Abstract
SoxB is an essential component of the bacterial Sox sulfur oxidation pathway. SoxB contains a di-manganese(II) site and is proposed to catalyze the release of sulfate from a protein-bound cysteine S-thiosulfonate. A direct assay for SoxB activity is described. The structure of recombinant Thermus thermophilus SoxB was determined by x-ray crystallography to a resolution of 1.5 Å. Structures were also determined for SoxB in complex with the substrate analogue thiosulfate and in complex with the product sulfate. A mechanistic model for SoxB is proposed based on these structures.
The oxidation of reduced inorganic sulfur species by sulfur bacteria is an important component of the biogeochemical sulfur cycle and has practical applications in biomining, agriculture, biocorrosion, fuel desulfuration, and waste treatment (1, 2). Sulfur bacteria use the electrons liberated in sulfur oxidation reactions as the reductant for carbon dioxide fixation and/or as donors to respiratory electron transport chains.
The Sox (sulfur oxidizing) system is one of the most widely distributed sulfur oxidation pathways and is found in both photosynthetic and nonphotosynthetic sulfur-oxidizing eubacteria (3). Substrates of the Sox system are reported to include thiosulfate, sulfide, elemental sulfur, sulfite, and tetrathionate (4–6). The Sox pathway has been best characterized in the α-Proteobacterium Paracoccus pantotrophus. In this bacterium thiosulfate is oxidized to sulfate by the four periplasmic protein complexes SoxYZ, SoxAX, SoxB, and SoxCD (3, 7, 8). Intermediates in the pathway are covalently bound to a cysteine residue located in a conserved Gly-Gly-Cys-Gly-Gly sequence at the C terminus of the SoxY protein (9). This C-terminal peptide acts as a swinging arm enabling the cysteine and its bound adducts to enter the active sites of the other pathway components (10). In the current pathway model the heme protein SoxAX (11) oxidatively conjugates thiosulfate to the SoxY swinging arm to form a cysteine S-thiosulfonate, which is then degraded by a combination of SoxB and SoxCD.
The electrons produced in the two oxidative steps are fed into the electron transfer chain via a small c-type cytochrome. Many bacteria with a Sox system lack the SoxCD complex found in P. pantotrophus and are instead thought to feed the sulfane group of thiosulfate into other sulfur oxidation pathways (12–14).
The reaction assigned to SoxB in the Sox pathway model is the hydrolysis of a sulfur-sulfur bond. This is an unusual enzymatic reaction that has only otherwise been suggested for enzymes designated as trithionate or tetrathionate hydrolases (15–18). The thiosulfohydrolase activity proposed for SoxB has yet to be directly demonstrated. It is, instead, inferred from two key observations. First, in vitro pathway reconstitution experiments show that SoxB catalyzes a nonoxidative reaction (7). Second, SoxB has sequence similarity to the 5′-nucleotidase family of enzymes (19). Because 5′-nucleotidases catalyze the hydrolytic cleavage of phosphate groups from nucleotides, this sequence similarity suggests that SoxB also carries out a hydrolytic reaction.
Catalytically active SoxB purified from P. pantotrophus or the closely related bacterium Paracoccus versutus contains up to two atoms of manganese but only traces of other metal ions (20, 21). EPR studies suggest that the manganese ions are present in the form of a dinuclear Mn(II) cluster with bis(μ-hydroxo) (μ-carboxylato) bridging ligands (20, 22).
In phylogenetic and environmental studies the presence of a soxB gene has been used as a marker for the presence of the Sox pathway and as an indicator of the ability of the organism to oxidize thiosulfate (23, 24).
Here we report experiments aimed at establishing a direct assay of SoxB activity. We have used x-ray crystallography to determine the structure of recombinant SoxB from the thermophilic bacterium Thermus thermophilus. This is the first structure of an enzyme catalyzing the hydrolysis of a sulfur-sulfur bond. We have also obtained structures of T. thermophilus SoxB in complex with mechanistically relevant ligands. Based on these structures, we propose a model for the SoxB mechanism.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification of T. thermophilus SoxB
T. thermophilus HB27 SoxB was expressed in the cytoplasm of Escherichia coli with a Strep II tag replacing the N-terminal Tat signal peptide. The soxB gene was amplified from genomic DNA using the forward primer 5′-GCTAGCTGGAGCCACCCGCAGTTCGAAAAAGGCGCCCTGGAGGACCCCAGGTCC-3′ and the reverse primer 5′-ACGCCTGGTACCTCATC CCGTCACCTCCGG-3′. The amplicon was used as the target in a second round of PCR using the same reverse primer and 5′-CAGGACGAATTCATTAAAGAGGAGAAATTAACTATGGCTAGCTGGAGCCACCCG-3′ as the forward primer. This second amplicon was digested with EcoRI and KpnI and ligated into the same sites in pQE80L (Qiagen) to produce the expression plasmid pVS048.
E. coli strain Rosetta 2 (DE3) (Novagen) containing pVS048 was cultured aerobically at 30 °C in 2 liters of LB medium (25) that had been supplemented with 1 mm MnCl2, 100 μg ml−1 ampicillin, and 25 μg ml−1 chloramphenicol. When the culture reached an A600 nm of 0.6, soxB expression was induced with 100 μm isopropyl β-d-thiogalactopyranoside, and growth was continued for a further 17 h. The bacteria were harvested by centrifugation and resuspended in 100 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm MnCl2, with EDTA-free Complete protease inhibitors (Roche Applied Science) and a few crystals each of lysozyme and DNase I (both Sigma-Aldrich). The resuspended cells were broken by three passages through a French Press at 8000 p.s.i. The lysate was centrifuged at 155,000 × g for 30 min at 4 °C. The supernatant was collected and heat-treated at 65 °C for 20 min. Denatured material was removed by centrifugation at 10,000 × g at 4 °C for 15 min, and the supernatant was loaded onto a 2.5-ml Strep-Tactin Superflow column (IBA) previously equilibrated with 100 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm MnCl2. The column was washed with 60 ml of the equilibration buffer. SoxB was then eluted with 10 ml of 100 mm Tris-HCl, pH 8, 150 mm NaCl, 1 mm MnCl2, 2.5 mm dethiobiotin. SoxB fractions were concentrated and further purified on a Superdex 200 10/300 size exclusion column (GE Healthcare) that had been equilibrated in 30 mm Tris-HCl, pH 8.0, 150 mm NaCl.
Cloning, Expression, and Purification of the T. thermophilus SoxYZ Complex and Derivatives
The T. thermophilus HB27 SoxYZ complex was expressed in the cytoplasm of E. coli with a hexahistidine tag replacing the N-terminal signal peptide of SoxY. The soxY gene was amplified from genomic DNA using the primers 5′-CCTGCGGGATCCCAGGGCCTCGAGGGCGAG-3′ and 5′-GCCGCAGGTACCTTAACCGCAGCCCCCCACG-3′. The resulting amplicon was digested with BamHI and KpnI and cloned into the same sites in pQE80L to produce plasmid pVS054. The soxZ gene was amplified from genomic DNA using the primers 5′-ACCGTGGGTACCATTAAAGAGGAGAAATTAACTATGCCTTTTAGGACCATCGCG-3′ and 5′-CGGGCAAAGCTTTTAGGCCAGCTCCAGCTTGA-3′. The resulting amplicon was digested with KpnI and HindIII and cloned into the same sites in pVSO54 to produce plasmid pVS056.
