Abstract
Whereas it is now clear that human bone marrow stromal cells (BMSCs) can be immunosuppressive and escape cytotoxic lymphocytes (CTLs) in vitro and in vivo, the mechanisms of this phenomenon remain controversial. Here, we test the hypothesis that BMSCs suppress immune responses by Fas-mediated apoptosis of activated lymphocytes and find both Fas and FasL expression by primary BMSCs. Jurkat cells or activated lymphocytes were each killed by BMSCs after 72 h of co-incubation. In comparison, the cytotoxic effect of BMSCs on non-activated lymphocytes and on caspase-8(−/−) Jurkat cells was extremely low. Fas/Fc fusion protein strongly inhibited BMSC-induced lymphocyte apoptosis. Although we detected a high level of Fas expression in BMSCs, stimulation of Fas with anti-Fas antibody did not result in the expected BMSC apoptosis, regardless of concentration, suggesting a disruption of the Fas activation pathway. Thus BMSCs may have an endogenous mechanism to evade Fas-mediated apoptosis. Cumulatively, these data provide a parallel between adult stem/progenitor cells and cancer cells, consistent with the idea that stem/progenitor cells can use FasL to prevent lymphocyte attack by inducing lymphocyte apoptosis during the regeneration of injured tissues.
Human bone marrow stromal cells (BMSCs)2 (also referred to as mesenchymal stem cells (MSCs)) (1) contain a subset of multipotent, non-hematopoietic stem/progenitor cells. BMSCs can differentiate into hematopoiesis-supporting stromal tissue, adipocytes, osteoblasts, and chondrocytes (2, 3). In addition, they may be able to transdifferentiate into hepatocytes, myocytes, neuroectodermal cells, and endothelial cells, (4–6) although proof of such differentiation is not definitive to date. BMSCs have immunosuppressive potential, as recently demonstrated in both in vitro (7) and in vivo (8, 9) systems, including clinical studies (10, 11). However, the mechanisms by which BMSCs suppress immune responses are unresolved. Soluble factor-mediated immunosuppressive effects are beginning to come to light, (10, 12), and in addition there are as yet unexplained effects of cell-to-cell contact.
In the present study, we hypothesize that BMSC-mediated cytotoxicity of lymphocytes involves the FasL-activated apoptotic machinery. FasL is a type II transmembrane protein belonging to the tumor necrosis factor (TNF) family. FasL interacts with its receptor, Fas (CD95/APO-1) and triggers a cascade of subcellular events culminating in apoptotic cell death. FasL and Fas are key regulators of apoptosis in the immune system. In addition, FasL is expressed by cells in immune-privileged sites, such as cancer cells, neurons, eyes, cytotrophoblasts of the placenta, and reproductive organs (13–17). In neurons, FasL expression specifically protects against T cell-mediated cytotoxicity (16).
The discovery that FasL is also expressed by a variety of tumor cells raises the possibility that FasL may mediate immune privilege in human tumors (18). Activated T cells expressing Fas are sensitive to Fas-mediated apoptosis. Thus, up-regulation of FasL expression by tumor cells may enable tumorigenesis by targeting apoptosis in infiltrating lymphocytes. In the present work, we show that BMSCs can mediate immunosuppressive activity by FasL-induced killing of activated lymphocytes. Thus, BMSCs have properties of immune-privileged cells.
EXPERIMENTAL PROCEDURES
Cell Culture
Normal human BMSCs were isolated according to a previously published protocol (1) or were purchased (passage 1) from Cambrex Bio Science (Baltimore, MD). Cells were positive for CD105, CD166, CD29, and CD44 and negative for CD14, CD34, and CD45 (>95% of total cell number). BMSCs were cultured as a monolayer in mesenchymal stem cell growth medium (MSCGM; Cambrex Bio Science) containing 10% fetal bovine serum and antibiotics (Cambrex). When the cells had grown to 70–80% confluence, they were detached with trypsin/EDTA (Invitrogen).
Wild-type Jurkat cells (TIB-152 ATCC) and caspase-8-negative (I 9.2; ATCC) Jurkat cells with a mutation in the cysteine protease, caspase-8/FLICE, were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). The cells were split every third day, ensuring that the cell density in the culture did not exceed 5 × 105 cells/ml. All cells were grown at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Human peripheral blood lymphocytes from healthy donors were separated by Ficoll gradient centrifugation. The lymphocytes were resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin at a density of 105/ml in 6-well plates. Phytohemagglutinin (PHA) (Sigma-Aldrich) was then added to each well at a concentration of 10 μg/ml. Cells were incubated at 37 °C in 5% CO2 for 48 h.
Human skin fibroblasts were grown from skin biopsies and cultured in monolayer cultures in Eagle's Minimum Essential Medium (EMEM, ATCC) containing 10% fetal bovine serum and antibiotics (Invitrogen).
