Abstract
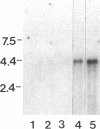
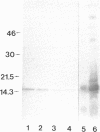
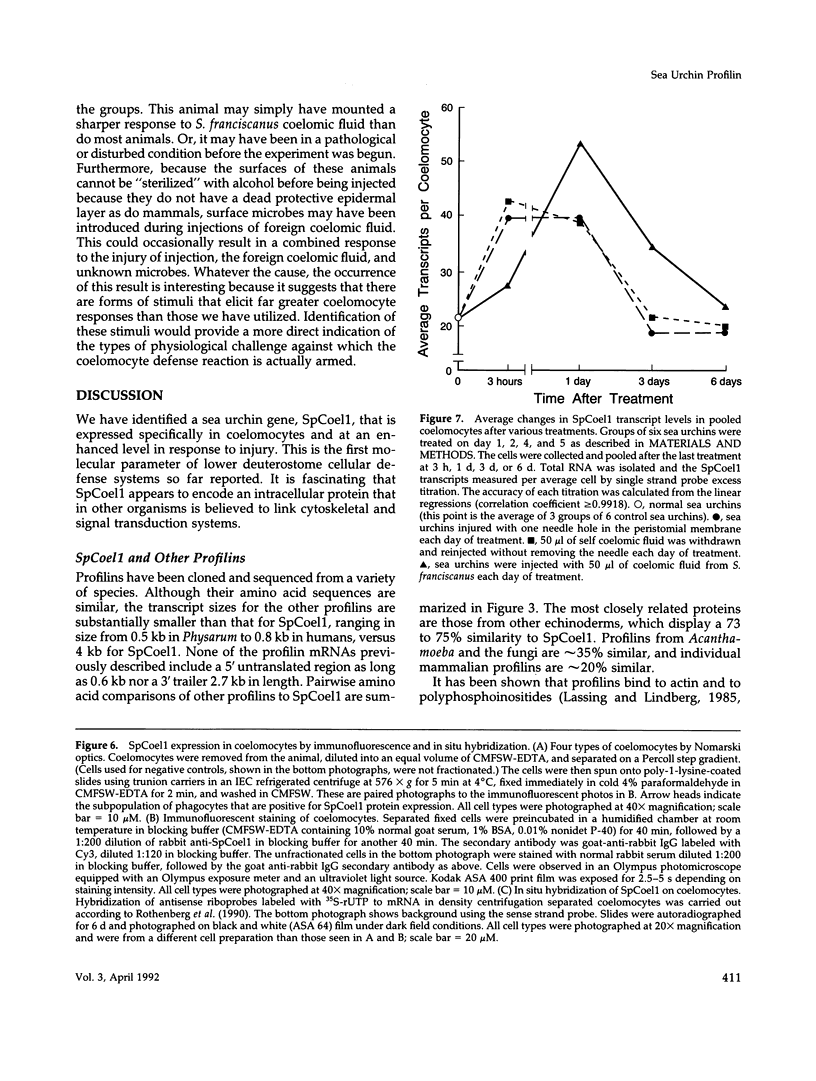
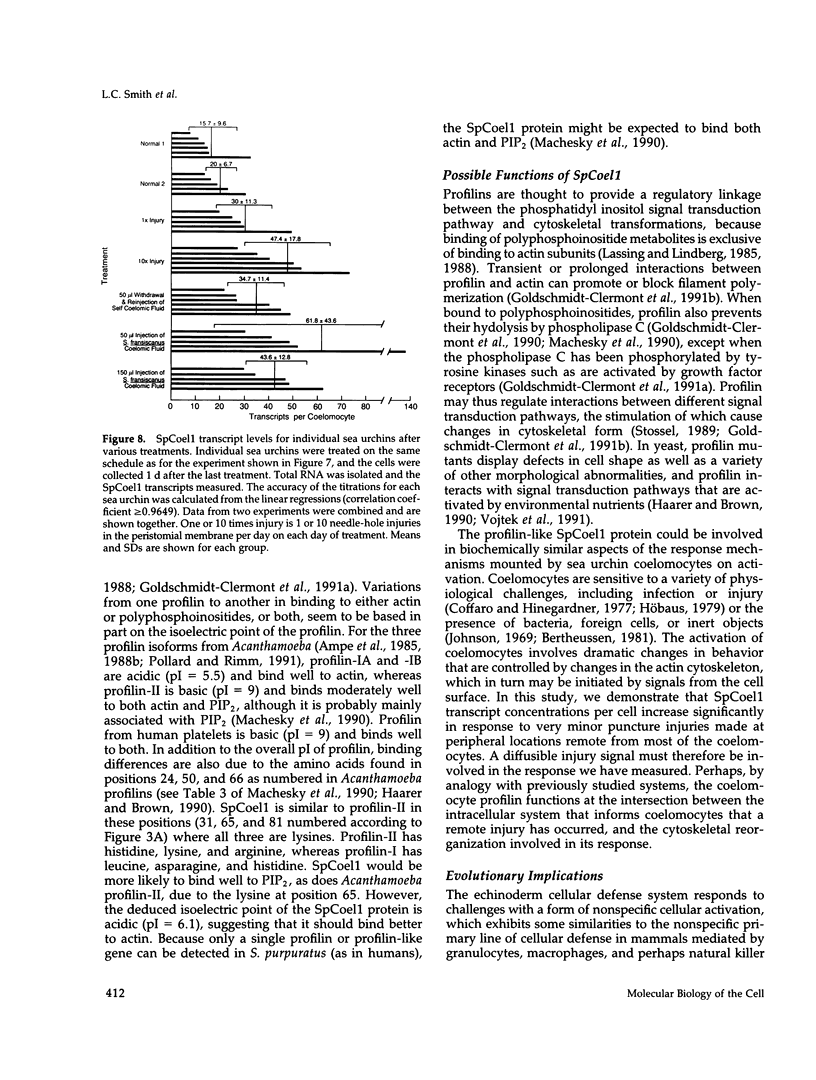
SpCoel1 is a single copy gene that is specifically expressed in most of the coelomocytes of the adult purple sea urchin, Strongylocentrotus purpuratus. The 4-kb transcript from this gene has a relatively short (426 nucleotide) open reading frame (ORF) with long 3' and 5' untranslated regions. The ORF encodes a protein that has strong amino acid sequence similarity to profilins from yeast to mammals. Transcript titrations of SpCoel1 show significant increases per coelomocyte in animals that have been physiologically challenged. Increases in transcript levels are of similar magnitudes between animals receiving different treatments, such as injuries from needle punctures or from injections of foreign cells. The evidence presented here implies a molecular mechanism by which this lower deuterostome defense system responds to external insult, viz that an external "injury signal" activates a signal transduction system, which in turn mediates the alterations in cytoskeletal state that are required for coelomocyte activation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampe C., Markey F., Lindberg U., Vandekerckhove J. The primary structure of human platelet profilin: reinvestigation of the calf spleen profilin sequence. FEBS Lett. 1988 Feb 8;228(1):17–21. doi: 10.1016/0014-5793(88)80575-1. [DOI] [PubMed] [Google Scholar]
- Ampe C., Sato M., Pollard T. D., Vandekerckhove J. The primary structure of the basic isoform of Acanthamoeba profilin. Eur J Biochem. 1988 Jan 4;170(3):597–601. doi: 10.1111/j.1432-1033.1988.tb13739.x. [DOI] [PubMed] [Google Scholar]
- Ampe C., Vandekerckhove J., Brenner S. L., Tobacman L., Korn E. D. The amino acid sequence of Acanthamoeba profilin. J Biol Chem. 1985 Jan 25;260(2):834–840. [PubMed] [Google Scholar]
- Bertheussen K. Endocytosis by echinoid phagocytosis in vitro. I. Recognition of foreign matter. Dev Comp Immunol. 1981 Spring;5(2):241–250. doi: 10.1016/0145-305x(81)90031-8. [DOI] [PubMed] [Google Scholar]
- Bertheussen K. The cytotoxic reaction in allogeneic mixtures of echinoid phagocytes. Exp Cell Res. 1979 May;120(2):373–381. doi: 10.1016/0014-4827(79)90397-5. [DOI] [PubMed] [Google Scholar]
- Binette F., Bénard M., Laroche A., Pierron G., Lemieux G., Pallotta D. Cell-specific expression of a profilin gene family. DNA Cell Biol. 1990 Jun;9(5):323–334. doi: 10.1089/dna.1990.9.323. [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Britten R. J., Davidson E. H. Expression of two actin genes during larval development in the sea urchin Strongylocentrotus purpuratus. Mol Reprod Dev. 1989;1(3):149–155. doi: 10.1002/mrd.1080010302. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Coffaro K. A., Hinegardner R. T. Immune response in the sea urchin Lytechinus pictus. Science. 1977 Sep 30;197(4311):1389–1390. doi: 10.1126/science.331476. [DOI] [PubMed] [Google Scholar]