E. coli strain Rosetta 2 (DE3) containing pVS056 was cultured, and a heat-treated soluble extract was prepared using the same protocol that was employed in the purification of recombinant SoxB except that the growth medium was not supplemented with MnCl2. The heat-treated extract was supplemented with 1 mm DTT4 and loaded onto a 5-ml His-Trap column (GE Healthcare) previously equilibrated in 50 mm Tris-HCl, pH 8.0, 0.5 m NaCl, 10 mm imidazole, 2 mm DTT. The column was washed with equilibration buffer containing 25 mm imidazole and then developed with a linear 25–210 mm imidazole gradient. SoxYZ fractions were concentrated by ultrafiltration and further purified on a Superdex 75 10/300 size exclusion column (GE Healthcare) in 30 mm Tris-HCl, pH 8.0, 0.2 m NaCl, 2 mm DTT.
N-terminal sequencing and mass spectrometry of the purified SoxYZ complex showed that the N-terminal methionine residue of the recombinant SoxZ protein had been removed. The N-terminal amino acid of SoxY released by the Edman reaction ran at the same chromatographic position as phenylalanine. In addition, the recombinant SoxY protein had a mass 14 Da greater than the expected mass value of 14,668 Da. These observations are consistent with the N-terminal methionine of SoxY being methylated, as has been reported previously for proteins that have a positively charged residue as the second amino acid (this residue is arginine in T. thermophilus SoxY) (26).
To produce DTT-free reduced SoxYZ, the purified protein was supplemented with a further 5 mm DTT to ensure complete reduction of the active site cysteine. Excess DTT was then removed by gel filtration on a Superdex 75 10/300 column in 10 mm Tris-HCl, pH 8.0.
To produce the S-thiosulfonate derivative of SoxYZ, the purified protein was dialyzed against 10 mm Tris-HCl, pH 8.0, 50 mm potassium tetrathionate for 16 h at 22 °C. Excess tetrathionate together with the thiosulfate reaction product were removed from the protein by gel filtration as described above.
Electrospray Ionization Mass Spectrometry of SoxB Reactions
The samples were desalted by a 30-min dialysis against 0.1% formic acid using a 0.025-μm pore floating membrane (Millipore). After dialysis the samples were diluted with acetonitrile to a SoxYZ concentration of 10 μm. Electrospray mass spectrometry data were obtained using a QTof1 (Micromass) operated using MassLynx v4.0. The samples were sprayed from borosilicate emitters (Proxeon Biosystems) using a capillary voltage tuned between 1.5 and 2.1 kV to obtain a stable spray. Cone voltage was maintained at 40 V. Raw spectra were combined and processed using MaxEnt1 (Micromass).
Crystallization of SoxB and SoxB-Ligand Complexes
Crystals were grown at 20 °C by the sitting drop vapor diffusion method by mixing 1 μl of SoxB at 6.5 mg/ml in 10 mm Tris-HCl, pH 8.0, 0.2 m NaCl, and 1 μl of 24–26% (v/v) tert-butanol, and 0.1 m Tris acetate, pH 8.5. To ensure that the two metal sites were well occupied with manganese, 2 mm MnCl2 was added to the mother liquor for some structures. For crystallization in the presence of sulfur compounds, 2 mm disodium thiosulfate or diammonium sulfate was added directly to the protein prior to setting up the drops.
X-ray Data Collection, Structure Solution, and Refinement
The crystal used for the phasing was cryoprotected in the crystallization solution by the addition of 25% (v/v) glycerol to the mother liquor. The structure derived from this crystal showed glycerol molecules occupying the active site. To avoid interference of cryoprotectant with the active site, 25% (v/v) PEG 400 replaced glycerol for the cryoprotection of all other crystals analyzed. 100 mm disodium thiosulfate or diammonium sulfate were added to the cryosolutions for crystals grown in the presence of these chemicals.
All of the data sets were collected at 100 K either in-house using a Bruker MicroStar microfocus x-ray generator with Montel optics and a Bruker platinum 135 CCD detector or at the European Synchrotron Radiation Facility (ID14-1, Grenoble, France) (Table 1).
TABLE 1.
Data collection, phasing, and refinement statistics for T. thermophilus SoxB
| SoxB-glycerol | SoxB-Mn2+ | SoxB-Mn2+-thiosulfate | SoxB-Mn2+-sulfate | |
|---|---|---|---|---|
| Additive(s) | Mn2+ | Sodium thiosulfate and Mn2+ | Ammonium sulfate and Mn2+ | |
| Cryoprotectant agent | Glycerol | PEG 400 | PEG 400 | PEG s400 |
| X-ray source (wavelength) | In-house (1.54 Å) | In-house (1.54 Å) | In-house (1.54 Å) | ESRF ID14-1 (0.93 Å) |
| Space group | P212121 | P212121 | P212121 | P212121 |
| Cell dimensions (Å) | a = 71.3, b = 86.5, c = 96.0 | a = 70.4, b = 86.7, c = 95.1 | a = 70.8, b = 86.7, c = 95.4 | a = 70.8, b = 86.6, c = 96.1 |
| Resolution (Å) | 57.3-1.5 (1.54-1.50) | 64.1-2.1 (2.16-2.10) | 64.2-1.85 (1.89-1.85) | 39.5-1.50 (1.54-1.50) |
| Number of unique reflections | 90,719 | 32,817 | 48,244 | 86,525 |
| Redundancy | 9.8 (1.2) | 13.0 (10.2) | 7.6 (3.0) | 6.0 (3.0) |
| Completeness (%) | 100 (100) | 99.9 (99.4) | 100 (100) | 95.8 (87.1) |
| Rmerge (%) | 4.4 (35.3) | 8.4 (39.5) | 6.0 (37.9) | 5.8 (23.0) |
| Average I/σI | 26 (1.6) | 25.6 (6.4) | 21.5 (2.9) | 8.7 (3.3) |
| Refinement statistics | ||||
| R (%) | 17.1 (22.7) | 18.6 (21.9) | 20.0 (27.0) | 18.2 (23.2) |
| Rfree (%) | 19.1 (26.1) | 23.1 (26.8) | 23..4 (29.0) | 20.7 (28.3) |
| Root mean square deviation from idealized covalent geometry | ||||
| Bonds (Å) | 0.008 | 0.010 | 0.009 | 0.009 |
| Angles (Å) | 1.21 | 1.23 | 1.13 | 1.23 |
| Protein residues in model | 32–573 | 30–573 | 29–573 | 31–573 |
| Number of atoms in model | 5079 | 4778 | 4841 | 5222 |
| Number of protein atoms | 4437 | 4440 | 4375 | 4599 |
| Number of nonprotein atoms | 2 Mn2+ (1 at 100% and 1 at 75% occupancy), 564 H2O, 30 glycerol (5), 30 tert-butanol (6), 16 acetate (4) | 2 Mn2+, 336 H2O | 2 Mn2+, 439 H2O, 20 tert-butanol (4),5 S2O32− (1) | 2 Mn2+, 592 H2O, 10 sulfate (2 molecules at 25% occupancy each), 15 tert-butanol (3), 4 acetate (1) |
| Mean <B> protein (Å2) | 13.9 | 20.7 | 18.2 | 14.8 |
| Mean <B> nonprotein (Å2) | 30.9 | 28.7 | 28.9 | 29.9 |
| Residues in most favorite Ramachandran regions (%) | 98.0 | 97.6 | 96.5 | 97.41 |
| Ramachandran outliers (%) | 0.0 | 0.0 | 0.92 | 0.0 |
The images were processed using SAINT (27) for the in-house data and MOSFLM (28) for the synchrotron data. The in-house data were processed with XPREP (27) to obtain a set of structure factors with anomalous scattering, which in turn were used in the program ShelxD (29) to find the two manganese sites (f″ = 2.8 e− at λ = 1.54 Å) and five sulfur atoms. DANO/SIGMA values from SHELXD ranged from 4 to 1.5 in the resolution range 50–2.5 Å. XPREP was then rerun keeping Friedel mates intensities separate, and these intensities were processed through CCP4-combat and then CCP4-truncate (30) to produce a file of structure factor amplitudes for the phasing program SHARP (31). The heavy atom model eventually comprised two manganese and six sulfur sites. Starting from the SHARP phase distribution solvent flattening in the program Solomon using a 47.5% solvent content produced a 1.8-Å map that enabled autobuilding in the program ArpWarp (32). Residues before Tyr32 were not seen. All other minor gaps in the autobuilt model were easily built manually (11 residues in total). An autoBUSTER refinement5 was performed on the model for SoxB residues 32–573 with the two active site manganese atoms assigned a full occupancy. After 10 autoBUSTER cycles with water addition, the R factor was 23.5% and the Rfree was 25.3%. The structure was further refined with Refmac5 (33), and building was completed with Coot (34). Further rounds of refinement and model building used Refmac5 (33) and Coot (34) or X-fit (35). Pocket parameters were calculated with CASTp (36). The coordinate root mean square deviation values were calculated with Swiss-Pdb viewer (37) and Dali-Lite (38). Sequence conservation was mapped onto SoxB using ConSurf (39, 40).
