levels of C-reactive protein (CRP) have been found to correlate positively with the number of plaque ruptures19 and may reflect the activity of these macrophages.20
Plaque Disruption, Thrombosis, and ACS
The pathogenesis of ACS involves an intricate interplay among the endothelium, the inflammatory cells, and the thrombogenicity of the blood.21,22 Angiographically, noncritical coronary lesions (<50% stenosis in the diameter of the vessel) may be associated with abrupt progression to severe or total occlusion and may eventually account for as many as two-thirds of cases of ACS.23,24 Factors such as the lipid and tissue factor content of the plaque, the severity of the plaque rupture, the degree of inflammation at the site, the blood flow in the area, and the patient's antithrombotic and prothrombotic balance are important in controlling the degree of thrombus formation and determining whether a given plaque rupture will result in ACS.25-27 Studies using intravascular ultrasonography have shown that at least 80% of patients with ACS exhibit multiple plaque ruptures distinct from the culprit lesion.28
Autopsy studies have shown that plaque rupture causes approximately 75% of fatal MIs, whereas superficial endothelial erosion accounts for the remaining 25%.17,29 After either plaque rupture or endothelial erosion, the subendothelial matrix (which is rich in tissue factor, a potent procoagulant) is exposed to the circulating blood; this exposure leads to platelet adhesion followed by platelet activation and aggregation and the subsequent formation of a thrombus. Two types of thrombi can form: a platelet-rich clot (referred to as a white clot) that forms in areas of high shear stress and only partially occludes the artery, or a fibrin-rich clot (referred to as a red clot) that is the result of an activated coagulation cascade and decreased flow in the artery. Red clots are frequently superimposed on white clots, and this characteristic causes total occlusion. Several lines of evidence support the central role of thrombosis in the pathogenesis of ACS.30-32
Therapeutic Goals and Approaches for ACS
The severity of findings on coronary angiography and angioscopy parallels the clinical severity of ACS. Although only white clots are found in patients with UA/NSTEMI,33 red clots form in patients with STEMI.34 The differences in the underlying pathophysiology of UA/NSTEMI and STEMI call for different therapeutic goals and approaches. In UA/NSTEMI, the goal of antithrombotic therapy is to prevent further thrombosis and to allow endogenous fibrinolysis to dissolve the thrombus and reduce the degree of coronary stenosis35-39; revascularization is frequently used to increase blood flow and prevent reocclusion or recurrent ischemia.40 In contrast, in STEMI, the infarct-related artery is usually totally occluded, and immediate pharmacological or catheter-based reperfusion is the initial approach, with the goal of obtaining normal coronary blood flow.41 Other therapies, such as anti-ischemic and lipid-lowering therapies, are used in all cases to stabilize plaques over the long term.
EARLY ASSESSMENT
The symptoms of UA/NSTEMI and STEMI are similar, and differentiating the two requires medical evaluation and 12-lead electrocardiography (ECG). The 2007 guidelines for managing UA/NSTEMI, released by the American College of Cardiology (ACC) and the American Heart Association (AHA), state that patients with symptoms suggestive of ACS should be instructed to call 9-1-1 and should be referred to a facility that has capabilities for 12-lead ECG recording, biomarker determination, and evaluation by a physician (eg, an emergency department [ED]).42 Patients who have previously been given a prescription for nitroglycerin should be instructed to promptly take 1 dose of nitroglycerin sublingually for chest discomfort or pain. If no relief occurs, or if symptoms worsen 5 minutes after 1 dose of nitroglycerin has been taken, the patient should immediately call 9-1-1.42 Patients at increased risk of ACS, such as those with known coronary artery disease (CAD), peripheral vascular disease, cerebral vascular disease, diabetes, or a 10-year Framingham risk of CAD of 20% or higher, should be targeted by health care professionals and should be educated about recognizing the symptoms of ACS and calling 9-1-1 promptly if such symptoms occur.43
All patients presenting to the ED with chest discomfort or other symptoms suggestive of ACS should be considered high-priority triage cases. Evaluation and treatment should follow a predetermined, institution-specific protocol for chest pain. If the initial diagnosis and treatment plan are unclear to the ED physician, immediate cardiology consultation is advisable. Each year in the United States, 6 to 7 million persons present to EDs with the symptom of chest pain or other symptoms suggestive of possible ACS; of these, approximately 20% to 25% receive a final diagnosis of UA or MI.44 The differential diagnosis of patients with chest pain is shown in Table 1.
TABLE 1.
Differential Diagnosis of Patients With Chest Pain
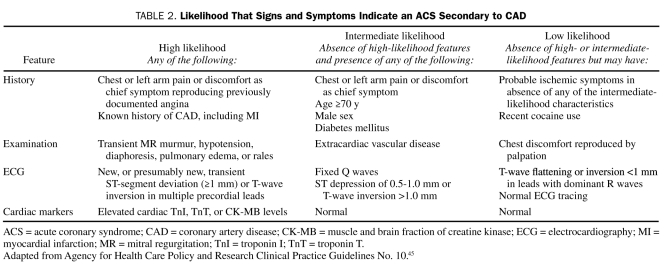
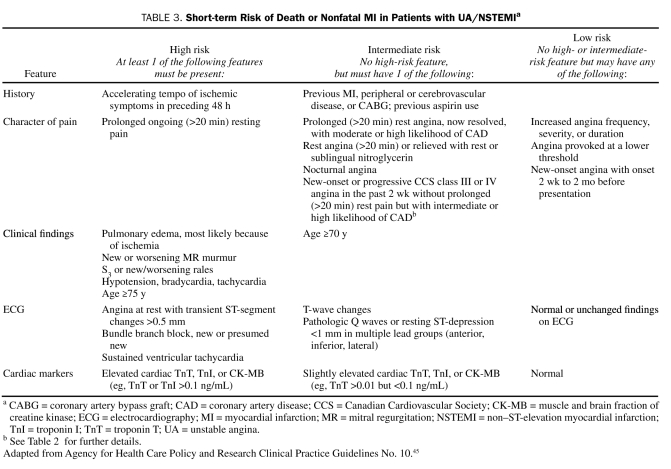
The 2007 ACC/AHA guidelines for managing UA/NSTEMI state that the first step in assessing patients with chest discomfort or other symptoms suggestive of ACS is determining the likelihood that the symptoms and signs represent ACS secondary to obstructive CAD (Table 2). The second step is determining the risk of an adverse clinical outcome for those patients with an intermediate or high likelihood of ACS (ie, risk stratification; Table 3).42 Early risk assessment is based on initial findings from the history and physical examination and the results of ECG and cardiac biomarker measurements.
TABLE 2.
Likelihood That Signs and Symptoms Indicate an ACS Secondary to CAD
TABLE 3.
Short-term Risk of Death or Nonfatal MI in Patients with UA/NSTEMIa
CLINICAL PRESENTATION
History and Physical Examination Findings
Careful and focused history taking and physical examination are essential both to assessing the likelihood that the presenting illness is ACS and to determining the risk of an adverse outcome. Although patients typically describe stable angina as deep, poorly localized chest or arm discomfort that is exacerbated by activity or emotional stress and relieved by rest, nitroglycerin, or both, the discomfort associated with UA is more severe, occurs at rest, and is usually described as frank pain. Often located in the substernal region (sometimes the epigastric area), the pain or pressure frequently radiates to the neck, jaw, left shoulder, and left arm. Some patients may present with symptoms other than chest discomfort; such “anginal equivalent” symptoms include dyspnea (most common), nausea and vomiting, diaphoresis, and unexplained fatigue.46 Atypical presentations are more common among women and elderly people. Rarely, syncope may be the presenting symptom of ACS. Pain that is sharp, stabbing or pleuritic, reproducible with palpation or with movement, or able to be localized at the tip of 1 finger is usually not ischemic. Chest pain that resolves with the administration of sublingual nitroglycerin in the ED setting is not predictive of ACS. The 5 most important history-related factors that help identify ischemia due to CAD, ranked in order of importance, are the nature of the anginal symptoms (Table 2), a history of CAD, male sex, older age, and the number of traditional risk factors present.47,48 Traditional cardiac risk factors (eg, hypertension, hypercholesterolemia, cigarette smoking, diabetes, and family history of premature CAD) have actually been found to be weak predictors of the likelihood of acute ischemia,49 although their presence relates to poor outcomes for patients with established ACS.
The primary goals of the physical examination are to identify any precipitating causes of myocardial ischemia and to assess the hemodynamic consequences of the acute ischemic event. Physical examination findings that indicate a large area of ischemia and high risk include diaphoresis; pale, cool skin; sinus tachycardia; a third or fourth heart sound; basilar rales; and hypotension. The physical examination may also provide clues that can help in determining the differential diagnosis. For example, unequal pulses or a murmur of aortic regurgitation indicates possible aortic dissection, whereas a pericardial friction rub suggests acute pericarditis.
Electrocardiography
The ACC/AHA guidelines state that an experienced emergency physician should review the results of 12-lead ECG within no more than 10 minutes after the arrival in the ED of a patient with chest discomfort or other symptoms suggestive of ACS.42 The value of the ECG is 2-fold: to support a clinical diagnosis of ACS and to aid in risk stratification. Electrocardiography, however, has several limitations. For example, it does not adequately represent the posterior, lateral, and apical walls of the left ventricle. Additionally, normal findings do not exclude the possibility of ACS.
Findings on ECG associated with UA include ST-segment depression, transient ST-segment elevation, T-wave inversion, or some combination of these factors; depending on the severity of the clinical presentation, these findings are present in 30% to 50% of patients.39,50,51 New ST-segment deviation, even of only 0.05 mV, is an important and specific measure of ischemia and prognosis.50-52 T-wave inversion is sensitive for ischemia but is less specific, unless it is marked (≥0.3 mV).39 An ST-segment elevation of 0.1 mV or more, if present in at least 2 contiguous leads, indicates acute MI in 90% of patients, as confirmed by serial measurements of cardiac biomarkers.53 It is important to compare current and previous findings on ECG because studies suggest that patients with no ECG changes are at a lower risk of complications than those with ECG changes.54
Because the process of myocardial ischemia is quite dynamic and a single 12-lead ECG provides only a snapshot view of this process, the ACC/AHA guidelines recommend that patients hospitalized for UA/NSTEMI undergo serial ECG tracings or continuous ST-segment monitoring.42,55,56
Cardiac Biomarkers of Necrosis
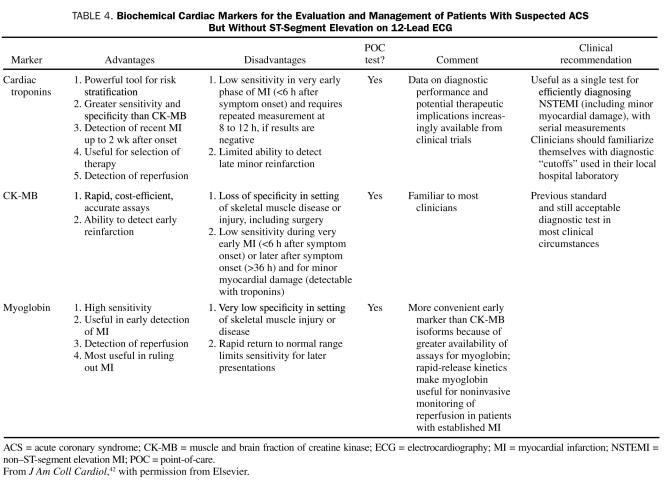
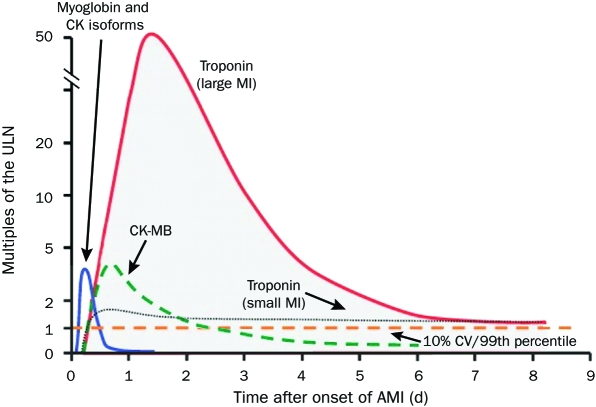
Cardiac biomarkers should be measured for all patients who present with chest discomfort or other symptoms suggestive of ACS. Measurements of the cardiac-specific troponins T and I allow for highly accurate, sensitive, and specific determination of myocardial injury in the context of ischemic symptoms; these troponins have replaced CK-MB as the preferred marker for the detection of myocardial necrosis. However, troponin measurements have some drawbacks. Troponin levels usually do not increase until at least 6 hours after the onset of symptoms; therefore, a negative result obtained within this period should prompt a repetition of the assay 8 to 12 hours after the onset of symptoms. Because troponin levels remain elevated for a prolonged period (5 to 14 days) after myocardial necrosis, their usefulness in detecting recurrent myocardial damage is limited. However, they are helpful in detecting myocardial damage in a patient who presents for assessment several days after the onset of symptoms. Because of the shorter half-life of CK-MB, the levels of this isoenzyme are useful for diagnosing infarct extension (reinfarction) and periprocedural MI. Point-of-care assays for bedside detection of biomarkers are being developed so that the time delay can be minimized and treatment decisions can be made quickly, but the use of such assays is currently limited.57 The advantages and disadvantages of the various biomarkers are shown in Table 4, and the timing of their release after acute MI is shown in Figure 1.58
TABLE 4.
