Abstract
Transient receptor potential channels have recently been implicated in physiological functions in a urogenital system. In this study, we investigated the role of transient receptor potential vanilloid 4 (TRPV4) channels in a stretch sensing mechanism in mouse primary urothelial cell cultures. The selective TRPV4 agonist, 4α-phorbol 12,13-didecanoate (4α-PDD) evoked Ca2+ influx in wild-type (WT) urothelial cells, but not in TRPV4-deficient (TRPV4KO) cells. We established a cell-stretch system to investigate stretch-evoked changes in intracellular Ca2+ concentration and ATP release. Stretch stimulation evoked intracellular Ca2+ increases in a stretch speed- and distance-dependent manner in WT and TRPV4KO cells. In TRPV4KO urothelial cells, however, the intracellular Ca2+ increase in response to stretch stimulation was significantly attenuated compared with that in WT cells. Stretch-evoked Ca2+ increases in WT urothelium were partially reduced in the presence of ruthenium red, a broad TRP channel blocker, whereas that in TRPV4KO cells did not show such reduction. Potent ATP release occurred following stretch stimulation or 4α-PDD administration in WT urothelial cells, which was dramatically suppressed in TRPV4KO cells. Stretch-dependent ATP release was almost completely eliminated in the presence of ruthenium red or in the absence of extracellular Ca2+. These results suggest that TRPV4 senses distension of the bladder urothelium, which is converted to an ATP signal in the micturition reflex pathway during urine storage.
Transient receptor potential vanilloid 4 (TRPV4),3 a member of the TRP superfamily of cation channels, is a Ca2+-permeable channel activated by a wide variety of physical and chemical stimuli (1, 2). TRPV4 was originally viewed as an osmo- or mechano-sensor, because the channel opens in response to hypotonicity-induced cell swelling (3–5) and shear stress (6). Alternatively, TRPV4 can be activated by diverse chemical stimuli such as synthetic phorbol ester 4α-phorbol 12,13-didecanoate (4α-PDD) (7), a botanical agent (bisandrographolide A), anandamide metabolites such as arachidonic acid and epoxyeicosatrienoic acids, as well as moderate warmth (>27 °C) (8–10). TRPV4 is widely expressed throughout the body, including renal epithelium, auditory hair cells, skin keratinocytes, hippocampus neurons, endothelial cells, and urinary bladder epithelium, thereby contributing to numerous physiological processes such as osmoregulation (11, 12), hearing (13), thermal and mechanical hyperalgesia (14, 15), neural activity in the brain (16), skin barrier recovery (17), and cell volume regulation (18). Therefore, the TRPV4 channel is now considered a multimodal transducer in various tissues and cells.
Non-neuronal cells within the urinary bladder wall (notably the transitional epithelial cells (urothelial cells)) function as a barrier against ions, solutes, and infection and also participate in the detection of physical and chemical stimuli (19–21). The urothelium expresses various sensory receptors and channels (bradykinin receptors, adrenergic/cholinergic receptors, nerve growth factor receptors, purinergic receptors, amiloride-sensitive Na+ channels, and TRP channels), all of which are substantially implicated in modulating bladder functions (22).
Recently, the potential roles of TRP channels have been explored in the bladder. Thus far, expression of TRPV1, TRPV2, TRPV4, TRPA1, and TRPM8 has been reported in different regions of urogenital tracts (21). TRPV1 is reportedly expressed in the epithelial cells lining the urothelium, in interstitial cells, and in sensory nerve terminals. TRPV1-deficient mice displayed a higher frequency of low amplitude non-voiding bladder contractions in comparison with wild-type (WT) mice (22), suggesting that TRPV1 is required for detection of bladder stretch, which involves stretch-evoked release of ATP and nitric oxide. The release of both mediators was reduced in the bladders of TRPV1-deficient mice. In a clinical setting, capsaicin or resiniferatoxin reduces bladder overactivity through desensitization of bladder afferents by acting on TRPV1 (23). Expression of other TRP channels, e.g. TRPM8 and TRPA1, was found in sensory C fibers in the bladder (24–27). The diagnostic ice water test is utilized to determine whether disturbance of bladder function involves neurogenic components, one of which could be related to TRPM8 function, in patients with spinal cord lesion (28). TRPA1 in sensory afferents is activated by several known ligands (allyl isothiocyanate and cinnamaldehyde), thereby inducing bladder overactivity (26). TRPV2 is expressed by several cell types in the rat bladder (29); however, its physiological function has not yet been investigated. TRPV4 is expressed in the urothelium and in smooth muscle cells of the urinary bladder (30, 31). Activation of the channel by specific ligands leads to augmentation of bladder contraction amplitude in cystometry and induction of bladder overactivity in vivo. In a separate cystometry analysis in conjunction with behavioral experiments, the intermicturitional interval was elongated and storage urine volume was increased in TRPV4-deficient mice compared with WT mice (32). Thus, TRPV4 may contribute to bladder function, especially to mediating bladder distention signals to primary afferent nerves during urine storage. However, whether urothelial TRPV4 is required for sensing mechanical stretch, or to what extent urothelial TRPV4 contributes to stretch-evoked ATP release, has not been precisely determined.
In the present study, we examined the functional contribution of TRPV4 to stretch-dependent urothelial cell responses and stretch-evoked ATP release in vitro. We first established a primary cell culture for mouse urothelium and retention of TRPV4 expression was confirmed. Because urothelial cells are physically extended during urine storage in vivo, we reproduced this phenomenon in an in vitro experiment using the uni-axial cell stretch system. All the experiments were performed by comparing urothelial cells obtained from WT mice and TRPV4-deficient mice to evaluate the correlation between TRPV4 expression and stretch responses. We demonstrated that urothelial cells sense mechanical stretch stimuli via TRPV4 channels, which induces robust Ca2+ influx and contributes to ATP release upon extension.
EXPERIMENTAL PROCEDURES
Animals
Wild-type (C57BL/6Cr) mice and TRPV4-deficient mice (12) backcrossed on a C57BL/6Cr background were used. All experiments were performed using 8- to 12-week-old male mice. All procedures were conducted in accordance with the policies of the Institutional Animal Care and Use Committee, National Institute for Physiological Sciences and University of Yamanashi.
