Abstract
STIM1 and Orai1 have been reported to interact upon store depletion culminating in Ca2+ release-activated Ca2+ current activation. Recently, the essential region has been identified within the STIM1 C terminus that includes the second coiled-coil domain C-terminally extended by ∼50 amino acids and exhibits a strong binding to the Orai1 C terminus. Based on the homology within the Orai family, an analogous scenario might be assumed for Orai2 as well as Orai3 channels as both are activated in a similar STIM1-dependent manner. A combined approach of electrophysiology and Foerster resonance energy transfer microscopy uncovered a general mechanism in the communication of STIM1 with Orai proteins that involved the conserved putative coiled-coil domains in the respective Orai C terminus and the second coiled-coil motif in the STIM1 C terminus. A coiled-coil single mutation in the Orai1 C terminus abrogated communication with the STIM1 C terminus, whereas an analogous mutation in Orai2 and Orai3 still allowed for their moderate activation. However, increasing coiled-coil probability by a gain of function deletion in Orai1 or by generating an Orai1-Orai3 chimera containing the Orai3 C terminus recovered stimulation to a similar extent as with Orai2/3. At the level of STIM1, decreasing probability of the second coiled-coil domain by a single mutation within the STIM1 C terminus abolished activation of Orai1 but still enabled partial stimulation of Orai2/3 channels. A double mutation within the second coiled-coil motif of the STIM1 C terminus fully disrupted communication with all three Orai channels. In aggregate, the impairment in the overall communication between STIM1 and Orai channels upon decreasing probabilities of either one of the putative coiled-coil domains in the C termini might be compatible with the concept of their functional, heteromeric interaction.
Store-operated Ca2+ entry is a key to cellular regulation of short term responses such as contraction and secretion as well as long term processes like proliferation and cell growth (1). The prototypic and best characterized store-operated channel is the Ca2+ release-activated Ca2+ (CRAC)5 channel (2–6). However, its molecular components have remained elusive until 4 years ago; the STIM1 (stromal interacting molecule 1) (7, 8) and later on Orai1 (9–11) have been identified as the two limiting components for CRAC activation. STIM1 is an ER-located Ca2+ sensor, and store depletion triggers its aggregation into punctae close to the plasma membrane, resulting in stimulation of CRAC currents (12, 13). Its N terminus is located in the ER lumen and contains an EF-hand Ca2+-binding motif, which senses the ER Ca2+ level, and a sterile α-motif, which is suggested to mediate homomeric STIM1 aggregation (14–16). In the cytosolic STIM1 C terminus, two coiled-coil regions overlapping with the ezrin-radixin-moesin-like domain and a lysine-rich region are essential for CRAC activation (14, 17, 18). Three recent studies have independently identified the ezrin-radixin-moesin domain as the essential Orai activating domain, named SOAR (STIM1 Orai-activating region) (20) which represents so far the shortest active fragment, OASF (Orai-activating small fragment) (21) or CAD (CRAC-activating domain) (22), which includes the second, more C terminally located coiled-coil domain and the following ∼55 amino acids. The latter amino acids are suggested to contain an additional cytosolic homomerization domain indispensable for OASF homomerization and Orai activation (21).
The Orai family includes three highly Ca2+-selective ion channels (Orai1–3) that locate to the plasma membrane, and each protein contains four predicted transmembrane segments with cytosolic N and C termini (10). All three Orai proteins possess a conserved putative coiled-coil domain in the C terminus (23, 24), whereas only the N terminus of Orai1 consists of a proline/arginine-rich region (25). Orai1 has been assumed to act in concert with STIM1 (10, 27)-activating inward Ca2+ currents after store depletion. The two other members of the Orai family, Orai2 and Orai3, display similar but smaller store-operated inward Ca2+ currents when co-expressed with STIM1 with distinct inactivation profiles, permeability properties, and 2-aminoethoxydiphenyl borate sensitivity (28–32). Recently, we have provided evidence for a store depletion-induced, dynamic coupling of STIM1 to Orai1 that involves the putative coiled-coil domain in the C terminus of Orai1 (33). Furthermore, the C terminus of STIM1, in particular the essential cytosolic region 344–442 as narrowed down by SOAR, OASF, and CAD (20–22), has been established as the key fragment for CRAC as well as Orai1 activation, because its expression alone, without the necessity to deplete ER store, is sufficient for constitutive current activation (18, 32, 33). These fragments SOAR, OASF, and CAD when co-expressed with Orai1 (20–22) exhibit enhanced plasma membrane localization in comparison with the complete STIM1 C terminus in the presence of Orai1. Specificity of interaction of SOAR to the Orai1 C terminus has been shown by its disruption (20) employing the Orai1 L273S mutant (33). Park et al. (22) have provided additional, conclusive evidence for a direct binding by combining multiple biochemical approaches demonstrating CAD interaction with Orai1.
This study focused specifically on the role of the putative coiled-coil domains of STIM1 as well as Orai proteins in their coupling. Coiled-coils generally function as protein-protein interaction sites with the ability of dynamic protein assembly and disassembly (35–37). We suggest the C-terminal, putative coiled-coil domains in all three Orai proteins and the second coiled-coil motif of STIM1 as essential for STIM1/Orai communication. Moreover, the single point coiled-coil STIM1 L373S mutant allowed for differential activation of Orai channels partially stimulating Orai2 as well as Orai3 but not Orai1.
MATERIALS AND METHODS
Molecular Cloning and Mutagenesis
Human Orai1 (Orai1; accession number NM_032790) was kindly provided by the A. Rao laboratory (Harvard Medical School). Human Orai2 (Orai2; accession number NM_032831.1) and Orai3 (Orai3; accession number NM_152288.1) were courtesy of the Lutz Birnbaumer laboratory (NIEHS, National Institutes of Health, Research Triangle Park, NC). N-terminally tagged Orai1 constructs were cloned via SalI and SmaI restriction sites of pECFP-C1 and pEYFP-C1 expression vectors (Clontech). For N-terminally tagged Orai2 constructs, the restriction sites KpnI and XbaI and for Orai3 BamHI and XbaI were used. pECFP/pEYFP-C1/Orai1 served as a template for the generation of the coiled-coil mutant L273S and the Orai1 Δ277–279 construct. Suitable primers exchanged the corresponding codon from GAG to TCG (L273S) or deleted the three-amino acid sequence AEF (Orai1 Δ277–279) using the QuikChange XL site-directed mutagenesis kit (Stratagene). Orai1-Orai3 C-terminal chimera was cloned via overlap extension PCR into pcDNA3.1V5 His TOPO and subcloned into pECFP/pEYFP-C1 via internal BamHI and XbaI restriction sites. For Orai2 coiled-coil mutants L237S and L244S, the corresponding codon was changed from CTC to GCC (L237S) and from CTG to TCG (L244S) using the QuikChange XL site-directed mutagenesis kit (Stratagene). Similarly, pEYFP/pECFP-C1 constructs served as templates for Orai3 coiled-coil mutants L285S and L285S/L292S by exchanging the codon from CTG to TCG (L285S + L292S).
