It has been said that retirement consists of going from “Who's who?” to “Who's he?” My invitation to contribute to the Journal of Biological Chemistry (JBC) Reflections is an honor and timely as well in that as an emeritus professor, I have the time and an opportunity to beat the clock to “Who was he?” In a Venn diagram of biological sciences in which circles representing biochemistry and neuroscience overlap, the lens-shaped area can be defined as neurochemistry or, more recently, as molecular neuroscience and best describes my research area. My career in neuroscience is summarized in an autobiographical series recently published under the auspices of the Society for Neuroscience (1). In it, I detail my research on neuroplasticity, the ability of the nervous system to undergo change as a result of external inputs. This includes interventive studies on the role of protein synthesis in memory formation and correlative studies on optic nerve regeneration, both in teleost fish (1). This JBC Reflections article encapsulates my biochemical career, with emphasis on the neurochemical significance of myo-inositol, one of nine positional isomers of hexahydroxycyclohexane, and its phosphates.
My early years are described in Ref. 1. Briefly, I was born in Detroit in 1926, graduated from Cass Technical High School in 1944, entered a Navy premedical officer training program at the University of Michigan, and received an M.D. from Wayne State Medical School. I cannot resist sharing a recollection. In 1946, as a 20-year-old freshman medical student, I found myself surrounded by returning World War II veterans who were not only older than I, but understandably a bit rustier in college chemistry. In our freshman biochemistry class, I volunteered to go to the blackboard to derive and discuss the Henderson-Hasselbach equation. I returned to my seat, satisfied that I had done a clear and concise job. The 30- or so year-old returning veteran sitting next to me whispered, “Are you going to end up as a biochemistry professor?” Fishing for a compliment, I said, “No, why?” He answered, “The way you got right up there and mumbled into the blackboard, you just can't fake that!” He became a psychiatrist, and I, a biochemist. I interned in Sayre, PA, and had a postdoctoral fellowship with F. O. Schmitt in the Department of Biology at the Massachusetts Institute of Technology, until I was recalled by the Navy for a 2-year stint at the National Naval Medical Center in Bethesda, after which I moved to the nearby National Institutes of Health (NIH) in 1952.
The “PI Effect”
Now a research scientist in the Section of Lipid Chemistry in the intramural program of the National Institute of Neurological Diseases and Blindness (NINDB, presently NINDS) and searching for a suitable research problem, I was influenced by the reports of Mabel and Lowell Hokin that 32Pi incorporation into phospholipids in pigeon pancreas and guinea pig brain slices was stimulated by the presence of carbamylcholine and was blocked in the presence of atropine (2). The labeled lipids were identified as phosphatidic acid (PtdOH, PA) and phosphatidylinositol (PtdIns, PI). At the time, there was some question as to the relevance of the finding because maximal stimulation required excessively high (millimolar) concentrations of carbamylcholine. Nevertheless, 10 μm atropine was sufficient to block the effect. Another potential drawback was the then prevalent view that PA was an uninteresting lipid because it was best known as a degradation product of the action of cabbage phospholipase D on phospholipids. Even so, it had already been shown by this time that PA could be biosynthesized from long-chain acyl-CoA and sn-glycerol 3-phosphate (3). I was additionally drawn to the Hokins' findings of the involvement of PI by virtue of its phosphorylated homologs (4), later to be identified as PI(4)P and PI(4,5)P2 (5, 6). Their prominence in brain suggested a possible relevance of the stimulated labeling to nerve function. Eugene Kennedy's laboratory had at that time discovered the critical role of cytidine nucleotide derivatives in the biosynthesis of phosphatidylcholine and phosphatidylethanolamine (7). The biosynthetic pathways for PI remained unknown. Could there be a CDP-inositol analogous to CDP-choline and CDP-ethanolamine that reacted with 1,2-diacylglycerol to form PI? Around this time, I learned that Roy Bradley, a former Navy laboratory technician, was now a civilian and looking for employment, and I succeeded in getting him hired as my technician. He was a superb associate, and we worked together for my remaining 4 years at NIH. I began to look for the appearance of radiolabeled acidic products of tissue slices and homogenates following incubation with 3H-labeled inositol, searching for an acidic product that might be the predicted CDP-inositol. Guinea pig kidney homogenates produced a labeled acidic substance from 3H-labeled inositol that looked promising. Just at that time, an abstract appeared in the upcoming FASEB (Federation of American Societies for Experimental Biology) meeting proceedings, eventually published in JBC (8), in which Frixos Charalampous reported the oxidation of myo-inositol into glucuronic acid in kidney preparations. Recognizing that I had been pursuing a dead end, I switched to seeking other products of [3H]CMP. I soon found labeled lipid-soluble material in incubated guinea pig kidney preparations that did not accumulate in the presence of added inositol. The deacylation product of the labeled lipid co-migrated upon chromatography with known CDP-glycerol, and on that basis, I proposed the existence of a novel substance, CDP-diacylglycerol (CDP-DG), coined a “liponucleotide,” as the precursor of PI (9).