All of the atomic coordinates and experimental structure factors of the structures described in this paper have been deposited in the Protein Data Bank with the accession codes 2WDC for SoxB-glycerol, 2WDF for SoxB-Mn2+, 2WDE for SoxB-Mn2+-thiosulfate, and 2WDD for SoxB-Mn2+-sulfate.
MicroPIXE Analysis of the Elemental Composition of SoxB Crystals
MicroPIXE measurements were carried out at the Ion Beam Centre (University of Surrey) on a beamline arranged as described by Grime et al. (41). Two SoxB crystals were dried onto 2-μm-thick mylar films in aluminum target holders (42, 43). A 2.5-MeV proton beam 3 μm in diameter was used to induce characteristic x-ray emission from the dried crystal under vacuum. The X-rays were detected in a solid state lithium drifted silicon detector with high energy resolution. The proton beam was then scanned spatially in the x and y dimensions. Spatial maps were obtained of all elements heavier than neon that were present in the sample. Quantitative information was obtained by collecting spectra at selected points on the crystals and also at a point on the backing film. These spectra were analyzed using GUPIX (44) to determine the amount of each element of interest in the sample relative to the sulfur signal from the six methionine residues found in the recombinant T. thermophilus SoxB protein.
RESULTS
Analysis of SoxB Activity
The SoxB protein from the thermophilic bacterium T. thermophilus HB27 was expressed in E. coli. The identity of the recombinant protein was confirmed by N-terminal sequencing and mass spectrometry. The protein was a monomer in solution as judged by size exclusion chromatography (supplemental Fig. S1).
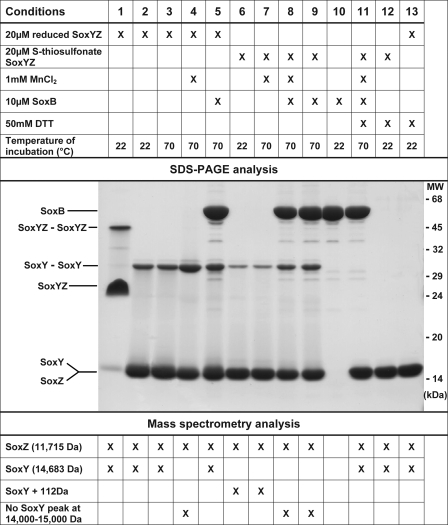
To establish that the recombinant T. thermophilus SoxB was enzymatically active and as a means to substantiate the proposed SoxB reaction, we attempted to assay SoxB activity directly. Recombinant T. thermophilus SoxYZ was produced in E. coli, and the active site cysteine residue of the SoxY subunit was chemically modified to the S-thiosulfonate (-S-SO3−) derivative. The authenticity of this modification was confirmed by mass spectrometry showing that the derivatized SoxY protein had a molecular mass 113 Da greater than that of the native protein (the mass of thiosulfate is 112 Da) (Fig. 1, compare samples 2 and 6). The additional mass could be removed by DTT treatment. This indicated that the S-thiosulfonate group had formed a disulfide linkage with the protein and must therefore be conjugated through the sulfane sulfur to the unique active site cysteine residue in SoxY (Fig. 1, compare samples 6 and 12).
FIGURE 1.
Assay of SoxB activity. The components present in each reaction are shown in the top panel. The assay buffer was 10 mm Hepes-NaOH, pH 7.5. The reactions were incubated for 3 h at the indicated temperature. Where DTT is specified, it was added to the sample at the end of the incubation. Reduced SoxYZ has a free active site cysteine. In the S-thiosulfonate adduct the cysteine is covalently linked to thiosulfate. An analysis of the reaction products by SDS-PAGE is shown in the middle panel. Reactions 2–13 were placed at 100 °C for 2 min prior to loading, whereas reaction 1 was kept at 22 °C. The identities of the observed species are indicated to the left of the gel. A summary of the electrospray ionization mass spectrometry results for the same samples is shown in the bottom panel. The original mass data are shown in supplemental Fig. S2. Complementary analysis of the reaction products by size exclusion chromatography is given in supplemental Fig. S1.
The S-thiosulfonate SoxYZ protein was mixed with SoxB and incubated for 3 h at 70 °C, which is the optimum growth temperature of T. thermophilus. The reaction products were then analyzed by mass spectrometry. Following incubation, the mass peak corresponding to the S-thiosulfonate SoxY derivate had disappeared (Fig. 1, compare samples 6 and 9), whereas the SoxZ mass peak was unchanged from the start of the reaction. Identical results were obtained for a duplicate reaction that had been supplemented with Mn2+ to guard against the possibility that recombinant SoxB had not been fully loaded with metal cofactors (Fig. 1, compare samples 8 and 9). If SoxB was omitted from the reaction, no alteration of the S-thiosulfonate SoxY peak was seen even if Mn2+ was present (Fig. 1, sample 7). Taken together these observations showed that SoxB had specifically converted the SoxY derivative into a new species. The nature of the resultant species was not, however, immediately apparent because no new mass peaks were detected. Insight into the nature of the product species was instead obtained from SDS-PAGE analysis.
