Abstract
In this study, murine peritoneal macrophages from naïve lavage were found to generate four phospholipids that contain 12-hydroxyeicosatetraenoic acid (12-HETE). They comprise three plasmalogen and one diacyl phosphatidylethanolamines (PEs) (16:0p, 18:1p, 18:0p, and 18:0a at sn-1) and are absent in macrophages from 12/15-lipoxygenase (12/15-LOX)-deficient mice. They are generated acutely in response to calcium mobilization, are primarily cell-associated, and are detected on the outside of the plasma membrane. Levels of 12-HETE-PEs in naïve lavage are in a similar range to those of free 12-HETE (5.5 ± 0.2 ng or 18.5 ± 1.03 ng/lavage for esterified versus free, respectively). In healthy mice, 12/15-LOX-derived 12-HETE-PEs are found in the peritoneal cavity, peritoneal membrane, lymph node, and intestine, with a similar distribution to 12/15-LOX-derived 12-HETE. In vivo generation of 12-HETE-PEs occurs in a Th2-dependent model of murine lung inflammation associated with interleukin-4/interleukin-13 expression. In contrast, in Toll receptor-dependent peritonitis mediated either by live bacteria or bacterial products, 12-HETE-PEs are rapidly cleared during the acute phase then reappear during resolution. The human homolog, 18:0a/15-HETE-PE inhibited human monocyte generation of cytokines in response to lipopolysaccharide. In summary, a new family of lipid mediators generated by murine macrophages during Th2 inflammation are identified and structurally characterized. The studies suggest a new paradigm for lipids generated by 12/15-LOX in inflammation involving formation of esterified eicosanoids.
12/15-Lipoxygenase (12/15-LOX)2 belongs to a family of lipid-peroxidizing enzymes that catalyze the oxygenation of polyunsaturated fatty acids to their corresponding hydroperoxy derivatives (1). They are best known for generation of free acid eicosanoids, comprising positional isomers of hydroperoxyeicosatetraenoic acid, which are subsequently converted into secondary products, including hydroxyeicosatetraenoic acid (HETE). The human homolog, 15-LOX1, is the most highly induced gene product in response to IL-4/IL-13 suggesting a potential role in Th2-driven immune responses such as autoimmune disease and allergy (2). Indeed, two recent studies indicate that mice deficient in 12/15-LOX are protected against Th2-dependent lung allergic disease (3, 4).
Deficiency of 12/15-LOX in peritoneal macrophages (MΦ) alters their in vitro phenotype resulting in decreased IL-4 induction of scavenger receptor CD36, decreased stimulation of IL-12 synthesis by LPS and attenuated phagocytosis of apoptotic cells (5–7). However, the identities of the LOX products that regulate these processes are not clear, because several known products are unable to bypass the requirement for enzyme expression (7–10). Collectively, the studies infer the involvement of further uncharacterized 12/15-LOX products and indicate that the identification of novel lipids derived from this pathway is important.
We recently reported that 15-LOX1 could generate 15-HETE-PE in response to calcium ionophore (11). In this study, we characterize generation of similar lipids by murine 12/15-LOX in vitro and in vivo. These new studies extend the previous findings to temporal generation of these lipids in immunologically distinct models of inflammation, as well as identifying potential biological mechanisms of action.
EXPERIMENTAL PROCEDURES
Animals
All animal experiments were performed in accordance with the United Kingdom Home Office Animals (Scientific Procedures) Act of 1986. 12/15-LOX knock-out mice generated as described previously and wild-type male C57BL/6 mice (25–30 g) from Charles River, UK, were kept in constant temperature cages (20–22 °C) and given free access to water and standard chow (12).
Isolation and Activation of Peritoneal Cells
Peritoneal lavage was obtained using 2 ml of ice-cold PBS. Unless otherwise stated, macrophages were isolated by 2-h adhesion in culture, then washed. They were then harvested by scraping, diluted to 4 × 106/ml in Krebs buffer, activated using 10 μm A23187 for 15 min at 37 °C. To determine cellular localization, total lavage was spun at 800 × g for 10 min to pellet cells, then 100,000 × g for 1 h to remove “vesicles.” Lipids were extracted as described below. For determination of externalization of PE, 1 mg/ml Sulfo-NHS-biotin was added for 10 min at room temperature, followed by 1 mm SnCl2 for 10 min. Cells were pelleted to remove excess Sulfo-NHS-biotin. In some experiments, water in buffers was replaced with H218O (CK Gas, UK). Lipids were extracted as described below.