Human STIM1 (STIM1; accession number NM_003156) N-terminally enhanced CFP-tagged and enhanced YFP-tagged were kindly provided by the T. Meyer laboratory, Stanford University. C-terminally EYFP-tagged STIM1 was purchased from GeneCopoeiaTM (catalogue number EX-S0521-M02). STIM1 C terminus (aa 233–685) was cloned into the T/A site of pcDNA3.1V5 His TOPO by PCR and subcloned into pECFP-N1 and pEYFP-N1 via their internal restriction sites HindIII and SacII. pECFP-C1 and pEYFP-C1 STIM1 C terminus were used as templates for the generation of the STIM1-OASF fragment by introducing a stop codon at position 475 (aa 233–474) using the QuikChangeXL site-directed mutagenesis kit (Stratagene).
The second putative coiled-coil region of STIM1 was mutated by changing the codon for leucine (CTG) into a serine (TCG) (L373S) or the codon for alanine (GCC) into serine (TCC) (A376S). The integrity of all resulting clones was confirmed by sequence analysis.
Electrophysiology
Electrophysiological recordings comparing characteristics of 2–3 constructs were carried out by paired comparison on the same day. Expression pattern and levels of the various constructs were carefully monitored by confocal fluorescence microscopy and were not significantly changed by the introduced mutations. Experiments were performed at 20–24 °C, using the patch clamp technique in the whole-cell recording configuration. For STIM1/Orai as well as C-terminal STIM1/Orai current measurements, voltage ramps were usually applied every 5 s from a holding potential of 0 mV, covering a range of −90 to 90 mV over 1 s. The internal pipette solution contained (in mm) 3.5 MgCl2, 145 cesium methane sulfonate, 8 NaCl, 10 HEPES, 10 EGTA, pH 7.2. Extracellular solution consisted of (in mm) 145 NaCl, 5 CsCl, 1 MgCl2, 10 HEPES, 10 glucose, 10 CaCl2, pH 7.4. Currents were leak-corrected by subtracting the leak current obtained in the presence of 10 μm LaCl3.
Confocal Förster Resonance Energy Transfer (FRET) Fluorescence Microscopy
Confocal FRET microscopy was performed similarly as in Ref. 38. In brief, a QLC100 real time confocal system (VisiTech International Ltd., UK) was used for recording fluorescence images connected to two Photometrics CoolSNAPHQ monochrome cameras (Roper Scientific) and a dual port adapter (dichroic, 505lp; cyan emission filter, 485/30; yellow emission filter, 535/50; Chroma Technology Corp.). This system was attached to an Axiovert 200 M microscope (Zeiss, Germany) in conjunction with an argon ion multiple wavelength (457, 488, and 514 nm) laser (Spectra Physics). The wavelengths were selected by an Acousto Optical Tunable Filter (VisiTech International Ltd., UK). MetaMorph 5.0 software (Universal Imaging Corp.) was used to acquire images and to control the confocal system. Illumination times of about 900–1500 ms were typically used for CFP, FRET, and YFP images that were consecutively recorded with minimum delay. Prior to the calculation the images had to be corrected due to cross-talk as well as cross-excitation. For this, the appropriate cross-talk calibration factors were determined for each of the constructs on the day the FRET experiments were performed. The corrected FRET image (NFRET) was calculated on a pixel to pixel basis after background subtraction and threshold determination using custom-made software (39) integrated in MatLab 7.0.4 according to the method published by (40). The local ratio between CFP and YFP might vary because of different localizations of diverse protein constructs, which could lead to the calculation of false FRET values (41). Accordingly, the analysis was limited to pixels with a CFP:YFP molar ratio between 1:10 and 10:1 to yield reliable results (41).
Statistics
Mean ± S.E. values are shown throughout. Significance analysis was performed with the two-tailed Mann-Whitney test.
RESULTS
STIM1 C Terminus Constitutively Activates All Three Orai Proteins
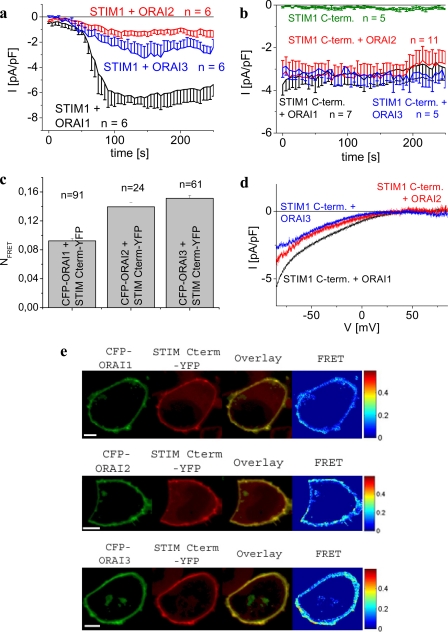
The cytosolic STIM1 C terminus (aa 233–685) as well as shorter cytosolic fragments alone have been proven sufficient to constitutively activate inwardly rectifying Orai1 Ca2+ as well as CRAC currents independent of store depletion (18, 20–22, 32, 33, 42). Moreover, STIM1 C terminus as well as CAD interacts directly with the C terminus of Orai1 in pulldown experiments (22, 33). Thus the STIM1 C terminus, in the absence of ER depletion-induced processes (33), is considered as a functional surrogate for full-length STIM1 to study key characteristics of the STIM1/Orai coupling (42, 43). To extend such experiments onto Orai2 as well as Orai3 channels, we initially demonstrated activation of whole-cell currents from all three Orai proteins each co-expressed in human embryonic kidney (HEK) 293 cells with full-length STIM1. Passive store depletion by 10 mm EGTA in the pipette induced Ca2+ inward currents in a 10 mm Ca2+-containing bath solution from HEK293 cells co-expressing STIM1 with Orai1, -2, and -3 proteins. Current densities of Orai2 and Orai3 channels reached ∼3- and ∼2-fold lower levels than that of Orai1 (Fig. 1a), in line with previous reports (28, 29, 44). To evaluate if the STIM1 C terminus is able to constitutively couple to and activate Orai2 and Orai3 in a similar manner as with Orai1, we utilized a combined approach of electrophysiology and confocal FRET microscopy employing N-terminally CFP-labeled Orai proteins and C-terminally YFP-labeled STIM1 co-expressed in HEK293 cells. The STIM1 C terminus alone turned out as sufficient in constitutively stimulating Orai2 as well as Orai3 Ca2+ currents comparable with Orai1 (Fig. 1, b and d). Confocal FRET microscopy revealed clear co-localization of the STIM1 C terminus and all respective Orai proteins (Fig. 1e) in the plasma membrane region correlating with a robust FRET (Fig. 1c). The significantly smaller FRET between the STIM1 C terminus and Orai1 compared with Orai2/3 might in part account for the reduced stimulatory efficiency of the STIM1 C terminus on Orai1 activity compared with that of the full-length form. Nevertheless, OASF (aa 233–450) lacking the last 235 aa of the STIM1 C terminus efficiently stimulates Orai1–3 current densities to comparable ratios as those obtained with full-length STIM1 (21) suggesting that clustering of full-length STIM1 induced by store depletion might compensate in an avidity-based mechanism for a possibly lower affinity of the STIM1 C terminus to Orai1 than to Orai2/3. In conclusion, the STIM1 C terminus is principally sufficient to couple to and activate all three Orai proteins setting the basis to further characterize molecular determinants of the communication between Orai and the STIM1 C terminus. In the beginning we focused at the Orai C termini containing a putative coiled-coil domain as a key site that interacts with the STIM1 C terminus (20–22).
