Abstract
Sphingosine 1-phosphate (S1P) is a bioactive lipid signal transmitter present in blood. Blood plasma S1P is supplied from erythrocytes and plays an important role in lymphocyte egress from lymphoid organs. However, the S1P export mechanism from erythrocytes to blood plasma is not well defined. To elucidate the mechanism of S1P export from erythrocytes, we performed the enzymatic characterization of S1P transporter in rat erythrocytes. Rat erythrocytes constitutively released S1P without any stimulus. The S1P release was reduced by an ABCA1 transporter inhibitor, glyburide, but not by a multidrug resistance-associated protein inhibitor, MK571, or a multidrug resistance protein inhibitor, cyclosporine A. Furthermore, we measured S1P transport activity using rat erythrocyte inside-out membrane vesicles (IOVs). Although the effective S1P transport into IOVs was observed in the presence of ATP, this activity was also supported by dATP and adenosine 5′-(β,γ-imido)triphosphate. The rate of S1P transport increased depending on S1P concentration, with an apparent Km value of 21 μm. Two phosphorylated sphingolipids, dihydrosphingosine 1-phosphate and ceramide 1-phosphate, did not inhibit S1P transport. Similar to the intact erythrocytes, the uptake of S1P into IOVs was inhibited by glyburide and vanadate but not by the other ABC transporter inhibitors. These results suggest that S1P is exported from the erythrocytes by a novel ATP-dependent transporter.
Sphingosine 1-phosphate (S1P),2 a bioactive lipid molecule present in the blood, plays an important role in diverse cellular responses, such as migration, proliferation, and differentiation (1, 2). These processes are triggered by the binding of S1P to its specific receptors (3), of which five subtypes (S1P1-S1P5) have been identified in endothelial and immune cells (4). Studies using S1P1 receptor-deficient mice showed abnormalities in lymphocyte egress from lymph nodes, spleen, and thymus (5, 6). Whereas blood plasma contains a basal level of S1P from the nanomolar to the micromolar range (7–12), lymphoid tissues maintain a low S1P environment through the activity of S1P lyase (13). It has been proposed that a higher concentration of S1P in the blood plasma than in the lymphoid organs establishes an essential gradient along which lymphocytes expressing the S1P1 receptor on cell surfaces migrate (2, 5, 6, 13–15).
The source of plasma S1P remains unclear despite its importance in the cellular responses of endothelial cells and lymphocytes. Unlike most cells, blood cells, astrocytes, and vascular endothelial cells are reported to release S1P (8, 16–18). These cells contain sphingosine kinase, which synthesizes S1P through the phosphorylation of sphingosine (16, 18, 19). Whereas platelets and mast cells release S1P in a stimulus-dependent manner (17, 20), erythrocytes, neutrophils, and mononuclear cells release S1P in a stimulus-independent manner (16). The roles of S1P derived from erythrocytes, the most abundant of these blood cells, have not been elucidated. However, recent reports suggest that S1P released from erythrocytes is a major source of plasma S1P (7, 9) and promotes lymphocyte egress to blood (9).
Previously, we showed that S1P is released from rat platelets upon stimulation by thrombin or Ca2+ (21). We proposed that an ATP-dependent transporter plays a key role in S1P release from platelets (21). However, the detailed mechanism of S1P release is unclear because there is no way to assay the transport of S1P across the membrane. In this study we compared the properties of S1P release from erythrocytes with that of platelets and showed that S1P release from erythrocytes does not require any stimuli. We then established an assay to measure the ATP-dependent S1P uptake into inside-out membrane vesicles (IOVs) prepared from rat erythrocytes and characterized S1P transport in erythrocytes.
EXPERIMENTAL PROCEDURES
Materials
AMP, ADP, ATP, ATPγS, AMP-PNP, BSA (fatty acid-free), thrombin, TPA, A23187, ceramide 1-phosphate, glyburide, and cyclosporine A were obtained from Sigma. CTP, GTP, UTP, and dNTPs were from GE Healthcare, MK571 was from Calbiochem, S1P was from Avanti, and dihydrosphingosine 1-phosphate (DHS1P) was from Biomol. [3H]Sphingosine and [33P]S1P were purchased from American Radiolabeled Chemicals, Inc. [3H]cGMP was from PerkinElmer Life Sciences, anti-Na+-K+ ATPase mAb (05–369) was from Millipore, and anti-ABCA1 mAb (ab18180) and anti-MRP1 mAb (ab32574) were obtained from Abcam. Other chemicals were of reagent grade and were obtained from Wako Pure Chemical or Nacalai Tesque.
Isolation of Rat Erythrocytes
Wistar rats (9–14 weeks old, female) were anesthetized, and whole blood was collected from their hearts using an acid citrate-dextrose solution as an anti-coagulant. Erythrocytes were prepared by centrifugation at 500 × g for 15 min. For the S1P release assay erythrocytes were washed twice with a mixture of buffer A (20 mm HEPES-NaOH (pH 7.4), 3.3 mm NaH2PO4, 2.9 mm KCl, 1 mm MgCl2, 138 mm NaCl, and 1 mg/ml glucose) containing 1% BSA followed by immediate resuspension in the same buffer.
Measurement of [3H]S1P Release from Erythrocytes
Erythrocyte suspensions (180 μl, 1 × 107 erythrocytes/ml) in buffer A containing 1% BSA were preincubated at 37 °C for 5 min followed by a calcium chelator or an inhibitor treatment for 10 min. Then assay buffer containing 0.2 μm [3H]sphingosine (40 nCi/10 μl) in buffer A and 1% BSA was added to each suspension (final concentration of sphingosine, 10 nm) and incubated at 37 °C. After an indicated incubation period, erythrocytes and the assay buffer were separated by centrifugation for 5 s at 12,000 × g. Lipids were extracted from the supernatant and erythrocytes and developed by TLC in butanol-acetic acid-water (3:1:1). Radioactive bands were quantified with a FLA-3000G Bioimaging Analyzer (Fuji Film Co., Tokyo, Japan).