Preparation of Primary Urothelial Cell Cultures
Whole bladders were taken from anesthetized mice, and the urothelial cells were prepared by modified procedures (33–35). Briefly, the bladder was inverted by pushing the dome downward through the bladder neck with a blunt 18-gauge needle. A suture was tied around the neck, and the inverted bladder was inflated with 100 μl of phosphate-buffered saline (PBS) through the needle. As the needle was removed, the suture was tightened to produce a distended “everted ball bladder” in which the urothelial surface was exposed. The everted ball bladder was incubated with 0.05% trypsin-EDTA for 30 min on a shaker at 37 °C. After replacing with PBS, urothelial cells were harvested and collected with a cell scraper. After pipetting, all urothelial cells were centrifuged (1000 rpm for 5 min), and the cell pellet was resuspended with keratinocyte serum-free medium (KSFM, Invitrogen) (34, 36). This medium was supplemented with epidermal growth factor (5 ng/ml), bovine pituitary extract (50 μg/ml), cholera toxin (30 ng/ml), penicillin (100 units/ml), and streptomycin (1 μg/ml) (all from Invitrogen). The urothelial cells were suspended in supplemented KSFM at 1 × 106 cells/ml. Drops (50 μl) of the cell suspension were seeded on 60-mm dishes or 0.01% collagen type I (Sigma)-coated coverslips (diameter, 12 mm, Warner Instruments Inc., Hamden, CT). The cells were incubated in a humidified atmosphere of 5% CO2 at 37 °C for 2 h to allow cells to attach, and the dishes were filled with additional KSFM medium. All the experiments with urothelial cells were performed after they had formed clusters, ∼72 h after cultivation.
RT-PCR and Real-time PCR of TRP Channels and CK7
Total RNA was isolated and purified from WT and TRPV4-deficient primary urothelial cell cultures using RNeasy micro kits (Qiagen) according to the manufacturer's instructions. Reverse transcription (RT)-PCR was performed with TaqMan 1step RT-PCR Master Mix Reagents (Takara, Kusatsu, Japan). The reverse transcription was performed at 55 °C for 60 min, followed by inactivation at 70 °C for 15 min. The temperature profile consisted of 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and elongation at 60 °C for 1 min. Real-time PCR analysis was performed using a Smart Cycler System (Cepheid, Sunnyvale, CA). The temperature profile consisted of 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 20 s, and elongation at 72 °C for 10 s. The gene-specific primers for these channels were designed with the on-line program Primer 3. The primer sequences for these channels are shown in Table 1. Intron-spanning primer pairs were used, except for trpv4 and trpa1 primer pairs for the real-time PCR analysis. Amplified PCR products were confirmed by electrophoresis on 1% agarose gel containing ethidium bromide. The amplified DNA fragments by PCR were identified as trpv4 fragments by DNA sequencing.
TABLE 1.
Genes and primers used in this study
| Gene | Primer | Product size |
|---|---|---|
| bp | ||
| Primers for RT-PCR | ||
| mTRPV1 | F: 5′-AACTCCACCCCACACTGAAG-3′ | 548 |
| R: 5′-TCGCCTCTGCAGGAAATACT-3′ | ||
| mTRPV4 | F: 5′-ACAACACCCGAGAGAACACC-3′ | 404 |
| R: 5′-CCCAAACTTACGCCACTTGT-3′ | ||
| mTRPA1 | F: 5′-AACTCCTCAACCACCCTGTG-3′ | 577 |
| R: 5′-CTGAGGCCAAAAGCCAGTAG-3′ | ||
| mTPPM8 | F: 5′-ATATGAGACCCGAGCAGTGG-3′ | 412 |
| R: 5′-GAGCAGCACATAGGCAAACA-3′ | ||
| mCK7 | F: 5′-TGCCAAGTTAGAGTCCAGCA-3′ | 350 |
| R: 5′-TTGATGGAATAGGCCCTGAG-3′ | ||
| mGAPDH | F: 5′-TGAAGGGTGGAGCCAAAAGG-3′ | 545 |
| R: 5′-GGAAGAGTGGGAGTTGCTGTTG-3′ | ||
| Primers for real-time PCR | ||
| mTRPV1 | F: 5′-CGGAAGACAGATAGCCTGAA-3′ | 134 |
| R: 5′-GCTCCATTCTCCACCAAGAG-3′ | ||
| mTRPV4 | F: 5′-TCACCTTCGTGCTCCTGTTG-3′ | 84 |
| R: 5′-AGATGTGCTTGCTCTCCTTG-3′ | ||
| mTRPA1 | F: 5′-GCTGGTGATGGATGAAGACA-3′ | 105 |
| R: 5′-ATGGACACATTGAAGCCAAG-3′ | ||
| mTPPM8 | F: 5′-GGCTCATCCACATTTTCACC-3′ | 113 |
| R: 5′-CACCATCCACACAGCAAAGA-3′ | ||
| mCK7 | Same as above | |
| mGAPDH | Same as above | |
Immunostaining
For immunohistochemistry, whole bladders were excised from male mice (WT and TRPV4-deficient) and fixed at 4 °C for 2 h in 4% paraformaldehyde in PBS, followed by cryoprotection at 4 °C in 20% sucrose, then 30% sucrose, each overnight. The samples were embedded with tissue-TEK OCT compound (Sakura, Torrance, CA) and frozen in liquid nitrogen. Cryostat sections (16 μm) mounted on glass slides were washed (3 × 5 min) with PBS supplemented with 0.3% Triton X-100 and blocked for 1 h at room temperature with Block Ace (Yukijirushi, Sapporo, Japan). The samples were incubated overnight at 4 °C with the first antibody (rabbit anti-TRPV4 antibody, a generous gift from Dr. B. Nilius) diluted 1:500 in blocking solution, washed (3 × 5 min) with PBS supplemented with 0.3% Triton X-100, and then reacted with the second antibody (Alexa Rb488, Molecular Probes, Eugene, OR) for 1 h at room temperature. For immunocytochemistry, primary urothelial cell cultures were fixed with 4% paraformaldehyde for 10 min and washed in PBS. Cells were incubated with the first antibody (rabbit anti-TRPV4 antibody, 1:500 and mouse monoclonal anti-cytokeratin 7 (CK7) antibody, 1:25, Dako Denmark A/S, Glostrup, Denmark) dissolved in Block Ace overnight at 4 °C and washed (4 × 15 min) with PBS supplemented with 0.3% Triton X-100, then covered with the second antibody (Alexa Rb488 and Ms546, Molecular Probe) for 1 h at room temperature. A different anti-TRPV4 antibody raised in our laboratory (16) was used in an attempt to stain primary culture cells; however, it showed high background with nonspecific nuclear staining (data not shown). The images were obtained with a fluorescence microscope (Olympus, Tokyo, Japan).