FIGURE 1.
STIM1 C terminus constitutively couples to and activates all three Orai1/2/3 channels. a, time course of whole-cell inward currents at −74 mV activated by passive store depletion (10 mm EGTA pipette solution) of HEK293 cells expressing STIM1 with Orai1, Orai2, or Orai3. b, time course of constitutive whole-cell inward currents at −74 mV of HEK293 cells expressing STIM1 C terminus with Orai1, Orai2, or Orai3 in comparison with the STIM C terminus (C-term) alone (p > 0.05). c, average FRET of STIM1 C terminus with Orai1 was significantly (p < 0.01) different from that with Orai2 and Orai3. d, current-voltage relationships corresponding to b. e, localization, overlay, and calculated FRET of CFP-Orai1 (upper panel), CFP-Orai2 (middle panel), and CFP-Orai3 (bottom panel) co-expressed with STIM1 C terminus YFP. Scale bars correspond to 5 μm.
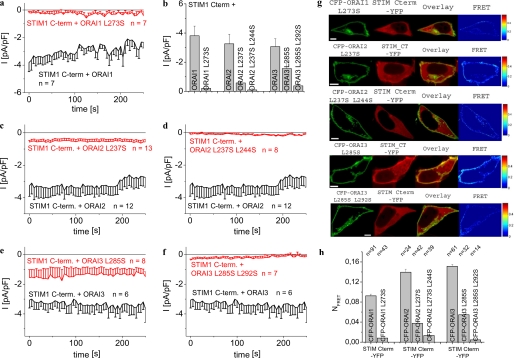
A Single Point Mutation in the C Terminus of Orai Proteins Abrogates Orai1, yet Allows for Moderate Activation of Orai2 as Well as Orai3 Channels
All three Orai proteins contain in their C termini a conserved, putative coiled-coil domain, a common motif involved in homomeric as well as heteromeric protein-protein interactions (36). Based on bioinformatic prediction methods (45), the probability of a putative coiled-coil in the C terminus of Orai2 (aa 222–254; 67.1%) and Orai3 (aa 266–295; 74.6%) is apparently ∼15-fold higher compared with Orai1 (aa 263–285; 4.2%). Orai2 as well as Orai3 proteins display 5 heptad repeats in contrast to Orai1 that only possesses 2–3 repeats. Two contiguous heptad repeats have been defined as the minimum number required for stable coiled-coil folding where particularly lipophilic interactions (e.g. via leucines) play a dominant role (36). We have previously demonstrated (33) that a single Leu to Ser mutation in the putative coiled-coil domain of Orai1 L273S reducing coiled-coil probability to 2.5% is sufficient to fully disrupt its communication with STIM1. Hence, in an analogous approach, we generated single Leu mutations within the respective coiled-coil domains in Orai2 and Orai3 to evaluate their impact on STIM1 C-terminally mediated current activation. Mutation of a hydrophobic Leu at position “a” or “d” of the central coiled-coil heptad (aa 237 in Orai2 and aa 285 in Orai3) to a hydrophilic Ser was expected to impair hydrophobic interactions (36) as the calculated coiled-coil probability was ∼3-fold reduced (Orai2 L237S, 19%; Orai3 L285S, 25.1%; see also Table 1). Although the STIM1 C terminus failed to generate constitutively activated Ca2+ currents when co-expressed with the single point mutant Orai1 L273S (Fig. 2, a and b), it retained some although clearly reduced activity to induce constitutive currents of the single point Orai2 L237S and Orai3 L285S mutants (Fig. 2, b, c, and e). Hence, reducing putative coiled-coil probability in Orai2 as well as Orai3 C terminus by this single Leu to Ser mutation was apparently not sufficient to fully inhibit coupling with and activation by the STIM1 C terminus. Introduction of a second Leu to Ser substitution in Orai2/Orai3 double mutants (Orai2 L237S/L2442S, 4.2%; Orai3 L285S/L292S, 8.9%) further reduced coiled-coil probability (Table 1), thereby abolishing the ability of the STIM1 C terminus to constitutively activate respective Ca2+ currents (Fig. 2, b, d, and f). These electrophysiological data are in good correlation with confocal FRET microscopy measurements that failed to detect a clear co-localization of the STIM1 C terminus and Orai2 as well as Orai3 double coiled-coil mutants in the plasma membrane region together with almost abolished FRET values compared with those from wild-type Orai proteins (Fig. 2, g and h). Consistent results were obtained with full-length STIM1 that following store depletion exhibited reduced ability to activate Ca2+ currents of Orai2 and Orai3 single coiled-coil mutants, but remained essentially ineffective when co-expressed with the double coiled-coil Orai2/3 mutants (supplemental Fig. 1). Thus, the observed decrease of the calculated probabilities from the Orai C-terminal putative coiled-coil domain with the reduction of Orai channel currents as well as interaction with the STIM1 C terminus led us to hypothesize a communication pathway with a corresponding coiled-coil domain within the STIM1 C terminus.
TABLE 1.
