Abstract
The neurodegenerative disorder spinal and bulbar muscular atrophy or Kennedy disease is caused by a CAG trinucleotide repeat expansion within the androgen receptor (AR) gene. The resulting expanded polyglutamine tract in the N-terminal region of the receptor renders AR prone to ligand-dependent misfolding and formation of oligomers and aggregates that are linked to neuronal toxicity. How AR misfolding is influenced by post-translational modifications, however, is poorly understood. AR is a target of SUMOylation, and this modification inhibits AR activity in a promoter context-dependent manner. SUMOylation is up-regulated in response to multiple forms of cellular stress and may therefore play an important cytoprotective role. Consistent with this view, we find that gratuitous enhancement of overall SUMOylation significantly reduced the formation of polyglutamine-expanded AR aggregates without affecting the levels of the receptor. Remarkably, this effect requires SUMOylation of AR itself because it depends on intact AR SUMOylation sites. Functional analyses, however, indicate that the protective effects of enhanced AR SUMOylation are not due to alterations in AR transcriptional activity because a branched protein structure in the appropriate context of the N-terminal region of AR is necessary to antagonize aggregation but not for inhibiting AR transactivation. Remarkably, small ubiquitin-like modifier (SUMO) attenuates AR aggregation through a unique mechanism that does not depend on critical features essential for its interaction with canonical SUMO binding motifs. Our findings therefore reveal a novel function of SUMOylation and suggest that approaches that enhance AR SUMOylation may be of clinical use in polyglutamine expansion diseases.
Spinal and bulbar muscular atrophy (SBMA),2 or Kennedy disease, is an inherited degenerative disorder of lower motor neurons (1, 2). SBMA is characterized by muscle cramps and fasciculations followed by progressive weakness and atrophy of the proximal limb and bulbar muscles (3–5). The causative genetic alteration is an expansion in the length of a CAG trinucleotide repeat within the coding sequence of the androgen receptor (AR) gene, leading to an expanded polyglutamine tract in the N-terminal transcriptional regulatory domain of the receptor. A similar expansion within the coding sequence of a set of additional genes is responsible for other members of the polyglutamine class of protein folding diseases (6, 7), which include Huntington disease, several autosomal dominant spinocerebellar ataxias (SCAs) (8), and dentatorubral-pallidoluysian atrophy (9, 10).
The length of the CAG repeat within AR is correlated to the severity of SBMA. Although the normal repeat length is highly polymorphic and ranges between 9 and 36 copies, overt disease is associated with lengths in the range of 38–62 repeats. The presence of an expanded polyglutamine tract within AR renders the protein prone to hormone-dependent misfolding, oligomerization, and aggregation and to the formation of microscopically visible nuclear and/or cytosolic inclusions in neurons and in neuronal processes (11). Although such large inclusions were initially proposed to be the proximal cause of neurodegeneration, their presence is not always correlated with the disease (12–16), leading to the distinction between aggregation and formation of inclusions. In fact, inclusions may represent a protective mechanism to sequester pathologically misfolded forms into a more innocuous form.
Multiple mechanisms of neurotoxicity have been proposed, including alterations in transcriptional programs, mitochondrial dysfunction, and proteotoxic stress due to excessive demands placed on the ubiquitin-proteasome pathway. In addition, alterations in cellular transport mechanisms, which are critical to the function of motoneurons, have been also implicated (3, 4). Although the dominant inheritance of SBMA indicates the gain of a toxic function, an expanded polyglutamine tract in AR is also associated with a partial loss of function in its transcriptional properties (17, 18), and other polyglutamine diseases are also associated with alterations in transcriptional programs, presumably by altering the activity of transcriptional coregulators (10, 19, 20).
There is significant interest in understanding SBMA and other polyglutamine diseases because they provide a window to the cellular mechanisms responsible for coping with the stress of misfolded proteins. As in the case of other signaling processes, post-translational modifications are likely to play an important regulatory role in the adaptive responses to polyglutamine-induced aggregation. Their identity and mechanism, however, remain to be defined. In this regard, SUMOylation has been implicated in the pathogenesis of neurodegenerative diseases (21, 22). Thus, enhanced neuronal immunoreactivity for SUMO1 is observed in dentatorubral-pallidoluysian atrophy, SCA-1, Machado-Joseph disease (also known as SCA-3), and Huntington disease (23). Although these studies implicate SUMOylation in neurodegeneration, the mechanisms involved and how these pathways exert effects on protein aggregation and/or degradation are unclear. The fact that AR is a well characterized target of SUMOylation, however, suggested that this modification might play an important role in SBMA.
The conjugation of small ubiquitin-like modifier proteins or SUMOylation is a post-translational modification process that shares common ancestry and core enzymological features with ubiquitination but exerts distinct functional roles. Conjugation of SUMO requires SUMO-specific E1-activating (SAE1/SAE2) and E2-conjugating (UBC9) enzymes. UBC9, which interacts directly with specific substrates (24), catalyzes the formation of an isopeptide bond between the C terminus of SUMO and the amino group of the target lysine. This step is facilitated by SUMO E3 ligases such as RanBP2 and members of the protein inhibitor of activated STAT (PIAS) family (25–27). SUMOylation is reversible, and specific isopeptidases release the SUMO moiety (28). Four mammalian SUMO isoforms have been identified (SUMO1, -2, -3, and -4). SUMO2 and -3 are closely related, whereas SUMO1 shares 48% identity to either SUMO2 or SUMO3 (29, 30). A more recently identified gene encodes a fourth isoform very similar to SUMO2/3 (31). This form harbors a Pro residue at position 90 that prevents initial processing by known SUMO protease enzymes and subsequent conjugation (32). Whether this member functions through non-covalent interactions only remains to be determined. Notably, SUMO2 and -3 harbor a consensus SUMOylation site in their N-terminal region and can form SUMO chains. Recent data indicate that SUMO chains can have specific functions such as facilitating the recruitment of ubiquitin ligases, which in turn can target poly-SUMOylated and polyubiquitinated proteins for degradation (33–35). Such chains, however, appear to be dispensable for the transcriptional functions of SUMO (36).
AR was one of the first transcription factors shown to be SUMOylated (37), and the two main sites of conjugation (Lys-385 and Lys-518) lie in the N-terminal region (see Fig. 1). One of the prominent functional roles of SUMOylation in AR is to mediate the regulatory effects of synergy control or SC motifs. These short regulatory sequences (see Fig. 1) were first identified in members of the steroid receptor family such as the glucocorticoid receptor and AR but are found in multiple transcription factors (38–40). SC motifs exert a promoter context-dependent inhibitory effect and selectively restrain the transcriptional activation of factors when stably bound to multiple, closely spaced instances of their cognate response element. In contrast, SC motifs are functionally silent when activators are bound to a single site (36, 40, 41). Through multiple approaches, we (26, 36, 39, 42) and others (37, 43–53) have demonstrated that SC motifs exert their effects by serving as sites for SUMO modification. Furthermore, recent functional and structural studies by our group as well as concordant findings of others indicate that once conjugated, individual SUMO isoforms mediate their transcriptional effects through a conserved effector surface (42, 54–57). This pocket is thought to facilitate the recruitment of additional factors by virtue of its ability to directly bind short Val/Leu-rich SUMO-interacting motifs, or SIMs (54, 55, 58). The identity of the SUMO interacting factors responsible for the effects of SC motifs, however, remains to be fully defined.
FIGURE 1.