Biochemical Cardiac Markers for the Evaluation and Management of Patients With Suspected ACS But Without ST-Segment Elevation on 12-Lead ECG
FIGURE 1.
Timing of release of various biomarkers after acute myocardial infarction (AMI). The biomarkers are plotted showing the multiples of the cutoff for AMI over time. The dashed horizontal line shows the upper limit of normal (ULN, defined as the 99th percentile from a normal reference population without myocardial necrosis; the coefficient of variation [CV] of the assay should be 10% or less). The earliest rising biomarkers are myoglobin and creatine kinase (CK) isoforms (leftmost curve). The muscle and brain fraction of CK (CK-MB, dashed curve) rises to a peak of 2 to 5 times the ULN and typically returns to the normal range within 2 to 3 d after AMI. The cardiac-specific troponins show small elevations above the ULN in small infarctions (eg, as is often the case with non—ST-segment elevation MI) but rise to 20 to 50 times the ULN in the setting of large infarctions (eg, as is typically the case in ST-segment elevation MI). The troponin levels may stay elevated above the ULN for 7 d or more after AMI.
Adapted from Mayo Clinic Cardiology: Concise Textbook, 3rd ed.58
Other Laboratory Tests
A chest radiograph is usually obtained at the time of admission so that the patient can be evaluated for other causes of chest pain and screened for pulmonary congestion, which implies an adverse prognosis.59 A full lipid profile should be obtained within 24 hours of the onset of ACS, as recommended by the National Cholesterol Education Program Adult Treatment Panel III60 and by the 2007 ACC/AHA guidelines.42 Selected patients should be assessed for secondary causes of ACS: for example, thyroid function should be evaluated when a patient presents with symptoms of ACS and has persistent tachycardia. Measurement of other circulating markers of increased risk may also be considered (see Troponins and Other Markers).
Diagnostic Pathways in the ED
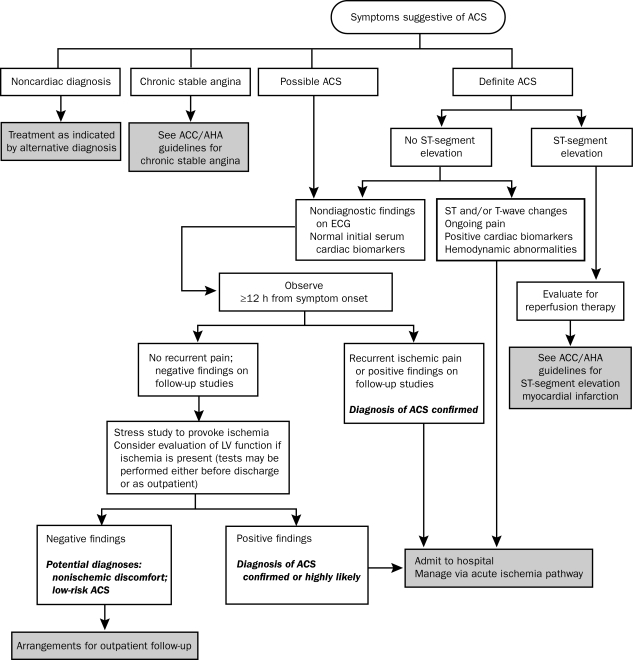
The current ED pathways for assessing and managing patients who may have ACS rely on 4 main diagnostic tools: clinical history, ECG results, levels of cardiac markers, and the results of stress testing. On the basis of the initial information, patients are assigned to one of 4 categories: a noncardiac diagnosis, chronic stable angina, possible ACS, or definite ACS.42 The pathway proposed by the ACC/AHA guidelines is shown in Figure 2.
FIGURE 2.
Algorithm for evaluation and management of patients with suspected acute coronary syndrome (ACS). ACC = American College of Cardiology; AHA = American Heart Association; ECG = electrocardiography; LV = left ventricular.
From J Am Coll Cardiol,42 with permission from Elsevier.
Patients with definite ACS are admitted to the hospital for further treatment. Admission to the critical care unit is recommended if there is evidence of active, ongoing ischemia or injury or of hemodynamic or electrical instability; otherwise, placing patients in a telemetry step-down unit is reasonable. Patients with persistent ST-segment elevation should be assessed for immediate reperfusion therapy. Patients with clearly atypical chest pain and evidence of a noncardiac diagnosis (eg, gastrointestinal or musculoskeletal disorders) can be discharged home and instructed to follow up with their primary physician (chronic stable angina may also be diagnosed in this setting). The remaining patients, those with possible ACS, should be observed in a facility with cardiac monitoring capabilities (eg, a chest pain unit, an ED, or a hospital telemetry ward), and ECG (or continuous 12-lead ECG monitoring) and cardiac biomarker measurements should be repeated at predetermined, specified time intervals. If new ST-segment abnormalities or elevations in the levels of cardiac markers are noted, the diagnosis of ACS is considered highly likely, and the patient is taken off the pathway and admitted to the hospital. If the patient remains pain-free and the results of ECG and cardiac marker tests are negative, an early stress test should be performed either before discharge or on an outpatient basis within 72 hours. Patients with negative results from diagnostic testing can be discharged with specific instructions for activity, medications, and additional testing. Patients with evidence on stress testing of ischemia or left ventricular (LV) dysfunction should be admitted to the hospital and managed according to an acute ischemia pathway.
RISK STRATIFICATION
The ACC/AHA guidelines state that risk stratification is an integral prerequisite to decision-making.42 The outcomes of patients with ACS span the entire risk spectrum: data from a global registry indicate that the 30-day mortality rate ranges from 1.7% for patients with UA to 7.4% for patients with NSTEMI to 11.1% for those with STEMI.61 Early risk stratification is useful for selecting the site of care (coronary care unit or monitored step-down unit), selecting therapy (such as glycoprotein [GP] IIb/IIIa inhibitors62,63 and early invasive strategy40,64), and estimating prognosis.
High-Risk Clinical Subgroups
Certain clinical characteristics are associated with a substantial increase in adverse outcomes for patients with ACS: older age,50,65 diabetes (diabetic patients with UA/NSTEMI are at an approximately 50% higher risk of adverse outcomes than nondiabetic patients),66,67 extracardiac vascular disease,68 evidence of congestive heart failure (CHF; Killip class II or higher),59,65 and presentation with ACS despite long-term aspirin therapy.69
Electrocardiography
The admission ECG is a strong predictor of both early and long-term prognosis. In the Thrombolysis in Myocardial Infarction (TIMI) III Registry of patients with UA/NSTEMI, an ST deviation of as little as 0.05 mV increased the risk of death or MI by approximately 2-fold both at 30 days and at 1 year.50 Another study found that ST depression of 0.05 mV or more on the admission ECG was related to 4-year mortality rates; the risk of death increased as ST depression increased.52 In contrast, T-wave inversion of 0.1 mV or more was associated with only a modest increase or no increase at all in the subsequent risk of death or MI.52 The number of leads demonstrating ST elevation has been a useful risk marker for patients with STEMI.70
Troponins and Other Markers
Troponin is a powerful instrument for risk stratification across the spectrum of patients presenting with symptoms of acute cardiac ischemia. Even a minor elevation of troponin signifies an adverse prognosis and permits the determination of high-risk patients who will benefit from specific therapies, such as GP IIb/IIIa inhibitors, an early invasive strategy, or both.71 In addition, a quantitative relationship exists between the degree of elevation of troponin levels and the risk of death.72
The past decade has seen an increasing recognition of the central role of inflammatory mechanisms in the pathogenesis of atherosclerosis and its complications. Recently, attention has focused on the potential role of plasma markers of inflammation as risk predictors for patients with ACS; of these markers, CRP has been the most extensively studied. Elevated CRP levels detected by a high-sensitivity CRP test relate to an increased risk of mortality. C-reactive protein levels allowed a differentiation between high-risk and low-risk groups among patients with normal troponin levels, for whom the overall 14-day mortality rate was only 1.5%. When these patients had an elevated CRP level, the mortality rate increased to 5.8%, whereas when they had a normal CRP level, the mortality rate was only 0.4%.73 Of note, the cutoff point for the CRP level in the ACS setting is nearly 5 times higher (>15 mg/L) (to convert to nmol/L, multiply by 9.524) than that in the stable CAD setting (>3 mg/L). The white blood cell count is another simple marker of inflammation: for patients with UA/NSTEMI, an elevated count was associated with higher mortality rates and recurrent MI.74,75 One study involving 1090 patients with ACS found that myeloperoxidase was an independent prognostic factor for death or recurrent MI at 6 months.76
B-type natriuretic peptide (BNP) provides powerful prognostic information across the entire spectrum of patients with ACS. The GUSTO-IV (Global Utilization of Strategies To Open Occluded Arteries IV) trial, which involved 6809 patients with UA/NSTEMI, found that the risk of short-term and long-term mortality increased proportionately with rising levels of N-terminal proBNP.77 The OPUS-TIMI 16 (Orbofiban in Patients with Unstable Coronary Syndromes—Thrombolysis In Myocardial Infarction 16) trial found that the risk of death at 10 months was 2-fold to 3-fold higher for patients with ACS who had elevated levels of BNP (>80 pg/mL) (to convert to ng/L, multiply by 1.0) than for those with normal levels.78 Elevated levels of BNP were associated with a higher short-term risk of mortality for patients with STEMI.79 The peak level of BNP has been found to increase proportionately with the size of the myocardial infarct.80
A multimarker approach using several biomarkers has been advocated for improving risk stratification and enhancing patient outcomes. One study used a combination of troponin I, CRP, and BNP to assess risk and found that each marker was an independent predictor of the composite of death, MI, or heart failure. Notably, the mortality risk nearly doubled as the number of elevated markers increased.81
Multivariable Risk-Assessment Scores
Several groups have developed an integrated approach that combines many predictor variables to arrive at a multivariable risk model that provides a comprehensive assessment of risk and an accurate method of prognostication for patients with ACS. The TIMI risk score combines 7 independent risk factors: age of 65 years or older, at least 3 risk factors for CAD, documented CAD at catheterization, ST deviation of 0.5 mm or more, at least 2 episodes of angina in the previous 24 hours, aspirin taken within the previous week, and elevated levels of cardiac markers. When this scoring system was used, patients could be stratified across a 10-fold gradient of risk ranging from 4.7% to 40.9% (P<.001).82 Thus, the TIMI risk score enables identification of or allows detection of high-risk patients, who have been shown to reap more benefit from newer, potent therapies such as GP IIb/IIIa inhibitors83 and an early invasive strategy.40,84 Other risk scores (ie, the GRACE [Global Registry of Acute Coronary Events] risk score and the PURSUIT [Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy] risk score) have greater value in predicting mortality.65,85,86 There are separate risk scores to predict the likelihood of mortality for patients with STEMI.87,88
UNSTABLE ANGINA/NSTEMI
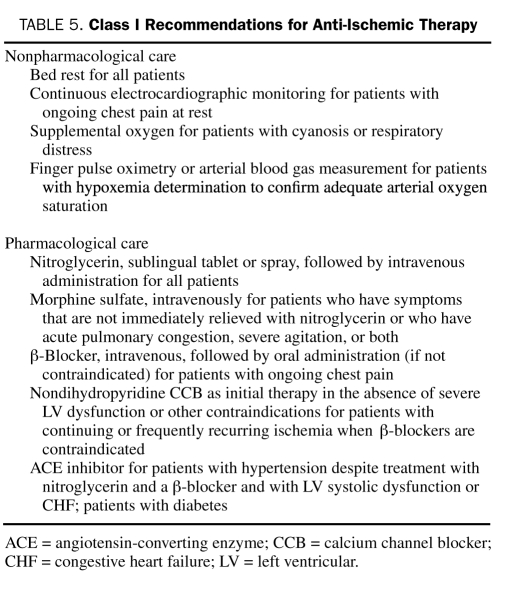
The 2007 ACC/AHA guidelines state that the goal of immediate treatment of patients with UA/NSTEMI is to provide relief of ischemia and to prevent the recurrence of adverse ischemic events.42 Treatment with anti-ischemic, antiplatelet, and anticoagulant agents is fundamental to achieving this goal. In addition to aggressive medical therapy, 2 treatment pathways have emerged for treating UA/NSTEMI patients: an early invasive strategy and an initial conservative strategy. Risk stratification helps to determine how aggressive we should be with respect to both medical therapy (Table 5) and treatment strategy.