Preparation of Inside-out Membrane Vesicles of Erythrocytes
IOVs were prepared according to a modified procedure of Steck and Kant (22). Rat erythrocytes were collected by centrifugation (500 × g for 10 min) of whole blood prepared as described above, washed twice with one volume of buffer A containing 1% BSA and 0.4 volume of acid citrate-dextrose solution, and washed once with 5 volumes of buffer A containing 1% BSA and 1.2 volume of acid citrate-dextrose solution. Then erythrocytes were lysed in 20 volumes of ice-cold lysis buffer (20 mm Tris-HCl (pH 7.4), 1 mm EDTA) and subsequently centrifuged at 28,000 × g for 10 min at 4 °C. The supernatant was removed, and the pelleted ghosts were resuspended in ice-cold lysis buffer. This step was repeated three times. After the last wash, the pelleted ghost was resuspended in 40 volumes of ice-cold vesiculation buffer containing 0.5 mm Tris-HCl, 0.1% BSA (pH 8.1 at 4 °C) and incubated on ice overnight. When we prepared the IOVs without BSA in the lumen, the vesiculation was performed using the vesiculation buffer without BSA. After the incubation, the suspension was centrifuged (30,000 × g, 30 min, 4 °C), and the pellet was resuspended in one volume of vesiculation buffer. The suspension was then passed through a ¾-inch 27-gauge needle 5 times. The mixture of vesicles and ghosts was layered onto a dextran 70 solution (4.46% in 0.5 mm Tris-HCl (pH 8.1) at 4 °C) and centrifuged at 30,000 × g at 4 °C for 40 min. The vesicles at the interface were washed with 40 volumes of vesiculation buffer and sedimented at 30,000 × g at 4 °C for 30 min. The wash was repeated once, and the vesicles were diluted with a buffer containing 10 mm Tris-HCl, 0.1% BSA (pH 7.4). The vesicles were stored at −80 °C until further use.
Vesicular Transport Assay
For the S1P uptake studies, membrane vesicles (50 μg of protein) were incubated with 1 or 10 μm [33P]S1P in different concentrations of ATP in the incubation buffer (10 mm Tris-HCl (pH 7.4), 10 mm MgCl2, and 0.1% BSA) at 37 °C. The incubations were terminated by the addition of ice-cold stop buffer (10 mm Tris-HCl (pH 7.4), 10 mm EDTA, 10 mm vanadate, and 1% BSA). The mixture was centrifuged at 100,000 × g at 4 °C for 5 min. The amount of [33P]S1P in the pelleted vesicles was determined by liquid scintillation counting.
Reverse flow of the S1P from the IOVs was measured as follows. First, membrane vesicles were incubated with or without 2 mm ATP in the incubation buffer containing 10 μm [33P]S1P at 37 °C as described above. After the incubation for 20 min to remove the S1P in the assay buffer, ice-cold buffer (10 mm Tris-HCl (pH 7.4), 10 mm EDTA, and 1% BSA) was added, and the resulting solution was centrifuged at 29,000 × g at 4 °C for 5 min. Vesicles were resuspended in the S1P-free incubation buffer with or without 2 mm ATP and incubated at 37 °C for 20 min. Then the ice-cold stop buffer was added, and the resulting solution was centrifuged at 29,000 × g at 4 °C for 5 min. The amount of [33P]S1P in the vesicles was determined as described above.
Western Blotting
Cells were sonicated with phosphate-buffered saline supplemented with a protease inhibitor mixture (Nacalai) and centrifuged at 800 × g for 10 min at 4 °C. Rat liver was homogenized and centrifuged at 800 × g for 10 min at 4 °C. The supernatant was collected and centrifuged at 100,000 × g for 1 h at 4 °C to obtain the membrane fraction. Rat platelets were collected in the platelet-rich plasma by centrifugation of whole blood at 500 × g for 15 min. The platelet-rich plasma was centrifuged at 1500 × g for 10 min, and the platelets were lysed in the hypotonic buffer (10 mm NaH2PO4 (pH 7.6), 10 mm EDTA) containing a protease inhibitor mixture by sonication and freeze-thawing. Subsequently, the membrane fraction was obtained by centrifugation at 100,000 × g for 1 h at 4 °C. Samples were electrophoresed on an SDS-polyacrylamide gel and immunodetected with the protein-specific antibodies. Rat monoclonal antibodies against mouse ABCA7 were developed by immobilization of the ABCA7 carboxyl-terminal domain (1841 to 2170 amino acids) fused with maltose-binding protein.
RESULTS
Rat Erythrocytes Synthesize and Release S1P
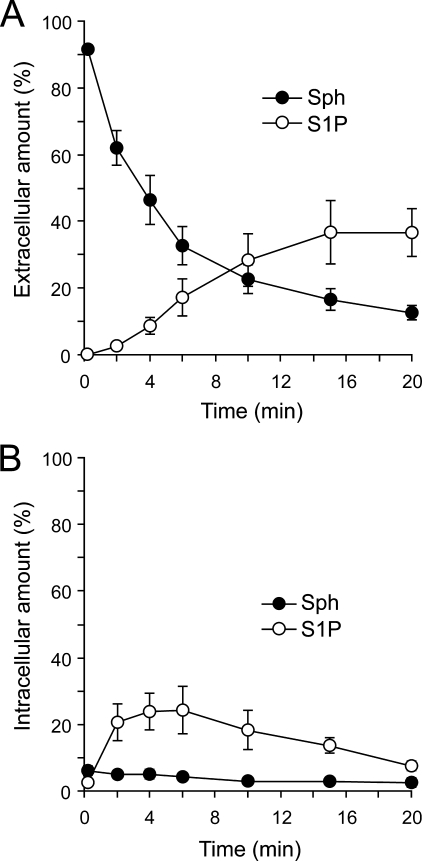
S1P export from erythrocytes was recently reported, suggesting that it plays an important role in lymphocyte egress into blood (9). To further investigate this possibility, we measured the S1P release from rat erythrocytes (Fig. 1). When [3H]sphingosine was added to the medium, it was taken up into rat erythrocytes and immediately converted to [3H]sphingosine 1-phosphate (Fig. 1B). The amount of synthesized S1P in the erythrocyte reached its maximum around 5 min after the addition of [3H]sphingosine. [3H]S1P release was observed beginning at 2 min and increased in a time-dependent manner (Fig. 1A). The addition of thrombin, TPA, and Ca2+, which stimulate the S1P release in platelets (21), did not change the amount of [3H]S1P released from erythrocytes, indicating that cell stimulation is not necessary for secretion of the S1P from these cells (Table 1). Previously, we showed that ATP- and Ca2+-dependent transporters independently release S1P from rat platelets (21). In erythrocytes, [3H]S1P release was not changed by the addition of Ca2+ chelators, an MRP inhibitor, MK571, or a multidrug resistance protein inhibitor, cyclosporine A, but it was inhibited by an ABCA1 inhibitor, glyburide (Table 1). These results suggest that an ABCA-like transporter, but not a Ca2+-dependent transporter, may play a role in S1P secretion from erythrocytes.
FIGURE 1.
Rat erythrocytes take up sphingosine and release sphingosine 1-phosphate. Rat erythrocytes (1.8 × 106 cells) were incubated with [3H]sphingosine for the indicated times at 37 °C. Then the assay buffer and erythrocytes were separated by centrifugation. The amounts of S1P in the assay buffer (A, extracellular) and erythrocytes (B, intracellular) were determined as described under “Experimental Procedures.” Closed and open circles indicate sphingosine (Sph) and S1P, respectively. The total amounts of sphingosine and S1P at 0.2 min were set at 100%. Experiments were performed more than three times, and the error bars indicate the S.D.
TABLE 1.