T. thermophilus SoxYZ is sufficiently thermostable that the two subunits do not dissociate in 2% (w/v) SDS at room temperature (Fig. 1, sample 1). However, if the temperature is increased to 100 °C, SoxY and SoxZ appear to become separated and migrate with identical mobility under SDS-PAGE (Fig. 1, compare samples 1 and 2). This interpretation of the electrophoretic behavior of T. thermophilus SoxYZ was confirmed by expressing and analyzing each subunit individually (data not shown).
If the cysteine residue of SoxY is initially in the reduced state, it has a strong tendency to form an intermolecular disulfide bridge with the cysteine residue of another SoxY protein molecule. This disulfide-linked dimer is evident as a species of 31 kDa on SDS-PAGE, which disappears in the presence of DTT (Fig. 1, compare samples 2 and 13). Formation of this disulfide-linked SoxY dimer is enhanced by incubation at 70 °C in the presence of Mn2+ (Fig. 1, compare samples 3 and 4). Divalent metal ions are known to act as a catalyst for disulfide bond shuffling (45, 46).
The S-thiosulfonate-SoxYZ preparation contained very little disulfide-linked SoxY dimer, and the concentration of the dimer was not altered by incubation with Mn2+ at 70 °C (Fig. 1, compare samples 6, 7, and 12). However, incubation with SoxB at 70 °C led to a significant increase in SoxY dimers with or without supplementary Mn2+ (Fig. 1, compare sample 7 with samples 8 and 9). The SoxB-dependent formation of SoxYZ complex dimers was confirmed by size exclusion chromatography (supplemental Fig. S1). The addition of DTT to the SoxB-containing reaction led to the disappearance of the SoxY dimer band on SDS-PAGE and of the SoxYZ complex dimer peak on the size exclusion column (Fig. 1, compare samples 8 and 11; supplemental Fig. S1). These changes were associated with the appearance of a mass peak corresponding to unmodified SoxY (Fig. 1, compare samples 8 and 11). Thus the SoxB reaction leads to the partial conversion of the S-thiosulfonate SoxY derivative to a species in which SoxYZ complexes form a disulfide-linked dimer through the SoxY cysteine residue.
At first sight this conclusion is at odds with the current proposal that the product of the SoxB reaction should be S-sulfane SoxY rather than a disulfide-linked SoxY dimer. However, taking into account the propensity of the reduced SoxY cysteine to form disulfide links to other SoxY molecules nonenzymatically, we believe that the observed SoxB-dependent products are still consistent with SoxB catalyzing a thiosulfohydrolase reaction. Removal of the terminal sulfonate group in the SoxB reaction produces a reactive sulfane derivative that we suggest would nonenzymatically form disulfide linkages via reactions such as the following.
In summary, the assay described above shows that the recombinant T. thermophilus SoxB protein is enzymatically active and can use the S-thiosulfonate derivative of SoxY as a substrate. Although the reaction catalyzed by SoxB could not be directly demonstrated, the observed pattern of products is consistent with the proposal that SoxB is a SoxY thiosulfohydrolase.
SoxB Has a 5′-Nucleotidase Fold
Recombinant T. thermophilus SoxB was crystallized, and the native structure was determined by single-wavelength anomalous diffraction using an in-house x-ray source with CuKα radiation (λ = 1.5418 Å). Structures were also determined for SoxB in crystals that had been grown in the presence of Mn2+, of Mn2+ and thiosulfate, or of Mn2+ and sulfate (see below), with the native structure being used as the starting point in the refinements. All of the refined models contain a single SoxB polypeptide in the asymmetric unit. The streptavidin tag added to the recombinant protein and the following five to seven residues of the N terminus of the protein are disordered.
The overall shape of SoxB approximates to a prolate spheroid with dimensions of 65 × 50 × 50 Å. It is composed of a large N-terminal domain (residues 30/32–353, the numbering refers to the precursor protein) linked by an α-helix (residues 354–372) to a smaller C-terminal domain (residues 373–573) (Fig. 2A). The linking helix has limited interactions with the rest of the protein. The N-terminal domain has a five-layered α/β-β-β-α-α structure, including two sandwiched mixed β-sheets, whereas the C-terminal domain has a four-layered α/β-β-α-β structure. The SoxB active site is located at the end of a cavity formed at the interface of the two domains. The active site contains two metal ions that are coordinated solely by residues from the N-terminal domain.
FIGURE 2.
Structure of T. thermophilus SoxB and the effect of binding the substrate analogue thiosulfate. A, cartoon representation of the overall structure of SoxB using the SoxB-glycerol coordinates. The N-terminal domain is shown in blue with the N-terminal loop subdomain in green. The interdomain helix is shown in yellow, the C-terminal domain is in red, and the manganese ions are in purple. The route of access to the active site is indicated with an arrow. B, a representation of the residues that are critical for the relative orientation of the substrate analogue thiosulfate and the reactive bridging hydroxide ion based on the SoxB-Mn2+-thiosulfate structure. The manganese ions are in purple, and the oxygen of the reactive bridging hydroxide is in red. Thiosulfate is shown in stick representation. The footprint of the thiosulfate S–S bond on the protein surface is green. Distances between selected atoms (indicated by dashed lines) are given. C, the two conformations of loop 465–476. The closed conformation observed when the active site is empty (SoxB-Mn2+ structure) is shown in yellow, and the open conformation adopted upon binding of the substrate analogue thiosulfate at the active site (SoxB-Mn2+-thiosulfate structure) is in red. The manganese ions are shown in purple, and the thiosulfate molecule present in the open conformation in a stick representation.
The T. thermophilus SoxB structure is grossly similar to the fold of E. coli 5′-nucleotidase (19) (Protein Data Bank entry 1USH), a protein with which it shares 20% sequence identity (Fig. 3). The root mean square deviation in the Cα positions of the individual domains of the two proteins is 1.9 Å for 263 aligned residues of the N-terminal domain and 2.4 Å for 171 aligned residues of the C-terminal domain (supplemental Fig. S3A). However, the active site region of the C-terminal domain of SoxB (residues 415–418, 438–441, 464–475, and 521–524) has a different arrangement from that found in the 5′-nucleotidase (supplemental Fig. S3A, panel ii).
FIGURE 3.
Sequence alignment of T. thermophilus SoxB with other SoxB proteins and E. coli 5′-nucleotidase. The proteins analyzed are T. thermophilus HB27 SoxB (Tthe), Aquifex aeolicus VF5 SoxB (Aaeo), P. pantotrophus SoxB (Ppan), Chlorobium tepidum TLS SoxB (Ctep), and E. coli 5′-nucleotidase (5-NT). Numbering is that of the precursor proteins. However, only the mature amino acid sequences are shown. Residues that are invariant between the SoxB proteins in the alignment are shown in white on a black background. Residues that are similar between SoxB proteins in the alignment at a threshold of 75% using the BLOSUM62 substitution matrix (64) have a gray background. Secondary structure elements above the alignment are those of T. thermophilus SoxB, and those below the alignment are those of E. coli 5′-nucleotidase (Protein Data Bank entry 1HPU). Rectangles denote α-helices, and arrows show β-strands. The numbering of these elements follows that of the E. coli 5′-nucleotidase structure. *, ligands of metal site A. ●, ligands of metal site B. §, second sphere metal ligands in SoxB. ▼, residues directly or indirectly involved in substrate binding.
