Abstract
We have purified and characterized a specific CTP:molybdopterin cytidylyltransferase for the biosynthesis of the molybdopterin (MPT) cytosine dinucleotide (MCD) cofactor in Escherichia coli. The protein, named MocA, shows 22% amino acid sequence identity to E. coli MobA, the specific GTP:molybdopterin guanylyltransferase for molybdopterin guanine dinucleotide biosynthesis. MocA is essential for the activity of the MCD-containing enzymes aldehyde oxidoreductase YagTSR and the xanthine dehydrogenases XdhABC and XdhD. Using a fully defined in vitro assay, we showed that MocA, Mo-MPT, CTP, and MgCl2 are required and sufficient for MCD biosynthesis in vitro. The activity of MocA is specific for CTP; other nucleotides such as ATP and GTP were not utilized. In the defined in vitro system a turnover number of 0.37 ± 0.01 min−1 was obtained. A 1:1 binding ratio of MocA to Mo-MPT and CTP was determined to monomeric MocA with dissociation constants of 0.23 ± 0.02 μm for CTP and 1.17 ± 0.18 μm for Mo-MPT. We showed that MocA was also able to convert MPT to MCD in the absence of molybdate, however, with only one catalytic turnover. The addition of molybdate after one turnover gave rise to a higher MCD production, revealing that MCD remains bound to MocA in the absence of molybdate. This work presents the first characterization of a specific enzyme involved in MCD biosynthesis in bacteria.
The biosynthesis of the molybdenum cofactor (Moco)2 is an ancient, ubiquitous, and highly conserved pathway leading to the biochemical activation of molybdenum. In Moco the molybdenum atom is coordinated to the dithiolene group of the 6-alkyl side chain of a pterin called molybdopterin (MPT). Moco biosynthesis has been extensively studied in Escherichia coli by using a combination of biochemical, genetic, and structural approaches (1, 2). The biosynthesis of Moco has been divided into four major steps in Escherichia coli: (i) formation of precursor Z (3, 4), (ii) formation of MPT from precursor Z (5, 6), (iii) insertion of molybdenum to form Moco via an adenylylated MPT intermediate (7–9), and (iv) additional modification by covalent addition of GMP to the C4′ phosphate of MPT via a pyrophosphate bond, forming the molybdopterin guanine dinucleotide (MGD) cofactor (10, 11). In E. coli, GMP attachment to Moco is catalyzed by the MobA and MobB proteins (12). Although MobA was shown to be essential for this reaction and acts as a GTP:molybdopterin guanylyltransferase (11), the role of MobB still remains uncertain. From the crystal structure, it was postulated that MobB is an adapter protein acting in concert with MobA to achieve the efficient biosynthesis and utilization of MGD (13). Although MobA was shown to bind MPT, Mo-MPT, and MGD (14), investigations of in vitro studies using purified MobA, MgCl2, GTP, and either MPT or Mo-MPT showed that MGD was only formed by MobA when the molybdenum atom was already ligated to MPT (15). The formation of bis-MGD is one of the most enigmatic steps in Moco biosynthesis in E. coli. It is still not known whether the two MGD molecules assemble on MobA or instead after the insertion into the respective target proteins like DMSO reductase or nitrate reductase A. In other bacteria like Arthrobacter nicotinovorans, Veillonella atypica, or Oligotropha carboxidovorans, Moco can be further modified by the attachment of CMP to the C4′ phosphate of MPT forming the molybdopterin cytosine dinucleotide (MCD) cofactor (16–18). A specific enzyme catalyzing the CTP:molybdopterin cytidylyltransferase reaction has not been identified so far. For A. nicotinovorans nicotine dehydrogenase and ketone dehydrogenase the involvement of a MobA homologous protein for MCD formation was reported (16); however, it was not shown whether the MobA protein was specifically required for MCD biosynthesis or whether it was also involved in the biosynthesis of MGD in this bacterium. Furthermore, enzymes binding MCD in bacteria usually contain an additional modification at the molybdenum site of Moco, where a terminal oxo-ligand is exchanged by a sulfido ligand, forming sulfurated or mono-oxo Moco (19). Recently, the MCD-containing protein YagTSR was identified and characterized in E. coli as a periplasmic aldehyde oxidoreductase which oxidizes a broad spectrum of aldehydes using ferredoxin as electron acceptor (20). It was shown that for the production of an active form of YagTSR, the YagQ protein was required, which is believed to be a MCD binding chaperone involved in the sulfuration of the Mo site and the insertion of sulfurated MCD into apoYagTSR (20). The majority of the other molybdoenzymes in E. coli were shown to bind the bis-MGD form of Moco, in which molybdenum is coordinated to two MGD moieties. The other exception is the YedY protein, being so far the only E. coli protein binding the Mo-MPT form of Moco (21). However, the physiological role of this protein still remains unclear.
Investigations on YagTSR showed that MCD was inserted into YagR independent of the function of MobA, indicating that a so-far unidentified protein is involved in MCD biosynthesis in E. coli (20). Here, we report the identification of the specific CTP:molybdopterin cytidylyltransferase, which we named MocA (formerly named YgfJ by the E. coli nomenclature of genes with unknown function). Purified MocA was shown to catalyze the formation of MCD from Mo-MPT and CTP in vitro. Additionally, we report that a disruption in the mocA gene impaired MCD biosynthesis in E. coli, resulting in an inactive YagTSR protein devoid of Moco.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Media, and Growth Conditions
Strains and plasmids used in this study are listed in Table 1. Cell strains containing expression vectors were grown aerobically in LB medium at 22 or 30 °C in the presence of either 150 μg/ml ampicillin or 25 μg/ml kanamycin. Sodium molybdate was added to the expression cultures of TP1000 × pMN100 (YagTSR) and TP1000 × pTG818 (Moco-containing human sulfite oxidase Moco domain (hSO-MD)) at a concentration of 1 mm.
TABLE 1.