Predicted probabilities of putative coiled-coil domains within C termini of Orai1/2/3 and STIM1 as calculated by webmarcoil software (45)
| Protein | Amino acid stretch | Coiled-coil probability |
|---|---|---|
| % | ||
| Orai1 | 265–287 | 4.2 |
| Orai1 L273S | 273–287 | 2.5 |
| Orai1 Δ277–279 | 273–289 | 14.6 |
| Orai2 | 215–255 | 67.1 |
| Orai2 L237S | 216–255 | 19.0 |
| Orai2 L237S/L244S | 222–253 | 4.2 |
| Orai3 | 263–295 | 74.6 |
| Orai3 L285S | 263–295 | 25.1 |
| Orai3 L285S/L292S | 267–294 | 8.9 |
| STIM1, 1st coiled-coil | 233–340 | 100 |
| STIM1, 2nd coiled-coil | 352–394 | 62.4 |
| STIM1 L373S | 233–340 | 100 |
| 351–391 | 19.4 | |
| STIM1 L373S/A376S | 233–340 | 100 |
| 352–390 | 7.3 |
FIGURE 2.
Single point coiled-coil mutants of Orai2 and Orai3 contrary to Orai1 still allow for moderate coupling to as well as activation by STIM1 C terminus. Time course of constitutive whole-cell inward currents at −74 mV of HEK293 cells expressing STIM1 C terminus with the following: Orai1 L273S in comparison with Orai1 (a) and with Orai2 L237S (c), and Orai2 L237S/L244S in comparison with Orai2 (d) and with Orai3 L285S (e), and Orai3 L285S/L292S in comparison with Orai3 (f). b, average values of STIM1 C-terminally activated, constitutive current densities at t = 0 s from a and c–f. Results within each Orai subgroup were significantly different with p < 0.01 except p < 0.05 for Orai3 and Orai3 L285S. g, localization, overlay, and calculated FRET of STIM C terminus YFP co-expressed with the following: 1st panel, CFP-Orai1 L273S; 2nd panel, CFP-Orai2 L237S; 3rd panel, CFP-Orai2 L237S/L244S; 4th panel, CFP-Orai3 L285S; and 5th panel, CFP-Orai3 L285S/L292S. h, average FRET of STIM1 C terminus with Orai coiled-coil mutants. Results within each Orai subgroup were significantly different with p < 0.01. Scale bar corresponds to 5 μm.
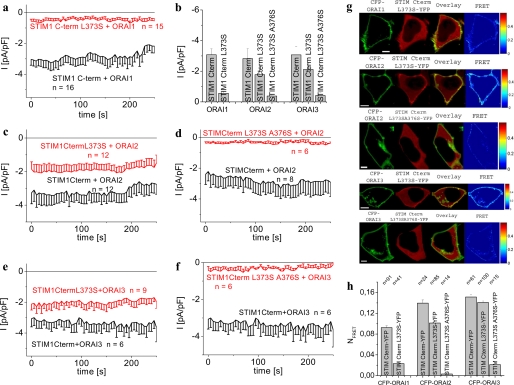
A Single Point Mutation within the Second Coiled-coil Domain of STIM1 C Terminus Allows for Differential Activation of Orai Channels
To evaluate if the two coiled-coil domains in the STIM1 C terminus are essential for the activation of Orai proteins, we similarly examined by bioinformatic analysis whether single point mutations of Leu at respective a and d positions might reduce coiled-coil probability in an attempt to interfere with hydrophobic interactions. Although single point mutations in the first coiled-coil domain (aa 233–340), which is predicted with 100% probability, emerged as less effective, the second coiled-coil domain (aa 352–394) appeared more susceptive in that the single point mutation L373S (predicted a position) resulted in a pronounced reduction of its probability from 62.4 to 19.4%. Moreover, this second coiled-coil domain is present within the essential key fragment CAD or SOAR that interacts with the Orai1 C terminus (20, 22). Indeed, the STIM1 C terminus L373S mutant failed to activate constitutive Orai1 currents (Fig. 3, a and b), yet still allowed for partial stimulation of constitutive Orai2 as well as Orai3 currents (Fig. 3, b, c, and e). These functional electrophysiological data were nicely correlated with a substantial co-localization of STIM1 C terminus L373S with Orai2 and Orai3 together with a clear FRET that was slightly reduced compared with wild-type STIM1 C terminus (Fig. 3, g, 2nd and 4th panels, and h). Furthermore, with the lack of co-localization a very low FRET was obtained for Orai1 and the STIM1 C terminus L373S mutant (Fig. 3, g, 1st panel, and h). Consistently, introduction of this L373S mutation in full-length STIM1 abrogated store-dependent activation of and co-clustering with Orai1 (supplemental Fig. 2, a and c), whereas Orai2 (data not shown) and Orai3 channels still exhibited partial stimulation as well as co-clustering (supplemental Fig. 2, d and f). Thus, the second coiled-coil domain in STIM1 and particularly Leu at position 373 represents a molecular determinant for the coupling to and activation of Orai channels. Moreover, it is tempting to speculate that the higher probability of coiled-coil domains in both Orai2 and Orai3 compared with Orai1 compensates for the decrease in the second coiled-coil domain of the STIM1 L373S mutant thereby still allowing for their partial coupling and activation.
FIGURE 3.
STIM1 C terminus L373S differentiates between Orai1 and Orai2/3 channels. Time course of constitutive whole-cell inward currents at −74 mV of HEK293 cells expressing STIM1 C terminus L373S with the following: a, Orai1; c, Orai2; e, Orai3 and STIM1 C terminus L373S/A376S with d, Orai2; f, Orai3 in comparison with STIM1. b, average values summarizing constitutive current densities at t = 0 s from a and c–f. Results within each Orai subgroup were significantly different with p < 0.01 except p < 0.05 for STIM1/Orai3 and STIM1 L373S/Orai3. g, localization, overlay, and calculated FRET of STIM1 C terminus L373S-YFP as follows: 1st panel, CFP-Orai1; 2nd panel, CFP-Orai2; 4th panel, CFP-Orai3 and STIM1 C terminus L373S/A376S-YFP with 3rd panel, CFP-Orai2; 5th panel, CFP-Orai3. h, average FRET of Orai1–3 with STIM1 C terminus coiled-coil mutants. Results within each Orai subgroup were significantly different with p < 0.01 except for comparison of STIM1 C terminus YFP/CFP-Orai3 with STIM1 C terminus L373S-YFP/CFP-Orai3 (p > 0.05). Scale bars correspond to 5 μm.
