Abstract
The reversible regulation of myosin light chain phosphatase (MLCP) in response to agonist stimulation and cAMP/cGMP signals plays an important role in the regulation of smooth muscle (SM) tone. Here, we investigated the mechanism underlying the inhibition of MLCP induced by the phosphorylation of myosin phosphatase targeting subunit (MYPT1), a regulatory subunit of MLCP, at Thr-696 and Thr-853 using glutathione S-transferase (GST)-MYPT1 fragments having the inhibitory phosphorylation sites. GST-MYPT1 fragments, including only Thr-696 and only Thr-853, inhibited purified MLCP (IC50 = 1.6 and 60 nm, respectively) when they were phosphorylated with RhoA-dependent kinase (ROCK). The activities of isolated catalytic subunits of type 1 and type 2A phosphatases (PP1 and PP2A) were insensitive to either fragment. Phospho-GST-MYPT1 fragments docked directly at the active site of MLCP, and this was blocked by a PP1/PP2A inhibitor microcystin (MC)-LR or by mutation of the active sites in PP1. GST-MYPT1 fragments induced a contraction of β-escin-permeabilized ileum SM at constant pCa 6.3 (EC50 = 2 μm), which was eliminated by Ala substitution of the fragment at Thr-696 or by ROCK inhibitors or 8Br-cGMP. GST-MYPT1-(697–880) was 5-times less potent than fragments including Thr-696. Relaxation induced by 8Br-cGMP was not affected by Ala substitution at Ser-695, a known phosphorylation site for protein kinase A/G. Thus, GST-MYPT1 fragments are phosphorylated by ROCK in permeabilized SM and mimic agonist-induced inhibition and cGMP-induced activation of MLCP. We propose a model in which MYPT1 phosphorylation at Thr-696 and Thr-853 causes an autoinhibition of MLCP that accounts for Ca2+ sensitization of smooth muscle force.
The contractile state of smooth muscle (SM)3 is driven by phosphorylation of the regulatory myosin light chain and reflects the balance of the Ca2+-calmodulin-dependent myosin light chain kinase and myosin light chain phosphatase (MLCP) activities (1). The stoichiometry between force and [Ca2+] varies with different agonists (2), reflecting other signaling pathways that modulate the MLCP or myosin light chain kinase activities (3–5). Agonist activation of G-protein-coupled receptors triggers Ca2+ release from the sarcoplasmic reticulum. Simultaneously, G-protein-coupled receptor signals are mediated by Ca2+-independent phospholipase A2 (6) and initiate kinase signals, such as PKC, phosphoinositide 3-kinase (7), and ROCK. These lead to inhibition of MLCP activity resulting in an increase in regulatory myosin light chain phosphorylation independent of a change in Ca2+ (Ca2+ sensitization) (for review, see Ref. 1). K+ depolarization can also activate RhoA in a Ca2+-dependent manner (8). Conversely, Ca2+ desensitization occurs when nitric oxide production and the activation of Gas elevate cGMP and cAMP levels in SM, leading to dis-inhibition and restoration of MLCP activity (9–15). Thus, MLCP plays a pivotal role in controlling phosphorylation of myosin, in response to physiological stimulation.
MLCP is a trimeric holoenzyme consisting of a catalytic subunit of protein phosphatase 1 (PP1) δ isoform and a regulatory complex of MYPT1 and an accessory M21 subunit (16). A PP1 binding site, KVKF38, is located at the N terminus of MYPT1 followed by an ankyrin-repeat domain. This N-terminal domain forms a part of the active site together with the catalytic subunit and controls the substrate specificity via allosteric interaction and targeting to loci (17). The C-terminal region of MYPT1 directly binds to substrates such as myosin and ezrin/radixin/moecin proteins as well as, under some conditions, the plasma membrane, tethering the catalytic subunit to multiple targets (18, 19). Furthermore, MYPT1 is involved in the regulation of MLCP activity. Alternative splicing of MYPT1 occurs in SM depending on the tissue and the developmental stage (20). An exon 13 splicing of MYPT1 is involved in Ca2+ sensitization that occurs in response to GTP (21), whereas a splice variant of MYPT1, containing the C-terminal Leu-zipper sequence, correlates with cGMP-dependent relaxation of smooth muscle (22). Direct binding of PKG to MYPT1 at the Leu-zipper domain and/or Arg/Lys-rich domain is involved in the activation of MLCP (23–25). In addition, a myosin phosphatase-Rho interacting protein (M-RIP) is directly associated with the MYPT1 C-terminal domain, proposed to recruit RhoA to the MLCP complex (26). The C-terminal region also binds to ZIP kinase, which phosphorylates MYPT1 at Thr-6964 (27). Thus, the C-terminal domain of MYPT1 functions as a scaffold for multiple phosphatase regulatory proteins.
Phosphorylation of MYPT1 at Thr-696 and Thr-853 and the phosphatase inhibitory protein CPI-17 at Thr-38 play dominant roles in the agonist-induced inhibition of MLCP (18, 28–34), yet the molecular mechanism(s) of MYPT1 inhibitory phosphorylation is poorly understood. Receptor activation induces biphasic contraction of SM, reflecting a sequential activation of PKC and ROCK. Phosphorylation of CPI-17 occurs first in parallel with Ca2+ release and the activation of a conventional PKC that causes Ca2+-dependent Ca2+ sensitization (35). A delayed activation of ROCK increases the phosphorylation of MYPT1 at Thr-853. These phosphorylation events maintain the sustained phase of contraction after the fall in [Ca2+]i (35). Phosphorylation of MYPT1 at Thr-853 is elevated in response to various agonists (35, 36). Unlike the Thr-853 site, phosphorylation of MYPT1 at Thr-696 is often spontaneously phosphorylated under resting conditions and insensitive to stimuli with most agonists (36). Nonetheless, up-regulation of MYPT1 phosphorylation at Thr-696 is reported in some types of hypertensive animals and patients, suggesting an importance of the site under pathological conditions (37–39). Phosphorylation of CPI-17 and MYPT1 at Thr-696 is reversed in response to nitric oxide production and cGMP elevation, which parallels relaxation (14, 15). Upon cGMP elevation, MYPT1 at Ser-695 is phosphorylated, and the Ser phosphorylation blocks the adjacent phosphorylation at Thr-696, causing dis-inhibition of MLCP (27, 40). However, Ser-695 phosphorylation does not cause the dephosphorylation at Thr-696 in intact cerebral artery (41). Thus, phosphorylation of MYPT1 governs Ca2+ sensitization and desensitization of SM, although the underlying mechanisms are still controversial. In addition, telokin, a dominant protein in visceral and phasic vascular SM tissues, is phosphorylated by PKG and PKA, activating MLCP by an unknown mechanism and inducing SM relaxation (42).
Multiple mechanisms have been suggested for the phosphorylation-dependent inhibition of MLCP. Thiophosphorylation of MYPT1 results in lower Vm and higher Km values of MLCP activity, suggesting that allosteric modulation of the active site is necessary for the thiophosphorylation-dependent inhibition of MLCP (43). On the other hand, translocation of MYPT1 to the plasma membrane region occurs in parallel with the phosphorylation of MYPT1 at Thr-696 (44, 45), but the amount translocated and the functional meaning remain controversial (41). Phosphorylation of MYPT1 at Thr-853 in vitro reduces its affinity for phospho-myosin, thus suppressing the phosphatase activity (18). It has also been demonstrated that reconstitution of thiophosphorylated MYPT1 at Thr-696 or Thr-853 with isolated PP1δ produces a less-active form of MLCP complex (46). This supports the kinetic analysis (43) that suggests an allosteric effect of MYPT1 phosphorylation on the phosphatase activity. In contrast, a thiophosphopeptide mimicking the phosphorylation site of MBS85, a homolog of MYPT1 and not present in SM, inhibits the activity of MBS85·PP1 complex, suggesting the direct interaction between the MBS85 site and PP1 (47). In the crystal structure model of MYPT1-(1–229). PP1δ complex, the electrostatic potential map at the MLCP active site complements amino acid profiles around the phosphorylation sites (17). Therefore, it is possible that the inhibitory phosphorylation sites directly dock at the active site of MLCP and inhibit the activity. Here, we examine mechanisms underlying the inhibition of MLCP through the phosphorylation of MYPT1 at Thr-696 and Thr-853 using GST fusion versions of various MYPT1 fragments including or excluding either or both of these phosphorylation sites. Phosphorylated MYPT1 fragments including either Thr-696 or Thr-853 potently and specifically inhibit MLCP purified from pig aorta and the enzyme associated with myofilaments in permeabilized ileum SM tissues. We further show that inhibition of MLCP in SM tissues is eliminated by activation of PKA/PKG, suggesting that the GST-MYPT1 fragments mimic agonist-induced autoinhibition and cAMP/cGMP-dependent dis-autoinhibition of MLCP in SM.