TABLE 5.
Class I Recommendations for Anti-Ischemic Therapy
Early Invasive Strategy or Initial Conservative Strategy
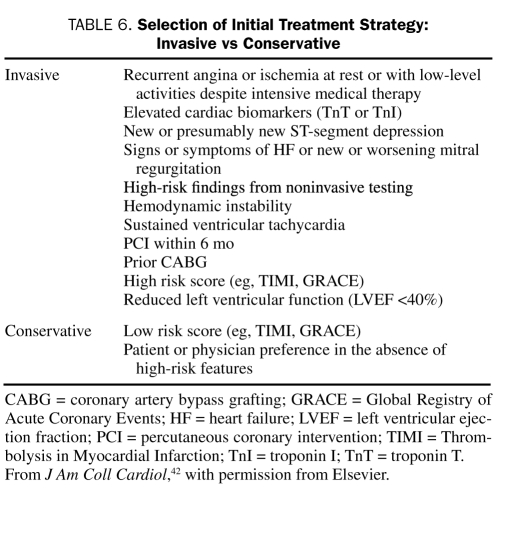
An early invasive strategy involves routine cardiac catheterization, generally within 4 to 24 hours after admission, followed by revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), as appropriate, depending on the coronary anatomy. A conservative strategy, in contrast, consists of initial medical management, followed by catheterization and revascularization only if ischemia recurs despite vigorous medical therapy, either when the patient is at rest or during a noninvasive stress test. The 2007 ACC/AHA guidelines have given the early invasive strategy a class I, level of evidence A recommendation for patients with UA/NSTEMI who are at high risk (Table 6).42 The guidelines recommend either a conservative or an invasive strategy for low-risk patients because the outcomes achieved by these approaches are similar for these patients. However, the guidelines give the conservative strategy a class I recommendation for women with low-risk characteristics.
TABLE 6.
Selection of Initial Treatment Strategy: Invasive vs Conservative
To date, 10 randomized trials have assessed these 2 general strategies. Although the first 3 trials and the most recent trial found no substantial differences between the strategies in outcomes, the remaining 6 trials have shown that an early invasive strategy provides substantial benefits.
The FRISC II (Framingham and Fast Revascularization During Instability in Coronary Artery Disease II) trial, which involved 2457 patients with UA/NSTEMI, found that the early invasive strategy achieved a significantly lower rate of the primary end point of death or MI at 6 months (9.4%) than did the conservative strategy (12.1%; P=.031).89 The TACTICS-TIMI 18 (Treat Angina With Aggrastat and Determine Cost of Therapy With an Invasive or Conservative Strategy—Thrombolysis in Myocardial Infarction 18) trial randomly assigned 2200 patients, who were treated with aspirin, heparin, and tirofiban, to an early invasive or a conservative strategy.40 At 6 months, the rate of the primary end point of death, MI, or rehospitalization for ACS was 19.4% for the conservative strategy group and 15.9% for the early invasive group (odds ratio, 0.78; P=.025).40 Patients with elevated troponin concentrations, ST-segment changes, and a high TIMI risk score (≥3) derived the most benefit from the early invasive strategy. The most recent trial, the ICTUS (Invasive Versus Conservative Treatment in Unstable Coronary Syndromes), randomly assigned 1200 patients with ACS to an early invasive strategy or a conservative strategy and found no significant differences between the groups at 1 year90 and at 3 years91 in the rate of the primary end point of death, MI, or rehospitalization for angina.
A meta-analysis of contemporary randomized trials of treatments for NSTEMI found that the early invasive strategy was associated with a statistically significant 25% lower incidence of all-cause mortality than was the conservative strategy (P=.001).92 Another meta-analysis of 8 randomized trials comparing invasive and conservative strategies for women and men with non—ST-segment elevation ACS found that an early invasive strategy was equally beneficial for men and for women who were considered to have high-risk disease on the basis of elevated levels of biomarkers of necrosis.93
A recent randomized trial, the TIMACS (Timing of Intervention in Patients With Acute Coronary Syndromes), compared the outcomes achieved by an early invasive strategy (intervention within 24 hours of presentation) and a delayed invasive strategy (intervention at any time >36 hours after presentation) for 3031 high-risk patients with UA/NSTEMI.94 The early invasive strategy was not superior to the delayed invasive strategy in reducing the primary end point of death, MI, or stroke at 6 months (9.6% vs 11.3%; hazard ratio [HR], 0.85; 95% confidence interval [CI], 0.68-1.06; P=.15), except for high-risk patients with a GRACE risk score higher than 140 (13.9% vs 21.0%; HR, 0.65; 95% CI, 0.48-0.89; P=.006).
ANTI-ISCHEMIC THERAPY
The ACC/AHA class I recommendations for anti-ischemic therapy are listed in Table 5 and include both nonpharmacological and pharmacological measures.
Nitroglycerin
Nitroglycerin is a vasodilator that reduces myocardial oxygen demand by decreasing ventricular preload via venodilation; it enhances myocardial oxygen delivery by dilating large coronary arteries and improving collateral flow to ischemic areas. Nitroglycerin should initially be given sublingually or by buccal spray (0.3-0.6 mg) every 5 minutes for a total of 3 doses. If pain persists, the administration of intravenous (IV) nitroglycerin should be initiated (initial rate of 5-10 μg/min with increases of 10 μg/min every 3 to 5 minutes until symptoms are relieved or if systolic blood pressure falls below 100 mm Hg). Topical or oral nitrates can be used if the episode of pain has resolved, and they may replace IV nitroglycerin if the patient has been pain-free for 12 to 24 hours. Absolute contraindications to the use of nitroglycerin are hypotension or the use of sildenafil within the previous 24 hours or of tadalafil within the previous 48 hours.95
Morphine and Other Analgesics
Morphine is recommended when ischemia-related symptoms are unrelieved after 3 doses of nitroglycerin or when such symptoms recur during treatment. In such cases, 1 to 5 mg of morphine sulfate can be administered intravenously every 5 to 30 minutes as needed, with careful monitoring of blood pressure and respiratory rate. Morphine acts as a potent analgesic and anxiolytic; in addition, its hemodynamic effects may be beneficial in treating UA/NSTEMI. The 2007 ACC/AHA guidelines downgraded the recommendation for the use of morphine for uncontrolled ischemic discomfort from class I to class IIa because data from a large observational registry, although subject to uncontrolled selection biases, suggested that the adjusted likelihood of death was higher when morphine was used.96
The ACC/AHA guidelines state that the use of nonsteroidal anti-inflammatory drugs, both nonselective agents and cyclooxygenase-2 selective agents (except for aspirin), should be discontinued when a patient presents with UA/NSTEMI because of the known cardiovascular risks associated with these agents,97 and also because the EXTRACT-TIMI 25 (Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment—Thrombolysis In Myocardial Infarction 25) trial found that these agents were associated with an increased risk of adverse cardiovascular events.98
β-Blockers
β-Blockers inhibit β-1 adrenergic receptors in the myocardium and decrease myocardial contractility and heart rate, thereby reducing myocardial oxygen demand. The 2007 ACC/AHA guidelines state that, in the absence of contraindications, therapy with oral β-blockers should be initiated within the first 24 hours after onset of ACS (class I recommendation).42 For all patients, the oral dose should be adjusted to achieve a target resting heart rate of 50 to 60 beats/min. It is reasonable to administer IV β-blockers to patients who are hypertensive at the time of presentation (class IIa recommendation). The COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction) trial found that the risk of cardiogenic shock was higher for patients treated with intravenous β-blockers than for those who were not (especially for patients with tachycardia, hypotension, or in Killip class II or III CHF). Because of this finding, the 2007 ACC/AHA guidelines suggest caution in the use of IV β-blockers.99 Contraindications to β-blockade include severe sinus bradycardia (heart rate <50 beats/min), marked first-degree atrioventricular block (ECG P-R interval >0.24 second) or any second-degree or third-degree atrioventricular block, persistent hypotension, pulmonary edema, history of bronchospasm, evidence of a low-output state (eg, oliguria), and increased risk of cardiogenic shock.42 Several placebo-controlled trials involving patients with UA/NSTEMI have demonstrated the benefit of β-blockers in reducing the incidence of subsequent MI, recurrent ischemia, or both.100-103
Calcium Channel Blockers
Calcium channel blockers inhibit the contraction of both the myocardium (thereby reducing myocardial oxygen demand) and the vascular smooth muscle (thereby causing coronary vasodilatation and improving myocardial blood flow). The ACC/AHA guidelines recommend these agents for patients with persistent or recurrent symptoms after treatment with full-dose nitrates and β-blockers, for patients with contraindications to β-blockade, and for patients with Prinzmetal variant angina.42 For such patients, calcium channel blockers that slow the heart rate (eg, diltiazem or verapamil) are recommended. These agents should not be administered to patients with severe LV dysfunction or pulmonary edema.104 DAVIT (Danish Verapamil Infarction Trial) is the largest randomized trial to date to have evaluated the efficacy of a calcium channel blocker for patients with ACS. The results showed a trend toward lower rates of death or MI when verapamil was administered to patients with suspected ACS.105,106 Similar decreases in the rates of MI and refractory angina have been found with diltiazem.107 Nifedipine, which does not reduce the heart rate, has been shown to be harmful to patients with acute MI when it is administered without the simultaneous administration of a β-blocker.108 The newer dihydropyridine calcium antagonists amlodipine and felodipine have not been evaluated specifically for administration to patients with ACS, but trials involving normotensive patients with CAD109 or hypertensive patients with cardiovascular risk factors110 have demonstrated that these agents provide significant benefits.