Effect of stimuli, calcium chelators and inhibitors on the S1P release from erythrocytes
Stimuli were added to erythrocytes preincubated with [3H]Sph for 2 min at 37 °C. After incubation for 10 min at 37 °C, erythrocyte suspensions were centrifuged. Calcium chelators and inhibitors were preincubated with erythrocytes for 10 min at 37 °C. Then [3H]Sph was added, and erythrocyte suspensions were incubated for 20 min at 37 °C. Release rate of S1P was calculated as (amount of medium)/(total amount of medium + erythrocytes). The S1P release rate with no addition of compounds is set at 100%. Values are means with the S.D. BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, tetrapotassium salt.
| Compound | Concentration | % of release |
|---|---|---|
| μm | ||
| Stimuli | ||
| None | 100.0 | |
| Thrombin | 5 NIH U/ml | 98.2 ± 2.5 |
| TPA | 0.16 | 114.0 ± 3.1 |
| Ca2+ + 2 μm A23187 | 2,000 | 100.5 ± 3.3 |
| Calcium chelators | ||
| EDTA | 100 | 100.3 ± 1.2 |
| EGTA | 100 | 100.5 ± 2.0 |
| BAPTA | 100 | 101.1 ± 1.9 |
| EDTA + 10 μm A23187 | 100 | 92.0 ± 5.3 |
| EGTA + 10 μm A23187 | 100 | 88.3 ± 2.3 |
| BAPTA + 10 μm A23187 | 100 | 89.7 ± 0.7 |
| Inhibitors | ||
| Glyburide | 500 | 69.4 ± 5.2 |
| MK571 | 50 | 97.9 ± 2.6 |
| Cyclosporine A | 10 | 97.4 ± 2.8 |
Establishment of an Assay for S1P Transport into the Rat Erythrocyte Inside-out Membrane Vesicles
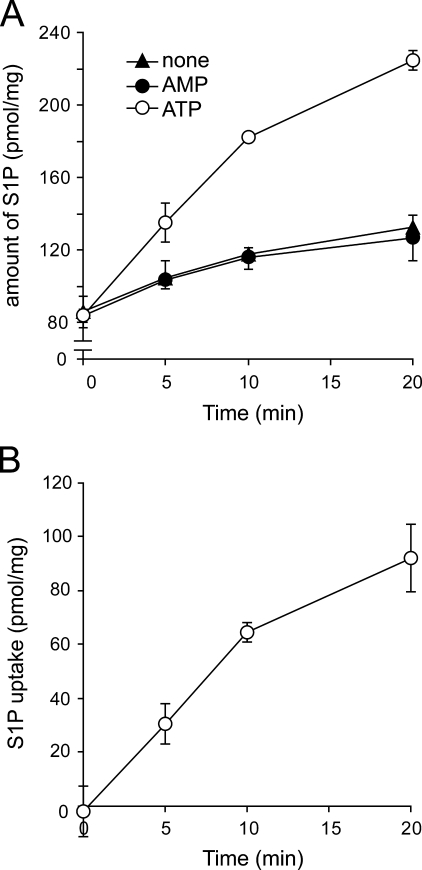
Although characterization of the S1P transporter in intact cells is difficult because of interference with the assay system from cytosolic factors, the use of the IOVs circumvents this problem. We were able to exploit the fact that erythrocytes secrete S1P without any stimulus, in contrast to platelets, and use IOVs from erythrocytes to measure S1P transport activity. [33P]S1P was transported into IOVs in the presence of ATP in a time-dependent manner but not in the presence of AMP or in the absence of ATP (Fig. 2). The amount of [33P]S1P was calculated from the [33P] radioactivity in the IOVs measured by the liquid scintillation counting. To demonstrate that the radioactive species transported into the IOVs were [33P]S1P, lipids were extracted and analyzed using TLC (supplemental Fig. 1A). Amounts of transported [33P]S1P calculated from the TLC method and the liquid scintillation counting were comparable (Fig. 2 and supplemental Fig. 1B), suggesting that most transported 33P-labeled species were [33P]S1P.
FIGURE 2.
ATP-dependent transport of S1P into IOVs prepared from rat erythrocytes. A, IOVs (50 μg of protein) were incubated with 1 μm [33P]S1P in the presence of 2 mm ATP (open circles) or AMP (closed circles) or the absence of nucleotide (closed triangles). After incubation for the indicated times at 37 °C, the amount of S1P trapped inside the IOVs was measured as described under “Experimental Procedures.” B, ATP-dependent S1P uptake was calculated as the difference between the S1P amounts in the presence and absence of ATP. Experiments were performed three times, and the error bars indicate the S.D.
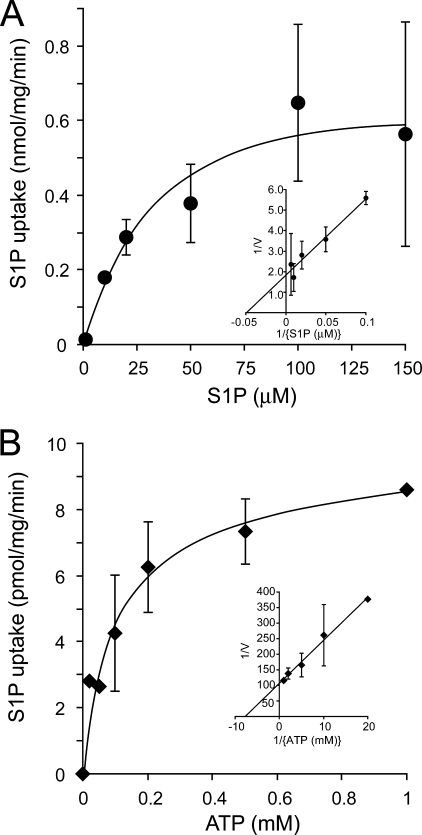
The ATP-dependent transport of [33P]S1P showed saturation kinetics with respect to the S1P and ATP concentrations (Fig. 3). Based on the Lineweaver-Burk plot (Fig. 3, inset), the apparent Km values were estimated to be 21 μm and 130 μm for S1P and ATP, respectively. However, relatively large errors were observed at high concentrations of S1P (Fig. 3A). This might be due to a lower solubility in the assay buffer and a detergent effect of S1P at a high concentration because of its amphiphilic nature. Reverse flow or leakage of the S1P from the vesicles was not observed in the presence or absence of ATP, suggesting that this ATP-dependent S1P transport was unidirectional (supplemental Fig. 2).
FIGURE 3.
Effects of S1P and ATP concentration on the rate of ATP-dependent S1P transport into the IOVs. A, ATP-dependent S1P transport inside the IOVs was calculated from the different concentrations of [33P]S1P in the presence or absence of 2 mm ATP at 37 °C for 5 min. B, different concentrations of ATP were used for ATP-dependent S1P transport. IOVs were incubated with 1 μm [33P]S1P at 37 °C for 5 min. S1P uptake was expressed as the difference between the S1P amounts in the presence and absence of ATP. The inset shows the Lineweaver-Burk plot of the rate of ATP-dependent S1P uptake.