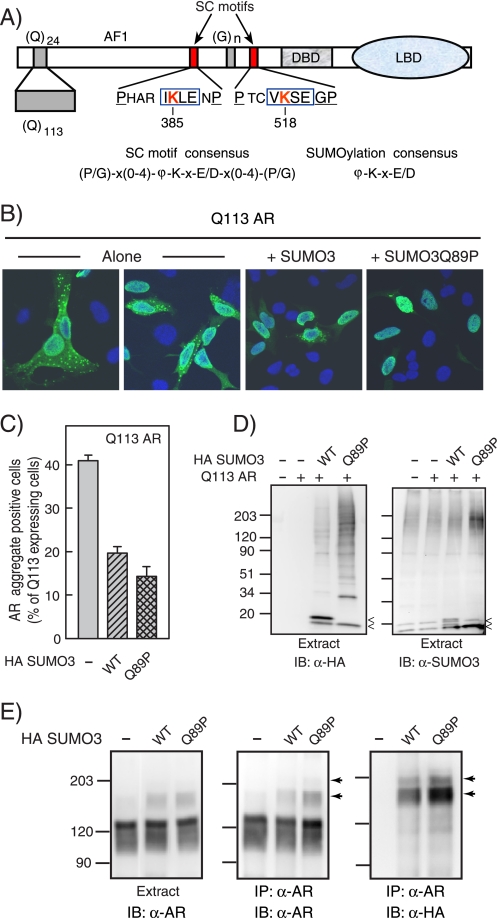
Enhanced SUMOylation inhibits polyglutamine-expanded androgen receptor aggregation. A, schema of human AR highlighting key structural features including the polyglutamine tract and the two SC motifs. The core SC motifs are boxed with lysines (Lys-385 and Lys-518) targeted for SUMOylation in red. DBD, DNA binding domain; LBD, ligand binding domain. The SC motif and SUMOylation consensus sequences are also shown. B, HeLa cells were co-transfected with 1 μg of the Gln113 WT AR and 0.3 μg of control, HA-SUMO3, or HA-SUMO3Q89P expression vectors. After a 20-h incubation with either vehicle or 10 nm R1881, AR aggregate-positive cells (detected only in R1881-treated samples) were visualized by indirect immunofluorescence using a fluorescein isothiocyanate-conjugated secondary antibody and DAPI nuclear counterstain. C, the fraction of AR aggregate-positive cells was determined as in B in at least 4 independent experiments performed in triplicate. The data represent the average ± S.E. D, extracts were resolved by SDS-PAGE and processed for immunoblotting (IB) using anti-HA or SUMO2/3 antibodies. The arrowheads indicate the precursor (top) and mature (bottom) unconjugated forms of SUMO. E, extracts or AR immunoprecipitates (IP) were analyzed by Western blot analysis with antibodies against AR or HA (SUMO). The arrowheads indicate the positions of SUMO-modified forms of AR.
In light of the established role of SUMO modification in regulating the transcriptional activity of AR, coupled with the potential for this modification to influence polyglutamine diseases, we investigate, in the present study, the role of SUMO modification in the aggregation behavior of polyglutamine-expanded AR. Our analysis reveals that SUMOylation significantly reduces AR aggregation. Notably, the mechanisms responsible are clearly distinct from those involved in regulating the transcriptional activity of AR and thus reveal a novel mode of action for this versatile post-translational modification.
EXPERIMENTAL PROCEDURES
Plasmids and Cell Culture
Expression vectors for AR forms are derivatives of the p5HB hAR cytomegalovirus-driven expression vector for WT human AR bearing a Gln24 tract. The SC motif mutants (p5HB hAR K385R/K518R) and (p5HB hAR I384N/V517N) were generated using the QuikChange Multi site-directed mutagenesis approach using p5HB hAR WT Gln24 as a template. To generate deletions of the first and second SC motifs (p5HB hAR ΔSC), 7 amino acids (Δ 382–388 in the first motif and Δ 515–521 in the second motif) were removed by seamless cloning through PCR and the type II restriction enzyme EarI, which cleaves outside its recognition site. The expanded (Gln113) glutamine tract (3) was transferred to p5HB hAR Gln24 as an EagI/AflII fragment to generate p5HB hAR Gln113. The mutant SC motif forms were transferred as RsrII/HindIII fragments from the Gln24 forms and ligated to the same sites of p5HB hAR Gln113. To generate the expression vector for the non-cleavable fusion of HA-SUMO3 at the N terminus of AR, p5HB hAR Gln113 K385R/K518R was first modified by site-directed mutagenesis to remove the second BamHI site located downstream of the AR coding sequence and to introduce an NheI site upstream of the AR coding sequence. This yielded p5HB NB hAR Gln113 AR KRKR. The HA-SUMO portion from pcDNA3 HA-SUMO3 (−Gly) Gal4 (36, 38) was then excised as an NheI/BamHI fragment and ligated into the same sites of p5HB NB hAR Gln113 AR KRKR. The HA-tagged version of the preprocessed protease-resistant form of SUMO3 (HA-SUMO3-Q89P-GGstop) and the surface mutant (HA-SUMO3-Q89P-K33E/K42E-GGstop) were generated by site-directed mutagenesis using as a template the pCMV-driven (pcDNA3) expression vector for HA-tagged SUMO3 described previously (36). The reporter plasmid pΔ(TAT)4-Luc harbors four copies of a minimal response element from the tyrosine aminotransferase (TAT) gene upstream of a minimal Drosophila distal alcohol dehydrogenase promoter (−33 to +55) driving the luciferase gene (40, 59). The FKBP5 luciferase reporter, which harbors a 500-bp genomic region centered on the AR binding region in the first intron of the human FKBP5 gene, was a kind gift of Dr Keith Yamamoto (60). The pCMV β-galactosidase and pRSV β-galactosidase, which are cytomegalovirus- and Rous sarcoma virus-driven β-galactosidase expression vectors, respectively, were used to correct for transfection efficiency. HeLa and human embryonic kidney 293T cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10 or 5% charcoal-stripped fetal bovine serum, respectively. In all transfection experiments, cells received equimolar amounts of each type of expression plasmid to control for promoter dosage effects.
In Vivo SUMOylation and Cell Fractionation
HeLa cells (7.5 × 104/well) were seeded onto 6-well plates and co-transfected 24 h later using FuGENE-6® transfection reagent with 1 μg of expression vectors for expression of the Gln113 AR with WT or mutant SC motifs together with 0.3 μg of the indicated HA-SUMO3 expression vectors. Cultures were supplemented with 10 nm R1881 or vehicle (0.1% ethanol) 24 h after transfection and harvested 20 h later. Cells were lysed for 15 min on ice with 300 μl of high salt lysis buffer (20 mm Hepes, pH 7.5, 400 mm NaCl, 5 mm EDTA, 1 mm EGTA, 1% Nonidet P-40) containing 20 mm N-ethylmaleimide and one tablet per 10 ml of CompleteTM protease inhibitors (Roche Diagnostics). After 5 min, N-ethylmaleimide was quenched by the addition of dithiothreitol to 40 mm final concentration and a further 2-min incubation on ice. A fraction (5%) of each sample was reserved for analysis, and the remaining cleared cell lysates were immunoprecipitated with 4 μl of rabbit polyclonal AR-N20 antibody (Santa Cruz Biotechnology) at 4 °C for 2 h. Complexes were recovered with 60 μl of 50% protein A-agarose (Invitrogen) at 4 °C for 2 h. The immunoprecipitates were washed three times in low salt lysis buffer (200 mm NaCl) and eluted in 4× Laemmli sample buffer. Samples were resolved by 7.5% SDS-PAGE and processed for immunoblotting as described below. For cell fractionation studies, quenched lysates were centrifuged at 15,000 × g for 15 min at 4 °C. Pellets were resuspended in a volume equal to the supernatant using low salt lysis buffer. Equal amounts of supernatant and pellet fractions were resolved by 7.5% SDS-PAGE and processed for immunoblotting as described below. As indicated, the supernatants were further fractionated by ultracentrifugation at 100,000 × g for 30 min at 4 °C. The supernatants were resolved by 4–20% gradient SDS-PAGE and processed for immunoblotting as described below.
Immunoblotting
For the in vivo SUMOylation experiments, immunoblots were probed with primary rabbit polyclonal AR-N20 (Santa Cruz Biotechnology), mouse monoclonal HA-11 (Covance), or rabbit polyclonal SUMO2/3 (Abcam) antibodies followed by goat anti-rabbit or mouse IgG peroxidase-conjugated (Bio-Rad) secondary antibodies. AR expression levels were confirmed by Western blotting. Cells were transfected as described for the functional assays and lysed in 4× SDS-PAGE sample buffer resolved by 7.5% SDS-PAGE, transferred to Immobilon-P membranes (Millipore) using a wet transfer apparatus, and processed for immunoblotting. Immunoreactive proteins were detected by chemiluminescence. Images were captured in a Kodak Image Station 440 CF using SuperSignal West Femto substrates (Pierce). All the experiments were performed at least thrice with similar results.