I put the PI project on hold at this in order to spend a year in the laboratory of Feodor Lynen, at the Max Planck Institute for Cell Chemistry in Munich, to participate in his exciting search to unravel the biosynthesis of cholesterol (10, 11). It was truly a memorable and productive experience. I was able during the year to meet at the University of Geneva with Theodore Posternak, who was a recognized authority on cyclitols and whom I had known through his encyclopedic treatise (12). During that year, Paulus and Kennedy synthesized CDP-diacylglycerol chemically and confirmed the biosynthetic step in studies using a chicken liver microsomal preparation (13).
During my NIH years, I collaborated with Harry Eagle on inositol biosynthesis in cultured cell lines (14), with John Burns' laboratory on inositol conversion to l-gulonate in rat liver (15), and with Spivey Fox on competition between dietary inositol and choline in growing chicks (16).
Back to Ann Arbor
In 1960, I accepted an offer from the University of Michigan for a joint appointment in the Department of Biological Chemistry and the Mental Health Research Institute, where my laboratory space was located. There were a number of attractions to the new position, not least of which was the opportunity to retain my identity as a biochemist and at the same time to pursue a growing interest in biochemical aspects of brain function. Thus began a dual research career and an unwillingness to give up either of my interests. I have urged my students and postdoctoral students to avoid the situation in which unrelated projects coexist in one's laboratory. Nevertheless, I probably would do it over, unable to resist the lure of each of the two disparate research areas (1).
Together with biochemistry graduate students, we perfected a chemical synthesis of CDP-DG (17), demonstrated its biosynthesis in embryonic chick brain particulates from CTP and PA (18), and established in a guinea pig brain microsomal preparation an enzymatic preference for myo-inositol in PI synthesis over the other cyclitols (19). We returned to CDP-DG many years later to clone the human gene (20) for the synthase after it had been demonstrated in Drosophila. Thought to be a substrate-limiting step in the PI cycle in signal transmission, its formation remains a possible site for pharmaceutical intervention.
Our interest in inositol phosphates led to the development of a low-pH high-voltage paper electrophoretic (HVE) separation technique (21). We separated a partial hydrolysate of plant IP6 (phytate) into multiple components using sodium oxalate buffer at pH 1.5, visualized with a phosphomolybdate spray. A slowly migrating band was seen for Pi as well as a rapid one for unhydrolyzed phytate. Between them were distinct regions for inositol monophosphate as well as for inositol di-, tri-, tetrakis-, and pentakisphosphates, within which could be seen partial separation of positional isomers. This later proved useful for us in many ways, but at the time, the HVE method was picked up primarily by soil chemists, who have noted that the inositol esters likely comprise the major organophosphorus mass on Earth (22). Many years later, in working out a gas chromatographic technique for inositol phosphates with my technician Ed Seguin, I used an aqueous methanolysis product of PIP2 that co-migrated upon HVE with one of the inositol 3-phosphate (Ins(3)P)) bands of the phytate hydrolysate and surmised it to be Ins(1,4,5)P3 (23). The biological activity of this preparation was eventually confirmed (M. J. Berridge, personal communication). In examining 32Pi-labeled mitochondrial lipid hydrolysates by HVE, Amiya Hajra and I were led to a novel intermediate, acyldihydroxyacetone phosphate, providing an alternate biosynthetic pathway to PA formation that bypasses sn-3-glycerophosphate (25, 26). Hajra later demonstrated that acyldihydroxyacetone phosphate is the precursor of the alkenylacyl and alkylacyl phospholipids (27).
