Abstract
According to the lipid raft theory, the plasma membrane contains small domains enriched in cholesterol and sphingolipid, which may serve as platforms to organize membrane proteins. Using methyl-β-cyclodextrin (MβCD) to deplete membrane cholesterol, many G protein-coupled receptors have been shown to depend on putative lipid rafts for proper signaling. Here we examine the hypothesis that treatment of HEK293 cells stably expressing FLAG-tagged μ-opioid receptors (HEK FLAG-μ) or δ-opioid receptors (HEK FLAG-δ) with MβCD will reduce opioid receptor signaling to adenylyl cyclase. The ability of the μ-opioid agonist [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin to acutely inhibit adenylyl cyclase or to cause sensitization of adenylyl cyclase following chronic treatment was attenuated with MβCD. These effects were due to removal of cholesterol, because replenishment of cholesterol restored [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin responses back to control values, and were confirmed in SH-SY5Y cells endogenously expressing μ-opioid receptors. The effects of MβCD may be due to uncoupling of the μ receptor from G proteins but were not because of decreases in receptor number and were not mimicked by cytoskeleton disruption. In contrast to the results in HEK FLAG-μ cells, MβCD treatment of HEK FLAG-δ cells had no effect on acute inhibition or sensitization of adenylyl cyclase by δ-opioid agonists. The differential responses of μ- and δ-opioid agonists to cholesterol depletion suggest that μ-opioid receptors are more dependent on cholesterol for efficient signaling than δ receptors and can be partly explained by localization of μ- but not δ-opioid receptors in cholesterol- and caveolin-enriched membrane domains.
Membrane cholesterol can alter the function of integral proteins, such as G protein-coupled receptors, through cholesterol-protein interactions and by changes in membrane viscosity (1). In addition, cholesterol interacts with other lipids found in the bilayer, particularly sphingolipids (2), which allows for tight and organized packing that can precipitate the formation of specialized domains within the plasma membrane (3). These domains have become an area of intense research interest and have been termed lipid or membrane rafts (4). The study of membrane rafts in intact cells is controversial, due in part to the limitations of the current methods used to study rafts (5, 6). Regardless, the membrane environment formed in regions of high cholesterol and sphingolipids may be such that certain proteins have an affinity for these regions, especially proteins with a propensity to interact with cholesterol.
Many G protein-coupled receptors and signaling proteins have been found to prefer cholesterol-enriched domains leading to the hypothesis that these domains can organize signaling molecules in the membrane to enhance or inhibit specific signaling events (7). This includes μ- (8, 9), δ- (10, 11), and κ-opioid receptors (12). In addition, Gαi (12–17), Gαo (16), and adenylyl cyclase isoforms 3 (18), 5/6 (9, 18, 19), and 8 (20) have been found to associate with cholesterol and/or the cholesterol-binding protein caveolin. Activated opioid receptors couple to Gαi/o proteins and acutely inhibit the activity of adenylyl cyclase. Longer term exposure to opioid agonists causes sensitization of adenylyl cyclase and a rebound overshoot of cAMP production upon withdrawal of the agonist (21). Consequently, we sought to assess the role of cholesterol depletion on the ability of μ- and δ-opioid receptor agonists to inhibit and cause sensitization of adenylyl cyclase.
There are conflicting data for the effect of changes in membrane cholesterol on opioid signaling. For example, an increase in plasma membrane microviscosity by addition of cholesteryl hemisuccinate to SH-SY5Y cell membranes increased μ-opioid receptor coupling to G proteins (22). Conversely, removal of membrane cholesterol from Chinese hamster ovary cells has been shown to either decrease (23) or increase (24) the coupling of μ-opioid receptors to G proteins, as measured by [35S]GTPγS3 binding stimulated by the μ-opioid agonist DAMGO. Furthermore, the effect of cholesterol removal on δ-opioid agonist-stimulated [35S]GTPγS binding varies by cell type (10, 25). In these previous studies, the variety of cell types utilized and the conflicting results make comparisons between opioid receptor types difficult. The objective of this study was to directly compare the role of membrane cholesterol in modulating acute and chronic μ- and δ-opioid signaling in the same cell systems using identical methods, including the following: 1) depletion of cholesterol by the cholesterol-sequestering agent methyl-β-cyclodextrin (MβCD); 2) separation of cholesterol-enriched membranes by sucrose gradient ultracentrifugation; and 3) clustering of lipid raft patches in whole cells with cholera toxin B subunit.
In initial experiments using human embryonic kidney (HEK) cells heterologously expressing μ- or δ-opioid receptors, we found that δ-opioid receptors were located in caveolin-poor fractions following 1% Triton X-100 homogenization and sucrose gradient ultracentrifugation. This differs from studies using a detergent-free method to identify lipid raft fractions (10, 11). In contrast, we found that the μ-opioid receptor was found in both caveolin-poor and caveolin-rich fractions, in accordance with previous literature (8, 9). This differential localization of opioid receptors led us to test the hypothesis that, in contrast to the μ-opioid receptor, the δ-opioid receptor would not be dependent on cholesterol for signaling. The results show that μ- but not δ-opioid receptors have a dependence on cholesterol for signaling to adenylyl cyclase and that this effect is much more pronounced following chronic exposure to opioids.
EXPERIMENTAL PROCEDURES
Materials
SNC80 ((+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide), DPDPE ([d-Pen2,5]-enkephalin), and naltrindole hydrochloride were obtained from the Narcotic Drug and Opioid Peptide Basic Research Center at the University of Michigan (Ann Arbor, MI). Lovastatin hydroxy acid was obtained from Cayman Chemical (Ann Arbor, MI). [3H]Diprenorphine, [3H]DAMGO ([d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin), and [35S]GTPγS were obtained from PerkinElmer Life Sciences. Tissue culture media, geneticin, fetal bovine serum, and trypsin were from Invitrogen. All other chemicals were obtained from Sigma unless otherwise stated.
Cell Culture
Human embryonic kidney 293 cells stably transfected with the N-terminal FLAG-tagged δ- (HEK FLAG-δ) or μ-opioid receptor (HEK FLAG-μ) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 0.8 mg/ml geneticin and 10% fetal bovine serum at 37 °C in 5% CO2. Receptor expression in HEK FLAG-δ cells (8.4 ± 1.5 pmol/mg protein; n = 5) and HEK FLAG-μ cells (9.7 ± 1.3 pmol/mg protein; n = 5) was similar (p > 0.05). Receptor expression was measured by saturation binding of the opioid antagonist [3H]diprenorphine as described previously (22). SH-SY5Y cells were grown as above but without geneticin. SH-SY5Y cells were differentiated by adding 10 μm retinoic acid (Calbiochem) 3–5 days prior to assay.
Cholesterol Modulation
HEK FLAG-μ or HEK FLAG-δ cells were grown to confluence in DMEM + 10% FBS. Media were replaced with serum-free DMEM with or without 2% (15 mm) MβCD (Sigma) for 1 h at 37 °C. SH-SY5Y cells were treated with 5 mm MβCD for 10 min. For cholesterol replenishment, cells were incubated with or without a 2% MβCD-cholesterol complex (MβCD-CH) in serum-free DMEM for 2 h following cholesterol depletion. MβCD-cholesterol complexes were formed in an 8:1 molar ratio as described previously (26). Briefly, cholesterol was dissolved in a 1:1 ratio by volume of chloroform/methanol in a glass tube. Following evaporation of the solvent, the dried cholesterol was reconstituted with a suitable volume of serum-free DMEM containing 2% MβCD, vortexed, sonicated for 30 s, and incubated overnight at 37 °C with shaking. For lovastatin experiments, cells were treated with serum-free Opti-MEM containing 10 μm lovastatin hydroxy acid or DMSO vehicle for 48 h. Cholesterol content from cell lysates was determined using the Amplex Red cholesterol assay kit (Invitrogen) following the manufacturer's instructions. Cholesterol content was normalized to protein content, as determined by the method of Bradford (27).
Stimulation of [35S]GTPγS Binding
Membranes were prepared from HEK FLAG-δ or FLAG-μ cells following treatment with or without 2% MβCD as described previously (28). Final membrane pellets were resuspended in 50 mm Tris-HCl buffer, aliquoted, and stored at −80 °C. Protein concentration was measured using the Bradford assay (27).
Membranes (30 μg of protein) were incubated with 0.1 nm [35S]GTPγS for 60 min at 25 °C with or without various concentrations of the δ-opioid agonist SNC80 or the μ-opioid agonist DAMGO in [35S]GTPγS binding buffer (50 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 100 mm NaCl, 1 mm EDTA, 2 mm dithiothreitol, and 30 μm GDP). Membranes with bound [35S]GTPγS were collected on GF/C filters (Whatman) using a Brandel harvester (MLR-24, Gaithersburg, MD) and rinsed three times with cold wash buffer (50 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 100 mm NaCl). Dried filters were saturated with EcoLume liquid scintillation mixture (MP Biomedicals, Solon, OH), and radioactivity was counted in a Wallec 1450 MicroBeta (PerkinElmer Life Sciences).
Cyclic AMP Accumulation Assays
Inhibition of adenylyl cyclase activity was measured in HEK FLAG-δ or FLAG-μ cells grown to confluence in 24-well poly-d-lysine-coated plates. Cells were washed with serum-free DMEM and incubated with various concentrations of the δ-opioid agonist SNC80 or the μ-opioid agonist DAMGO in the presence of 5 μm forskolin and 1 mm 3-isobutyl-1-methylxanthine in serum-free media for 10 min at 37 °C. The assay was stopped by replacing the media with 1 ml of ice-cold 3% perchloric acid. After at least 30 min at 4 °C, a 400-μl aliquot of sample was neutralized with 2.5 m KHCO3 and centrifuged at 13,000 × g. Cyclic AMP was measured from the supernatant using a [3H]cAMP assay system (GE Healthcare) following the manufacturer's instructions. Inhibition of cAMP formation was calculated as a percent of forskolin-stimulated cAMP accumulation in the absence of opioid agonist.
For adenylyl cyclase sensitization experiments, HEK cells were rinsed with serum-free DMEM and incubated in the presence or absence of SNC80, DPDPE, or DAMGO in serum-free DMEM for 30 min at 37 °C. SH-SY5Y cells, plated in uncoated 24-well plates (5 × 105 cells/well) and differentiated with 10 μm retinoic acid for 3–5 days prior to sensitization experiments, were incubated in the presence or absence of 1 μm DAMGO for 60 min at 37 °C. The drug-containing media were then removed and replaced with serum-free media containing 5 μm forskolin, 1 mm 3-isobutyl-1-methylxanthine, and 10 μm of the opioid antagonist naloxone for HEK FLAG-μ cells and SH-SY5Y cells, or 10 μm of the δ-opioid antagonist naltrindole for HEK FLAG-δ cells, to precipitate cAMP overshoot. After 10 min at 37 °C, the assay was stopped with ice-cold 3% perchloric acid, and cAMP accumulation was quantified as described above. Overshoot was calculated as a percent of forskolin-stimulated cAMP accumulation in the absence of opioid agonist.