In an attempt to test our hypothesis of a correlation between second coiled-coil domain probability and efficiency of STIM1 C terminus to activate the Orai channels, we introduced an additional mutation A376S (predicted d position) that resulted in a further decrease of the probability down to 7.3%. This STIM1 C terminus L373S/A376S double mutant completely failed to activate Ca2+ currents when co-expressed with either of these Orai proteins (Fig. 3, b, d, and f). Consistently, neither Orai1, Orai2, nor Orai3 displayed co-localization with this STIM1 C terminus coiled-coil double mutant nor yielded a substantial FRET (Fig. 3, g, 3rd and 5th panels, and h). Hence, further disruption of the second coiled-coil domain by a double Leu/Ala mutation completely abrogates STIM1 C terminus coupling to and activation of all three Orai channels.
Because STIM1 C terminus L373S was still able to partially activate wild-type Orai2 as well as Orai3 channels, we evaluated whether single point mutations in their respective putative coiled-coil domain further affected activability. Indeed, the STIM1 C terminus L373S failed to activate any of the Orai single point coiled-coil mutants (supplemental Fig. 2) suggesting that a combination of single point coiled-coil mutants of both STIM1 and Orai2/Orai3 apparently generated a synergistic effect in that communication between these proteins was disrupted.
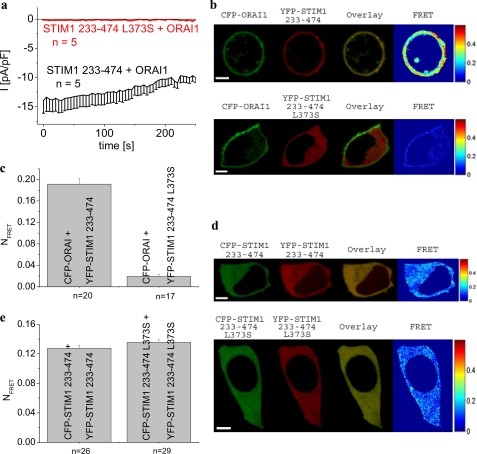
Leu-373 in the Second Coiled-coil Domain of OASF Represents a Molecular Determinant for Activation of Orai1 Channels
Recently it has been demonstrated that CAD, OASF, or SOAR, including the second coiled-coil domain, and 55 additional amino acids are key STIM1 fragments sufficient for Orai1 activation (20–22). In an attempt to strengthen our conclusion on L373S within the STIM1 C terminus as an essential molecular determinant, we investigated whether OASF (aa 233–474) containing the L373S mutation mimics the behavior of complete STIM1 C terminus. Co-expression of STIM1-OASF L373S with Orai1 failed to induce constitutive current in contrast to the strong activation observed in the presence of wild-type OASF (Fig. 4a). Accordingly, STIM1-OASF L373S lacked co-localization and FRET with Orai1 (Fig. 4, b and c).
FIGURE 4.
OASF L373S mutant exhibits disrupted coupling to as well as activation of Orai1, whereas its homomerization is preserved similar to wild-type OASF (aa 233–474). a, time course of constitutive whole-cell inward currents at −74 mV of HEK293 cells expressing Orai1 together with the OASF-L373S in comparison with currents mediated by OASF. Values at t = 0 s were significantly different within each STIM1 C terminus subgroup with p < 0.01. b, localization, overlay, and calculated FRET of CFP-Orai1 together with YFP-STIM1-(233–474) (upper panel) and YFP-STIM1-(233–474) L373S (lower panel); c, corresponding average FRET values (p < 0.01). d, localization, overlay, and calculated FRET of CFP-/YFP-STIM1-(233–474) (upper panel) in comparison with CFP-/YFP-STIM1-(233–474) L373S (lower panel); e, corresponding average FRET values (p > 0.05). Scale bars correspond to 5 μm.
This disrupted coupling to Orai1 might as well occur by an impairment of OASF homomerization as we have recently shown (21). However, STIM1-OASF L373S homomerization as judged by FRET was preserved to a comparable extent as with wild-type OASF (Fig. 4, d and e). It is noteworthy that clustering of the full-length STIM1 L373S mutant, which lacks coupling to Orai1 upon store depletion, occurred less efficiently in comparison with its co-expression with Orai3 where coupling was significantly preserved (supplemental Fig. 3, c and f). Hence, Leu-373 in the second coiled-coil domain of STIM1 represents a key determinant for coupling to Orai1 channels.
Increasing Coiled-coil Probability within the C Terminus of Orai1 Correlated with an Increase in Activation by STIM1 L373S
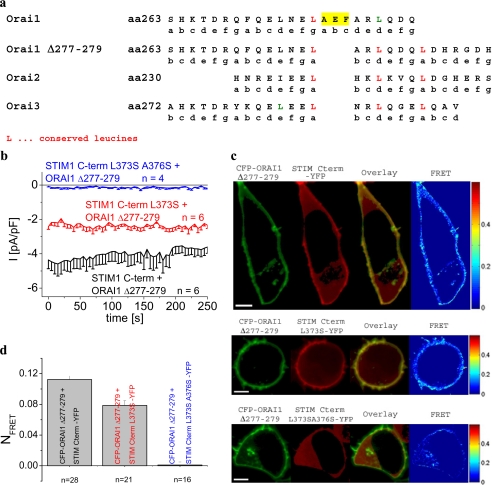
Comparison of the amino acid sequences of the putative coiled-coil domains of Orai1–3 (Fig. 5a) revealed an insert of three amino acids (277–279; AEF) in Orai1 that might be responsible for its strongly reduced coiled-coil probability by causing a shift in heptad repeats. Thus, deletion of amino acids 277–279 might therefore increase Orai1 coiled-coil probability as estimated to 14.6% (Table 1). This Orai1 Δ277–279 deletion mutant was constitutively activated by the STIM1 C terminus to a similar extent as wild-type Orai1. However, co-expression of the STIM1 C terminus L373S with this Orai1 deletion mutant (Fig. 5b) was now able to partially recover constitutive Ca2+ currents in contrast to our previous results on wild-type Orai1 (compare with Fig. 3a). Consistently, the STIM1 C terminus L373S/A376S mutant failed to activate currents from this Orai1 Δ277–279 deletion mutant. These functional electrophysiological data are in perfect correlation with the substantial co-localization of the STIM1 C terminus L373S with Orai1 Δ277–279 mutant along with a recovered FRET that was only slightly reduced as compared with wild-type STIM1 C terminus (Fig. 5, c and d). The STIM1 C terminus L373S/A376S correspondingly failed to co-localize with this deletion Orai1 mutant lacking a significant FRET.
FIGURE 5.