Electrophoresis and Western Blotting
Electrophoresis was performed separately on lysates from BMSCs and lymphocytes. Following SDS-PAGE, proteins were transferred overnight to 0.45-μm polyvinylidene difluoride membranes, and blots were blocked for 30 min at room temperature in a solution consisting of 140 mm NaCl, 10 mm NaPO4, 0.05% Tween 20, 50 mg/ml bovine serum albumin, pH 7.4. Blots were incubated overnight at 4 °C with anti-Fas or anti-FasL antibodies (Santa Cruz Biotechnology) diluted in blocking solution containing 20 mg/ml bovine serum albumin. Proteins were visualized using alkaline phosphatase-linked secondary antibody, enhanced chemifluorescence (Amersham Biosciences International), and quantified by Storm System Fluorescence Imaging (Molecular Dynamics, Sunnyvale, CA). Prestained molecular mass markers were from Novex (San Diego, CA).
Total RNA Extraction, RT-PCR Amplification, Subcloning, and Sequencing
Total RNA was extracted separately from normal BMSCs and activated and non-activated lymphocytes using RNeasyTM total RNA kit (Qiagen) according to the manufacturer's manual. cDNA was made from 1 μg of total RNA with oligo dT primers to synthesize the first-strand cDNA. RT was performed at 42 °C for 50 min followed by 70 °C for 15-min reaction termination, and 37 °C for 20 min RNase H digestion of RNA. The nested primer pairs for FAS ligand mRNA in this study were designed based on the cDNA sequence in GenBankTM (accession: NM_000639) and described in Table 1.
TABLE 1.
Primer pairs for FAS ligand mRNA
| Primer | Position | Oligo sequence |
|---|---|---|
| FASL-3 | 35–54 nt (external sense) | 5′-AGAGATCCAGCTTGCCTCCT-3′ |
| FASL-4 | 1028–1009 nt (external antisense) | 5′-TGGAAAGAATCCCAAAGTGC-3′ |
| FASL-5 | 136–151 nt (internal sense) | 5′-CTGGCCTGACTCACCA-3′ |
| FASL-6 | 653–636 nt (internal antisense) | 5′-AGGTGTCTTCCCATTCCA-3′ |
| FASL-7 | 465–482 nt (internal sense) | 5′-AGCTCTTCCACCTACAGA-3′ |
| FASL-8 | 980–964 nt (internal antisense) | 5′-ACGTCTGAGATTCCTCA-3′ |
The following thermocycle programs were used for nested PCR: 1 cycle of 94 °C for 3 min, 35 cycles of 94 °C for 45s, 58 °C (FASL-3 and FASL-4), or 50 °C (FASL-5 and FASL-6) or 48 °C (FASL-7 and FASL-8) for 45s, and 72 °C for 1 min, followed by an extension step of 72 °C for 10 min. The PCR products were analyzed in 1.5% SeaKem LE agarose gel electrophoresis, stained with ethidium bromide, and visualized by UV illumination.
Single bands of PCR product of FASL-7 and FASL-8 were cut from the agarose gel, purified with GeneClean kit, and subcloned into pCR2.1 vector (TOPOTM TA Cloning Kit, Invitrogen, CA). The insert size from a number of subclones was determined by KpnI restriction enzyme digestion analysis. Plasmid sequencing was performed by MTR Scientific Company using T7 and M13 primers.
Delivery of FasL siRNA
FasL siRNAs were obtained from Sigma-Aldrich. The target sequence used for FasL was 5′-GUCUACAUGAGGAACUCUATT-3′. hMSC cells was transfected with 25 nm siRNA duplexes (the final concentration in the cell culture medium) in the presence of the carrier Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol. After 48-h transfection with FasL siRNA duplexes or without FasL siRNA, hMSC cells were harvested for the study of cell interaction and the examination of FasL expression by Western blot.
Co-culture
For co-culture experiments, BMSCs were seeded on flasks or chamber glass slides (Nunc) at a density of 2,000/cm2 and allowed to recover for 15 h. Where indicated, BMSCs and Jurkat cells were prestained with 10 μm CellTracker Green CMFDA or CellTracker Red CMTPX (Invitrogen) for 20 min at 37 °C. Jurkat cells were added to BMSCs at a density of 20,000/cm2. Co-cultured cells and controls were cultured in a medium mixture containing MSCGM and RPMI (50:50, v/v). For apoptosis, medium and unattached cells were removed 24 h after co-culture initiation and replaced with fresh media mixture. Live Jurkat-BMSC attachment was observed using scanning laser microscopy (LSM 510; Zeiss).