The 5′-nucleotidase has been crystallized in multiple conformations that differ in the relative orientations of the N- and C-terminal domains (19, 47). Comparison of SoxB with the most open (Protein Data Bank entry 1HP1) and most closed (Protein Data Bank entry 1HPU) forms of the nucleotidase shows that SoxB is more tightly shut than the most closed nucleotidase conformation (supplemental Fig. S3B). The interdomain cavity in SoxB is ∼16 Å deep with a volume of ∼1900 Å3, while the interdomain cavity in the most closed form of the 5′-nucleotidase is considerably larger at ∼6125 Å3 (36).
The N-terminal domain of SoxB has an extensive insertion (Tyr56–Arg107) (green in Fig. 2A) compared with the 5′-nucleotidase structure (supplemental Fig. S3A, panel i). This forms a long loop followed by three helices. The loop runs along the interface of the two domains, covering the cleft between them (Fig. 2A). The loop has extensive interactions with both the C- and N-terminal domains. Indeed, it forms half of all contacts between them. The loop appears to stabilize the interaction between the N- and C-terminal domains, preventing the hinging between domains that is observed in the 5′-nucleotidase (47, 48). The more restricted active site access in SoxB presumably reflects the fact that the linear C-terminal peptide of SoxY is less bulky than substrates of the 5′-nucleotidase.
The C terminus of SoxB runs along the interdomain interface and also interacts with the N-terminal loop subdomain. This extended C-terminal polypeptide is only found in SoxB proteins from thermophilic organisms (supplemental Fig. S3A, panel ii). It may be an adaptation to high temperature that tightens the interdomain interface and protects the integrity of the active site cavity.
Structure of the Active Site
The initial SoxB structure was determined using a crystal that had been cryoprotected with glycerol. That structure showed strong electron density in the active site that could be most convincingly modeled as two overlapping glycerol molecules (Fig. 4A). To avoid the cryoprotectant interfering with the active site, all subsequent SoxB structures were determined from crystals that had been cryoprotected with PEG 400 rather than glycerol.
FIGURE 4.
Structure of the SoxB active site containing different ligands. The local environment of the SoxB active site is shown for the SoxB-glycerol (A), SoxB-Mn2+-sulfate (B), SoxB-Mn2+ (C), and SoxB-Mn2+-thiosulfate (D) structures. Two glycerol orientations are seen in the SoxB-glycerol structure. These are depicted in A as overlapping pink and cyan molecules. The sulfate ion in the SoxB-Mn2+-sulfate also has two orientations that are shown by overlapping sulfate molecules in B. The (2Fo − Fc) electron density maps have been contoured at the 1.0 σ level. Stereo views of these panels can be found in supplemental Fig. S4. Schematic representations are presented of the empty active site (E, SoxB-Mn2+ structure from C) and the active site occupied by the substrate analogue thiosulfate (F, SoxB-Mn2+-thiosulfate structure from D).
To establish the identities and amounts of the metal ions bound within the T. thermophilus SoxB protein, a microPIXE experiment was performed on two crystals grown under the same conditions as the glycerol-cryoprotected crystal used for initial structure determination. A metal content of 0.9 (±0.04) atoms of manganese, 0.10 (±0.02) atoms of zinc, and 0.09 (±0.02) atoms of iron/molecule of SoxB protein was determined. Combining these observations with the anomalous signal and refined occupancies of the metal in the crystal, one metal site (site A) was modeled as fully occupied by Mn2+, and the second metal site (site B) was only partially occupied with Mn2+ (Fig. 4A). Loss of the metal ion at site B has also been observed in 5′-nucleotidase (49). Mn2+ undergoes rapid ligand exchange and is often lost from its binding sites or substituted by other divalent metal ions during protein purification (50–52). In an attempt to increase the occupancy of metal site B in SoxB, the buffer used to grow the crystals was supplemented with Mn2+. The structure determined for crystals grown under these conditions exhibited two fully occupied metal sites (Fig. 4C). All further structures were therefore determined from crystals that had been grown in the presence of added Mn2+.
In the resting active site (i.e. without exogenous ligands) each manganese atom has five ligands that adopt a distorted trigonal bipyramidal conformation around the metal ion (Fig. 4, C and E). The manganese atoms are bridged both by a water molecule and the Oδ2 atom of the side chain of Asp143 in μ-1,1 coordination. The double Mn2+ coordination of the water molecule almost certainly ionizes it to the more reactive hydroxide ion. The remaining SoxB metal ligands are two histidines and an aspartate (His49, His299, and Asp47) at site A and three histidine residues at site B (His174, His274, and His297) (Figs. 3 and 4E). The Mn2+ ligand bond lengths are given in supplemental Table S1. An outer shell of amino acids provides hydrogen bonds to the metal-coordinating amino acid side chains to control their position in the active site (Figs. 3 and 4E). Notably, the main chain amide of site B ligand His297 is involved in the orientation of the carboxylate side chain of site A ligand Asp47.
The metal-coordinating residues are completely conserved between SoxB proteins (Fig. 3). The sequence positions of the SoxB metal ligands are retained in E. coli 5′-nucleotidase, but one of the nitrogen-donating histidine residues at each metal site is replaced by an oxygen-donating amide side chain (Fig. 3). The metal ligand set found in SoxB also occurs at the di-zinc site of hydrolases, having a metallo-β-lactamase-like fold including glyoxalase II (Protein Data Bank entry 1QH5 (53)), RNase Z (Protein Data Bank entry 1Y44 (54)), and N-acyl-l-homoserine lactone hydrolase (Protein Data Bank entry 2A7M (55)). However, the spatial arrangement of the ligands in these proteins is different from that found in SoxB, and one more of the histidine ligands uses the Nϵ2, rather than Nδ1, atom to coordinate the metal ions.
The two SoxB Mn2+ ions are separated by an average distance of 3.4 ± 0.1 Å in the four crystal structures. This compares with a distance of 3.3 ± 0.1 Å previously predicted from EPR studies of P. pantotrophus SoxB (18). A manganese-manganese separation of 3.3 Å is observed in the crystal structures of other di-manganese(II) enzymes that have μ-1,1 bridging carboxylate coordination (arginase, manganese-catalase, and the catalytic domain of the DNA repair protein MRE11) (56–58).
Structures of SoxB with Different Molecules Bound in the Active Site
To mimic the substrate complex, SoxB was crystallized in the presence of the substrate analogue thiosulfate (−S-SO3−). Unlike the physiological substrate, thiosulfate is kinetically stable to hydrolysis because breaking the sulfur-sulfur bond would require electron movement onto the negatively charged sulfane sulfur atom. The resulting structure revealed a thiosulfate molecule in the active site at full occupancy (Figs. 2, B and C, and 4, D and F), consistent with earlier data showing that the P. versutus enzyme associated with, but did not cleave, thiosulfate (59). The two SoxB Mn2+ ions are each coordinated by a thiosulfate oxygen atom giving both metals distorted octahedral coordination. The third thiosulfate oxygen participates in a hydrogen bond with the side chain of invariant Arg416. This side chain is also linked by a hydrogen bond to His297, a ligand of the site B Mn2+ ion (bond not shown in Fig. 4F for simplicity). The S–S bond of the thiosulfate molecule packs against the hydrophobic side chains of Val415, Trp417, and Trp175, as well as the nonpolar portion of the Arg416 side chain, and this contributes to the orientation of thiosulfate in the active site pocket (Fig. 2B). The residues at these positions are well conserved across SoxB sequences, with Trp175, Arg416, and Trp417 being invariant and Val415 substituted only by phenylalanine or tyrosine (Fig. 3). Trp175 is also involved in a hydrogen bond to the thiosulfate oxygen atom that coordinates the manganese ion at site B.