To investigate whether S1P is transported by primary pumps or by secondary ion-coupled transporters that are activated with ion-transporting ATPases, several of which are expressed in erythrocyte membranes, we examined the effect of ionophores and ATPase inhibitors on ATP-dependent [33P]S1P transport. Potassium and proton ionophores valinomycin and carbonyl cyanide p-chlorophenylhydrazone did not affect S1P transport; neither did the proton pump inhibitors dicyclohexylcarbodiimide and NaN3 or ouabain or strophanthidin, both known to inhibit Na+-K+ ATPase (Table 2). In contrast to these inhibitors, a V-ATPase inhibitor, bafilomycin A1, partially inhibited S1P transport activity in a dose-dependent manner (Table 2). However, carbonyl cyanide p-chlorophenylhydrazone and dicyclohexylcarbodiimide, which also effectively inhibit V-ATPase activity, did not inhibit S1P transport, suggesting a direct inhibition of the S1P transporter by bafilomycin A1. These results indicated that ATP-dependent S1P transport was mediated directly by primary transporters.
TABLE 2.
Effect of ionophores and ATPase inhibitors on the ATP-dependent uptake of S1P
Experiments were performed as described for Fig. 7. Values are the means with the S.D. CCCP, carbonyl cyanide p-chlorophenylhydrazone; DCCD, dicyclohexylcarbodiimide.
| Compound | Concentration | S1P uptake |
|---|---|---|
| μm | pmol/mg | |
| None | 104.4 ± 11.8 | |
| Valinomycin | 10 | 99.6 ± 14.2 |
| CCCP | 10 | 107.1 ± 9.9 |
| DCCD | 10 | 114.4 ± 19.1 |
| NaN3 | 100 | 100.2 ± 9.9 |
| Bafilomycin A1 | 1 | 89.6 ± 14.5 |
| 10 | 61.9 ± 6.0 | |
| Ouabain | 100 | 117.2 ± 11.1 |
| Strophanthidin | 100 | 104.6 ± 5.8 |
Expression of ABC Transporters in Rat Erythrocytes
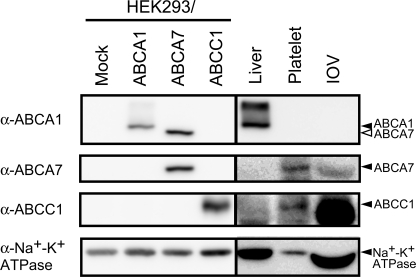
Recently, certain ABC transporters, ABCA1, ABCA7, and ABCC1, were reported as candidates for the S1P transporter (21, 23, 24). Because S1P was transported in an ATP-dependent manner, we examined the expression of these ABC transporters in rat erythrocytes. IOVs prepared from rat erythrocytes were studied by Western blot using monoclonal antibodies against ABCA1, ABCA7, and ABCC1 (Fig. 4). Anti-ABCA1 antibodies recognized both the ABCA1 protein (about 250 kDa) in HEK293/ABCA1 and the ABCA7 protein in HEK293/ABCA7 (Fig. 4, upper panel). ABCA1 is distinguishable from ABCA7 by its molecular size. ABCA1 was not detected in rat platelets or IOVs. The anti-ABCA7 and anti-ABCC1 antibodies specifically recognized the ABCA7 and ABCC1 proteins, respectively (Fig. 4, second and third panels), and ABCA7 and ABCC1 were detected in platelets and IOVs. These results suggest that the ABCA7 and ABCC1 proteins could participate in S1P transport in erythrocytes.
FIGURE 4.
Expression of ABCA1, ABCA7, and ABCC1 in rat erythrocytes. IOVs prepared from rat erythrocytes and membrane fractions from rat liver, platelet-rich plasma, and HEK293 cells expressing ABCA1, ABCA7, or ABCC1 were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane for Western blotting with anti-Na+-K+ ATPase mAb, anti-ABCA1 mAb, anti-ABCA7 mAb, and anti-ABCC1 mAb. Expression of the ubiquitous protein Na+-K+ ATPase was used for a loading control for each membrane fraction.
Characterization of ATP-dependent S1P Transporter
BSA, which is necessary for S1P release from platelets (21), was added to the medium as an S1P carrier in all experiments to increase the solubility of S1P in water. For the development of the assay, we investigated the effect of BSA on ATP-dependent S1P uptake into the IOVs. When BSA was added inside the IOVs, [33P]S1P uptake was increased more than 2-fold (supplemental Fig. 3A). However, the ATP-dependent uptake of cGMP, a water-soluble substrate for the erythrocyte MRPs, into IOVs was unaffected by BSA (supplemental Fig. 3B). These results indicate that BSA is required only for the uptake of lipophilic compounds, such as S1P, into the IOVs. In the absence of BSA inside the IOVs, [33P]S1P uptake activity was approximately halved (supplemental Fig. 3A), indicating a residual uptake possibly because of the presence of [33P]S1P residing in the inner leaflet of the IOV membranes. These results suggest that BSA plays an important role in the release of [33P]S1P from the erythrocyte membrane and in the effective transport of the [33P]S1P inside the vesicles.
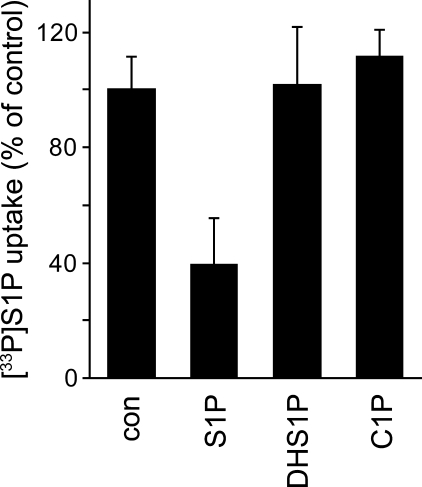
DHS1P is a phosphorylated sphingolipid with a similar structure to S1P. It is released from rat platelets after the uptake and phosphorylation of dihydrosphingosine.3 Dihydrosphingosine was also taken up by rat erythrocytes and phosphorylated to DHS1P (supplemental Fig. 4). This DHS1P was released to the medium in a time-dependent manner, raising the possibility that DHS1P might be released from erythrocytes through the S1P transporter. To investigate the substrate specificity of the S1P transporter in erythrocytes, cis-inhibitory effects against the uptake of 10 μm [33P]S1P into IOVs were examined with phosphorylated sphingolipids, which have a chemical structure similar to that of S1P (Fig. 5). Inhibition experiments were performed using 100 μm cold S1P, 100 μm DHS1P, and 50 μm ceramide 1-phosphate. Although the [33P]S1P uptake was reduced to 39% by cold S1P, DHS1P and ceramide 1-phosphate had no inhibitory effect on the transport of [33P]S1P. This result indicates that the erythrocyte ATP-dependent S1P transporter strictly recognizes the S1P structure as its substrate.
