Abstract
A surge in cytosolic calcium ion concentration by entry of extracellular Ca2+ is a hallmark of T cell activation. According to store-operated Ca2+ entry mechanism, the Ca2+ entry is preceded by activation of phospholipase C-γ1 (PLC-γ1) and the consequent mobilization of intracellular Ca2+. Using membrane vesicles expressing the mouse class I major histocompatibility complex, i.e. Ld plus costimulatory ligands, i.e. B7-1 and intercellular adhesion molecule-1 along with 2C T cell receptor transgenic T cells, we investigated the roles of CD28 and LFA-1 (lymphocyte function-associated antigen-1) in the activation of PLC-γ1 and Ca2+ signaling. Both CD28 and LFA-1 made significant and comparable contributions to the activation of PLC-γ1 as gauged by the level of its phosphorylation at tyrosine 783. In contrast, their roles in Ca2+ signaling were quite distinct so that LFA-1/intercellular adhesion molecule-1 interaction exerted a determining role, whereas CD28/B7-1 interaction played only a minimal role. In particular, when the T cells were activated by suboptimal T cell receptor stimulation, LFA-1 played an indispensable role in the Ca2+ signaling. Further experiments using Ca2+-free medium demonstrated that the entry of extracellular Ca2+ was not always accompanied by mobilization of intracellular Ca2+. Thus, intracellular Ca2+ mobilization was hardly detected under the condition that LFA-1 played the indispensable role in the entry of extracellular Ca2+, while a distinct level of intracellular Ca2+ mobilization was readily detected under the condition that LFA-1 played only the supporting role. These results ensure the unique role of LFA-1 in T cell Ca2+ signaling and reveal that LFA-1-dependent Ca2+ entry proceeds via a mechanism separate from store-operated Ca2+ entry.
The T cell encounter with antigen-presenting cells (APCs)2 carrying cognate peptide in the context of major histocompatibility complex (MHC) is followed by an increase in cytosolic Ca2+ ion concentration ([Ca2+]) by entry of extracellular Ca2+ (1). The extracellular Ca2+ entry is key for a myriad of physiological changes leading to cell cycle progression and development of effector functions of T cells. Downstream signaling events requiring an influx of extracellular Ca2+ involve activation of calcineurin/NF-AT and Ras/mitogen-activated protein kinase (MAPK) signaling pathways together resulting in transcriptional activation of multiple genes, including interleukin-2 (2, 3).
A well known Ca2+-signaling mechanism in T cells is store-operated Ca2+ entry (SOCE). According to that mechanism, phospholipase C-γ1 (PLC-γ1) activated by T cell receptor (TCR) stimulation catalyzes hydrolysis of phosphatidylinositol 4,5-bisphosphate to produce inositol 1,4,5-trisphosphate (IP3), which binds to the IP3 receptor in the endoplasmic reticulum to induce release (mobilization) of Ca2+ from the endoplasmic reticulum. As a result, Ca2+ channel in plasma membrane opens to allow entry of extracellular Ca2+. Recent advances in the field entail identification of specific proteins involved in the process such as STIM-1, a Ca2+ sensor in endoplasmic reticulum, and ORAI-1, a Ca2+ channel in plasma membrane (4).
LFA-1 (lymphocyte function-associated antigen-1), an integrin composed of αL (CD11a) and β2 (CD18) subunits, plays multiple roles in various stages of T cell immune responses, i.e. in activation of resting T cells, migration of activated effector T cells to the site of infection, and execution of their effector functions (5, 6). LFA-1 acts as both an adhesion and a signaling molecule so that interaction of LFA-1 with its ligand, intercellular adhesion molecule-1 (ICAM-1), not only facilitates firm contact between T cell and APC but also induces intracellular signaling events (7).
The importance of LFA-1 in Ca2+ signaling has been identified. Earlier studies showed that engagement of LFA-1 resulted in prolonged IP3 production and the sustained increase of intracellular [Ca2+] as well as stronger PLC-γ1 activation (8, 9). It was also shown that a specific motif (NPXF) in the intracellular domain of the CD18 subunit was responsible for the action of LFA-1 (10). In addition, a study by Bachmann et al. (11) showed that costimulation by LFA-1 lowered the threshold level of cognate peptide-MHC complex (pMHC) expression required for induction of extracellular Ca2+ entry during the T/APC interaction. It was also suggested that LFA-1 facilitated entry of extracellular Ca2+ by promoting the formation of immunological synapse by which production of IP3 could be amplified and stabilized (12).