Escherichia coli plasmids and strains
| Plasmid or strain | Genotype or relevant characteristics | Source or reference |
|---|---|---|
| Plasmids | ||
| pACYC-duet1 | CmR | Novagen |
| pET16b | ApR | Novagen |
| pET28a | KmR | Novagen |
| pMN100 | pTrcHis derivative carrying yagTSRQ | 20 |
| pMN106 | 582-bp NdeI-SalI fragment of mocA cloned into the NdeI-SalI sites of pET28a | This study |
| pMN125 | 582-bp NdeI-SalI fragment of mocA cloned into the NdeI-XhoI sites of pACYC-duet1 | This study |
| pMN125b | 582-bp NdeI-SalI fragment of mocA cloned into the NdeI-XhoI sites of pET16b | This study |
| pTG818 | pTrcHis derivative carrying hSO-MD | 26 |
| Strains | ||
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Novagen |
| TP1000 | F-ΔlacU169 araD139 rpsL150 relA1 ptsF rbsR flbB ΔmobAB ::Kan | 31 |
| RK5202 | araD139 Δ(argF-lac)U169 deoC1 flbB5201 ayrA219 non-9 ptsF25 relA1 rpsL150 chID ::Mu cts | 32 |
| BW25113 | lacIqrrnBT14 ΔlacZWJ16hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | 33 |
| JW2845 | mocA− mutant of BW25113 | 34 |
| JW0278 | yagR− mutant of BW25113 | 34 |
| JW2849 | xdhA− mutant of BW25113 | 34 |
| JW5462 | xdhD− mutant of BW25113 | 34 |
| JW2849/5462 | xdhA−xdhD− mutant of BW25113 | This study |
Construction of the E. coli xdhA xdhD Double Mutant Strain
Plasmid pCP20 was used to remove the kanamycin resistance cassette of strain JW2849 (xdhA−) (22). The mutation of strain JW5462 (xdhD−, kan) was transferred to JW2849 by P1 transduction (25), resulting in a xdhA−/xdhD− strain JW2849/5462. Integration of the kanamycin resistance gene at the correct position on the chromosome was verified by PCR.
Construction of mocA Expression Vectors
DNA fragments containing the coding regions for E. coli mocA (ygfJ) were amplified by PCR using primers NdeI-mocA (GGCATATGTCAGCCATCGACTGTATAATTCC) and mocA-SalI (GGGTCGACCTAAATTTCAGTATACCTTTCCTTCGC) introducing NdeI and SalI as flanking restriction sites.
Growth Experiments
Growth experiments after the functional expression of YagTSR in E. coli strains BW25113, JW2845 (mocA−), and JW2849/5462 (xdhA− xdhD−) were performed using 0.1 × LB, pH 4.0, in the presence or absence of 800 μm cinnamaldehyde as described previously (20). Functional expression of XdhABC and XdhD was followed after modified protocols described by Xi et al. (22) and Levine and Taylor (23). E. coli strains BW25113, JW2845 (mocA−), JW0278 (yagR−), JW2849 (xdhA−), JW5462 (xdhD−), and JW2849/5462 (xdhA−/xdhD−) were inoculated at an A600 nm of 0.1 in the absence and presence of 0.1% adenine in minimal medium after Vogel and Bonner (24) supplemented with 0.5% glucose (VB medium), cells were incubated at 37 °C and 150 rpm, and growth was followed by the absorbance at 600 nm.
To test the functional complementation of E. coli strain JW2845 (mocA−) with a plasmid expressing MocA, cells were transformed with plasmid pMN125 and tested for growth in 0.1 × LB medium, pH 4.0, or VB medium as described above supplemented with 50 μg/ml chloramphenicol.
Expression and Purification of E. coli MocA
For production of MocA, E. coli BL21(DE3) cells were transformed with plasmid pMN106. For expression, 6 × 1 liter of LB was inoculated with 10 ml of an overnight culture and incubated at 30 °C until an A600 nm of 0.3–0.5. The expression was induced with 100 μm isopropyl-β-d-thiogalactopyranoside, cells were harvested after an additional growth of 5 h, and the cell pellet was resuspended in 15 ml of phosphate buffer (50 mm NaH2PO4, 300 mm NaCl, pH 8.0) per liter of expression culture. Cell lysis was achieved after three passages through a TS Series Benchtop cell disruptor at 1350 bar in presence of DNase I (1 μg/ml). The cleared lysate was applied to 0.25 ml of nickel-nitrilotriacetic acid (NTA) per liter of culture. The column was washed with 20 column volumes of phosphate buffer containing sequentially 10, 20, and 50 mm imidazole. MocA was eluted with phosphate buffer containing 250 mm imidazole, dialyzed against 100 mm Tris, pH 7.2, and stored at −80 °C until further use. For the purification of MocA in a Moco-loaded form, MocA was expressed from pMN125b in E. coli RK5202 (modC−) cells, and the protein was purified by Ni-NTA using phosphate buffers described above without the supplementation of NaCl.
Expression and Purification of E. coli YagTSR and hSO
YagTSR was expressed in E. coli BW25113 and JW2845 (mocA−) from plasmid pMN100 and purified by Ni-NTA chromatography as described earlier (20). Moco-containing and MPT-containing hSO-MD were expressed from pTG818 in E. coli TP1000 (ΔmobAB) or RK5202 (modC−) cells, respectively, and purified as described by Temple et al. (26).
Cofactor Analysis of Purified YagTSR
Metal analysis was performed using PerkinElmer Life Sciences Optima 2100DV inductively coupled plasma optical emission spectrometer. Protein samples were incubated overnight in a 1:1 mixture with 65% nitric acid (Suprapur, Merck) at 100 °C. Samples were filled to a 10-fold volume with water before inductively coupled plasma-optical emission spectrometry analysis. As reference, the multi-element standard solution XVI (Merck) was used. Nucleotides were released from flavin adenine dinucleotide (FAD) and MCD by incubation at 95% for 15 min in the presence of 3% (v/v) sulfuric acid and analyzed as described earlier (20).
Enzyme Assays
The activity of YagTSR (units/mg) was determined by monitoring the reduction of ferricyanide at 420 nm in a buffer containing 126.3 mm disodium hydrogen phosphate and 36.9 mm citric acid, pH 6.0, with 500 μm vanillin as substrate. The specific activity is defined as the reduction of 1 μmol of vanillin per min and mg of protein.