FIGURE 5.
Effect of phosphorylated sphingolipid on S1P transport into IOVs. IOVs were preincubated with 10 μm [33P]S1P in the presence of additional 100 μm cold S1P, 100 μm DHS1P, 50 μm ceramide 1-phosphate (C1P), or the absence of these compounds (con) at 37 °C for 5 min and then incubated in the presence or absence of 2 mm ATP at 37 °C for 5 min. S1P uptake was calculated as the difference between the S1P amounts in the presence and absence of ATP. The S1P uptake in the absence of compounds (con) is set at 100%. Experiments were performed three times, and the error bars indicate the S.D.
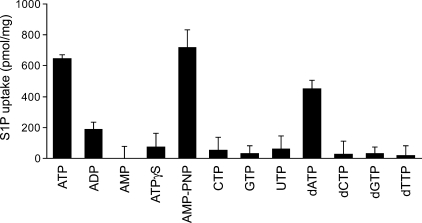
Furthermore, we used nucleotides and non-hydrolyzable ATP analogs to examine the nucleotide specificity and the necessity of ATP hydrolysis in S1P transport (Fig. 6). A significant uptake of S1P was observed in the presence of ATP, AMP-PNP, and dATP, whereas no or low S1P uptake was observed in the presence of the other nucleotides (Fig. 6). These results indicate that S1P transport is driven by some adenine nucleotides and that ATP-hydrolysis is not essential for transport.
FIGURE 6.
Effect of nucleotides on S1P transport into IOVs. Experiments were performed as described under “Experimental Procedures” except that the cold S1P concentration was 10 μm. IOVs were incubated in the presence of 2 mm concentrations of each nucleotide at 37 °C for 10 min. Experiments were performed more than three times, and the error bars indicate the S.D.
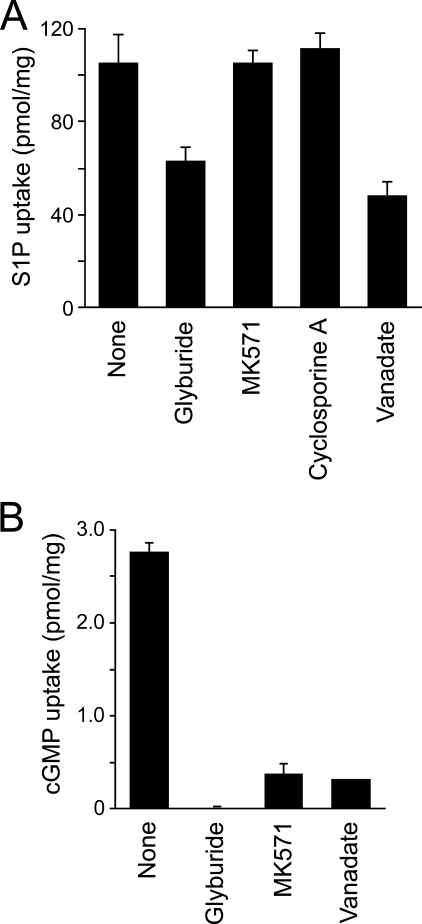
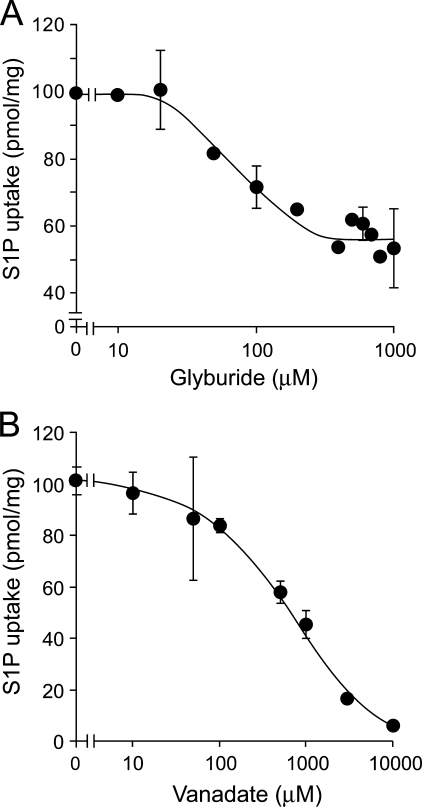
To further investigate the S1P transport mechanism in IOVs, we studied the effect of inhibitors on the uptake of [33P]S1P. Glyburide inhibited S1P release from intact erythrocytes (Table 1) and also inhibited the transport of [33P]S1P into the IOVs, whereas the other ABC transporter inhibitors, MK571 (MRP inhibitor) or cyclosporine A (a multidrug resistance protein inhibitor), had no effect in IOVs (Fig. 7A). At the same or lower concentrations of inhibitor, the uptake of cGMP, which is transported by MRP, was completely inhibited (Fig. 7B). Furthermore, a phosphate analog, vanadate, which inhibits a large number of ATPases, also efficiently reduced S1P uptake (Fig. 7A). Glyburide and vanadate reduced the [33P]S1P transport activity in a dose-dependent manner (Fig. 8). Despite the almost complete inhibition of the transport activity by vanadate at 10 mm, about half of the activity remained with glyburide treatment (up to 1 mm). This remaining S1P transport activity was additively inhibited by vanadate (supplemental Fig. 5). These results indicate that glyburide and vanadate affect different steps of the S1P transport mechanism and that an ATP-dependent glyburide- and vanadate-sensitive transporter is the S1P transporter in erythrocytes.
FIGURE 7.
Effects of ABC transporter inhibitors on the ATP-dependent transport of S1P into IOVs. A, IOVs were preincubated with 1 μm [33P]S1P in the presence of 100 μm glyburide, 20 μm MK571, and 10 μm cyclosporine A or 1 mm vanadate at 37 °C for 5 min and then incubated in the presence or absence of 2 mm ATP at 37 °C for 20 min. S1P uptake was expressed as the difference between the S1P amounts in the presence and absence of ATP. B, experiments were performed as described above except that 1 μm [3H]cGMP was used as the substrate instead of [33P]S1P.
FIGURE 8.
Effect of concentrations of glyburide and vanadate on the ATP-dependent transport of S1P into IOVs. A, inhibitory effects of indicated concentrations of glyburide were tested. ATP-dependent uptake of the [33P]S1P in IOVs was performed with 2 mm ATP at 37 °C for 20 min, as described under “Experimental Procedures.” B, inhibitory effects of indicated concentrations of vanadate were tested.
