Abstract
The orphan G-protein-coupled receptor GPR109B is the result of a recent gene duplication of the nicotinic acid and ketone body receptor GPR109A being found in humans but not in rodents. Like GPR109A, GPR109B is predominantly expressed in adipocytes and is supposed to mediate antilipolytic effects. Here we show that GPR109B serves as a receptor for the β-oxidation intermediate 3-OH-octanoic acid, which has antilipolytic activity on human but not on murine adipocytes. GPR109B is coupled to Gi-type G-proteins and is activated by 2- and 3-OH-octanoic acid with EC50 values of about 4 and 8 μm, respectively. Interestingly, 3-OH-octanoic acid plasma concentrations reach micromolar concentrations under conditions of increased β-oxidation rates, like in diabetic ketoacidosis or under a ketogenic diet. These data suggest that the ligand receptor pair 3-OH-octanoic acid/GPR109B mediates in humans a negative feedback regulation of adipocyte lipolysis to counteract prolipolytic influences under conditions of physiological or pathological increases in β-oxidation rates.
Triacylglycerols stored in the white adipose tissue serve as the major energy reserve in higher eukaryotes (1). Although they are constantly turned over by lipolysis and re-esterification, their mobilization and storage are precisely balanced by various hormones and other factors depending on the nutritional state (2). The net rate of lipolysis is increased during fasting or periods of increased energy demand. Fatty acids generated via lipolysis undergo β-oxidation in the muscle and liver to serve directly as a source of energy or as a precursor for ketone bodies (3). The major intracellular regulator of lipolysis is cyclic AMP, which stimulates cAMP-dependent kinase to activate lipolytic enzymes (2, 4–6). This lipolytic pathway is induced, for example, via β-adrenergic receptors that couple to the G-protein Gs and thereby stimulate adenylyl cyclase (7, 8). To adjust lipolysis at the appropriate rate, the effects of prolipolytic stimuli are balanced by various antilipolytic influences. Besides insulin, which promotes the degradation of cAMP via activation of phosphodiesterase 3B (2, 5, 7), several antilipolytic stimuli decrease cAMP levels by activation of Gi-coupled receptors, which mediate an inhibition of adenylyl cyclase (5, 8). One of these receptors, GPR109A, has recently been shown to mediate the anti-lipolytic effects of high concentrations of the ketone body 3-OH-butyrate thereby providing a negative feedback mechanism during fasting (9, 10). GPR109A also binds nicotinic acid (11–13) and mediates the anti-lipolytic effects of this anti-dyslipidemic drug (12).
GPR109B, a close relative of GPR109A, is the result of a recent gene duplication being present in humans but not in rodents and most other mammals (14). GPR109B differs from GPR109A in an extended C-terminal tail as well as in 16 amino acids (11, 13). Despite its high homology to GPR109A, GPR109B does not bind nicotinic acid or 3-OH-butyrate with reasonable affinity (10, 11, 13). Because GPR109A and GPR109B have very similar expression patterns (11, 13, 15) and are likely to have the same basic signaling properties, agonists of GPR109B are expected to have physiological and pharmacological effects comparable with those of the GPR109A agonist 3-OH-butyrate and nicotinic acid, respectively. Recently, several synthetic compounds as well as various aromatic d-amino acids have been shown to be selective agonists at GPR109B (16–18). However, endogenous physiological anti-lipolytic ligands of GPR109B are unknown.
In this study we tested endogenous carboxylic acids for their ability to activate GPR109B. We found that the fatty acid β-oxidation intermediate 3-OH-octanoic acid is a highly specific agonist of GPR109B. 3-OH-octanoic acid has anti-lipolytic activity, and its plasma concentration in humans reflects the β-oxidation flux. Our data suggest that 3-OH-octanoic acid and GPR109B mediate a negative feedback regulation of adipocyte lipolysis.
EXPERIMENTAL PROCEDURES
Materials
N6-(l-2-Phenylisopropyl)adenosine ((R)-PIA),3 (−)-isoproterenol hydrochloride, collagenase type II, and all carboxylic acid derivatives were obtained from Sigma except for 3-OH-carboxylic acids, which were from Epsilon Chimie. Pertussis toxin and adenosine deaminase were obtained from Calbiochem.