EPR experiments performed previously on P. pantotrophus SoxB in the presence of thiosulfate predicted bis(μ-hydroxo)(μ-carboxylato) coordination for the two Mn2+ ions (22). This prediction is in good agreement with the T. thermophilus SoxB-thiosulfate structure described here. One hydroxo group can be attributed to the monodentate bridging oxygen atom of the Asp143 side chain, and the second is assigned to the bridging water if this is in the form of a hydroxide ion. Lastly, the μ-sulfonate coordination observed in the structure resembles μ-carboxylato coordination. The EPR data therefore support the assignment of the bridging water molecule as a hydroxide ion.
The orientation of the bridging hydroxide is determined by a hydrogen bonding interaction with the main chain carbonyl of His297. The oxygen atom of the hydroxide is on the same axis as the S–S bond of thiosulfate and is at an equal distance from the three thiosulfate oxygen atoms (Figs. 2B and 4, D and F). Furthermore, the hydroxide oxygen-to-thiosulfate sulfur distance is similar to that found between the oxygen atoms and central sulfur atom in sulfate. The bridging hydroxide is thus ideally positioned to undertake an in-line nucleophilic attack on the sulfone sulfur atom of the substrate molecule.
SoxB was additionally crystallized in the presence of sulfate, which is a product of the SoxB reaction. In this structure a sulfate molecule is found at the active site. However, it adopts two different positions, each refined at 50% occupancy, which is indicative of only weak interactions with SoxB (Fig. 4B). Notably sulfate does not benefit from the favorable hydrophobic interactions with Val415, Trp417, and Trp175 that are involved in thiosulfate binding. The positioning, coordination, and stabilization of one of the sulfate orientations is similar to that of the thiosulfate molecule in the SoxB-Mn2+-thiosulfate structure. In the second orientation the sulfate molecule is inverted compared with the first conformer. The second orientation could thus resemble the position of the product sulfate after attack of the bridging hydroxide on the S-(thio)sulfonate cysteine of SoxY. In this second conformer one sulfate oxygen atom is coordinated by the site A manganese ion, and another is stabilized by the side chain of Trp175. A third oxygen atom of the sulfate is located very close to the bridging hydroxide, which has only 75% occupancy. Notably in the second conformer the Arg416 side chain is not involved in stabilizing the sulfate molecule and is oriented out of the active site.
General Structural Differences for Ligand-bound SoxB
All four structures determined in this work have the same general fold. The Cα root mean square deviation between the SoxB-glycerol and the SoxB-Mn2+, SoxB-Mn2+-thiosulfate, and SoxB-Mn2+-sulfate structures are, respectively, 0.16, 0.54, and 0.55 Å. The main difference between the structures is found in a conserved loop (residues 465–476) located on the surface of SoxB at the entrance to the active site cavity (Fig. 2C). In the SoxB-glycerol and SoxB-Mn2+ structures this loop is in a “closed” conformation. The entrance of the cavity in this conformation is ∼10 × 16 Å with a calculated cross-sectional area of 168 Å2. It adopts an “open” conformation in the SoxB-Mn2+-thiosulfate structure. The mouth of the cavity increases in size to an area of 191 Å2 with dimensions of ∼10 × 20 Å. However, the volume of the active site pocket diminishes slightly from 1900 to 1860 Å3 when adopting the open conformation. Both the open and the closed conformations are observed in the SoxB-Mn2+-sulfate structure with the open structure being associated with the orientation in which the sulfate molecule adopts a similar position to thiosulfate. Stabilization of the bound thiosulfate/sulfate by the side chain of Arg416 is only sterically possible in the open conformation, and it is this interaction that appears to induce the shift of the loop away from the active site. Specifically, when the side chain of Arg416 interacts with thiosulfate, it is no longer available to stabilize the position of the loop via interaction with the side chain of Asp476. As a consequence the loop moves to allow Asp476 to form new bonding interactions with Arg385.
Modeling studies show that a S-thiosulfonate derivative of the C-terminal Thr144-Arg-Val-Thr-Val-Gly-Gly-Cys-Gly152 peptide of T. thermophilus SoxY is easily accommodated within the SoxB active site in either the open or closed state. The S-thiosulfonate peptide is long enough to reach into the active site and coordinate the manganese atoms in the manner observed with thiosulfate. Intriguingly, the side chain of Arg416, which changes position on substrate binding, would be well positioned to form an ion pair with the peptide C-terminal carboxylate. An analysis of conserved SoxB surface residues shows that they cluster on the face of the protein containing the active site entrance (supplemental Fig. S5) and plausibly form the site of interaction with the SoxYZ partner.
DISCUSSION
SoxB Mechanism
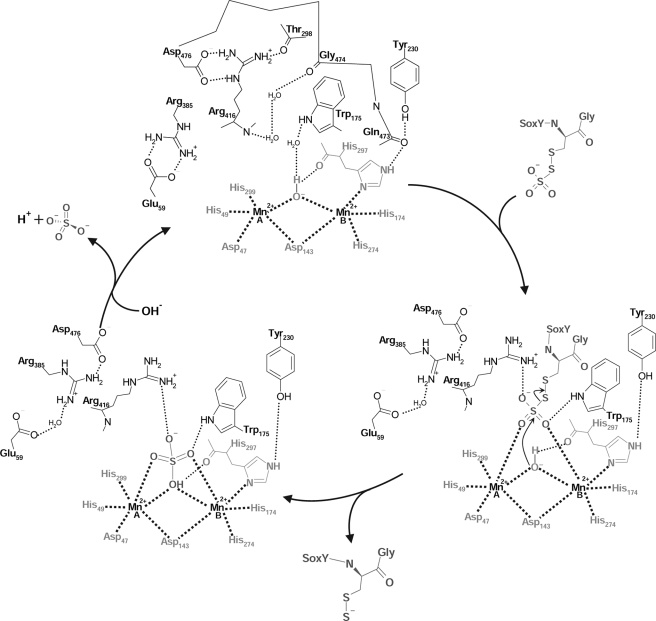
The structures determined here allow us to propose a straightforward model for the mechanism of SoxB (Fig. 5). The C-terminal peptide swinging arm of SoxY bearing the cysteine-conjugated sulfonate substrate inserts into the SoxB active site cavity. By analogy to the SoxB-Mn2+-thiosulfate structure, two oxygen atoms of the sulfonate group are held rigidly in place by symmetrical coordination to the two active site manganese ions. Substrate binding is further strengthened by interaction of the remaining oxygen atom of the sulfonate group with the side chain of Arg416. This interaction involves breaking the hydrogen bonds between the Arg416 side chain and Asp476 that are seen in the substrate-free SoxB-Mn2+ structure. The loss of these interactions allows movement of the 465–476 loop and a rearrangement of the hydrogen bonding network around Asp476. The substrate binding interactions are completed by a ring of hydrophobic amino acids that pack against the substrate sulfur-sulfur bond.