DISCUSSION
S1P in plasma is thought to play an important role in the induction of cellular responses in vascular endothelial cells and lymphocytes (5, 6, 25), and it is maintained at a constant concentration (26). Recently, several groups have shown that plasma S1P is supplied from erythrocytes (7, 9, 27). However, a detailed mechanism of the S1P release from the cells is still unclear. To demonstrate the direct evidence for the S1P transport across the erythrocyte membrane, we measured the ATP-dependent S1P transport into IOVs prepared from rat erythrocytes (Fig. 2, supplemental Fig. 1). BSA was required for the effective transport of S1P into the IOVs (supplemental Fig. 3) and for the extraction of S1P molecules from the membrane surface (21). In plasma most of the S1P binds to the carrier proteins, BSA and high density lipoprotein (28). Carrier proteins, therefore, most likely play an important role in maintaining the serum S1P level by protecting S1P from degradation enzymes and by extracting S1P from the membrane surface.
As shown in Fig. 2, about 130 pmol/mg of S1P was trapped in IOVs after a 20-min incubation without ATP, more than half of the S1P in presence of ATP. It was speculated that S1P, in the absence of ATP, was also transported into IOVs by diffusion. Although ATP-dependent uptake was enhanced with BSA inside the vesicles, the amount of the S1P trapped in IOVs without ATP was not changed with the addition of BSA (supplemental Fig. 3). Moreover, S1P transport is unidirectional, and S1P backflow from IOVs was not detected (supplemental Fig. 2). These results suggest that S1P is bound to the vesicle surface and does not diffuse into the IOVs without ATP.
Glyburide, an ABC transporter inhibitor, and vanadate, a global ATPase inhibitor, effectively inhibited the ATP-dependent S1P transport activity (Fig. 7). Several ABC transporters export lipid derivatives and are inhibited by glyburide or vanadate. For example, ABCA1 is involved in cholesterol efflux from cells and is inhibited by glyburide (29). Thus, an ABC transporter is a candidate for the S1P transporter in erythrocytes. However, the S1P transporter did not require ATP hydrolysis, as it was activated not only with the ATP but also with dATP and AMP-PNP (Fig. 6). In contrast to the AMP-PNP, another non-hydrolyzable ATP analog, ATPγS, did not support the S1P transport (Fig. 6). Among the adenine nucleotides, ATP, AMP-PNP, and dATP could support the S1P transport activity, whereas low or no transport activity was observed with ADP, AMP, and ATPγS. This difference might be due to the presence of a γ-phosphate group in the first group of nucleotides. Supporting this idea, a phosphate analog, vanadate, inhibited the ATP-dependent S1P transport activity (Fig. 8). These results suggest that ATP hydrolysis is not essential but that a phosphate group at the γ-position of ATP, dATP, and AMP-PNP is important for S1P transport activity. So far, most ABC transporters require ATP hydrolysis for the effective transport of their substrates. However, some members of the ABC proteins are activated by nonhydrolyzable ATP analogs. For example, the GSH flux mediated by cystic fibrosis transmembrane conductance regulator (ABCC7) is stimulated by ATP and AMP-PNP (30). It is possible that an ABC transporter could participate in S1P transport in erythrocytes without ATP hydrolysis.
In contrast to a previous report (7), our IOV preparations have some S1P dephosphorylation activity (data not shown), which can probably be attributed to differing methods of erythrocyte preparation. The previous report used purified erythrocytes, whereas we prepared erythrocyte IOVs using the conventional method (22), which may result in vesicles contaminated with phosphohydrolase activity from other cells such as platelets (7). Thus, it is possible that this phosphohydrolase activity was inhibited with ATP and affected the ATP-dependent S1P transport activity. This dephosphorylation activity was blocked by NaF and vanadate but not by the addition of ATP. Moreover, about 85% of the ATP-dependent transport activity was observed with the addition of 50 μm vanadate, sufficient for the complete inhibition of the S1P phosphohydrolase activity (Fig. 8). These results indicate that S1P dephosphorylation does not affect the S1P transport activity.
S1P release from the cells was initiated after increment of S1P concentration inside the erythrocyte cells (Fig. 1). This suggested that S1P transport follows the S1P concentration gradient and that ATP acts as a trigger to open the S1P transport pore of the transporter.
Based on previously reported results, we considered ABCA1, ABCA7, and ABCC1 as possible candidates for the S1P transporter (21, 23, 24). Because S1P release from erythrocytes and the ATP-dependent S1P transport in IOVs were inhibited by an ABCA1 inhibitor, glyburide, it was suggested that S1P is transported by ABCA1 in erythrocytes. However, ABCA1 was not detectable in the IOV (Fig. 4). Recently, Sato et al. (23) reported that ABCA1 plays an important role in S1P release from astrocytes. The calculated apparent Km value for ATP (130 μm) is also consistent with the reported Km value of ABCA1 (31). However, whereas the cholesterol efflux from ABCA1-expressing cells is dependent on apoA-I but not BSA (32, 33), S1P release from the platelets and erythrocytes was enhanced with BSA but not with apoA-1 (supplemental Fig. 3) (21). Furthermore, ABCA1-mediated cholesterol efflux and ATPase activity of ABCA1 were inhibited by glyburide but not by vanadate (29, 31). These results suggest that ABCA1 is not the S1P transporter of the blood cells.
Mitra et al. (24) reported that ABCC1 (MRP1) plays an important role in S1P release from mast cells. They showed that S1P release from mast cells was inhibited by an MRP inhibitor, MK571, and the down-regulation of ABCC1. Although ABCC1 was expressed in erythrocytes (Fig. 4), S1P release from erythrocytes and S1P uptake into IOVs were not affected by MK571 (Table 1, Fig. 7), indicating that S1P is not likely to be exported by ABCC1 (MRP1) in erythrocytes.
Previously, we showed that ABCA7, which has a high amino acid sequence homology to ABCA1, is preferentially expressed in platelets (34), suggesting that ABCA7 potentially participates in S1P release in platelets. In this study we showed that ABCA7 is expressed in rat platelets and erythrocytes (Fig. 4). Because ABCA7 has been reported to be involved in lipid release from transfected cells (35–40), it is possible that ABCA7 may function as an S1P transporter. However, Lee et al. (8) reported that serum S1P levels in ABCA1, ABCA7, and ABCC1 knock-out mice do not differ from those of wild-type mice.
During the preparation of this manuscript, we identified that spinster-like protein 2 (spns2) participates in S1P release from the cells and is essential for the zebrafish heart precursor migration (41). This protein is a candidate for the S1P transporter in erythrocyte, but little is known about the function of the spns2 in S1P secretion from the blood cells. Based on the amino acid sequences, spns2 is a member of the major facilitator superfamily-type transporter and does not have a typical ATP binding motif (41). The erythrocyte S1P transporter required ATP but not ATP hydrolysis for its activity. It will be interesting to examine whether spns2 requires the ATP binding for its activity. The identity of the protein that functions as the S1P transporter in blood cells is still to be determined. Different types of cells may have cell type-specific S1P transporters.