EXPERIMENTAL PROCEDURES
Reagents
Human MYPT1 cDNA was cloned from a HeLa cell cDNA library. Various parts of MYPT1 cDNA fragments were generated using PCR with a pair of mismatched primers with BamHI and EcoRI sites. The PCR product was cloned at the BamHI and EcoRI sites of pGEX4T-2 (GE Healthcare). Recombinant GST-MYPT1 fragments were induced for 24 h at 23 °C with 0.1 mm isopropyl-β-d-1-thiogalactopyranoside in Escherichia coli Rosetta2TM(DE3)pLysS strain transformed with the pGEX vector and purified from lysozyme-treated bacterial lysates by GSH-HiTrap affinity chromatography using AKTA Purifier chromatography system. After the elution with 10 mm glutathione, the protein solution was dialyzed against 50 mm MOPS-NaOH, pH 7.0, supplemented with 0.8 mm 4-(2-aminoethyl)-benzenesulfonyl fluoride (PefablocTM) and 0.5 mm Tris[2-carboxyethyl] phosphine and stored in −80 °C. Phosphorylation of GST-MYPT1 proteins (10 μm) was performed for 1 h at 30 °C with recombinant human ROCK-II (Millipore #14-338) (1.25 μg/ml) or PKA catalytic subunit (Sigma) in the presence of 25 mm MOPS-NaOH, pH 7.0, plus 1 mm ATP, 10 mm Mg(OAc)2, 0.5 mm Tris[2-carboxyethyl] phosphine, 0.4 mm PefablocTM, and 0.1 mg/ml bovine serum albumin. Thiophosphorylation was carried out using 1 mm ATPγS instead of ATP. The reaction mixture was subjected to buffer exchange to 10 mm Tris-HCl, pH 7.5, using a Protein Desalting Spin Column (Pierce) before the phosphatase assay. MLCP was prepared from pig aorta homogenate, as described previously (48). Monomeric PP1 subunit was prepared from rabbit skeletal muscle (49). Human red cell PP2A was a generously gift from Prof. Brautigan (University of Virginia). Okadaic acid, microcystin-LR, Y27632, H1152, and 8Br-cGMP were obtained from Biomol. Anti-MYPT1 and anti- PP1δ was obtained from Millipore. Anti-HA (12CA5 clone) was obtained from the monoclonal facility at University of Virginia. HEK293 and A7r5 cells were purchased from ATCC and harvested in Dulbecco's modified medium supplemented with 10% fetal bovine serum (CellGro).
Assays
Phosphatase activity was measured as described previously (50). Briefly, 32P-labeled chicken gizzard myosin regulatory light chain (0.5 μm) was added to the reaction mixture of phosphatase and inhibitors to initiate the dephosphorylation. After a 10-min incubation at 30 °C, the reaction was terminated by the addition of 10% trichloroacetic acid, and the radioactivity of released 32Pi was counted using Beckman LS-6500 scintillation counter. The phosphatase activities without enzyme and with phosphatase minus inhibitor were set as 0 and 100%, respectively. Mean value was obtained from duplicate assay. IC50 ± error value was calculated by a nonlinear regression curve-fitting program using an equation: activity (%) = 100 − (100 × [inhibitor]/(IC50 + [inhibitor])). A GST pulldown assay was performed using GST-MYPT1 fragment as a ligand. A7r5 rat aortic smooth muscle cells in a 10-cm dish were lysed with 1 ml of lysis buffer (50 mm MOPS-NaOH, pH 7.0, with 0.1 m NaCl, 1 mm EGTA, 0.1% Tween 20, 5% glycerol, 0.5 mm Tris[2-carboxyethyl] phosphine, 0.8 mm Pefabloc, and 0.5 μg/ml leupeptin). The cell lysates were split into four portions, with 1 μm microcystin LR added to two of them. Each solution was mixed with 20 μl of GSH-agarose slurry and 10 μg of GST-MYPT1 proteins and incubated for 90 min at 4 °C. The beads were collected by brief centrifugation and then washed with the lysis buffer 3 times. Proteins bound to the ligands were analyzed by immunoblotting. For HA-tagged PP1, HEK293 cells in a 6-cm dish were transiently transfected for 24 h with mammalian expression vector of HA-PP1δ (1 μg) plus Myc-MYPT1 (1 μg) using FuGene6TM transfection reagent (Roche Applied Science). The cells expressing the recombinant MLCP complex were used for a GST pulldown assay (51). Immunoblotting was performed using polyvinylidene difluoride membranes as described previously (50). The ECL signal was detected using a Fluorochem CCD camera imaging system and quantified using the attached software, AlphaEaseFC (Alpha-Inotech).
Tissue Preparation and Force Measurements
Force measurements were performed as described previously (42, 52). New Zealand White male rabbits were euthanized with halothane inhalation according to the animal protocol approved by the Animal Care and Use Committee at University of Virginia. Small strips (250 μm × 2 mm) were cut from the longitudinal smooth muscle layer of the ileum in Hepes-buffered saline (5 mm Hepes, pH 7.3, with 0.15 m NaCl, 4 mm KCl, 2 mm CaCl2, 2 mm MgCl2, 5.6 mm d-glucose). Muscle strips were tied with monofilament silk and mounted between a tungsten needle attached to a SensoNor AE801 force transducer and a stationary hook in a 400-μl bubble chamber with a stirring bar as reported previously (42). Before the assay, each strip was stimulated with 154 mm K+ to assess viability. Procedures were done at room temperature (22–24 °C). Muscle strips were permeabilized for 20 min with 75 μm β-escin in the cytoplasmic buffer (30 mm PIPES buffer, pH 7.1, with 74.1 mm potassium methanesulfonate, 3.3 mm magnesium methanesulfonate, 4.5 mm MgATP, and 10 mm creatine phosphate supplemented with 1 mm EGTA (pCa > 8) (42, 52). After permeabilization, intracellular Ca2+ stores were depleted by addition of 10 μm A23187 for 10 min. Muscles were partially contracted with pCa6.3 buffer (the cytoplasmic buffer plus 6.5 mm Ca2+ and 10 mm EGTA) supplemented with 1 μm chicken gizzard calmodulin that evokes ∼15% of the maximum contraction induced with pCa 4.5 buffer (the cytoplasmic buffer plus 10 mm Ca2+ and 10 mm EGTA). GST-MYPT1 fragments were intensively dialyzed against pCa6.3 buffer and concentrated using a Centricon tube, saving the filtrate as a control for contaminant Ca2+. GST-MYPT1 fragments or the “filtrate” controls were added to the bath, and the contraction was recorded. The mean value with the standard error (S.E.) was obtained from 3–5 independent experiments for each condition. Student's t test was performed using Microsoft Excel to test for significance.
RESULTS
Phosphorylation-dependent Inhibition of MLCP by GST-MYPT1-(654–880) Including Thr-696 and Thr-853
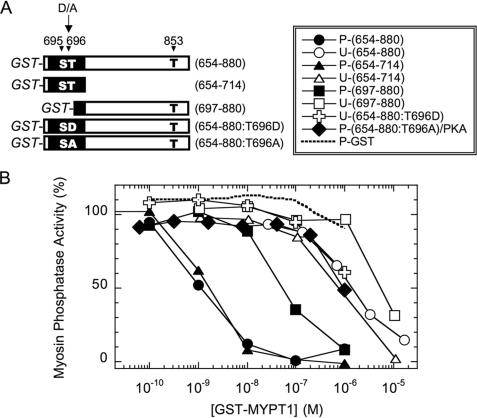
Murányi et al. (46) report that thiophosphorylation of MYPT1 at Thr-696 or Thr-853 suppresses the PP1 catalytic subunit upon reconstitution. We tested whether phosphorylation of MYPT1 induces an allosteric effect to suppress the activity or converts the region into autoinhibitory domains. We avoided the stable thiophosphorylation and prepared orthophosphorylated GST-tagged MYPT1 fragments (Fig. 1A) and asked whether the MYPT1 segment, including the phosphorylation sites, directly inhibits the activity of purified MLCP complex. The amino acid sequence around Thr-696, indicated by a black box in Fig. 1A, is conserved among the MYPT1 family, such as MYPT2 and MBS85. The sequence around Thr-853 is unique to MYPT1. There is charge complementarity around Thr-853 similar to the Thr-696 site and, interestingly, also around Ser-19 of regulatory myosin light chain 20 (RLC20) (17). Phosphatase assays were performed using the MLCP purified from pig aorta, consisting of PP1δ and the N-terminal 68-kDa fragment of MYPT1 (48) (Fig. 1B). Unphosphorylated GST-MYPT1-(654–880) inhibits the pig aorta MLCP with an apparent IC50 of 1.4 ± 0.2 μm (Fig. 1B, open circles). Phosphorylation of GST-MYPT1-(654–880) with recombinant ROCK (closed circles) increased the inhibitory potency about 1000-fold (IC50 = 1.1 ± 0.2 nm). In contrast, PKA phosphorylation of GST-MYPT1-(654–880:T696A) protein at Ser-695 yielded no increase in the potency (Fig. 1B, closed diamond), indicating that the phosphorylation at adjacent Ser-695 is not inhibitory. GST-alone incubated with ROCK (broken line) did not suppress the phosphatase activity. Importantly, the thiophospho form of GST-MYPT1-(654–880) was no more potent than the orthophospho form, indicating that MLCP is incapable of dephosphorylation of MYPT1 at Thr-696 and Thr-853 (data not shown).