Radioligand Binding Assays
In competition binding assays, membranes (5–12 μg of protein) from HEK FLAG-μ or -δ cells treated with or without 2% MβCD were incubated for 1 h with shaking at 25 °C with 0.2 nm [3H]diprenorphine and increasing concentrations of unlabeled ligand (DAMGO or SNC80) in 50 mm Tris-HCl, pH 7.4. Where indicated, a final concentration of 10 μm GTPγS and 100 mm NaCl was added to the incubation buffer. For saturation binding assays, membranes (20 μg of protein) from low expressing HEK FLAG-μ cells (1.6 ± 0.1 pmol/mg protein) were incubated with increasing concentrations of [3H]diprenorphine (0.08–5 nm) or [3H]DAMGO (0.06–12 nm) in 50 mm Tris buffer, pH 7.4, for 1 h with shaking at 25 °C. For whole cell binding, HEK FLAG-μ cells (1 × 105 cells/tube) were incubated for 1 h in a 37 °C shaking water bath with 4 nm [3H]diprenorphine ± 10 μm CTAP (d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2) in serum-free DMEM. Protein content from a representative aliquot was determined by the method of Bradford (27). For all binding assays, nonspecific binding was determined using 10 μm naloxone. All assays were stopped by rapid filtration through GF/C filters using a Brandel harvester (MLR-24, Gaithersburg, MD) and rinsed three times with ice-cold 50 mm Tris-HCl wash buffer, pH 7.4. Bound radioactivity was determined by liquid scintillation counting as described above.
Separation of Detergent-resistant Membranes
HEK FLAG-δ or FLAG-μ cells were grown to confluence in 10-cm2 dishes, washed, and resuspended in ice-cold phosphate-buffered saline (PBS). Cells were pelleted and homogenized with a Dounce homogenizer in 100 μl of MES-buffered saline (MBS) containing 1% Triton X-100. The homogenate was placed on the bottom of a 2.2-ml ultracentrifuge tube, adjusted to 40% by addition of 53.3% sucrose in MBS, and overlaid with 900 μl of 30% sucrose and 900 μl of 5% sucrose in MBS for a discontinuous gradient. The samples were centrifuged at 200,000 × g in a Beckman-Coulter (Fullerton, CA) Optima Max-E Ultracentrifuge using a swinging bucket rotor (TLS-55) for 16–24 h at 4 °C.
Equal volume (183 μl) fractions were collected from the top. Equal volume aliquots were taken from each fraction, mixed with sample buffer (63 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 0.008% bromphenol blue, 50 mm dithiothreitol), separated by SDS-PAGE on a 10% polyacrylamide gel, and transferred to nitrocellulose membranes (Pierce) for Western blotting. Antibodies used were monoclonal anti-FLAG M1 (1:2000; Sigma), polyclonal anti-caveolin (1:2000; BD Transduction Laboratories), and monoclonal anti-human TfR (1:2000; Zymed Laboratories Inc.). Secondary antibodies used were goat anti-mouse horseradish peroxidase or goat anti-rabbit horseradish peroxidase (1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA). The above antibodies were diluted in 5% milk in Tris-buffered saline, 0.05% Tween 20 (+1 mm CaCl2 for FLAG M1 antibody). SuperSignal West Pico chemiluminescent substrate (Pierce) was used to detect immunoreactivity. For detection of adenylyl cyclase (AC) 5/6, samples were separated on a 6% polyacrylamide gel and transferred to an Immobilon-P polyvinylidene fluoride membrane (Millipore, Billerica, MA). AC 5/6 was detected by rabbit anti-AC 5/6 (Santa Cruz Biotechnology) diluted 1:200 in 1% bovine serum albumin in Tris-buffered saline, 0.05% Tween 20.
Cholera Toxin B-induced Patching
HEK FLAG-δ or HEK FLAG-μ cells were plated on poly-d-lysine-coated coverslips in a 6-well plate (1 × 106 cells/well) 24 h prior to patching. Cells were incubated with AlexaFluor 488-conjugated cholera toxin B subunit (1 μg/μl in DMEM, 10% goat serum; Invitrogen) for 45 min at 4 °C to label endogenous ganglioside GM1. Lipid raft aggregation, or patching, was induced as described by Fra et al. (29) by incubating with goat anti-CTB antibody (1:250 in DMEM, 10% goat serum; Calbiochem) for 30 min at 4 °C, followed by 20 min at 37 °C. Cells were fixed with 4% paraformaldehyde for 20 min and incubated in monoclonal anti-FLAG M1 antibody (1:1000 in PBS, 5% milk; Sigma) for 1 h followed by AlexaFluor 594-conjugated goat anti-mouse antibody (1:1000 in PBS, 5% milk; Invitrogen) to stain the FLAG-tagged μ- or δ-opioid receptor. For TfR and caveolin staining, cells were permeabilized with 0.1% Triton X-100 for 10 min and incubated with monoclonal anti-TfR antibody (1:200 in PBS, 5% milk; Zymed Laboratories Inc.) or polyclonal anti-caveolin antibody (1:200 in PBS, 5% milk; BD Transduction Laboratories). Coverslips were mounted on slides using ProLong Gold (Invitrogen). Fluorescent images of 0.5-μm Z planes were captured using an Olympus FV-500 confocal microscope.
Quantification of colocalization was performed using the RG2B colocalization plug-in to ImageJ (version rsb.info.nih.gov). The minimum threshold pixel intensity was set to 50 for both channels, and the minimum ratio for pixel intensity between the channels was 50%. Colocalization pixels from individual Z planes were displayed as a stack of Z projections with maximum pixel intensity. Colocalization was reported as the average pixel density of colocalized pixels per cell.
Statistical Analysis
All data were analyzed using GraphPad Prism 4 software (San Diego, CA). All data points represent at least three separate experiments in duplicate and are presented as means ± S.E., unless otherwise noted. The effect of treatment on agonist responses at various concentrations was analyzed by two-way ANOVA with Bonferroni's post hoc test. EC50 values were calculated from individual concentration-effect curves using the fixed slope sigmoidal dose-response curve fit analysis in GraphPad Prism. Ki and Bmax/Kd values were calculated from individual binding experiments using one- or two-site competition or one-site hyperbola binding curve fit linear regression analysis, respectively. EC50, Ki, Bmax, and Kd values are expressed as mean ± S.E. and compared for statistical significance by unpaired, two-tailed Student's t test. Cholesterol repletion experiments were compared using one-way ANOVA with Bonferroni's post hoc test. All other statistical comparisons were made with unpaired, two-tailed Student's t test, unless otherwise indicated. For all tests significance was set at p < 0.05.
RESULTS
Effect of Cholesterol Depletion on Opioid Receptor Coupling to G Protein
HEK293 cells stably expressing either the FLAG-μ or FLAG-δ opioid receptor were treated with the cholesterol-sequestering agent MβCD (2%) for 1 h at 37 °C. This reduced cholesterol to 40 ± 5.6% of control, consistent with previously published results of 35–55% reductions (12, 30, 31), and induced a rounder cell morphology, although cells were still viable by trypan blue exclusion. This treatment has been shown to eliminate caveolae as determined by electron microscopy (30), and it has been commonly used to disrupt lipid rafts to study effects on opioid signaling (9–12). Therefore, we used this pharmacological tool to directly compare the effects of cholesterol depletion on μ- and δ-opioid receptor signaling.
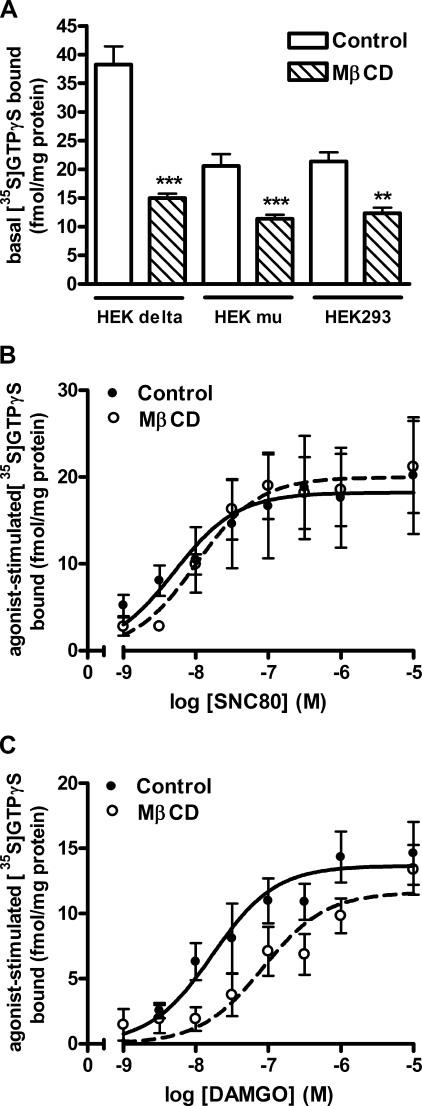
Agonist-activated opioid receptors couple to Gαi/o proteins and induce the exchange of GTP for GDP, which can be measured by the increase in binding of the guanine nucleotide analog [35S]GTPγS. Basal levels of [35S]GTPγS binding were similar in membranes from untransfected HEK293 cells and HEK cells expressing μ-opioid receptors but were higher in membranes from cells expressing δ-opioid receptors (Fig. 1A), which are thought to be tightly coupled to G proteins and show constitutive activity (32). Treatment of either HEK FLAG-μ cells or untransfected HEK293 cells with 2% MβCD for 1 h reduced basal [35S]GTPγS binding by 38 ± 5.5 or 39 ± 2.2%, respectively (Fig. 1A), suggesting an opioid receptor-independent effect. In contrast, treatment of HEK FLAG-δ cells with MβCD reduced basal levels of [35S]GTPγS binding by 61 ± 1.7%. However, the δ-opioid inverse agonist RTI-5989-25 (33) was able to reduce [35S]GTPγS binding by 18 ± 3.8% in control HEK FLAG-δ cells (data not shown). Therefore, in HEK FLAG-δ cells approximately one-third of the decrease in basal [35S]GTPγS binding caused by MβCD may be due to a loss of constitutively active receptors. The remaining decrease, which is similar to the decrease in HEK FLAG-μ or untransfected HEK293 cells, is likely because of a reduction in available, unoccupied Gα proteins themselves or a loss of constitutive activity of other G protein-coupled receptors endogenous to HEK293 cells.
FIGURE 1.
μ-Opioid but not δ-opioid agonist-mediated G protein activation is altered following MβCD treatment. A, basal [35S]GTPγS binding in membranes from HEK FLAG- δ, HEK FLAG-μ, or untransfected HEK293 cells treated with (hatched bars) or without (open bars) 2% MβCD in serum-free media for 1 h. **, p < 0.01; ***, p < 0.001 versus respective control by unpaired two-tailed Student's t test. B, stimulation of [35S]GTPγS binding by the δ-agonist SNC80 (1 nm to 10 μm) was unchanged in membranes from HEK FLAG-δ cells treated with MβCD. EC50 values are as follows: control = 4.9 ± 2.4 nm; MβCD = 5.2 ± 0.8 nm (p = 0.898). C, stimulation of [35S]GTPγS binding by the μ-agonist DAMGO (1 nm to 10 μm) was shifted in membranes from HEK FLAG-μ cells treated with MβCD. EC50 values are as follows: control = 16.8 ± 9.3 nm; MβCD = 73.0 ± 26.5 nm (p = 0.116). Data are presented as mean ± S.E. from 3 to 4 separate experiments, in duplicate.