Much of our work in which the PI effect was studied in brain utilized an isolated nerve-ending preparation (synaptosomes). Using this preparation, we were able to identify the form of the muscarinic cholinergic receptor that coupled to increased phosphoinositide turnover in response to carbamylcholine (28). Unexpectedly, even though it had been widely assumed that the increases in inositol lipid turnover occurred within presynaptic structures, results from hippocampal nerve-lesioning experiments conducted by Steve Fisher and Kirk Frey demonstrated that dendrite-derived structures present in the preparation were likely responsible for the observed changes. Thus, in common with other non-neural tissues, the PI effect was shown to be associated predominantly with activation of receptors on postsynaptic structures (29). Although we had characterized the nature of the muscarinic receptor binding and its relationship with the PI effect, we had made little progress in unraveling a connection with cellular responses, such as secretion and neurotransmission. When we examined the effect of thrombin on 32P-labeled platelets, we found a decrease in labeling of PIP2 and an increase in the appearance of radiolabeled IP3. Whereas more label was associated with IP2, the largest increases in labeling following thrombin addition were observed for IP3. We concluded that there was a labeling cycle and that stimulation by the thrombin occurred at the level of PIP2 cleavage (30). Later that year and 30 years after the Hokins initiated their studies, Streb, Irvine, Berridge, and Schultz answered the question of the significance of the PI effect by demonstrating that I(1,4,5)P3 is the second messenger that leads to intracellular Ca2+ release and to various physiological cell responses (31). During the long interim between the Hokins' identification of PA and PI as the lipids in the stimulated labeling (2) and elucidation of the inositol lipid-related signal transduction process (31), it can be seen through the “retroscope” that many investigators had solved bits and pieces of the PI cycle puzzle along the way, including the Hokins' work on diglyceride kinase, the work of our laboratory and Kennedy's on CDP-DG, and evidence of ligand-stimulated breakdown of PIP2 (30, 33–35). Michell had long proposed the existence of a link between PI labeling and elevated cellular Ca2+ (36). The role in signal transduction for activation of protein kinases by diacylglycerol, which, like Ins(1,4,5)P3, is produced in the cleavage of PI(4,5)P2, had been demonstrated by the Nishizuka laboratory (37). An additional major chapter in the myriad roles of inositol phosphates arose from the discovery of PI(3)P (38) and eventually many additional phosphoinositides: PI(3,4)P2, PI(4,5)P2, PI(3,5)P2, and PI(3,4,5)P3 (39).
Inositol Phosphates and the Turtle
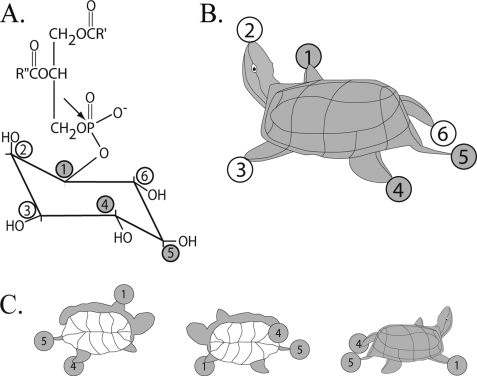
There are 63 possible phosphomonoesters of myo-inositol (6 each of IP1 and IP5, 15 each of IP2 and IP4, 20 of IP3, and IP6). About half have been identified thus far in eukaryotes. Confusion in numbering and in depicting the Haworth projections of the six hydroxyls is mitigated by use of a three-dimensional visual mnemonic (40) in the form of a turtle, in which the axial hydroxyl is its head, and the five equatorial hydroxyls serve as forelimbs, hind limbs, and the tail, as illustrated in the Fig. 1. Using the d numbering convention, one proceeds counterclockwise, from above. The hydroxyl of myo-inositol that is diesterified to diacylglycerol is at carbon d1, the right forelimb. The axial hydroxyl serves as the turtle's head at d2, and the left forelimb represents carbon d3, moving on to the left hind limb (d4), the tail (d5), and the right hind limb (d6). The less used l numbering system begins at the turtle's left forelimb (l1) and proceeds clockwise with the head at l2 and the right forelimb at l3, etc. The product of the action of inositol synthase on glucose 6-phosphate can thus be termed myo-inositol d3 monophosphate or myo-inositol l1 monophosphate.
FIGURE 1.