FIGURE 1.
Inhibition of MLCP by GST-MYPT1 fragments. A, schematic drawing of GST fusion version of MYPT1 fragments. GST tag is attached at the N terminus, and deduced residue numbers corresponding to the full-length human MYPT1 are indicated at the left. The closed box indicates the region of conserved amino acid sequence among MYPT1, MYPT2, and MBS85. Phosphorylation site Ser/Thr is indicated as S and T. B, inhibition of MLCP with various versions of GST-MYPT1 fragments. MLCP activity was measured using 32P-labeled myosin light chain as a substrate in the presence of various concentrations of GST-MYPT1 fragments. Recombinant proteins used are GST-MYPT1-(654–880) (circle), GST-MYPT1-(654–714) (triangle), (697–880) (square), and (654–880:T696D) (cross). Unphosphorylated (U) and ROCK-phosphorylated (P) proteins are indicated with open and closed symbols, respectively. PKA-phosphorylated GST-MYPT1-(654–880) and GST alone are indicated with closed diamonds and a dotted lines, respectively.
A shorter fragment including Thr-696 but lacking Thr-853, GST-MYPT1-(654–714) (Fig. 1B, closed and open triangles) was as effective as GST-MYPT1-(654–880) in inhibiting MLCP activity. Therefore, the phosphorylation of Thr-696 is sufficient to produce maximum potency for the inhibition of GST-MYPT1-(654–880). Asp-substitution at Thr-696, yielding GST-MYPT1-(654–880:T696D), did not enhance the inhibitory potency (Fig. 1B, open crosses). In addition to the phosphorylation at Thr-696, the phosphorylation of GST-MYPT1-(697–880) at Thr-853 also enhanced the inhibitory potency to the purified MLCP, 100-fold (IC50 = 60 ± 10 nm (phosphorylated, closed squares) and 6.1 ± 1.7 μm (unphosphorylated, open squares) (Fig. 1B). Thus, our phospho-MYPT1 fragments mimic the effect of thiophosphorylation of MYPT1 at Thr-696 and Thr-853, yielding a less active form of reconstituted MLCP complex (46). These results suggest that interdomain interaction between the phosphorylation site and the catalytic domain seems to account for the phosphorylation-dependent autoinhibition of MLCP. Neither point mutant at Thr-853 replaced with Ala or Asp was successfully prepared from bacterial lysates because of unexpected instability of the proteins (data not shown).
Specificity of GST-MYPT1-(654–880) for the Inhibition of Phosphatases
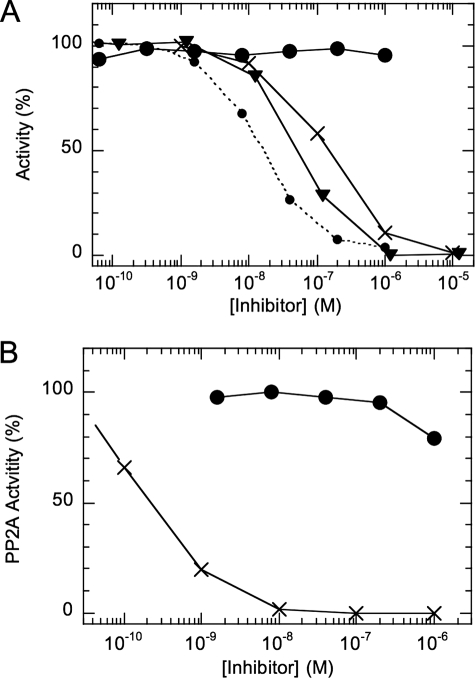
Fig. 2 shows inhibition of the monomeric catalytic subunit of PP1 from rabbit skeletal muscle (panel A) and PP2A from human red cells (panel B) with thiophospho (thioP)-GST-MYPT1-(654–880). Thiophosphoprotein was used to prevent dephosphorylation during the assay. Surprisingly, thioP-GST-MYPT1-(654–880) had no effect on the activity of the monomeric PP1 (large closed circle), even at 1 μm, whereas it inhibited MLCP complex with an IC50 of 16 nm (dotted line). Okadaic acid (×) and thioP-CPI-17 (triangle) fully suppressed the monomeric PP1 with IC50 of 51.7 and 152 nm, respectively, as reported previously (53, 54). PP2A was insensitive to thioP-GST-MYPT1-(654–880) but was potently inhibited with okadaic acid (Fig. 2B). The results suggest that the MYPT1 segment (654–880) specifically recognizes PP1 associated with MYPT1 but not PP1 alone.
FIGURE 2.
Effects of GST-MYPT1-(654–880) on the activities of monomeric PP1 and PP2A. Phosphatase activity was measured using monomeric PP1 (A) or PP2A (B), with 32P-labeled myosin light chain as a substrate. The assay was done in the presence of ROCK-thiophosphorylated (tP)-GST-MYPT1-(654–880) (closed circle), tP-CPI-17 (triangle), and okadaic acid (×). The dotted line in panel A indicates the results with MLCP in the presence of thioP-GST-MYPT1-(654–880).
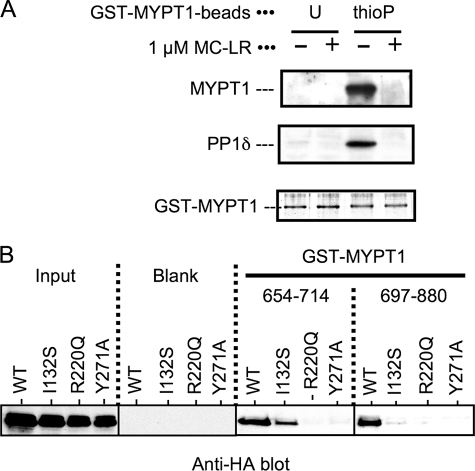
Interaction of the Autoinhibitory Domain Fragment with MLCP
We examined the direct interaction of the MYPT1 fragments with the active site of MLCP using a GST-pulldown method. Rat aortic smooth muscle cell lysates were used as the source of native MLCP consisting of the full-length MYPT1 and PP1δ. Unphospho- or thioP-GST-MYPT1-(654–880) beads were mixed with the lysates, and the bound MLCP was analyzed by immunoblotting (Fig. 3A). The intact MYPT1 and PP1δ were associated with thioP-GST-MYPT1 but not with the unphosphoprotein (Fig. 3A). The binding of the thioP-GST-MYPT1 fragment with MYPT1-PP1 complex was eliminated by the addition of microcystin-LR, a potent PP1/PP2A inhibitor compound. Because microcystin-LR is known to dock at the active site of PP1 (55), the results imply a competition of thioP-GST-MYPT1 fragment with MC-LR at the active site of MLCP. We further examined the interaction of P-Thr-696 and P-Thr-853 with the active site of MLCP using a GST pulldown assay with recombinant MLCP complexes with point mutations at the active site (Fig. 3B). HA-tagged PP1δ proteins, wild type, I132S, K221Q, and Y271A were transiently co-expressed with Myc-tagged MYPT1-(1–300), and cell lysates were used as the recombinant MLCP complex (Fig. 3B, left). These mutations at the active site had no effects on the interaction of HA-PP1δ with MYPT1-(1–300) (51), and this cotransfection method yields active MLCP complex in cells (56). HA-PP1δ wild type protein was coprecipitated with both thioP-GST-MYPT1-(654–714) and -(697–880), indicating that the two domains can bind MLCP independently (Fig. 3B). The binding of thioP-Thr-696 fragment was impaired by either mutation at Arg-220 or Tyr-271, whereas a mutation at Ile-132 yielded moderate effect on the binding. Similarly, thioP-Thr-853 fragment failed to associate with HA-PP1 mutants of R220Q, Y271A, and I132A. These results demonstrate that each MYPT1 domain around Thr-696 or -853 directly docks at the active site of MLCP complex, resulting in the inhibition of the activity. Phosphorylated CPI-17 failed to bind to HA-PP1 R220Q and Y271A, whereas the I132S mutation of HA-PP1 did not affect the interaction with phosphorylated CPI-17 (51). Therefore, the overall mechanisms of the phosphorylation-dependent inhibition of MLCP are similar between MYPT1 Thr-696, Thr-853, and CPI-17 Thr-38, although the residues at the PP1 active site involved in the docking are distinguishable.
FIGURE 3.
Interaction of GST-MYPT1 fragments with MLCP. A, GST pulldown assay was performed with GST-MYPT1-(654–880) as a ligand. Unphospho (U)- or thioP-GST-MYPT1-(654–880) was immobilized on glutathione beads and mixed with smooth muscle cells lysates in the presence and absence of 1 μm microcystin (MC)-LR. Proteins bound to the beads were analyzed by immunoblotting using anti-MYPT1 (top), anti-PP1δ (middle), and Coomassie stain (bottom). B, HA-PP1 wild type (WT) and mutants, I132S, R220Q, and Y271A, were transiently expressed in HEK293 cells with Myc-tagged full-length MYPT1. Each cell lysate (indicated as Input) was subjected to a GST pulldown assay with GST alone (Blank), and thioP-GST-MYPT1-(654–714) and -(697–880). The recombinant MLCP complex was detected by immunoblotting with anti-HA. The results were reproduced in two independent experiments.