Inhibitors of the Renin-Angiotensin-Aldosterone System
The 2007 ACC/AHA guidelines recommend that, in the absence of hypotension or other known contraindications, an angiotensin-converting enzyme (ACE) inhibitor (or an angiotensin II receptor blocker for patients who cannot tolerate ACE inhibitors) should be administered orally within the first 24 hours to patients with pulmonary congestion or an LV ejection fraction of 40% or lower (class I recommendation) and should be considered for administration to patients without these features (class IIa recommendation).42 The recommendation for ACE inhibitor therapy is based on the results of several large trials showing that mortality rates were substantially reduced when ACE inhibitors were initiated within 24 hours of MI.111,112 The angiotensin II receptor blocker valsartan was found to be as effective as captopril for patients at high risk of cardiovascular events after MI; however, administering a combination of the 2 agents was found to be harmful.113 Long-term use of ACE inhibitors is indicated for many patients with high-risk chronic CAD.114,115
EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) found that the selective aldosterone receptor blocker eplerenone reduced morbidity and mortality rates for patients with MI complicated by LV dysfunction and either CHF or diabetes mellitus.116 Long-term administration of eplerenone is indicated for such patients in the absence of severe renal dysfunction or hyperkalemia.42
Other Anti-Ischemic Therapies
Ranolazine is a recently approved anti-ischemic agent that is indicated for use alone or in combination with nitrates, β-blockers, or amlodipine for the treatment of chronic refractory angina. The MERLIN-TIMI 36 (Metabolic Efficiency with Ranolazine for Less Ischemia in Non—ST-Elevation Acute Coronary Syndromes—Thrombolysis in Myocardial Infarction 36) trial demonstrated that ranolazine had a benefit over placebo in reducing the incidence of recurrent ischemia (HR, 0.87; 95% CI, 0.76-0.99; P=.03) when administered within 48 hours of the onset of UA/NSTEMI. However, ranolazine had no effect on the composite end point of cardiovascular death, MI, or recurrent ischemia (HR, 0.92; 95% CI, 0.83-1.02; P=.11).117,118
ANTITHROMBOTIC THERAPY
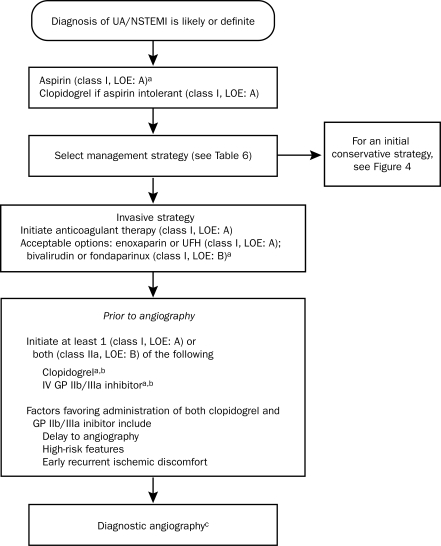
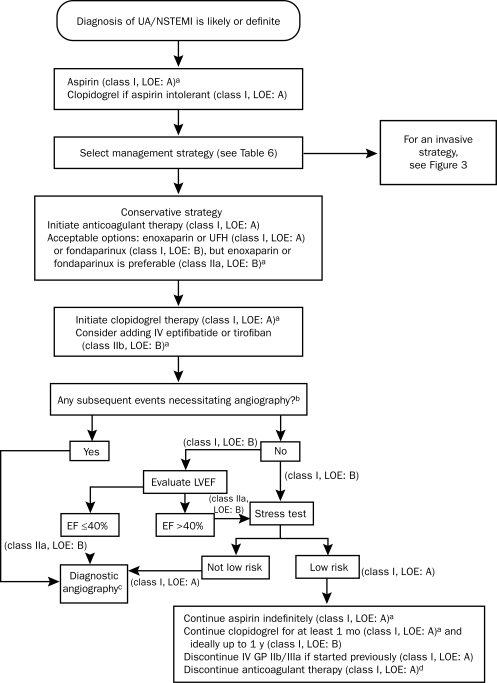
Antithrombotic therapy is the cornerstone of treatment for patients with UA/NSTEMI. It has 2 components: (1) antiplatelet therapy, which reduces platelet activation and aggregation, integral steps in the formation of a thrombus after plaque disruption, and (2) anticoagulant therapy, which targets the clotting cascade to prevent the deposition of fibrin strands in the clot. The ACC/AHA guidelines recommend tailoring the specific antithrombotic agents to the treatment strategy selected. Figure 3 shows the algorithm for choosing agents for patients managed with an invasive strategy, and Figure 4 shows the algorithm for patients managed with a conservative strategy.
FIGURE 3.
Algorithm for patients with UA/NSTEMI managed by an initial invasive strategy. When multiple drugs are listed, they are in alphabetical order and not in order of preference. GP = glycoprotein; IV = intravenous; LOE = level of evidence; NSTEMI = non—ST-segment elevation myocardial infarction; UA = unstable angina; UFH = unfractionated heparin.
a For full dosing information, see Table 13 in reference 42.
b Evidence exists that GP IIb/IIIa inhibitors may not be necessary if the patient received a preloading dose of at least 300 mg of clopidogrel at least 6 h earlier (class I, LOE: B for clopidogrel administration) and bivalirudin is selected as the anticoagulant (class IIa, LOE: B).
cFor more details on management of patients with UA/NSTEMI after diagnostic angiography, see Figure 9 of reference 42.
From J Am Coll Cardiol,42 with permission from Elsevier.
FIGURE 4.
Algorithm for patients with UA/NSTEMI managed by an initial conservative strategy. When multiple drugs are listed, they are in alphabetical order and not in order of preference. EF = ejection fraction; GP = glycoprotein; LOE = level of evidence; LVEF = left ventricular ejection fraction; NSTEMI = non—ST-segment elevation myocardial infarction; UA = unstable angina; UFH = unfractionated heparin.
a For full dosing information, see Table 13 in reference 42.
b For example, recurrent symptoms/ischemia, heart failure, or serious arrhythmia.
c For more details on management of patients with UA/NSTEMI after diagnostic angiography, see Figure 9 of reference 42.
d See recommendations in section 3.2.3 of reference 42.
From J Am Coll Cardiol,42 with permission from Elsevier.
Antiplatelet Therapy
Aspirin. Aspirin blocks the synthesis of thromboxane A2 by irreversibly inhibiting cyclooxygenase 1, thereby diminishing platelet aggregation. Four randomized trials have each demonstrated that, compared with placebo, aspirin reduces the risk of death or MI by more than 50% for patients presenting with UA/NSTEMI.35,36,119,120 The ACC/AHA guidelines recommend an initial daily dose of 162 to 325 mg, followed by a daily dose of 75 to 162 mg for long-term secondary prevention.42 Absolute contraindications to aspirin therapy include documented aspirin allergy (eg, asthma or anaphylaxis), active bleeding, or a known platelet disorder. Clopidogrel is a recommended alternative for patients who cannot tolerate aspirin.42
Clopidogrel. Clopidogrel is a thienopyridine derivative that blocks the P2Y12 adenosine diphosphate (ADP) receptor on platelets. This action decreases platelet activation and aggregation, increases bleeding time, and reduces blood viscosity. Therapy with clopidogrel and aspirin is recommended for essentially all patients with UA/NSTEMI.
The CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial randomly assigned 12,562 patients to receive either aspirin alone (75-325 mg/d) or aspirin plus clopidogrel (300-mg loading dose, then 75 mg/d).121 The incidence of the primary end point of cardiovascular death, MI, or stroke was 20% lower for both low-risk and high-risk patients with UA/NSTEMI who received aspirin plus clopidogrel (11.4%) than for those who received aspirin alone (9.3%; P<.0001).38 Benefit was seen as early as 24 hours after the initiation of treatment (the Kaplan-Meier curves began diverging after just 2 hours) and continued throughout the trial's 1-year treatment period. Clopidogrel was associated with substantially more instances of major bleeding but not with more instances of life-threatening bleeding. The prespecified subgroup analysis, PCI-CURE, found that treatment with clopidogrel before PCI was also associated with a substantial benefit: the reduction in cardiac events was 31% at 30 days and at 1 year.122
On the basis of the results of the PCI-CURE trial, the CREDO (Clopidogrel for the Reduction of Events During Observation) trial and the CLARITY-TIMI 28 (Clopidogrel as Adjunctive Reperfusion Therapy—Thrombolysis in Myocardial Infarction 28) trial, together with the results of a meta-analysis (which found that, in comparison with no pretreatment, clopidogrel pretreatment reduced the incidence of cardiovascular death, MI, or stroke from randomization through 30 days by 41%; P=.001123), the 2005 guidelines from the ACC, the AHA, and the Society for Coronary Angiography and Interventions contain a class I, level of evidence A recommendation for clopidogrel pretreatment before PCI.124,125
The risk of major bleeding was increased when patients had received clopidogrel within 5 days before undergoing CABG.38 Therefore, the ACC/AHA guidelines recommend discontinuing the administration of clopidogrel at least 5 days before surgery, if possible.42,126 The current practice in most hospitals is either to initiate clopidogrel administration at the time of admission (this action affords the benefits of reducing the incidence of early ischemic events and of pretreatment before PCI) or to delay treatment until after coronary angiography has been performed, in which case the drug can be either administered while PCI is carried out or withheld until after CABG has been performed.
Newer P2Y12 ADP Inhibitors. A high rate of recurrent atherothrombotic events despite the administration of dual-antiplatelet therapy with aspirin and clopidogrel has sparked great interest in finding more potent inhibitors of the P2Y12 ADP receptor.
Prasugrel is an irreversible P2Y12 ADP receptor antagonist that was recently approved by the US Food and Drug Administration. Several studies have shown that prasugrel achieves much higher (nearly double) levels of platelet inhibition than does daily clopidogrel dosing of 75 mg or even 150 mg.127 The TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction 38) trial administered prasugrel (a 60-mg loading dose and a 10-mg daily maintenance dose) or clopidogrel to 13,608 high-risk patients with ACS who were scheduled for PCI.128 The incidence of the primary end point of cardiovascular death, MI, or stroke at 6 to 15 months was significantly lower in the prasugrel group (9.9%) than in the clopidogrel group (12.1%; P<.001). The incidence of stent thrombosis was 52% lower with prasugrel (1.1%) than with clopidogrel (2.4%; P<.001). The risk of TIMI major bleeding, including the risk of fatal bleeding, was higher for the patients receiving prasugrel (2.4%) than for those receiving clopidogrel (1.8%; P=.03).
Ticagrelor (AZD6140) is a reversible oral P2Y12 receptor antagonist with a half-life of approximately 12 hours. The recently completed PLATO (Study of Platelet Inhibition and Patient Outcomes) randomized 18,624 patients with ACS to either ticagrelor (loading dose of 180 mg followed by 90 mg twice daily) or clopidogrel for up to 12 months.129 The primary end point of death from vascular causes, MI, or stroke occurred in 9.8% of patients receiving ticagrelor vs 11.7% of those receiving clopidogrel (HR, 0.84; 95% CI, 0.77-0.92; P<.001). The rate of death from any cause was also reduced with ticagrelor vs clopidogrel (4.5% vs 5.9%; P<.001). The rates of major bleeding overall were similar between the ticagrelor and clopidogrel groups (11.6% vs 11.2%; P=.43).