Mechanical Stretch Experiment
An elastic silicone chamber (STB-CH-04, STREX, Osaka, Japan) was attached to two pieces of glass by an adhesive agent, in which a slit 1-mm wide (from glass edge to edge) was formed in the center of the observation area. This customized design enables only part of the chamber to be extended upon stretching (supplemental Fig. S1, A and B). To reduce glue-derived cell toxicity, the following procedures were performed. After adhesion, the silicone chamber was dried at 50 °C overnight. The dried chamber was autoclaved in distilled water at 120 °C for 20 min. The chamber was left in autoclaved dH2O for 3 days, after which the chamber was ready for use. Primary urothelial cell suspensions (1 × 106 cells/ml, 50 μl) were added to the collagen-coated silicone chamber, followed by incubation at 37 °C for 2–3 h. Medium was added up to 200 μl, and cells were further cultured for 72 h. The chamber was carefully attached to an extension device (modified version of STB150, STREX) on the microscope stage (supplemental Fig. S1, C and D). Stretch stimulation was applied with preset stretch speed and distance.
Measurement of [Ca2+]i
primary cultures of urothelial cells were loaded with the fluorescent Ca2+ indicator (5 μm fura-2-acetoxymethyl ester, Molecular Probes) and nonionic surfactant (0.02% pluronic F-127, Sigma) in KSFM at 37 °C for 60 min. The cells were washed in an extracellular solution containing (in mm): 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose, pH 7.4, adjusted with NaOH. The chemical stimulants were applied by perfusion, or applied directly to the chamber when the cell stretch system was used. Ionomycin (5 μm, Sigma) was applied at the final step in each experiment for normalization and to check cell viability. Measurement of [Ca2+]i was performed by ratiometric imaging with fura-2 at 340 nm and 380 nm, and the emitted light signal was read at 510 nm. F340/F380 was calculated and acquired with an imaging processing system (IP-Lab, Scanalytics Inc., Rockville, MD). Changes in ratio (Δ) were calculated by subtracting basal values from peak values. 4α-PDD-responsive cells were defined as cells having >20% increase in Δ4α-PDD/Δionomycin.
Photon Imaging of ATP Release
Stretch-evoked ATP release from primary urothelial cell cultures was detected with a luciferin-luciferase bioluminescence assay. The stretch chamber and an extension device were set on a photon imaging system, and the chamber medium was replaced with extracellular solution containing a luciferase reagent (ATP bioluminescence assay kit CLS II, Roche Diagnostics, Basel, Switzerland). ATP bioluminescence during stretch stimulation was detected and visualized with a VIM camera (C2400-35, Hamamatsu Photonics, Hamamatsu, Japan) using an integration time of 30 s (37). The absolute ATP concentration was estimated using a standard ATP solution (Roche Diagnostics). The standard calibration curve yielded a correlation coefficient for bioluminescence versus ATP concentration of 0.9903 over a concentration range of 0 nm to 1 μm (supplemental Fig. S2). Data were imaged with Aquacosmos software (Hamamatsu Photonics) and analyzed with ImageJ 1.41 software (rsbweb.nih.gov/ij/).
Statistical Analysis
The experimental results were expressed as means ± S.E. The statistical significance of differences between two groups was determined by Student's t test or Fisher's protected least significant difference, and a p value of <0.05 was considered significant.
RESULTS
TRPV4 Is Highly Expressed in Mouse Bladder Epithelium
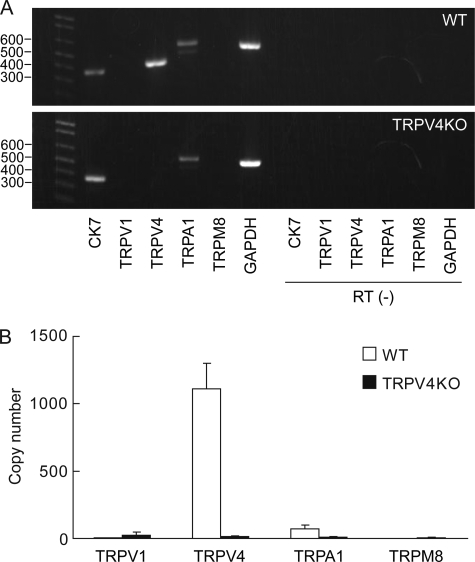
Several thermosensitive TRP channels, including TRPV4, are expressed in urinary bladder epithelium (20, 21). We, therefore, first confirmed gene expression of these TRP channels and a positive marker of primary urothelial cell cultures (Fig. 1). Primary 3-day urothelial cell cultures from both WT and TRPV4-deficient mice revealed expression of ck7, which is an intermediate filament protein present in all urothelial layers and considered a urothelial marker (38) (Fig. 1A). trpv4 expression was much more abundant than other thermosensitive TRP channels in WT urothelium, whereas no trpv4 expression was detected in TRPV4-deficient cells (Fig. 1, A and B). trpa1 seemed to be present in both cell types, while the expression of trpv1 and trpm8 genes was undetectable, unlike previous reports (21, 29, 39). We also confirmed expression of these genes in freshly dissected urothelium after trypsin treatment by a RT-PCR method, which showed no essential difference from data with culture cells (data not shown).
FIGURE 1.
Gene expression of TRP channels in mouse primary urothelial cell cultures. A, RT-PCR shows expression of trpv4 and trpa1 in wild-type (WT, upper panel) and expression of trpa1 in TRPV4-deficient (TRPV4KO, lower panel) primary urothelial cell cultures from mice. The cytokeratin 7 (ck7) gene is detected in both WT and TRPV4KO cells. In RT (−) lanes, reverse transcriptase enzyme was omitted (negative control). B, real-time PCR analysis shows that trpv4 is uniquely abundant in primary urothelial cell cultures. Copy numbers were calculated by standard curves of specific primer sets and normalized by the copy numbers of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1 × 106 copies).
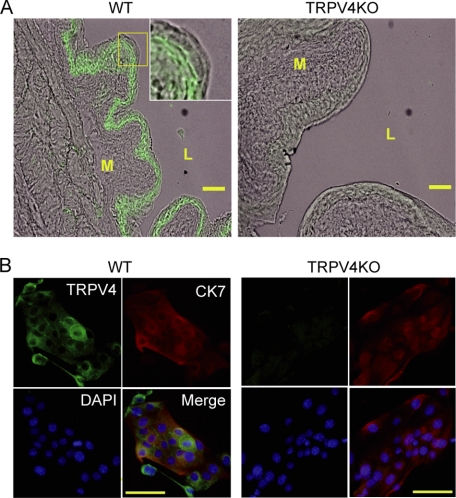
We performed a TRPV4 immunofluorescent study for whole bladder tissues and primary urothelial cell cultures. As previously reported (32), TRPV4-like immunoreactivity was present in basal and intermediate urothelial cell layers, but to a much lesser extent in an apical umbrella cell layer and a detrusor muscle suburothelial space in WT tissue (Fig. 2A, left). In contrast, TRPV4-like immunoreactivity was lacking in the bladder of TRPV4-deficient mice (Fig. 2A, right). Immunocytochemical analysis of primary urothelial cells obtained from both WT and TRPV4-deficient mice revealed that these cultured cells originated from urothelial cells with positive signals for CK7, and WT urothelial cells were stained with an anti-TRPV4 antibody as did the tissues, but TRPV4-deficient cells were negative for TRPV4 staining (Fig. 2B). The population of TRPV4-positive cells was ∼66.2% (2145 positive cells out of 3241 total cells). Contaminating interstitial cells (e.g. fibroblasts and muscle cells) showed no staining for either anti-CK7 or anti-TRPV4 antibody (supplemental Fig. S3). The contamination level of interstitial cells was ∼3.6% (48 cells out of 1350 total cells), and these cells were clearly distinguished from urothelial cells by their shapes, even without staining for CK7 and TRPV4. Therefore, we omitted the interstitial cells from analysis in the following experiments.