Orai1 Δ277–279 deletion mutant exhibits increased coiled-coil probability and recovered activation by STIM1 C terminus L373S. a, comparison of corresponding portions of the C-terminally located coiled-coil domains from Orai1–3. b, time course of constitutive whole-cell inward currents at −74 mV of HEK293 cells expressing Orai1 Δ277–279 deletion mutant together with STIM1 C terminus, STIM1 C terminus L373S, and STIM1 C terminus L373S/A376S. Values at t = 0 s were significantly different within each STIM1 C-terminal subgroup with p < 0.01. c, localization, overlay, and calculated FRET of CFP-Orai1 Δ277–279 deletion mutant together with STIM1 C terminus YFP, STIM1 C terminus L373S-YFP, and STIM1 C terminus L373S/A376S-YFP; d, corresponding average FRET values (p < 0.01). Scale bars correspond to 5 μm.
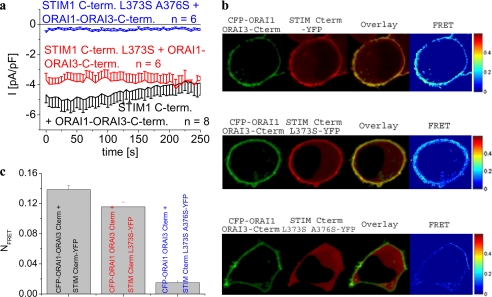
In a further approach to support the relation of the Orai C-terminal coiled-coil domain probability and Orai channel activation, we constructed an Orai1 chimera containing the Orai3 C terminus (Orai1-Orai3 C terminus) thereby enhancing coiled-coil probability to that of wild-type Orai3. The Orai1-Orai3 C terminus was constitutively stimulated by the STIM1 C terminus to a comparable extent as observed with wild-type Orai1 (Fig. 6a). Co-expression of this Orai1-Orai3 chimera with the STIM1 C terminus L373S resulted in nearly fully recovered constitutive Ca2+ currents, although STIM1 C terminus L373S/A376S failed to substantially activate the Orai1-Orai3 C terminus (Fig. 6a). Electrophysiological results were again perfectly in line with co-localization of the STIM1 C terminus L373S and Orai1-Orai3 C terminus chimera and their robust FRET that was only marginally reduced compared with the wild-type STIM1 C terminus (Fig. 6, b and c). As expected, STIM1 C terminus L373S/A376S failed to co-localize and lacked robust FRET with the Orai1 chimera (Fig. 6, b and c). In aggregate, increasing coiled-coil probability in the Orai1 C terminus by deleting amino acids 277–279 or substituting the whole C terminus by that of Orai3 restored both coupling to as well as activation of Orai1 by the STIM1 C terminus L373S mutant.
FIGURE 6.
Orai1-Orai3 C terminus chimera recovers activation by STIM1 C terminus L373S. a, time course of constitutive whole-cell inward currents at −74 mV of HEK293 cells expressing Orai1-Orai3 C terminus chimera together with STIM1 C terminus, STIM1 C terminus L373S, and STIM1 C terminus L373S/A376S. Values at t = 0 s were significantly different within each STIM1 C terminus subgroup with p < 0.01. b, localization, overlay, and calculated FRET of CFP-Orai1-Orai3 C terminus chimera together with STIM1 C terminus YFP, STIM1 C terminus L373S-YFP, and STIM1 C terminus L373S/A376S-YFP; c, corresponding average FRET values (p < 0.01). Scale bars correspond to 5 μm.
DISCUSSION
This study identifies molecular determinants within the second coiled-coil domain in STIM1 and the C-terminally located coiled-coil domain in all Orai proteins that are essential for their coupling and channel activity. Moreover, a single point mutation L373S within the STIM1 coiled-coil region allowed for differentiation between Orai1/2/3 channels resulting in selective coupling to as well as activation of Orai2 and Orai3 without affecting Orai1. Complete disruption of STIM1/Orai communication was observed either with single coiled-coil mutants of both STIM1 and Orai or with double coiled-coil mutants of either protein.
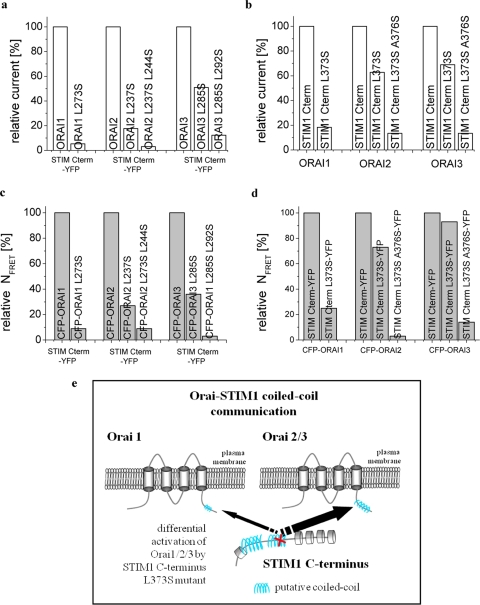
We have recently demonstrated (33) that the putative coiled-coil region in the Orai1 C terminus conserved among all Orai proteins is crucial in the process coupling STIM1 to Orai1. As the latter displays a very low coiled-coil probability, a single point mutation there is apparently sufficient to fully abrogate STIM1-mediated Orai1 activation in contrast to those of Orai2 as well as Orai3 (Fig. 7a). It appears that functional coupling of the STIM1 C terminus with Orai proteins generally decreased the more the coiled-coil domain probabilities declined (Fig. 7c). Thus probability of coiled-coil domain in the Orai C terminus somehow determined communication with STIM1. A certain limitation in this hypothesis is that Orai1 with a coiled-coil probability of about 4% was already sufficient to interact with the STIM1 C terminus, although Orai2 and Orai3 double mutants with predicted coiled-coil probabilities of 4 and 8%, respectively, already lacked coupling to STIM1. It could be, however, that coiled-coil probability in the Orai1 C terminus was underestimated, as common bioinformatics software only recognized heptad repeats and would not take into account “stutters” or “stammers” representing breaks in the periodicity of heptad repeats (35). Another explanation might be that STIM1-Orai1 coupling is supported by an additional interaction site within Orai1 that is missing in Orai2 or Orai3 proteins. Consistently, besides its strong interaction with the Orai1 C terminus, CAD has been reported (22) to also exhibit weaker binding to its N terminus. Hence, additional regions besides coiled-coil domains may contribute, and/or a third component somewhat stabilizing the STIM1-Orai1 coupling complex might be involved (46, 47).
FIGURE 7.