Apoptosis
To induce apoptosis, BMSCs were treated with anti-Fas antibody (clone CH-11) (10–100 ng/ml) and cultured for 18 h. Soluble Fas/Fc chimera) and Fc fragment (R&D Systems) were used for blocking Fas-mediated apoptosis. Cell viability was examined using WST-1 assay, and the apoptotic rate of BMSC was detected by annexin V staining or PI staining followed by flow cytometry analysis. We used Jurkat cells as a positive control for apoptosis detection.
Annexin Staining
Phosphatidylserine exposure in Jurkat cells was detected in co-culture conditions by staining with annexin V (AV) Alexa Fluor 488 conjugate (Invitrogen) for 15 min at room temperature and taking observations with a fluorescent microscope (Axiovert 25; Zeiss). Non-attached Jurkat cells were then removed with three washes of RPMI and Jurkat cells attached to BMSCs were removed with trypsin/EDTA. Where indicated, unattached and attached cell populations were simultaneously stained with annexin V Alexa Fluor 488 conjugate and 1 μg/ml propidium iodide (PI) (Invitrogen) for 15 min at room temperature. The number of early apoptotic (AV+/PI−) and necrotic/late apoptotic (AV+/PI+) cells were expressed as a percentage of total Jurkat cells. In some experiments, Jurkat cells were prelabeled with Cell Tracker Red, and at various time points after co-culture initiation, stained with AV. All samples were analyzed using a FACS Calibur Flow Cytometer (Becton Dickinson).
WST-1 Assay
A viability analysis of cells was performed using a commercial colorimetric assay (Roche Applied Science) based on the cleavage of WST-1 tetrazolium salt by mitochondrial dehydrogenases of viable cells. Briefly, 10 μl of WST-1 was added to each of the 96-well culture plates containing 100 μl of cell suspension. After 40 min of incubation at 37 °C and 5% CO2, plates were evaluated using an ELISA reader and reference wavelengths of 450 and 650 nm. Data were expressed as the mean percentage of three replicates relative to untreated controls.
Cell Cycle and Apoptosis Analysis by Propidium Iodide Staining
BMSCS were fixed in 70% ethanol for at least 7 days, treated with 0.5 mg/ml RNase (Sigma) and 0.1% Triton in phosphate-buffered saline and incubated at 37 °C for 30 min before staining with 20 μg/ml PI (Sigma). Cells were then analyzed for DNA content by a FACSCalibur Flow Cytometer. The pre-G1 population corresponded to that of apoptotic cells. The acquired histograms were analyzed using Cell Quest Software (Becton Dickinson) (19).
RESULTS
To investigate the nature of BMSC lymphocyte interaction, we performed co-culture experiments using BMSCs and purified lymphocytes or Jurkat cells. Strong attachment of lymphocytes to stromal cells was detected immediately after initiation of co-culture (Fig. 1A). Apoptosis of attached lymphocytes was detected 18 h after co-culture initiation and reached 90% by 72 h (Fig. 1, B and C), as shown by annexin V labeling. To prove that annexin V-labeled cells are lymphocytes we used different approaches, including prelabeling of lymphocytes with cell trackers, double labeling with anti-CD3 antibody (data not shown) and annexin V, and morphological and size differences between lymphocytes and bone marrow stromal cells.
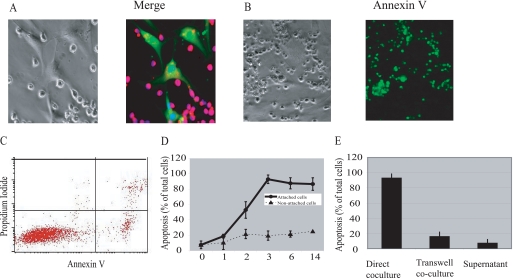
FIGURE 1.
Lymphocyte apoptosis induced by BMSCs. A, Jurkat cells and BMSCs were labeled with CellTracker Red and Green, respectively. Jurkat cells were incubated with a monolayer of BMSCs. Two hours following incubation, the samples were stained with Hoechst, a nuclear dye, and analyzed by confocal microscopy. BMSCs induce apoptosis in lymphocytes through cell-cell contact. Jurkat cells were added to the monolayer of BMSCs in a ratio of 1:10 directly (B–D) or separated by the transwell inserts, or supernatant from BMSCs-Jurkat co-culture was collected 18 h after co-culture initiation and added to control Jurkat cells (E). After the indicated time periods, non-attached Jurkat cells were removed from the co-culture chambers. The percentage of annexin V-positive cells among attached cells was detected by fluorescent microscopy (B) and among non-attached cells, by flow cytometry (C). The results of three independent experiments have been summarized ± S.E. (D and E).
Non-attached lymphocytes reached only 20% apoptosis despite 72 h of co-incubation (Fig. 1, C and D). To prove contact dependence of BMSC-induced lymphocyte apoptosis, we performed transwell experiments. As shown in Fig. 1E, lymphocytes separated from BMSCs by 3-μm pore inserts reached only 15% apoptosis after 72 h of co-incubation. The supernatant collected from BMSC lymphocyte co-culture was not able to initiate apoptosis of control Jurkat cells (Fig. 1E).