“The turtle.” A, PI(4,5)P2 and its cleavage at hydroxyl D1 of inositol by phospholipase C; B, I(1,4,5)P3 depicted as a turtle; C, views of the turtle from different vantage points (modified from Ref. 41). Use of the turtle is suggested by the International Union of Biochemistry Nomenclature Committee (42).
IP6 through IP9
Phytate (IP6), a major component of plant seeds, was named phytin in 1903 by S. Posternak, father of Theodore Posternak. Phytate was removed from grain flour in the 1920s on the basis that it was “rachitogenic,” that is, by binding dietary calcium, could cause or aggravate rickets, a disease of bone malformation in growing children in the pre-vitamin D supplementation days. The disease is now rare, and there are some who now praise phytate's virtues; for example, it binds dietary iron and thereby acts as an antioxidant. Its multivalent ionic environment may affect its solubility and chelating ability (43). Phytate and I(1,3,4,5,6)P5 that additionally have varying amounts of pyrophosphates on the d1, d3, or d5 position are referred to as IP7 and IP8, and there is even an IP9. At present, 15 of these pyrophosphorylated inositol phosphates been identified as enzyme products (44). Some have been shown to phosphorylate protein, and their possible physiological significance is being actively explored (45, 46).
Brain Inositol and Lithium
Free inositol in mammalian brains is ∼6 mm, serving as a non-ionic osmolyte (47). Scyllitol, with all six of its hydroxyls equatorial, is present in brain at a level about one-tenth that of the myo-isomer. Although enzymes can oxidize myo- and scyllo-inositol to the 2-inosose and interconvert them via reduction, the origin of scyllitol in brain is thought to be dietary. d-myo-Inositol 3-phosphate can be synthesized from glucose 6-phosphate by a synthase and cleaved to free inositol in the meningeal chorioid bodies (48), but inositol enters the brain principally via an active transport system. The enzyme is blocked by Li+, and this proved to be very useful in the experiments that helped define the phosphoinositide cycle (32). Lithium (LiCl or Li2CO3) is widely prescribed for the treatment of bipolar psychiatric disease, even though it is not a profitable pharmaceutical, and its mechanism(s) of therapeutic action is not well understood (49). The possibility that the therapeutic action of Li+ is related to its block of inositol monophosphatase led to the search for an effective drug that, like lithium, would block the monophosphatase but lack some of the side effects of Li+. These efforts have not proven successful thus far (50).
Inositol, a Prebiotic Molecule?
Taking advantage of the disinhibiting effect of an invitation to reflect, I offer a speculation: that terrestrial inositol preceded the existence of living organisms. A number of investigators who study the origin of living matter have addressed the question as to when inositol and relevant enzymes for its synthesis and utilization appeared during evolution (51, 52). I propose that cyclitols, as has been proposed for amino acids (53), be considered prebiotic “Ur” molecules (in the sense that they may have preceded the existence of living matter, not that they are self-replicating). My hypothesis harks back to the formose reaction, reported by Butlerow in 1861 (54), in which formaldehyde in the presence of aqueous calcium hydroxide produced a high yield of a syrupy sugar-like material, rich in dl-ketohexoses. Emil Fischer's source of reducing sugars for his famous crystallization of their phenylhydrazine osazones was formose reaction products. The possibility that photosynthesis was mediated by reduction of carbon dioxide to formaldehyde was later investigated and abandoned, as were many unsuccessful attempts over the years to produce nutritional products from formaldehyde or glycolaldehyde (55). The possible role of carbohydrates arising from formose, specifically ribose, as a prebiotic molecule has been proposed (56) and criticized based on the conclusion that it would not be sufficiently stable over long time periods thought to be required for the emergence of living matter (57). Unlike reducing sugars or acyclic polyols, myo-inositol is remarkably stable to degradation by temperature or oxidation. Its stability was in fact the basis of a chemical method for determination of inositol in tissue, in which other polyols such as glycerol were oxidized by periodate, whereas inositol oxidation required a much higher pH and temperature (9). Could then inositol, a most stable carbohydrate, have sat by, waiting patiently over the aeons, as a prebiotic energy source and/or building block? Cyclitols have not been reported as products of the formose reaction, but the presence of polyols including inositols has been reported in meteorites (58), lending credence to this hypothesis. In addition, it has been proposed that inositol lipid harks back to early beginnings in the origin of life (52). Pyrophosphates must certainly have been present prebiotically as well (24), and hexose phosphoesterification could have facilitated cyclization to cyclitol phosphates. The prebiotic existence of inositol phosphates thus could have preceded that of free inositols. It may have been “turtles all the way”: up (alluding to an ancient cosmological belief that the world rests on a giant turtle that in turn rests on a still larger turtle, that “it's turtles all the way down”; folk lore cited by William James, Thomas Huxley, Bertrand Russell, and more recently by Stephen Hawking (59)).