Overview depicting normalized current densities and corresponding FRET values of the various Orai and STIM1 C terminus constructs. Respective values were normalized to those obtained with the wild-type forms. Normalized current densities of STIM1 C terminus + Orai coiled-coil mutants (a) and STIM1 C-terminal coiled-coil mutants + Orai proteins (b) with corresponding normalized FRET values of STIM1 C terminus + Orai coiled-coil mutants (c) and STIM1 C terminus coiled-coil mutants + Orai proteins (d). e, model of Orai-STIM1 coiled-coil mediated coupling.
The coiled-coil domains in the STIM1 C terminus have been suggested as important domains affecting Orai1 activation (14, 33, 43, 48) and are included within the recently identified key fragments CAD or SOAR. Coiled-coil deletion mutants of STIM1 display pronounced tubular vesicular shape arrangement in comparison with wild-type STIM1, do not form puncta following store depletion, and fail to activate Orai1-derived Ca2+-influx (14). Our present study revealed that a single Leu to Ser mutation decreasing the probability of the second coiled-coil domain within STIM1 was sufficient for the abrogation of STIM1-Orai1 coupling. The higher probability of the coiled-coil domain in Orai2/3 C terminus apparently allowed for partial stimulation by this single point coiled-coil STIM1 mutant that was already ineffective for Orai1. Accordingly, increasing coiled-coil probability in the Orai1 Δ277–279 deletion mutant and Orai1-Orai3 C terminus chimera recovered its activability by this single coiled-coil STIM1 mutant to a similar extent as with Orai2/3. The double point coiled-coil mutant of STIM1 C terminus was unable to activate any Orai protein (Fig. 7, b and d).
As the single point mutant STIM1 C terminus L373S allowed for differential activation of Orai2 as well as Orai3 but not Orai1, it might represent a novel tool to specifically address these channels in native cells. However, because of the fact that Orai proteins are able to form homomeric as well as heteromeric assemblies, it still has to be investigated to what extent the specific action of the STIM1 C terminus L373S is conserved among potential heteromeric Orai channels.
As the communication between the STIM1 C terminus and Orai was increasingly impeded, the more respective coiled-coil domain probabilities either in the Orai proteins or in STIM1 were destabilized, it is tempting to speculate that a heteromeric coiled-coil interaction might be involved in this process. Although we cannot exclude that our single or double mutations within coiled-coil domains affect additional interactions within the Orai-STIM1 complexes that might hinder their coupling, a coiled-coil interaction would represent the simplest model for STIM1-Orai activation coupling. This is also compatible with a recent report (22) demonstrating direct and strong binding of CAD to the Orai1 C terminus. Indeed, the ability of a small STIM1 C-terminal fragment (OASF) to constitutively activate and couple Orai1 was disrupted by the single point mutation L373S. As recently reported, electrostatic interactions (49) based on three glutamates (aa 272, 275, and 278) and three aspartates (aa 284, 287, and 291) in the Orai1 C terminus might additionally play a role in the coupling to STIM1 C terminus. It is known that electrostatic effects can mediate oligomerization states and can also define heterospecificity of coiled-coiled interactions (36, 50). An interaction of these negative amino acids in Orai1 with the polybasic cluster at the very end of STIM1, as also suggested in Ref. 49, appears, however, less likely, as its presence is required to gate TRPC1 but not Orai1 channels (22, 51). An involvement of heteromeric coiled-coil interactions has been reported for the vesicle fusion process, where coiled-coil domains of SNARE proteins in the vesicle and the target membrane entwine to draw the two membranes together (19). Homomeric as well as heteromeric coiled-coil interactions have also been presented for various other ion channels (26, 34) mediating channel formation. Further studies are needed to investigate the function of the first coiled-coil domain in STIM1 that cannot be easily disrupted by point mutations and because of the fact that its deletion in the STIM1 C terminus substantially alters its cellular distribution (data not shown).
In conclusion, we report on molecular determinants within putative coiled-coil domains that mediate STIM1-Orai coupling via their C termini. The single STIM1 C terminus L373S mutant with a partially decreased probability of its second coiled-coil domain provides a novel tool (Fig. 7e) that is able to distinguish between Orai1 and Orai2/3 probably based on their distinct predicted coiled-coil probabilities. The observed impairment in overall communication between STIM1 and Orai channels upon destabilization of either one of the putative coiled-coil interaction domains in the C termini of these proteins is compatible with their functional, heteromeric interaction as a pivotal element of the STIM/Orai coupling process providing the flexibility of a dynamic assembly and disassembly upon store depletion. Future crystallographic studies are certainly required to demonstrate coiled-coil mediated STIM1-Orai interaction.
Supplementary Material
Acknowledgments
We thank S. Buchegger and B. Kenda for excellent technical assistance.
This work was supported by the Austrian Science Foundation Project P19820 (to K. G.) and Projects P18169, P21118, and Subproject 11 of the Ph.D. Programme “Molecular Bioanalytics” (to C. R.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- CRAC
- Ca2+ release-activated Ca2+
- FRET
- Förster resonance energy transfer
- YFP
- yellow fluorescent protein
- CFP
- cyan fluorescent protein
- aa
- amino acid
- ER
- endoplasmic reticulum
- CAD
- CRAC-activating domain
- OASF
- Orai-activating small fragment
- HEK
- human embryonic kidney.