GST-MYPT1-(654–880) Evoked Ca2+-sensitized Force of Permeabilized Rabbit Ileum Smooth Muscle
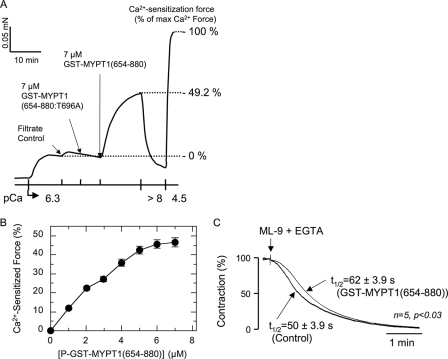
We examined the role of MLCP autoinhibition in the regulation of SM contraction using GST-MYPT1 fragments. Unphosphorylated GST-MYPT1 fragments were added to rabbit ileum SM strips permeabilized with β-escin. This permeabilization protocol retains receptor coupling and signaling for the generation of Ca2+-sensitized force in response to GTPγS or agonist stimulation at constant Ca2+ concentration (42, 52). Fig. 4A demonstrates a typical force measurement trace from a permeabilized ileum strip. GST-MYPT1-(654–880) increased contraction of permeabilized strips at a submaximal calcium concentration of pCa 6.3. The Ca2+-sensitizated force reached 49% that of the maximum force induced with pCa4.5 and was reversed by withdrawing Ca2+ with EGTA (pCa <8). Buffer control (filtrate) induced no effect on the contraction (Fig. 4A). The Ca2+-sensitized force induced with GST-MYPT1-(654–880) was increased dose-dependently to 50% that of maximum force, with an apparent EC50 value of 3 μm (Fig. 4B), which is consistent with the expression level of MLCP, estimated as 2–3 μm in phasic smooth muscles (57, 58). Fig. 4C shows an in situ MLCP activity assay where the relaxation rate of a permeabilized SM strip was measured in the presence and absence of GST-MYPT1-(654–880). Maximum contraction of the permeabilized ileum strip was induced with pCa4.5 buffer. Then myosin light chain kinase activity was arrested by the addition of ML-9 (200 μm), and removal of Ca2+ with EGTA and MLCP-dependent relaxation was monitored. The half-time of 50 ± 3.9 s in the control was significantly faster than the 62 ± 3.9 s in the presence of 7 μm GST-MYPT1-(654–880) (Fig. 4C), consistent with inhibition of endogenous MLCP by GST-MYPT1-(654–880).
FIGURE 4.
Contraction of permeabilized ileum smooth muscle strip induced by addition of GST-MYPT1-(654–880) at constant Ca2+. A, representative force trace of GST-MYPT1-(654–880)-induced Ca2+ sensitization. The smooth muscle strip was permeabilized with β-escin on the force transducer, and partial contraction was elicited with pCa6.3 buffer. At pCa6.3-induced force, the filtrate control, 7 μm GST-MYPT1-(654–880:T696A) and GST-MYPT1-(654–880) were sequentially added to the bath. The strip was then relaxed with pCa > 8 solution and re-contracted with pCa4.5 buffer as a measure of maximal force. The levels of force at pCa6.3 and pCa4.5 were set as 0 and 100% that of the Ca2+ sensitization force, respectively. B, dose-dependent Ca2+ sensitization force with GST-MYPT1-(654–880). GST-MYPT1-(654–880) was sequentially added to the bath, and the contraction was read after reaching a plateau level. S.E. was obtained from independent assays (n = 5). C, effects of GST-MYPT1-(654–880) on the relaxation of permeabilized smooth muscle. Maximum contraction of β-escin-permeabilized ileum strip was induced with pCa4.5 buffer in the presence or absence of 7 μm GST-MYPT1-(654–880). After reaching the plateau, the buffer was switched to pCa > 8 buffer plus 200 μm ML-9 with or without 7 μm GST-MYPT1-(654–880). Thick and thin lines indicate the force trace of control and in the presence of GST-MYPT1-(654–880), respectively. S.E. of the half-time for the relaxation (t½) was obtained from five independent experiments.
Phosphorylation by ROCK Is Required for the Potency of GST-MYPT1-(654–880)
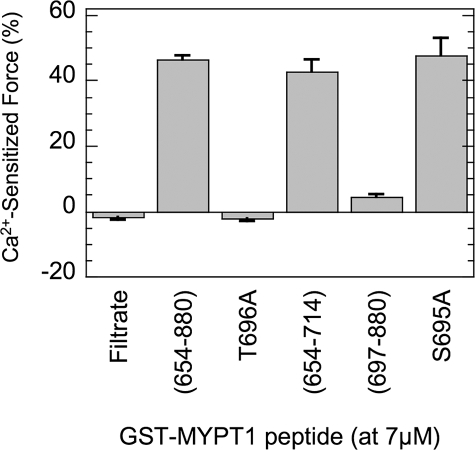
Fig. 5 summarizes the results of Ca2+ sensitized force induced with various versions of the GST-MYPT1 fragment. Ala substitution of GST-MYPT1-(654–880) at Thr-696 (T696A) failed to induce Ca2+-sensitized force, indicating that phosphorylation of GST-MYPT1-(654–880) at Thr-696 in the permeabilized tissue is needed to produce the inhibitory potency for the endogenous MLCP (also see Fig. 4A). The negative value seen with filtrate and T696A was because of a slow fall of the pCa 6.3 force over the time of incubation with the filtrate (Fig. 4A). The shorter fragment, GST-MYPT1-(654–714), induced a similar level of Ca2+-sensitized force as induced with GST-MYPT1-(654–880). Thus, the sequence conserved among the MYPT1 family is sufficient to produce Ca2+ sensitization of permeabilized SM. The other part of the MYPT1 fragment, GST-MYPT1-(697–880), induced Ca2+-sensitized force but only by 10% that of the level achieved with Thr-696 fragments. This is consistent with the results of higher IC50 values of GST-MYPT1-(697–880) compared with GST-MYPT1-(654–714), shown in Fig. 1. These results suggest that both phosphorylation sites of MYPT1, Thr-696 and Thr-853, are capable of inducing Ca2+-sensitized force via inhibition of MLCP in SM, albeit with markedly different potencies.
FIGURE 5.
Ca2+ sensitization force induced with GST-MYPT1 fragments. β-Escin-permeabilized smooth muscle strips were partially contracted with pCa6.3 solution, and then 7 μm GST-MYPT1 fragments added as indicated under the bar graph. Negative value with filtrate control indicates phasic response of the contraction. S.E. are obtained from independent assay (n = 3–5).
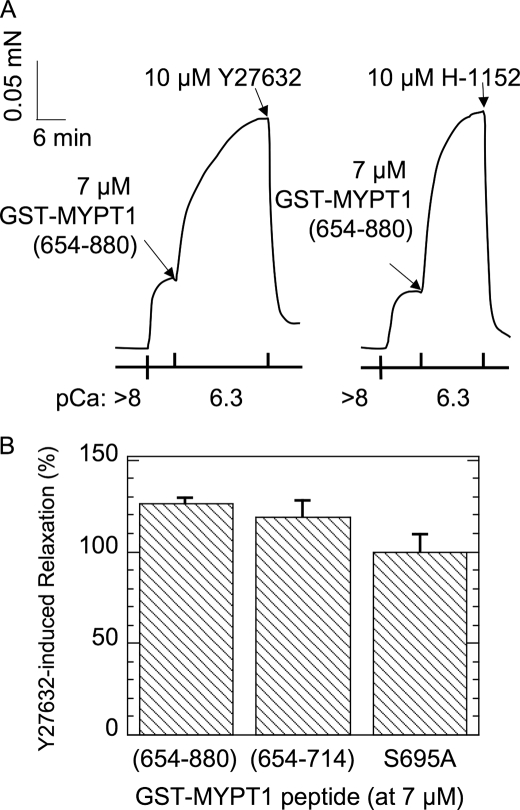
The MYPT1 Thr-853 site is reported as a ROCK phosphorylation site (18, 28), whereas multiple kinases, such as ROCK (29), integrin-linked kinase (30), zipper-interacting kinase (31), and p21-activated kinase (32) are capable of phosphorylating Thr-696. As shown in Fig. 6, the GST-MYPT1-(654–880)-induced contraction was completely eliminated by the addition of both ROCK inhibitors, Y27632 and H1152. Y27632 also canceled the contraction evoked by GST-MYPT1-(654–714) (Fig. 6B). There results indicate that endogenous ROCK in permeabilized ileac SM is at least partially active under submaximum Ca2+ concentrations without agonist stimulation and is involved in the phosphorylation of MYPT1 at Thr-696 to evoke Ca2+ sensitized force. Because ROCK inhibitors suppress the contraction at pCa6.3, the extent of the relaxation of the GST-MYPT1-(654–880) induced by Y27632 treatment was >100%. Ca2+ is known to activate RhoA/ROCK (8), so that the submaximal pCa6.3 possibly induces the partial activation of ROCK, which is responsible for the basal level of contraction at pCa6.3 and the phosphorylation of GST-MYPT1-(654–880) reported below.