Cell Transfection and Determination of [Ca2+]i
CHO-K1 cells stably transfected with a calcium-sensitive bioluminescent fusion protein consisting of aequorin and green fluorescent protein (29) were seeded in 96-well plates and were transfected with the indicated cDNAs or control DNA (60 ng/well) using FuGENE 6 reagent (Roche Applied Science). Determination of [Ca2+]i was performed by using a luminometer plate reader (Luminoskan Ascent, Labsystems) as described (12, 19).
Neutrophils isolated from whole blood using a Histopaque gradient according to the manufacturer's instructions (Sigma) were washed and loaded with fluo-4 (10 μm) for 15 min at 37 °C. After washing, calcium mobilization was determined with a fluorescence microplate reader (Flexstation 3, MDS Analytical Technologies) in 100-μl samples containing 106 cells/ml.
GTPγS Binding
To analyze receptor-mediated G-protein activation, plasma membranes were prepared from HEK-293T cells transfected with cDNAs encoding the indicated receptors, and the binding of [35S]GTPγS was determined in the absence and presence of the indicated ligands. Briefly, 50 μg of membrane protein were incubated for 60 min at 25 °C in a total volume of 100 μl of buffer containing 100,000 cpm (0.4 nm) [35S]GTPγS, 1 mm EDTA, 5 mm MgCl2, 1 mm dithiothreitol, 100 mm NaCl, 10 μm GDP, and 50 mm Tris-HCl, pH 7.4. Incubations were terminated by filtration over GF/B glass fiber filters (Whatman) followed by two 4-ml washes with ice-cold buffer (50 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 0.02% CHAPS). Bound GTPγS was determined by counting the filters in a liquid scintillation counter (Beckman).
Mutagenesis, Determination of cAMP Levels, and Phosphorylation of ERK
Generation of mutated versions of GPR109A and GPR109B receptors and determination of ERK phosphorylation and cAMP levels have been described (12, 19).
Preparation of Mouse and Human Adipocytes and Determination of Lipolysis
Adipocytes from mouse epididymal fat or from human visceral fat were isolated according to the method of Rodbell (30). Isolated adipocytes were incubated in the presence or absence of the indicated ligands and/or isoproterenol at 37 °C in a shaking water bath. After 2 h medium samples were taken, and glycerol release from isolated adipocytes was measured using a colorimetric assay kit (Randox).
Analysis of 3-OH-Octanoic Acid and 3-OH-Butyrate Levels
Human Plasma and Fat Tissue Samples
Patients with diabetic ketoacidosis were from the general hospital population, and the samples were de-identified excess volumes from samples that had been drawn for the measurement of 3-OH-butyrate. Patients under ketogenic diet were treated for severe drug-refractory epilepsy of unknown cause (patient 1) or for epilepsy because of glucose transporter type 1 deficiency (patient 2). Ketogenic diet was performed according to a standard protocol (31). Visceral fat tissue samples were collected from patients undergoing abdominal surgery. The use of human tissue or blood for the analysis was approved by the local ethics committee (University of Heidelberg and Technical University Munich, Germany), and written informed consent was obtained from the patients or their parents.