Immunofluorescence and Quantitation of AR Aggregation
HeLa cells were transfected as described above for the in vivo SUMOylation experiments and seeded onto chambered slides 18 h after transfection. Cultures were supplemented with 10 nm R1881 or vehicle (0.1% ethanol) 6 h later and incubated a further 20 h. Cells were then fixed at −20 °C with methanol for 10 min, washed in phosphate-buffered saline, and blocked in 5% goat serum for 60 min at room temperature. Slides were incubated with primary rabbit polyclonal (AR-N20, 1:250) or mouse monoclonal (HA-11, 1:100) antibodies for 2 h at room temperature. Donkey anti-rabbit IgG conjugated to fluorescein and donkey anti-mouse IgG conjugated to Texas Red (from Jackson ImmunoResearch Laboratories) were used as secondary antibodies at 1:500 and 1:250 dilutions, respectively. After a 45-min incubation, slides were washed, and nuclei were counterstained with DAPI. Confocal images were captured using a Zeiss LSM 510 microscope and a ×63 water immersion objective. For quantitation, cells were examined using a Zeiss Axioplan 2 imaging system under a ×40 objective. AR aggregation was scored by determining the percentage of transfected cells with visible protein aggregates. For each experiment, transfections were performed in triplicate, and between 100 and 150 cells were scored in a blinded manner for each sample. Each experimental condition was then repeated in separate occasions between three and seven times. The data are expressed as the average ± S.E. Statistical analysis was carried out using the two-tailed Student's t test. Differences remained significant even after arcsine square root transformation of the proportions or when tested with the non-parametric χ2 test.
AR Transcriptional Activity Assays
For functional assays in HeLa cells (see Fig. 3), cells were seeded onto 24-well plates (1.8 × 104/well) and co-transfected 24 h later with 1 ng of AR expression vector, the indicated amounts of expression vector for HA-SUMO3Q89P, 100 ng of reporter plasmid, and 50 ng of the control pCMV β-galactosidase plasmid. The total amount of DNA was supplemented to 0.4 μg/well with pBSKS(−). For functional assays in human embryonic kidney 293T cells (see Fig. 4), cells (5 × 103/well) were seeded in 96-well plates and transfected 24 h later. For the data in panel A, cells received 1 ng of AR expression plasmid and 30 ng of pΔ(TAT)4-Luc reporter plasmid. For panel B, the cells received 0.1 ng of AR expression plasmid and 3 ng of the FKBP5 luciferase reporter. In all cases, cells received 1 ng of the control pRSV β-galactosidase plasmid. The total amount of DNA was supplemented to 90 ng/well with pBSKS(−) and empty vector pCMV-5 to maintain an equimolar amount of expression vector. After 16 h, cells were exchanged into medium containing 10 nm R1881 or vehicle (0.1% ethanol) and lysed 20 h later. Luciferase and β-galactosidase activities were determined as described (59).
FIGURE 3.
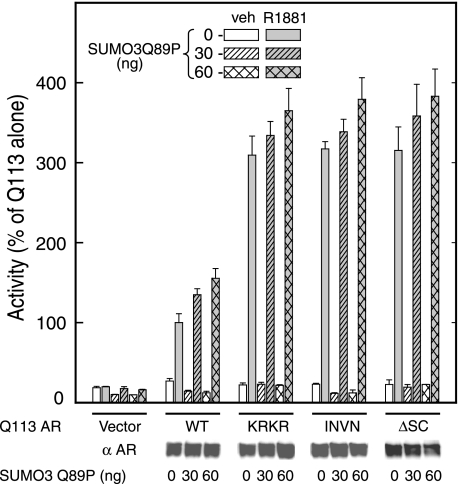
Effects of SUMO expression on the transcriptional activity of polyglutamine-expanded AR with intact or disrupted SC motifs. HeLa cells were co-transfected, as described under “Experimental Procedures” with expression vectors for Gln113 WT AR or the indicated Gln113 AR SC motif mutants together with the indicated amounts of HA-SUMO3 Q89P expression plasmid. Equimolar amounts of expression vector were maintained using empty expression vector. Cells also received a minimal luciferase reporter plasmid driven by four copies of a 15-bp response element from the tyrosine aminotransferase gene (pΔTAT4-Luc) as well as a control pCMV β-galactosidase plasmid. Cells were treated 24 h after transfection with 10 nm R1881 or vehicle (veh) and processed as described. The data represent the average ± S.E. of at least 3 independent experiments performed in triplicate and are expressed as a percentage of the activity of Gln113 AR alone (0.93 ± 0.10). Lysates from the same cells were analyzed by Western blot with antibodies against AR to examine receptor expression.
FIGURE 4.
Colinear N-terminal fusion of SUMO restores synergy control but has no effect on AR aggregation. Human embryonic kidney 293T cells were co-transfected in 96-well plates as described under “Experimental Procedures” with expression vectors for AR forms bearing normal (Gln24) or expanded (Gln113) glutamine tracts with intact (WT) or disrupted (KRKR) SC motifs and in the absence or presence of an N-terminal fusion of SUMO3 (S3). Cells also received the constitutive pRSV β-galactosidase plasmid as well as a minimal luciferase reporter plasmid driven by either four copies of a 15-bp response element from the TAT gene (ARE-4, panel A) or a 500-bp intronic region of the AR-responsive human FKBP5 gene (panel B). Cells were treated with 10 nm R1881 or vehicle, and activity was measured as described. The data represent the average ± S.E. of at least 4 independent experiments performed in triplicate and are expressed as a percentage of the Gln24 WT AR activity at each promoter, which corresponds to 25.62 ± 1.92 and 1.95 ± 0.13 for the ARE-4 and FKBP5 reporters, respectively. Expression of AR variants in 293T cells was confirmed by immunoblotting using anti-AR-N20 antibody as described under “Experimental Procedures.” C, HeLa cells expressing the indicated AR expression plasmid were treated with either vehicle or 10 nm R1881 for 20 h. The incidence of AR aggregate-positive cells was determined as in Fig. 1. Data represent the means ± S.E. of 3–4 independent experiments performed in triplicate.
RESULTS
Expression of SUMO Reduces AR Aggregation
A growing body of evidence indicates that in response to multiple forms of cellular stress, including temperature shifts and exposure to reactive oxygen species, the overall extent of protein SUMOylation is rapidly induced (61–63). This suggests that SUMOylation may form part of an adaptive mechanism to protect cellular proteins from the structural alterations induced by such insults. Because polyglutamine expansion renders AR particularly vulnerable to aggregation, we hypothesized that gratuitous enhancement of SUMOylation in the absence of cellular stress may provide a protective effect. To test this hypothesis, we took advantage of a well characterized cell culture model of Kennedy disease (3, 4). As can be seen in Fig. 1B and consistent with previous reports (3, 4), agonist treatment of HeLa cells expressing a polyglutamine-expanded form of AR leads to the appearance of punctate cytoplasmic inclusions in a substantial fraction (∼40%) of AR-expressing cells. In addition, significant diffuse staining of AR, likely reflecting smaller scale aggregates, is detectable in the cytoplasm. The formation of large inclusions is ligand-dependent because it is absent in vehicle-treated cells. This effect and the persistent diffuse cytoplasmic staining are polyglutamine expansion-dependent because in cells expressing AR with a short (24 amino acids) glutamine tract, inclusions are absent and AR is fully nuclear upon agonist treatment (Refs. 3 and 20 and data not shown). To enhance cellular SUMOylation, we co-expressed HA tagged full-length WT SUMO3. Our choice of isoform is based on our observations that SUMO3 accumulates to higher levels than either SUMO1 or SUMO2. As can be seen in Fig. 1D, co-expression of SUMO3 led to the appearance of precursor and mature free forms of SUMO (marked by arrowheads) as well as a broad spectrum of HA-immunoreactive SUMO-modified proteins. Remarkably, co-expression of SUMO3 reduced the fraction of AR aggregate-positive cells by half to ∼20% (p < 0.001) and led to a reduction in the polyglutamine-dependent diffuse cytoplasmic staining (Fig. 1, B and C). To further enhance the extent of SUMOylation, we took advantage of the observation that the presence of a critical Pro residue in SUMO4 is responsible for its resistance to cellular SUMO proteases. We therefore expressed a mutant form of SUMO3 bearing a proline at the equivalent position (Q89P). To bypass the need for SUMO proteases for the initial processing of SUMO, we expressed a form with a mature C terminus. As can be seen in Fig. 1D, expression of Q89P SUMO3 led to the appearance of only the mature form of free SUMO3 as well as to a significant enhancement in the accumulation of SUMO conjugates. Consistent with the enhanced overall SUMOylation, expression of this form further reduced AR aggregation to ∼14% (Fig. 1, B and C).