GP IIb/IIIa Inhibitors. The platelet GP IIb/IIIa inhibitors are potent and specific inhibitors of platelet aggregation. They act by interrupting the final common pathway of fibrinogen-mediated cross-linkage of platelets. Several large trials involving patients with UA/NSTEMI have shown that the GP IIb/IIIa inhibitors are of substantial benefit for patients at high risk, those undergoing PCI, or both.39,130 Three agents are currently available for use: abciximab, eptifibatide, and tirofiban. Abciximab is indicated only if angiography will not be appreciably delayed and PCI is likely to be performed; otherwise, IV eptifibatide or tirofiban is the preferred choice. The main risk associated with GP IIb/IIIa inhibitors is an increased rate of hemorrhage, usually at the site of vascular intervention. Therefore, patients should be monitored closely for bleeding, and complete blood cell counts should be determined regularly.
The benefit of GP IIb/IIIa inhibition appears to be greatest for patients at higher risk of complications, eg, those with elevated troponin concentrations,62,63 diabetes,66 ST-segment changes,39 recurrent angina,39,131 previous aspirin use,132 or a TIMI risk score of 4 or higher.83 The benefit of GP IIb/IIIa inhibition has been confirmed even for patients who have been pretreated with clopidogrel.133 The optimal timing for the initiation of GP IIb/IIIa inhibitors has been debated. The EARLY ACS (Early Glycoprotein IIb/IIIa Inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome) trial involved 9492 patients who were randomly assigned either to early GP IIb/IIIa inhibition or to the provisional use of GP IIb/IIIa inhibitors after angiography. The results showed that early eptifibatide exerted no statistically significant benefit in reducing the composite end point of adverse cardiovascular events but was associated with a statistically significant increase in bleeding rates.134
The 2007 ACC/AHA guidelines recommend that, for patients with UA/NSTEMI who will be treated initially according to an invasive strategy, either an intravenous GP IIb/IIIa inhibitor or clopidogrel should be added to aspirin and anticoagulant therapy (upstream) before diagnostic angiography is performed (class I recommendation). They also state that adding both agents is reasonable (class IIa recommendation).42
Anticoagulant Therapy
The 2007 ACC/AHA UA/NSTEMI guidelines recommend the initiation of anticoagulant therapy for all patients (without contraindications) as soon as possible after presentation (class I recommendation). The guidelines recommend 4 agents as options: unfractionated heparin (UFH), enoxaparin, fondaparinux, and bivalirudin (approved only for patients managed according to an invasive strategy).
Unfractionated Heparin. The results of several randomized trials suggest that UFH is associated with lower rates of death or MI than is aspirin alone.36,120,135 The anticoagulant effects of UFH are variable.136 The ACC/AHA guidelines recommend weight-adjusted dosing of UFH (60 U/kg bolus and 12 U/kg/hr infusion), frequent monitoring of activated partial thromboplastin time (every 6 hours until 2 consecutive values are within the target range, and every 24 hours thereafter), and titration of UFH according to a standardized nomogram with a target range of activated partial thromboplastin time between 1.5 and 2.0 times that of control, or approximately 50 to 70 seconds.42 Administration of UFH should continue for at least 48 hours after presentation with UA/NSTEMI.42
Complete blood cell counts should be determined at least daily during therapy with UFH. Autoimmune heparin-induced thrombocytopenia in association with thrombosis is a rare but dangerous complication of UFH administration (incidence is <0.2%).137 When clinical findings suggest that this complication has occurred, all heparin therapy should be immediately discontinued.
Low—Molecular-Weight Heparin. Because the rates of recurrence of ischemic events remain high even when UFH is administered, low—molecular-weight heparins (LMWHs) were developed with the goal of providing improved anticoagulation. They are active against both factor Xa and factor IIa; therefore, they inhibit both the action and the generation of thrombin. Their other advantages over UFH include a lower rate of thrombocytopenia,138 more bioavailability, and less binding to plasma proteins, a factor that renders monitoring the level of anticoagulation unnecessary.
Various LMWHs (dalteparin, enoxaparin, and nadroparin) have been compared with UFH for the treatment of UA/NSTEMI, but only enoxaparin has been found to have a clear benefit. Early trials, such as ESSENCE (Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events) and the TIMI 11B, showed that, compared with UFH, enoxaparin achieved a 20% reduction in the incidence of death, MI, recurrent ischemia, or some combination of these factors.139 The SYNERGY (Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors) trial found that enoxaparin was not inferior to UFH in the setting of an early invasive strategy.140 However, enoxaparin achieved a clear benefit over UFH in the setting of a conservative strategy, as shown by the older trials and the more recent A-to-Z (Aggrastat to Zocor) trial.141 The 2007 ACC/AHA guidelines contain a class IIa recommendation stating that enoxaparin or fondaparinux (see Factor Xa Inhibitors) is preferable to UFH as anticoagulant therapy for UA/NSTEMI patients who will be treated conservatively, unless CABG is planned within 24 hours.42 The benefit of enoxaparin is greater for patients at higher risk, such as those with ST-segment deviation,142 elevated troponin concentrations,143 and high TIMI risk scores.82 The rates of major bleeding associated with LMWHs have been found to be similar to those associated with UFH, with 1 exception: the SYNERGY trial found a statistically significant increase in the incidence of major bleeding in association with enoxaparin administration.140
Direct Thrombin Inhibitors. Direct thrombin inhibitors have several potential advantages over indirect thrombin inhibitors (such as UFH or LMWH): they do not require a cofactor such as antithrombin for their action and can thus directly inhibit clot-bound thrombin; they do not interact with plasma proteins; and they do not cause thrombocytopenia.
The administration of bivalirudin to patients with UA/NSTEMI was recently studied in the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial, which randomly assigned 13,819 patients with ACS managed with an early invasive strategy to one of 3 antithrombotic regimens: UFH (or enoxaparin) plus a GP IIb/IIIa inhibitor, bivalirudin plus a GP IIb/IIIa inhibitor, or bivalirudin alone.144 No differences in the rates of the primary end point (composite of death, MI, unplanned revascularization for ischemia, and major bleeding at 30 days) were observed between the group receiving UFH plus a GP IIb/IIIa inhibitor and the group receiving bivalirudin plus a GP IIb/IIIa inhibitor. However, the 30-day net clinical outcomes were significantly better for the group receiving bivalirudin alone than for the group receiving UFH plus a GP IIb/IIIa inhibitor (rates of primary end point, 10.1% vs 11.7%; P=.015); this difference was due primarily to a substantially reduced rate of major bleeding. The ACC/AHA guidelines have given bivalirudin a class I recommendation for the treatment of patients with UA/NSTEMI selected for an early invasive strategy. The guidelines further state that it is reasonable to omit the administration of an intravenous GP IIb/IIIa antagonist if a thienopyridine is administered simultaneously with bivalirudin (class IIa recommendation).42
The 2007 ACC/AHA guidelines recommend the use of other direct thrombin inhibitors, such as lepirudin (recombinant hirudin) and argatroban, only for patients with heparin-induced thrombocytopenia.42
Factor Xa Inhibitors. Fondaparinux is a synthetic pentasaccharide that is an indirect factor Xa inhibitor and requires antithrombin for its action. The OASIS-5 (Fifth Organization to Assess Strategies in Acute Ischemic Syndromes) trial, which involved 20,078 patients with high-risk UA/NSTEMI, compared subcutaneous fondaparinux at a once-daily dose of 2.5 mg with standard-dose enoxaparin.145 Fondaparinux was found to be not inferior to enoxaparin in reducing the incidence of the primary outcomes of death, MI, or refractory ischemia at 9 days. The rate of major bleeding, however, was almost 50% lower in the fondaparinux arm than in the enoxaparin arm, and analyses using the composite variable of the primary outcome and major bleeding at 9 days demonstrated an advantage of fondaparinux over enoxaparin (incidence, 7.3% vs 9.0%; HR, 0.81; P<.001). Fondaparinux was also associated with a statistically significant reduction in 30-day and 6-month mortality rates. In the subset of patients undergoing PCI, the risk of catheter-related thrombi was more than 3 times higher in the fondaparinux arm than in the enoxaparin arm; supplemental UFH at the time of catheterization appeared to minimize the risk of this complication.
The 2007 ACC/AHA guidelines contain a class I recommendation for fondaparinux as treatment for patients with UA/NSTEMI who will be managed by either a conservative strategy or an early invasive strategy, unless CABG is planned within 24 hours.42 They further state that fondaparinux is preferred over other anticoagulants for patients who are selected for a conservative treatment strategy and who are at an increased risk of bleeding (class I recommendation).
Oral Anticoagulation. Trials of oral anticoagulation with warfarin after ACS have demonstrated the benefit of the combination of warfarin plus aspirin over aspirin alone, provided a sufficient degree of anticoagulation was achieved.146-148 However, a similar degree of benefit is seen with clopidogrel plus aspirin rather than with aspirin alone, without the drawback of monitoring the international normalized ratio, as is necessary with warfarin therapy. In addition, the use of clopidogrel is well established for patients with ACS who undergo PCI and stenting. Thus, the clinical use of aspirin plus warfarin is limited. Occasionally, an indication for warfarin, in addition to aspirin and clopidogrel, arises after UA/NSTEMI (eg, for patients with atrial fibrillation, a mechanical prosthetic valve, or LV thrombus).
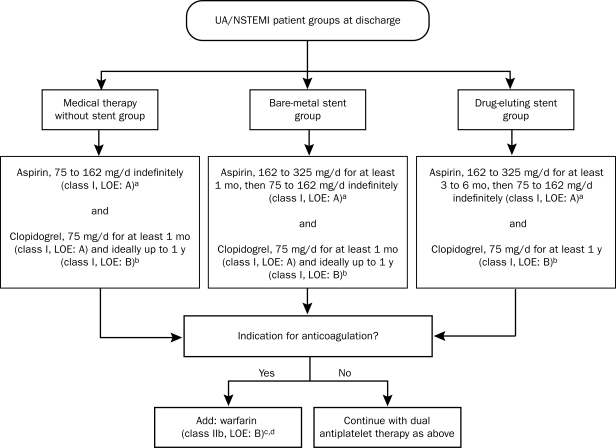
Discharge Antithrombotic Therapy. The 2007 ACC/AHA guidelines provide clear recommendations for antithrombotic therapy at the time of discharge; these recommendations are based on the management strategy (Figure 5). The benefits and risks of triple antithrombotic therapy with aspirin, clopidogrel, and warfarin have not been clearly established. Such therapy should be selected only when clear indications are present and should be administered for the shortest possible time and at the lowest effective doses: aspirin, 81 mg; warfarin, titrated to the dosage necessary to sustain an international normalized ratio of 2.0 to 2.5 (class IIb recommendation).42
FIGURE 5.
Long-term antithrombotic therapy at hospital discharge after unstable angina (UA)/non—ST-segment elevation myocardial infarction (NSTEMI). LOE = level of evidence.
a For patients allergic to aspirin, use clopidogrel alone indefinitely, or try aspirin desensitization.
b For patients allergic to clopidogrel, use ticlopidine, 250 mg by mouth twice daily.
c Continue aspirin indefinitely and warfarin longer term as indicated for such specific conditions as atrial fibrillation; left ventricular thrombus; and cerebral, venous, or pulmonary emboli.
d When warfarin is added to aspirin plus clopidogrel, an international normalized ratio of 2.0 to 2.5 is recommended.
From J Am Coll Cardiol,42 with permission from Elsevier.
LIPID-LOWERING THERAPY
In the absence of contraindications, lipid-lowering therapy with statins should be initiated for all patients with UA/NSTEMI, regardless of baseline LDL cholesterol levels. If the LDL cholesterol concentration is 100 mg/dL (to convert to mmol/L, multiply by 0.0259) or higher, cholesterol-lowering therapy should be initiated or intensified with the goal of achieving an LDL cholesterol concentration lower than 100 mg/dL. An update to both the Adult Treatment Panel III guidelines149 and the 2007 ACC/AHA guidelines42 states that further titration to a dose necessary to sustain an LDL cholesterol concentration of 70 mg/dL or lower is reasonable (class IIa recommendation).