RESULTS
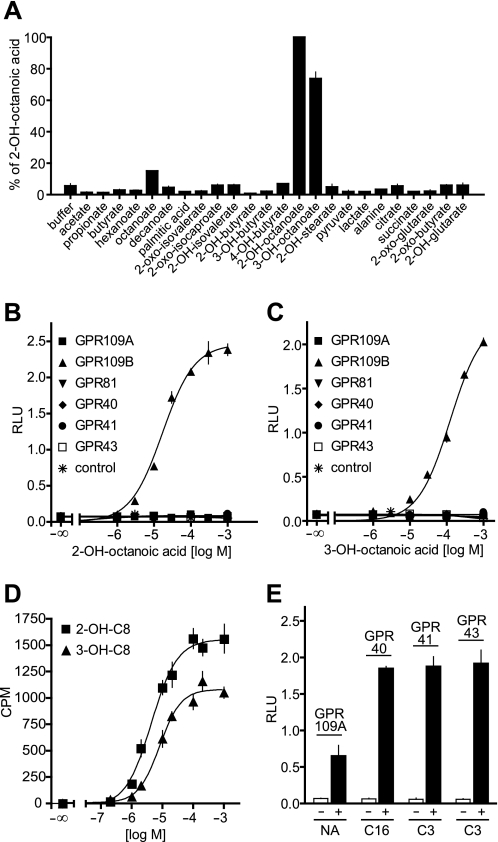
The different affinity of GPR109A and GPR109B for nicotinic acid has been shown to be due to different amino acid residues especially in the first and second extracellular loop of the receptor (19). However, an arginine residue at position 111, which is supposed to function as an anchor for the carboxylic acid ligands of GPR109A, is conserved in GPR109A and GPR109B. Given the fact that synthetic ligands of GPR109B are characterized by a carboxylic acid residue (13, 16, 17), we tested a variety of naturally occurring carboxylic acids for their ability to activate GPR109B (Fig. 1A). Using a heterologous expression system employing the promiscuous G-protein α-subunit Gα15 (20), we found that 2- and 3-OH-octanoic acid activated GPR109B in a dose-dependent manner (Fig. 1, A–C). The GPR109B-dependent activation of endogenous G-proteins by 2- and 3-OH-octanoic acid as determined by GTPγS binding occurred with EC50 values of 4 and 8 μm, respectively (Fig. 1D). To test whether this activation was specific for GPR109B, we tested the activity of 2- and 3-OH-octanoic acid on closely related receptors like GPR109A and GPR81 as well as on the fatty acid receptors FFA1 (GPR40), FFA2 (GPR43), and FFA3 (GPR41). Although GPR109A and the free fatty acid receptors were activated by nicotinic acid and free fatty acids, respectively (Fig. 1E), none of the receptors responded to 2- or 3-OH-octanoic acid at concentrations up to 1 mm (Fig. 1, B and C).
FIGURE 1.
Identification and characterization of GPR109B agonists. A, effects of naturally occurring carboxylic acids (1 mm) on [Ca2+]i in CHO-K1 cells expressing GPR109B and a promiscuous G-protein α-subunit. Relative light units (RLU) were expressed as percent of the maximal effect obtained with 2-OH-octanoic acid. B, C, and E, effect of increasing concentrations of 2-OH-octanoic acid (B) and 3-OH-octanoic acid (C) or of 10 μm nicotinic acid (NA), 30 μm palmitate (C16), or 300 μm propionate (C3) on [Ca2+]i in CHO-K1 cells transfected with cDNA encoding a promiscuous G-protein α-subunit alone or together with empty vector (control) or vectors encoding GPR109A, GPR109B, GPR81, GPR40, GPR41, or GPR43 (E). D, effect of 2-OH-octanoic acid (2-OH-C8) and 3-OH-octanoic acid (3-OH-C8) on the binding of GTPγS in membranes prepared from HEK-293 cells transfected with a vector encoding GPR109B. Shown are mean values ± S.D. of at least three independently performed experiments.
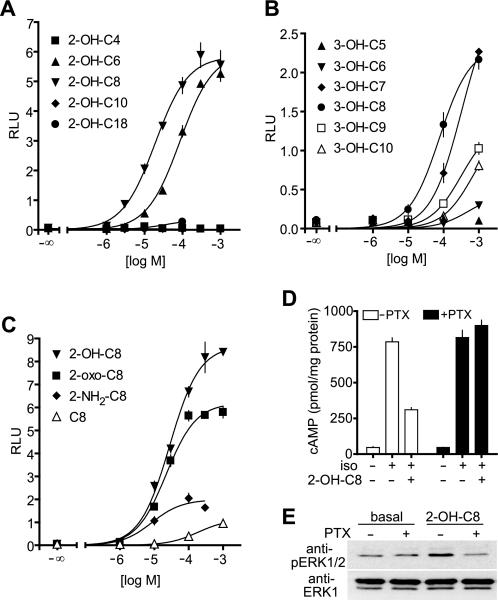
An increase as well as a decrease in the chain length of 2- and 3-OH-octanoic acids resulted in a strong reduction of activity (Fig. 2, A and B), and a substitution in the 2- or 3-position of octanoic acid appeared to be crucial for the activity as octanoic acid itself had hardly any effect on GPR109B. An amino group instead of the 2-OH group severely reduced the potency on GPR109B, whereas an oxo group instead of the hydroxyl group did not change the potency but resulted in reduction in efficacy (Fig. 2C). Thus, GPR109B is specifically activated by medium-chain carboxylic acids carrying an oxo or hydroxy group in the 2- or 3-position.