FIGURE 2.
Immunofluorescent analyses with mouse bladder and cultured urothelial cells. A, TRPV4 staining in mouse whole bladder tissue merged with a phase-contrast image. TRPV4-like immunoreactivity is mainly detected in basal and intermediate urothelial cell layers in wild-type (WT), but not in TRPV4-deficient (TRPV4KO) cells. A muscle layer, a suburothelial layer, and an urothelial apical cell layer (umbrella cells) appear to express much less TRPV4 in WT. An inset in the WT image was expanded from the square box to show TRPV4 localization in urothelial cell layers. M, muscle; L, lumen. Scale bars: 100 μm. B, immunocytochemical analysis of primary urothelial cell cultures. Cytokeratin 7 (CK7)-like immunoreactivity indicates that the cells are urothelial origin both in WT and TRPV4KO cell clusters, and TRPV4 signals are present in WT cells, but not in TRPV4KO cells. Green, TRPV4; red, CK7; and blue, 4′,6-diamidino-2-phenylindole. Scale bars: 100 μm.
Primary Urothelial Cell Cultures Retain Functional TRPV4
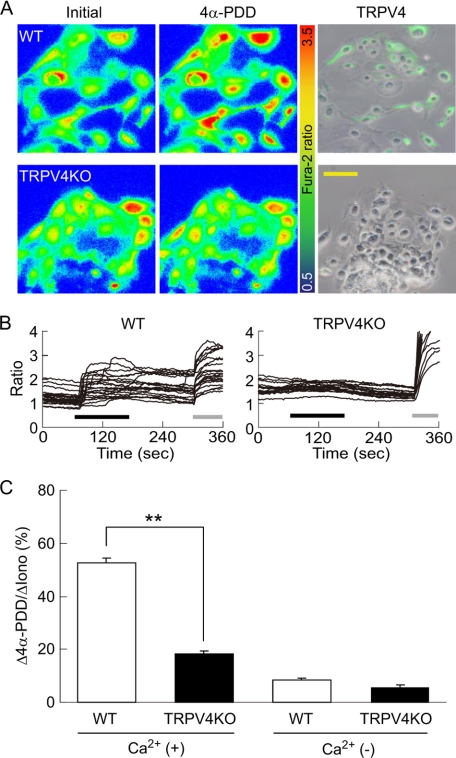
To determine whether TRPV4 expressed in urothelium was functional, we performed a Ca2+-imaging experiment to examine the effect of the TRPV4-selective agonist, 4α-PDD, on primary urothelial cell cultures. 4α-PDD evoked a prominent increase in intracellular Ca2+ concentration ([Ca2+]i) in WT urothelium, whereas the Ca2+ response to 4α-PDD was absent in TRPV4-deficient cells (Fig. 3, A (left and middle panels), B, and C). The population of 4α-PDD-responsive cells was ∼77.9% (141 positive cells out of 181 total cells), being not much different from that of TRPV4-positive cells in immunocytochemical analysis (Fig. 2B). After the Ca2+-imaging experiment, the urothelial cells were fixed and stained with an anti-TRPV4 antibody. In WT mice, the cells which showed the [Ca2+]i increase in response to 4α-PDD corresponded to TRPV4-positive cells (Fig. 3A, right). In contrast, TRPV4-deficient cells showed no TRPV4 immunoreactivity. Some TRPV4-negative cells were present in WT urothelium, possibly because primary urothelial cell cultures consisted of all the urothelial layers with faint TRPV4 expression in the apical cell layer (Fig. 2A). In the absence of extracellular Ca2+, WT and TRPV4-deficient urothelium showed minimal [Ca2+]i increase upon 4α-PDD stimulation (Fig. 3C), indicating that the [Ca2+]i increase is due to Ca2+ influx via TRPV4 channels. These results clearly show that primary urothelial cell cultures from WT mice conserve functional TRPV4 expression.
FIGURE 3.
Ca2+ imaging in response to 4α-PDD in primary urothelial cell cultures. A, representative pseudo-color images show that 4α-PDD stimulation causes robust [Ca2+]i increases in wild-type (WT) urothelial cells (upper left and middle panels), whereas TRPV4-deficient (TRPV4KO) cells fail to respond to 4α-PDD (lower left and middle panels). The right panels show the TRPV4-positive cells (shown in “green”) merged with a phase-contrast image after a Ca2+-imaging experiment. Scale bars: 100 μm. B, quantification of [Ca2+]i changes in individual cells displays 4α-PDD-evoked [Ca2+]i increases in WT cells, but not in TRPV4KO cells. All traces were obtained from the cells shown in A both in WT and TRPV4KO cells. Black bars: 10 μm 4α-PDD application; gray bars: 5 μm ionomycin application. C, the average peak [Ca2+]i increases in response to 10 μm 4α-PDD are significantly reduced in TRPV4KO cells in the presence of extracellular Ca2+ (Ca2+ (+)), but Ca2+ responses are negligible in both cell types in the absence of extracellular Ca2+ (Ca2+ (−)). All data are normalized to the responses to ionomycin as 100% and presented as means ± S.E. (n > 6). **, p < 0.005 (Student's t test).