Because of this decrease in basal [35S]GTPγS binding, data were graphed as femtomoles of agonist-stimulated [35S]GTPγS bound/mg of protein rather than as percent change over basal, as has been reported previously (10, 24, 25). Treatment of HEK FLAG-δ or FLAG-μ cells with MβCD did not alter maximal stimulation of [35S]GTPγS by the δ-opioid agonist, SNC80 (p = 0.557), or the μ-opioid agonist, DAMGO (p = 0.200), respectively (Fig. 1, B and C). The potency of SNC80 to stimulate [35S]GTPγS binding was also similar in control (EC50 = 4.9 ± 2.4 nm) and MβCD-treated cells (EC50 = 5.2 ± 0.8 nm) (p = 0.898; Fig. 1B). In contrast, MβCD treatment caused a significant rightward shift in the ability of DAMGO to stimulate [35S]GTPγS binding (Fig. 1C) (treatment, F(1,92) = 9.838, p = 0.002). This was reflected by a 4-fold increase in EC50 from 16.8 ± 9.3 nm in control cells to 73.0 ± 26.5 nm in MβCD-treated cells, although variability precluded significance (p = 0.116). Even though this difference in G protein activation between μ- and δ-opioid receptors in response to cholesterol removal is small, it does disagree with previous reports using heterologous systems that show an effect of MβCD on δ-opioid agonist-stimulated [35S]GTPγS binding (10, 24, 25). This could be due to cell types utilized or methods of data analysis because the basal level of [35S]GTPγS binding does change. Regardless, the trend shown here suggests that cholesterol aids in efficient coupling of μ-opioid receptors to G proteins, but it is not necessary for efficient δ-opioid receptor coupling.
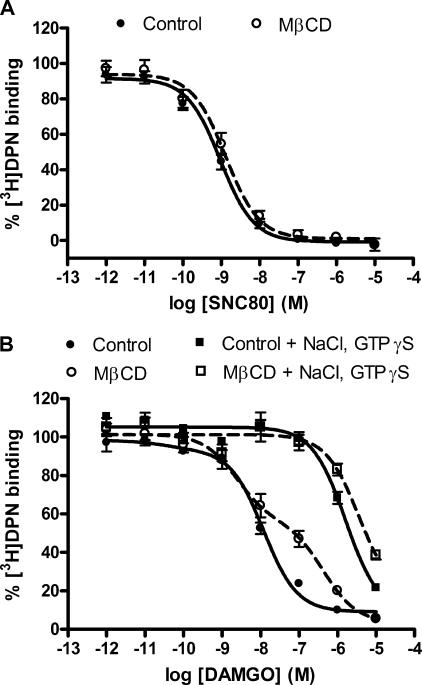
The reduced ability of the μ-opioid agonist DAMGO to stimulate [35S]GTPγS binding may be due to a loss of receptor-G protein coupling following cholesterol depletion. To test this hypothesis, we used a ligand binding approach. Receptors were labeled with the nonselective opioid antagonist [3H]diprenorphine and displaced by the δ-opioid agonist SNC80 or the μ-opioid agonist DAMGO, as appropriate. The δ-opioid agonist SNC80 was best fit by a single, high affinity binding site model (Ki = 0.78 ± 0.21 nm), although the slope of the displacement curve was less than unity (−0.77 ± 0.09) suggesting a population of δ-opioid receptors with slightly different agonist affinities. Regardless, this high affinity binding was retained even after removal of cholesterol from the plasma membrane of HEK FLAG-δ cells with MβCD (Ki = 0.87 ± 0.09 nm; slope = −0.72 ± 0.12) (Fig. 2A). Similarly, the μ-opioid agonist DAMGO displayed high affinity binding in control membranes (Ki = 4.9 ± 1.2 nm; slope = −0.76 ± 0.03). In contrast, DAMGO displacement (slope = −0.45 ± 0.05) was best fit to two affinity sites following removal of cholesterol from HEK FLAG-μ cells (Ki high = 1.9 ± 0.3 nm; Ki low = 244 ± 49 nm) (Fig. 2B). This MβCD-induced low affinity site in HEK FLAG-μ cells had a similar affinity to the site induced by uncoupling the receptor from G proteins with sodium ions and GTPγS (Ki = 768 ± 82 nm). Moreover, the Ki value induced by sodium ions and GTPγS was not significantly reduced further in membranes from cells treated with MβCD (Ki = 1887 ± 712 nm). These results confirm the [35S]GTPγS experiments and indicate that removal of membrane cholesterol by MβCD generates two affinity states of the μ-opioid receptor, likely due to uncoupling a proportion of the μ-opioid receptors from G proteins, thus resulting in a loss of stimulatory activity of agonist on G proteins.
FIGURE 2.
Cholesterol removal by MβCD induces low affinity binding in μ- but not δ-opioid receptor-expressing cells. Competition binding experiments in membranes prepared from HEK FLAG-δ (A) or HEK FLAG-μ cells (B) treated with (open circles) or without (filled circles) 2% MβCD for 1 h. Unlabeled agonist (SNC80 or DAMGO) was added in increasing concentrations in competition with 0.2 nm [3H]diprenorphine (DPN). Nonspecific binding was determined using 10 μm naloxone. A, Ki values for SNC80 using one-site competition linear regression are as follows: control = 0.78 ± 0.21 nm; MβCD = 0.87 ± 0.09 nm (p = 0.767). Slope of concentration displacement curve was unchanged as follows: control = −0.77 ± 0.09; MβCD = −0.72 ± 0.12 (p = 0.788). B, 100 mm NaCl and 10 μm GTPγS was added in the Tris incubation buffer as indicated to uncouple G proteins. Ki values for DAMGO are as follows: control = 4.9 ± 1.2 nm, one-site model; MβCD = 1.9 ± 0.3 nm (Ki high), 244 ± 49 nm (Ki low), two-site model. Slope of concentration displacement curve was reduced as follows: control = −0.76 ± 0.03; MβCD = −0.45 ± 0.05 (p = 0.0004). Ki values after NaCl and GTPγS addition are as follows: control = 768 ± 82 nm; MβCD = 1887 ± 712 nm. Data are presented as a percent of the total specifically bound [3H]diprenorphine in the absence of competing agonist and are from three separate experiments, in duplicate. Ki values and slopes were compared using two-tailed, unpaired Student's t tests.
Effect of Cholesterol Depletion on Opioid Inhibition of Adenylyl Cyclase
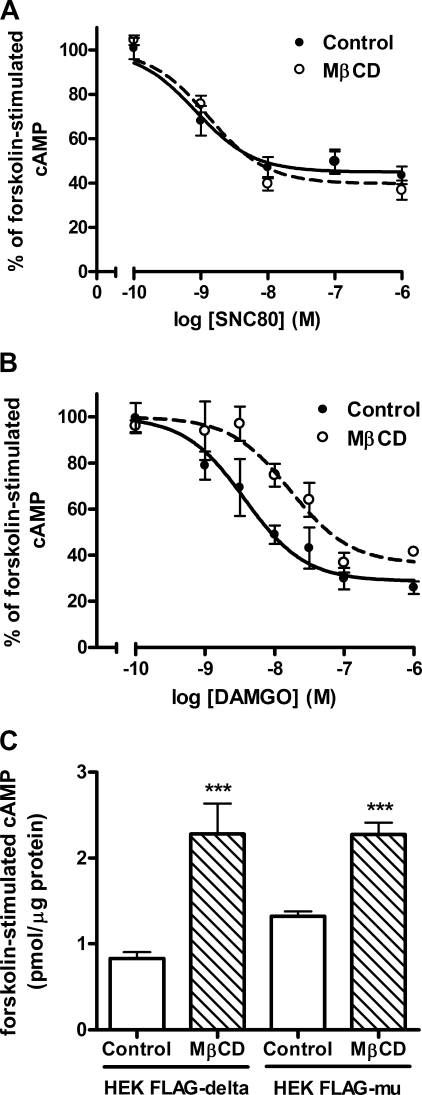
To examine if a decrease in G protein activation by agonists translates to decreases in downstream responses within the cell, inhibition of adenylyl cyclase by μ- and δ-opioid agonists was measured following cholesterol removal. Treatment of HEK FLAG-δ cells with 2% MβCD did not alter the ability of the δ-opioid agonist SNC80 to inhibit adenylyl cyclase (Fig. 3A) (EC50 of control = 1.05 ± 0.32 nm; MβCD-treated = 1.51 ± 0.07 nm; p = 0.229). In contrast, MβCD treatment of HEK FLAG-μ cells significantly affected acute inhibition of adenylyl cyclase by the μ-opioid agonist DAMGO (Fig. 3B) (treatment, F(1,54) = 17.39, p = 0.0001) with a significant 4-fold rightward shift in the potency of DAMGO from an EC50 of 2.49 ± 1.01 nm in control cells to 9.24 ± 1.15 nm in MβCD-treated cells (p = 0.023). Together, these results are consistent with [35S]GTPγS and binding results, indicating that cholesterol or cholesterol-dependent membrane effects modulate efficient μ-opioid receptor signaling more than δ-opioid receptor signaling.
FIGURE 3.
μ- but not δ-opioid agonist-mediated inhibition of adenylyl cyclase is altered following MβCD treatment. A and B, concentration-response curves for acute adenylyl cyclase inhibition by the δ-agonist SNC80 (0.1 nm to 1 μm) or the μ-agonist DAMGO (1 nm to 1 μm) in HEK FLAG-δ (A) or HEK FLAG-μ (B) cells pretreated with (open circles) or without (filled circles) 2% MβCD for 1 h. Data are presented as a percent of the forskolin-stimulated cAMP accumulation in the absence of agonist (n = 3, in duplicate). A, EC50 values for SNC80 were not changed as follows: control = 1.05 ± 0.32 nm; MβCD = 1.51 ± 0.07 nm (p = 0.229). B, MβCD caused a 4-fold rightward shift in the potency of DAMGO as follows: control = 2.49 ± 1.01 nm; MβCD = 9.24 ± 1.15 nm (p = 0.023). C, cAMP production by 5 μm forskolin in HEK FLAG-δ or FLAG-μ cells treated with (hatched bars) or without (open bars) 2% MβCD for 1 h. ***, p < 0.001 compared with respective control by unpaired two-tailed Student's t test. Data presented as mean ± S.E. from six separate experiments, in duplicate.
To measure inhibition of adenylyl cyclase by opioid agonists, the enzyme is stimulated directly with forskolin. Consistent with previous reports (31, 34), the level of cAMP produced by forskolin was enhanced ∼2-fold by MβCD treatment in both HEK FLAG-δ (control = 0.83 ± 0.07 pmol of cAMP/μg of protein; MβCD-treated = 2.3 ± 0.35 pmol of cAMP/μg of protein, p = 0.0004) and FLAG-μ cells (control = 1.3 ± 0.06 pmol of cAMP/μg of protein; MβCD-treated = 2.3 ± 0.14 pmol/μg of protein, p < 0.0001) (Fig. 3C). This could be due to decreased inhibitory input on adenylyl cyclase by the lower basal Gαi/o protein activity that was observed in [35S]GTPγS binding experiments. To investigate if this was due to decreased constitutive activity of the opioid receptor, cells were treated with pertussis toxin overnight to block receptor-Gαi/o protein coupling. Pertussis toxin treatment (100 ng/ml) did not lead to a significant increase of basal or forskolin-stimulated adenylyl cyclase activity in control or MβCD-treated HEK FLAG-δ cells (data not shown), suggesting that the decreased base-line activity of the G protein following MβCD treatment is either independent of coupling to the receptor or is not responsible for the increased forskolin response.