Acknowledgment
I thank my wife, Raquel (Ricky), whom I met and married in 1957, for lives shared.
REFERENCES
- 1.Agranoff B. W. (2008) in History of Neuroscience in Autobiography (Squire L. R. ed) Vol. 6, pp. 1–31, Oxford University Press, New York [Google Scholar]
- 2.Hokin L. E., Hokin M. R. (1955) Effects of acetylcholine on the turnover of phosphoryl units in individual phospholipids of pancreas slices and brain cortex slices. Biochim. Biophys. Acta 18, 102–110 [DOI] [PubMed] [Google Scholar]
- 3.Kornberg A., Pricer W. E., Jr. (1953) Enzymatic esterification of α-glycerophosphate by long chain fatty acids. J. Biol. Chem. 204, 345–357 [PubMed] [Google Scholar]
- 4.Folch J. (1949) Brain phosphoinositide, a new phosphatide having inositol metadiphosphate as a constituent. J. Biol. Chem. 177, 505–519 [PubMed] [Google Scholar]
- 5.Dittmer J. C., Dawson R. M. (1961) The isolation of a new lipid, triphosphoinositide, and monophosphoinositide from ox brain. Biochem. J. 81, 535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grado C., Ballou C. E. (1961) Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J. Biol. Chem. 236, 54–60 [PubMed] [Google Scholar]
- 7.Kennedy E. P., Weiss S. B. (1956) The function of cytidine coenzymes in the biosynthesis of phospholipids. J. Biol. Chem. 222, 193–214 [PubMed] [Google Scholar]
- 8.Charalampous F. C., Lyras C. (1957) Biochemical studies on inositol. IV. Conversion of inositol to glucuronic acid by rat kidney extracts. J. Biol. Chem. 228, 1–13 [PubMed] [Google Scholar]
- 9.Agranoff B. W., Bradley R. M., Brady R. O. (1958) The enzymatic synthesis of inositol phosphatide. J. Biol. Chem. 233, 1077–1083 [PubMed] [Google Scholar]
- 10.Agranoff B. W., Eggerer H., Henning U., Lynen F. (1959) Isopentenolpyrophosphate isomerase. J. Am. Chem. Soc. 81, 1254 [Google Scholar]
- 11.Agranoff B. W., Eggerer H., Henning U., Lynen F. (1960) Biosynthesis of terpenes. VII. Isopentenyl pyrophosphate isomerase. J. Biol. Chem. 235, 326–332 [PubMed] [Google Scholar]
- 12.Posternak T. (1965) The Cyclitols, Holden-Day, Inc., San Francisco [Google Scholar]
- 13.Paulus H., Kennedy E. P. (1960) The enzymatic synthesis of inositol monophosphatide. J. Biol. Chem. 235, 1303–1311 [PubMed] [Google Scholar]
- 14.Eagle H., Agranoff B. W., Snell E. E. (1960) The biosynthesis of meso-inositol by cultured mammalian cells, and the parabiotic growth of inositol-dependent and inositol-independent strains J. Biol. Chem. 235, 1891–1893 [PubMed] [Google Scholar]
- 15.Burns J. J., Trousof N., Evans C., Papadopoulos N., Agranoff B. W. (1959) Conversion of myo-inositol to D-glucuronic acid and L-gulonic acid in the rat. Biochim. Biophys. Acta 33, 215–219 [DOI] [PubMed] [Google Scholar]
- 16.Agranoff B. W., Fox M. R. S. (1959) Antagonism of choline and inositol. Nature 183, 1259–1260 [DOI] [PubMed] [Google Scholar]
- 17.Agranoff B. W., Suomi W. (1963) Cytidine diphosphate-DL-dipalmitin. Biochem. Prep. 10, 47–51 [Google Scholar]
- 18.Petzold G. L., Agranoff B. W. (1967) The synthesis of cytidine diphosphate diglyceride by embryonic chick brain. J. Biol. Chem. 242, 1187–1191 [PubMed] [Google Scholar]
- 19.Benjamins J. A., Agranoff B. W. (1969) Distribution and properties of CDP-diglyceride:inositol transferase from brain. J. Neurochem. 16, 513–527 [DOI] [PubMed] [Google Scholar]
- 20.Heacock A. M., Uhler M. D., Agranoff B. W. (1996) Cloning of CDP-diacylglycerol synthase from a human neuronal cell line. J. Neurochem. 67, 2200–2203 [DOI] [PubMed] [Google Scholar]