Both Jurkat cells and activated lymphocytes were much more sensitive to BMSC-mediated apoptosis than non-activated lymphocytes (Fig. 2A). BMSCs demonstrated some level of annexin V binding, which was not increased during co-culture with lymphocytes.
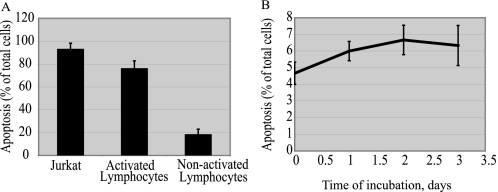
FIGURE 2.
Apoptosis is specific for BMSCs and activated lymphocytes co-culture. PHA-activated and non-activated lymphocytes were added to monolayer of BMSCs. Non-attached lymphocytes were removed after 24 h of co-incubation. The percentage of annexin V-positive attached lymphocytes was detected by fluorescent microscopy (A). Jurkat cells were added to the monolayer of skin fibroblasts in a ratio of 1:10. After the indicated time periods, non-attached Jurkat cells were removed from the co-culture chambers. The percentage of annexin V-positive cells among attached cells was detected by fluorescent microscopy (B). The results of three independent experiments are presented as mean ± S.E.
To prove that lymphocyte apoptosis requires contact with BMSCs, co-incubation of Jurkat with human skin fibroblasts was performed. As shown in Fig. 2B, co-incubation with fibroblasts did not cause significant apoptosis of Jurkat cells. Incubation with Chinese Hamster Ovary cell line (CHO K-1), and hamster kidney fibroblast cell line (BHK 21) also did not result in Jurkat cell apoptosis (data not shown).
The observation that lymphocyte apoptosis requires cell-cell contact led us to propose that the death receptor-activated pathway is involved in the apoptosis of Jurkat cells under co-culture conditions. Therefore, we tested the expression of FasL on BMSCs. Strong expression of FasL was detected as the protein (Fig. 3A) and the mRNA (Fig. 3B) by Western blot analysis and RT-PCR, respectively. This finding was inconsistent with previously published flow cytometry data showing no FasL expression on BMSCs (20). To confirm our results, we performed flow cytometry analysis of permeabilized and non-permeabilized BMSCs. As shown in Fig. 3C, the majority of FasL demonstrated intracellular localization. These data explain the discrepancy between our findings and the previously published data (20). Moreover, only under co-culture conditions, we were able to detect FasL on non-permeabilized BMSCs by fluorescent microscopy (Fig. 3D).
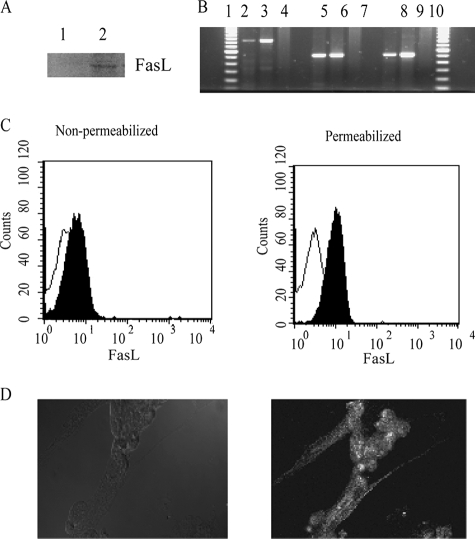
FIGURE 3.
Expression of FasL by BMSCs. A, immunoblot detection of FasL in BMSC lysates. Expression of FasL was assessed by Western blot analysis using a polyclonal rabbit anti-human FasL antibody. Cells lysates were prepared separately from BMSCs and lymphocytes, not under co-culture conditions. B, RT-PCR analysis of human Fas ligand in BMSCs. Lanes 1 and 11 correspond to the 100 bp DNA ladder from Invitrogen. Lanes 2–4 correspond to almost the full-length Fas ligand (845 base pairs) with primers FASL5 and FASL8. Lanes 5–7 correspond to the first half of the full-length Fas ligand (518 base pairs) with primers FASL 5 and FASL 6. Lanes 8–10 correspond to the second half of the full-length Fas ligand (516 base pairs) with primers FASL 7 and FASL 8. C, flow cytometry analysis of FasL expression on permeabilized and non-permeabilized BMSCs using fluorescein isothiocyanate-conjugated monoclonal anti-FasL antibody. D, fluorescent microscopy analysis of FasL expression on BMSC following 24 h co-culture with Jurkat cells.