Effect of Cholesterol Depletion on Opioid-induced Adenylyl Cyclase Sensitization
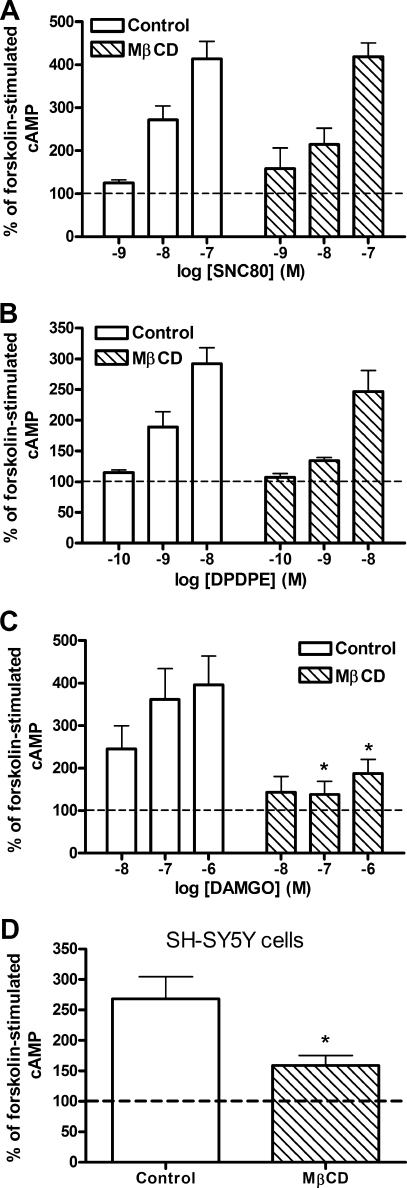
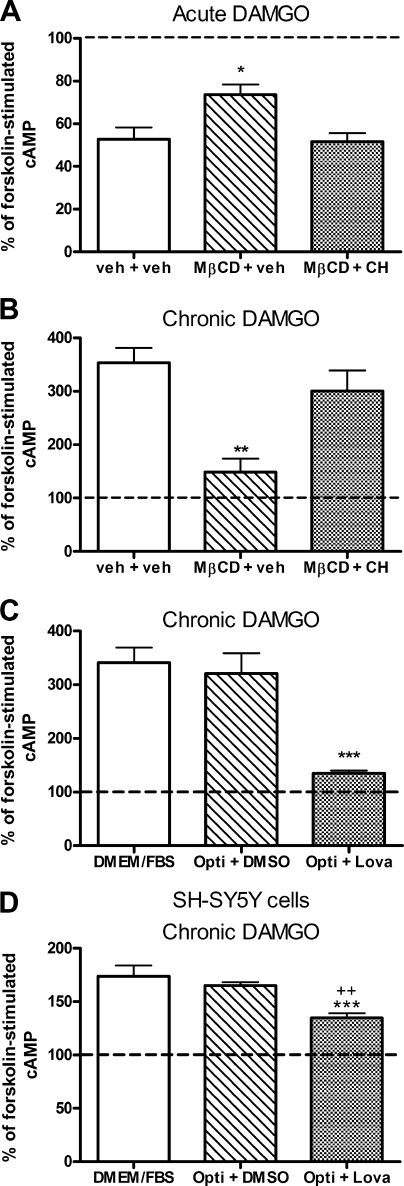
Chronic administration of opioid agonists results in a dependent state in both animal and cell models, which is characterized by withdrawal following removal of the agonist. In cells, the chronic inhibition of adenylyl cyclase by agonists for Gαi/o-coupled receptors causes a sensitization of the enzyme. Upon withdrawal of the agonist (usually by addition of a competitive antagonist) and subsequent stimulation of adenylyl cyclase by forskolin, cAMP production is increased over that of forskolin alone, termed cAMP overshoot (21). Treatment of HEK FLAG-δ cells with 2% MβCD for 1 h, prior to chronic (30 min) exposure to varying concentrations of either the nonpeptidic δ-opioid agonist, SNC80 (1–100 nm), or the peptidic δ-opioid agonist, DPDPE (0.1–10 nm), did not alter the degree of cAMP overshoot precipitated by the δ-opioid antagonist naltrindole at any of the agonist concentrations tested (Fig. 4, A and B). However, similar treatment of HEK FLAG-μ cells with MβCD reduced the resultant cAMP overshoot induced by incubation with various concentrations (10–1000 nm) of the μ-opioid agonist, DAMGO (Fig. 4C) (treatment F(1,32) = 16.39, p = 0.0003), consistent with results published previously (9). These results suggest that cholesterol removal by MβCD selectively blocks μ, rather than δ, agonist-induced sensitization of adenylyl cyclase. Furthermore, this alteration in chronic signaling is much more robust than effects of MβCD on the acute signaling to Gαi/o proteins and adenylyl cyclase.
FIGURE 4.
MβCD treatment blocks μ- but not δ-opioid agonist-mediated adenylyl cyclase sensitization. Adenylyl cyclase overshoot was precipitated with antagonist (naltrindole (A and B) or naloxone (C)) in the presence of 5 μm forskolin following chronic (30 min) treatment with the nonpeptidic δ-agonist SNC80 (A), the peptidic δ-agonist DPDPE (B), or the μ-agonist DAMGO (C) in HEK FLAG-δ (A and B) or HEK FLAG-μ cells (C). Pretreatment with 2% MβCD (hatched bars) for 1 h prior to agonist exposure prevented overshoot produced by the μ-agonist DAMGO (*, p < 0.05 by two-way ANOVA with Bonferroni's post hoc test) but not by either δ-agonist. D, retinoic acid-differentiated SH-SY5Y cells were treated with 5 mm MβCD (hatched bar) for 10 min prior to incubation with 1 μm DAMGO for 60 min. Overshoot was precipitated with naloxone as above. *, p < 0.05 by unpaired two-tailed Student's t test. Dashed lines indicate cAMP production with forskolin alone. Data are presented as mean ± S.E. from three separate experiments, in duplicate.
To determine whether the effect of MβCD on μ-opioid-induced adenylyl cyclase sensitization was restricted to a heterologous expression system, we repeated the sensitization experiments with SH-SY5Y neuroblastoma cells that endogenously express μ-opioid receptors. SH-SY5Y cells were differentiated with retinoic acid prior to sensitization experiments to create a more neuronal model (35, 36). SH-SY5Y cells were treated with 5 mm MβCD in serum-free DMEM for 10 min, which depleted cholesterol by 30.6 ± 4.4% (control = 43.3 ± 2.2 μg/mg protein; MβCD = 29.9 ± 2.0 μg/mg protein, p = 0.002, n = 5), similar to a previous report (37). This treatment paradigm had no effect on cAMP production by 5 μm forskolin (control = 1.27 ± 0.12 pmol of cAMP/μg of protein; MβCD = 1.27 ± 0.16 pmol of cAMP/μg of protein). However, adenylyl cyclase sensitization by 1 μm DAMGO was significantly attenuated in SH-SY5Y cells treated with MβCD (Fig. 4D, p < 0.01). Therefore, the robust effect of MβCD on μ-opioid agonist-mediated adenylyl cyclase sensitization is not limited to HEK293 cells but also occurs in this neuronal model expressing endogenous receptors.
Alteration of μ-Opioid Receptor Signaling by MβCD Is Because of Removal of Cholesterol
Despite the common use of MβCD for its cholesterol-sequestering properties, it may also have other nonspecific effects on a cell due in part to changes in cell morphology. To ensure that the effects on μ-opioid signaling observed following MβCD treatment were related to the removal of cholesterol from the membrane, membrane cholesterol was replenished using 2% MβCD pre-conjugated to cholesterol (MβCD-CH) in an 8:1 MβCD/cholesterol molar ratio (9, 12, 26). In these experiments, HEK FLAG-μ cells were treated first with 2% MβCD for 1 h to deplete membrane cholesterol. Cholesterol was then reintroduced to some cells by incubation with serum-free media containing 2% MβCD-CH for 2 h, whereas other MβCD-treated cells were incubated for 2 h with serum-free media alone. As expected, although cholesterol levels in MβCD-treated cells remained low, cholesterol levels in cells incubated with MβCD-CH returned to a level similar to that of control cells (p > 0.05) (control = 21.1 ± 3.3 μg of cholesterol/mg of protein; MβCD-treated = 7.8 ± 1.0 μg of cholesterol/mg of protein; MβCD-CH-treated = 30.6 ± 3.4 μg of cholesterol/mg of protein; n = 6).
The replenishment of cholesterol restored acute inhibition of adenylyl cyclase by 10 nm DAMGO (Fig. 5A) and sensitization of adenylyl cyclase by 100 nm DAMGO (Fig. 5B) to levels similar to control cells. Restoration of membrane cholesterol by MβCD-CH also allowed cell morphology to return to normal. Incubation with serum-free media or MβCD-CH for 2 h produced similar levels of forskolin-stimulated cAMP as control cells (control = 1.73 ± 0.10 pmol of cAMP/μg of protein; MβCD = 2.16 ± 0.33; MβCD-CH = 1.53 ± 0.20 pmol of cAMP/μg of protein), indicating that effects of MβCD on the forskolin response are transient.
FIGURE 5.
Effects of MβCD on μ-opioid signaling are due to removal of cholesterol from the membrane. A, acute adenylyl cyclase inhibition by 10 nm DAMGO in HEK FLAG-μ cells following cholesterol depletion without (MβCD + veh) or with cholesterol replenishment (MβCD + CH). B, adenylyl cyclase overshoot following chronic (30 min) treatment with 100 nm DAMGO in HEK FLAG-μ cells following cholesterol depletion without (MβCD + veh) or with replenishment (MβCD + CH). Dashed lines indicate cAMP production with forskolin alone. *, p < 0.05; **, p < 0.01 compared with control (veh + veh) following one-way ANOVA and Bonferroni's post hoc test. Forskolin stimulation was similar across all groups in each experiment (p > 0.05 by two-way ANOVA and Bonferroni's post-test: veh + veh = 1.75 ± 0.13; MβCD + veh = 2.06 ± 0.22; MβCD + CH = 1.53 ± 0.11 pmol of cAMP/μg of protein). C and D, HEK FLAG-μ or SH-SY5Y cells, respectively, were grown in regular growth media (DMEM/FBS), Opti-MEM containing DMSO vehicle (Opti + DMSO), or Opti-MEM containing the cholesterol synthesis inhibitor lovastatin hydroxy acid (10 μm) (Opti + Lova) for 48 h. Opti-MEM had no effect on overshoot compared with normal growth media (DMEM/FBS). C, adenylyl cyclase overshoot following chronic (30 min) treatment with 100 nm DAMGO in HEK FLAG-μ cells grown in each of the above conditions. ***, p < 0.001 compared with regular media (DMEM/FBS) or Opti-MEM + DMSO controls by one-way ANOVA with Bonferroni's post-test. Forskolin stimulation was similar among groups (DMEM/FBS = 1.06 ± 0.03; Opti + DMSO = 1.30 ± 0.04; Opti + Lova = 1.02 ± 0.12 pmol of cAMP/μg of protein). D, adenylyl cyclase overshoot following chronic (60 min) treatment with 1 μm DAMGO in differentiated SH-SY5Y cells grown in each of the above conditions plus 10 μm retinoic acid. ***, p < 0.001 versus DMEM/FBS; ++, p < 0.01 versus Opti + DMSO by one-way ANOVA and Bonferroni's post-test. DMEM/FBS and Opti + DMSO were not different (p > 0.05). Forskolin stimulation was not different in Opti + DMSO (0.93 ± 0.07 pmol/μg protein) and Opti + Lova (0.89 ± 0.10 pmol/μg protein). Dashed lines indicate cAMP production with forskolin alone. All data are presented as mean ± S.E. (n = 3 or 4, in duplicate).