FIGURE 5.
Proposed mechanism for SoxB. The three steps of the cycle depicted here are based on the SoxB-Mn2+, SoxB-Mn2+-thiosulfate, and SoxB-Mn2+-sulfate (conformer 2) structures.
We propose that the sulfur-sulfur bond of the bound substrate molecule is broken in a straightforward SN2 reaction involving in-line nucleophilic attack of the bridging hydroxide ion on the sulfone sulfur atom. The bonding interactions of the sulfone oxygen atoms will assist the reaction by withdrawing negative charge from the central sulfur atom. The SoxB-Mn2+-sulfate structure shows that the product sulfate molecule is only weakly bound in the active site. Thus, in the final step in the reaction mechanism, the manganese-bound sulfate is displaced by a water molecule.
The mechanism we suggest here for SoxB differs from that proposed for the related 5′-nucleotidase. In the 5′-nucleotidase the substrate only coordinates one of the active site metal ions, the nucleophile is a water molecule bound to the other metal atom and not the bridging hydroxide molecule, and a histidine/aspartate catalytic pair that is absent from SoxB has a critical role (60). In addition, catalysis by the 5′-nucleotidase, in contrast to SoxB, is thought to involve opening and closing of the active site by whole scale domain rotation (48).
Selection of Mn2+ as the SoxB Cofactor
Why has evolution selected Mn2+ as the catalytic cofactor for SoxB rather than more widely used divalent metal cations such as Zn2+ or Fe2+? Mn2+ is a hard metal ion with a relatively low affinity for soft ligands such as sulfide and thiolates (61). Using Mn2+ as the SoxB cofactor would avoid the thiolate reaction product (cysteine S-sulfane or cysteine) binding to the active site metal ions and inhibiting enzyme turnover. Employing Mn2+ would also avoid active site inhibition by sulfide and other thiolate compounds that are present in many environments where bacterial sulfur oxidation is taking place.
Mn2+ has a lower affinity for protein ligands than other divalent first row transition metal ions (61, 62). This raises the question of how SoxB is able to bind Mn2+ in the face of competition from other divalent metal cations present in the periplasm. One possibility is that in environments rich in sulfide Mn2+ has a higher bioavailability than other divalent metals ions because manganese has the lowest propensity to precipitate as sulfide complexes (50, 62). However, the manganese-containing SoxB proteins purified from P. versutus and P. pantrophus were isolated from cells growing under nonsulfidic conditions, indicating that bioavailability is not the sole determinant of metal specificity (16, 17). An alternative possibility is suggested by a recent study in which a periplasmic Mn2+ protein was proposed to receive its metal cofactor in the cytoplasm, where metal speciation can be controlled, before being exported to the periplasm in a folded state by the Tat protein transport pathway (63). Strikingly, all SoxB proteins have a Tat-targeting signal peptide (5, 8). However, our crystallographic and PIXE data show that the metal ion at site B in T. thermophilus SoxB is easily exchanged, which would seem to obviate any advantage in preloading the site with Mn2+. It is, instead, most likely that it is the arrangement of ligands in the active site that allows specific Mn2+ binding to SoxB. In particular, Mn2+ is a large ion that will be favored by sites with long metal-ligand bond distances.
Supplementary Material
Acknowledgments
We thank Prof. G. Gottschalk for providing the T. thermophilus HB27 genomic DNA, Dr. E. D. Lowe for help with the in-house data collection, and Prof. R. J. P. Williams for discussions. We thank Gérard Bricogne and the Global Phasing Consortium for access to beta versions of BUSTER-TNT, autoBUSTER, SHARP, and autoSHARP.
This work was supported by Biotechnology and Biological Sciences Research Council Grant P15195 and Medical Research Council Grant G0400775.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S5.
The atomic coordinates and structure factors (codes 2WDC, 2WDD, 2WDE, and 2WDF) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
C. Vonrhein, personal communication.
- DTT
- dithiothreitol
- PEG
- polyethylene glycol.
REFERENCES
- 1.Dahl C., Friedrich C. G. (eds) ( 2007) Microbial Sulfur Metabolism, Springer-Verlag [Google Scholar]
- 2.Lengeler J. W., Drews G., Schlegel H. G. (eds) ( 1999) Biology of the Prokaryotes, Blackwell Science, Oxford, UK [Google Scholar]
- 3.Friedrich C. G., Bardischewsky F., Rother D., Quentmeier A., Fischer J. (2005) Curr. Opin. Microbiol. 8, 253–259 [DOI] [PubMed] [Google Scholar]
- 4.Wodara C., Bardischewsky F., Friedrich C. G. (1997) J. Bacteriol. 179, 5014–5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appia-Ayme C., Little P. J., Matsumoto Y., Leech A. P., Berks B. C. (2001) J. Bacteriol. 183, 6107–6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyaya P. N., Deb C., Lahiri C., Roy P. (2000) J. Bacteriol. 182, 4278–4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rother D., Henrich H. J., Quentmeier A., Bardischewsky F., Friedrich C. G. (2001) J. Bacteriol. 183, 4499–4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedrich C. G., Rother D., Bardischewsky F., Quentmeier A., Fischer J. (2001) Appl. Environ. Microbiol. 67, 2873–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quentmeier A., Friedrich C. G. (2001) FEBS Lett. 503, 168–172 [DOI] [PubMed] [Google Scholar]
- 10.Sauvé V., Bruno S., Berks B. C., Hemmings A. M. (2007) J. Biol. Chem. 282, 23194–23204 [DOI] [PubMed] [Google Scholar]
- 11.Bamford V. A., Bruno S., Rasmussen T., Appia-Ayme C., Cheesman M. R., Berks B. C., Hemmings A. M. (2002) EMBO J. 21, 5599–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hensen D., Sperling D., Trüper H. G., Brune D. C., Dahl C. (2006) Mol. Microbiol. 62, 794–810 [DOI] [PubMed] [Google Scholar]
- 13.Beller H. R., Chain P. S., Letain T. E., Chakicherla A., Larimer F. W., Richardson P. M., Coleman M. A., Wood A. P., Kelly D. P. (2006) J. Bacteriol. 188, 1473–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa T., Furusawa T., Nomura R., Seo D., Hosoya-Matsuda N., Sakurai H., Inoue K. (2008) J. Bacteriol. 190, 6097–6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meulenberg R., Pronk J. T., Frank J., Hazeu W., Bos P., Kuenen J. G. (1992) Eur. J. Biochem. 209, 367–374 [DOI] [PubMed] [Google Scholar]