To further support the possible role of FasL in BMSC-initiated apoptosis of lymphocytes, we performed a co-culture experiment of BMSCs and mutated Jurkat cells, in which the extrinsic apoptotic pathway has been disabled at the level of caspase-8. As shown in Fig. 4A, the apoptotic rate of attached mutated Jurkat cells was significantly lower than the rate of normal Jurkat cells. In addition, a rise in caspase-3 level was detected in attached lymphocytes as soon as 18 h after co-culture initiation (Fig. 4C).
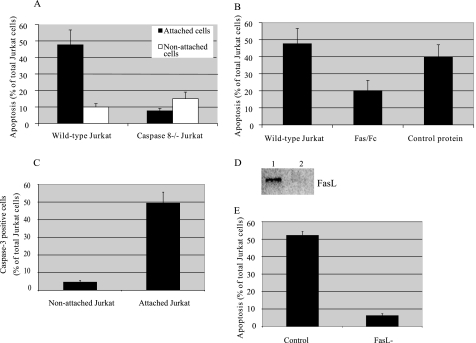
FIGURE 4.
Role of death receptors in Jurkat apoptosis initiated in co-culture with BMSCs. Wild type (WT) and caspase-8-negative Jurkat cells were incubated with a monolayer of BMSCs at a ratio of 1:100 for 48 h (A). Four hours after initiation of co-culture, non-attached Jurkat cells were removed from the co-culture chambers. At the end of co-incubation, all attached cells were trypsinized and labeled with annexin V and anti-CD3 antibody. The percentage of annexin V-positive cells among CD3-positive, attached cells was detected by flow cytometry. Separately, Jurkat cells were co-incubated with BMSCs for 48 h, non-attached cells were removed, and annexin V labeling of non-attached cells was detected by flow cytometry. Fas Fc fusion protein (1 μg/ml) and control Fc protein (1 μg/ml) were added to BMSCs 10 min prior to co-culture initiation (B). Caspase-3 activity of attached Jurkat cells was evaluated using the fluorescent substrate for caspase-3 24 h after co-culture initiation (C). BMSCs were transiently transfected with FasL siRNA or negative control siRNA. Down-regulation of FasL expression by specific siRNA was detected by Western blot (D). Jurkat cells were incubated with a monolayer of wild type and FasL siRNA-transfected BMSCs at a ratio of 1:100 for 48 h. Four hours after initiation of co-culture, the percentage of annexin V-positive cells among CD3-positive, attached cells was detected by microscopy (E).
We then added Fas/Fc chimera and control peptide prior to co-culture initiation to further examine whether BMSC-mediated apoptosis of lymphocytes involves Fas-FasL interaction. As shown in Fig. 4B, lymphocytes exposed to recombinant human Fas/Fc chimera (1 μg/ml), but not to control antibody, had been protected from apoptosis.
To provide additional evidence that FasL plays a role in BMSC-mediated apoptosis of lymphocytes, we suppressed FasL expression on BMSCs by siRNA delivery. The efficacy of FasL antisense RNA in inhibiting FasL protein expression was then tested by Western blotting (Fig. 4C). Western blot analysis found a complete inhibition in FasL protein expression in the BMSCs. Treatment of cells with the FasL siRNA significantly reversed the ability of BMSCs to induce apoptosis of Jurkat cells (Fig. 4D).
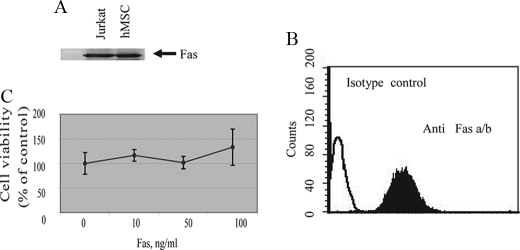
Expression of functional FasL by BMSCs calls into question the expression and functionality of the extrinsic apoptotic pathway in these cells. For this reason, we examined the expression of Fas, TRAIL, and TNF receptor II on BMSCs. As demonstrated in Fig. 5, significant expression of Fas (Fig. 5, A and B), but not TNF receptor II or TRAIL receptors (data not shown) was detected in BMSCs. Despite our finding that BMSCs demonstrate a high level of expression of Fas protein (Fig. 5, A and B), ligation of this receptor by an anti-Fas antibody was unable to initiate cell death and had no effect on the rate of BMSC proliferation (Fig. 5C). In addition, anti-Fas treated BMSCs were found to be negative for major apoptotic indicators, such as PS exposure, caspase-3 activation, and pre-G1 subpopulation formation (data not shown). To detect the stage in apoptotic pathway that was blocked in BMSCs, we examined apoptotic events upstream to mitochondria. Our data indicated no caspase-8 activation following BMSCs incubation with anti-Fas-activating antibody at a concentration of 100 ng/ml. These data indicate that blockage of the Fas-mediated apoptotic pathway happened upstream to caspase-8 activation, probably on the level of DISC formation. Nevertheless, we were able to activate the intrinsic apoptotic pathway by different stimuli, including growing BMSCs under unattached conditions (anoikis) and UV irradiation (data not shown). Thus, while we conclude that BMSCs co-express Fas and FasL, we propose that the Fas pathway in BMSCs is not active such that BMSCs can evade autocrine and/or T-cell-induced apoptosis by FasL.