In Vitro Analysis of CTP:Molybdopterin Cytidylyltransferase Activity
Moco (Mo-MPT) and MPT were obtained from purified hSO-MD expressed in E. coli TP1000 or RK5202 cells, respectively, after heat treatment as described previously (26, 27). For standard in vitro MCD production, 1 μm MocA was incubated with 100 μm concentrations of either Mo-MPT or MPT, 1 mm MgCl2, and 1 mm CTP in a total volume of 400 μl of 100 mm Tris, pH 7.2. 1 mm Na2MoO4 was included in incubation mixtures containing Mo-MPT. Additionally, to analyze the role of MgCl2 in the incubation mixtures, MgCl2 was replaced by 1 mm Co(NO3)2, NiSO4, or MnCl2. The effect of 1 mm MgSO4 and 1 mm KNO3 on MCD production was also analyzed but had no influence on the efficiency of the reaction (data not shown). For time-dependent analyses, the reactions were stopped at different time points by the addition of 50 μl of acidic iodine (1% I2, 2% KI, 1 m HCl). In reaction mixtures containing MPT, up to 1 mm molybdate was added after 60 min of incubation, and samples were incubated for another 30 min.
The kinetic parameters were determined by varying the CTP concentration from 0–100 μm after an incubation time of 12 min in the presence of either 1 mm MgCl2 or 1 mm MnCl2. The formation of MCD during the reaction was quantified after an overnight oxidation of the acidic iodine-containing reaction mixtures, whichs result in the release of MCD and the conversion to its fluorescent degradation product Form A-CMP. Form A-CMP was detected as described previously (20). In-line fluorescence was monitored by an Agilent 1100 series detector with excitation at 383 nm and emission at 450 nm. For quantification, the Form A-CMP peak was collected, and the height of the Form A-CMP peak was correlated to the CMP amount released after acidic heat treatment. The ratio of bound Moco or MCD was determined after their conversion to either Form A or Form A-CMP by acidic iodine treatment followed by a separation on Q-Sepharose chromatography as described previously (20, 27).
Binding of Mo-MPT, MPT, CTP, and CMP to MocA
KD values for Mo-MPT and MPT binding to MocA were determined by ultrafiltration as described previously (27). Binding assays contained 6 μm MocA and 0–24 μm Mo-MPT or MPT. Mo-MPT or MPT was quantified as described earlier (27). KD values for CTP and CMP were determined by ultrafiltration using 10 μm MocA and 0–20 μm CTP and CMP. CTP and CMP were quantified after the method described by Hänzelmann and Meyer (28) with some modifications. A Nucleosil 100–10 SB ion exchange column (Macherey & Nagel) was equilibrated with 95 mm ammonium phosphate, pH 3.4. 20 μl of sample or standard was injected and eluted with a linear gradient of 95–500 mm ammonium phosphate, pH 3.4, over 30 ml followed by 5 min of 500 mm ammonium phosphate, pH 3.4, at a flow rate of 1 ml/min. The column temperature was set to 25 °C. CTP and CMP calibration curves were used for the quantification. An Agilent 1100 series diode array detector was used to monitor the absorbance at 260 and 280 nm. To investigate the influence of Mg2+ on the binding constants, 1 mm MgCl2 was added to the incubation mixtures. The binding constants of CTP were additionally determined in the presence of 50 μm MPT or Mo-MPT and the constants of Mo-MPT in the presence of 50 μm CTP or CMP, respectively.
RESULTS
Identification of an E. coli Protein with Amino Acid Sequence Identities to MobA
Genes coding for proteins with amino acid sequence similarities to the E. coli MobA protein have been identified in a number of organisms. It was shown that the A. nicotinovorans megaplasmid pAO1 contains a coding region involved in MCD biosynthesis, which was investigated by gene disruptions. The putative protein encoded by the megaplasmid shares amino acid sequence identities to E. coli MobA (16). Thus, it seemed likely that the E. coli protein involved in MCD biosynthesis is a MobA homologue. A bioinformatic approach was used to identify sequences in the E. coli genome sharing amino acid sequence homologies to MobA. This approach identified the gene sequence ygfJ, which codes for a protein with an amino acid sequence identity of 22% to E. coli MobA (supplemental Fig. S1). Amino acid sequence analysis of YgfJ revealed that the protein belongs to the NTP-transferase superfamily of proteins with an NTP binding domain at the N-terminal domain (National Center for Biotechnology Information). YgfJ is similar in length to E. coli MobA; however, the amino acid identities are mainly located at the N terminus, and a number of conserved amino acid substitutions are found at the C terminus. In general MobA proteins from different organisms show a high amino acid sequence identity of 15–30%. The A. nicotinovorans MobA protein shows an identity of 18% to E. coli YgfJ. Because YgfJ is a MobA homologue, we renamed the protein to MocA after the nomenclature of proteins involved in Moco biosynthesis.