To further verify that the effect of MβCD on μ-opioid-mediated overshoot was due to reduction in cellular cholesterol, we used the cholesterol-lowering drug lovastatin, which inhibits the rate-limiting enzyme in cholesterol synthesis, 3-hydroxy-3-methylglutaryl-CoA reductase. HEK FLAG-μ cells were treated with the activated open ring form of lovastatin (lovastatin hydroxy acid) for 48 h to lower cholesterol by 28.3 ± 0.07% (p = 0.02) as compared with vehicle-treated cells. Under this treatment paradigm, lovastatin abolished the ability of the μ-opioid agonist DAMGO (100 nm) to elicit overshoot (Fig. 5C; p < 0.001). To prevent cellular uptake of cholesterol from the serum present in the normal growth media (DMEM with 10% FBS), cells were grown in lovastatin or DMSO vehicle containing Opti-MEM for 48 h. Cells were 70–80% viable as assessed by trypan blue exclusion after 48 h in Opti-MEM. Furthermore, the overshoot elicited by the μ-agonist DAMGO was similar in cells cultured in Opti-MEM or regular growth media (DMEM with 10% FBS) (Fig. 5C). Additionally, there was no effect of media type or lovastatin treatment on the forskolin response (DMEM/FBS = 1.06 ± 0.03 pmol of cAMP/μg of protein; Opti-MEM + DMSO = 1.30 ± 0.04 pmol of cAMP/μg of protein; Opti-MEM + lovastatin = 1.02 ± 0.12 pmol of cAMP/μg of protein).
Similarly, differentiated SH-SY5Y cells were treated with lovastatin hydroxy acid for 48 h to measure the effect on overshoot mediated by endogenous μ-opioid receptors. Lovastatin treatment reduced cholesterol by 17 ± 2.4% compared with vehicle-treated controls, which was not as robust as the reduction observed with MβCD or with lovastatin in HEK cells. However, there was a significant reduction in the ability of the μ-opioid agonist DAMGO (1 μm, 1 h) to cause adenylyl cyclase overshoot (Fig. 5D). Production of cAMP by 5 μm forskolin was similar in lovastatin and vehicle-treated SH-SY5Y cells (vehicle = 0.93 ± 0.07 pmol/μg protein; lovastatin = 0.89 ± 0.10 pmol/μg protein). Together, the data obtained from replenishment of cholesterol and reduction of cholesterol using the synthesis inhibitor lovastatin indicate that MβCD prevents μ-opioid agonist sensitization of adenylyl cyclase by a mechanism dependent on lowered cholesterol.
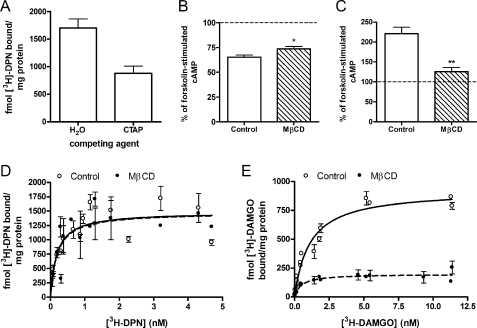
Receptor Number Is Not Responsible for Alterations in μ-Opioid Receptor Signaling by MβCD
Both the HEK FLAG-μ and HEK FLAG-δ cells used in this study express a high number of opioid receptors (9.7 ± 1.3 pmol/mg protein and 8.4 ± 1.5 pmol/mg protein, respectively), so it is unlikely that the difference observed between μ- and δ-agonists is due to different expression levels. Furthermore, HEK FLAG-μ cells treated with 2% MβCD for 1 h retained a similar level of cell surface μ-opioid receptors as vehicle-treated cells (92.2 ± 3.1%; p = 0.107; n = 2, in triplicate) as determined by immunoassays directed toward the extracellular FLAG epitope performed as described previously (38). Of the total μ-opioid receptors in HEK FLAG-μ cells, 51 ± 4% of the receptors are on the cell surface as determined by competition binding in whole cells with the membrane-impermeable μ-opioid antagonist CTAP and the cell-permeable antagonist [3H]diprenorphine (Fig. 6A). To further confirm that the high receptor number is not responsible for the alteration in μ-opioid signaling to adenylyl cyclase, we repeated experiments using a HEK FLAG-μ clone that expressed six times less μ-opioid receptors (1.6 ± 0.1 pmol/mg protein). Similar to results in the high expressing clone, treatment of this lower receptor-expressing HEK FLAG-μ clone with 2% MβCD reduced the ability of the μ-opioid agonist DAMGO to acutely inhibit adenylyl cyclase (Fig. 6B; p < 0.05) and greatly blocked its ability to cause adenylyl cyclase sensitization (Fig. 6C; p < 0.01). Furthermore, treatment of these cells with MβCD did not reduce total receptor number as determined by saturation binding of the opioid antagonist [3H]diprenorphine (Bmax of control = 1560 ± 108 fmol/mg; MβCD = 1580 ± 64 fmol/mg; Fig. 6D). Similarly, treatment of SH-SY5Y cells with 5 mm MβCD for 10 min did not alter maximal (4 nm) [3H]diprenorphine binding (control = 879 ± 51 fmol/mg protein; MβCD = 858 ± 43 fmol/mg protein; p > 0.05; n = 2, in triplicate).
FIGURE 6.
Alterations in μ-opioid signaling are not due to changes in receptor number. A, surface expression of μ-opioid receptors was determined in whole cells (1 × 105 cells/tube) by displacement of 4 nm [3H]DPN by the cell-impermeable antagonist CTAP (10 μm). Nonspecific binding was determined using the cell-permeable antagonist naloxone (10 μm). B and C, HEK FLAG-μ cells expressing only 1.6 fmol/mg receptor were treated with (hatched bars) or without (open bars) 2% MβCD for 1 h prior to acute cyclase inhibition (B) or adenylyl cyclase overshoot (C) experiments with 10 nm DAMGO or 100 nm DAMGO, respectively. Data are graphed as percent of forskolin-stimulated cAMP, represented by the hatched line (n = 3, in duplicate). *, p < 0.05; **, p < 0.01 by unpaired, two-tailed Student's t test. D and E, saturation binding experiments with the opioid antagonist [3H]DPN (D) or the μ-opioid agonist [3H]DAMGO (E) in membranes from low expressing HEK FLAG-μ cells treated with (closed circles) or without (open circles) 2% MβCD for 1 h. Control cells (open circles) in E were incubated with vehicle for 3 h. Nonspecific binding was determined using 10 μm naloxone. Data are presented as mean ± S.E. (n = 3, in duplicate). D, [3H]diprenorphine binding was unchanged following cholesterol depletion as follows: Bmax, control = 1560 ± 108 fmol/mg; MβCD = 1580 ± 64 fmol/mg; Kd, control = 0.20 ± 0.07 nm; MβCD = 0.18 ± 0.01 nm. E, [3H]DAMGO Bmax decreased (control = 944 ± 85 fmol/mg; MβCD = 205 ± 24 fmol/mg; p = 0.0019 by unpaired two-tailed Student's t test) with no change in Kd (control = 0.86 ± 0.33 nm; MβCD = 0.60 ± 0.24 nm).
The antagonist [3H]diprenorphine can recognize all affinity states of the μ-opioid receptor, but as an agonist [3H]DAMGO will only label high affinity sites at the concentrations used in saturation binding assays. Therefore, in HEK FLAG-μ cells the maximum number of receptors bound by [3H]DAMGO (944 ± 85 fmol/mg protein; Fig. 6E) was expectedly less than those bound by [3H]diprenorphine. Furthermore, treatment of these cells with MβCD significantly reduced the Bmax of [3H]DAMGO to 205 ± 24 (p = 0.002), indicating a loss of μ-opioid receptors in a high affinity state. This is consistent with the increase in low affinity agonist binding observed in competition binding assays following cholesterol depletion by MβCD (Fig. 2 B). These results support the previous observations from a high receptor-expressing model and suggest that the effects of MβCD on decreasing μ-opioid receptor-G protein coupling and downstream signaling to adenylyl cyclase is not merely the result of a loss of receptors.
Effect of Cytoskeletal Disruption on μ-Opioid-induced Adenylyl Cyclase Sensitization
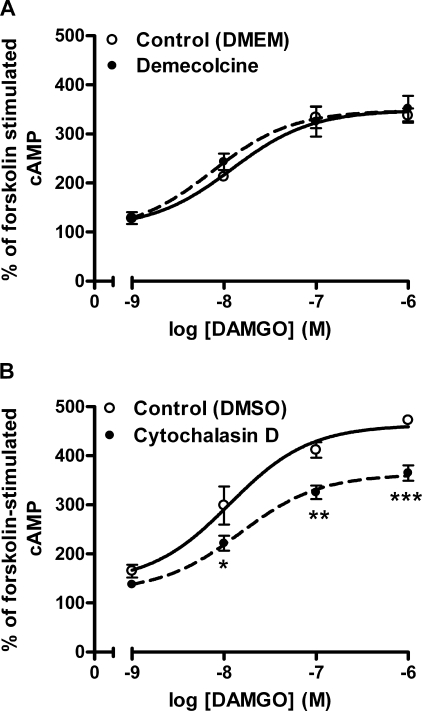
Disruption of either actin or tubulin has been shown to perturb caveolae and raft microdomains in rodent cardiac myocytes and S49 T-lymphoma cells (39). To address the effects of caveolae and raft disruption using cytoskeletal inhibitors, HEK FLAG-μ-cells were treated with demecolcine to prevent microtubule polymerization or cytochalasin D to disrupt actin filament organization. Demecolcine is a close analog of colchicine, which has been shown to disrupt rafts (39). Demecolcine (1 μg/ml) for 16–20 h produced rounding cell morphology similar to 1 h of MβCD treatment, but it did not inhibit μ-opioid agonist-induced sensitization of adenylyl cyclase (Fig. 7A). In contrast, cytochalasin D (20 μm, 1.5 h), which has previously been shown to disrupt rafts (39), caused slimming of the cells and decreased the ability of DAMGO to sensitize adenylyl cyclase (treatment, F(1,34) = 42.14, p < 0.0001). Cytochalasin D altered the efficacy of DAMGO to produce sensitization at maximal concentrations (p < 0.01 at 0.1 μm and p < 0.001 at 1 μm DAMGO), but it did not shift the potency of DAMGO-mediated sensitization (EC50 after DMSO vehicle = 13.4 ± 8.4 nm and after cytochalasin D = 17.8 ± 5.7 nm, p = 0.676; Fig. 7B). Neither demecolcine nor cytochalasin D treatment affected cAMP produced by forskolin alone (control = 1.14 ± 0.09 pmol/μg versus demecolcine = 1.13 ± 0.07 pmol/μg; DMSO = 1.06 ± 0.05 pmol/μg versus cytochalasin D = 1.08 ± 0.08 pmol/μg). These data demonstrate that although the effect of MβCD on DAMGO-mediated signaling was due to removal of cholesterol, it could not be mimicked using a tubulin inhibitor, even though tubulin inhibitors have been shown to disrupt rafts (39). However, the actin inhibitor cytochalasin D did attenuate μ-opioid overshoot, indicating a potential role for actin in adenylyl cyclase sensitization.
FIGURE 7.