Extraction of Lipids from Peritoneal Lavage or Macrophages
Lipid products were determined using LC/MS/MS. 12-HETE-d8 (10 ng) and/or 10 ng of di-14:0-phosphatidylethanolamine (DMPE) was added to each lavage sample before extraction, as internal standards. Hydroperoxides were then reduced to their corresponding stable alcohols using 1 mm SnCl2. Lipids were extracted by adding a solvent mixture (1 m acetic acid/2-isopropanol/hexane (2:20:30, v/v) to the sample at a ratio of 2.5 ml of solvent mixture/1 ml of sample, vortexing, and then adding 2.5 ml of hexane. Following vortexing and centrifugation, lipids were recovered in the upper hexane layer. The samples were then re-extracted by addition of an equal volume of hexane, followed by vortexing and centrifugation. The combined hexane layers were dried under N2 flow resuspended in 100 μl of methanol and stored at −80 °C until analysis.
Harvesting and Lipid Extraction of Organs
Mice were sacrificed, and organs were obtained by dissection and snap frozen in liquid nitrogen. Weighed tissue from organs was ground in liquid nitrogen using a mortar and pestle, in PBS (pH 7.4) supplemented with butylated hydroxytoluene (100 μm) and diethylenetriamine pentaacetic acid (100 μm), then extracted using hexane:isopropanol:acetic acid as detailed above, following addition of DMPE and 12-HETE-d8 as internal standards.
LC/MS/MS of PE-HETEs and Free HETEs
Lipid extracts were separated on a C18 Luna(2), 3 μm, 150-mm × 2-mm column (Phenomenex) using conditions as described in supplemental materials and methods. Products were analyzed in the multiple reaction monitoring (MRM) mode monitoring transitions from the parent ion to a daughter ion of m/z 319.2 (HETE, [M-H]−) and m/z 179 (12-HETE characteristic ion) every 200 ms with a collision energy of −45 V. Peaks containing both a 319 and 179 daughter ion confirmed the presence of 12-HETE and the area under the curve for the parent-319.2 transition was integrated and normalized to the internal standard (DMPE). Product ion scans were also generated at the apex of the peak for each parent-319.2 transition scanning from 150 to 800 atomic mass unit over 0.65 s, with a linear ion trap fill time of 200 ms. Free HETEs were determined using MRM transitions specific to each positional isomer as described in the supplemental materials.
Staphylococcus epidermidis Cell-free Supernatant-induced Peritoneal Inflammation
Peritoneal inflammation was established in mice through intraperitoneal administration of a defined 500-μl dose of S. epidermidis-derived cell-free supernatant (SES), prepared from a clinical isolate of S. epidermidis as described previously (13). At defined intervals following SES administration, animals were sacrificed, and peritoneal cavity was lavaged with 2 ml of ice-cold PBS. Lipids were extracted as described for peritoneal lavage above and analyzed using LC/MS/MS with 12-HETE-PE expressed relative to internal standard, DMPE.
Live Bacteria Infection
An inoculum of live dose S. epidermidis ATCC 12228 (5 × 108 cfu/mouse) was prepared from bacteria harvested during log phase growth under sterile conditions. Anesthetized mice were inoculated by intraperitoneal injection following aseptic preparation. At specific time intervals (3, 6, 12, and 18 h) mice were sacrificed, and the peritoneal cavity was lavaged using sterile PBS. Lipids were extracted as described for peritoneal lavage above and analyzed using LC/MS/MS with 12-HETE-PE expressed relative to internal standard, DMPE.
Ovalbumin (OVA) Model of Lung Inflammation
Acute allergic airway inflammation was induced in female BALB/c mice by sensitizing with 0.5% OVA/aluminum hydroxide intraperitoneally on days 0 and 10. Mice given intraperitoneal injections with PBS/aluminum hydroxide served as control. From day 20, mice were given 6 consecutive daily aerosols of 5% OVA/PBS for 20 min and culled on day 26. The lung was homogenized using a solution of 1× Hanks' balanced salt solution and protease inhibitor tablet (Roche Applied Science) at a concentration of 50 mg/ml, centrifuged at 1600 rpm for 20 min, and supernatant was collected and analyzed (14). Lipids were extracted as described for peritoneal lavage above and analyzed using LC/MS/MS with 12-HETE-PE expressed relative to internal standard, DMPE.
Generation of Cytokines by Human Peripheral Monocytes
18:0a/15-HETE-PE was synthesized as described in the supplemental materials. Human peripheral monocytes were isolated by adhesion as previously described (11). Cells were incubated with 18:0a/15-HETE-PE or 18:0a/20:4-PE (Avanti, AL), with or without 10 ng/ml LPS for 24 h. The supernatant was then removed and spun at 800 × g for 5 min. Cytokine generation was analyzed using enzyme-linked immunosorbent assay kits for granulocyte colony-stimulating factor or tumor necrosis factor-α (R&D).