Growth of E. coli Wild-type and mocA Mutant Strain in the Presence and Absence of Cinnamaldehyde or Adenine
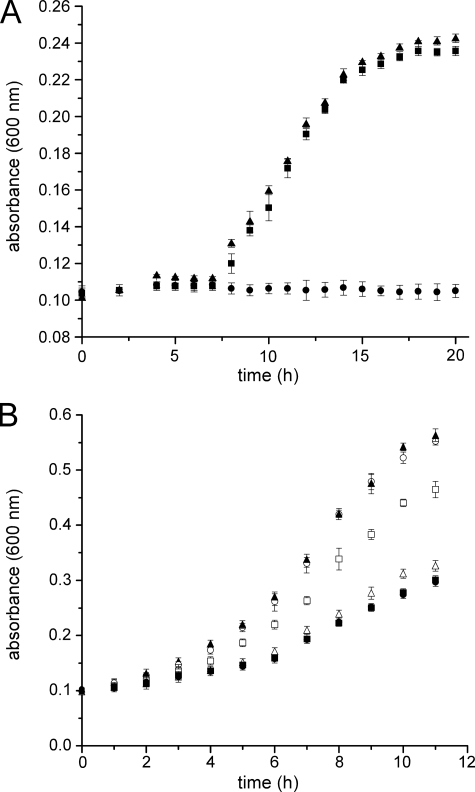
To investigate the role of MocA for MCD biosynthesis, we analyzed the influence of an E. coli mocA mutation on the activity of the MCD-containing aldehyde oxidoreductase YagTSR and the putative xanthine dehydrogenases XdhABC and XdhD in E. coli. YagTSR was shown to be essential for growth of E. coli on a low pH medium in the presence of cinnamaldehyde (20), whereas E. coli xdhA or xdhD mutant strains showed an increased sensitivity to adenine (22). Growth curves were recorded of E. coli wild-type and the mocA mutant strain in the presence or absence of cinnamaldehyde using 0.1 × LB medium at pH 4.0. In the absence of cinnamaldehyde, the growth of the mocA mutant strain and the corresponding wild-type strain were identical (data not shown). The addition of 800 μm cinnamaldehyde resulted in an impaired cell growth of wild-type E. coli, prolonging the lag phase from 4 to 7 h, with maximal optical density reduced to 65% (Fig. 1A and data not shown). In comparison, the disruption of the mocA gene resulted in complete growth inhibition (Fig. 1A). We could show that the phenotype of the mocA mutant strain was not based on polar effects of the introduced kanamycin cassette into BW25113, as introduction of a plasmid expressing MocA into strain JW2845 (mocA−) complemented the phenotype in direct comparison to BW25113 transformed with pACYC-duet1 vector as control (data not shown). Because the putative E. coli xanthine dehydrogenases XdhABC and XdhD are also likely to contain the MCD form of Moco, we analyzed the growth of a double mutant strain lacking xdhA and xdhD (JW2849/5462) under the same conditions. These growth experiments should exclude any overlapping effects of the mocA mutant on the activity of the two putative xanthine-oxidizing proteins, which also might be able to oxidize aldehydes as substrates. As shown in Fig. 1A, the xdhA/xdhD double mutant strain showed the same growth phenotype in the presence of cinnamaldehyde in comparison to wild-type strain BW25113. In contrast, experiments by Xi et al. (22) showed an increased sensitivity to adenine in xdhA or xdhD mutant strains. To analyze the influence of the mocA mutation on XdhABC and XdhD activity, we analyzed the growth of the xdhA and xdhD single and double mutant strains in VB medium in the presence or absence of adenine. In the absence of adenine, the growth of the mutant strains was not affected in comparison to the corresponding wild-type strain (data not shown). The addition of 0.1% adenine to the VB medium resulted in an impaired cell growth of all E. coli strains (Fig. 1B). However, in comparison to the wild type, the effect on growth of the mutant strains in mocA, xdhA, and/or xdhD was even more impaired in the presence of 0.1% adenine (Fig. 1B). After a growth time of 11 h, the A600 nm of the xdhD mutant strain was reduced to 83% compared with the wild type, whereas the growth of the xdhA mutant strain was reduced to 57%. The strongest growth inhibition of about 53% was observed for the mocA and the xdhA/xdhD double mutant strains. In contrast, a deletion of yagTSR under these growth conditions did not influence cell growth. Thus, the effects of the mocA deletion on growth in the presence of adenine can be attributed to a loss of function of XdhABC and XdhD, two enzymes which were suggested to bind MCD. Additionally, we could show that the phenotype of the mocA mutant strain was not based on polar effects of the introduced kanamycin cassette, as a plasmid containing the mocA gene was able to restore the phenotype completely under the respective growth conditions with adenine (data not shown).
FIGURE 1.
The influence of cinnamaldehyde and adenine on growth of E. coli mutant strains in mocA, xdhA, xdhD, and yagR. A, growth curves of E. coli BW25113 (filled triangles, reproduced from (20)), JW2845 (mocA−, filled circles), and JW2849/5462 (xdhA− xdhD−, filled squares) in 0.1 × LB, pH 4.0 containing 800 μm cinnamaldehyde. B, growth curves of E. coli BW25113 (filled triangles), JW2845 (mocA−, filled circles), JW2849 (xdhA−, open triangles), JW5462 (xdhD−, open squares), JW0278 (yagR−, open circles), and JW2849/5462 (xdhA− xdhD−, filled squares) in the presence of 0.1% adenine in VB medium.
Characterization of YagTSR Purified from the E. coli mocA Mutant Strain
YagTSRQ was expressed in E. coli mocA− (JW2845) cells and the corresponding wild-type strain BW25113 from plasmids pMN100. After expression, YagTSR was purified and analyzed for activity and cofactor content. The purified protein from E. coli JW2845 was shown to be completely inactive (Table 2). Analysis of the metal content revealed that although the purified protein was completely saturated with iron (4.06 ± 0.06 molecules per YagTSR trimer), it was devoid of molybdenum (Table 2). To quantify the MCD and FAD content of YagTSR, the protein was incubated for 15 min at 95 °C in the presence of 5% sulfuric acid releasing AMP from FAD and CMP of the MCD cofactor. Nucleotide analysis was performed using the respective nucleotides AMP and CMP as standards. The AMP content determined for YagTSR purified from the mocA mutant strain was related to a complete FAD saturation (98.2 ± 6.4%); however, no detectable CMP was released after acidic heat treatment (Table 2). Samples were incubated in the presence of acidic iodine to convert bound Mo-MPT or MPT to Form A; however, a fluorescent oxidation product was not detectable after the treatment. After heat treatment at pH 7.5 or SDS denaturation, no other nucleotides like GTP, ATP, CTP, GDP, ADP, CDP, AMP, or GMP were identified to be bound to the protein. YagTSR purified from the mocA mutant strain was found to be rather unstable, as during further purification (e.g. Q-Sepharose chromatography or Superdex 200 chromatography) the loss of the YagR subunit devoid of Moco was observed.
TABLE 2.
Cofactor content of YagTSR expressed in E. coli BW25113 and JW2845 (mocA−)
| E. coli strain | Activitya | Mob | Feb | AMPc | CMPc |
|---|---|---|---|---|---|
| Units/mg | % | % | % | % | |
| BW25113 | 21.9 ± 0.4 | 49.6 ± 1.4 | 99.4 ± 1.8 | 98.4 ± 5.3 | 48.6 ± 1.2 |
| JW2845 (BW25113 mocA−) | NDd | ND | 101.7 ± 1.4 | 98.2 ± 6.4 | ND |
a Specific enzyme activity (units/mg) is defined as the oxidation of 1 μmol of vanillin/min/mg in phosphate-citrate buffer, pH 6.0, at room temperature using ferricyanide as electron acceptor.
b Molybdenum (μm molybdenum/μm YagTSR) and iron (μm 2[2Fe2S]/μm YagTSR) contents were determined by ICP-OES (“Experimental Procedures”) and related to a fully saturated enzyme.
c Nucleotide content (μm CMP or AMP/μm protein) content was analyzed after release of CMP from MCD and AMP from FAD by acidic heat treatment as described in “Experimental Procedures.”
d ND, none detectable.