FIGURE 5.
Expression and functionality of Fas on BMSCs. A, immunoblot detection of Fas in BMSC lysates. Expression of FasL was assessed by Western blot analysis using a monoclonal anti-human FasL antibody. B, flow cytometric analysis of surface Fas expression on BMSC using PE-conjugated monoclonal anti-human Fas antibody. C, BMSCs were incubated with different concentrations of CH-11 anti-Fas antibody for 18 h. Cell viability was assessed by the WST-1 dye assay.
DISCUSSION
In the present study, we show that BMSCs counterattack activated lymphocytes using the same mechanism as cancer cells: Fas-mediated apoptosis. Fas and FasL are co-expressed on primary BMSCs. Moreover, BMSCs kill co-cultured lymphocytes and Jurkat cells. As expected, both Jurkat cells and activated lymphocytes were susceptible to induction of apoptosis by BMSCs, and non-activated lymphocytes were notably spared. Caspase-8 deficiency inhibited apoptosis of Jurkat cells, and blocking with Fas Fc protein prevented BMSC-induced apoptosis by 60%. These results show that death receptors are responsible for lymphocyte cell death and that BMSC-associated FasL inhibited apoptosis signaling by Fas on lymphocytes, rather than induction of the Jurkat cells to kill themselves.
Previous reports concerning BMSC-induced apoptosis in general and FasL expression on BMSC (MSC) in particular, are controversial. Benvenuto et al. (21) demonstrated a protective effect of BMSCs on lymphocytic apoptosis. A possible explanation of this apparent contradiction with our present report is the fact that the two studies use different populations of lymphocytes: Benvenuto et al. collected non-attached lymphocytes for flow cytometry analysis, while we studied apoptosis only of lymphocytes that were attached to BMSCs. In addition, recent studies support our data, showing that BMSCs induce apoptosis in lymphocytes. FasL is found on cells derived from the Banna Minipig Inbred-line (BMI-MCS) (22). However, Ryan et al. (20) demonstrated no FasL expression on BMSCs. Plumas et al. (8) demonstrated that MSCs induce apoptosis of activated lymphocytes, but had a different explanation from the one that we present here: that tryptophan is converted into kynurenine by indoleamine 2,3-dioxygenase expressed by MSCs in the presence of IFNγ. In the present work, we demonstrated that blocking the Fas-mediated apoptotic pathway does not completely inhibit BMSC-mediated apoptosis, suggesting that additional mechanisms might play a role in this process. The intracellular localization of FasL in BMSC may explain the inconsistency concerning FasL expression, since flow cytometry does not generally pick up intracellular epitopes without permeabilization. Moreover, our results are consistent with data showing that FasL is required for the graft survival of donor bone marrow transplant from C3H-gld mice (23). C3H-gld mice have a FasL defect because of a point mutation that does not inhibit expression but renders it incapable of inducing apoptosis through Fas. In addition, the putative externalization of FasL to induce lymphocyte apoptosis can explain the fact that lymphocytes showed a peak of apoptosis 3 days after co-culture initialization. The way that FasL is externalized remains to be elucidated. One possible mechanism involves FasL-positive exosome formation, as is now indicated for cancer cells (24), or FasL externalization during activation by lymphocytes. Inducibility of the FasL externalization in BMSCs is in line with recent data describing induction of soluble factors that mediate immunosupression by BMSCs. Supernatants from non-activated BMSCs did not have any suppressive capacity on lymphocyte proliferation; however, co-culture with lymphocytes, with or without transwell separation, induced the suppressive activity of BMSC (25). In addition, controversy in this field can be explained by heterogeneity of isolated BMSC populations. In the present work, we used both commercially available (Cambrex) and BMSCs isolated in our laboratories; the detailed description of the latter was recently published (26). Results were similar regardless of which source was used. In parallel to our results, Pluchino et al. (27) have demonstrated that adult neural multipotent (stem) precursor cells induce apoptosis of infiltrating pro-inflammatory Th1 cells. The lymphocyte apoptosis occurred via multiple distinct pathways, with the death receptor-mediated pathway likely to predominate.