- 21.Seiffert U. B., Agranoff B. W. (1965) Isolation and separation of inositol phosphates from hydrolysates of rat tissues. Biochim. Biophys. Acta 98, 574–581 [DOI] [PubMed] [Google Scholar]
- 22.Cosgrove D. J. (1980) Inositol Phosphates, p. 15, Elsevier Science Publishers B.V., Amsterdam [Google Scholar]
- 23.Agranoff B. W., Seguin E. B. (1974) Preparation of inositol triphosphate from brain: GLC of trimethylsilyl derivative. Prep. Biochem. 4, 359–366 [DOI] [PubMed] [Google Scholar]
- 24.Kornberg A. (1994) Inorganic polyphosphate: a molecular fossil come to life. In: Phosphate in Microorganisms: Cellular and Molecular Biology (Torriani-Gorini A., Silver S., Yagil E. eds) American Society for Microbiology, Washington, D. C. [Google Scholar]
- 25.Hajra A. K., Agranoff B. W. (1968) Acyl dihydroxyacetone phosphate. Characterization of a 32P-labeled lipid from guinea pig liver mitochondria. J. Biol. Chem. 243, 1617–1622 [PubMed] [Google Scholar]
- 26.Agranoff B. W., Hajra A. K. (1971) The acyldihydroxyacetone phosphate pathway for glycerolipid biosynthesis in mouse liver and Ehrlich ascites tumor cells. Proc. Natl. Acad. Sci. U.S.A. 68, 411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis P. A., Hajra A. K. (1979) Stereochemical specificity of the biosynthesis of the alkyl ether bond in ether lipids. J. Biol. Chem. 254, 4760–4763 [PubMed] [Google Scholar]
- 28.Fisher S. K., Klinger P. D., Agranoff B. W. (1983) Muscarinic agonist binding and phospholipid turnover in brain. J. Biol. Chem. 258, 7358–7363 [PubMed] [Google Scholar]
- 29.Fisher S. K., Frey K. A., Agranoff B. W. (1981) Loss of muscarinic receptors and of stimulated phospholipid labeling in ibotenate-treated hippocampus. J. Neurosci. 12, 1407–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agranoff B. W., Murthy P., Seguin E. B. (1983) Thrombin-induced phosphodiesteratic cleavage of phosphatidyl inositol bisphosphate in human platelets. J. Biol. Chem. 258, 2076–2078 [PubMed] [Google Scholar]
- 31.Streb H., Irvine R. F., Berridge M. J., Schultz I. (1983) Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306, 67–69 [DOI] [PubMed] [Google Scholar]
- 32.Berridge M. J. (2005) Unlocking the secrets of cell signaling. Annu Rev. Physiol. 67, 1–21 [DOI] [PubMed] [Google Scholar]
- 33.Durell J., Garland J. T., Friedel R. O. (1969) Acetylcholine action: biochemical aspects. Science 65, 861–864 [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Latif A. A., Akhtar R. A., Hawthorne J. N. (1977) Acetylcholine increases the breakdown of triphosphoinositide of rabbit iris muscle prelabelled with [32P] phosphate. Biochem. J. 162, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher S. K., Agranoff B. W. (1981) Enhancement of the muscarinic synaptosomal phospholipid labeling effect by the ionophore A23187. J. Neurochem. 37, 968–977 [PubMed] [Google Scholar]
- 36.Michell R. H. (1975) Inositol phospholipids and cell surface receptor function. Biochim. Biophys. Acta 415, 81–147 [DOI] [PubMed] [Google Scholar]
- 37.Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. (1980) Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J. Biol. Chem. 255, 2273–2276 [PubMed] [Google Scholar]
- 38.Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. (1988) Type 1 phosphatidylinositol kinase makes a novel inositol lipid, phosphatidylinositol-3-phosphate. Nature 332, 644–646 [DOI] [PubMed] [Google Scholar]
- 39.Cantley L. C. (2007) From kinase to cancer. The Scientist 21, 44–49 [Google Scholar]