For comparison, a UV-visible absorption spectrum was recorded for YagTSR purified from the mocA mutant strain and the corresponding wild-type strain (supplemental Fig. S2). Significant differences in the UV-visible absorption spectra were observed in the range of 320–325 nm where Moco typically absorbs. In addition, absorption spectra of the oxidized and reduced enzymes with benzaldehyde and sodium dithionite were recorded. From the reduction spectra, the amount of active YagTSR purified from BW25113 was calculated to be 49%. In contrast, YagTSR purified from the mocA mutant strain was reducible with sodium dithionite but not with benzaldehyde. A difference spectrum of both YagTSR purifications was recorded; however, because of structural changes based on the instable YagR subunit, it does not completely reflect the absorption spectrum of MCD (supplemental Fig. S2).
Expression and Purification of MocA
For purification of MocA, a fusion protein was generated containing an N-terminal His6 tag. After homologous expression in E. coli BL21(DE3) cells, MocA was purified by affinity chromatography. After elution, one major band was displayed on Coomassie Brilliant Blue R-stained SDS-polyacrylamide gels, corresponding to the molecular mass of the His6 fusion protein of 23.5 kDa (supplemental Fig. S3). The purification procedure yielded 0.8 mg of MocA per liter E. coli culture. Gel filtration experiments using an analytical size exclusion column (Superdex 200) showed that under these conditions MocA is present as a monomer in its native state (supplemental Fig. S3).
In Vitro MCD Production of MocA
The mocA gene product was shown to be essential for the activity of MCD-containing enzymes; thus, it was of interest to determine the role of MocA as a CTP:molybdopterin cytidylyltransferase. For this purpose, we determined whether MocA is able to produce MCD in vitro from Mo-MPT or MPT in a CTP-dependent manner. The in vitro system consisted of MocA, MgCl2, CTP, and either Mo-MPT or MPT to determine the minimal components required for MCD production. After an incubation time of 60 min, produced MCD was converted to Form A-CMP by oxidation with acidic iodine at room temperature, and Form A-CMP was quantified by its fluorescence after reversed-phase HPLC on a Hypersil C-18 column. MgCl2 was shown to be essential for MCD production. However, Mn2+ was able to functionally replace Mg2+ with a 50% reduced efficiency, whereas no MCD was produced with other divalent cations like Co2+ or Ni2+ (data not shown).
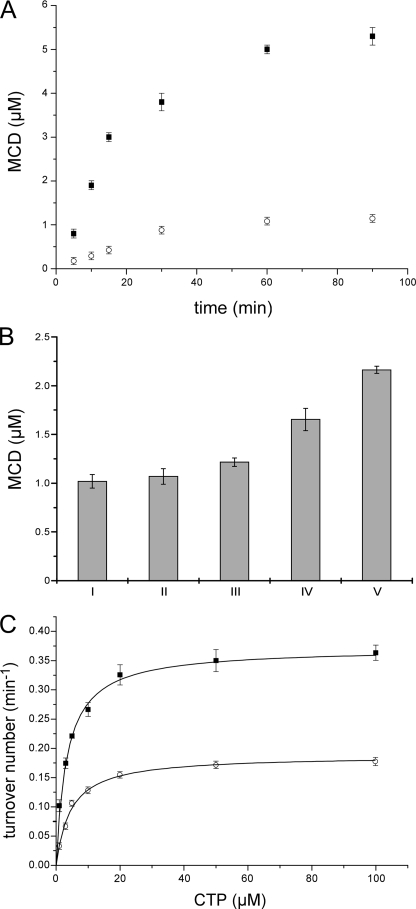
To analyze the time-dependent production of MCD, an in vitro system consisting of MocA, MgCl2, CTP, and Mo-MPT or MPT was used. As shown in Fig. 2A, the Form A-CMP production was linear over a time range of 15 min, and a half-maximal turnover was obtained at 11.3 min from the reaction mixture containing Mo-MPT. After a reaction time of 90 min, more than 5 μm Form A-CMP was produced in the reaction mixture containing 1 μm MocA, revealing that a multiple turnover of the reaction is possible in the absence of an acceptor protein. Form A-CMP was also produced in the reaction mixture containing MPT, however, with a slower rate and only one catalytic turnover (Fig. 2A). To analyze the basis for the inhibited multiple-turnovers of the protein in the absence of molybdate, we analyzed whether produced MPT-CMP remains bound to MocA, and thus, a product inhibition occurs. A reaction mixture containing 1 μm MocA, 1 mm MgCl2, 1 mm CMP, and either 100 μm Mo-MPT or MPT was incubated for 60 min before unbound cofactor was removed by gel filtration using a Nick column. The eluted protein was analyzed for the ratio of bound MPT and MPT-CMP. The ratio of both cofactors was determined to be 0.5 MPT to 1 MPT-CMP after an incubation time of 60 min. After a prolonged incubation time of 8 h, a ratio of 0.41 MPT to 1 MPT-CMP was determined, showing that the protein was still active; however, a substrate turnover occurred at very low rates (supplemental Fig. S4). In contrast, in incubation mixtures containing Mo-MPT, the ratio of the bound cofactors was determined to be 4.4 MPT to 1 MCD molecules (after 60 min of incubation) with a similar total cofactor saturation. Thus, a product inhibition is the likely rate-limiting step in the incubation mixtures, which is more apparent in the mixtures devoid of molybdate.
FIGURE 2.