- Edds K. T., Chambers C., Allen R. D. Coelomocyte motility. Cell Motil. 1983;3(2):113–121. doi: 10.1002/cm.970030202. [DOI] [PubMed] [Google Scholar]
- Edds K. T. Dynamic aspects of filopodial formation by reorganization of microfilaments. J Cell Biol. 1977 May;73(2):479–491. doi: 10.1083/jcb.73.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edds K. T. The formation and elongation of filopodia during transformation of sea urchin coelomocytes. Cell Motil. 1980;1(1):131–140. doi: 10.1002/cm.970010110. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Kim J. W., Machesky L. M., Rhee S. G., Pollard T. D. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991 Mar 8;251(4998):1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Baldassare J. J., Pollard T. D. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990 Mar 30;247(4950):1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Doberstein S. K., Pollard T. D. Mechanism of the interaction of human platelet profilin with actin. J Cell Biol. 1991 Jun;113(5):1081–1089. doi: 10.1083/jcb.113.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goustin A. S., Wilt F. H. Protein synthesis, polyribosomes, and peptide elongation in early development of Strongylocentrotus purpuratus. Dev Biol. 1981 Feb;82(1):32–40. doi: 10.1016/0012-1606(81)90426-7. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS T. CHEMICAL DISSOLUTION AND IN VITRO RECONSTRUCTION OF SPONGE CELL ADHESIONS. I. ISOLATION AND FUNCTIONAL DEMONSTRATION OF THE COMPONENTS INVOLVED. Dev Biol. 1963 Aug;8:27–47. doi: 10.1016/0012-1606(63)90024-1. [DOI] [PubMed] [Google Scholar]
- Haarer B. K., Brown S. S. Structure and function of profilin. Cell Motil Cytoskeleton. 1990;17(2):71–74. doi: 10.1002/cm.970170202. [DOI] [PubMed] [Google Scholar]
- Johnson P. T. The coelomic elements of sea urchins (Strongylocentrotus). 3. In vitro reaction to bacteria. J Invertebr Pathol. 1969 Jan;13(1):42–62. doi: 10.1016/0022-2011(69)90237-7. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Bruns G. A. Human profilin. Molecular cloning, sequence comparison, and chromosomal analysis. J Biol Chem. 1988 Apr 25;263(12):5910–5915. [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex. J Cell Biochem. 1988 Jul;37(3):255–267. doi: 10.1002/jcb.240370302. [DOI] [PubMed] [Google Scholar]
- Leahy P. S. Laboratory culture of Strongylocentrotus purpuratus adults, embryos, and larvae. Methods Cell Biol. 1986;27:1–13. doi: 10.1016/s0091-679x(08)60339-8. [DOI] [PubMed] [Google Scholar]