Because the formation of AR aggregates is positively correlated with the levels of expressed AR and most manipulations that reduce AR aggregation also reduce the total amount of cellular AR, we examined whether this was also the case for enhanced SUMOylation. Remarkably, expression of WT or Q89P SUMO3 reduced AR aggregation without significant alterations in the overall expression of the receptor (Fig. 1E, left). Notably, the expression of SUMO also allowed the detection of slower migrating, AR-immunoreactive species in the extracts. Given that AR is a target for SUMOylation, we examined whether expression of these SUMO isoforms also enhanced SUMO conjugation to polyglutamine-expanded AR. As can be seen in Fig. 1E (center), analysis of AR immunoprecipitates also revealed slower migrating AR-immunoreactive species (indicated by the arrowheads), which are particularly enhanced upon co-expression of SUMO3 forms. These species are also HA-immunoreactive (Fig. 1E, right), confirming their identity as SUMO-modified forms of AR. Consistent with the overall SUMOylation pattern, a significant enhancement in AR SUMOylation is observed in the case of the deconjugation-resistant Q89P form of SUMO3. This indicates, on the one hand, that the presence of an expanded polyglutamine tract in AR does not interfere with its SUMOylation, and on the other hand, that the extent of AR SUMOylation correlates with reduced aggregation. Taken together, the data indicate that a significant attenuation of the aggregation of polyglutamine-expanded AR can be achieved by expression of SUMO forms that enhance SUMOylation of cellular proteins, including AR itself.
The Anti-aggregation Effect of Enhanced SUMOylation Depends on Modification of AR
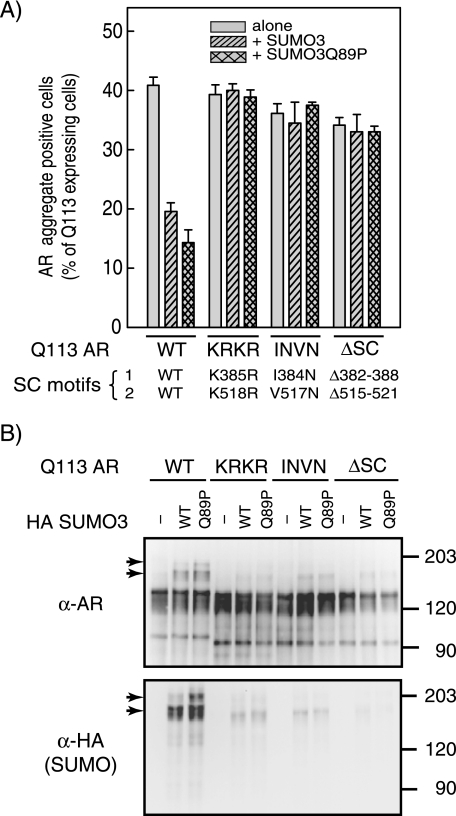
The above results suggested that the reduced AR aggregation in response to enhanced SUMOylation could be due to a direct effect of SUMO conjugation to AR. To examine this possibility, we examined the effect of SUMO expression on the aggregation propensity of AR forms defective in SUMOylation. As can be seen in Fig. 2A, disruption of the two SUMOylation/SC motif sites in the N-terminal region of AR by replacing the acceptor lysines by arginine residues (KRKR) completely eliminated the protective effect of enhanced SUMOylation on the aggregation of AR (Fig. 2A) and led to a concomitant loss of AR SUMO modification (Fig. 2B). Because these mutations can impair lysine-directed modifications other than SUMOylation, we also examined a mutant bearing Asn substitutions at the conserved hydrophobic residue of the SC motifs. As in the case of other proteins, these substitutions severely compromised AR SUMOylation (Fig. 2B), and importantly, also negated the reduced AR aggregation afforded by SUMO expression (Fig. 2A). A similar loss of SUMO modification and protection toward aggregation was observed for a mutant AR deleted of its SC motifs. Taken together, these remarkable results indicate that enhanced SUMOylation significantly decreases the formation of polyglutamine-expanded AR aggregates. Moreover, because this reduction in aggregation is dependent on intact AR SUMOylation sites, this suggests that enhanced SUMOylation of AR itself exerts a direct protective effect to counteract its aggregation.
FIGURE 2.
The protective effect of enhanced SUMOylation depends on modification of AR. A, HeLa cells were co-transfected with 1 μg of Gln113 WT AR or the indicated SC motif mutants and 0.3 μg of control, HA-SUMO3, or HA-SUMO3Q89P expression vectors. AR aggregate-positive cells were quantitated as in Fig. 1. Data represent the means ± S.E. of at least 3–4 independent experiments performed in triplicate. B, AR immunoprecipitates were analyzed by Western blot with antibodies against AR and HA (SUMO). The arrowheads indicate the positions of SUMO-modified forms of AR.
The Anti-aggregation Effects of Enhanced SUMOylation Are Independent of AR Transcriptional Activity
A growing body of evidence indicates that SUMO modification of the SC motifs in AR and many other sequence-specific transcription factors exerts a promoter context-dependent inhibitory effect. We therefore examined whether the transcriptional effects of AR SUMOylation are preserved in the presence of an expanded glutamine tract. We also determined whether the anti-aggregation effects of enhanced SUMOylation are correlated with its effects on AR transcriptional activity. As can be seen in Fig. 3, disruption of the SC motifs in Gln113 AR, using the same mutations as in Fig. 2, leads to a robust 3-fold enhancement of AR activity at a target gene harboring four AREs. This indicates that the basal SUMOylation levels are sufficient to exert a significant inhibitory effect on transcription. This is in contrast to the data of Fig. 2 because loss of basal Gln113 AR SUMOylation upon disruption of the SC motifs does not by itself enhance AR aggregation. This suggests that although the low basal level of AR SUMOylation exerts a strong inhibitory effect on transcription, it is not sufficient to provide a measurable effect toward aggregation.
Upon expression of the protease-resistant Q89P form of SUMO3, however, the data of Fig. 2 indicate that this leads to a significant enhancement in Gln113 AR SUMOylation and a concomitant reduction in its aggregation. Functionally, expression of Q89P SUMO3 led to a small, dose-dependent enhancement of Gln113 AR transcriptional activity (Fig. 3, hatched bars). Unlike the effect on aggregation, however, this stimulation is unlikely to be due to alterations in AR SUMOylation because a similar enhancement is observed for AR forms bearing disrupted or deleted SC motifs (Fig. 3). This behavior is similar to what has been observed for AR bearing a normal Gln tract (64) and likely reflects alterations in transcriptional components other than AR itself. These observations indicate that the reduced aggregation afforded by enhanced SUMOylation is unlikely to be a consequence of altered AR transcriptional activity. Taken together, these data indicate that the effects of AR SUMOylation on aggregation are distinct from its transcriptional effects. Thus, whereas the former require a significant level of AR SUMOylation and are independent of AR activity, the latter are detectable at the low SUMOylation levels observed under basal conditions.