MCD production by MocA. A, time-dependent MCD production using Mo-MPT, MPT, MgCl2, and CTP. Reaction mixtures contained 1 μm MocA, 1 mm CTP, 1 mm MgCl2, and 100 μm Mo-MPT (filled squares) or MPT (open circles). Reactions were stopped after 5, 10, 15, 30, 60, and 90 min by the addition of acidic iodine. Produced MCD was converted to Form A-CMP and quantified by fluorescence after reverse phase HPLC using a C18 column. B, increased MCD production of MocA from MPT after the addition of molybdate. Reaction mixtures containing 1 μm MocA, 1 mm CTP, 1 mm MgCl2, and 100 μm MPT were incubated for 60 min before the addition of different concentrations of molybdate (panel I, 0 μm; panel II, 1 μm; panel III, 10 μm; panel IV, 100 μm; panel V, 1 mm). The reactions were stopped after a further incubation step of 30 min by the addition of acidic iodine, and produced MCD was quantified as described above. C, effects of CTP concentrations on in vitro MCD production. Incubation mixtures containing 1 μm MocA, 100 μm Mo-MPT, and 1 mm MgCl2 (filled squares) or MnCl2 (open circles) were incubated in the presence of 1, 3, 5, 10, 20, 50, or 100 μm CTP. The reactions were stopped by the addition of acidic iodine after 12 min, and produced MCD was quantified as described above. Data were fitted nonlinear using Origin 6.0 software (Microcal) according to the Michaelis-Menten equation.
To additionally analyze the influence of molybdate on the release of MCD from MocA in samples containing bound MPT and MPT-CMP, molybdate was added at concentrations of 1 μm–1 mm to the reaction mixture after the first incubation step. As shown in Fig. 2B, a 100- and 1000-fold excess of molybdate resulted in a 2-fold increase of MCD production. To show that MCD was released after the addition of molybdate, the incubation mixture was passed through a Microcon ultrafiltration unit, and the flow-through was analyzed for MCD. After ultrafiltration, MCD was only determined in incubation mixtures where molybdate was added (data not shown), showing that molybdate can be inserted into protein-bound MPT-CMP and produced MCD is readily released from the protein.
Additional analysis of the CTP dependence of the reaction containing Mo-MPT, MgCl2, and different CTP concentrations resulted in a typical hyperbolic saturation curve, with a kcat of 0.37 ± 0.01 min−1 and a KmCTP of 3.4 ± 0.4 μm (Fig. 2C). When Mg2+ was replaced by Mn2+, the kcat was 2-fold reduced to 0.18 ± 0.01 min−1, whereas the KmCTP was increased to 4.6 ± 0.5 μm (Fig. 2C). It was not possible to replace CTP with ATP or GTP in the incubation mixtures (data not shown), showing that MocA is a specific CTP:molybdopterin cytidylyltransferase.
We were further interested to purify MocA in a cofactor loaded from E. coli extracts after expression. For this purpose, MocA was expressed in a modC-deficient E. coli strain, which is impaired in molybdate transport. Thus, the lack of molybdate should result in an impaired MCD production so that possibly MPT-CMP remains bound to MocA. We could show that MocA purified from the E. coli modC mutant strain was loaded with Moco; however, a detailed analysis of the cofactor content showed that the cofactors were bound in a ratio of 1 MCD to 25 MPT (data not shown). Thus, in the cell there seems to be an additional control mechanism, which prevents the conversion of MPT to MPT-CMP in the absence of molybdate.
Dissociation Constants for MPT, Mo-MPT, CTP, or CMP
We were interested to determine the dissociation constants for MPT, Mo-MPT, CTP, and CMP to MocA (Table 3). For this purpose MocA was incubated for 15 min at varying concentrations of Mo-MPT, MPT, CMP, and CTP at 4 °C, and unbound ligands were subsequently separated by ultrafiltration using a membrane with a molecular mass cutoff of 10 kDa. The Mo-MPT and MPT concentrations in the flow-through fractions were quantified after conversion to the stable, fluorescent oxidation product Form A by a reversed-phase HPLC. CMP and CTP were quantified in comparison to the corresponding nucleotide standards after separation with an ion exchange column. The KD values were calculated according to the determined free and total ligand concentrations in relation to the total MocA concentration. Fitting procedures revealed that MocA binds CTP, CMP, Mo-MPT, and MPT in a 1:1 ratio (Table 3). The determined KD values showed the strongest binding affinity for CTP (KD = 0.23 ± 0.02 μm), whereas the dissociation constant for CMP was 20-fold higher (KD = 4.66 ± 0.89 μm). The determined KD for MPT (8.66 ± 0.47 μm) was 7.5 times higher as the KD for Mo-MPT (1.17 ± 0.18 μm), showing in total a preferred binding of CTP and Mo-MPT to MocA.
TABLE 3.
KD values for the binding of CTP, CMP, Mo-MPT, and MPT to MocA
| Ligand | Cosubstratea | Bmaxb | KDc |
|---|---|---|---|
| CTPd | —f | 0.98 ± 0.04 | 0.23 ± 0.02 |
| Mg2+ | 0.95 ± 0.08 | 0.18 ± 0.01 | |
| Mo-MPT | 1.04 ± 0.02 | 0.81 ± 0.06 | |
| MPT | 1.03 ± 0.03 | 0.82 ± 0.06 | |
| CMPd | — | 1.08 ± 0.09 | 4.66 ± 0.89 |
| Mg2+ | 1.06 ± 0.04 | 4.41 ± 0.17 | |
| Mo-MPTe | — | 0.89 ± 0.08 | 1.17 ± 0.18 |
| Mg2+ | 0.95 ± 0.03 | 1.70 ± 0.22 | |
| CTP | 1.09 ± 0.13 | 12.83 ± 1.07 | |
| CMP | 1.06 ± 0.03 | 1.69 ± 0.19 | |
| MPTe | — | 0.90 ± 0.07 | 8.66 ± 0.47 |
| Mg2+ | 0.94 ± 0.05 | 8.98 ± 0.64 |
aKD values were determined in the presence of 1 mm MgCl2, 50 μm Mo-MPT, MPT, CTP, or CMP as indicated.
b Bmax describes the maximum saturation of MocA revealed by a 1:1 fitting procedure following the law of mass action.
c KD values were determined by ultrafiltration as described by Neumann et al. (27).
d CTP and CMP were used in a concentration range of 0–20 μm in the presence of 10 μm MocA and quantified as described under “Experimental Procedures.”
e Mo-MPT and MPT were used in a concentration range of 0–24 μm in the presence of 6 μm MocA and quantified as described earlier (27).
f −, no addition.
Additionally, the influence of MgCl2 on the binding constants was analyzed. The results in Table 3 show that the presence of Mg2+ resulted only in a slightly decreased affinity of Mo-MPT to MocA, whereas the binding of MPT, CTP, or CMP was in the same range in the presence or absence of Mg2+ ions.