Statistical Analysis
Statistics used Student's t test, with p < 0.05 being considered significant.
RESULTS
Characterization of Structures of Esterified 12-HETEs Generated by Murine Peritoneal Macrophages
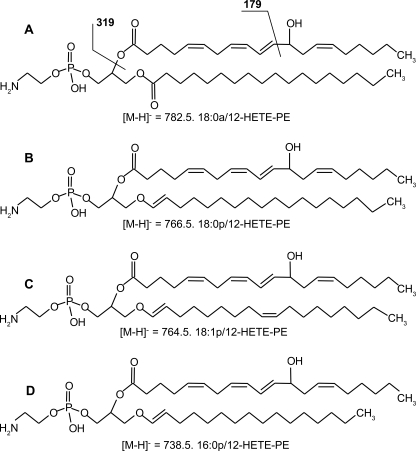
Using precursor scanning LC/MS/MS, four distinct molecular species of esterified 12S-HETE were found, consisting of three plasmalogen (16:0p, 18:0p, and 18:1p) and one diacyl (18:0a) lipids (Scheme 1). Their full structural characterization is presented as supplemental data (see supplemental Results for full description of this data, including supplemental Figs. S1–S4).
SCHEME 1.
Structures of 12-HETE-PE detected in murine peritoneal lavage during inflammation.
12S-HETE-PE Is Acutely Generated and Externalized on the Outside of the Cells
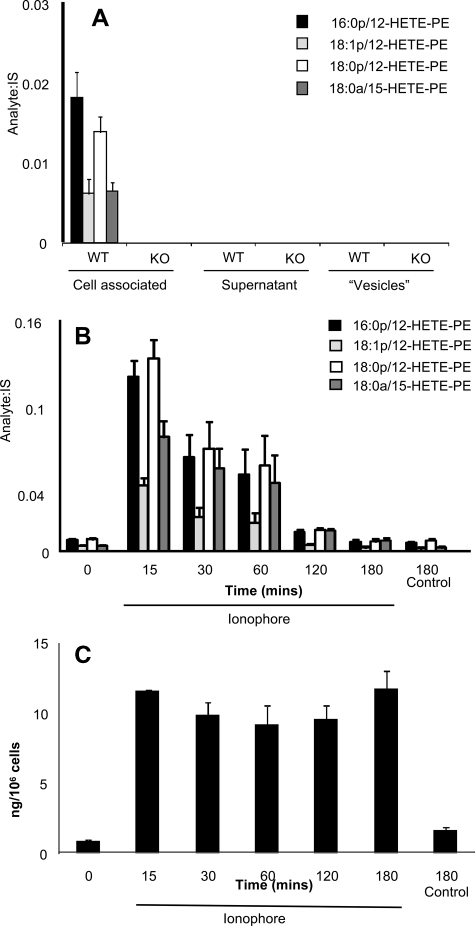
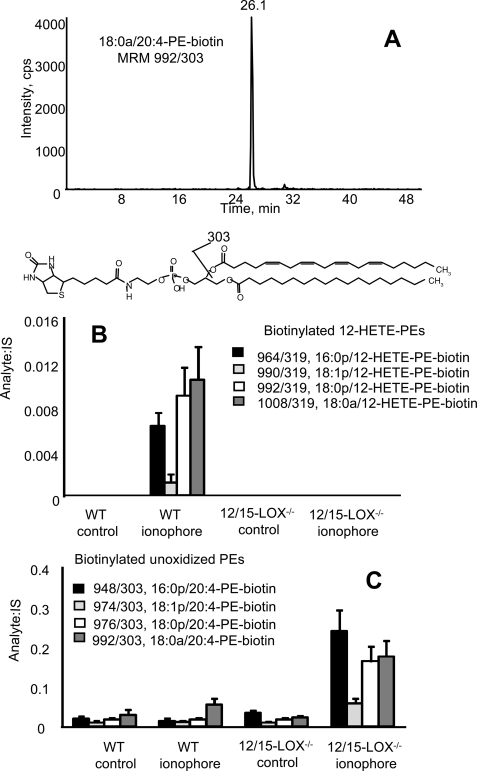
Free acid eicosanoids are secreted from the cells, however, in contrast, 12S-HETE-PEs were retained, and were absent from lavage from 12/15-LOX−/− mice (Fig. 1A). Stimulation of isolated lavage cells in vitro using calcium ionophore led to acute and transient increases in levels of 12-HETE-PEs, which were back to baseline by 3 h (Fig. 1B). In contrast, free 12-HETE was stable following its generation (Fig. 1C). Because we do not yet have purified standards for quantitation, levels of esterified 12S-HETE were determined by quantitation of free 12-HETE before and after saponification. Using this method, we estimate that murine lavage from naïve mice contains ∼5.5 ± 0.2 ng of 12-HETE-PEs compared with 18.5 ± 1.03 ng of free 12-HETE per mouse (mean ± S.E., n = 3). After ionophore activation, these levels increase to 98.8 ± 17 ng of 12-HETE-PEs, versus 123 ± 11 ng of free 12-HETE per mouse (mean ± S.E., n = 3). These data confirm that their formation is an enzymatic process regulated by cell signaling events and esterified eicosanoids account for a significant proportion of 12/15-LOX products generated by these cells. Although PE is primarily on the inside of the plasma membrane, it can be exposed on the surface during cell activation or apoptosis (16, 17). To determine whether 12S-HETE-PEs are externalized, primary amines on the outside of the cells were biotinylated using a cell-impermeable reagent, Sulfo-NHS-biotin. Addition of the biotin group causes a mass shift of 226 atomic mass unit, and derivatized lipids are detected using LC/MS/MS where they elute around 24–26 min as distinct peaks, based on MRM of parent mass to sn-2 fatty acid (e.g. m/z 319 or 303 for 12-HETE or arachidonate, respectively) (Fig. 2A). Without cell activation, no external PE was detected, even though small amounts of 12-HETE-PE were present (Figs. 1B and 2B). In contrast, ionophore stimulation of wild-type cells, but not 12/15-LOX−/−, caused appearance of biotinylated 12-HETE-PEs (Fig. 2B). The externalization of native PE was also determined using this