Effect of cytoskeleton inhibitors on μ-opioid-mediated adenylyl cyclase sensitization. HEK FLAG-μ cells were treated with (filled circles) or without (open circles) the tubulin inhibitor demecolcine (1 μg/ml, overnight) (A) or the actin inhibitor cytochalasin D (20 μm, 1.5 h) (B) prior to induction of adenylyl cyclase sensitization by the μ-agonist DAMGO as described under “Experimental Procedures.” Data are presented as mean ± S.E. (n = 3, in duplicate). *, p < 0.05; **, p < 0.01; ***, p < 0.001 by two-way ANOVA with Bonferroni's post hoc test. Forskolin stimulation was not affected by cytoskeleton disruption in either experiment (p > 0.05 by two-way ANOVA and Bonferroni's post hoc test): control = 1.14 ± 0.09 pmol/μg versus demecolcine = 1.13 ± 0.07 pmol/μg; DMSO = 1.06 ± 0.05 pmol/μg versus cytochalasin D = 1.08 ± 0.08 pmol/μg.
Differential Membrane Localization of Opioid Receptors and Adenylyl Cyclase
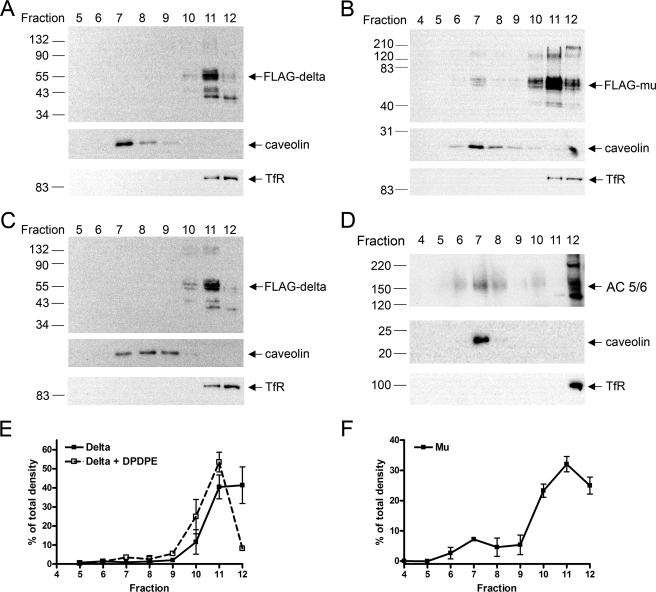
The disparate importance of cholesterol for μ- but not for δ-opioid receptor signaling described above could be explained by direct interaction of the receptor with cholesterol or differing localization of receptors and signaling proteins in putative cholesterol-enriched membrane rafts. Membrane rafts are identified by their buoyancy and insolubility in non-ionic detergents, such as Triton X-100 (40). To assess the localization of μ- and δ-opioid receptors in putative membrane rafts in HEK293 cells, a detergent-based method was employed similar to that described by Brady et al. (41). HEK FLAG-δ or HEK FLAG-μ cells were homogenized in 1% Triton X-100 and separated on a 5:30:40% discontinuous sucrose gradient, after which 12 fractions were collected from the top. Fractions 7–9 contained a cloudy band that floated at the interface between the 5 and 30% sucrose layers, consistent with the definition of membrane rafts. These fractions were also enriched in the cholesterol-binding protein caveolin (Fig. 8). Transferrin receptor, which is found in clathrin-coated pits and is excluded from caveolin-enriched fractions (41, 42), was used as a marker to identify the cholesterol-poor, detergent-soluble membrane fractions. These solubilized membranes are found in the dense 40% sucrose fractions located at the bottom of the discontinuous gradient as evidenced by transferrin receptor immunoreactivity found in fractions 11 and 12 (Fig. 8). The separation between the caveolin-containing fractions and the transferrin receptor-containing fractions indicates the quality of the preparation and efficient separation of the putative raft and non-raft membrane types. Under these conditions, the δ-opioid receptor was observed only in the transferrin receptor-containing fractions (Fig. 8, A and E), whereas the μ-opioid receptor was found in both caveolin-containing and transferrin receptor-containing fractions (Fig. 8, B and F). The δ-opioid receptor was not observed in the caveolin-containing fractions even after overexposure of the blot (data not shown). Although this separation method is inherently nonquantitative for the amount of protein in detergent-resistant fractions (43), analysis of the signal in each fraction from multiple experiments illustrated the less exclusive distribution of μ-opioid receptors in both caveolin-enriched and nonenriched fractions compared with δ-opioid receptors (Fig. 8, E and F).
FIGURE 8.
Localization of μ- but not δ-opioid receptors in caveolin-enriched fractions, in addition to transferrin receptor-enriched fractions. Detergent-resistant membranes were prepared from HEK FLAG-δ cells (A, C, and D) or HEK FLAG-μ cells (B) as described under “Experimental Procedures.” Equal volume-loaded samples were assessed for localization of opioid receptor (using anti-FLAG M1 antibody), adenylyl cyclase 5/6 (AC 5/6), caveolin (a raft marker), or transferrin receptor (TfR, a non-raft marker) as indicated. C, treatment of HEK FLAG-δ cells with the δ-agonist, DPDPE (10 μm, 5 min), prior to homogenization did not change the localization of δ receptors compared with untreated cells (A). E and F, the mean pixel density of the FLAG-δ or FLAG-μ-opioid receptor (55-kDa band) in each fraction of a separation experiment was quantified and compared with the total mean pixel density in all lanes for an experiment. Note that equal volume loading and presence of intracellular receptors in soluble fractions (11 and 12) precludes truly representative quantification. Results are presented as a percent of the total pixel density in each fraction from 2 to 7 separate experiments.
Many of the signaling proteins that δ-opioid receptors activate have been identified in cholesterol-enriched membranes, including Gαi (12) and adenylyl cyclase isoforms 3 and 5/6 (18). Consequently, we tested the hypothesis that δ-opioid receptors would move into cholesterol-enriched membranes and therefore associate with these signaling proteins upon agonist stimulation. Such movement into rafts has been reported previously for muscarinic (M2) and purinergic A1 receptors (7). However, treatment of HEK FLAG-δ cells with a 10 μm concentration of the δ-opioid agonist DPDPE for 5 min did not change the localization of the receptors (Fig. 8, C and E).
Because δ-opioid receptor signaling to adenylyl cyclase is unaffected by cholesterol depletion, this raises the question of which adenylyl cyclase isoform the δ-opioid receptor is coupled to, especially as adenylyl cyclase enzymes that are inhibited by the δ-opioid receptor have been found in rafts, including AC 5/6 (18). However, it is likely that the propensity for the adenylyl cyclases to localize only to cholesterol-enriched domains is not absolute and that cyclases are also present in other areas of the plasma membrane. In HEK293 cells, endogenous AC 5/6 was detected in transferrin receptor-containing fractions, in addition to the caveolin-containing fractions, following detergent solubilization (Fig. 8D). Therefore, δ receptors would not need to reside in high cholesterol regions of the membrane to effectively signal to adenylyl cyclase.
Cholera Toxin B-induced Patching
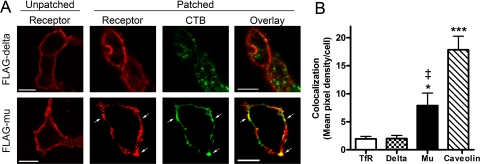
CTB-induced patching was used to further examine the membrane localization characteristics of μ- and δ-opioid receptors in intact cells using a method that does not rely on cell disruption or detergent solubilization. This method has been used previously to identify raft-associated proteins (42). With an estimated size of 10–200 nm (4), putative membrane rafts are too small for observation by conventional light microscopy but can be clustered into patches by CTB, which binds with high affinity as a pentamer to the raft-associated ganglioside GM1 (42). Further clustering with an anti-CTB antibody aggregated the CTB-GM1 complexes into patches visible by confocal microscopy (Fig. 9A).
FIGURE 9.
ä-Opioid receptor is not colocalized with CTB subunit patches. A, HEK FLAG-δ or FLAG-μ cells were plated on poly-d-lysine-coated coverslips 24 h prior to patching with cholera toxin B subunit conjugated to AlexaFluor 488 at 4 °C to label endogenous ganglioside GM1, enriched in lipid rafts. Lipid raft clustering was induced by incubating with goat anti-CTB for 30 min at 4 °C, followed by 20 min at 37 °C. Cells were fixed with 4% paraformaldehyde, and δ or μ receptors were stained using mouse anti-FLAG M1 antibody followed by goat anti-mouse AlexaFluor 594. Fluorescent images of 0.5-μm Z planes were captured using confocal microscopy. Scale bar, 10 μm. B, quantification of colocalization with CTB from at least 25 cells from three separate experiments is presented as mean pixel density of colocalization per cell ± S.E. *, p < 0.05; ***, p < 0.001 compared with TfR or δ by two-tailed unpaired Student's t test; ‡, p = 0.005 compared with caveolin by two-tailed unpaired Student's t test.
Staining of the FLAG epitope on the extracellular N terminus of δ-opioid receptors following patching showed that δ-opioid receptors on the cell surface were poorly colocalized with CTB-clustered raft patches. In fact, upon quantification of over 25 cells from at least three separate experiments, δ-opioid receptors colocalized with CTB to the same degree as the non-raft marker protein transferrin receptor (Fig. 9B). In contrast, μ-opioid receptors colocalized with many CTB patches, as indicated by arrows (Fig. 9A). Upon quantification, μ-opioid receptors were colocalized with CTB to a higher degree than δ receptors (μ = 7.89 ± 2.22 versus δ = 2.00 ± 0.56 pixel density/cell, p = 0.01) or transferrin receptors (1.93 ± 0.46 pixel density/cell, p = 0.01) but to a significantly lesser degree than caveolin (17.85 ± 2.41 pixel density/cell, p = 0.005). Together, these localization and cell signaling experiments indicate that in HEK293 cells μ-opioid receptors tend to depend on cholesterol and can interact with cholesterol-rich domains of the cell membrane more than δ-opioid receptors.
DISCUSSION
In this study we have used FLAG-tagged μ- and δ-opioid receptors expressed in HEK293 cells to ask questions concerning the localization and signaling of these two highly homologous plasma membrane proteins. Two conclusions can be drawn from this study. First, μ-opioid receptor signaling is sensitive to the cholesterol content of the cell membrane, with a much greater effect on signaling following chronic agonist exposure. This conclusion was reached by removal and replenishment of plasma membrane cholesterol using the cholesterol-sequestering agent MβCD and by cholesterol synthesis inhibition by lovastatin. Furthermore, the decrease in μ-opioid signaling appears to be due to an uncoupling of the μ-opioid receptor from its G protein and is not due to a decrease in receptor number. The effect of MβCD on adenylyl cyclase sensitization is also not a product of using a heterologous expression system or a high receptor number as shown by experiments using SH-SY5Y cells and HEK cells expressing a lower level of μ-opioid receptors. Under the same conditions, δ-opioid receptor signaling and G protein coupling are not affected. Second, using fractionation of the cell membrane and CTB patching of whole cells, we demonstrate the localization of δ-opioid receptors outside of cholesterol-enriched domains, but a less exclusive localization of μ-opioid receptors in both cholesterol-enriched and cholesterol-poor membrane regions. This differential distribution pattern supports the differential sensitivity of μ- and δ-opioid signaling to cholesterol.