- Leahy P. S., Tutschulte T. C., Britten R. J., Davidson E. H. A large-scale laboratory maintenance system for gravid purple sea urchins (Strongylocentrotus purpuratus). J Exp Zool. 1978 Jun;204(3):369–380. doi: 10.1002/jez.1402040308. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Calzone F. J., Britten R. J., Angerer R. C., Davidson E. H. Activation of sea urchin actin genes during embryogenesis. Measurement of transcript accumulation from five different genes in Strongylocentrotus purpuratus. J Mol Biol. 1986 Mar 20;188(2):173–183. doi: 10.1016/0022-2836(86)90302-5. [DOI] [PubMed] [Google Scholar]
- Machesky L. M., Goldschmidt-Clermont P. J., Pollard T. D. The affinities of human platelet and Acanthamoeba profilin isoforms for polyphosphoinositides account for their relative abilities to inhibit phospholipase C. Cell Regul. 1990 Nov;1(12):937–950. doi: 10.1091/mbc.1.12.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechsner U., Magdolen V., Bandlow W. The cDNA and deduced amino acid sequence of profilin from Saccharomyces cerevisiae. Nucleic Acids Res. 1987 Nov 11;15(21):9078–9078. doi: 10.1093/nar/15.21.9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Rimm D. L. Analysis of cDNA clones for Acanthamoeba profilin-I and profilin-II shows end to end homology with vertebrate profilins and a small family of profilin genes. Cell Motil Cytoskeleton. 1991;20(2):169–177. doi: 10.1002/cm.970200209. [DOI] [PubMed] [Google Scholar]
- Reichstein E., Korn E. D. Acanthamoeba profilin. A protein of low molecular weight from Acanpthamoeba castellanii that inhibits actin nucleation. J Biol Chem. 1979 Jul 10;254(13):6174–6179. [PubMed] [Google Scholar]
- Reinisch C. L., Bank F. B. Cell recognition: reactions of the sea star (Asterias vulgaris) to the injection of amebocytes of sea urchin (Arbacia punctulata). Cell Immunol. 1971 Oct;2(5):496–503. doi: 10.1016/0008-8749(71)90058-x. [DOI] [PubMed] [Google Scholar]
- Rothenberg E. V., Diamond R. A., Pepper K. A., Yang J. A. IL-2 gene inducibility in T cells before T cell receptor expression. Changes in signaling pathways and gene expression requirements during intrathymic maturation. J Immunol. 1990 Mar 1;144(5):1614–1624. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G. D., Altschul S. F., Lipman D. J. A workbench for multiple alignment construction and analysis. Proteins. 1991;9(3):180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Takagi T., Mabuchi I., Hosoya H., Furuhashi K., Hatano S. Primary structure of profilins from two species of Echinoidea and Physarum polycephalum. Eur J Biochem. 1990 Sep 24;192(3):777–781. doi: 10.1111/j.1432-1033.1990.tb19289.x. [DOI] [PubMed] [Google Scholar]
- Vojtek A., Haarer B., Field J., Gerst J., Pollard T. D., Brown S., Wigler M. Evidence for a functional link between profilin and CAP in the yeast S. cerevisiae. Cell. 1991 Aug 9;66(3):497–505. doi: 10.1016/0092-8674(81)90013-1. [DOI] [PubMed] [Google Scholar]
- Widada J. S., Ferraz C., Liautard J. P. Total coding sequence of profilin cDNA from Mus musculus macrophage. Nucleic Acids Res. 1989 Apr 11;17(7):2855–2855. doi: 10.1093/nar/17.7.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]