technique. In contrast, activation only slightly increased external aminophospholipid in wild-type macrophages but caused a robust and significant increase of external PE in 12/15-LOX−/− cells (Fig. 2C). Because ionophore is not a receptor-dependent stimulus, we screened several bacterial and fungal products for their ability to acutely activate 12-HETE-PE generation. However, neither LPS, zymosan, β-glucan, flagellin, or PAM3CSK4 caused a response indicating that acute activation of this pathway is not via these ligands (data not shown).
FIGURE 1.
12-HETE-PEs are primarily cell-associated, generated in response to agonist stimulation, and absent in 12/15-LOX−/− mice. A, 12-HETE-PEs are cell-associated. Cells and vesicles were removed from wild-type or 12/15-LOX−/− lavage by centrifugation then 12-HETE-PEs determined using LC/MS/MS as described under “Experimental Procedures” (n = 4, mean ± S.E.). B, calcium ionophore stimulates formation of 12-HETE-PEs by macrophages. Peritoneal macrophages isolated by adhesion were stimulated for various times with 10 μm A23187, before extraction of lipids and analysis using LC/MS/MS (n = 3, mean ± S.E.). C, formation of 12-HETE by macrophages. Peritoneal macrophages isolated by adhesion were stimulated for various times with 10 μm A23187, before extraction of lipids and analysis using LC/MS/MS (n = 3, mean ± S.E.).
FIGURE 2.
Activation of macrophages induces externalization of 12-HETE-PEs on the surface of macrophages. Murine macrophages were activated with ionophore and externally exposed PE biotinylated and detected as described under “Experimental Procedures.” A, LC/MS/MS of biotinylated 18:0a/20:4-PE-biotin from platelets. Lipid extracts from activated platelets derivatized with Sulfo-NHS-biotin were separated using LC/MS/MS as described under “Experimental Procedures,” monitoring for MRM 992/303. B, biotinylated 12-HETE-PEs were determined as described under “Experimental Procedures,” before and after ionophore activation of wild-type or 12/15-LOX−/− peritoneal macrophages (n = 3, mean ± S.E.). C, biotinylated native PEs were determined as for 12-HETE-PEs, above (n = 3, mean ± S.E.).
12S-HETE-PE Is Generated via Direct Oxidation of Intact Phospholipid by 12/15-LOX
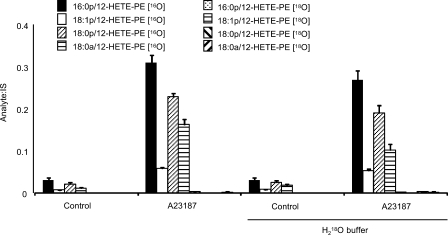
12S-HETE-PE may be formed either by esterification of free 12S-HETE, or by direct phospholipid oxidation. To distinguish, cells were activated in buffer generated using H218O. When arachidonate is hydrolyzed by phospholipase A2, an atom of oxygen from H2O is incorporated. Thus, 12S-HETE generated in the presence of H218O will have a mass increase of 2 atomic mass units (18). During re-esterification, there is a 50% chance that the label will be lost, thus 50% of the 12S-HETE-PE generated by this mechanism will be labeled. However, in this case none of the 12S-HETE-PEs contained 18O indicating they arise through direct oxidation by 12/15-LOX (Fig. 3).