TRPV4 Mediates the Response of Urothelium to Mechanical Stretch Stimulation
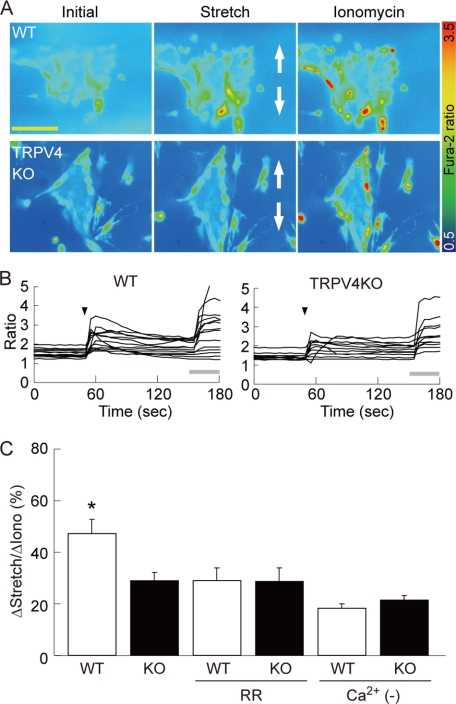
Recent investigations using TRPV4-deficient mice have revealed the involvement of TRPV4 in urinary bladder functions, such as distention-induced micturition (32). It has been proposed that TRPV4 is activated upon hypotonicity-induced cell swelling (3–5) and shear stress (6), suggesting that TRPV4 is mechanosensitive. We, therefore, established a uni-axial cell-stretch system for in vitro experiments (see “Experimental Procedures” and supplemental Fig. S1). Primary urothelial cells were seeded in an elastic silicone chamber, and the Ca2+ responses to stretch stimulation were examined in various combinations of stretch speeds and distances after 3 days of cultivation (supplemental Fig. S4). When the stretch distance was fixed at 500 μm, [Ca2+]i increased in a stretch speed-dependent manner in both WT and TRPV4-deficient urothelium. However, the [Ca2+]i increase was significantly lower in TRPV4-deficient cells at a stretch speed of 400 μm/s (supplemental Fig. S4A). When the stretch speed was fixed at 400 μm/s, [Ca2+]i increased in a stretch distance-dependent manner in both WT and TRPV4-deficient urothelium, but significant differences in [Ca2+]i increase were observed at stretch distances of 500 and 700 μm (Fig. 4, A–C, and supplemental Fig. S4B). A stretch distance of 500–700 μm theoretically induces 150–170% elongation of 1 mm width slit in the silicone chamber, but the actual extents of cell elongation in the chamber were 127 ± 1.3% at 500- and 138 ± 2.1% at 700-μm stretch (supplemental Fig. S4C). WT urothelial cells showed a transient [Ca2+]i increase immediately after stretching, followed by a sustained [Ca2+]i increase (Fig. 4B). The transient [Ca2+]i increase was small, whereas a sustained increase was retained in TRPV4-deficient cells. We next evaluated the effects of ruthenium red (RR, 10 μm), a broad TRP channel blocker, in the stretch experiments. In WT urothelial cells, RR significantly attenuated the stretch-evoked [Ca2+]i increase to the level of TRPV4-deficient cells, whereas no further reduction of [Ca2+]i response was observed in TRPV4-deficient urothelium (Fig. 4C). Furthermore, the stretch-evoked [Ca2+]i increase was significantly reduced in WT cells in the absence of extracellular Ca2+; however, TRPV4-deficient cells did not show such a reduction (Fig. 4C). These data strongly indicate the involvement of Ca2+ influx via TRPV4 in the mechanical stretch-evoked [Ca2+]i increase in primary urothelial cell cultures.
FIGURE 4.
Ca2+ responses to stretch stimulation in primary urothelial cell cultures. A, [Ca2+]i increases upon stretch stimulation by STREX in wild-type (WT) urothelial cells (upper panels), but the response is poor in TRPV4-deficient (TRPV4KO) cells (lower panels). All images were taken from cells seeded on the 1-mm slit area in the stretch chamber (refer to supplemental Fig. S1). The stretch speed was 400 μm/s, and the distance was 500 μm. Cells were extended to a vertical axis (indicated by arrows) in both cell types. Scale bars: 100 μm. B, quantification of [Ca2+]i changes in individual cells. Stretch evokes transient [Ca2+]i increases in WT cells, whereas only small and sustained [Ca2+]i increases are observed in TRPV4KO cells. All traces were obtained from the cells shown in A both in WT and TRPV4KO cells. Black arrowheads denote the onset of stretch. Gray bars: 5 μm ionomycin application. C, the average peak of [Ca2+]i increases is significantly reduced in TRPV4KO (KO) urothelium upon stretching. Ruthenium red (RR, 10 μm) attenuates the stretch-evoked [Ca2+]i increase in WT cells to the level achieved in TRPV4KO cells without RR treatment, but shows no effect on TRPV4KO cells. [Ca2+]i increases are similar between WT and TRPV4KO cells in the absence of extracellular Ca2+ (Ca2+ (−)). All the data are normalized to the values induced by 5 μm ionomycin application as 100% and presented as means ± S.E. (n > 7). An asterisk indicates significant difference between WT and the other five groups. *, p < 0.05 (Fisher's protected least significant difference).
Stretch-induced ATP Release from Urothelial Cells via TRPV4 Channel
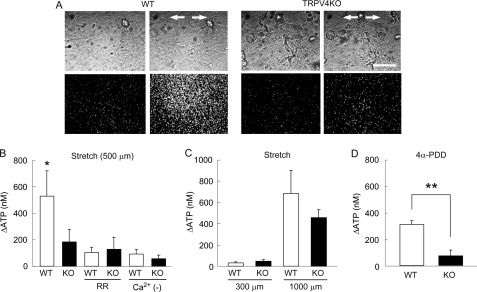
Bladder epithelium releases ATP upon distention, hypotonic stimulation, or stretch stimulation, which mediates signaling to primary afferent neurons via purinergic receptors (32, 40). To determine whether activation of TRPV4 is required for the stretch-evoked ATP release from urothelial cells, we modified the luciferin-luciferase chemiluminescence bioassay to detect released ATP levels by using a high sensitivity single photon counting camera in which photon counts correlate with extracellular ATP levels. Urothelial cells in a stretch chamber were extended using the same conditions as the Ca2+-imaging experiment (stretch distance: 500 μm = ∼127%, stretch speed: 400 μm/s). Upon stretch stimulation, prominent ATP release was observed in WT urothelial cells, whereas ATP released from TRPV4-deficient cells was significantly less (Fig. 5, A and B). Consistent with the Ca2+-imaging experiment, the ATP release was dramatically suppressed by application of RR or chelating extracellular Ca2+ in WT cells (Fig. 5B). RR treatment or Ca2+ chelation slightly affected the amount of the stretch-evoked ATP release in TRPV4-deficient cells; however, there was no significant reduction (Fig. 5B). When cells on the silicone chamber were extended for 300 μm, during which only small [Ca2+]i increases were observed in WT and TRPV4-deficient cells (supplemental Fig. S4B), small amounts of ATP were released from both WT and TRPV4-deficient cells (Fig. 5C). A 1000-μm stretch, which causes a maximal [Ca2+]i response both in WT and TRPV4-deficient urothelium (supplemental Fig. S4B), induced robust ATP release from WT and TRPV4-deficient cells (Fig. 5C). Interestingly, the amount of released ATP was higher in WT urothelium than that in TRPV4-deficient cells after a 1000-μm stretch, although there was no significant difference. We also examined ATP release upon 4α-PDD stimulation (Fig. 5D). 4α-PDD-evoked ATP release was significantly higher in WT urothelium than that in TRPV4-deficient cells. Collectively, these results suggest that TRPV4 channel activation and the resultant [Ca2+]i increase critically contribute to ATP release upon stretch stimulation in primary urothelial cell cultures.