We also investigated the influence of the presence of either CTP or CMP on Mo-MPT binding and the presence of either Mo-MPT or MPT on CTP binding to MocA. The results in Table 3 show that the KD of Mo-MPT binding is increased 10-fold in presence of CTP, whereas the KD value remained in the same range in the presence of CMP. A similar result was obtained for CTP binding, where the presence of both Mo-MPT and MPT increased the KD value for CTP 4-fold.
DISCUSSION
We have purified and characterized the monomeric MocA protein from E. coli, a protein that shares 22% sequence identity to the E. coli MobA congener. MocA was shown to catalyze the addition of CMP to the C4′ phosphate atom of MPT via a pyrophosphate bond, demonstrating that the protein is a specific CTP:molybdopterin cytidylyltransferase. Using a fully defined in vitro assay, we showed that MocA, Mo-MPT, CTP, and MgCl2 are required and sufficient for MCD biosynthesis in vitro. Mn2+ was able to replace Mg2+ to some extend in our assay. The activity of MocA is specific for CTP as other nucleotides such as ATP or GTP were not converted by MocA. The apparent Km for CTP was determined to be in the low micromolar range. A 1:1 binding ratio of MocA to Mo-MPT and CTP was determined. The binding affinity for the two substrates Mo-MPT and CTP were the highest for MocA. However, the presence of CTP resulted in a decrease in the binding affinity for Mo-MPT possibly because of the absence of Mg2+ in our assays. In the defined in vitro system using purified compounds, a turnover number of 0.37 ± 0.01 min−1 was obtained. In contrast, a fully defined in vitro system for MGD biosynthesis using E. coli MobA, MPT, GTP, and MgCl2 produced significant amounts of MGD only after an incubation for 3 h at 37 °C (15). Thus, in vitro some factors seem to be missing for the efficient synthesis of either MCD or MGD at a physiological turnover rate. The presence of an acceptor protein, like the appropriate apoprotein or a Moco binding chaperone, might speed up the turnover rate of the reaction (29). Unfortunately, we could not analyze MCD insertion into YagTSR, as apo-YagTSR was unstable, and the YagR subunit was partially lost during purification. We showed that MocA was also able to convert MPT to MCD in the absence of molybdate; however, with only one catalytic turnover. The addition of molybdate after one turnover gave rise to a higher MCD production, showing that MPT-CMP remains bound to MocA in the absence of molybdate and, thus, results in a product inhibition of the reaction. We believe that a catalytic turnover for MocA is only obtained when Mo-MCD is produced. This is in accordance with E. coli MobA, where it was shown that metal chelation to MPT strictly has to precede dinucleotide attachment. However, our observation that molybdate could be inserted into MCD when bound to MocA does not rule out the possibility that molybdate is added after dinucleotide attachment to MPT. Under our in vitro conditions, unphysiological high concentrations of molybdate were required for the release of MCD from MocA. In vivo this reaction might be performed under physiological molybdate concentrations, e.g. by a specific molybdate insertase. Under physiological molybdate concentrations, the E. coli MoeA protein was shown to mediate molybdenum ligation to MPT-AMP produced by MogA. Because MocA produces MPT-CMP, it remains to be determined whether MoeA can also mediate molybdenum ligation to MPT-CMP under physiological molybdate concentrations. Because only a very low amount of MPT-CMP was determined on MocA expressed in an E. coli modC mutant strain, this might suggest that in the cell an additional control mechanism exists to ensure that MCD is only efficiently produced from Mo-MPT rather that MPT and that MoeA inserts molybdate only into MPT-AMP.
The growth phenotypes in medium containing adenine of different mutant strains in the putative E. coli XDHs xdhABC and xdhD in comparison to the mocA mutant strain showed that both proteins also bind the MCD form of Moco. Thus, E. coli contains three proteins that bind MCD, the aldehyde oxidoreductase YagTSR and the putative XDHs XdhABC and XdhD. Another well characterized MCD-containing enzyme is CO dehydrogenase from Hydrogenophaga pseudoflava. It has been reported previously that cell extracts from H. pseudoflava grown in the absence of molybdate did not produce MCD (28). In addition, CO dehydrogenase expressed under these conditions was devoid of MCD or MPT, whereas the protein still contained stoichiometric amounts of cytidine moieties, like CDP, dCDP, CMP, dCMP, CTP, or dCTP (28). These observations can now be explained by our results with MocA. Assuming that H. pseudoflava also contains a MocA homologue, MPT-CMP is not produced by MocA in the absence of molybdate and, thus, is not inserted into CO dehydrogenase.
Analysis of genome sequence data from different organisms revealed that bacteria like Aeromonas hydrophila, Photobacterium profundum, Shewanella halifaxensis, Shigella sonnei, or Bacillus subtilis also contain two genes, encoding for a MocA and a MobA homologue (KEGG GENES data base). Amino acids sequence comparisons of putative MocA and MobA proteins from different organisms showed conserved differences between both proteins (supplemental Fig. S1). The most significant differences between MocA and MobA are observed in two conserved motifs at the C-terminal domain of the proteins. The co-structure of MobA with bound GTP showed that the nucleotide binding site is located at the N terminus of the protein (30). In this domain MobA proteins share a characteristic and highly conserved LAGG amino acid sequence motif. This sequence is altered in MocA homologues to a conserved (T/P/L)AAG motif. The crystal structure of MobA also showed that the conserved Gly-82 of a highly conserved GP(L/M)(A/G)G motif is located in vicinity of the guanine ring, however, without direct contact to the base (30). In MocA homologues this amino acid sequence is exchanged for a conserved G(L/M/Q)X(S/T)S motif. Because the crystal structure of MobA with bound MPT or MGD is not available, it has only been speculated which residues are involved in binding of the MPT moiety. MobA residues Asn-180 and Asn-182 are believed to coordinate O4 and N5 of MPT (30). These residues are replaced by aspartates in MocA homologues. We speculate that these conserved amino acid exchanges might determine the specificity of MocA and MobA for CTP and GTP, respectively, and might also distinguish between MPT and Mo-MPT. Future studies including site-directed mutagenesis will give more insights into the specificity of the two nucleotide transferases in E. coli.