FIGURE 3.
12-HETE-PEs are generated via direct oxidation of PE by 12/15-LOX. Cells were activated with ionophore in buffer made with/without H218O, and lipids were extracted and analyzed either for 16O- or 18O-containing 12-HETE-PEs using LC/MS/MS.
Tissue Distribution of 12S-HETE-PEs in Mice
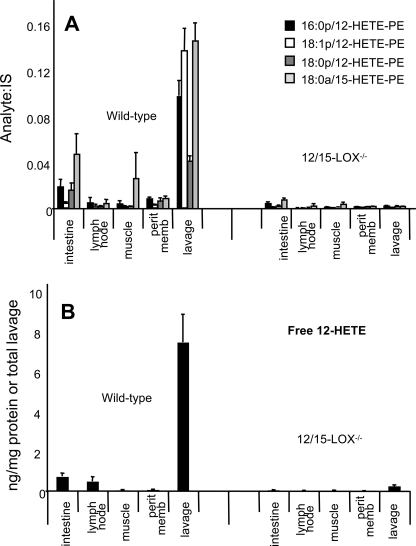
All major organs from healthy wild-type and 12/15-LOX-deficient mice were obtained, and relative levels of 12-HETE-PE and 12-HETE were determined using LC/MS/MS. Results show that peritoneal lavage, lymph node, muscle, intestine, and peritoneal membrane have detectable levels of 12-HETE-PE derived from this pathway (Fig. 4, A and B). In other organs, 12-HETE-PEs were either absent (liver, kidney, adipose tissue, and brain) or were present also in knock-out tissue indicating they originated from a different LOX isoform (spleen and lung) (data not shown).
FIGURE 4.
Tissue distribution of 12-HETE-PEs (A) and free 12-HETE (B) in murine organs. Lipids were extracted from wild-type or 12/15-LOX−/− mice and analyzed using LC/MS/MS. Levels of 12-HETE-PEs in organs are expressed as analyte:internal standard, after normalization for grams of wet weight (n = 10–11, mean ± S.E.). For lavage, levels are normalized for total lavage.
Formation of 12-HETE-PEs during in Vivo Inflammation
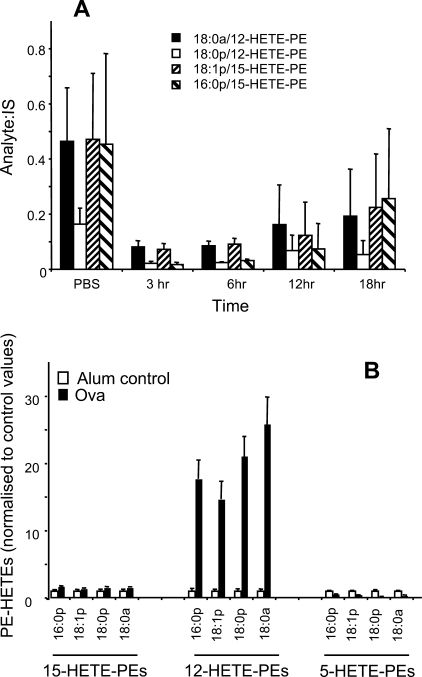
To characterize how 12-HETE-PE levels changes during acute inflammation, three in vivo models were utilized. Two related models of peritonitis comprised either (i) intraperitoneal administration of either live S. epidermidis, one of the principal causative agents of human peritonitis in peritoneal dialysis patients, or (ii) (intraperitoneal) SES, prepared from a clinical isolate of S. epidermidis. In both, peritoneal lavage 12-HETE-PE levels decreased during acute inflammation, recovering at later time points (Figs. 5 and 6A). In contrast, during Th2-driven lung inflammation induced by OVA sensitization followed by OVA administration to the lung, 12-HETE-PEs were dramatically elevated at day 6 in lung homogenate (Fig. 6B). In contrast, there was no increase in isomers oxidized at either C15 or C5 indicating that the 12-HETE-PE lipids originate from 12/15-LOX.