FIGURE 6.
Effects of ROCK inhibitors on the Ca2+ sensitization force induced with GST-MYPT1-(654–880). Permeabilized smooth muscle strips were contracted with 7 μm GST-MYPT1-(654–880) under the pCa6.3 condition. ROCK inhibitors Y27632 (10 μm) or H-1152 (10 μm) were added to the bath (panel A), and the magnitude of the decrease in the contraction is indicated as relaxation (%), where 100% is set as the extent of Ca2+ sensitization force induced with each GST-MYPT1 fragment (n = 3–5) (panel B).
8Br-cGMP-induced Relaxation of Ca2+-sensitizing Force Evoked by GST-MYPT1-(654–880)
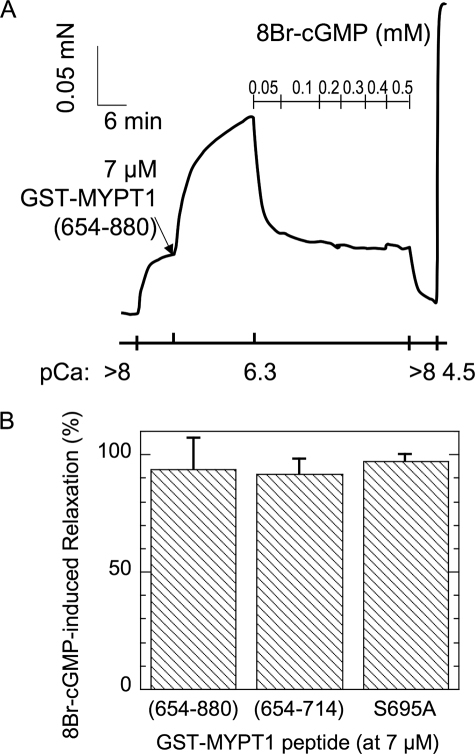
The addition of 8Br-cGMP, a stable form of cGMP, evoked relaxation of GST-MYPT1-(654–880)-induced contraction at pCa6.3 (Fig. 7A). The extent of relaxation at 100 μm 8Br-cGMP reached nearly 100% that of the Ca2+ sensitization force (Fig. 7). Similarly, 8Br-cGMP suppressed the contraction induced by GST-MYPT1-(654–714). Therefore, cGMP-mediated signaling can eliminate the contraction induced by the autoinhibition of MLCP.
FIGURE 7.
Effects of 8Br-cGMP on the Ca2+ sensitization force induced with GST-MYPT1-(654–880). Permeabilized SM strips were contracted with 7 μm GST-MYPT1-(654–880) under the pCa6.3 condition. The relaxation was evoked by a serial addition of 8Br-cGMP (A). The magnitude of the decrease in the contraction with 50 μm 8Br-cGMP is indicated as relaxation (%), where 100% is set as the extent of Ca2+ sensitization force induced with each GST-MYPT1 fragment (n = 3) (panel B).
Recent reports suggest a critical role of the phosphorylation of MYPT1 at Ser-695 adjacent to Thr-696 on cGMP-mediated relaxation of smooth muscle by blocking the phosphorylation at Thr-696 (27, 40). We asked whether the phosphorylation at Ser-695 is necessary for the 8Br-cGMP-induced elimination of the inhibition of MLCP by GST-MYPT1-(654–880) using an Ala-substituted S695A mutant. GST-MYPT1-(654–880:S695A) induced Ca2+ sensitization force as effectively as the Ser-695 version (Fig. 5). The contraction induced with GST-MYPT1-(654–880:S695A) was suppressed by the addition of Y27632 (Fig. 6B). Importantly, the addition of 8Br-cGMP fully eliminated the Ca2+ sensitization force induced with GST-MYPT1-(654–880:S695A) (Fig. 7B). 8Br-cGMP is known to activate PKG as well as PKA. Phosphorylation of CREB (cAMP-response element-binding protein), a PKA substrate, also occurred, so that both PKA and PKG are activated in response to 100 μm 8Br-cGMP (data not shown). Thus, phosphorylation of GST-MYPT1-(654–880) at Ser-695 is not involved in 8Br-cGMP-mediated elimination of the Ca2+-sensitized force.
In Situ Phosphorylation of GST-MYPT1 Fragments and Endogenous MYPT1 in Ileum SM Strips
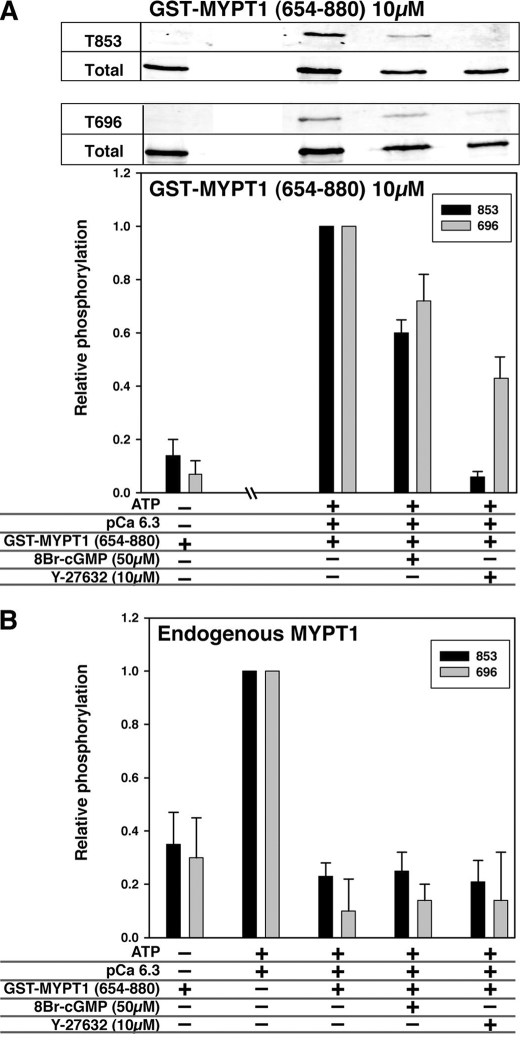
Fig. 8 shows the phosphorylation of GST-MYPT1-(654–880) (panel A) and endogenous MYPT1 (panel B) in the permeabilized ileum. After an incubation for 5 min with 4.0 mm ATP, both Thr-696 and Thr-853 of GST-MYPT1-(654–880) were phosphorylated in the permeabilized ileum at pCa6.3 (Fig. 8A). The phosphorylation was reduced to 60–70% maximum by the addition of 8Br-cGMP. Y27632 treatment potently reduced the phosphorylation at Thr-853 and halved that at Thr-696 (Fig. 8A). As shown in Fig. 8B, the phosphorylation of endogenous MYPT1 at both sites was increased by the addition of ATP-pCa6.3 solution, in parallel to the contraction that reached about 50% of GTPγS-induced force. Interestingly, the phosphorylation of endogenous MYPT1 was reduced to the basal level by the addition of GST-MYPT1-(654–880). There was no further reduction of MYPT1 phosphorylation in the presence of 8Br-cGMP or Y27632 (Fig. 8B). These results suggest that phosphorylation of GST-MYPT1-(654–880) at Thr-696 and Thr-853 spontaneously occurs in the permeabilized ileum tissues, and this accounts for the Ca2+ sensitization force. The reduction in phosphorylation of endogenous MYPT1 by GST-MYPT1-(654–880) indicates a competition between ectopic (10 μm) and endogenous (1–2 μm) MYPT1 for the kinase. Thus, the Ca2+-sensitized force induced by the addition of GST-MYPT1 is because of the inhibition of endogenous MLCP by the 10-fold higher concentration of phospho-ectopic protein. Therefore, the change in the phosphorylation of endogenous MYPT1 is negligible and does not contribute to the GST-MYPT1-(654–880)-induced Ca2+ sensitized force.
FIGURE 8.
Phosphorylation of GST-MYPT1-(654–880) (A) and endogenous MYPT1 (B) in permeabilized ileum SM tissues. β-Escin-permeabilized SM strips were incubated under the conditions indicated at bottom of the graph. The samples were subjected to trichloroacetic acid-acetone treatment and immunoblotting. Representing blots with anti-phospho-MYPT1(Thr-853) and (Thr-696) are shown at the top along with the blots stained with anti-GST as loading control. The extent of phosphorylation was normalized with the loading control. Gray and black bars indicate the relative extent of MYPT1 phosphorylation at Thr-696 and Thr-853, respectively.