FIGURE 2.
Structure activity relationship of GPR109B activation by hydroxy/oxo-carboxylic acids and involvement of Gi-type G-proteins. A–C, effect of increasing concentrations of 2-hydroxylated (A) and 3-hydroxylated (B) carboxylic acids with the indicated chain lengths or of octanoic acid and derivatives with the indicated substitutions in the 2- or 3-position (C) on [Ca2+]i in CHO-K1 cells expressing GPR109B. Shown are mean values ± S.D. D and E, CHO-K1 cells cotransfected with GPR109B and β2-adrenergic receptor were untreated or treated overnight with 100 ng/ml pertussis toxin (PTX). Cells were then incubated in the absence or presence of 200 μm 2-OH-octanoic acid (2-OH-C8) and/or 1 μm isoproterenol (iso), and cAMP levels were determined as described under “Experimental Procedures” (D). Shown are mean values of triplicates ± S.D. Alternatively, ERK1/2 phosphorylation was determined in cell lysates separated by SDS-PAGE and immunoblotted with anti-phospho-ERK1/2 antibodies (anti-pERK1/2) (E). RLU, relative light units.
The analysis of GPR109B ligands was performed in cells expressing the promiscuous G-protein α-subunit Gα15, which couples receptors to phospholipase C β-isoforms. In the absence of Gα15, activation of GPR109B by 2-OH-octanoic acid had no effect on [Ca2+]i. However, GPR109B mediated 2-OH-octanoic acid-induced activation of ERK1/2 and inhibition of isoproterenol-stimulated adenylyl cyclase activity in a pertussis toxin-sensitive manner (Fig. 2, D and E). This indicates that GPR109B is coupled to Gi-type G-proteins as shown previously for GPR109A (11–13).
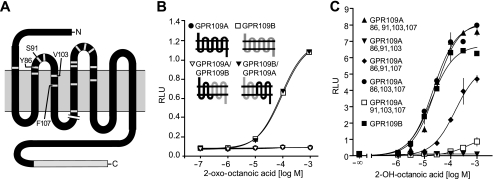
In addition to the extended C terminus, GPR109B differs from GPR109A in only 16 amino acids, of which 12 are nonconservative changes that are clustered around extracellular loops 1 and 2 (Fig. 3A). Interestingly, a chimeric receptor in which the first three transmembrane regions, including the first extracellular loop, were from GPR109B and the C-terminal four transmembrane regions, including the second extracellular loop, were from GPR109A (GPR109B/GPR109A; Fig. 3B) was activated by 2-OH-octanoic acid with the same potency and efficacy as GPR109B, whereas the GPR109A/GPR109B chimera was unresponsive (Fig. 3B). This clearly indicates that GPR109B-specific amino acid residues in the N-terminal half of the receptor mediate the specific activation of GPR109B by 2-OH-octanoic acid. We therefore introduced GPR109B-specific amino acids into the N-terminal half of GPR109A and found that GPR109A (N86Y,W91S,M103V,L107F) gained the ability to be activated by 2-OH-octanoic acid (Fig. 3C). Although reintroduction of the GPR109A-specific tryptophan in position 91 did not alter the responsiveness of the GPR109A (N86Y,W91S,M103V,L107F) mutant, reversion of each of the other three residues into the GPR109A-specific amino acid resulted in considerable or complete loss of activation (Fig. 3C). Thus, we conclude that a tyrosine residue in position 86 as well as valine 103 and phenylalanine 107 in the third transmembrane region are critically involved in forming the selective binding site for OH-octanoic acid in GPR109B. Mutation of the conserved arginine residue in position 111 to alanine completely abrogated the activation of GPR109B by OH-octanoic acid (data not shown), suggesting that similar to GPR109A arginine 111 in the third transmembrane helix functions as an important anchor point for the carboxylic acid ligands of both receptors (19).
FIGURE 3.