Co-expression of Fas and FasL by BMSCs calls into question the functionality of the extrinsic apoptotic pathway in these cells. Although we detected high levels of Fas expression on BMSCs, stimulation of Fas with different concentrations of anti-Fas antibody did not result in any apoptotic response. Although we were not able at this stage to identify the exact molecular mechanism of Fas-mediated pathway inactivation in BMSCs, we could narrow it to the step that includes events between Fas protein trimerization to caspase-8 activation. We speculate that similar to previously demonstrated cancer cells models (17), transformation of intracellular domain of Fas protein expressed in hMBSCs prevents trimerization of the receptor and blocks activation of apoptotic pathway activation. Our speculation based on the observation that we were not able to detect Fas with antibodies against the intracellular domain, but had a very strong specific bond with antibodies against the extracellular domain (data not shown). However, to prove this hypothesis additional experiments should be performed. Our finding regarding expression of nonfunctional Fas on BMSCs consistent with previous studies performed on different adult stem cells (SCs). All examined adult stem cells, including neural and satellite SCs, are shown to express at least one type of death receptor (28, 29). Moreover, SCs are shown to express all components of the Fas/TNF apoptotic pathway: surface FAS, TNFR1, TNFR2, Fas-associated protein with death domain, and caspase-8. Fas treatment of neural stem cells (NSCs) activates the extracellular signal-regulated protein kinase (ERK) pathway, which may have an anti-apoptotic as well as a growth-stimulating role. However, the exposure of SCs to death receptor agonists failed to induce apoptosis. As was shown in NSCs, the mitochondrial pathway was not affected, and caspase-3 served as an executioner caspase in the apoptotic machinery. Similarly, the differentiation of putative rat liver hepatic progenitor (RLE) cells to hepatocytes turns on a TNF-α-mediated apoptotic pathway. It was shown that 50% of the differentiated cells underwent apoptosis after 6 h of TNF-α treatment, whereas control RLE cells were resistant.
Concomitant FasL expression and insensitivity to Fas-mediated apoptosis have been demonstrated in different types of cancer, suggesting a mechanism for immune evasion by tumor cells (18). The expression of FasL on tumor cells is so well established that it is considered by some as a marker for cancer prognosis. Moreover, previous studies show that many malignant cells are of mesenchymal origin, such as those observed in osteosarcomas. The expression of FasL in these cells may explain the aggressive progression of this tumor type. These observations, taken together with our data, draw a direct parallel between adult stem cells and cancer cells.
The discovery that allogenic BMSC preferentially engraft at tumor sites and contribute to stromal tissue formation (30) suggests that BMSCs (with or without gene modification) may be useful in targeting cancer therapy. The ability of BMSCs to activate FasL-mediated apoptosis may open new strategies for targeted treatment of Fas-sensitive cancers.
In conclusion, FasL is expressed on BMSCs where it is plays role in the anti-immune activity of these cells against lymphocytes. Taken together with the rapidly accumulating data on the capacity of BMSCs to facilitate tissue regeneration, these data suggest that the unique success of BMSCs during transplantation may result from their ability to neutralize the innate immunity of the original tissue. Furthermore, the combined expression of non-functional Fas and functional FasL in BMSCs may create an immune-protected niche for these cells at the site of tissue injury.
Acknowledgments
We thank Drs. Jonathan D. Ashwell and Rocky S. Tuan for critical evaluation of this work and important suggestions for experiments and Drs. Svetlana Glushakova and Paul Blank for comments and discussion. We thank Elena Mekhedov for technical assistance.
This work was supported, in whole or in part, by the National Institutes of Health intramural programs of the NICHD and NIDCR. This work was also supported by Department of Health and Human Services and Breast Cancer Stamp funds.
- BMSC
- human bone marrow stromal cells
- MSC
- mesenchymal stem cells
- PHA
- phytohemagglutinin
- RT
- reverse transcriptase
- TNF
- tumor necrosis factor
- FasL
- Fas ligand
- PI
- propidium iodide.