DISCUSSION
In this study we demonstrate that the MYPT1 fragment, inclusive of the Thr-696 or Thr-853 phosphorylation sites, inhibits MLCP and induces Ca2+-sensitized force in SM upon phosphorylation through basal activity of endogenous ROCK. Because of unexpected instability of T853A/D mutants, we could not test whether the phosphorylation of Ser-852, which is phosphorylated in fibroblasts (28), is involved in the inhibition of MLCP. The inhibition with P-GST-MYPT1-(654–880) was highly specific to the MLCP complex, such that the isolated PP1 monomer from rabbit skeletal muscle was insensitive to the MYPT1 fragment, indicating that neither phospho-Thr-696 nor phospho-Thr-853 is capable of inhibiting monomeric PP1. In contrast to our data, Murányi et al. (30) demonstrated that the MYPT1 fragment (residues 514–963) inhibits the isolated PP1 catalytic subunit with an IC50 around 100 nm. Although the protein was thiophosphorylated with recombinant zipper-interacting kinase or integrin-linked kinase, not with ROCK, the phosphorylation site was identified at Thr-696. Our phospho-MYPT1-(654–880) inhibited the MLCP complex with an IC50 of 1.6 nm, so that clearly the isolated PP1 is less sensitive when compared with the MLCP complex. Tan et al. (47) demonstrated that the 50-residue peptide mimicking the conserved region of MBS85, corresponding to MYPT1 Thr-696, binds to the recombinant HA-tagged PP1δ purified from COS7 cells and inhibits the activity of MBS85·PP1 complex. There are only minor differences between two sequences of MYPT1 and MBS85 in the phosphorylation site domain. However, overall sequence of MBS85 shows less than a 40% homology to MYPT1, and the length between the N-terminal ankyrin repeats and the phosphorylation site of MBS85 is 135 residues shorter than that of MYPT1, which could have significant effects on folding and conformation in the inhibitory mechanism. Importantly, the thiophosphopeptide of MBS85 was used for the inhibition assay (47). Generally, Ser/Thr phosphatases cannot catalyze a hydrolysis of thiophospho ester, so that the thioP-protein forms a stable complex with phosphatase and suppresses the activity. Indeed, thioP-myosin inhibits MLCP activity with an IC50 of 35 nm, whereas orthophosphoprotein does not affect the activity at all (data not shown). Therefore, the inhibitory effect of the previous MYPT1 fragment possibly reflects stability of the thiophospho ester but not the autoinhibition. The present study provides the first evidence that phosphorylation of MYPT1 converts the regions into an autoinhibitory domain under physiological conditions. In this study we show that our preparations of PP1 and PP2A are active using okadaic acid and CPI-17. Further evidence that phospho-MYPT1-(695–880) docks at the PP1 site in the MYPT1·PP1 complex is based on our PP1 mutation assay. The residues in the active site of MLCP play dominant roles in the interaction with MYPT1 autoinhibitory domains. The N-terminal domain of MYPT1-(1–300) includes a PP1 binding motif plus 8× ankyrin repeats. Crystallographic studies of this N-terminal domain and PP1 showed that the formation of the complex produces an extended catalytic cleft that determines substrate specificity via this allosteric interaction (17). The surface electropotential mapping of the active site of MLCP shows a charge complementarity around the Thr-696 and Thr-853 of MYPT1, resembling those around Ser-19 of the myosin light chain (17). Therefore, the autoinhibitory domain of MYPT1 likely recognizes the active site modulated by the allosteric regulation with the N-terminal domain of MYPT1. Interestingly, the N-terminal 19 residues of MYPT1, located near the PP1 active site, are directly associated with phosphorylated CPI-17 (59). Therefore, it is also possible that the autoinhibitory domain recognizes the MYPT1 N-terminal segment as a marker of the active site. In addition to Thr-696 site, the sequence in MYPT1-(1–300) is conserved in MYPT2 and MBS85. Thus, the autoinhibitory regulation via Thr-696 site of MYPT1 seems to be a common theme in the regulation of PP1 complexed with MYPT1, MYPT2, and MBS85.
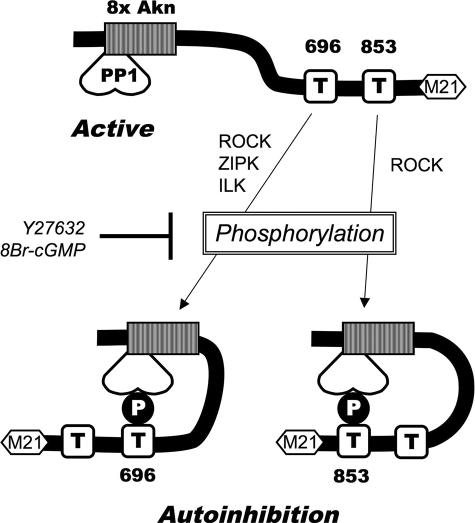
The role of Thr-853 phosphorylation on the regulation of MLCP has been controversial. Velasco et al. (18) reported that a MYPT1 fragment (714–1004) is coprecipitated with myosin filaments under low ionic strength condition. The phosphorylation of MYPT1-(714–1004) with ROCK blocked the interaction with myosin filaments, accounting for the reduction in the activity (18). Our experimental conditions are significantly different from those used in that study. Instead of whole myosin molecules, we used isolated myosin light chains for the inhibition assay to omit the effect of the direct binding between MYPT1 and the substrate. Furthermore, the MLCP used for the assay lacks the C-terminal myosin binding site of MYPT1. Therefore, the inhibition of MLCP with P-GST-MYPT1-(697–880) occurs without interference from the binding of myosin. Murányi et al. (46) reported that recombinant full-length MYPT1 thiophosphorylated at Thr-696 or Thr-853 inhibits monomeric PP1, using myosin light chain and myosin as substrates. Based on our results and the results with the MBS85 peptide, we presume that the N-terminal domain of the full-length recombinant MYPT1 associates with monomeric PP1, which makes the active site accessible for the autoinhibitory domains. Taken together, we propose a model for the autoinhibitory regulation of MLCP mediated by two individual phosphorylation sites, Thr-696 and Thr-853 (Fig. 9). Phosphorylation at Thr-696 produces a potent autoinhibitory site, docking at the active site of MLCP. Phosphorylation of MYPT1 at Thr-853 also results in the inactive form of MLCP, although this is less potent. Our study does not address the release of MYPT1 from the myosin filament upon phosphorylation at Thr-853, which may play a more prominent role in myosin binding than in catalytic activity. The IC50 value of GST-MYPT1-(697–880) was 50-fold higher that that of GST-MYPT1-(654–714). However, because in our model the autoinhibition is driven by intramolecular interaction, the affinity of each inhibitory domain with the active site will not affect the potency in the intact holoenzyme. In fact, each thiophosphorylation at Thr-696 or Thr-853 of the full-length MYPT1 is reported to suppress the phosphatase activity equally (46).
FIGURE 9.
Schematic drawing of the autoinhibition of MLCP. The catalytic subunit of PP1 is associated with MYPT1 near 8× ankylin (Akn) repeat domain (striped box). With unphosphorylated MYPT1, the PP1 active site is accessible to its substrate. Upon the phosphorylation of Thr-696 or Thr-853, the phosphorylation site directly docks at the active site of PP1 and suppresses the phosphatase activity. Treatment with Y27632 and 8Br-cGMP reduces MYPT1 phosphorylation and attenuates the autoinhibition. ZIPK, zipper-interacting kinase; ILK, integrin-linked kinase.
In the model, illustrated in Fig. 9, the direct docking of the phosphorylation sites with the active site of MLCP is a determinant of MLCP activity. Ichikawa et al. (43) demonstrated that thiophosphorylation of purified MLCP complex causes inhibition of the activity. The kinetic analysis shows an increase in Km value and a decrease in Vm value upon thiophosphorylation. In addition, the thioP-MLCP complex retains 20–30% activity (43, 46). These results led to the conclusion that the phosphorylation of MYPT1 causes allosteric inhibition of the phosphatase activity through conformational change. Our data with MYPT1 fragments suggest another mechanism underlying MLCP regulation. In this mechanism the phospho-MYPT1 fragment with either Thr-696 or Thr-853 directly docks at the active site of MLCP and inhibits the activity completely. This indicates competitive autoinhibition upon the phosphorylation of MYPT1. An analogy can be made with another Ser/Thr phosphatase, calcineurin (CaN). The active domain of CaN is followed by an inhibitory domain and calmodulin binding domain (for review, see Klee et al. (60)). The kinetic analysis demonstrated that calmodulin binding of CaN increases the Vm value but does not affect the Km value, suggesting allosteric activation of the phosphatase (61). On the other hand, the peptide mimicking the inhibitory domain causes a competitive inhibition of CaN active fragment, suggesting a direct binding of the inhibitory domain to the active site (62). Later, x-ray crystallography solved the three-dimensional structure of CaN with the autoinhibitory domain, showing that the alpha-helical inhibitory domain locates along the active site groove of CaN, supporting the autoinhibitory mechanism (63). Thus, we presume that Michaelis-Menten type kinetic analysis cannot be applied to the intramolecule regulation of MLCP. For example, structural hindrance may give a restraint to the interaction between the phosphorylation site of MYPT1 and the active site, so that activity is not suppressed completely. Our study using MYPT1 fragments points out the possibility of the autoinhibition of MLCP upon the phosphorylation. Recently, Wang et al. (64) reported that multiple loci in CaN are involved in the autoregulation of CaN. Thus, it is possible that multiple factors, including multi-interactions and conformational changes, are orchestrated to control the MLCP activity in addition to the simplified autoinhibition model in Fig. 9. Structural insights into the regulation are needed for further discussion of the underlying mechanism.