Colinear SUMO Fusions Inhibit AR Activity but Not Aggregation
The functional data above indicate that loss of SUMOylation influences the activity of polyglutamine-expanded AR. We have therefore compared the functional consequences of AR SUMOylation in the context of a normal or expanded polyglutamine tract. Consistent with previous data, the transcriptional activity of AR at a target gene driven by four AREs (Fig. 4A) is reduced in the presence of an expanded polyglutamine tract. Nevertheless, disruption of the SC motifs leads to a comparable more than 2-fold enhancement of AR activity irrespective of the polyglutamine tract length. We also examined AR activity driven by an endogenous 500-bp intronic enhancer region of the FKBP5 gene. This region harbors two closely spaced AREs that are occupied by AR in vivo (65). As can be seen in Fig. 4B, we observed a similar pattern of activity at this natural regulatory region.
Analysis of multiple transcription factors such as the glucocorticoid receptor and SP-3 has revealed that stable recruitment of SUMO in the form of a colinear N-terminal fusion recapitulates the transcriptional properties of SC motifs (36, 48). Consistent with this view, we find that fusion of SUMO3 to AR bearing non-functional SC motifs reduces the transcriptional activity to levels comparable with that of the WT receptor. Notably, this effect is observed for AR forms with normal or pathologically expanded polyglutamine tracts. These data indicate that the transcriptional effects of AR SUMOylation are preserved irrespective of the polyglutamine tract length and that stable N-terminal recruitment of SUMO is sufficient to recapitulate the transcriptional effects of AR SUMOylation. In light of these findings, we examined whether fusing SUMO3 to polyglutamine-expanded AR also attenuated AR aggregation. Remarkably, and in contrast to the effects of enhanced AR SUMOylation at the native SC motifs, we find that the N-terminal fusion of SUMO3 does not alter the aggregation propensity of polyglutamine-expanded AR (Fig. 4C). These surprising results indicate that a branched protein structure or modification in the appropriate context of the N-terminal region is necessary to antagonize aggregation. Furthermore, the lack of correlation with the transcriptional effects further supports the view that the anti-aggregation effects of AR SUMOylation are independent of its effects on transcriptional activity.
SUMOylation Selectively Excludes AR from Polyglutamine Aggregates
The data above indicate that elevated SUMOylation attenuates polyglutamine-expanded AR aggregation. The fact that SUMOylation of AR itself is required for this effect suggests that SUMOylated forms of AR may be excluded from the aggregates. We therefore examined whether SUMO co-localizes with AR within the aggregates. Consistent with our hypothesis, co-immunostaining of cells expressing polyglutamine-expanded AR and SUMO3 (Fig. 5A) revealed that the receptor-positive cytoplasmic inclusions (labeled in green) are not enriched for SUMO (labeled in red). To examine more specifically the effects of SUMOylation on the solubility of AR, we have used a biochemical fractionation approach. As can be seen in Fig. 5B, fractionation of whole cell extracts from cells expressing WT AR indicate that nearly all of the AR protein remains soluble after a 15,000 × g centrifugation. In contrast, and consistent with the microscopy studies, analysis of cells expressing Gln113 AR indicates that nearly 60% of the AR is insoluble (Fig. 5, B and C). Upon co-expression of SUMO3, the fraction of Gln113 AR found in the pellet decreases significantly and is further reduced in the case of the protease-resistant form of SUMO3. Notably, examination of the solubility of SUMOylated versus non-modified forms of AR revealed that nearly 80% of the conjugated forms of Gln113 AR remain soluble in the case of the Q89P form of SUMO3 (Fig. 5C). As for the microscopy data, when SUMO conjugation to AR is prevented by disruption of the SC motifs, the ability of SUMO expression to enhance the solubility of AR is abrogated.
FIGURE 5.
SUMO-modified forms of AR are excluded from aggregates and have increased solubility. A, HeLa cells co-expressing Gln113 AR and HA-SUMO3 were treated with 10 nm R1881 for 20 h. Localization of AR and SUMO was assessed by indirect immunofluorescence using fluorescein isothiocyanate- (AR) and Texas-red (HA)-conjugated secondary antibodies. Nuclei were counterstained with DAPI. B, HeLa cells were co-transfected with 1 μg of expression vectors for AR forms bearing normal (Gln24) or expanded (Gln113) glutamine tracts with intact (WT) or disrupted (KRKR) SC motifs. Cells also received 0.3 μg of control, HA-SUMO3, or HA-SUMO3Q89P expression vectors. After a 20-h treatment with 10 nm R1881, samples were harvested as described under “Experimental Procedures.” Cell lysates were centrifuged at 15,000 × g for 15 min at 4 °C. Equivalent pellet (P) and supernatant (S) fractions were analyzed by immunoblotting with AR antibody. The closed arrowheads indicate the SUMO-modified forms of AR. C, quantitative analysis. Data represent the means ± S.E. of 4 independent experiments. D, supernatant fractions were further subjected to ultracentrifugation at 100,000 × g for 30 min, resolved by gradient SDS-PAGE, and probed with antibody against AR. The closed arrowheads indicate the SUMO-modified forms of AR, and the bracket delineates the spread of AR oligomeric species. IB, immunoblot.
It has recently been proposed that the appearance of AR macromolecular oligomeric complexes that remain soluble after ultracentrifugation correlate well with the development of neuronal toxicity in a mouse model of SBMA (66). We therefore analyzed supernatants from a 100,000 × g centrifugation by SDS-PAGE in gradient gels. Under these conditions, soluble oligomeric species can be revealed as material that spreads in the upper section of the gel or that fails to enter the gel. As can be seen in Fig. 5D, a significant amount of such material is visible in samples from cells expressing Gln113 AR. In contrast, co-expression of SUMO3 leads to the accumulation of SUMO-modified forms of AR and a concomitant reduction in oligomeric species, especially in the case of the protease-resistant form of SUMO3. As in the previous assays, disruption of the SC motifs in AR led to the loss of conjugated forms of AR and the reappearance of oligomeric material. Taken together, the data clearly show that conjugation of SUMO to AR enhances the solubility of polyglutamine-expanded AR and prevents the formation of insoluble aggregates as well as soluble oligomeric species.
The Main Effector Surface of SUMO Is Dispensable for Reducing AR Aggregation
Studies by our group (42) have identified a critical surface in SUMO required for its transcriptional inhibitory effects. Structural and biochemical data (54–58, 67) indicate that this region serves as a binding site for short SUMO-interacting motifs or SIMs found in multiple SUMO binding partners. To examine whether this effector surface is required for the observed SUMOylation-mediated reduction in AR aggregation, we have taken advantage of a double mutant in this surface (K33E/K42E) that severely compromises the ability of SUMO to inhibit transcription (42). We thus examined the effect of expressing protease-resistant forms of SUMO3 bearing an intact or disrupted effector surface on the incidence of aggregates in cells expressing Gln113 AR. As can be seen in Fig. 6A, we find that disruption of the effector surface in SUMO does not alter its ability to reduce aggregation of Gln113 AR. In fact, this mutant appears to be slightly more effective than the WT counterpart is and reduced the fraction of cells with aggregates to ∼7%. The effect of this mutant, however, remains dependent on AR SUMOylation because no reduction in aggregation is observed for the SUMOylation-deficient SC motif mutant AR.
FIGURE 6.
The effector surface of SUMO is dispensable for its protective effect on AR aggregation. A, HeLa cells were co-transfected with 1 μg of expression vectors for Gln113 AR bearing intact (WT) or disrupted (KRKR) SC motifs and 0.3 μg of control vector or vectors for the expression of HA-SUMO3 Q89P bearing an intact or disrupted (K33E/K42E (KEKE)) effector surface. AR aggregate-positive cells were quantitated as in Fig. 1. Data represent the means ± S.E. of at least 3 independent experiments performed in triplicate. B, AR immunoprecipitates (IP) were analyzed by Western blot with antibodies against AR or HA (SUMO). The arrowheads indicate the positions of SUMO-modified forms of AR.
Given the inverse correlation between the extent of SUMO modification and aggregation, the enhanced protection afforded by the effector surface mutant could be due to an enhanced stoichiometry of SUMOylation. Analysis of the extent of AR modification, however, revealed slightly lower degrees of conjugation for the effector surface mutant (Fig. 6B). Thus, the data indicate that although the effector surface of SUMO is essential for its transcriptional functions, it is dispensable for its anti-aggregation effects. This in turn implies that the latter are exerted through novel and distinct mechanisms.