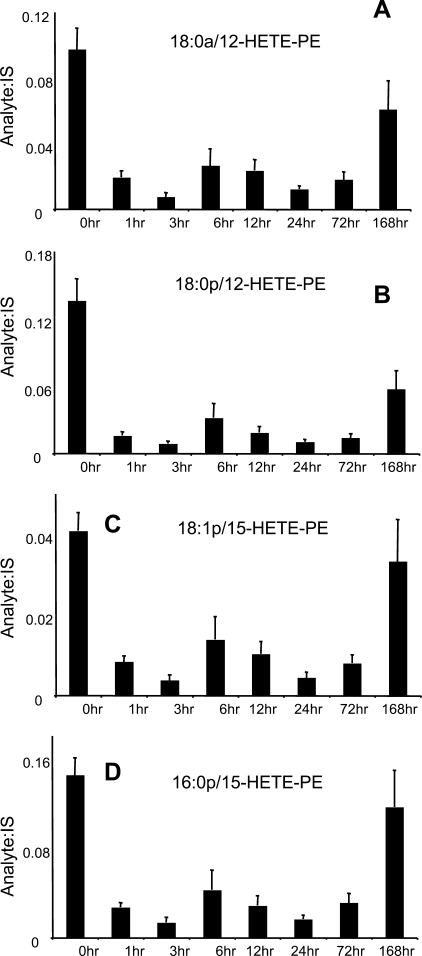
FIGURE 5.
12-HETE-PEs decrease in SES peritonitis during inflammation, then recover by day 7. Relative levels of PE-HETE species were determined by LC/MS/MS as described under “Experimental Procedures,” by comparison of ion intensity with internal standard (di14:0-PE) added before extraction of lipids (n = 5, mean ± S.E.). A–D, time courses for individual 12-HETE-PE lipids generated by 12/15-LOX during peritoneal inflammation.
FIGURE 6.
12-HETE-PEs decrease in live bacterial peritonitis, but are markedly elevated in OVA lung allergy. A, lipid extracts from peritoneal lavage of S. epidermidis-infected mice were profiled for 12-HETE-PEs at various times post infection, using LC/MS/MS as described under “Experimental Procedures” (n = 3–5, mean ± S.E.). B, lung homogenate from OVA-sensitized mice were profiled for 12-HETE-PEs after 6 days of OVA challenge (intranasally), using LC/MS/MS as described under “Experimental Procedures” (n = 3, mean ± S.E.).
18:0a/15S-HETE-PE Inhibits Cytokine Generation in Human Monocytes
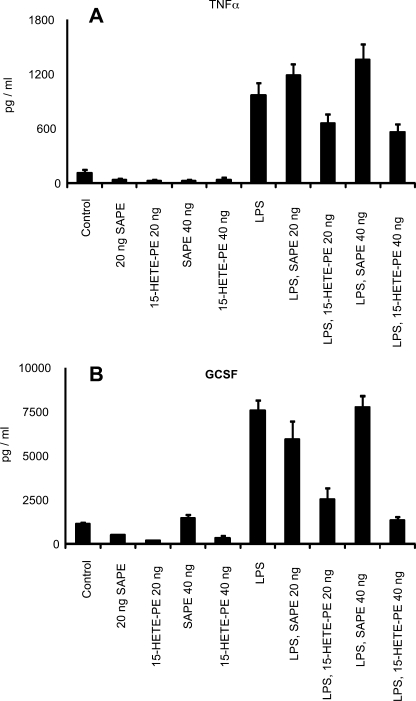
To examine whether HETE-PEs could modulate inflammatory activation of monocytic cells, we examined the action of a human homolog containing 15S-HETE on LPS activation of human monocytes. This lipid was chosen, because it is possible to synthesize and purify large amounts using soybean 15-LOX. Although 18:0a/15-HETE-PE had no effect on basal generation, LPS stimulation of tumor necrosis factor-α or granulocyte colony-stimulating factor was effectively inhibited. In contrast, the parent lipid containing arachidonate instead of 15-HETE was without effect (Fig. 7).
FIGURE 7.
15-HETE-PE inhibits LPS-stimulated cytokine generation by human monocytes. Human monocytes were incubated with 18:0a/15-HETE-PE or 18:0a/20:4-PE (20 or 40 ng/500 ul) with or without 10 ng/ml lipopolysaccharide (LPS) for 24 h. The supernatant was then analyzed using enzyme-linked immunosorbent assay kits for tumor necrosis factor-α (A) or GCSF (B).
DISCUSSION
12/15-LOX regulates macrophage function in vitro and in vivo, and the human homolog has been implicated in Th2-dependent inflammation; however, the lipid-signaling mediators responsible are unclear (6, 8, 19, 20). Precursor MS/MS scanning of lipid extracts from resident peritoneal lavage, the site of greatest basal 12/15-LOX expression in the mouse, identified four novel products as one diacyl (18:0a) and three plasmalogen (18:0p, 18:1p, and 16:0p) PEs that contain 12S-HETE (supplemental Figs. S1–S4). The enzymatic generation of these lipids is confirmed by several observations: (i) they contained primarily 12S-HETE enantiomer, (ii) they are absent from several tissues and organs in 12/15-LOX−/− mice, including peritoneal lavage, intestine and peritoneal membrane, and (iii) they are generated acutely on ionophore stimulation, with levels similar to free 12S-HETE. We previously showed that similar lipids are formed by ionophore-activated human peripheral monocytes, however in that case, oxidation was at C-15 and mediated by 15-LOX1 (11). Herein, we extended our studies to murine cells and characterized formation of 12S-HETE-containing lipids both in vitro and in vivo in three models of inflammation, as well as studying the tissue and cellular localization of the lipids.