REFERENCES
- 1.Robey P. G., Bianco P. (2006) J. Am. Dent. Assoc. 137, 961–972 [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein A. J., Piatetzky-Shapiro , II, Petrakova K. V. (1966) J. Embryol. Exp. Morphol. 16, 381–390 [PubMed] [Google Scholar]
- 3.Kemp K. C., Hows J., Donaldson C. (2005) Leuk. Lymphoma 46, 1531–1544 [DOI] [PubMed] [Google Scholar]
- 4.Ferrari G., Cusella-De Angelis G., Coletta M., Paolucci E., Stornaiuolo A., Cossu G., Mavilio F. (1998) Science 279, 1528–1530 [DOI] [PubMed] [Google Scholar]
- 5.Prockop D. J. (1997) Science 276, 71–74 [DOI] [PubMed] [Google Scholar]
- 6.Oh S. H., Miyazaki M., Kouchi H., Inoue Y., Sakaguchi M., Tsuji T., Shima N., Higashio K., Namba M. (2000) Biochem. Biophys. Res. Commun. 279, 500–504 [DOI] [PubMed] [Google Scholar]
- 7.Bartholomew A., Sturgeon C., Siatskas M., Ferrer K., McIntosh K., Patil S., Hardy W., Devine S., Ucker D., Deans R., Moseley A., Hoffman R. (2002) Exp. Hematol. 30, 42–48 [DOI] [PubMed] [Google Scholar]
- 8.Plumas J., Chaperot L., Richard M. J., Molens J. P., Bensa J. C., Favrot M. C. (2005) Leukemia 19, 1597–1604 [DOI] [PubMed] [Google Scholar]
- 9.Jiang X. X., Zhang Y., Liu B., Zhang S. X., Wu Y., Yu X. D., Mao N. (2005) Blood 105, 4120–4126 [DOI] [PubMed] [Google Scholar]
- 10.Rasmusson I. (2006) Exp. Cell Res. 312, 2169–2179 [DOI] [PubMed] [Google Scholar]
- 11.Lee S. T., Jang J. H., Cheong J. W., Kim J. S., Maemg H. Y., Hahn J. S., Ko Y. W., Min Y. H. (2002) Br. J. Haematol. 118, 1128–1131 [DOI] [PubMed] [Google Scholar]
- 12.Uccelli A., Pistoia V., Moretta L. (2007) Trends Immunol. 28, 219–226 [DOI] [PubMed] [Google Scholar]
- 13.Green D. R., Ferguson T. A. (2001) Nat. Rev. Mol. Cell Biol. 2, 917–924 [DOI] [PubMed] [Google Scholar]
- 14.Griffith T. S., Ferguson T. A. (1997) Immunol. Today 18, 240–244 [DOI] [PubMed] [Google Scholar]
- 15.Griffith T. S., Brunner T., Fletcher S. M., Green D. R., Ferguson T. A. (1995) Science 270, 1189–1192 [DOI] [PubMed] [Google Scholar]
- 16.Medana I., Li Z., Flügel A., Tschopp J., Wekerle H., Neumann H. (2001) J. Immunol. 167, 674–681 [DOI] [PubMed] [Google Scholar]
- 17.O'Connell J., Houston A., Bennett M. W., O'Sullivan G. C., Shanahan F. (2001) Nat. Med 7, 271–274 [DOI] [PubMed] [Google Scholar]
- 18.O'Connell J., O'Sullivan G. C., Collins J. K., Shanahan F. (1996) J. Exp. Med. 184, 1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacombe F., Belloc F. (1996) Hematol. Cell Ther. 38, 495–504 [DOI] [PubMed] [Google Scholar]
- 20.Ryan J. M., Barry F. P., Murphy J. M., Mahon B. P. (2005) J. Inflamm. (Lond.) 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benvenuto F., Ferrari S., Gerdoni E., Gualandi F., Frassoni F., Pistoia V., Mancardi G., Uccelli A. (2007) Stem. Cells 25, 1753–1760 [DOI] [PubMed] [Google Scholar]
- 22.Liu J., Lu X. F., Wan L., Li Y. P., Li S. F., Zeng L. Y., Zeng Y. Z., Cheng L. H., Lu Y. R., Cheng J. Q. (2004) Transplant Proc. 36, 3272–3275 [DOI] [PubMed] [Google Scholar]
- 23.George J. F., Sweeney S. D., Kirklin J. K., Simpson E. M., Goldstein D. R., Thomas J. M. (1998) Nat. Med. 4, 333–335 [DOI] [PubMed] [Google Scholar]
- 24.Abrahams V. M., Straszewski S. L., Kamsteeg M., Hanczaruk B., Schwartz P. E., Rutherford T. J., Mor G. (2003) Cancer Res. 63, 5573–5581 [PubMed] [Google Scholar]
- 25.Maitra B., Szekely E., Gjini K., Laughlin M. J., Dennis J., Haynesworth S. E., Koç O. N. (2004) Bone Marrow Transplant 33, 597–604 [DOI] [PubMed] [Google Scholar]
- 26.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P. G., Riminucci M., Bianco P. (2007) Cell 131, 324–336 [DOI] [PubMed] [Google Scholar]
- 27.Pluchino S., Zanotti L., Rossi B., Brambilla E., Ottoboni L., Salani G., Martinello M., Cattalini A., Bergami A., Furlan R., Comi G., Constantin G., Martino G. (2005) Nature 436, 266–271 [DOI] [PubMed] [Google Scholar]
- 28.Ceccatelli S., Tamm C., Sleeper E., Orrenius S. (2004) Toxicol Lett. 149, 59–66 [DOI] [PubMed] [Google Scholar]
- 29.Sánchez A., Factor V. M., Espinoza L. A., Schroeder I. S., Thorgeirsson S. S. (2004) Hepatology 40, 590–599 [DOI] [PubMed] [Google Scholar]
- 30.Studeny M., Marini F. C., Champlin R. E., Zompetta C., Fidler I. J., Andreeff M. (2002) Cancer Res. 62, 3603–3608 [PubMed] [Google Scholar]