The LIPID (Long-Term Intervention with Pravastatin in Ischaemic Disease) trial demonstrated that, compared with placebo, pravastatin achieved a 26% reduction in mortality rates (P=.004) for patients with UA, as well as statistically significant reductions in the incidence of subsequent MI, coronary revascularization, and stroke.150 The PROVE IT (Pravastatin or Atorvastatin Evaluation and Infection Therapy)-TIMI 22 trial found that, compared with moderate lipid lowering after ACS with standard-dose pravastatin (40 mg/d), intensive lipid lowering with high-dose atorvastatin (80 mg/d) achieved a 16% reduction in the primary composite end point of all-cause death, MI, UA requiring rehospitalization or revascularization, and stroke.151 The benefit was linked to statistically significant reductions in both LDL cholesterol and CRP concentrations.152
CONCLUSION
During the past quarter of a century, huge advances have been made in our understanding of the pathophysiology of ACS, and these advances have been accompanied by important breakthroughs in the management of this condition. Accurate diagnosis of ACS has life-saving implications and requires a careful assessment of both the patient's history and the findings on physical examination, 12-lead ECG, and cardiac biomarker assays. The initial management of UA/NSTEMI involves both aggressive medical therapy and revascularization. Early risk stratification permits the identification of high-risk patients who stand to gain the most from potent therapies such as GP IIb/IIIa inhibitors and early invasive strategies. Platelets play a crucial role in ACS, and newer antiplatelet drugs continue to be developed with the goal of maximizing the reduction in atherothrombotic events while minimizing bleeding complications.
Supplementary Material
On completion of this article, you should be able to (1) define the pathophysiology of acute coronary syndrome, including the role of inflammation and thrombosis; (2) recognize the currently available tools for the diagnosis and risk stratification of acute coronary syndrome; and (3) describe the importance of anti-ischemic and antithrombotic therapy and identify the relative benefits of an invasive vs a conservative strategy for managing unstable angina/non—ST-segment elevation myocardial infarction.
Footnotes
Dr Cannon is a senior investigator for the Thrombolysis in Myocardial Infarction Study Group. He has received research grants and support from Accumetrics, AstraZeneca, Bristol-Myers Squibb/sanofi Partnership, GlaxoSmithKline, Merck, and the Merck/Schering Plough Partnership. He is also a clinical advisor and has equity interest in Automedics Medical Systems.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2009 update. a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee [published correction appears in Circulation. 2009;119(3):e182] Circulation 2009January27;119(3):480-486 Epub 2008 Dec 15 [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Badimon L, Cohen M, Ambrose JA, Badimon JJ, Chesebro J. Insights into the pathogenesis of acute ischemic syndromes. Circulation 1988;77(6):1213-1220 [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992;326(5):310-318 [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 2001;104(3):365-372 [DOI] [PubMed] [Google Scholar]
- 5.Corti R, Fuster V, Badimon JJ, Hutter R, Fayad ZA. New understanding of atherosclerosis (clinically and experimentally) with evolving MRI technology in vivo. Ann N Y Acad Sci. 2001;947:181-195 [DOI] [PubMed] [Google Scholar]
- 6.Kinlay S, Libby P, Ganz P. Endothelial function and coronary artery disease. Curr Opin Lipidol. 2001;12(4):383-389 [DOI] [PubMed] [Google Scholar]
- 7.Webster MWI, Chesebro JH, Smith HC, et al. Myocardial infarction and coronary artery occlusion: a prospective 5-year angiographic study. J Am Coll Cardiol. 1990;15:218A [Google Scholar]
- 8.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262-1275 [DOI] [PubMed] [Google Scholar]
- 9.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes: implications for plaque rupture. Circulation 1994;90(2):775-778 [DOI] [PubMed] [Google Scholar]
- 10.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89(1):36-44 [DOI] [PubMed] [Google Scholar]
- 11.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69(5):377-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukhova GK, Schönbeck U, Rabkin E, et al. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation 1999;99(19):2503-2509 [DOI] [PubMed] [Google Scholar]
- 13.Herman MP, Sukhova GK, Libby P, et al. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation 2001;104(16):1899-1904 [DOI] [PubMed] [Google Scholar]
- 14.Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000;101(6):598-603 [DOI] [PubMed] [Google Scholar]
- 15.von Birgelen C, Klinkhart W, Mintz GS, et al. Plaque distribution and vascular remodeling of ruptured and nonruptured coronary plaques in the same vessel: an intravascular ultrasound study in vivo. J Am Coll Cardiol. 2001;37(1):1864-1870 [DOI] [PubMed] [Google Scholar]
- 16.Rauch U, Osende JI, Fuster V, Badimon JJ, Fayad Z, Chesebro JH. Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences. Ann Intern Med. 2001;134:224-238 [DOI] [PubMed] [Google Scholar]
- 17.Davies MJ. A macro and micro view of coronary vascular insult in ischemic heart disease. Circulation 1990;82(3) (suppl):II38-II46 [PubMed] [Google Scholar]
- 18.Lendon CL, Davies MJ, Born GV, Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis 1991;87(1):87-90 [DOI] [PubMed] [Google Scholar]
- 19.Tanaka A, Shimada K, Sano T, et al. Multiple plaque rupture and C-reactive protein in acute myocardial infarction. J Am Coll Cardiol. 2005May17;45(10):1594-1599 Epub 2005 Apr 25 [DOI] [PubMed] [Google Scholar]
- 20.Sano T, Tanaka A, Namba M, et al. C-reactive protein and lesion morphology in patients with acute myocardial infarction. Circulation 2003July22;108(3):282-285 Epub 2003 Jun 30 [DOI] [PubMed] [Google Scholar]
- 21.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 2003;108(14):1664-1672 [DOI] [PubMed] [Google Scholar]
- 22.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part II. Circulation 2003;108(15):1772-1778 [DOI] [PubMed] [Google Scholar]
- 23.Lüscher TF, Tanner FC, Noll G. Lipids and endothelial function: effects of lipid-lowering and other therapeutic interventions. Curr Opin Lipidol. 1996;7(4):234-240 [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Chester MR, Crook R, Kaski JC. Differential progression of complex culprit stenoses in patients with stable and unstable angina pectoris. J Am Coll Cardiol. 1996;28(3):597-603 [DOI] [PubMed] [Google Scholar]
- 25.Moreno PR, Bernardi VH, López-Cuéllar J, et al. Macrophages, smooth muscle cells, and tissue factor in unstable angina: implications for cell-mediated thrombogenicity in acute coronary syndromes. Circulation 1996;94(12):3090-3097 [DOI] [PubMed] [Google Scholar]
- 26.Fosang AJ, Smith PJ. Human genetics: to clot or not. Nature 2001;413:475-476 [DOI] [PubMed] [Google Scholar]
- 27.Weiss EJ, Bray PF, Tayback M, et al. A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis. N Engl J Med. 1996;334(17):1090-1094 [DOI] [PubMed] [Google Scholar]
- 28.Rioufol G, Finet G, Ginon I, et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation 2002;106(7):804-808 [DOI] [PubMed] [Google Scholar]
- 29.Conti CR, Brawley RK, Griffith LS, et al. Unstable angina pectoris: morbidity and mortality in 57 consecutive patients evaluated angiographically. Am J Cardiol. 1973;32(6):745-750 [DOI] [PubMed] [Google Scholar]
- 30.Mizuno K, Satumo K, Miyamoto A, et al. Angioscopic evaluation of coronary artery thrombi in acute coronary syndromes. N Engl J Med. 1992;326(5):287-291 [DOI] [PubMed] [Google Scholar]
- 31.The TIMI IIIA Investigators Early effects of tissue-type plasminogen activator added to conventional therapy on the culprit lesion in patients presenting with ischemic cardiac pain at rest: results of the Thrombolysis in Myocardial Ischemia (TIMI IIIA) Trial. Circulation 1993;87(1):38-52 [DOI] [PubMed] [Google Scholar]
- 32.Sullivan E, Kearney M, Isner JM, Topol EJ, Losorda DW. Pathology of unstable angina: analysis of biopsies obtained by directional coronary atherectomy. J Thromb Thrombolysis 1994;1(1):63-71 [DOI] [PubMed] [Google Scholar]
- 33.Sherman CT, Litvack F, Grundfest W, et al. Coronary angioscopy in patients with unstable angina pectoris. N Engl J Med. 1986;315(15):913-919 [DOI] [PubMed] [Google Scholar]
- 34.DeWood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303(16):897-902 [DOI] [PubMed] [Google Scholar]
- 35.Lewis HD, Davis JW, Archibald DG, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. N Engl J Med. 1983;309(7):396-403 [DOI] [PubMed] [Google Scholar]
- 36.Théroux P, Ouimet H, McCans J, et al. Aspirin, heparin or both to treat unstable angina. N Engl J Med. 1988;319(17):1105-1111 [DOI] [PubMed] [Google Scholar]
- 37.Cohen M, Demers C, Gurfinkel EP, et al. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. N Engl J Med. 1997;337(7):447-452 [DOI] [PubMed] [Google Scholar]
- 38.Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation [published corrections appear in N Engl J Med. 2001;345(23):1716 and 2001;345(20):1506] N Engl J Med. 2001;345(7):494-502 [DOI] [PubMed] [Google Scholar]
- 39.Platelet Receptor Inhibition for Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Trial Investigators Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction [published correction appears in N Engl J Med. 1998;339(6):415] N Engl J Med. 1998;338(21):1488-1497 [DOI] [PubMed] [Google Scholar]
- 40.Cannon CP, Weintraub WS, Demopoulos LA, et al. TACTICS-Thrombolysis in Myocardial Infarction 18 Investigators Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344(25):1879-1887 [DOI] [PubMed] [Google Scholar]
- 41.Cannon CP, Braunwald E. Time to reperfusion: the critical modulator in thrombolysis and primary angioplasty. J Thromb Thrombolysis 1996;3(2):117-125 [DOI] [PubMed] [Google Scholar]
- 42.Anderson JL, Adams CD, Antman EM, et al. Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol. 2007;50(7):e1-e157 [DOI] [PubMed] [Google Scholar]
- 43.McDermott MM, Mandapat AL, Moates A, et al. Knowledge and attitudes regarding cardiovascular disease risk and prevention in patients with coronary or peripheral arterial disease. Arch Intern Med. 2003;163(18):2157-2162 [DOI] [PubMed] [Google Scholar]
- 44.Pope JH, Ruthazer R, Beshansky JR, Griffith JL, Selker HR. Clinical features of emergency department patients presenting with symptoms suggestive of acute cardiac ischemia: a multicenter study. J Thromb Thrombolysis 1998;6(1):63-74 [DOI] [PubMed] [Google Scholar]
- 45.Braunwald E, Mark DB, Jones RH, et al. Unstable angina: diagnosis and management Rockville, MD: Agency for Health Care Policy and Research and the National Heart, Lung, and Blood Institute, US Public Health Service, US Dept of Health and Human Services, 1994. AHCPR Publication No. 94-0602 (124) [Google Scholar]
- 46.Abidov A, Rozanski A, Hachamovitch R, et al. Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Engl J Med. 2005;353(18):1889-1898 [DOI] [PubMed] [Google Scholar]
- 47.Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118(2):81-90 [DOI] [PubMed] [Google Scholar]
- 48.Morise AP, Haddad WJ, Beckner D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med. 1997;102(4):350-356 [DOI] [PubMed] [Google Scholar]