Supplementary Material
Acknowledgments
We thank Julia Lücke for help with the MocA experiments. We thank KV Rajagopalan (Duke University) for helpful discussions. We also thank Dennis Dean (Virginia Tech) for the ongoing support of our research.
This work was supported by Deutsche Forschungsgemeinschaft Grant LE1171/3-3. The exchange of researchers among laboratories involved in the work was funded by the DAAD PROCOPE program (to C. I.-N. and S. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- Moco
- molybdenum cofactor
- MPT
- molybdopterin
- MGD
- molybdopterin guanine dinucleotide cofactor
- MCD
- molybdopterin cytosine dinucleotide cofactor
- hSO-MD
- human sulfite oxidase Moco domain
- NTA
- nitrilotriacetic acid
- FAD
- flavin adenine dinucleotide
- HPLC
- high performance liquid chromatography
- XDH
- xanthine dehydrogenase.
REFERENCES
- 1.Rajagopalan K. V., Johnson J. L. (1992) J. Biol. Chem. 267, 10199–10202 [PubMed] [Google Scholar]
- 2.Rajagopalan K. V. (1996) in Escherichia coli and Salmonella. Cellular and Molecular Biology (Neidhardt F. C. ed) pp. 674–679, American Society for Microbiology, Washington, DC [Google Scholar]
- 3.Hänzelmann P., Schindelin H. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12870–12875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wuebbens M. M., Rajagopalan K. V. (1995) J. Biol. Chem. 270, 1082–1087 [DOI] [PubMed] [Google Scholar]
- 5.Gutzke G., Fischer B., Mendel R. R., Schwarz G. (2001) J. Biol. Chem. 276, 36268–36274 [DOI] [PubMed] [Google Scholar]
- 6.Pitterle D. M., Johnson J. L., Rajagopalan K. V. (1993) J. Biol. Chem. 268, 13506–13509 [PubMed] [Google Scholar]
- 7.Joshi M. S., Johnson J. L., Rajagopalan K. V. (1996) J. Bacteriol. 178, 4310–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols J. D., Rajagopalan K. V. (2005) J. Biol. Chem. 280, 7817–7822 [DOI] [PubMed] [Google Scholar]
- 9.Nichols J. D., Xiang S., Schindelin H., Rajagopalan K. V. (2007) Biochemistry 46, 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson J. L., Bastian N. R., Rajagopalan K. V. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3190–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson J. L., Indermaur L. W., Rajagopalan K. V. (1991) J. Biol. Chem. 266, 12140–12145 [PubMed] [Google Scholar]
- 12.Palmer T., Vasishta A., Whitty P. W., Boxer D. H. (1994) Eur. J. Biochem. 222, 687–692 [DOI] [PubMed] [Google Scholar]
- 13.McLuskey K., Harrison J. A., Schuttelkopf A. W., Boxer D. H., Hunter W. N. (2003) J. Biol. Chem. 278, 23706–23713 [DOI] [PubMed] [Google Scholar]
- 14.Guse A., Stevenson C. E., Kuper J., Buchanan G., Schwarz G., Giordano G., Magalon A., Mendel R. R., Lawson D. M., Palmer T. (2003) J. Biol. Chem. 278, 25302–25307 [DOI] [PubMed] [Google Scholar]
- 15.Temple C. A., Rajagopalan K. V. (2000) J. Biol. Chem. 275, 40202–40210 [DOI] [PubMed] [Google Scholar]
- 16.Sachelaru P., Schiltz E., Brandsch R. (2006) Appl. Environ. Microbiol. 72, 5126–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith S. T., Rajagopalan K. V., Handler P. (1967) J. Biol. Chem. 242, 4108–4117 [PubMed] [Google Scholar]
- 18.Schübel U., Kraut M., Mörsdorf G., Meyer O. (1995) J. Bacteriol. 177, 2197–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hille R. (2002) Trends Biochem. Sci. 27, 360–367 [DOI] [PubMed] [Google Scholar]
- 20.Neumann M., Mittelstädt G., Iobbi-Nivol C., Saggu M., Lendzian F., Hildebrandt P., Leimkühler S. (2009) FEBS J. 276, 2762–2774 [DOI] [PubMed] [Google Scholar]
- 21.Loschi L., Brokx S. J., Hills T. L., Zhang G., Bertero M. G., Lovering A. L., Weiner J. H., Strynadka N. C. (2004) J. Biol. Chem. 279, 50391–50400 [DOI] [PubMed] [Google Scholar]
- 22.Xi H., Schneider B. L., Reitzer L. (2000) J. Bacteriol. 182, 5332–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine R. A., Taylor M. W. (1982) J. Bacteriol. 149, 923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogel H. J., Bonner D. M. (1956) J. Biol. Chem. 218, 97–106 [PubMed] [Google Scholar]
- 25.Miller J. H. (1972) Experiments in Molecular Genetics, pp. 201–205, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 26.Temple C. A., Graf T. N., Rajagopalan K. V. (2000) Arch. Biochem. Biophys. 383, 281–287 [DOI] [PubMed] [Google Scholar]
- 27.Neumann M., Schulte M., Jünemann N., Stöcklein W., Leimkühler S. (2006) J. Biol. Chem. 281, 15701–15708 [DOI] [PubMed] [Google Scholar]
- 28.Hänzelmann P., Meyer O. (1998) Eur. J. Biochem. 255, 755–765 [DOI] [PubMed] [Google Scholar]
- 29.Genest O., Neumann M., Seduk F., Stöcklein W., Méjean V., Leimkühler S., Iobbi-Nivol C. (2008) J. Biol. Chem. 283, 21433–21440 [DOI] [PubMed] [Google Scholar]
- 30.Lake M. W., Temple C. A., Rajagopalan K. V., Schindelin H. (2000) J. Biol. Chem. 275, 40211–40217 [DOI] [PubMed] [Google Scholar]
- 31.Palmer T., Santini C. L., Iobbi-Nivol C., Eaves D. J., Boxer D. H., Giordano G. (1996) Mol. Microbiol. 20, 875–884 [DOI] [PubMed] [Google Scholar]
- 32.Miller J. B., Scott D. J., Amy N. K. (1987) J. Bacteriol. 169, 1853–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datsenko K. A., Wanner B. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Mol. Syst. Biol. 2, 2006.0008, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.