FIGURE 5.
Visualization of stretch-evoked ATP release from primary urothelial cell cultures. A, ATP is robustly released from wild-type (WT) urothelial cells, but poorly released from TRPV4-deficient (TRPV4KO) cells upon stretching. The upper panels show urothelial cells in phase-contrast images, and the lower panels show photon count images (white dots) in the same field. The stretch speed was 400 μm/s, and the distance was 500 μm. Cells were extended transversely (indicated by arrows). Scale bars: 100 μm. B, the average amount of ATP released from TRPV4KO cells in response to mechanical stretch stimulation is significantly smaller than that in WT cells. ATP release is diminished by RR treatment or by the absence of extracellular Ca2+ (Ca2+ (−)) in WT cells. Data presented as means ± S.E. (n > 6). An asterisk indicates a significant difference between WT and the other five groups. *, p < 0.05 (Fisher's protected least significant difference). C, ATP release is minimal at a stretch distance of 300 μm and saturated at 1000 μm in both cell types (also refer to supplemental Fig. S4). Data are presented as means ± S.E. (n = 6). D, 4α-PDD (10 μm) induces robust ATP release by WT cells, but not by TRPV4KO cells. Data are presented as means ± S.E. (n = 6). Significant difference: **, p < 0.005 (Student's t test).
DISCUSSION
Here, we provide evidence that the TRPV4 channel is a candidate molecule involved in mechanosensory transduction in the urinary bladder. First, we demonstrated that trpv4 mRNA is uniquely abundant among the TRP channels that reportedly exist in urinary bladder epithelium. In the immunohistochemical study, TRPV4 was localized in basal and intermediate urothelial cell layers of mice bladders, and TRPV4 expression was also confirmed in primary cell cultures. Second, we observed the functional expression of TRPV4 in urothelial cells using a selective agonist, 4α-PDD in Ca2+-imaging experiments. Third, we showed that in vitro mechanical stretch stimulation of urothelial cells activated TRPV4, leading to increased [Ca2+]i and ATP release. These results clearly indicate that the TRPV4 channel participates in the mechanosensory pathway in urinary bladder.
TRPV4 has been implicated in a number of physiological processes, including osmoregulation (11, 12), hearing (13), thermal and mechanical hyperalgesia (14, 15), neural activity in the brain (16), skin barrier recovery (17), and cell volume regulation (18). Many of these processes require mechanosensitive molecules, and TRPV4 is a proposed mechanoreceptor responding to cell swelling (3–5) and shear stress (6). Nevertheless, the mechanosensitivity of this channel has been unclear. Upon cell swelling, activation of the phospholipase A2 and arachidonic acid signaling cascade is required prior to TRPV4 activation, which implies that the channel is not the initial mechanosensor in the signaling pathway (41). To our knowledge, this is the first study to reveal the activation of TRPV4 upon mechanical stretch in primary urothelium culture. In our cell-stretch system, extension-evoked Ca2+ responses were significantly diminished by TRPV4 blockade or by TRPV4-deficiency, and the levels were similar to those in the absence of extracellular Ca2+ in both genotypes (Fig. 4). These results suggest that TRPV4 constitutes the primary Ca2+ influx pathway in the mechanical stretch response in mouse urothelial cells. A recent report claimed that TRPV4 interacts with actin, which is important for regulatory volume decrease after cell swelling (42). The authors showed that hypotonicity-mediated [Ca2+]i increase was almost absent after disruption of actin fibers in trpv4-transfected cells. It was speculated that TRPV4 is linked to the actin cytoskeleton forming a mechanosensory complex, although they failed to detect direct interaction between TRPV4 and actin. Whether TRPV4 is directly gated by membrane stretch, or activated by upstream molecules remains to be clarified. Alternatively, the TRPV4-independent [Ca2+]i increase (Fig. 4 and supplemental Fig. S4) suggests that there could be other mechanosensitive molecules. Multiple mechanosensory systems with different thresholds might regulate urinary bladder responses upon distension.
Stretch-evoked ATP release also appeared to be TRPV4-dependent. The amount of released ATP was dramatically reduced following RR treatment of WT urothelial cells or in TRPV4-deficient cells, and the stretch-evoked ATP release in WT cells required extracellular Ca2+ (Fig. 5). Given that TRPV4 causes major Ca2+ influx (Fig. 4), channel activation is critical for ATP release upon stretch stimulation. This is supported by the fact that pharmacological activation of TRPV4 resulted in robust ATP release in WT urothelial cells, but not in TRPV4-deficient cells (Fig. 5). The amount of 4α-PDD-evoked ATP release was less than that evoked by stretch stimulation in WT cells, and TRPV4-deficient cells displayed small amount of ATP release upon stretch stimulation. These results imply the existence of TRPV4-independent ATP-releasing pathway(s) responsible for stretch stimulation. So far, two general mechanisms for ATP release by epithelia have been proposed. One is conductive release through ion channels (43, 44), and the other is exocytotic release with ATP-enriched vesicles (45), which is regulated by intracellular Ca2+ levels. The mechanism for TRPV4-mediated ATP release remains unclear, and several pathways could be involved (46). For example, Ca2+ influx via TRPV4 might activate anion channels through which ATP could be released (43), or connexin hemi channels (47). Alternatively, ATP could also be released through vesicular exocytosis by a phosphatidylinositol 3-kinase-mediated pathway (45, 46).
ATP released from the urothelium is important for paracrine signaling to neighboring cells such as afferent nerve endings, surrounding muscle cells, and umbrella cells, because ATP regulates various bladder functions, including micturition reflex, non-voiding contractions, and increase of membrane capacitance (40). It is very interesting that TRPV4 is expressed in both the urothelial basal and intermediate layers in the urinary bladder, because primary afferent nerves and detrusor smooth muscles are presented adjacent to the urothelium. Transmitter signals such as ATP from urothelium could be directly conveyed to these nerves and muscles. Indeed, investigation using ionotropic ATP receptor 3 (P2X3)-null mice demonstrated that ionotropic ATP receptor 3 (P2X3)-deficiency caused a significant increase in intermicturition interval and total void volume (48), and TRPV4-deficient mice showed similar phenotypes (32). Thus, these data clearly indicate that mechanical stimulus-dependent activation of TRPV4 in urothelial cell layers is a key event for ATP signaling in the micturition reflex pathway.