12S-HETE-PEs were primarily cell-associated and were metabolized within 3 h (Fig. 1). This is distinct to free acid products that are secreted and were stable over this time period. This may indicate phospholipase A2 hydrolysis within the plasma membrane, although this remains to be studied. Studies using H218O indicated that they form by direct oxidation by 12/15-LOX, similar to the corresponding human lipids generated by IL-4-treated monocytes (11). Following ionophore activation, 12-HETE-PEs were detected on the outside of the cells (Fig. 2). The lack of 12-HETE-PE externalization in unstimulated cells that already contain detectable amounts of these lipids indicates that both their formation and externalization are stimulated by ionophore. In wild-type cells, unoxidized PE was already present on the outside, but its levels did not increase on cell activation (Fig. 2, B and C). In contrast, in 12/15-LOX−/− macrophages, ionophore caused a large increase in PE externalization. This shows that in wild-type macrophages, externalization of PE is targeted toward oxidized species. However, in the absence of 12-HETE-PEs, the equivalent native PE lipids become externalized instead. Recent studies have proposed that extracellular exposure of oxidized phospholipids, including those containing hydroxy/oxy short-chain aldehydes derived from hydroperoxide decomposition, facilitates their physical contact with pattern recognition receptors (21). This is termed the lipid whisker model. Thus it is possible that 12-HETE-PEs, formed and then externalized in response to agonist activation, may participate in similar processes, although this remains to be determined.
Culture of human monocytes with 18:0a/15-HETE-PE effectively inhibited LPS stimulation of tumor necrosis factor-α and GCSF generation. This is consistent with a recent report showing that similar concentrations of oxidized PC, including a hydroxylipid, can inhibit LPS stimulation of cytokine gene expression (22). The mechanism involved binding of the lipid with LPS-binding protein and CD14 and a similar mechanism for 15-HETE-PE is likely (Fig. 7).
Detection of 12-HETE and PE-HETE in lysates of resident peritoneal cells indicates that 12/15-LOX is basally active in vivo without inflammatory activation. Although further stimulation is achieved using ionophore in vitro, several bacterial and fungal mediators were without effect. Furthermore, two in vivo peritonitis models that mediate cell activation by Toll receptor-dependent mechanisms did not elevate 12-HETE-PE. Instead, 12-HETE-PEs were cleared during acute inflammation and reappeared during resolution (Fig. 4). This parallels the time course of clearance of 12/15-LOX-expressing macrophages and 12-HETE from the peritoneal cavity and their re-emergence, as recently demonstrated (20). In contrast, 12-HETE-PEs were specifically and significantly elevated in a Th2-dependent model of lung allergy. Importantly, the time point where the lipids were detected coincided with the peak of IL-4/IL-13 generation (23). These data indicate that 12/15-LOX-dependent phospholipid peroxidation is associated with the Th2 and not Th1 inflammatory profile in vivo, consistent with previous reports showing induction of the human homolog, 15-LOX1 in human monocytes by IL-4/IL-13 in vitro (15, 24–26).
In summary, esterified eicosanoids acutely generated by 12/15-LOX were characterized in vitro and in a model of Th2-dependent allergy in vivo. The studies define a class of lipid formed by this pathway that may represent novel mediators of 12/15-LOX bioactivity, and experiments are underway to address this idea.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- 12/15-LOX
- 12/15-lipoxygenase
- HETE
- hydroxyeicosatetraenoic acid
- IL
- interleukin
- LPS
- lipopolysaccharide
- PBS
- phosphate-buffered saline
- PE
- phosphatidylethanolamine
- LC/MS/MS
- liquid chromatography
- DMPE
- di-14:0-phosphatidylethanolamine
- SES
- S. epidermidis-derived cell-free supernatant
- MRM
- multiple reaction monitoring
- OVA
- ovalbumin.