There are two hypotheses to explain the differential dependence of μ- and δ-opioid receptors on membrane cholesterol. First, the partial localization of μ-opioid receptors in detergent-insoluble domains may result from a higher propensity for these receptors to bind to cholesterol or caveolin than the δ-opioid receptor. Although both μ- and δ-opioid receptors contain one of the proposed caveolin-binding motifs (ΦXΦXXXXΦ; where Φ = Phe, Trp, or Tyr) (44) at the interface of TM7 and the C-terminal tail, the μ-opioid receptor contains more aromatic and hydrophobic residues in the area immediately distal to this region. This difference may be especially important for interactions with cholesterol in the membrane because it is in the same region of the receptor that was shown to interact with cholesterol in the β2-adrenergic receptor crystal structure (45) and includes the conserved palmitoylated cysteine. Even though the δ-opioid receptor contains a caveolin binding domain, this may not be enough for the receptor to bind tightly to caveolin, because the binding domain is a rather general sequence that could be found on many proteins. For example, Gαi2 contains a caveolin binding domain (44) but has not always been shown to associate with caveolin (8).
Alternatively, the δ-opioid receptor may not need the extra membrane rigidity provided by cholesterol to support signaling because it is known to couple tightly to G proteins (32, 46). In fact, Andre et al. (25) conclude that δ-opioid receptors can form a high affinity state that is stabilized by G proteins rather than cholesterol. The μ receptor, however, is less tightly coupled to G proteins (47) and may depend on cholesterol to aid in this coupling even in non-raft regions of the plasma membrane. This is supported by evidence that cholesterol alone can stabilize μ receptors in a high affinity state (23), and increasing membrane viscosity with a cholesterol analog has been shown to improve the potency of the μ-opioid receptor agonist DAMGO to stimulate [35S]GTPγS binding (22).
The heterogeneous distribution of μ-opioid receptors in caveolin-enriched and caveolin-poor fractions may explain the rather small effect of cholesterol depletion on acute μ-opioid signaling (either [35S]GTPγS binding or adenylyl cyclase inhibition). However, this effect was greatly amplified when cells were treated chronically with a μ-opioid agonist to induce adenylyl cyclase sensitization. The complexity of adenylyl cyclase sensitization (21) may explain the more dramatic effect of cholesterol depletion (either by MβCD or lovastatin) on adenylyl cyclase sensitization versus acute inhibition of adenylyl cyclase by DAMGO. Besides the previously mentioned G proteins, many of the other signaling pathways postulated to play a role in adenylyl cyclase sensitization have also been shown to be associated with cholesterol-enriched domains, such as Raf-1 (48), protein kinase C (49), and Src kinase (50). Recently, Src kinase has been implicated as an important mediator of μ-opioid adenylyl cyclase sensitization in putative rafts (51). In addition, many of the adenylyl cyclase isoforms that can be sensitized (AC 5, 6, and 8 (21)) have also been found in cholesterol-enriched domains (AC 5/6 (9, 18, 19) and 8 (20)). Exceptions to this include AC 1, which can be sensitized by a variety of agonists (52–54) but has not been found in rafts (18), and AC 3, which is preferentially found in cholesterol-enriched membranes (18) but is not sensitized by several agonists, including the μ-opioid agonist morphine (52, 53). Additionally, sensitization caused by chronic agonist treatment may require a redistribution of the heterotrimeric G protein to detergent-insoluble domains. In a study using Chinese hamster ovary-μ cells, chronic morphine treatment increased the amount of Gαi and Gβ1 in the cholate-insoluble cellular fraction (with a corresponding decrease of G protein in the cholate-soluble fraction) in a time-dependent manner that was similar to the time course of adenylyl cyclase sensitization (55). This redistribution of Gαi and Gβ1 was reversed following removal of morphine in a similar time frame as the loss of sensitization. Therefore, cholesterol depletion may prevent the development of sensitization by preventing the shift of heterotrimeric G proteins to detergent-insoluble microdomains.
Although a percentage of μ-opioid receptors were found in detergent-insoluble domains, most were found in putative non-raft domains. Because the effect of cholesterol depletion by MβCD or lovastatin on adenylyl cyclase sensitization was very robust, this may suggest a loss of cholesterol function on μ-opioid receptors, rather than, or in addition to, disruption of rafts. The cytoskeleton, including both actin and tubulin, has also been shown to be important for the formation of lipid rafts and caveolae (39). Therefore, the lack of effect of the tubulin inhibitor demecolcine on μ-agonist-mediated adenylyl cyclase overshoot would point away from a raft mechanism. Even so, with the actin inhibitor cytochalasin D, we do see a decrease in the ability of the μ-opioid agonist DAMGO to cause overshoot. However, differences between MβCD and cytochalasin D, including morphology changes and the lack of effect of cytochalasin D on forskolin-stimulated cAMP production, argue against a contributing role of actin disruption in the mechanism of action of MβCD. Therefore, it appears most likely that cholesterol and actin are both important independently for μ-opioid overshoot. To our knowledge this is the first report of a role for actin in adenylyl cyclase sensitization, although there is evidence of increased actin cycling following withdrawal from chronic morphine treatment in rats (56).
The increase in forskolin-stimulated cAMP production observed immediately following cholesterol removal by MβCD has been observed by others (31, 34). However, this enhanced forskolin effect is lost if a 2-h incubation in serum-free media is included following cholesterol removal, even though cholesterol levels remain depleted. Furthermore, chronic inhibition of cholesterol synthesis using lovastatin for 48 h did not affect the forskolin response. Therefore, the effect of cholesterol removal on forskolin appears transient as the cell has to rapidly adapt to the loss of cholesterol. In addition, basal and isoproterenol activation of adenylyl cyclase through Gαs has been shown to increase following cholesterol depletion by MβCD or cholesterol oxidase (31, 34). Thus, the increased forskolin response could be due to increased synergistic activation of adenylyl cyclase with Gαs. Results from experiments by us and others (31) using pertussis toxin suggest that the increase in forskolin or isoproterenol response does not involve receptor-activated Gαi/o proteins.
The results from the sucrose gradient membrane separation experiments are consistent with reports of the localization of μ-opioid receptors in caveolin-enriched membrane fractions as well as caveolin-poor fractions (9, 23). In contrast, we found δ-opioid receptors only in the same cellular fractions as transferrin receptor, a non-raft marker. This finding differs from previously published reports of δ-opioid receptor localization in putative raft fractions (10, 11). These conflicting data may relate to the method used for isolation of membrane microdomains, about which there is much debate (6, 7). Previous studies of δ-opioid receptor localization used high molarity sodium carbonate buffer, pH 11, for homogenization prior to sucrose gradient ultracentrifugation to separate cholesterol-enriched from cholesterol-deficient membranes. Although this method has been commonly employed to study protein content in putative lipid rafts (7, 57), there are some problems intrinsic to this method. The primary concern with the nondetergent method is a lack of specificity for isolating putative raft proteins, because even non-plasma membrane proteins, such as mitochondrial proteins, are found in the cholesterol-enriched fractions (15). Using the sodium carbonate method, we have also identified δ-opioid receptors in more buoyant, caveolin-containing fractions. However, these fractions also contained significant amounts of the non-raft marker transferrin indicating a lack of separation between putative raft and non-raft membranes (data not shown). Thus, we are not confident using this method to identify raft proteins. Previous studies (10, 11) did not include a negative control, such as transferrin receptor, for comparison.
The detergent method used in this study also has limitations, but it is more stringent for identification of cholesterol-binding proteins (15). Recently, δ-opioid receptor localization has been studied using the detergent Triton X-100 in various concentrations (25). Although this study identified a small percentage (∼5%) of δ receptors in raft-like fractions, the majority of δ receptors was found in the non-raft pellet, which generally agrees with our findings. In addition, our identification of δ-opioid receptors in caveolin- and cholesterol-poor fractions was supported by the lack of association of δ-opioid receptors with raft domains as labeled by CTB patching of intact cells. Furthermore, these localization results are consistent with the δ-opioid signaling data in this study demonstrating that cholesterol depletion by MβCD did not affect agonist-mediated δ-opioid receptor signaling.
Although the evidence presented here suggests that δ-opioid receptors are excluded from cholesterol-enriched regions, many signaling molecules, including Gαi/o proteins (12–17), known to be activated by the δ-opioid receptor have been found in cholesterol-enriched fractions or associated with caveolin. One possible explanation we have excluded is that the δ-opioid receptor moves into cholesterol-enriched fractions upon agonist treatment. A more likely possibility is that although many signaling proteins are found in rafts, this relationship is not exclusive, and these proteins are also functioning outside of raft domains. For example, Gαi proteins have also been found in cholesterol-poor fractions in cardiomyocytes (8) and in these HEK cells (data not shown) so that δ-opioid receptors could signal through these Gαi proteins. In addition, adenylyl cyclase isoforms 3 and 5/6 are primarily, but not exclusively, found in caveolin-containing fractions (18). In our preparations, adenylyl cyclase 5/6 is found in transferrin receptor-containing fractions. Moreover, δ-opioid receptors may also signal through adenylyl cyclase 1, which has not been found in cholesterol-enriched domains (18, 58), is readily sensitized (21), and is present in HEK293 cells (59).
In conclusion, these studies demonstrate differences in signaling and localization properties of μ- and δ-opioid receptors, using identical methods in the same HEK cell system, and are supported by studies in SH-SY5Y neuroblastoma cells that endogenously express opioid receptors. δ-Opioid receptors, which were not found in cholesterol-enriched membranes, were not as affected by changes in the microenvironment of the membrane. In contrast, μ-opioid receptors, to which agonists cause robust dependence clinically, are significantly altered by cholesterol modulation, with a greater effect on chronic signaling than acute signaling. Although these effects are due to cholesterol removal, these studies cannot confirm that they are due to lipid raft disruption and may suggest a need for cholesterol to provide efficient μ-opioid receptor signaling in non-raft regions of the plasma membrane. Regardless, these findings have implications for cellular signaling in general, especially in light of the recent, somewhat controversial (60, 61) lowering of clinical cholesterol guidelines (62).
Acknowledgments
We thank Faye A. Bradbury and Christine S. Zelnik for help with cell surface receptor assays and Alexander A. Harris for assistance with binding assays. We thank Dr. Lee-Yuan Liu-Chen at Temple University School of Medicine, Philadelphia, PA, for providing the FLAG-tagged δ-opioid receptor cDNA and Dr. Lakshmi Devi at Mt. Sinai School of Medicine, New York, for providing the FLAG-tagged μ-opioid receptor cDNA.
This work was supported, in whole or in part, by National Institutes of Health (National Institute on Drug Abuse) Grants R01 DA04087 (to J. R. T.), R03 DA22377 (to J. R. T. and M. J. C.), F31 DA023339 (to E. S. L.), and HL0270973 (to J. R. M.). Parts of this work were previously presented at the Experimental Biology Meeting, April 28 to May 2, 2007, Washington, D. C.; the Experimental Biology Meeting, April 5–9, 2008, San Diego, CA; the Experimental Biology Meeting, April 18–22, 2009, New Orleans, LA; and the 39th International Narcotics Research Conference, July 13–18, 2008, Charleston, SC.