The extent of TRPV4 involvement seems different in Ca2+ influx and ATP release. TRPV4 involvement in total Ca2+ influx upon stretching is modest, whereas ATP release critically depends on TRPV4 activation upon stretching (Figs. 4 and 5). This raises a possibility that there are physical interactions and/or functional relays between TRPV4 and an ATP-releasing system, by which Ca2+ influx via TRPV4 specifically promotes ATP release, while intracellular Ca2+ increase through other mechanisms has only a minor contribution to the releasing system. This hypothesis is supported by the fact that ATP release was lower in TRPV4-deficient cells when the urothelium was stretched for 1000 μm (Fig. 5C), whereas [Ca2+]i increases evoked by the same stretch distance were indistinguishable between WT and TRPV4-deficient cells (supplemental Fig. S4B). However, we should be careful when interpreting our results to consider the contribution of TRPV4 channels in vivo. In our in vitro system, cells must be extended >120% at 400 μm/s to induce TRPV4 activation, whereas bladder is probably stretched more slowly upon urine storage. One explanation for this inconsistency is that our mechanical stimulation system stretches cells in a uni-axial direction, whereas bladder undergoes multidirectional distension with hydrostatic pressure. Alternatively, stretching experiments were done at room temperature because of technical reasons, whereas bladder is kept at core body temperature, at which warmth-activated TRPV4 channels could be more sensitive to other stimuli. Such physiological conditions might be more effective for TRPV4 activation with less extension.
Another TRP channel implicated in urinary bladder function is TRPV1, which contributes to bladder sensation with its expression in the urothelium and bladder afferent nerves (22, 29). However, we failed to detect trpv1 gene expression in our primary urothelial cell cultures even after acute disaggregation with 30-min trypsin treatment. On the other hand, trpa1 expression was observed in primary urothelial cells (Fig. 1). Some reports have claimed that TRPA1 has mechanosensitivity, although the data are not conclusive. In our study, TRPV4-independent Ca2+ influx during stretch stimulation was small (Fig. 4), and one or more TRPV4-independent pathways were not primal in ATP release (Fig. 5). These results suggest that mechanosensory systems independent of TRPV4 (including one involving TRPA1) make only minor contributions to stretch-induced ATP release in urothelial cells.
In a clinical setting, overactive bladder, which is a urine storage disorder defined as urinary urgency with little urine accumulation, has been thought to correlate with urothelial pathogenesis. Recently, it has been speculated that distension during urine storage stimulates urothelial cells to release various chemical mediators (e.g. ATP, prostaglandin, NO, and acetylcholine), which then transfer signals to afferent nerves or muscle cells (19). Some abnormalities in this pathway could cause overactive bladder (20, 39, 49). We currently have no evidence for the involvement of mechanical stretch stimulation in the induced release of other chemical mediators from primary urothelial cell cultures, which should be examined in the future. Given that TRPV4 is critically involved in the sensing mechanism in the bladder, development of chemicals modulating TRPV4 activity would hopefully lead to the treatment of bladder disorders such as overactive bladder.
Supplementary Material
Acknowledgment
We greatly appreciate the gift of rat anti-TRPV4 antibody from Dr. Bernd Nilius (Katholieke Universiteit Leuven, Dept. of Molecular Cell Biology, Laboratory Ion Channel Research).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- TRPV4
- transient receptor potential vanilloid 4
- 4α-PDD
- 4α-phorbol 12,13-didecanoate
- CK7
- cytokeratin 7
- RR
- ruthenium red
- OAB
- overactive bladder
- WT
- wild type
- PBS
- phosphate-buffered saline
- KSFM
- keratinocyte serum-free medium
- RT
- reverse transcription.
REFERENCES
- 1.Nilius B., Watanabe H., Vriens J. (2003) Pflugers Arch. 446, 298–303 [DOI] [PubMed] [Google Scholar]
- 2.Nilius B., Owsianik G., Voets T., Peters J. A. (2007) Physiol. Rev. 87, 165–217 [DOI] [PubMed] [Google Scholar]
- 3.Strotmann R., Harteneck C., Nunnenmacher K., Schultz G., Plant T. D. (2000) Nat. Cell Biol. 2, 695–702 [DOI] [PubMed] [Google Scholar]
- 4.Liedtke W., Choe Y., Martí-Renom M. A., Bell A. M., Denis C. S., Sali A., Hudspeth A. J., Friedman J. M., Heller S. (2000) Cell 103, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilius B., Prenen J., Wissenbach U., Bödding M., Droogmans G. (2001) Pflugers Arch. 443, 227–233 [DOI] [PubMed] [Google Scholar]
- 6.Gao X., Wu L., O'Neil R. G. (2003) J. Biol. Chem. 278, 27129–27137 [DOI] [PubMed] [Google Scholar]
- 7.Watanabe H., Davis J. B., Smart D., Jerman J. C., Smith G. D., Hayes P., Vriens J., Cairns W., Wissenbach U., Prenen J., Flockerzi V., Droogmans G., Benham C. D., Nilius B. (2002) J. Biol. Chem. 277, 13569–13577 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H., Vriens J., Suh S. H., Benham C. D., Droogmans G., Nilius B. (2002) J. Biol. Chem. 277, 47044–47051 [DOI] [PubMed] [Google Scholar]
- 9.Güler A. D., Lee H., Iida T., Shimizu I., Tominaga M., Caterina M. (2002) J. Neurosci. 22, 6408–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilius B., Vriens J., Prenen J., Droogmans G., Voets T. (2004) Am. J. Physiol. Cell Physiol. 286, C195–C205 [DOI] [PubMed] [Google Scholar]
- 11.Liedtke W., Friedman J. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13698–13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuno A., Matsumoto N., Imai M., Suzuki M. (2003) Am. J. Physiol. Cell Physiol. 285, C96–C101 [DOI] [PubMed] [Google Scholar]