- 16.De Jong G. A., Hazeu W., Bos P., Kuenen J. G. (1997) Eur. J. Biochem. 243, 678–683 [DOI] [PubMed] [Google Scholar]
- 17.Rzhepishevska O. I., Valdés J., Marcinkeviciene L., Gallardo C. A., Meskys R., Bonnefoy V., Holmes D. S., Dopson M. (2007) Appl. Environ. Microbiol. 73, 7367–7372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanao T., Kamimura K., Sugio T. (2007) J. Biotechnol. 132, 16–22 [DOI] [PubMed] [Google Scholar]
- 19.Knöfel T., Sträter N. (1999) Nat. Struct. Biol. 6, 448–453 [DOI] [PubMed] [Google Scholar]
- 20.Cammack R., Chapman A., Lu W. P., Karagouni A., Kelly D. P. (1989) FEBS Lett. 253, 239–243 [Google Scholar]
- 21.Friedrich C. G., Quentmeier A., Bardischewsky F., Rother D., Kraft R., Kostka S., Prinz H. (2000) J. Bacteriol. 182, 4677–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epel B., Schäfer K. O., Quentmeier A., Friedrich C., Lubitz W. (2005) J. Biol. Inorg. Chem. 10, 636–642 [DOI] [PubMed] [Google Scholar]
- 23.Petri R., Podgorsek L., Imhoff J. F. (2001) FEMS Microbiol. Lett. 197, 171–178 [DOI] [PubMed] [Google Scholar]
- 24.Meyer B., Imhoff J. F., Kuever J. (2007) Environ. Microbiol. 9, 2957–2977 [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J., Russell D. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., Vol. 3, p. A2.2, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 26.Apostol I., Aitken J., Levine J., Lippincott J., Davidson J. S., Abbott-Brown D. (1995) Protein Sci. 4, 2616–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruker (2000) SMART, SAINT, SADABS and XPREP Software Reference Manual, Bruker AXS Inc., Madison, WI [Google Scholar]
- 28.Leslie A. G. (1992) Joint CCP4 + ESF-EAMCB Newsletter on Protein Crystallography 26, STFC Daresbury Laboratory, Warrington, England, UK [Google Scholar]
- 29.Sheldrick G. M., Schneider T. R. (1997) Methods Enzymol. 277, 319–343 [PubMed] [Google Scholar]
- 30.Winn M. D., Ashton A. W., Briggs P. J., Ballard C. C., Patel P. (2002) Acta Crystallogr. D Biol. Crystallogr. 58, 1929–1936 [DOI] [PubMed] [Google Scholar]
- 31.De La Fortelle E., Bricogne G. (1997) Methods Enzymol. 276, 472–494 [DOI] [PubMed] [Google Scholar]
- 32.Perrakis A., Morris R., Lamzin V. S. (1999) Nat. Struct. Biol. 6, 458–463 [DOI] [PubMed] [Google Scholar]
- 33.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 34.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 35.Cheary R. W., Coelho A. A. (1996) in CCP4 Powder Diffraction Library, Engeneering and Physical Sciences Research Council, Daresbury Laboratory, Warrington, UK [Google Scholar]
- 36.Binkowski T. A., Naghibzadeh S., Liang J. (2003) Nucleic Acids Res. 31, 3352–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guex N., Peitsch M. C. (1997) Electrophoresis 18, 2714–2723 [DOI] [PubMed] [Google Scholar]
- 38.Holm L., Park J. (2000) Bioinformatics 16, 566–567 [DOI] [PubMed] [Google Scholar]
- 39.Glaser F., Pupko T., Paz I., Bell R. E., Bechor-Shental D., Martz E., Ben-Tal N. (2003) Bioinformatics 19, 163–164 [DOI] [PubMed] [Google Scholar]
- 40.Landau M., Mayrose I., Rosenberg Y., Glaser F., Martz E., Pupko T., Ben-Tal N. (2005) Nucleic Acids Res. 33, W299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grime G. W., Dawson M., Marsh M., McArthur I. C., Watt F. (1991) Nucl. Instrum. Methods Phys. Res. B 54, 52–63 [Google Scholar]
- 42.Garman E. (1999) Structure 7, R291–R299 [DOI] [PubMed] [Google Scholar]
- 43.Garman E. F., Grime G. W. (2005) Prog. Biophys. Mol. Biol. 89, 173–205 [DOI] [PubMed] [Google Scholar]
- 44.Johansson S. A. E., Campbell J. L., Malmqvist K. G. (1995) Particle-Induced X-Ray Emission Spectrometry, John Wiley & Sons, Inc., New York [Google Scholar]
- 45.Fan Q. R., Long E. O., Wiley D. C. (2000) J. Biol. Chem. 275, 23700–23706 [DOI] [PubMed] [Google Scholar]
- 46.Builder S., Hart R., Lester P., Reifsnyder D. ( September15, 1998) U. S. Patent 5808006 [Google Scholar]
- 47.Knöfel T., Sträter N. (2001) J. Mol. Biol. 309, 255–266 [DOI] [PubMed] [Google Scholar]
- 48.Schultz-Heienbrok R., Maier T., Sträter N. (2005) Biochemistry 44, 2244–2252 [DOI] [PubMed] [Google Scholar]
- 49.McMillen L., Beacham I. R., Burns D. M. (2003) Biochem. J. 372, 625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams R. J. P., Frausto da Silva J. J. (2000) Coord. Chem. Rev. 200–202, 247–348 [Google Scholar]
- 51.Schilling O., Wenzel N., Naylor M., Vogel A., Crowder M., Makaroff C., Meyer-Klaucke W. (2003) Biochemistry 42, 11777–11786 [DOI] [PubMed] [Google Scholar]
- 52.Marasinghe G. P., Sander I. M., Bennett B., Periyannan G., Yang K. W., Makaroff C. A., Crowder M. W. (2005) J. Biol. Chem. 280, 40668–40675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cameron A. D., Ridderström M., Olin B., Mannervik B. (1999) Structure 7, 1067–1078 [DOI] [PubMed] [Google Scholar]
- 54.de la Sierra-Gallay I. L., Pellegrini O., Condon C. (2005) Nature 433, 657–661 [DOI] [PubMed] [Google Scholar]
- 55.Liu D., Lepore B. W., Petsko G. A., Thomas P. W., Stone E. M., Fast W., Ringe D. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 11882–11887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopfner K. P., Karcher A., Craig L., Woo T. T., Carney J. P., Tainer J. A. (2001) Cell 105, 473–485 [DOI] [PubMed] [Google Scholar]
- 57.Antonyuk S., Melik-Adamyan V., Popov A., Lamzin V., Hempstead P., Harrison P., Artymyuk P., Barynin V. (2000) Crystallogr. Rep. 45, 105–116 [Google Scholar]
- 58.Di Costanzo L., Sabio G., Mora A., Rodriguez P. C., Ochoa A. C., Centeno F., Christianson D. W. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13058–13063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu W. P., Kelly D. P. (1983) J. Gen. Microbiol. 129, 3549–3564 [Google Scholar]
- 60.Knöfel T., Sträter N. (2001) J. Mol. Biol. 309, 239–254 [DOI] [PubMed] [Google Scholar]
- 61.Irving H., Williams R. J. P. (1948) Nature 162, 746–747 [Google Scholar]
- 62.Frausto da Silva J. J. R., Williams R. J. P. (2001) The Biological Chemistry of the Elements: The Inorganic Chemistry of Life ( 2nd Ed.) Oxford University Press, Oxford, UK [Google Scholar]
- 63.Tottey S., Waldron K. J., Firbank S. J., Reale B., Bessant C., Sato K., Cheek T. R., Gray J., Banfield M. J., Dennison C., Robinson N. J. (2008) Nature 455, 1138–1142 [DOI] [PubMed] [Google Scholar]
- 64.Henikoff S., Henikoff J. G. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 10915–10919 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.