- 49.Jayes RL, Jr, Beshansky JR, D'Agostino RB, Selker HP. Do patients' coronary risk factor reports predict acute cardiac ischemia in the emergency department? A multicenter study. J Clin Epidemiol. 1992;45(6):621-626 [DOI] [PubMed] [Google Scholar]
- 50.Cannon CP, McCabe CH, Stone PH, et al. The electrocardiogram predicts one-year outcome of patients with unstable angina and non-Q wave myocardial infarction: results of the TIMI III Registry ECG Ancillary Study. J Am Coll Cardiol. 1997;30(1):133-140 [DOI] [PubMed] [Google Scholar]
- 51.Savonitto S, Ardissino D, Granger CB, et al. Prognostic value of the admission electrocardiogram in acute coronary syndromes. JAMA 1999;281(8):707-713 [DOI] [PubMed] [Google Scholar]
- 52.Hyde TA, French JK, Wong CK, Straznicky IT, Whitlock RM, White HD. Four-year survival of patients with acute coronary syndromes without ST-segment elevation and prognostic significance of 0.5-mm ST-segment depression. Am J Cardiol. 1999;84(4):379-385 [DOI] [PubMed] [Google Scholar]
- 53.Yusuf S, Pearson M, Sterry H, et al. The entry ECG in the early diagnosis and prognostic stratification of patients with suspected acute myocardial infarction. Eur Heart J. 1984;5(9):690-696 [DOI] [PubMed] [Google Scholar]
- 54.Brush JE, Jr, Brand DA, Acampora D, Chalmer B, Wackers FJ. Use of the initial electrocardiogram to predict in-hospital complications of acute myocardial infarction. N Engl J Med. 1985;312(18):1137-1141 [DOI] [PubMed] [Google Scholar]
- 55.Braunwald E, Jones RH, Mark DB, et al. Diagnosing and managing unstable angina. Circulation 1994;90(1):613-622 [DOI] [PubMed] [Google Scholar]
- 56.Ryan TJ, Anderson JL, Antman EM, et al. ACC/AHA guidelines for the management of patients with acute myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J Am Coll Cardiol. 1996;28(5):1328-1428 [DOI] [PubMed] [Google Scholar]
- 57.McCord J, Nowak RM, McCullough PA, et al. Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I. Circulation 2001;104(13):1483-1488 [DOI] [PubMed] [Google Scholar]
- 58.Shapiro BP, Babuin L, Jaffe AS. Cardiac biomarkers. In: Murphy JG, Lloyd MA, eds. Mayo Clinic Cardiology: Concise Textbook 3rd ed.Rochester, MN: Mayo Clinic Scientific Press; and New York: Informa Healthcare USA; 2007:773-780 [Google Scholar]
- 59.Jaber WA, Prior DL, Marso SP, Houghtaling PL, Menon V, Harrington RA. CHF on presentation is associated with markedly worse outcomes among patients with acute coronary syndromes: PURSUIT trial findings. Circulation 1999;100(suppl I):I433 [Google Scholar]
- 60.Steinhubl SR, Berger PB, Mann JT, III, et al. CREDO Investigators Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial [published correction appears in JAMA. 2003;289(8):987] JAMA 2002;288(19):2411-2420 [DOI] [PubMed] [Google Scholar]
- 61.Batter A. The Euro Heart Survey of Acute Coronary Syndromes (EHSACS) Presented at: European Society of Cardiology Scientific Sessions Stockholm, Sweden; September1-5, 2001 [Google Scholar]
- 62.Hamm CW, Heeschen C, Goldmann B, et al. c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) Study Investigators Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels [published correction appears in N Engl J Med. 1999;341(7):548] N Engl J Med. 1999;340(21):1623-1629 [DOI] [PubMed] [Google Scholar]
- 63.Heeschen C, Hamm CW, Goldmann B, PRISM Study Investigators Troponin concentrations for stratification of patients with acute coronary syndromes in relation to therapeutic efficacy of tirofiban. Lancet 1999;354(9192):1757-1762 [DOI] [PubMed] [Google Scholar]
- 64.FRagmin and Fast Revascularisation during InStability in Coronary artery disease (FRISC II) Investigators Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet 1999;354(9180):708-715 [PubMed] [Google Scholar]
- 65.Boersma E, Pieper KS, Steyerberg EW, et al. PURSUIT Investigators Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation: results from an international trial of 9461 patients. Circulation 2000;101(22):2557-2567 [DOI] [PubMed] [Google Scholar]
- 66.Théroux P, Alexander J, Jr, Pharand C, et al. Glycoprotein IIb/IIIa receptor blockade improves outcomes in diabetic patients presenting with unstable angina/non-ST-elevation myocardial infarction: results from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) study. Circulation 2000;102(20):2466-2472 [DOI] [PubMed] [Google Scholar]
- 67.Roffi M, Chew DP, Mukherjee D, et al. Platelet glycoprotein IIb/IIIa inhibitors reduce mortality in diabetic patients with non-ST-segment-elevation acute coronary syndromes. Circulation 2001;104(23):2767-2771 [DOI] [PubMed] [Google Scholar]
- 68.Cotter G, Cannon CP, McCabe CH, Charlesworth A, Caspi A, Braunwald E. Prior peripheral vascular disease and cerebrovascular disease are independent predictors of increased 1 year mortality in patients with acute coronary syndromes: results from OPUS-TIMI 16 [abstract]. J Am Coll Cardiol. 2000;35(suppl A):410A [Google Scholar]
- 69.Santopinto J, Gurfinkel EP, Torres V, et al. Prior aspirin users with acute non-ST-elevation coronary syndromes are at increased risk of cardiac events and benefit from enoxaparin. Am Heart J. 2001;141(4):566-572 [DOI] [PubMed] [Google Scholar]
- 70.Mauri F, Franzosi MG, Maggioni AP, Santoro E, Santoro L. Clinical value of 12-lead electrocardiography to predict the long-term prognosis of GISSI-1 patients. J Am Coll Cardiol. 2002;39(10):1594-1600 [DOI] [PubMed] [Google Scholar]
- 71.Morrow DA, Cannon CP, Rifai N, et al. TACTICS-TIMI 18 Investigators Ability of minor elevations of troponin I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction: results from a randomized trial. JAMA 2001;286(19):2405-2412 [DOI] [PubMed] [Google Scholar]
- 72.Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335(18):1342-1349 [DOI] [PubMed] [Google Scholar]
- 73.Morrow DA, Rifai N, Antman EM, et al. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11A substudy. J Am Coll Cardiol. 1998;31(7):1460-1465 [DOI] [PubMed] [Google Scholar]
- 74.Cannon CP, McCabe CH, Wilcox RG, Bentley JH, Braunwald E, OPUS-TIMI 16 Investigators Association of white blood cell count with increased mortality in acute myocardial infarction and unstable angina pectoris. Am J Cardiol. 2001;87(5):636-639, A10 [DOI] [PubMed] [Google Scholar]
- 75.Sabatine MS, Morrow DA, Cannon CP, et al. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 substudy. J Am Coll Cardiol. 2002;40(10):1761-1768 [DOI] [PubMed] [Google Scholar]
- 76.Baldus S, Heeschen C, Meinertz T, et al. CAPTURE Investigators Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 2003September23;108(12):1440-1445 Epub 2003 Sep 2 [DOI] [PubMed] [Google Scholar]
- 77.James SK, Lindahl B, Siegbahn A, et al. N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)-IV substudy. Circulation 2003;108(3):275-281 Epub 2003 Jul 7 [DOI] [PubMed] [Google Scholar]
- 78.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345(14):1014-1021 [DOI] [PubMed] [Google Scholar]
- 79.Mega JL, Morrow DA, De Lemos JA, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: an ENTIRE-TIMI-23 substudy. J Am Coll Cardiol. 2004;44(2):335-339 [DOI] [PubMed] [Google Scholar]
- 80.Arakawa N, Nakamura M, Aoki H, Hiramori K. Relationship between plasma level of brain natriuretic peptide and myocardial infarct size. Cardiology 1994;85(5):334-340 [DOI] [PubMed] [Google Scholar]
- 81.Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation 2002;105(15):1760-1763 [DOI] [PubMed] [Google Scholar]
- 82.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284(7):835-842 [DOI] [PubMed] [Google Scholar]
- 83.Morrow DA, Antman EM, Snapinn SM, McCabe CH, Theroux P, Braunwald E. An integrated clinical approach to predicting the benefit of tirofiban in non-ST elevation acute coronary syndromes: application of the TIMI Risk Score for UA/NSTEMI in PRISM-PLUS. Eur Heart J. 2002;23(3):223-229 [DOI] [PubMed] [Google Scholar]
- 84.James SK, Lindbäck J, Tilly J, et al. Troponin-T and N-terminal pro-B-type natriuretic peptide predict mortality benefit from coronary revascularization in acute coronary syndromes: a GUSTO-IV substudy. J Am Coll Cardiol. 2006;48(6):1146-1154 Epub 2006 Aug 28 [DOI] [PubMed] [Google Scholar]
- 85.Eagle KA, Lim MJ, Dabbous OH, et al. GRACE Investigators A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004;291(22):2727-2733 [DOI] [PubMed] [Google Scholar]
- 86.Granger CB, Goldberg RJ, Dabbous O, et al. Global Registry of Acute Coronary Events Investigators Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345-2353 [DOI] [PubMed] [Google Scholar]
- 87.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000;102(17):2031-2037 [DOI] [PubMed] [Google Scholar]
- 88.Wiviott SD, Morrow DA, Frederick PD, et al. Performance of the thrombolysis in myocardial infarction risk index in the National Registry of Myocardial Infarction-3 and -4: a simple index that predicts mortality in ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2004;44(4):783-789 [DOI] [PubMed] [Google Scholar]
- 89.CIBIS-II Investigators and Committees The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353(9146):9-13 [PubMed] [Google Scholar]
- 90.de Winter RJ, Windhausen F, Cornel JH, et al. Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) Investigators Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med. 2005;353(11):1095-1104 [DOI] [PubMed] [Google Scholar]
- 91.Hirsch A, Windhausen F, Tijssen JG, Verheugt FW, Cornel JH, de Winter RJ, Invasive versus Conservative Treatment in Unstable coronary Syndromes (ICTUS) investigators Long-term outcome after an early invasive versus selective invasive treatment strategy in patients with non-ST-elevation acute coronary syndrome and elevated cardiac troponin T (the ICTUS trial): a follow-up study. Lancet 2007;369(9564):827-835 [DOI] [PubMed] [Google Scholar]
- 92.Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol. 2006October3;48(7):1319-1325 Epub 2006 Sep 12 [DOI] [PubMed] [Google Scholar]
- 93.O'Donoghue M, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. JAMA 2008;300(1):71-80 [DOI] [PubMed] [Google Scholar]
- 94.Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009;360(21):2165-2175 [DOI] [PubMed] [Google Scholar]
- 95.Cheitlin MD, Hutter AM, Jr, Brindis RG, et al. Use of sildenafil (Viagra) in patients with cardiovascular disease [published correction appears in J Am Coll Cardiol. 1999;34(6):1850] J Am Coll Cardiol. 1999;33(1):273-282 [DOI] [PubMed] [Google Scholar]
- 96.Meine TJ, Roe MT, Chen AY, et al. CRUSADE Investigators Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149(6):1043-1049 [DOI] [PubMed] [Google Scholar]
- 97.Gislason GH, Jacobsen S, Rasmussen JN, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation 2006June27;113:2906-2913 Epub 2006 Jun 19 [DOI] [PubMed] [Google Scholar]
- 98.Gibson CM, Pride YB, Aylward PE, et al. Association of non-steroidal anti-inflammatory drugs with outcomes in patients with ST-segment elevation myocardial infarction treated with fibrinolytic therapy: an ExTRACT-TIMI 25 analysis. J Thromb Thrombolysis 2009January;27(1):11-17 Epub 2008 Aug 10 [DOI] [PubMed] [Google Scholar]
- 99.COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group Early intravenous then oral metoprolol in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366(9497):1622-1632 [DOI] [PubMed] [Google Scholar]