The contraction evoked by the addition of GST-MYPT1-(654–714) was eliminated by ROCK inhibitors as well as 8Br-cGMP. Cyclic GMP is known to activate MLCP activity in arterial and ileum SM (12, 65). Phosphorylation of MYPT1 at Thr-696 is reduced upon 8Br-cGMP stimulation through the adjacent phosphorylation at Ser-695 (27, 40). In our assay, because the Ala substitution at Ser-695 was incapable of eliminating the effect of 8Br-cGMP, the phosphorylation of GST-MYPT1-(654–880) at Ser-695 is unlikely the cause of the relaxation in these experiments. On the other hand, our results do not exclude the possibility that the phosphorylation of endogenous MYPT1 at Ser-695 alters the sensitivity against GST-MYPT1 fragments. A simple explanation for the 8Br-cGMP-induced inactivation of the GST-MYPT1 fragment is the inhibition of ROCK by cGMP signaling. PKG has been reported to phosphorylate RhoA at Ser-188, causing translocation and inactivation of RhoA (66). It is possible that 8Br-cGMP induces phosphorylation of endogenous RhoA or possibly an activation of a RhoGAP with subsequent inactivation of ROCK that triggers in situ dephosphorylation of GST-MYPT1-(654–880). In addition, an unidentified Thr-696 phosphatase is possibly activated in response to the cGMP signal. PKG is reported to associate with the C-terminal domain of MYPT1 and is involved in SM relaxation (23, 25). The phosphorylation of telokin by PKG activates MLCP through an unknown mechanism (42, 65). Totsukawa et al. (67) showed that phosphorylation of MYPT1 at Ser-434 causes activation of the phosphatase activity during mitosis. Neither the C-terminal PKG binding sites nor Ser-434 is included in GST-MYPT1-(654–880), so that these signals are unlikely involved in the effect of 8Br-cGMP on GST-MYPT1 fragments. Nonetheless, because the inhibition of MLCP relies on the interaction between the active site and the autoinhibitory sites, the autoinhibition could be cancelled through a physical interference of the interaction without affecting the phosphorylation status. Our results encourage further study into mechanisms of de-autoinhibition of MLCP in cGMP-induced relaxation.
Agonist-induced Ca2+ sensitization of SM contraction is mediated by three sites of phosphorylation; MYPT1 at Thr-696, Thr-853, and CPI-17 at Thr-38. There are no significant similarities in the overall sequence within the inhibitory domains of MYPT1 and CPI-17 except for the residues around their phosphorylation sites, which are relatively enriched with basic residues (17). The apparent IC50 value of phosphorylated CPI-17 is similar to that of P-GST-MYPT1-(654–714) (59). Besides, Asp substitution at the phosphorylation site does not increase the potency, suggesting a similar important role of the phosphate group in the inhibition of MLCP (68). Thus, MYPT1 and CPI-17 share a common mechanism of inhibition, where upon phosphorylation the inhibitory domain of MYPT1 or CPI-17 directly docks at the active site of MLCP. However, there are differences. Phosphorylated CPI-17 is dephosphorylated by monomeric PP1 or PP1 complexed with other regulatory subunits; thereby, CPI-17 selectively inhibits MLCP rather than other PP1 complexes in cells (69). On the other hand, we demonstrate that the thiophosphorylated MYPT1 autoinhibitory domain cannot inhibit monomeric PP1. This indicates that the specificity of the autoinhibition relies on the allosteric regulation of PP1 through its complexing with the MYPT1 N-terminal domain as well as a spatial restraint in intramolecular interaction. Various agonists elicit diverse signals in SM cells and activate multiple kinases such as PKC, ROCK, integrin-linked kinase, zipper-interacting kinase, and others. The diverse signals converge on the active site of MLCP through the direct interaction with phosphorylated MYPT1 at Thr-696 and Thr-853 and CPI-17 at Thr-38. Thus, the interaction between the active site of MLCP with the inhibitory phosphorylation sites is the determinant of Ca2+ sensitivity of SM contraction. The autoinhibitory model offers a new focal point for further study of the regulation of smooth muscle contraction and relaxation.
Acknowledgments
We appreciate the critical discussions with Drs. David L. Brautigan, Robert Nakamoto, and Zygmunt Derewenda.
This work was supported, in whole or in part, by National Institutes of Health Grants PO1 HL48807, PO1 HL19242 (to A. V. S.), RO1 HL83261, and PO1 48807 (to M. E.). This work was also supported by a Pennsylvania CURE grant (to M. E.). A conflict of interest exists in Millipore products (for M. E.).
Residue numbering of MYPT1 is based on the amino acid sequence deduced from the longest splice variant of human MYPT1.
- SM
- smooth muscle
- PKC
- protein kinase C
- PKA
- cAMP-dependent kinase
- ROCK
- Rho-associated coiled coil-containing protein kinase
- MLCP
- myosin light chain phosphatase
- MYPT1
- myosin phosphatase targeting subunit
- PKG
- cGMP-dependent kinase
- PP1
- protein phosphatase 1
- PP1δ
- delta isoform of PP1
- PP2A
- protein phosphatase 2A
- CPI-17
- PKC-activated PP1 inhibitor, Mr = 17
- GST
- glutathione S-transferase
- thioP
- thiophospho
- CaN
- calcineurin
- MOPS
- 4-morpholinepropanesulfonic acid
- PIPES
- 1,4-piperazinediethanesulfonic acid
- HA
- hemagglutinin
- ATPγS
- adenosine 5′-O-(thiotriphosphate).
REFERENCES
- 1.Somlyo A. P., Somlyo A. V. (2003) Physiol. Rev. 83, 1325–1358 [DOI] [PubMed] [Google Scholar]
- 2.Rembold C. M. (1992) Hypertension 20, 129–137 [DOI] [PubMed] [Google Scholar]
- 3.Bradley A. B., Morgan K. G. (1987) J. Physiol. 385, 437–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himpens B., Casteels R. (1990) Pflugers Arch. 416, 28–35 [DOI] [PubMed] [Google Scholar]
- 5.Himpens B., Kitazawa T., Somlyo A. P. (1990) Pflugers Arch. 417, 21–28 [DOI] [PubMed] [Google Scholar]
- 6.Guo Z., Su W., Ma Z., Smith G. M., Gong M. C. (2003) J. Biol. Chem. 278, 1856–1863 [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., Yoshioka K., Azam M. A., Takuwa N., Sakurada S., Kayaba Y., Sugimoto N., Inoki I., Kimura T., Kuwaki T., Takuwa Y. (2006) Biochem. J. 394, 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakurada S., Takuwa N., Sugimoto N., Wang Y., Seto M., Sasaki Y., Takuwa Y. (2003) Circ. Res. 93, 548–556 [DOI] [PubMed] [Google Scholar]
- 9.Himpens B., Matthijs G., Somlyo A. P. (1989) J. Physiol. 413, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitazawa T., Somlyo A. P. (1990) Biochem. Biophys. Res. Commun. 172, 1291–1297 [DOI] [PubMed] [Google Scholar]
- 11.Wu J., Kleiner U., Brautigan D. L. (1996) Biochemistry 35, 13858–13864 [DOI] [PubMed] [Google Scholar]
- 12.Lee M. R., Li L., Kitazawa T. (1997) J. Biol. Chem. 272, 5063–5068 [DOI] [PubMed] [Google Scholar]
- 13.Sausbier M., Schubert R., Voigt V., Hirneiss C., Pfeifer A., Korth M., Kleppisch T., Ruth P., Hofmann F. (2000) Circ. Res. 87, 825–830 [DOI] [PubMed] [Google Scholar]
- 14.Etter E. F., Eto M., Wardle R. L., Brautigan D. L., Murphy R. A. (2001) J. Biol. Chem. 276, 34681–34685 [DOI] [PubMed] [Google Scholar]
- 15.Bonnevier J., Arner A. (2004) J. Biol. Chem. 279, 28998–29003 [DOI] [PubMed] [Google Scholar]
- 16.Hartshorne D. J., Ito M., Erdödi F. (2004) J. Biol. Chem. 279, 37211–37214 [DOI] [PubMed] [Google Scholar]
- 17.Terrak M., Kerff F., Langsetmo K., Tao T., Dominguez R. (2004) Nature 429, 780–784 [DOI] [PubMed] [Google Scholar]