- 40.Agranoff B. W. (1978) Cyclitol confusion. Trends Biochem. Sci. 3, N283–N285 [Google Scholar]
- 41.Agranoff B. W., Fisher S. K. (1991) Phosphoinositides and their stimulated breakdown in inositol phosphates and derivatives: synthesis, biochemistry, and therapeutic potential. Am. Chem. Soc. Symp. Ser. 463, 20–32 [Google Scholar]
- 42.Nomenclature Committee of the International Union of Biochemistry ( 1989) Numbering of atoms in myo-inositol. Biochem. J. 258, 1–2 [PMC free article] [PubMed] [Google Scholar]
- 43.Irvine R. F. (2005) Inositide evolution-toward turtle domination? J. Physiol. 566, 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Draskovic P., Saiardi A., Bhandari R., Burton A., Ilc G., Kovacevic M., Snyder S. H., Podobnik M. (2008) Inositol hexaphosphate kinase products contain diphosphate and triphosphate groups. Chem. Biol. 15, 274–386 [DOI] [PubMed] [Google Scholar]
- 45.Mulugu S., Bai W., Fridy P. C., Bastidas R. J., Otto J. C., Dollins D. E., Haystead T. A., Ribeiro A. A., York J. D. (2007) A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science 316, 106–109 [DOI] [PubMed] [Google Scholar]
- 46.Shears S. B. (2001) Assessing the omnipotence of inositol hexaphosphate. Cell. Signal. 13, 151–158 [DOI] [PubMed] [Google Scholar]
- 47.Fisher S. K., Novak J. E., Agranoff B. W. (2002) Inositol and inositol phosphates in neural tissues: homeostasis, metabolism, and functional significance. J. Neurochem. 82, 734–754 [DOI] [PubMed] [Google Scholar]
- 48.Wong Y. H., Kalmbach S. J., Hartman B. K., Sherman W. R. (1987) Immunohistochemical staining and enzyme activity measurements show myo-inositol-1-phosphate synthase to be localized in the vasculature of brain. J. Neurochem. 48, 1434–1442 [DOI] [PubMed] [Google Scholar]
- 49.Agranoff B. W., Fisher S. K. (2001) Inositol, lithium, and the brain. Psychopharmacol. Bull. 35, 5–18 [PubMed] [Google Scholar]
- 50.Atack J. R. (1996) Inositol monophosphatase, the putative therapeutic target for lithium. Brain Res. Rev. 2, 183–190 [PubMed] [Google Scholar]
- 51.Majumder A. L., Chatterjee A., Dastidar K. G., Majee M. (2003) Diversification and evolution of L-myo-inositol 1-phosphate synthase. FEBS Lett. 553, 3–10 [DOI] [PubMed] [Google Scholar]
- 52.Michell R. H. (2008) Inositol derivatives: evolution and functions. Nat. Rev. Mol. Cell Biol. 9, 151–161 [DOI] [PubMed] [Google Scholar]
- 53.Miller S. L. (1953) Production of amino acids under possible primitive earth conditions. Science 117, 528–529 [DOI] [PubMed] [Google Scholar]
- 54.Butlerow A. (1861) Formation synthetique d'une substance sucree. C. R. Acad. Sci. 53, 145–147 [Google Scholar]
- 55.Mizuno T., Weiss A. H. (1974) Synthesis and utilization of formose sugars. Adv. Carbohydr. Chem. Biochem. 29, 173–227 [Google Scholar]
- 56.Springsteen G., Joyce G. F. (2004) Selective derivatization and sequestration of ribose from a prebiotic mix. J. Am. Chem. Soc. 126, 9578–9583 [DOI] [PubMed] [Google Scholar]
- 57.Larrade R., Robertson M. P., Miller S. L. (1995) Rates of decomposition of ribose and other sugars: implications for chemical evolution. Proc. Natl. Acad. Sci. U.S.A. 92, 8158–8160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cooper G., Kimmich N., Belisle W., Sarinana J., Brabham K., Garrel L. (2001) Carbonaceous meteorites as a source of sugar-related organic compounds for the early earth. Nature 414, 879–883 [DOI] [PubMed] [Google Scholar]
- 59.Hawking S. (1988) A Brief History of Time, Bantam Books, New York [Google Scholar]