DISCUSSION
SUMOylation as a Protective Mechanism in SBMA
Although it is clear that an up-regulation of SUMO conjugation is a common response to multiple stressful stimuli, our understanding of the role of this modification in cellular protective mechanisms is limited. In this study, the finding that up-regulation of cellular SUMO conjugation can antagonize the development of polyglutamine-expanded AR aggregates and oligomers reveals that this post-translational modification is an important component of the cellular strategies to cope with unstable and aggregation-prone proteins. Although up-regulation of cellular SUMOylation leads to the modification of many proteins, the requirement for functional SC motifs in AR clearly indicates that modification of AR itself is essential for counteracting aggregation.
Our data clearly argue that SUMOylation is protective and are consistent with genetic data that indicate that expression of a catalytically deficient mutant form of the SUMO-activating enzyme (E1) greatly enhanced degeneration in an SBMA model in Drosophila (68). It is interesting to note that in the case of a model of Huntington disease, it has been argued that SUMOylation may exert a pro-aggregation effect (23). Whether this reflects an intrinsic difference between the diseases remains to be determined.
AR SUMOylation Reduces Aggregation Independently of Its Transcriptional Effects
SUMO modification of AR and many other SC motif-bearing transcription factors leads to a significant context-dependent inhibition of their transcriptional activation potential (36, 39, 42). Multiple observations in this study argue, however, that the anti-aggregation effect of AR SUMOylation is independent of its transcriptional effects. Firstly, although SUMO up-regulation alters AR activity, this effect does not depend on AR SUMOylation and therefore reflects alterations in other cellular components. Secondly, protection from aggregation depends on a significant stoichiometry of modification, whereas the transcriptional effects of AR SUMOylation are visible even at the low basal levels of modification. Thirdly, recruitment of SUMO to AR as an N-terminal fusion recapitulates many of the transcriptional effects of SUMO, yet it does not protect AR from aggregation. Finally, the critical effector surface of SUMO, which is essential for its transcriptional regulatory effects, is dispensable for SUMO inhibition of polyglutamine-expanded AR aggregation.
Taken together, the data clearly reveal a novel mechanism of action for SUMO modification. Conceivably, this modification could act by serving a signaling role in the cell to induce global protective mechanisms. This would lead to protection of the entire population of AR molecules irrespective of their modification (protection in trans). Alternatively, SUMOylation may provide a specific barrier to the incorporation of modified AR into aggregates. In this view, only the SUMOylated forms of AR would be protected (protection in cis). The fact that enhanced SUMOylation preferentially increases the solubility of the modified pool (Fig. 5B) argues for a cis acting mechanism. This is reminiscent of the role for SUMO in the function of A40R. This vaccinia virus protein is quantitatively SUMOylated, and SUMOylation is essential to prevent the protein from otherwise forming insoluble aggregates (69). The substoichiometric modification of AR coupled with the effects of non-modified AR, however, argues that additional in trans mechanisms may be at play. Regardless of the exact mechanism, it is clear that the ability of AR SUMOylation to mitigate aggregation demands significantly higher stoichiometries than those required to regulate the transcriptional properties of AR.
The aggregation of polyglutamine-expanded AR is clearly concentration-dependent. Binding of agonists such as dihydrotestosterone reduces AR degradation and therefore increases AR levels and facilitates aggregation. A reduction in AR concentration is the likely basis for the ameliorating effects of androgen blockade in mouse models of SBMA (70–72). A reduction in ligand binding and stabilization is also the likely mechanism for the recently described protective effects of IGF1-mediated AR phosphorylation by AKT (73). Other approaches, such as inhibition of HSP90-dependent transport, also appear to protect against AR aggregation by facilitating the degradation and turnover of the receptor (4). In contrast, our findings indicate that enhanced SUMO modification does not alter the levels of AR and that therefore SUMOylation is still protective even in the face of elevated levels of polyglutamine-expanded AR. This argues that SUMO is unlikely to operate by facilitating AR turnover.
Enhanced SUMOylation also reduced the polyglutamine expansion-dependent diffuse cytoplasmic AR staining. Although this likely reflects the reduced formation of smaller scale aggregates, this may also indicate that SUMOylation-dependent alterations in nucleocytoplasmic transport could contribute to the protective effects. Enhanced nuclear translocation, however, is not directly consistent with the reduced aggregation observed upon inhibition of HSP90-dependent transport (4).
The fact that enhanced AR SUMOylation leads to a reduction in soluble oligomeric species coupled with the enhanced solubility of SUMO-modified forms of AR argues that SUMO may exert its effects at an early stage rather than simply preventing the formation of large inclusions or aggresomes (16). It is therefore possible that SUMOylation functions at least in part by directly preventing the misfolding of the receptor. Structural studies indicate that polyglutamine segments can form various forms of extended β-sheet arrangements with a variable number and length of strands. Moreover, strands from multiple molecules appear to incorporate into these arrays (74, 75). Our findings indicate that the protective effects of SUMO depend on a branched protein structure and cannot be mimicked by an N-terminal fusion of SUMO. This may indicate that such a branched structure may function by serving as a steric impediment to the formation of higher order polyglutamine β-sheet structures. An analogous effect has been observed for flanking polyproline tracts, which can inhibit β-sheet structure in polyglutamine segments (76). Alternatively, SUMO modification of the SC motifs in AR may prevent proteolytic steps that are thought to be involved in the generation of toxic polyglutamine-containing fragments (77, 78). In this regard, SUMOylation could antagonize the phosphorylation-dependent caspase 3 cleavage of AR, which is closely associated with neuronal toxicity (79).
It is notable that most functional effects of SUMO modification to date appear to depend on the ability of SUMO to facilitate protein-protein interactions by binding SUMO-interacting motifs in partner proteins. Although it remains possible that SUMO protects AR from aggregation by recruiting additional factors, the mechanism is unlikely to involve the described interaction between the effector surface of SUMO and the SIMs that bind to it. Whether other binding modes are involved, however, remains to be determined. In this regard, analysis of the extensive collection of SUMO mutants we have generated (42) will be highly instructive.
SUMOylation as a Therapeutic Target in SBMA
A consequence of clinical relevance of our findings is that pharmacologic manipulation of AR SUMOylation could be of use in therapeutic strategies for SBMA. As suggested by the protease-resistant form of SUMO, this could be achieved through the use of small molecule inhibitors of the SUMO proteases responsible for de-SUMOylating AR. The significant diversity in specificity, expression, and subcellular localization of this group of enzymes could allow for selective targeting of the relevant isoform(s). Conversely, or in addition, enhanced SUMOylation could be achieved by regulating the expression or activity of the SUMO E3 ligases that target AR such as certain members of the protein inhibitor of activated STAT family of proteins (80, 81). Furthermore, the fact that other proteins involved in polyglutamine diseases such as Ataxin-1 (82), Huntingtin (23, 83), and Atrophin-1 (84) are SUMO-modified or co-localize with SUMO suggests that the protective effect of SUMO on AR aggregation may extend to multiple proteins and that similar therapeutic strategies may be effective in other polyglutamine diseases.
Clearly, the demonstration that SUMOylation can prevent the aggregation of polyglutamine-expanded AR though a novel mechanism has revealed a new facet of SUMOylation. The findings extend the extraordinarily diverse functions of this versatile post-translational modification and reinforce the view that SUMOylation plays a key role in the pathobiology of multiple diseases.
Supplementary Material
Acknowledgment
Core facility use was provided through U.S. Public Health Service Grant P60 DK20572.
This work was supported, in whole or in part, by National Institutes of Health Grants DK61656–01 (to J. A. I.) and NS055746 (to A. P. L.) from the USPHS.
This article was selected as a Paper of the Week.
- SBMA
- spinal and bulbar muscular atrophy
- AR
- androgen receptor
- hAR
- human AR
- ARE
- androgen-response element
- SUMO
- small ubiquitin-like modifier
- SC
- synergy control
- SCA
- spinocerebellar ataxia
- WT
- wild type
- HA
- hemagglutinin
- DAPI
- 4′,6-diamidino-2-phenylindole
- STAT
- signal transducers and activators of transcription
- E1
- ubiquitin-activating enzyme
- E2
- ubiquitin carrier protein
- E3
- ubiquitin-protein isopeptide ligase.