Structural requirements for specific activation of GPR109B by 2- and 3-OH-octanoic acid. A, secondary structure of GPR109B. Gray lines indicate the position of the 16 amino acids that are different from GPR109A. Shown also is the 24-amino acid C-terminal extension of GPR109B when compared with GPR109A. B and C, CHO-K1 cells expressing GPR109A, GPR109B, the indicated GPR109A/GPR109B chimeric receptors (B), or the indicated mutants of GPR109A (C) were incubated with increasing concentrations of 2-OH-octanoic acid. RLU, relative light units. Shown are mean values ± S.D. of at least three independently performed experiments. The indicated mutants are as follows: GPR109A 86, 91, 103, 107, GPR109A(N86Y, W91S, M103V, L107F); GPR109A 86, 91, 103, GPR109A(N86Y, W91S, M103V); GPR109A 86, 91,107, GPR109A(N86Y, W91S, L107F); GPR109A 86, 103, 107, GPR109A(N86Y, M103V, L107F), and GPR109A 91, 103, 107, GPR109A(W91S, M103V, L107F).
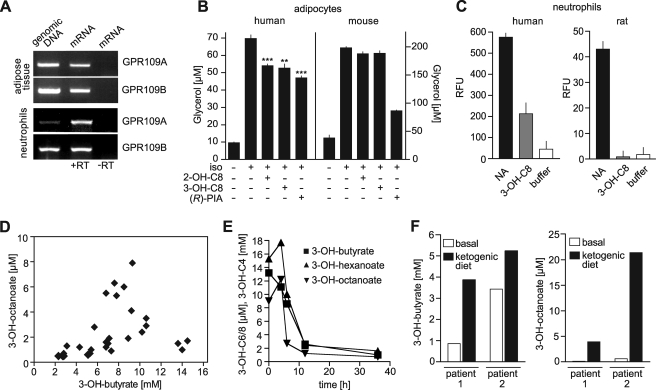
GPR109A and GPR109B appear to have very similar expression patterns (11, 13, 15). Consistent with this, we found GPR109B coexpressed with GPR109A both in human adipose tissue and in neutrophils (Fig. 4A). However, in contrast to GPR109A, GPR109B is encoded only in the human but not in the genome of rodents. Although the adenosine receptor agonist (R)-PIA induced an inhibition of lipolysis both in human and mouse adipocytes, OH-octanoic acids were able to inhibit lipolysis only in human fat tissue (Fig. 4B). Similarly, nicotinic acid was able to induce increases in intracellular free [Ca2+] both in human and rat neutrophils, although only human cells responded to 3-OH-octanoic acid (Fig. 4C). This is consistent with the idea that the human receptor GPR109B is responsible for the antilipolytic and other effects of OH-octanoic acids in human cells.
FIGURE 4.
Effect of OH-octanoic acids on lipolytic activity of human adipocytes and plasma levels of 3-OH-octanoic acid under different conditions. A, expression of GPR109A and GPR109B in human adipose tissue and neutrophils as determined by reverse transcription (RT)-PCR. B, white adipocytes were isolated from human or mouse, and glycerol release was determined after incubation without or with 50 nm (−)-isoproterenol (iso) and 500 μm 2-OH-octanoic acid (2-OH-C8), 500 μm 3-OH-octanoic acid (3-OH-C8), or 1 μm (R)-PIA. Shown are mean values of triplicates ± S.D.; **, p < 0.01; ***, p < 0.001 (compared with isoproterenol alone). C, effect of 100 μm nicotinic acid (NA) or 3-OH-octanoic acid (3-OH-C8) on [Ca2+]i in human or rat neutrophils. RFU, relative fluorescence units. D, plasma 3-OH-butyrate and 3-OH-octanoic acid levels in patients with various levels of diabetic ketoacidosis. Each point in the graph represents one patient. E, 3-OH-butyrate, 3-OH-octanoic, and 3-OH-hexanoic acid levels in a patient with diabetic ketoacidosis before (time 0) and after treatment. F, 3-OH-butyrate (left) and 3-OH-octanoic acid plasma concentrations (right) in two patients before (basal) and 8 (patient 1) or 40 days (patient 2) after initiation of a ketogenic diet.