REFERENCES
- 1.Kühn H., Thiele B. J. (1999) FEBS Lett. 449, 7–11 [DOI] [PubMed] [Google Scholar]
- 2.Chaitidis P., O'Donnell V., Kuban R. J., Bermudez-Fajardo A., Ungethuem U., Kühn H. (2005) Cytokine 30, 366–377 [DOI] [PubMed] [Google Scholar]
- 3.Andersson C. K., Claesson H. E., Rydell-Törmänen K., Swedmark S., Hällgren A., Erjefält J. S. (2008) Am. J. Respir. Cell Mol. Biol. 39, 648–656 [DOI] [PubMed] [Google Scholar]
- 4.Hajek A. R., Lindley A. R., Favoreto S., Jr., Carter R., Schleimer R. P., Kuperman D. A. (2008) J. Allergy Clin. Immunol. 122, 633–9.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J. T., Welch J. S., Ricote M., Binder C. J., Willson T. M., Kelly C., Witztum J. L., Funk C. D., Conrad D., Glass C. K. (1999) Nature 400, 378–382 [DOI] [PubMed] [Google Scholar]
- 6.Middleton M. K., Rubinstein T., Puré E. (2006) J. Immunol. 176, 265–274 [DOI] [PubMed] [Google Scholar]
- 7.Zhao L., Cuff C. A., Moss E., Wille U., Cyrus T., Klein E. A., Praticó D., Rader D. J., Hunter C. A., Puré E., Funk C. D. (2002) J. Biol. Chem. 277, 35350–35356 [DOI] [PubMed] [Google Scholar]
- 8.Miller Y. I., Chang M. K., Funk C. D., Feramisco J. R., Witztum J. L. (2001) J. Biol. Chem. 276, 19431–19439 [DOI] [PubMed] [Google Scholar]
- 9.Shankaranarayanan P., Nigam S. (2003) J. Immunol. 170, 887–894 [DOI] [PubMed] [Google Scholar]
- 10.Shappell S. B., Gupta R. A., Manning S., Whitehead R., Boeglin W. E., Schneider C., Case T., Price J., Jack G. S., Wheeler T. M., Matusik R. J., Brash A. R., Dubois R. N. (2001) Cancer Res. 61, 497–503 [PubMed] [Google Scholar]
- 11.Maskrey B., Bermúdez-Fajardo A., Morgan A., Stewart-Jones E., Dioszeghy V., Taylor G. W., Baker P. R., Coles B., Coffey M. J., Kühn H., O'Donnell V. B. (2007) J. Biol. Chem. 282, 20151–20163 [DOI] [PubMed] [Google Scholar]
- 12.Cyrus T., Witztum J. L., Rader D. J., Tangirala R., Fazio S., Linton M. F., Funk C. D. (1999) J. Clin. Invest. 103, 1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie R. K., Coles G. A., Williams J. D. (1991) J. Infect. Dis. 163, 837–842 [DOI] [PubMed] [Google Scholar]
- 14.Kearley J., Barker J. E., Robinson D. S., Lloyd C. M. (2005) J. Exp. Med. 202, 1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu B., Bhattacharjee A., Roy B., Xu H. M., Anthony D., Frank D. A., Feldman G. M., Cathcart M. K. (2003) Mol. Cell Biol. 23, 3918–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emoto K., Kobayashi T., Yamaji A., Aizawa H., Yahara I., Inoue K., Umeda M. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 12867–12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sessions A., Horwitz A. F. (1983) Biochim. Biophys. Acta 728, 103–111 [DOI] [PubMed] [Google Scholar]
- 18.Brash A. R., Ingram C. D. (1986) Prostaglandins Leukot. Med. 23, 149–154 [DOI] [PubMed] [Google Scholar]
- 19.Middleton M. K., Zukas A. M., Rubinstein T., Jacob M., Zhu P., Zhao L., Blair I., Puré E. (2006) J. Exp. Med. 203, 2529–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dioszeghy V., Rosas M., Maskrey B. H., Colmont C., Topley N., Chaitidis P., Kühn H., Jones S. A., Taylor P. R., O'Donnell V. B. (2008) J. Immunol. 181, 6514–6524 [DOI] [PubMed] [Google Scholar]
- 21.Greenberg M. E., Li X. M., Gugiu B. G., Gu X., Qin J., Salomon R. G., Hazen S. L. (2008) J. Biol. Chem. 283, 2385–2396 [DOI] [PubMed] [Google Scholar]
- 22.von Schlieffen E., Oskolkova O. V., Schabbauer G., Gruber F., Blüml S., Genest M., Kadl A., Marsik C., Knapp S., Chow J., Leitinger N., Binder B. R., Bochkov V. N. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 356–362 [DOI] [PubMed] [Google Scholar]
- 23.McMillan S. J., Lloyd C. M. (2004) Clin. Exp. Allergy 34, 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinckmann R., Kühn H. (1997) Adv. Exp. Med. Biol. 400B, 599–604 [PubMed] [Google Scholar]
- 25.Roy B., Cathcart M. K. (1998) J. Biol. Chem. 273, 32023–32029 [DOI] [PubMed] [Google Scholar]
- 26.Schnurr K., Borchert A., Kühn H. (1999) FASEB J. 13, 143–154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.