REFERENCES
- 1.Kennedy W. R., Alter M., Sung J. H. (1968) Neurology 18, 671–680 [DOI] [PubMed] [Google Scholar]
- 2.La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. (1991) Nature 352, 77–79 [DOI] [PubMed] [Google Scholar]
- 3.Thomas M., Dadgar N., Aphale A., Harrell J. M., Kunkel R., Pratt W. B., Lieberman A. P. (2004) J. Biol. Chem. 279, 8389–8395 [DOI] [PubMed] [Google Scholar]
- 4.Thomas M., Harrell J. M., Morishima Y., Peng H. M., Pratt W. B., Lieberman A. P. (2006) Hum. Mol. Genet. 15, 1876–1883 [DOI] [PubMed] [Google Scholar]
- 5.Lieberman A. P., Fischbeck K. H. (2000) Muscle Nerve 23, 843–850 [DOI] [PubMed] [Google Scholar]
- 6.Palazzolo I., Gliozzi A., Rusmini P., Sau D., Crippa V., Simonini F., Onesto E., Bolzoni E., Poletti A. (2008) J. Steroid Biochem. Mol. Biol. 108, 245–253 [DOI] [PubMed] [Google Scholar]
- 7.Fischbeck K. H. (2001) Brain Res. Bull. 56, 161–163 [DOI] [PubMed] [Google Scholar]
- 8.Lieberman A. P., Trojanowski J. Q., Leonard D. G., Chen K. L., Barnett J. L., Leverenz J. B., Bird T. D., Robitaille Y., Malandrini A., Fischbeck K. H. (1999) Ann. Neurol. 46, 271–273 [DOI] [PubMed] [Google Scholar]
- 9.Taylor J. P., Hardy J., Fischbeck K. H. (2002) Science 296, 1991–1995 [DOI] [PubMed] [Google Scholar]
- 10.Zoghbi H. Y., Orr H. T. (2000) Annu. Rev. Neurosci. 23, 217–247 [DOI] [PubMed] [Google Scholar]
- 11.Cummings C. J., Zoghbi H. Y. (2000) Annu. Rev. Genomics Hum. Genet. 1, 281–328 [DOI] [PubMed] [Google Scholar]
- 12.Saudou F., Finkbeiner S., Devys D., Greenberg M. E. (1998) Cell 95, 55–66 [DOI] [PubMed] [Google Scholar]
- 13.Klement I. A., Skinner P. J., Kaytor M. D., Yi H., Hersch S. M., Clark H. B., Zoghbi H. Y., Orr H. T. (1998) Cell 95, 41–53 [DOI] [PubMed] [Google Scholar]
- 14.Simeoni S., Mancini M. A., Stenoien D. L., Marcelli M., Weigel N. L., Zanisi M., Martini L., Poletti A. (2000) Hum. Mol. Genet. 9, 133–144 [DOI] [PubMed] [Google Scholar]
- 15.Arrasate M., Mitra S., Schweitzer E. S., Segal M. R., Finkbeiner S. (2004) Nature 431, 805–810 [DOI] [PubMed] [Google Scholar]
- 16.Taylor J. P., Tanaka F., Robitschek J., Sandoval C. M., Taye A., Markovic-Plese S., Fischbeck K. H. (2003) Hum. Mol. Genet. 12, 749–757 [DOI] [PubMed] [Google Scholar]
- 17.Mhatre A. N., Trifiro M. A., Kaufman M., Kazemi-Esfarjani P., Figlewicz D., Rouleau G., Pinsky L. (1993) Nat. Genet. 5, 184–188 [DOI] [PubMed] [Google Scholar]
- 18.Kazemi-Esfarjani P., Trifiro M. A., Pinsky L. (1995) Hum. Mol. Genet. 4, 523–527 [DOI] [PubMed] [Google Scholar]
- 19.Orr H. T. (2001) Genes Dev. 15, 925–932 [DOI] [PubMed] [Google Scholar]
- 20.Thomas M., Yu Z., Dadgar N., Varambally S., Yu J., Chinnaiyan A. M., Lieberman A. P. (2005) J. Biol. Chem. 280, 21264–21271 [DOI] [PubMed] [Google Scholar]
- 21.Lieberman A. P. (2004) Exp. Neurol. 185, 204–207 [DOI] [PubMed] [Google Scholar]
- 22.Dorval V., Fraser P. E. (2007) Biochim. Biophys. Acta 1773, 694–706 [DOI] [PubMed] [Google Scholar]
- 23.Steffan J. S., Agrawal N., Pallos J., Rockabrand E., Trotman L. C., Slepko N., Illes K., Lukacsovich T., Zhu Y. Z., Cattaneo E., Pandolfi P. P., Thompson L. M., Marsh J. L. (2004) Science 304, 100–104 [DOI] [PubMed] [Google Scholar]
- 24.Sampson D. A., Wang M., Matunis M. J. (2001) J. Biol. Chem. 276, 21664–21669 [DOI] [PubMed] [Google Scholar]
- 25.Sachdev S., Bruhn L., Sieber H., Pichler A., Melchior F., Grosschedl R. (2001) Genes Dev. 15, 3088–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun T. H., Itoh H., Subramanian L., Iñiguez-Lluhí J. A., Nakao K. (2003) Circ. Res. 92, 1201–1208 [DOI] [PubMed] [Google Scholar]
- 27.Pichler A., Gast A., Seeler J. S., Dejean A., Melchior F. (2002) Cell 108, 109–120 [DOI] [PubMed] [Google Scholar]
- 28.Yeh E. T., Gong L., Kamitani T. (2000) Gene 248, 1–14 [DOI] [PubMed] [Google Scholar]
- 29.Saitoh H., Hinchey J. (2000) J. Biol. Chem. 275, 6252–6258 [DOI] [PubMed] [Google Scholar]
- 30.Su H. L., Li S. S. (2002) Gene 296, 65–73 [DOI] [PubMed] [Google Scholar]
- 31.Bohren K. M., Nadkarni V., Song J. H., Gabbay K. H., Owerbach D. (2004) J. Biol. Chem. 279, 27233–27238 [DOI] [PubMed] [Google Scholar]
- 32.Owerbach D., McKay E. M., Yeh E. T., Gabbay K. H., Bohren K. M. (2005) Biochem. Biophys. Res. Commun. 337, 517–520 [DOI] [PubMed] [Google Scholar]
- 33.Weisshaar S. R., Keusekotten K., Krause A., Horst C., Springer H. M., Göttsche K., Dohmen R. J., Praefcke G. J. (2008) FEBS Lett. 582, 3174–3178 [DOI] [PubMed] [Google Scholar]
- 34.Mullen J. R., Brill S. J. (2008) J. Biol. Chem. 283, 19912–19921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatham M. H., Geoffroy M. C., Shen L., Plechanovova A., Hattersley N., Jaffray E. G., Palvimo J. J., Hay R. T. (2008) Nat. Cell Biol. 10, 538–546 [DOI] [PubMed] [Google Scholar]
- 36.Holmstrom S., Van Antwerp M. E., Iñiguez-Lluhí J. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15758–15763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poukka H., Karvonen U., Janne O. A., Palvimo J. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 14145–14150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chupreta S., Brevig H., Bai L., Merchant J. L., Iñiguez Lluhí J. A. (2007) J. Biol. Chem. 282, 36155–36166 [DOI] [PubMed] [Google Scholar]
- 39.Subramanian L., Benson M. D., Iñiguez-Lluhí J. A. (2003) J. Biol. Chem. 278, 9134–9141 [DOI] [PubMed] [Google Scholar]
- 40.Iñiguez-Lluhí J. A., Pearce D. (2000) Mol. Cell Biol. 20, 6040–6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmstrom S. R., Chupreta S., So A. Y., Iñiguez Lluhí J. A. (2008) Molecular endocrinology 22, 2061–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chupreta S., Holmstrom S., Subramanian L., Iñiguez-Lluhí J. A. (2005) Mol. Cell Biol. 25, 4272–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel-Hafiz H., Takimoto G. S., Tung L., Horwitz K. B. (2002) J. Biol. Chem. 277, 33950–33956 [DOI] [PubMed] [Google Scholar]