Although it is unclear whether 2-OH-octanoic acid plays a role in human metabolism, 3-OH-octanoic acid can be found at micromolar levels in the plasma of patients with fatty acid β-oxidation disorders (21, 22). To test whether an increase in the rate of β-oxidation results in 3-OH-octanoic acid plasma levels sufficient to activate GPR109B, we analyzed patients with acute diabetic ketoacidosis (Fig. 4D). 21 of 28 ketoacidotic patients showed strongly increased 3-OH-octanoic acid levels ranging from 1 to 8 μm. In most patients, the increase in 3-OH-octanoic acid levels was accompanied by an increase in 3-OH-butyrate plasma concentrations (Fig. 4D). Upon treatment of the diabetic ketoacidosis, the elevated 3-OH-octanoic acid as well as 3-OH-hexanoic acid plasma levels dropped in parallel with the elevated 3-OH-butyrate plasma concentration (Fig. 4E). Also patients who received a ketogenic diet, which results in an increased rate of β-oxidation (23), showed strongly elevated plasma levels of 3-OH-butyrate as well as of 3-OH-octanoic acid (Fig. 4F).
DISCUSSION
The utilization of fatty acids as energy source requires their lipolytic formation from triacylglycerols stored in adipocytes and their subsequent degradation to acetyl CoA via β-oxidation cycles in mitochondria. Each fatty acid oxidation cycle involves the formation of various intermediates, including 3-OH fatty acids (3). In particular, medium chain 3-OH fatty acids have been shown to reach micromolar plasma levels under conditions of defective fatty acid oxidation or increased β-oxidation rates (21, 22, 24) (see Fig. 4). Here we show that 3-OH-octanoic acid is an endogenous ligand of the orphan receptor GPR109B, a G-protein-coupled receptor expressed predominantly in adipocytes of humans but not rodents and closely related to the nicotinic acid and ketone body receptor GPR109A. GPR109B is coupled to Gi-type G-proteins mediating an anti-lipolytic effect in adipocytes. We show that 3-OH-octanoic acid inhibits lipolysis and that the 3-OH-octanoic acid plasma concentrations are increased to levels sufficient to activate GPR109B under conditions of increased rates of fatty acid oxidation both under physiological and pathophysiological conditions. Thus, 3-OH-octanoic acid appears to serve as a negative feedback mediator via activation of the anti-lipolytic receptor GPR109B on adipocytes to counteract prolipolytic stimuli in situations of increased fatty acid oxidation.
GPR109A is not activated by 3-OH-octanoic acid, whereas the GPR109A ligands 3-OH-butyrate and nicotinic acid have no activity on GPR109B. There are only a few amino acid differences between GPR109A and GPR109B, which can account for the different ligand selectivity. By systematic mutagenesis, we identified Tyr-86, Val-103, and Phe-107 as the most critical residues for the specific binding of 2-OH-octanoic acid to GPR109B. This is strongly indicated by the fact that a GPR109A mutant in which the corresponding residues are changed into those of GPR109B (GPR109A(N86Y,M103V,L107F)) is activated by 2-OH-octanoic acid with the same potency as GPR109B. The subtle difference in positions 103 and 107 of transmembrane helix 3, a change from methionine to valine and from leucine to phenylalanine, may be required to accommodate the hydroxyl group. The longer side chain of the tyrosine residue in position 86 of GPR109B may stabilize the binding of the aliphatic chain of 2-OH-octanoic acid, although it would interfere with the more bulky ring structure of nicotinic acid.
2-OH fatty acids have been shown to be abundant in mammalian nervous and epidermal tissues and to be components of sphingolipids (25, 26). In addition, several enzymes have been described that function as 2-OH acid oxidases and are expressed in various human tissues (27). However, the potential role of 2-OH fatty acids in mammalian metabolism is unclear, and 2-OH fatty acid concentrations high enough to activate GPR109B have not been described.