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- SNC80
- (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide
- DPDPE
- [d-Pen2,5]-enkephalin
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin
- DPN
- diprenorphine
- CTAP
- d-Phe-[Cys-Tyr-d-Trp-Arg-Thr-Pen]Thr-NH2
- MβCD
- methyl-β-cyclodextrin
- CTB
- cholera toxin B subunit
- AC
- adenylyl cyclase
- TfR
- transferrin receptor
- ANOVA
- analysis of variance
- HEK
- human embryonic kidney; FLAG epitope (DYKDDDDK)
- HEK FLAG-δ
- HEK cells stably expressing FLAG-δreceptor
- HEK FLAG-μ
- HEK cells stably expressing FLAG-μreceptor
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- MBS
- MES-buffered saline
- MES
- 2-(N-morpholino)ethanesulfonic acid
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Gimpl G., Burger K., Fahrenholz F. (1997) Biochemistry 36, 10959–10974 [DOI] [PubMed] [Google Scholar]
- 2.Slotte J. P. (1999) Chem. Phys. Lipids 102, 13–27 [DOI] [PubMed] [Google Scholar]
- 3.Simons K., Ikonen E. (1997) Nature 387, 569–572 [DOI] [PubMed] [Google Scholar]
- 4.Pike L. J. (2006) J. Lipid Res. 47, 1597–1598 [DOI] [PubMed] [Google Scholar]
- 5.Munro S. (2003) Cell 115, 377–388 [DOI] [PubMed] [Google Scholar]
- 6.Shogomori H., Brown D. A. (2003) Biol. Chem. 384, 1259–1263 [DOI] [PubMed] [Google Scholar]
- 7.Allen J. A., Halverson-Tamboli R. A., Rasenick M. M. (2007) Nat. Rev. Neurosci. 8, 128–140 [DOI] [PubMed] [Google Scholar]
- 8.Head B. P., Patel H. H., Roth D. M., Lai N. C., Niesman I. R., Farquhar M. G., Insel P. A. (2005) J. Biol. Chem. 280, 31036–31044 [DOI] [PubMed] [Google Scholar]
- 9.Zhao H., Loh H., Law P. (2006) Mol. Pharmacol. 69, 1421–1432 [DOI] [PubMed] [Google Scholar]
- 10.Huang P., Xu W., Yoon S. I., Chen C., Chong P. L., Liu-Chen L. Y. (2007) Biochem. Pharmacol. 73, 534–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel H. H., Head B. P., Petersen H. N., Niesman I. R., Huang D., Gross G. J., Insel P. A., Roth D. M. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H344– 350 [DOI] [PubMed] [Google Scholar]
- 12.Xu W., Yoon S. I., Huang P., Wang Y., Chen C., Chong P. L., Liu-Chen L. Y. (2006) J. Pharmacol. Exp. Ther. 317, 1295–1306 [DOI] [PubMed] [Google Scholar]
- 13.Moffett S., Brown D. A., Linder M. E. (2000) J. Biol. Chem. 275, 2191–2198 [DOI] [PubMed] [Google Scholar]
- 14.Quinton T. M., Kim S., Jin J., Kunapuli S. P. (2005) J. Thromb. Haemost. 3, 1036–1041 [DOI] [PubMed] [Google Scholar]
- 15.Foster L. J., De Hoog C. L., Mann M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S., Okamoto T., Chun M., Sargiacomo M., Casanova J. E., Hansen S. H., Nishimoto I., Lisanti M. P. (1995) J. Biol. Chem. 270, 15693–15701 [DOI] [PubMed] [Google Scholar]
- 17.Li S., Song K. S., Lisanti M. P. (1996) J. Biol. Chem. 271, 568–573 [PubMed] [Google Scholar]
- 18.Ostrom R. S., Liu X., Head B. P., Gregorian C., Seasholtz T. M., Insel P. A. (2002) Mol. Pharmacol. 62, 983–992 [DOI] [PubMed] [Google Scholar]
- 19.Fagan K. A., Smith K. E., Cooper D. M. (2000) J. Biol. Chem. 275, 26530–26537 [DOI] [PubMed] [Google Scholar]
- 20.Smith K. E., Gu C., Fagan K. A., Hu B., Cooper D. M. (2002) J. Biol. Chem. 277, 6025–6031 [DOI] [PubMed] [Google Scholar]
- 21.Watts V. J. (2002) J. Pharmacol. Exp. Ther. 302, 1–7 [DOI] [PubMed] [Google Scholar]
- 22.Emmerson P. J., Clark M. J., Medzihradsky F., Remmers A. E. (1999) J. Neurochem. 73, 289–300 [DOI] [PubMed] [Google Scholar]
- 23.Gaibelet G., Millot C., Lebrun C., Ravault S., Sauliere A., Andre A., Lagane B., Lopez A. (2008) Mol. Membr. Biol. 25, 423–435 [DOI] [PubMed] [Google Scholar]
- 24.Huang P., Xu W., Yoon S. I., Chen C., Chong P. L., Unterwald E. M., Liu-Chen L. Y. (2007) Brain Res. 1184, 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.André A., Gaibelet G., Le Guyader L., Welby M., Lopez A., Lebrun C. (2008) Biochim. Biophys. Acta 1778, 1483–1492 [DOI] [PubMed] [Google Scholar]
- 26.Christian A. E., Haynes M. P., Phillips M. C., Rothblat G. H. (1997) J. Lipid Res. 38, 2264–2272 [PubMed] [Google Scholar]
- 27.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 28.Clark M. J., Harrison C., Zhong H., Neubig R. R., Traynor J. R. (2003) J. Biol. Chem. 278, 9418–9425 [DOI] [PubMed] [Google Scholar]
- 29.Fra A. M., Williamson E., Simons K., Parton R. G. (1994) J. Biol. Chem. 269, 30745–30748 [PubMed] [Google Scholar]
- 30.Rodal S. K., Skretting G., Garred O., Vilhardt F., van Deurs B., Sandvig K. (1999) Mol. Biol. Cell 10, 961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pontier S. M., Percherancier Y., Galandrin S., Breit A., Galés C., Bouvier M. (2008) J. Biol. Chem. 283, 24659–24672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa T., Klinz F. J., Vachon L., Herz A. (1988) Mol. Pharmacol. 34, 744–754 [PubMed] [Google Scholar]
- 33.Zaki P. A., Keith D. E., Jr., Thomas J. B., Carroll F. I., Evans C. J. (2001) J. Pharmacol. Exp. Ther. 298, 1015–1020 [PubMed] [Google Scholar]
- 34.Rybin V. O., Xu X., Lisanti M. P., Steinberg S. F. (2000) J. Biol. Chem. 275, 41447–41457 [DOI] [PubMed] [Google Scholar]
- 35.Encinas M., Iglesias M., Liu Y., Wang H., Muhaisen A., Ceña V., Gallego C., Comella J. X. (2000) J. Neurochem. 75, 991–1003 [DOI] [PubMed] [Google Scholar]
- 36.Påhlman S., Ruusala A. I., Abrahamsson L., Mattsson M. E., Esscher T. (1984) Cell Differ. 14, 135–144 [DOI] [PubMed] [Google Scholar]
- 37.Cheema T. A., Fisher S. K. (2008) J. Pharmacol. Exp. Ther. 324, 648–657 [DOI] [PubMed] [Google Scholar]
- 38.Divin M. F., Bradbury F. A., Carroll F. I., Traynor J. R. (2009) Br. J. Pharmacol. 156, 1044–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Head B. P., Patel H. H., Roth D. M., Murray F., Swaney J. S., Niesman I. R., Farquhar M. G., Insel P. A. (2006) J. Biol. Chem. 281, 26391–26399 [DOI] [PubMed] [Google Scholar]
- 40.Brown D. A., London E. (1998) Annu. Rev. Cell Dev. Biol. 14, 111–136 [DOI] [PubMed] [Google Scholar]
- 41.Brady J. D., Rich T. C., Le X., Stafford K., Fowler C. J., Lynch L., Karpen J. W., Brown R. L., Martens J. R. (2004) Mol. Pharmacol. 65, 503–511 [DOI] [PubMed] [Google Scholar]
- 42.Janes P. W., Ley S. C., Magee A. I. (1999) J. Cell Biol. 147, 447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown D. A. (2006) Physiology 21, 430–439 [DOI] [PubMed] [Google Scholar]
- 44.Couet J., Li S., Okamoto T., Ikezu T., Lisanti M. P. (1997) J. Biol. Chem. 272, 6525–6533 [DOI] [PubMed] [Google Scholar]
- 45.Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Kuhn P., Weis W. I., Kobilka B. K., Stevens R. C. (2007) Science 318, 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polastron J., Jauzac P., Meunier J. C. (1992) Eur. J. Pharmacol. 226, 133–139 [DOI] [PubMed] [Google Scholar]
- 47.Chakrabarti S., Yang W., Law P. Y., Loh H. H. (1997) Mol. Pharmacol. 52, 105–113 [PubMed] [Google Scholar]
- 48.Mineo C., James G. L., Smart E. J., Anderson R. G. (1996) J. Biol. Chem. 271, 11930–11935 [DOI] [PubMed] [Google Scholar]
- 49.Weerth S. H., Holtzclaw L. A., Russell J. T. (2007) Cell Calcium. 41, 155–167 [DOI] [PubMed] [Google Scholar]
- 50.Mukherjee A., Arnaud L., Cooper J. A. (2003) J. Biol. Chem. 278, 40806–40814 [DOI] [PubMed] [Google Scholar]
- 51.Zhang L., Zhao H., Qiu Y., Loh H. H., Law P. Y. (2009) J. Biol. Chem. 284, 1990–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avidor-Reiss T., Nevo I., Saya D., Bayewitch M., Vogel Z. (1997) J. Biol. Chem. 272, 5040–5047 [DOI] [PubMed] [Google Scholar]
- 53.Nevo I., Avidor-Reiss T., Levy R., Bayewitch M., Heldman E., Vogel Z. (1998) Mol. Pharmacol. 54, 419–426 [DOI] [PubMed] [Google Scholar]
- 54.Rhee M. H., Nevo I., Avidor-Reiss T., Levy R., Vogel Z. (2000) Mol. Pharmacol. 57, 746–752 [DOI] [PubMed] [Google Scholar]
- 55.Bayewitch M. L., Nevo I., Avidor-Reiss T., Levy R., Simonds W. F., Vogel Z. (2000) Mol. Pharmacol. 57, 820–825 [DOI] [PubMed] [Google Scholar]
- 56.Toda S., Shen H. W., Peters J., Cagle S., Kalivas P. W. (2006) J. Neurosci. 26, 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song K. S., Li Shengwen, Okamoto T., Quilliam L. A., Sargiacomo M., Lisanti M. P. (1996) J. Biol. Chem. 271, 9690–9697 [DOI] [PubMed] [Google Scholar]
- 58.Ostrom R. S., Insel P. A. (2004) Br. J. Pharmacol. 143, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Premont R. T. (1994) Methods Enzymol. 238, 116–127 [DOI] [PubMed] [Google Scholar]
- 60.Pitt B. (2005) N. Engl. J. Med. 352, 1483–1484 [DOI] [PubMed] [Google Scholar]
- 61.LaRosa J. C., Grundy S. M., Waters D. D., Shear C., Barter P., Fruchart J. C., Gotto A. M., Greten H., Kastelein J. J., Shepherd J., Wenger N. K. (2005) N. Engl. J. Med. 352, 1425–1435 [DOI] [PubMed] [Google Scholar]
- 62.Smith S. C., Jr., Allen J., Blair S. N., Bonow R. O., Brass L. M., Fonarow G. C., Grundy S. M., Hiratzka L., Jones D., Krumholz H. M., Mosca L., Pearson T., Pfeffer M. A., Taubert K. A. (2006) J. Am Coll. Cardiol. 47, 2130–2139 [DOI] [PubMed] [Google Scholar]