- 18.Velasco G., Armstrong C., Morrice N., Frame S., Cohen P. (2002) FEBS Lett. 527, 101–104 [DOI] [PubMed] [Google Scholar]
- 19.Fukata Y., Kimura K., Oshiro N., Saya H., Matsuura Y., Kaibuchi K. (1998) J. Cell Biol. 141, 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dirksen W. P., Vladic F., Fisher S. A. (2000) Am. J. Physiol. Cell Physiol. 278, C589–600 [DOI] [PubMed] [Google Scholar]
- 21.Richards C. T., Ogut O., Brozovich F. V. (2002) J. Biol. Chem. 277, 4422–4427 [DOI] [PubMed] [Google Scholar]
- 22.Khatri J. J., Joyce K. M., Brozovich F. V., Fisher S. A. (2001) J. Biol. Chem. 276, 37250–37257 [DOI] [PubMed] [Google Scholar]
- 23.Surks H. K., Mochizuki N., Kasai Y., Georgescu S. P., Tang K. M., Ito M., Lincoln T. M., Mendelsohn M. E. (1999) Science 286, 1583–1587 [DOI] [PubMed] [Google Scholar]
- 24.Huang Q. Q., Fisher S. A., Brozovich F. V. (2004) J. Biol. Chem. 279, 597–603 [DOI] [PubMed] [Google Scholar]
- 25.Given A. M., Ogut O., Brozovich F. V. (2007) Am. J. Physiol. Cell Physiol. 292, C432–439 [DOI] [PubMed] [Google Scholar]
- 26.Surks H. K., Richards C. T., Mendelsohn M. E. (2003) J. Biol. Chem. 278, 51484–51493 [DOI] [PubMed] [Google Scholar]
- 27.Wooldridge A. A., MacDonald J. A., Erdodi F., Ma C., Borman M. A., Hartshorne D. J., Haystead T. A. (2004) J. Biol. Chem. 279, 34496–34504 [DOI] [PubMed] [Google Scholar]
- 28.Kawano Y., Fukata Y., Oshiro N., Amano M., Nakamura T., Ito M., Matsumura F., Inagaki M., Kaibuchi K. (1999) J. Cell Biol. 147, 1023–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. (1996) Science 273, 245–248 [DOI] [PubMed] [Google Scholar]
- 30.Murányi A., MacDonald J. A., Deng J. T., Wilson D. P., Haystead T. A., Walsh M. P., Erdodi F., Kiss E., Wu Y., Hartshorne D. J. (2002) Biochem. J. 366, 211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald J. A., Borman M. A., Murányi A., Somlyo A. V., Hartshorne D. J., Haystead T. A. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2419–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takizawa N., Koga Y., Ikebe M. (2002) Biochem. Biophys. Res. Commun. 297, 773–778 [DOI] [PubMed] [Google Scholar]
- 33.Eto M., Karginov A., Brautigan D. L. (1999) Biochemistry 38, 16952–16957 [DOI] [PubMed] [Google Scholar]
- 34.Koyama M., Ito M., Feng J., Seko T., Shiraki K., Takase K., Hartshorne D. J., Nakano T. (2000) FEBS Lett. 475, 197–200 [DOI] [PubMed] [Google Scholar]
- 35.Dimopoulos G. J., Semba S., Kitazawa K., Eto M., Kitazawa T. (2007) Circ. Res. 100, 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitazawa T., Eto M., Woodsome T. P., Khalequzzaman M. (2003) J. Physiol. 546, 879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seko T., Ito M., Kureishi Y., Okamoto R., Moriki N., Onishi K., Isaka N., Hartshorne D. J., Nakano T. (2003) Circ. Res. 92, 411–418 [DOI] [PubMed] [Google Scholar]
- 38.Hilgers R. H., Todd J., Jr., Webb R. C. (2007) J. Hypertens 25, 1687–1697 [DOI] [PubMed] [Google Scholar]
- 39.Guilluy C., Sauzeau V., Rolli-Derkinderen M., Guérin P., Sagan C., Pacaud P., Loirand G. (2005) Br. J. Pharmacol. 146, 1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura K., Koga Y., Sakai H., Homma K., Ikebe M. (2007) Circ. Res. 101, 712–722 [DOI] [PubMed] [Google Scholar]
- 41.Neppl R. L., Lubomirov L. T., Momotani K., Pfitzer G., Eto M., Somlyo A. V. (2009) J. Biol. Chem.284, 6348–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choudhury N., Khromov A. S., Somlyo A. P., Somlyo A. V. (2004) J. Muscle Res. Cell. Motil. 25, 657–665 [DOI] [PubMed] [Google Scholar]
- 43.Ichikawa K., Ito M., Hartshorne D. J. (1996) J. Biol. Chem. 271, 4733–4740 [DOI] [PubMed] [Google Scholar]
- 44.Shin H. M., Je H. D., Gallant C., Tao T. C., Hartshorne D. J., Ito M., Morgan K. G. (2002) Circ. Res. 90, 546–553 [DOI] [PubMed] [Google Scholar]
- 45.Bolz S. S., Vogel L., Sollinger D., Derwand R., de Wit C., Loirand G., Pohl U. (2003) Circulation 107, 3081–3087 [DOI] [PubMed] [Google Scholar]
- 46.Murányi A., Derkach D., Erdodi F., Kiss A., Ito M., Hartshorne D. J. (2005) FEBS Lett. 579, 6611–6615 [DOI] [PubMed] [Google Scholar]
- 47.Tan I., Ng C. H., Lim L., Leung T. (2001) J. Biol. Chem. 276, 21209–21216 [DOI] [PubMed] [Google Scholar]
- 48.Eto M., Ohmori T., Suzuki M., Furuya K., Morita F. (1995) J. Biochem. 118, 1104–1107 [DOI] [PubMed] [Google Scholar]
- 49.Martin B. L., Shriner C., Brautigan D. L. (1994) Protein Expression Purif. 5, 211–217 [DOI] [PubMed] [Google Scholar]
- 50.Eto M., Leach C., Tountas N. A., Brautigan D. L. (2003) Methods Enzymol. 366, 243–260 [DOI] [PubMed] [Google Scholar]
- 51.Matsuzawa F., Aikawa S. I., Ohki S. Y., Eto M. (2005) J. Biochem. 137, 633–641 [DOI] [PubMed] [Google Scholar]
- 52.Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. (1989) J. Biol. Chem. 264, 5339–5342 [PubMed] [Google Scholar]
- 53.Eto M., Senba S., Morita F., Yazawa M. (1997) FEBS Lett. 410, 356–360 [DOI] [PubMed] [Google Scholar]
- 54.Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. (1989) Biochem. Biophys. Res. Commun. 159, 871–877 [DOI] [PubMed] [Google Scholar]
- 55.Goldberg J., Huang H. B., Kwon Y. G., Greengard P., Nairn A. C., Kuriyan J. (1995) Nature 376, 745–753 [DOI] [PubMed] [Google Scholar]
- 56.Eto M., Kirkbride J. A., Brautigan D. L. (2005) Cell Motil. Cytoskeleton 62, 100–109 [DOI] [PubMed] [Google Scholar]
- 57.Gong M. C., Cohen P., Kitazawa T., Ikebe M., Masuo M., Somlyo A. P., Somlyo A. V. (1992) J. Biol. Chem. 267, 14662–14668 [PubMed] [Google Scholar]
- 58.Woodsome T. P., Eto M., Everett A., Brautigan D. L., Kitazawa T. (2001) J. Physiol. 535, 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eto M., Kitazawa T., Matsuzawa F., Aikawa S., Kirkbride J. A., Isozumi N., Nishimura Y., Brautigan D. L., Ohki S. Y. (2007) Structure 15, 1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klee C. B., Ren H., Wang X. (1998) J. Biol. Chem. 273, 13367–13370 [DOI] [PubMed] [Google Scholar]
- 61.Stewart A. A., Ingebritsen T. S., Cohen P. (1983) Eur. J. Biochem. 132, 289–295 [DOI] [PubMed] [Google Scholar]
- 62.Perrino B. A., Ng L. Y., Soderling T. R. (1995) J. Biol. Chem. 270, 340–346 [DOI] [PubMed] [Google Scholar]
- 63.Kissinger C. R., Parge H. E., Knighton D. R., Lewis C. T., Pelletier L. A., Tempczyk A., Kalish V. J., Tucker K. D., Showalter R. E., Moomaw E. W., Louis N. G., Habuka N., Chen X. M., Maldonado F., Barker J. E., Bacquet R., Villafranca J. E. (1995) Nature 378, 641–644 [DOI] [PubMed] [Google Scholar]
- 64.Wang H., Du Y., Xiang B., Lin W., Li X., Wei Q. (2008) Biochemistry 47, 4461–4468 [DOI] [PubMed] [Google Scholar]
- 65.Wu X., Haystead T. A., Nakamoto R. K., Somlyo A. V., Somlyo A. P. (1998) J. Biol. Chem. 273, 11362–11369 [DOI] [PubMed] [Google Scholar]
- 66.Sauzeau V., Le Jeune H., Cario-Toumaniantz C., Smolenski A., Lohmann S. M., Bertoglio J., Chardin P., Pacaud P., Loirand G. (2000) J. Biol. Chem. 275, 21722–21729 [DOI] [PubMed] [Google Scholar]
- 67.Totsukawa G., Yamakita Y., Yamashiro S., Hosoya H., Hartshorne D. J., Matsumura F. (1999) J. Cell Biol. 144, 735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohki S., Eto M., Shimizu M., Takada R., Brautigan D. L., Kainosho M. (2003) J. Mol. Biol. 326, 1539–1547 [DOI] [PubMed] [Google Scholar]
- 69.Eto M., Kitazawa T., Brautigan D. L. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 8888–8893 [DOI] [PMC free article] [PubMed] [Google Scholar]