The effects of ABC transporter inhibitors on S1P release from erythrocytes and S1P uptake into erythrocyte membrane vesicles were similar to that of platelets (Table 1, Fig. 7) (21). Therefore, it is likely that the transporters contributing to ATP-dependent S1P transport in erythrocytes and platelets are closely related proteins. However, there is a difference in the requirement of the stimulus on S1P release between erythrocytes and platelets (Table 1) (21). Platelets store the synthesized S1P inside the cells and secrete it depending on various stimuli (17). However, erythrocytes secreted the newly formed S1P immediately after its synthesis (7, 16). This difference could be explained by the source of the secreted S1P and the difference in the activation of S1P transporters in erythrocytes and platelets. The S1P transporter in erythrocytes may be stimulated constitutively by a modification of the transporter, whereas the transporter in platelets is activated depending on the stimulus and modification, such as phosphorylation. We demonstrated that ATP-dependent S1P release from the semi-intact platelets was activated by thrombin treatment, which activates the protein kinase C through the G-protein-coupled thrombin receptor (21). Some ABC transporters, such as ABCA1 and ABCC2, are activated depending on phosphorylation by protein kinase A and protein kinase C, respectively (42, 43). Similar activation mechanisms may be present in the S1P transporter in blood cells.
We propose that an ATP-dependent transport participates in S1P release from erythrocytes (Fig. 9). To elucidate the physiological significance of the ATP-dependent S1P transport, we calculated the contribution of the ATP-dependent transport to the S1P release from intact erythrocytes. The rate of the S1P release from the erythrocytes was calculated from values in human erythrocytes reported by Ito et al. (7). When the amount of S1P inside the erythrocytes (108 cells) was about 38 pmol, the rate of S1P release was about 1.6 pmol/min. The erythrocyte volume and the mass of IOVs prepared from one erythrocyte cell were assumed to be 90 fl and 8.56 × 10−11 mg, respectively. Thus, the S1P concentration inside the erythrocyte (108 cells) was 4.2 μm (7), and the rate of S1P release was calculated to be 0.19 nmol/mg/min. Because the rate of S1P uptake is linear up to 10 μm S1P (Fig. 3), the rate of the S1P release from intact erythrocytes at 10 μm S1P was expected to be 0.45 nmol/mg/min. In the IOVs, the S1P uptake rate divided by the rate of membrane vesicle sidedness (∼0.5) was calculated to be 0.36 nmol/mg/min at 10 μm S1P. Therefore, the rate of the S1P uptake into IOVs was comparable with that of the released S1P from erythrocytes, indicating that S1P was released from erythrocytes mainly through the ATP-dependent transporter.
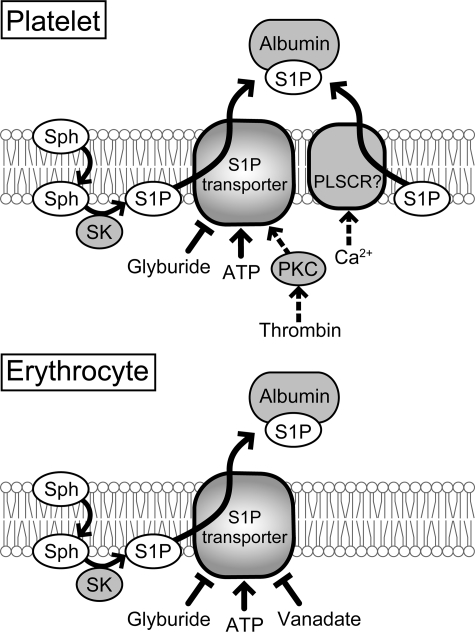
FIGURE 9.
Schematic model of S1P synthesis and export in platelets and erythrocytes. S1P is synthesized via the phosphorylation of sphingosine (Sph) by sphingosine kinase (SK). S1P was exported by ATP- and Ca2+-dependent transporters in platelets. However, thrombin-stimulated S1P export was mainly performed by the ATP-dependent transporter (21). Erythrocyte S1P transport systems do not have a Ca2+-dependent transporter and are not activated by any stimuli. PKC, protein kinase C; PLSCR, phospholipid scramblase.
In summary, our results provide direct evidence that S1P is unidirectionally transported across the membrane. This transport is ATP-dependent, but it did not require ATP hydrolysis for the S1P transport activity. Detailed analysis of the properties of the erythrocyte transporter indicated that S1P is specifically transported by an ATP-dependent glyburide- and vanadate-sensitive transporter. This is the first detailed characterization of the S1P transporters.
Supplementary Material
This study was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Takeda Science Foundation (to T. N. and A. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
N. Kobayashi and T. Nishi, unpublished result.
- S1P
- sphingosine 1-phosphate
- IOV
- inside-out membrane vesicle
- ABC
- ATP binding cassette
- AMP-PNP
- adenosine 5′-(β,γ-imido)triphosphate
- MRP
- multidrug resistance-associated protein
- TPA
- 12-O-tetradecanoylphorbol-13-acetate
- DHS1P
- dihydrosphingosine 1-phosphate
- ATPγS
- adenosine 5′-O-(thiotriphosphate)
- BSA
- bovine serum albumin
- mAb
- monoclonal antibody.