In contrast, 3-OH fatty acids are produced during β-oxidation of fatty acids in various mammalian tissues (3). Under basal conditions, the plasma concentrations of 3-hydroxylated medium-chain fatty acids are below 0.4 μm (24, 28). Although the plasma concentrations may underestimate local levels of 3-OH-octanoic acid, these concentrations are most likely too low to induce an activation of GPR109B. However, various disorders due to defects in mitochondrial fatty acid β-oxidation are characterized by elevated 3-OH-octanoic acid levels. In patients with long-chain, medium-chain, and short-chain acyl-CoA dehydrogenase defects, 3-OH-octanoic acid plasma concentrations as high as 10 μm have been reported (21, 22). It has been noted that independently of the block in free fatty acid oxidation, plasma levels of medium chain 3-OH fatty acids, in particular 3-OH-hexanoic and 3-OH-octanoic acid, are usually increased (21). Thus, they may be indicators of increased free fatty acid β-oxidation. Consistent with this, in patients suffering from diabetic ketoacidosis with strongly increased β-oxidation rates, 3-OH-octanoic acid plasma levels are in the micromolar range and correlate with increased 3-OH-butyrate plasma levels (24) (see Fig. 4D). Thus, under pathological conditions with increased β-oxidation flux, 3-OH-octanoic acid plasma concentrations reach levels that activate GPR109B. To analyze their potential physiological role, we determined 3-OH-octanoic acid levels in patients that received a ketogenic diet. Under this condition, which goes along with strongly elevated β-oxidation rates (23), a robust increase in 3-OH-octanoic acid plasma concentrations reaching up to 20 μm could be observed.
These data clearly indicate that increased 3-OH-octanoic acid plasma levels strongly correlate with increased rates of fatty acid β-oxidation both under physiological and pathophysiological conditions. Under conditions of high β-oxidation rates 3-OH-octanoic acid levels reach plasma concentrations in the lower micromolar range, a concentration sufficient to activate GPR109B. GPR109B is expressed on adipocytes where it couples via Gi-type G-proteins in an inhibitory fashion to adenylyl cyclase and thereby mediates anti-lipolytic effects. We think that GPR109B has evolved in higher primates to sense elevated concentrations of 3-OH-octanoic acid as an indicator of high β-oxidation rates, which by activation of GPR109B would inhibit free fatty acid release and thereby lower the substrate supply for β-oxidation. Therefore, GPR109B may mediate a negative feedback loop that counterbalances prolipolytic stimuli in situations of strongly increased lipolysis and fatty acid oxidation to avoid an excessive lipolysis resulting in the waste of energy.
Supplementary Material
Acknowledgments
We gratefully acknowledge K. Meyer and P. Feyh for excellent technical assistance and R. LeFaucheur for expert secretarial work.
This work was supported in part by a grant from the German Research Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental text and additional references.
- (R)-PIA
- N6-(l-2-phenylisopropyl)adenosine
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- ERK
- extracellular signal-regulated kinase.
REFERENCES
- 1.Gesta S., Tseng Y. H., Kahn C. R. (2007) Cell 131, 242–256 [DOI] [PubMed] [Google Scholar]
- 2.Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. (2007) Annu. Rev. Nutr. 27, 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton S., Bartlett K., Pourfarzam M. (1996) Biochem. J. 320, 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ducharme N. A., Bickel P. E. (2008) Endocrinology 149, 942–949 [DOI] [PubMed] [Google Scholar]
- 5.Langin D. (2006) Pharmacol. Res. 53, 482–491 [DOI] [PubMed] [Google Scholar]
- 6.Zechner R., Strauss J. G., Haemmerle G., Lass A., Zimmermann R. (2005) Curr. Opin. Lipidol. 16, 333–340 [DOI] [PubMed] [Google Scholar]