REFERENCES
- 1.Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 2.Hoth M., Penner R. (1992) Nature 355, 353–356 [DOI] [PubMed] [Google Scholar]
- 3.Zweifach A., Lewis R. S. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 6295–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis R. S., Cahalan M. D. (1990) Annu. Rev. Physiol. 52, 415–430 [DOI] [PubMed] [Google Scholar]
- 5.Parekh A. B., Putney J. W., Jr. (2005) Physiol. Rev. 85, 757–810 [DOI] [PubMed] [Google Scholar]
- 6.Prakriya M., Lewis R. S. (2003) Cell Calcium 33, 311–321 [DOI] [PubMed] [Google Scholar]
- 7.Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Veliçelebi G., Stauderman K. A. (2005) J. Cell Biol. 169, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. (2005) Curr. Biol. 15, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006) Nature 441, 179–185 [DOI] [PubMed] [Google Scholar]
- 10.Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., Kinet J. P. (2006) Science 312, 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S. L., Yeromin A. V., Zhang X. H., Yu Y., Safrina O., Penna A., Roos J., Stauderman K. A., Cahalan M. D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9357–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luik R. M., Wu M. M., Buchanan J., Lewis R. S. (2006) J. Cell Biol. 174, 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M. M., Buchanan J., Luik R. M., Lewis R. S. (2006) J. Cell Biol. 174, 803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baba Y., Hayashi K., Fujii Y., Mizushima A., Watarai H., Wakamori M., Numaga T., Mori Y., Iino M., Hikida M., Kurosaki T. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16704–16709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stathopulos P. B., Li G. Y., Plevin M. J., Ames J. B., Ikura M. (2006) J. Biol. Chem. 281, 35855–35862 [DOI] [PubMed] [Google Scholar]
- 16.Stathopulos P. B., Zheng L., Li G. Y., Plevin M. J., Ikura M. (2008) Cell 135, 110–122 [DOI] [PubMed] [Google Scholar]
- 17.Dziadek M. A., Johnstone L. S. (2007) Cell Calcium 42, 123–132 [DOI] [PubMed] [Google Scholar]
- 18.Huang G. N., Zeng W., Kim J. Y., Yuan J. P., Han L., Muallem S., Worley P. F. (2006) Nat. Cell Biol. 8, 1003–1010 [DOI] [PubMed] [Google Scholar]
- 19.Taylor C. W. (2006) Trends Biochem. Sci. 31, 597–601 [DOI] [PubMed] [Google Scholar]
- 20.Yuan J. P., Zeng W., Dorwart M. R., Choi Y. J., Worley P. F., Muallem S. (2009) Nat. Cell Biol. 11, 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muik M., Fahrner M., Derler I., Schindl R., Bergsmann J., Frischauf I., Groschner K., Romanin C. (2009) J. Biol. Chem. 284, 8421–8426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park C. Y., Hoover P. J., Mullins F. M., Bachhawat P., Covington E. D., Raunser S., Walz T., Garcia K. C., Dolmetsch R. E., Lewis R. S. (2009) Cell 136, 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frischauf I., Schindl R., Derler I., Bergsmann J., Fahrner M., Romanin C. (2008) Channels 2, 261–268 [DOI] [PubMed] [Google Scholar]
- 24.Cahalan M. D., Zhang S. L., Yeromin A. V., Ohlsen K., Roos J., Stauderman K. A. (2007) Cell Calcium 42, 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi Y., Murakami M., Watanabe H., Hasegawa H., Ohba T., Munehisa Y., Nobori K., Ono K., Iijima T., Ito H. (2007) Biochem. Biophys. Res. Commun. 356, 45–52 [DOI] [PubMed] [Google Scholar]
- 26.Schindl R., Romanin C. (2007) Biochem. Soc. Trans. 35, 84–85 [DOI] [PubMed] [Google Scholar]
- 27.Mercer J. C., Dehaven W. I., Smyth J. T., Wedel B., Boyles R. R., Bird G. S., Putney J. W., Jr. (2006) J. Biol. Chem. 281, 24979–24990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindl R., Bergsmann J., Frischauf I., Derler I., Fahrner M., Muik M., Fritsch R., Groschner K., Romanin C. (2008) J. Biol. Chem. 283, 20261–20267 [DOI] [PubMed] [Google Scholar]
- 29.Lis A., Peinelt C., Beck A., Parvez S., Monteilh-Zoller M., Fleig A., Penner R. (2007) Curr. Biol. 17, 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peinelt C., Lis A., Beck A., Fleig A., Penner R. (2008) J. Physiol. 586, 3061–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeHaven W. I., Smyth J. T., Boyles R. R., Bird G. S., Putney J. W., Jr. (2008) J. Biol. Chem. 283, 19265–19273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S. L., Kozak J. A., Jiang W., Yeromin A. V., Chen J., Yu Y., Penna A., Shen W., Chi V., Cahalan M. D. (2008) J. Biol. Chem. 283, 17662–17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muik M., Frischauf I., Derler I., Fahrner M., Bergsmann J., Eder P., Schindl R., Hesch C., Polzinger B., Fritsch R., Kahr H., Madl J., Gruber H., Groschner K., Romanin C. (2008) J. Biol. Chem. 283, 8014–8022 [DOI] [PubMed] [Google Scholar]
- 34.Howard R. J., Clark K. A., Holton J. M., Minor D. L., Jr. (2007) Neuron 53, 663–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason J. M., Arndt K. M. (2004) ChemBioChem. 5, 170–176 [DOI] [PubMed] [Google Scholar]
- 36.Woolfson D. N. (2005) Adv. Protein Chem. 70, 79–112 [DOI] [PubMed] [Google Scholar]
- 37.Strauss H. M., Keller S. (2008) Handb. Exp. Pharmacol. 186, 461–482 [DOI] [PubMed] [Google Scholar]
- 38.Singh A., Hamedinger D., Hoda J. C., Gebhart M., Koschak A., Romanin C., Striessnig J. (2006) Nat. Neurosci. 9, 1108–1116 [DOI] [PubMed] [Google Scholar]
- 39.Derler I., Hofbauer M., Kahr H., Fritsch R., Muik M., Kepplinger K., Hack M. E., Moritz S., Schindl R., Groschner K., Romanin C. (2006) J. Physiol. 577, 31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Z., Liu Y. (2001) Biophys. J. 81, 2395–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berney C., Danuser G. (2003) Biophys. J. 84, 3992–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji W., Xu P., Li Z., Lu J., Liu L., Zhan Y., Chen Y., Hille B., Xu T., Chen L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13668–13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penna A., Demuro A., Yeromin A. V., Zhang S. L., Safrina O., Parker I., Cahalan M. D. (2008) Nature 456, 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeHaven W. I., Smyth J. T., Boyles R. R., Putney J. W., Jr. (2007) J. Biol. Chem. 282, 17548–17556 [DOI] [PubMed] [Google Scholar]
- 45.Delorenzi M., Speed T. (2002) Bioinformatics 18, 617–625 [DOI] [PubMed] [Google Scholar]
- 46.Várnai P., Tóth B., Tóth D. J., Hunyady L., Balla T. (2007) J. Biol. Chem. 282, 29678–29690 [DOI] [PubMed] [Google Scholar]
- 47.Csutora P., Peter K., Kilic H., Park K. M., Zarayskiy V., Gwozdz T., Bolotina V. M. (2008) J. Biol. Chem. 283, 14524–14531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spassova M. A., Hewavitharana T., Fandino R. A., Kaya A., Tanaka J., Gill D. L. (2008) J. Biol. Chem. 283, 14938–14945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calloway N., Vig M., Kinet J. P., Holowka D., Baird B. (2009) Mol. Biol. Cell 20, 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fairman R., Chao H. G., Lavoie T. B., Villafranca J. J., Matsueda G. R., Novotny J. (1996) Biochemistry 35, 2824–2829 [DOI] [PubMed] [Google Scholar]
- 51.Zeng W., Yuan J. P., Kim M. S., Choi Y. J., Huang G. N., Worley P. F., Muallem S. (2008) Mol. Cell 32, 439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.