REFERENCES
- 1.Kihara A., Mitsutake S., Mizutani Y., Igarashi Y. (2007) Prog. Lipid Res. 46, 126–144 [DOI] [PubMed] [Google Scholar]
- 2.Rosen H., Goetzl E. J. (2005) Nat. Rev. Immunol. 5, 560–570 [DOI] [PubMed] [Google Scholar]
- 3.Hla T. (2004) Semin. Cell Dev. Biol. 15, 513–520 [DOI] [PubMed] [Google Scholar]
- 4.Spiegel S., Milstien S. (2003) Nat. Rev. Mol. Cell Biol. 4, 397–407 [DOI] [PubMed] [Google Scholar]
- 5.Matloubian M., Lo C. G., Cinamon G., Lesneski M. J., Xu Y., Brinkmann V., Allende M. L., Proia R. L., Cyster J. G. (2004) Nature 427, 355–360 [DOI] [PubMed] [Google Scholar]
- 6.Allende M. L., Dreier J. L., Mandala S., Proia R. L. (2004) J. Biol. Chem. 279, 15396–15401 [DOI] [PubMed] [Google Scholar]
- 7.Ito K., Anada Y., Tani M., Ikeda M., Sano T., Kihara A., Igarashi Y. (2007) Biochem. Biophys. Res. Commun. 357, 212–217 [DOI] [PubMed] [Google Scholar]
- 8.Lee Y. M., Venkataraman K., Hwang S. I., Han D. K., Hla T. (2007) Prostaglandins Other Lipid Mediat. 84, 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappu R., Schwab S. R., Cornelissen I., Pereira J. P., Regard J. B., Xu Y., Camerer E., Zheng Y. W., Huang Y., Cyster J. G., Coughlin S. R. (2007) Science 316, 295–298 [DOI] [PubMed] [Google Scholar]
- 10.Butter J. J., Koopmans R. P., Michel M. C. (2005) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 824, 65–70 [DOI] [PubMed] [Google Scholar]
- 11.Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., Mandala S., Spiegel S., Proia R. L. (2004) J. Biol. Chem. 279, 52487–52492 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt H., Schmidt R., Geisslinger G. (2006) Prostaglandins Other Lipid Mediat. 81, 162–170 [DOI] [PubMed] [Google Scholar]
- 13.Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y., Cyster J. G. (2005) Science 309, 1735–1739 [DOI] [PubMed] [Google Scholar]
- 14.Lo C. G., Xu Y., Proia R. L., Cyster J. G. (2005) J. Exp. Med. 201, 291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cyster J. G. (2005) Annu. Rev. Immunol. 23, 127–159 [DOI] [PubMed] [Google Scholar]
- 16.Yang L., Yatomi Y., Miura Y., Satoh K., Ozaki Y. (1999) Br. J. Haematol. 107, 282–293 [DOI] [PubMed] [Google Scholar]
- 17.Yatomi Y., Ruan F., Hakomori S., Igarashi Y. (1995) Blood 86, 193–202 [PubMed] [Google Scholar]
- 18.Anelli V., Bassi R., Tettamanti G., Viani P., Riboni L. (2005) J. Neurochem. 92, 1204–1215 [DOI] [PubMed] [Google Scholar]
- 19.Ancellin N., Colmont C., Su J., Li Q., Mittereder N., Chae S. S., Stefansson S., Liau G., Hla T. (2002) J. Biol. Chem. 277, 6667–6675 [DOI] [PubMed] [Google Scholar]
- 20.Prieschl E. E., Csonga R., Novotny V., Kikuchi G. E., Baumruker T. (1999) J. Exp. Med. 190, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi N., Nishi T., Hirata T., Kihara A., Sano T., Igarashi Y., Yamaguchi A. (2006) J. Lipid Res. 47, 614–621 [DOI] [PubMed] [Google Scholar]
- 22.Steck T. L., Kant J. A. (1974) Methods Enzymol. 31, 172–180 [DOI] [PubMed] [Google Scholar]
- 23.Sato K., Malchinkhuu E., Horiuchi Y., Mogi C., Tomura H., Tosaka M., Yoshimoto Y., Kuwabara A., Okajima F. (2007) J. Neurochem. 130, 2610–2619 [DOI] [PubMed] [Google Scholar]
- 24.Mitra P., Oskeritzian C. A., Payne S. G., Beaven M. A., Milstien S., Spiegel S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16394–16499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yatomi Y., Ohmori T., Rile G., Kazama F., Okamoto H., Sano T., Satoh K., Kume S., Tigyi G., Igarashi Y., Ozaki Y. (2000) Blood 96, 3431–3438 [PubMed] [Google Scholar]
- 26.Yatomi Y., Igarashi Y., Yang L., Hisano N., Qi R., Asazuma N., Satoh K., Ozaki Y., Kume S. (1997) J. Biochem. 121, 969–973 [DOI] [PubMed] [Google Scholar]
- 27.Hänel P., Andréani P., Gräler M. H. (2007) FASEB J. 21, 1202–1209 [DOI] [PubMed] [Google Scholar]
- 28.Aoki S., Yatomi Y., Ohta M., Osada M., Kazama F., Satoh K., Nakahara K., Ozaki Y. (2005) J. Biochem. 138, 47–55 [DOI] [PubMed] [Google Scholar]
- 29.Wang N., Silver D. L., Thiele C., Tall A. R. (2001) J. Biol. Chem. 276, 23742–23747 [DOI] [PubMed] [Google Scholar]
- 30.Kogan I., Ramjeesingh M., Li C., Kidd J. F., Wang Y., Leslie E. M., Cole S. P., Bear C. E. (2003) EMBO J. 22, 1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi K., Kimura Y., Kioka N., Matsuo M., Ueda K. (2006) J. Biol. Chem. 281, 10760–10768 [DOI] [PubMed] [Google Scholar]
- 32.Wang N., Silver D. L., Costet P., Tall A. R. (2000) J. Biol. Chem. 275, 33053–33058 [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi A., Takanezawa Y., Hirata T., Shimizu Y., Misasa K., Kioka N., Arai H., Ueda K., Matsuo M. (2006) J. Lipid Res. 47, 1791–1802 [DOI] [PubMed] [Google Scholar]
- 34.Sasaki M., Shoji A., Kubo Y., Nada S., Yamaguchi A. (2003) Biochem. Biophys. Res. Commun. 304, 777–782 [DOI] [PubMed] [Google Scholar]
- 35.Wang N., Lan D., Gerbod-Giannone M., Linsel-Nitschke P., Jehle A. W., Chen W., Martinez L. O., Tall A. R. (2003) J. Biol. Chem. 278, 42906–42912 [DOI] [PubMed] [Google Scholar]
- 36.Ikeda Y., Abe-Dohmae S., Munehira Y., Aoki R., Kawamoto S., Furuya A., Shitara K., Amachi T., Kioka N., Matsuo M., Yokoyama S., Ueda K. (2003) Biochem. Biophys. Res. Commun. 311, 313–318 [DOI] [PubMed] [Google Scholar]
- 37.Abe-Dohmae S., Ikeda Y., Matsuo M., Hayashi M., Okuhira K., Ueda K., Yokoyama S. (2004) J. Biol. Chem. 279, 604–611 [DOI] [PubMed] [Google Scholar]
- 38.Linsel-Nitschke P., Jehle A. W., Shan J., Cao G., Bacic D., Lan D., Wang N., Tall A. R. (2005) J. Lipid Res. 46, 86–92 [DOI] [PubMed] [Google Scholar]
- 39.Kim W. S., Fitzgerald M. L., Kang K., Okuhira K., Bell S. A., Manning J. J., Koehn S. L., Lu N., Moore K. J., Freeman M. W. (2005) J. Biol. Chem. 280, 3989–3995 [DOI] [PubMed] [Google Scholar]
- 40.Hayashi M., Abe-Dohmae S., Okazaki M., Ueda K., Yokoyama S. (2005) J. Lipid Res. 46, 1703–1711 [DOI] [PubMed] [Google Scholar]
- 41.Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A., Mochizuki N. (2009) Science 323, 524–527 [DOI] [PubMed] [Google Scholar]
- 42.See R. H., Caday-Malcolm R. A., Singaraja R. R., Zhou S., Silverston A., Huber M. T., Moran J., James E. R., Janoo R., Savill J. M., Rigot V., Zhang L. H., Wang M., Chimini G., Wellington C. L., Tafuri S. R., Hayden M. R. (2002) J. Biol. Chem. 277, 41835–41842 [DOI] [PubMed] [Google Scholar]
- 43.Ito K., Wakabayashi T., Horie T. (2005) Life Sci. 77, 539–550 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.