- 44.Bies J., Markus J., Wolff L. (2002) J. Biol. Chem. 277, 8999–9009 [DOI] [PubMed] [Google Scholar]
- 45.Callewaert L., Verrijdt G., Haelens A., Claessens F. (2004) Mol. Endocrinol. 18, 1438–1449 [DOI] [PubMed] [Google Scholar]
- 46.Hirano Y., Murata S., Tanaka K., Shimizu M., Sato R. (2003) J. Biol. Chem. 278, 16809–16819 [DOI] [PubMed] [Google Scholar]
- 47.Murakami H., Arnheiter H. (2005) Pigment Cell Res. 18, 265–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross S., Best J. L., Zon L. I., Gill G. (2002) Mol. Cell 10, 831–842 [DOI] [PubMed] [Google Scholar]
- 49.Sapetschnig A., Rischitor G., Braun H., Doll A., Schergaut M., Melchior F., Suske G. (2002) EMBO J. 21, 5206–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tallec L. P., Kirsh O., Lecomte M. C., Viengchareun S., Zennaro M. C., Dejean A., Lombès M. (2003) Mol. Endocrinol. 17, 2529–2542 [DOI] [PubMed] [Google Scholar]
- 51.Tian S., Poukka H., Palvimo J. J., Jänne O. A. (2002) Biochem. J. 367, 907–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang S. H., Jaffray E., Hay R. T., Sharrocks A. D. (2003) Mol. Cell 12, 63–74 [DOI] [PubMed] [Google Scholar]
- 53.Zheng G., Yang Y. C. (2004) J. Biol. Chem. 279, 42410–42421 [DOI] [PubMed] [Google Scholar]
- 54.Song J., Durrin L. K., Wilkinson T. A., Krontiris T. G., Chen Y. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 14373–14378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reverter D., Lima C. D. (2005) Nature 435, 687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosendorff A., Sakakibara S., Lu S., Kieff E., Xuan Y., DiBacco A., Shi Y., Shi Y., Gill G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5308–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin D. Y., Huang Y. S., Jeng J. C., Kuo H. Y., Chang C. C., Chao T. T., Ho C. C., Chen Y. C., Lin T. P., Fang H. I., Hung C. C., Suen C. S., Hwang M. J., Chang K. S., Maul G. G., Shih H. M. (2006) Mol. Cell 24, 341–354 [DOI] [PubMed] [Google Scholar]
- 58.Hecker C. M., Rabiller M., Haglund K., Bayer P., Dikic I. (2006) J. Biol. Chem. 281, 16117–16127 [DOI] [PubMed] [Google Scholar]
- 59.Iñiguez-Lluhí J. A., Lou D. Y., Yamamoto K. R. (1997) J. Biol. Chem. 272, 4149–4156 [DOI] [PubMed] [Google Scholar]
- 60.So A. Y., Chaivorapol C., Bolton E. C., Li H., Yamamoto K. R. (2007) PLoS Genet. 3, e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang W., Sheng H., Warner D. S., Paschen W. (2007) J. Cereb. Blood Flow Metab. [DOI] [PubMed] [Google Scholar]
- 62.Bossis G., Melchior F. (2006) Mol. Cell 21, 349–357 [DOI] [PubMed] [Google Scholar]
- 63.Manza L. L., Codreanu S. G., Stamer S. L., Smith D. L., Wells K. S., Roberts R. L., Liebler D. C. (2004) Chem. Res. Toxicol. 17, 1706–1715 [DOI] [PubMed] [Google Scholar]
- 64.Zheng Z., Cai C., Omwancha J., Chen S. Y., Baslan T., Shemshedini L. (2006) J. Biol. Chem. 281, 4002–4012 [DOI] [PubMed] [Google Scholar]
- 65.Magee J. A., Chang L. W., Stormo G. D., Milbrandt J. (2006) Endocrinology 147, 590–598 [DOI] [PubMed] [Google Scholar]
- 66.Li M., Chevalier-Larsen E. S., Merry D. E., Diamond M. I. (2007) J. Biol. Chem. 282, 3157–3164 [DOI] [PubMed] [Google Scholar]
- 67.Hannich J. T., Lewis A., Kroetz M. B., Li S. J., Heide H., Emili A., Hochstrasser M. (2005) J. Biol. Chem. 280, 4102–4110 [DOI] [PubMed] [Google Scholar]
- 68.Azuma Y., Dasso M. (2002) Dev. Cell 2, 130–131 [DOI] [PubMed] [Google Scholar]
- 69.Palacios S., Perez L. H., Welsch S., Schleich S., Chmielarska K., Melchior F., Locker J. K. (2005) Mol. Biol. Cell 16, 2822–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chevalier-Larsen E. S., O'Brien C. J., Wang H., Jenkins S. C., Holder L., Lieberman A. P., Merry D. E. (2004) J. Neurosci. 24, 4778–4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katsuno M., Adachi H., Kume A., Li M., Nakagomi Y., Niwa H., Sang C., Kobayashi Y., Doyu M., Sobue G. (2002) Neuron 35, 843–854 [DOI] [PubMed] [Google Scholar]
- 72.Katsuno M., Adachi H., Doyu M., Minamiyama M., Sang C., Kobayashi Y., Inukai A., Sobue G. (2003) Nat. Med. 9, 768–773 [DOI] [PubMed] [Google Scholar]
- 73.Palazzolo I., Burnett B. G., Young J. E., Brenne P. L., La Spada A. R., Fischbeck K. H., Howell B. W., Pennuto M. (2007) Hum. Mol. Genet. 16, 1593–1603 [DOI] [PubMed] [Google Scholar]
- 74.Sharma D., Shinchuk L. M., Inouye H., Wetzel R., Kirschner D. A. (2005) Proteins 61, 398–411 [DOI] [PubMed] [Google Scholar]
- 75.Tanaka M., Morishima I., Akagi T., Hashikawa T., Nukina N. (2001) J. Biol. Chem. 276, 45470–45475 [DOI] [PubMed] [Google Scholar]
- 76.Darnell G., Orgel J. P., Pahl R., Meredith S. C. (2007) J. Mol. Biol. 374, 688–704 [DOI] [PubMed] [Google Scholar]
- 77.Ellerby L. M., Hackam A. S., Propp S. S., Ellerby H. M., Rabizadeh S., Cashman N. R., Trifiro M. A., Pinsky L., Wellington C. L., Salvesen G. S., Hayden M. R., Bredesen D. E. (1999) J. Neurochem. 72, 185–195 [DOI] [PubMed] [Google Scholar]
- 78.Shao J., Diamond M. I. (2007) Hum. Mol. Genet. 16, R115–123 [DOI] [PubMed] [Google Scholar]
- 79.LaFevre-Bernt M. A., Ellerby L. M. (2003) J. Biol. Chem. 278, 34918–34924 [DOI] [PubMed] [Google Scholar]
- 80.Kotaja N., Vihinen M., Palvimo J. J., Jänne O. A. (2002) J. Biol. Chem. 277, 17781–17788 [DOI] [PubMed] [Google Scholar]
- 81.Kotaja N., Karvonen U., Jänne O. A., Palvimo J. J. (2002) Mol. Cell Biol. 22, 5222–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riley B. E., Zoghbi H. Y., Orr H. T. (2005) J. Biol. Chem. 280, 21942–21948 [DOI] [PubMed] [Google Scholar]
- 83.Szebenyi G., Morfini G. A., Babcock A., Gould M., Selkoe K., Stenoien D. L., Young M., Faber P. W., MacDonald M. E., McPhaul M. J., Brady S. T. (2003) Neuron 40, 41–52 [DOI] [PubMed] [Google Scholar]
- 84.Terashima T., Kawai H., Fujitani M., Maeda K., Yasuda H. (2002) Neuroreport 13, 2359–2364 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.