- 13.Tabuchi K., Suzuki M., Mizuno A., Hara A. (2005) Neurosci. Lett. 382, 304–308 [DOI] [PubMed] [Google Scholar]
- 14.Todaka H., Taniguchi J., Satoh J., Mizuno A., Suzuki M. (2004) J. Biol. Chem. 279, 35133–35138 [DOI] [PubMed] [Google Scholar]
- 15.Grant A. D., Cottrell G. S., Amadesi S., Trevisani M., Nicoletti P., Materazzi S., Altier C., Cenac N., Zamponi G. W., Bautista-Cruz F., Lopez C. B., Joseph E. K., Levine J. D., Liedtke W., Vanner S., Vergnolle N., Geppetti P., Bunnett N. W. (2007) J. Physiol. 578, 715–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibasaki K., Suzuki M., Mizuno A., Tominaga M. (2007) J. Neurosci. 27, 1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denda M., Sokabe T., Fukumi-Tominaga T., Tominaga M. (2007) J. Invest. Dermatol. 127, 654–659 [DOI] [PubMed] [Google Scholar]
- 18.Arniges M., Vázquez E., Fernández-Fernández J. M., Valverde M. A. (2004) J. Biol. Chem. 279, 54062–54068 [DOI] [PubMed] [Google Scholar]
- 19.Araki I., Du S., Kobayashi H., Sawada N., Mochizuki T., Zakoji H., Takeda M. (2008) Int. J. Urol. 15, 681–687 [DOI] [PubMed] [Google Scholar]
- 20.Birder L. A., de Groat W. C. (2007) Nat. Clin. Pract. Urol. 4, 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everaerts W., Gevaert T., Nilius B., De Ridder D. (2008) Neurourol. Urodyn. 27, 264–273 [DOI] [PubMed] [Google Scholar]
- 22.Birder L. A., Nakamura Y., Kiss S., Nealen M. L., Barrick S., Kanai A. J., Wang E., Ruiz G., De Groat W. C., Apodaca G., Watkins S., Caterina M. J. (2002) Nat. Neurosci. 5, 856–860 [DOI] [PubMed] [Google Scholar]
- 23.Avelino A., Cruz F. (2006) Naunyn Schmiedebergs Arch. Pharmacol. 373, 287–299 [DOI] [PubMed] [Google Scholar]
- 24.Du S., Araki I., Kobayashi H., Zakoji H., Sawada N., Takeda M. (2008) Urology 72, 450–455 [DOI] [PubMed] [Google Scholar]
- 25.Du S., Araki I., Yoshiyama M., Nomura T., Takeda M. (2007) Urology 70, 826–831 [DOI] [PubMed] [Google Scholar]
- 26.Andrade E. L., Ferreira J., André E., Calixto J. B. (2006) Biochem. Pharmacol. 72, 104–114 [DOI] [PubMed] [Google Scholar]
- 27.Tsukimi Y., Mizuyachi K., Yamasaki T., Niki T., Hayashi F. (2005) Urology 65, 406–410 [DOI] [PubMed] [Google Scholar]
- 28.Mukerji G., Yiangou Y., Corcoran S. L., Selmer I. S., Smith G. D., Benham C. D., Bountra C., Agarwal S. K., Anand P. (2006) BMC Urol. 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birder L. A., Kanai A. J., de Groat W. C., Kiss S., Nealen M. L., Burke N. E., Dineley K. E., Watkins S., Reynolds I. J., Caterina M. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 13396–13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birder L., Kullmann F. A., Lee H., Barrick S., de Groat W., Kanai A., Caterina M. (2007) J. Pharmacol. Exp. Ther. 323, 227–235 [DOI] [PubMed] [Google Scholar]
- 31.Thorneloe K. S., Sulpizio A. C., Lin Z., Figueroa D. J., Clouse A. K., McCafferty G. P., Chendrimada T. P., Lashinger E. S., Gordon E., Evans L., Misajet B. A., Demarini D. J., Nation J. H., Casillas L. N., Marquis R. W., Votta B. J., Sheardown S. A., Xu X., Brooks D. P., Laping N. J., Westfall T. D. (2008) J. Pharmacol. Exp. Ther. 326, 432–442 [DOI] [PubMed] [Google Scholar]
- 32.Gevaert T., Vriens J., Segal A., Everaerts W., Roskams T., Talavera K., Owsianik G., Liedtke W., Daelemans D., Dewachter I., Van Leuven F., Voets T., De Ridder D., Nilius B. (2007) J. Clin. Invest. 117, 3453–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurzrock E. A., Lieu D. K., de Graffenried L. A., Isseroff R. R. (2005) J. Urol. 173, 281–285 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y. Y., Ludwikowski B., Hurst R., Frey P. (2001) In Vitro Cell Dev. Biol. Anim. 37, 419–429 [DOI] [PubMed] [Google Scholar]
- 35.Kreft M. E., Hudoklin S., Sterle M. (2005) Folia Biol. Praha 51, 126–132 [DOI] [PubMed] [Google Scholar]
- 36.Kreft M. E., Romih R., Sterle M. (2002) Cell Biol. Int. 26, 63–74 [DOI] [PubMed] [Google Scholar]
- 37.Koizumi S., Fujishita K., Inoue K., Shigemoto-Mogami Y., Tsuda M., Inoue K. (2004) Biochem. J. 380, 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erman A., Veranic P., Psenicnik M., Jezernik K. (2006) Tissue Cell 38, 293–301 [DOI] [PubMed] [Google Scholar]
- 39.Birder L. A. (2005) Am. J. Physiol. Renal Physiol. 289, F489–F495 [DOI] [PubMed] [Google Scholar]
- 40.Wang E. C., Lee J. M., Ruiz W. G., Balestreire E. M., von Bodungen M., Barrick S., Cockayne D. A., Birder L. A., Apodaca G. (2005) J. Clin. Invest. 115, 2412–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vriens J., Watanabe H., Janssens A., Droogmans G., Voets T., Nilius B. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker D., Bereiter-Hahn J., Jendrach M. (2009) Eur. J. Cell Biol. 88, 141–152 [DOI] [PubMed] [Google Scholar]
- 43.Sabirov R. Z., Okada Y. (2005) Purinergic Signal. 1, 311–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suadicani S. O., Brosnan C. F., Scemes E. (2006) J. Neurosci. 26, 1378–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coco S., Calegari F., Pravettoni E., Pozzi D., Taverna E., Rosa P., Matteoli M., Verderio C. (2003) J. Biol. Chem. 278, 1354–1362 [DOI] [PubMed] [Google Scholar]
- 46.Fitz J. G. (2007) Trans. Am. Clin. Climatol. Assoc. 118, 199–208 [PMC free article] [PubMed] [Google Scholar]
- 47.Stout C. E., Costantin J. L., Naus C. C., Charles A. C. (2002) J. Biol. Chem. 277, 10482–10488 [DOI] [PubMed] [Google Scholar]
- 48.Cockayne D. A., Hamilton S. G., Zhu Q. M., Dunn P. M., Zhong Y., Novakovic S., Malmberg A. B., Cain G., Berson A., Kassotakis L., Hedley L., Lachnit W. G., Burnstock G., McMahon S. B., Ford A. P. (2000) Nature 407, 1011–1015 [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura N., Kaiho Y., Miyazato M., Yunoki T., Tai C., Chancellor M. B., Tyagi P. (2008) Naunyn Schmiedebergs Arch. Pharmacol. 377, 437–448 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.