- 7.Carmen G. Y., Víctor S. M. (2006) Cell. Signal. 18, 401–408 [DOI] [PubMed] [Google Scholar]
- 8.Granneman J. G., Moore H. P. (2008) Trends Endocrinol. Metab. 19, 3–9 [DOI] [PubMed] [Google Scholar]
- 9.Senior B., Loridan L. (1968) Nature 219, 83–84 [DOI] [PubMed] [Google Scholar]
- 10.Taggart A. K., Kero J., Gan X., Cai T. Q., Cheng K., Ippolito M., Ren N., Kaplan R., Wu K., Wu T. J., Jin L., Liaw C., Chen R., Richman J., Connolly D., Offermanns S., Wright S. D., Waters M. G. (2005) J. Biol. Chem. 280, 26649–26652 [DOI] [PubMed] [Google Scholar]
- 11.Soga T., Kamohara M., Takasaki J., Matsumoto S., Saito T., Ohishi T., Hiyama H., Matsuo A., Matsushime H., Furuichi K. (2003) Biochem. Biophys. Res. Commun. 303, 364–369 [DOI] [PubMed] [Google Scholar]
- 12.Tunaru S., Kero J., Schaub A., Wufka C., Blaukat A., Pfeffer K., Offermanns S. (2003) Nat. Med. 9, 352–355 [DOI] [PubMed] [Google Scholar]
- 13.Wise A., Foord S. M., Fraser N. J., Barnes A. A., Elshourbagy N., Eilert M., Ignar D. M., Murdock P. R., Steplewski K., Green A., Brown A. J., Dowell S. J., Szekeres P. G., Hassall D. G., Marshall F. H., Wilson S., Pike N. B. (2003) J. Biol. Chem. 278, 9869–9874 [DOI] [PubMed] [Google Scholar]
- 14.Zellner C., Pullinger C. R., Aouizerat B. E., Frost P. H., Kwok P. Y., Malloy M. J., Kane J. P. (2005) Hum. Mutat. 25, 18–21 [DOI] [PubMed] [Google Scholar]
- 15.Knowles H. J., te Poele R. H., Te Poole R., Workman P., Harris A. L. (2006) Biochem. Pharmacol. 71, 646–656 [DOI] [PubMed] [Google Scholar]
- 16.Semple G., Skinner P. J., Cherrier M. C., Webb P. J., Sage C. R., Tamura S. Y., Chen R., Richman J. G., Connolly D. T. (2006) J. Med. Chem. 49, 1227–1230 [DOI] [PubMed] [Google Scholar]
- 17.Skinner P. J., Cherrier M. C., Webb P. J., Sage C. R., Dang H. T., Pride C. C., Chen R., Tamura S. Y., Richman J. G., Connolly D. T., Semple G. (2007) Bioorg. Med. Chem. Lett. 17, 6619–6622 [DOI] [PubMed] [Google Scholar]
- 18.Irukayama-Tomobe Y., Tanaka H., Yokomizo T., Hashidate-Yoshida T., Yanagisawa M., Sakurai T. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3930–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunaru S., Lättig J., Kero J., Krause G., Offermanns S. (2005) Mol. Pharmacol. 68, 1271–1280 [DOI] [PubMed] [Google Scholar]
- 20.Offermanns S., Simon M. I. (1995) J. Biol. Chem. 270, 15175–15180 [DOI] [PubMed] [Google Scholar]
- 21.Costa C. G., Dorland L., Holwerda U., de Almeida I. T., Poll-The B. T., Jakobs C., Duran M. (1998) Clin. Chem. 44, 463–471 [PubMed] [Google Scholar]
- 22.Jones P. M., Burlina A. B., Bennett M. J. (2000) J. Inherited Metab. Dis. 23, 745–750 [DOI] [PubMed] [Google Scholar]
- 23.Westman E. C., Mavropoulos J., Yancy W. S., Volek J. S. (2003) Curr. Atheroscler. Rep. 5, 476–483 [DOI] [PubMed] [Google Scholar]
- 24.Jones P. M., Tjoa S., Fennessey P. V., Goodman S. I., Bennett M. J. (2002) Clin. Chem. 48, 176–179 [PubMed] [Google Scholar]
- 25.Hoshi M., Williams M., Kishimoto Y. (1973) J. Neurochem. 21, 709–712 [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto Y., Radin N. S. (1963) J. Lipid Res. 4, 139–143 [PubMed] [Google Scholar]
- 27.Jones J. M., Morrell J. C., Gould S. J. (2000) J. Biol. Chem. 275, 12590–12597 [DOI] [PubMed] [Google Scholar]
- 28.Hagenfeldt L., von Döbeln U., Holme E., Alm J., Brandberg G., Enocksson E., Lindeberg L. (1990) J. Pediatr. 116, 387–392 [DOI] [PubMed] [Google Scholar]
- 29.Baubet V., Le Mouellic H., Campbell A. K., Lucas-Meunier E., Fossier P., Brúlet P. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 7260–7265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodbell M. (1964) J. Biol. Chem. 239, 375–380 [PubMed] [Google Scholar]
- 31.Nordli D. R., Koenigsberger D., Schroeder J., de Vivo D. C. (1992) in The Medical Treatment of Epilepsy (Resor S. R., Kutt H. eds) pp. 455–471, Marcel Dekker, Inc., New York [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.