- 100.Gottlieb SO, Weisfeldt ML, Ouyang P, et al. Effect of the addition of propranolol to therapy with nifedepine for unstable angina: a randomized, double-blind, placebo-controlled trial. Circulation 1986;73(2):331-337 [DOI] [PubMed] [Google Scholar]
- 101.The Holland Interuniversity Nifedipine/Metoprolol Trial (HINT) Research Group Early treatment of unstable angina in the coronary care unit: a randomised, double blind, placebo controlled comparison of recurrent ischaemia in patients treated with nifedipine or metoprolol or both. Br Heart J. 1986;56(5):400-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Théroux P, Taeymans Y, Morissette D, Bosch X, Pelletier GB, Waters DD. A randomized study comparing propranolol and diltiazem in the treatment of unstable angina. J Am Coll Cardiol. 1985;5(3):717-722 [DOI] [PubMed] [Google Scholar]
- 103.Rizik D, Timmis GC, Grines CL, Bakalyar D, Schreiber T. Immediate use of beta blockers, but not calcium blockers, improves prognosis in unstable angina [abstract]. Circulation 1991;84(suppl. II):II-345 [Google Scholar]
- 104.The Multicenter Diltiazem Postinfarction Trial Research Group The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med. 1988;319(7):385-392 [DOI] [PubMed] [Google Scholar]
- 105.The Danish Study Group on Verapamil in Myocardial Infarction Verapamil in acute myocardial infarction. Eur Heart J. 1984;5(7):516-528 [PubMed] [Google Scholar]
- 106.Pepine CJ, Faich G, Makuch R. Verapamil use in patients with cardiovascular disease: an overview of randomized trials. Clin Cardiol. 1998;21(9):633-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gibson RS, Boden WE, Theroux P, et al. Diltiazem and reinfarction in patients with non-Q wave myocardial infarction: results of a double-blind, randomized, multicenter trial. N Engl J Med. 1986;315(7):423-429 [DOI] [PubMed] [Google Scholar]
- 108.Wilcox RG, Hampton JR, Banks DC, et al. Trial of early nifedepine in acute myocardial infarction: the Trent study. Br Med J (Clin Res Ed) 1986;293(6556):1204-1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 2004;292(18):2217-2225 [DOI] [PubMed] [Google Scholar]
- 110.Jamerson K, Weber MA, Bakris GL, et al. ACCOMPLISH Trial Investigators Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417-2428 [DOI] [PubMed] [Google Scholar]
- 111.ISIS-4 Collaborative Group ISIS-4: randomized factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet 1995;345(8951):669-685 [PubMed] [Google Scholar]
- 112.Chinese Cardiac Study Collaborative Group Oral captopril versus placebo among 13,634 patients with suspected myocardial infarction: interim report from the Chinese Cardiac Study (CCS-1). Lancet 1995;345(8951):686-687 [PubMed] [Google Scholar]
- 113.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan in Acute Myocardial Infarction Trial Investigators Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both [published correction appears in N Engl J Med. 2004;350(2):203] N Engl J Med. 2003November13;349(20):1893-1906 Epub 2003 Nov 10 [DOI] [PubMed] [Google Scholar]
- 114.Gustafsson I, Torp-Pedersen C, Køber L, Gustafsson F, Hildebrandt P, Trace Study Group Effect of the angiotensin-converting enzyme inhibitor trandolapril on mortality and morbidity in diabetic patients with left ventricular dysfunction after acute myocardial infarction. J Am Coll Cardiol. 1999;34(1):83-89 [DOI] [PubMed] [Google Scholar]
- 115.Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients [published corrections appear in N Engl J Med. 2000;342(18):1376 and 2000;342(10):748] N Engl J Med. 2000;342:145-153 [DOI] [PubMed] [Google Scholar]
- 116.Pitt B, Remme W, Zannad F, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction [published correction appears in N Engl J Med. 2003 Apr 3;348(22):2271] N Engl J Med. 2003;348(14):1309-1321 Epub 2003 Mar 31 [DOI] [PubMed] [Google Scholar]
- 117.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Skene A, McCabe CH, Braunwald E, MERLIN-TIMI 36 Investigators Evaluation of a novel anti-ischemic agent in acute coronary syndromes: design and rationale for the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-elevation acute coronary syndromes (MERLIN)-TIMI 36 trial. Am Heart J. 2006;151(6):1186.e1-1186.e9 [DOI] [PubMed] [Google Scholar]
- 118.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. MERLIN-TIMI 36 Investigators Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA 2007;297(16):1775-1783 [DOI] [PubMed] [Google Scholar]
- 119.Cairns JA, Gent M, Singer J, et al. Aspirin, sulfinpyrazone, or both in unstable angina; results of a Canadian multicenter trial. N Engl J Med. 1985;313(22):1369-1375 [DOI] [PubMed] [Google Scholar]
- 120.RISC Group Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. Lancet 1990;336(8719):827-830 [PubMed] [Google Scholar]
- 121.CURE Study Investigators The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J. 2000;21(24):2033-2041 [DOI] [PubMed] [Google Scholar]
- 122.Mehta SR, Yusuf S, Peters RJ, et al. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358(9281):527-533 [DOI] [PubMed] [Google Scholar]
- 123.Sabatine MS, Cannon CP, Gibson CM, et al. Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005September14;294(10):1224-1232 Epub 2005 Sep 4 [DOI] [PubMed] [Google Scholar]
- 124.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention-Summary Article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervent ion). J Am Coll Cardiol. 2006;47(1):216-235http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=16386696&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 125.King SB, III, Smith SC, Jr, Hirshfeld JW, Jr, et al. 2007 Focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2008 Jan 15;117(6):e161] Circulation 2008;117(2):261-295 Epub 2007 Dec 13 [DOI] [PubMed] [Google Scholar]
- 126.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) [published correction appears in Circulation. 2005;111(15):2014] Circulation 2004;110(9):1168-1176 [DOI] [PubMed] [Google Scholar]
- 127.Wiviott SD, Trenk D, Frelinger AL, et al. PRINCIPLE-TIMI 44 Investigators Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007December18;116(25):2923-2932 Epub 2007 Dec 3 [DOI] [PubMed] [Google Scholar]
- 128.Wiviott SD, Braunwald E, McCabe CH, et al. TRITON-TIMI 38 Investigators Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007November15;357(20):2001-2015 Epub 2007 Nov 4 [DOI] [PubMed] [Google Scholar]
- 129.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. [epub before print.]. N Engl J Med. 2009;361 [DOI] [PubMed] [Google Scholar]
- 130.Boersma E, Harrington RA, Moliterno DJ, et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials [published correction appears in Lancet. 2002;359(9323):2120] Lancet 2002;359(9302):189-198 [DOI] [PubMed] [Google Scholar]
- 131.Klootwijk P, Meij S, Melkert R, Lenderink T, Simoons ML. Reduction of recurrent ischemia with abciximab during continuous ECG-ischemia monitoring in patients with unstable angina refractory to standard treatment (CAPTURE). Circulation 1998;98(14):1358-1364 [DOI] [PubMed] [Google Scholar]
- 132.Alexander JH, Harrington RA, Tuttle RH, et al. PURSUIT Investigators Prior aspirin use predicts worse outcomes in patients with non-ST-elevation acute coronary syndromes. Am J Cardiol. 1999;83(8):1147-1151 [DOI] [PubMed] [Google Scholar]
- 133.Kastrati A, Mehilli J, Neumann FJ, et al. Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006April;295(13):1531-1538 Epub 2006 Mar 13 [DOI] [PubMed] [Google Scholar]
- 134.Giugliano RP, White JA, Bode C, et al. EARLY ACS Investigators Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. 2009May21;360:2176-2190 Epub 2009 Mar 30 [DOI] [PubMed] [Google Scholar]
- 135.Théroux P, Waters D, Qiu S, McCans J, de Guise P, Juneau M. Aspirin versus heparin to prevent myocardial infarction during the acute phase of unstable angina. Circulation 1993;88(5, pt 1):2045-2048 [DOI] [PubMed] [Google Scholar]
- 136.Rich JD, Maraganore JM, Young E, et al. Heparin resistance in acute coronary syndromes. J Thromb Thrombolysis 2007April;23(2):93-100 Epub 2007 Jan 13 [DOI] [PubMed] [Google Scholar]
- 137.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001;135(7):502-506 [DOI] [PubMed] [Google Scholar]
- 138.Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332(20):1330-1335 [DOI] [PubMed] [Google Scholar]
- 139.Antman EM, Cohen M, Radley D, et al. Assessment of the treatment effect of enoxaparin for unstable Angina/Non-Q-wave myocardial infarction: TIMI 11B-ESSENCE meta-analysis. Circulation 1999;100(15):1602-1608 [DOI] [PubMed] [Google Scholar]
- 140.Ferguson JJ, Califf RM, Antman EM, et al. SYNERGY Trial Investigators Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 2004;292(1):45-54 [DOI] [PubMed] [Google Scholar]
- 141.de Lemos JA, Blazing MA, Wiviott SD, et al. A to Z Investigators Enoxaparin versus unfractionated heparin in patients treated with tirofiban, aspirin and an early conservative initial management strategy: results from the A phase of the A-to-Z trial. Eur Heart J. 2004;25(19):1688-1694 [DOI] [PubMed] [Google Scholar]
- 142.Antman EM, McCabe CH, Gurfinkel EP, et al. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction: results of the Thrombolysis In Myocardial Infarction (TIMI) 11B trial. Circulation 1999;100(15):1593-1601 [DOI] [PubMed] [Google Scholar]
- 143.de Lemos JA, Rifai N, Morrow DA, et al. Elevated baseline myoglobin is associated with increased mortality in acute coronary syndromes, even among patients with normal baseline troponin I: a TIMI 11B substudy. Circulation 1999;100(suppl I):I372-I373 [Google Scholar]
- 144.Stone GW, McLaurin BT, Cox DA, et al. ACUITY Investigators Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355(21):2203-2216 [DOI] [PubMed] [Google Scholar]
- 145.Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006April6;354(14):1464-1476 Epub 2006 Mar 14 [DOI] [PubMed] [Google Scholar]
- 146.Organization to Assess Strategies for Ischemic Syndromes (OASIS) Investigators Effects of long-term, moderate-intensity oral anticoagulation in addition to aspirin in unstable angina. J Am Coll Cardiol. 2001;37(2):475-484 [DOI] [PubMed] [Google Scholar]
- 147.van Es RF, Jonker JJC, Verheugt FWA, Deckers JW, Grobbee DE, Antithrombotics in the Secondary Prevention of Events in Coronary Thrombosis-2 (ASPECT-2) Research Group Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet 2002;360(9327):109-113 [DOI] [PubMed] [Google Scholar]
- 148.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969-974 [DOI] [PubMed] [Google Scholar]
- 149.Grundy SM, Cleeman JI, Bairey Merz CN, et al. Coordinating Committee of the National Cholesterol Education Program Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines [published correction appears in Circulation. 2004;110(6):763] Circulation 2004;110(2):227-239 [DOI] [PubMed] [Google Scholar]
- 150.Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357 [DOI] [PubMed] [Google Scholar]
- 151.Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators Intensive versus moderate lipid lowering with statins after acute coronary syndromes [published correction appears in N Engl J Med. 2006;354(7):778] N Engl J Med. 2004April8;350(15):1495-1504 Epub 2004 Mar 8 [DOI] [PubMed] [Google Scholar]
- 152.Ridker PM, Cannon CP, Morrow D, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22. (PROVE IT-TIMI 22) Investigators C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20-28 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.