We have been using nanometric membrane vesicles prepared from the purified plasma membrane fraction of Drosophila cells engineered to express Ld class I MHC plus B7-1 and ICAM-1, along with 2C TCR transgenic T cells, for studying membrane-proximal TCR signaling events leading to LFA-1 activation (13, 14). In this study, we investigated Ca2+ signaling of 2C T cells being cultured with the plasma membrane-derived membrane vesicles (pMVs), and we present data revealing that LFA-1-dependent Ca2+ entry, manifest in T cells undergoing the activation process after suboptimal TCR stimulation, proceeds via a mechanism separate from SOCE.
EXPERIMENTAL PROCEDURES
Animals
CD28−/− (B6) and β2-integrin−/− (LFA-1−/−) (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained at The Scripps Research Institute. Wild type, CD28−/−, and LFA-1−/− 2C TCR transgenic mice (15) were bred at The Scripps Research Institute.
Peptides, Chemicals, and mAbs
QL9 (QLSPFPFDL), P2Ca (LSPFPFDL), and P1A (LPYLGWLVF) peptides (13) were purchased from Invitrogen. PP2, Ly294002, wortmannin, and U73122 were purchased from EMD Bioscience.
Anti-CD28 (37.51) monoclonal Ab (mAb), anti-CD11a (M17/4) mAb, and rat mAb (RTK 2758) used as an isotype control, phycoerythrin-conjugated anti-Ld (28-14-8), anti-B7-1 (16-10A1), anti-ICAM-1 (YN1/1.7.4), and anti-B7-2 (GL-1) mAbs were purchased from Biolegend. Anti-CD8 mAb was prepared as an ascites form (a gift from Dr. Surh, Department of Immunology, The Scripps Research Institute).
Polyclonal Abs directed to pan-Akt, phospho-Akt (Thr308), PLC-γ1, and phospho-PLC-γ1 (Tyr783) were purchased from Cell Signaling Technology. Horseradish peroxidase-tagged goat anti-mouse IgG Ab was purchased from Santa Cruz Biotechnology.
T Cells, Cell Lines, and Culture Media
CD8+ 2C T cells were negatively purified from pooled lymph nodes using CD8a+ T cell isolation MACSTM kit (Miltenyi Biotec) followed by panning on anti-CD8 mAb (3.168)-coated plates (13). Drosophila APCs were maintained in Schneider's medium (Invitrogen) supplemented with 10% fetal bovine serum plus antibiotics (13).
Preparation of pMVs
The pMVs were prepared as described by Goldberg and Kornfeld (16) with modifications. In brief, Drosophila APCs induced to express transfected mouse immunomolecules were washed and resuspended in buffer A (15 mm KCl, 1.5 mm MgCl2, 1 mm dithiothreitol, 10 mm Hepes, pH 7.5) containing a mixture of protease inhibitors (Sigma) and lysed with Contes homogenizer (loose-fitting pestle). The cell lysate was spun down at low centrifugal force (1500 × g) to remove unlysed cells and nuclei. The supernatant was recovered and mixed with 0.5 volume of buffer B (375 mm KCl, 22.5 mm MgCl2, 1 mm dithiothreitol, 220 mm Hepes, pH 7.5) containing protease inhibitors and subjected to ultracentrifugation (1 h at 50,000 × g at 4 °C) to collect the crude membrane particles. The resulting pellet was resuspended in buffer C (40 mm Tris·Cl, 10 mm KCl, 10 mm NaCl, 0.2 mm EDTA, pH 7.2) and finely dispersed using Contes homogenizer (tight-fitting pestle). Resuspended membrane vesicles were fractionated by sucrose gradient ultracentrifugation, 200,000 × g for 2 h at 0 °C. Membrane vesicles derived from plasma membrane fractions were taken from the interface of 20:35% sucrose layers, aliquoted, and stored at −80 °C. The amount of pMVs was determined by measuring the protein concentration in the sample.
Western Blot Analysis
2C T cells cultured with peptide-loaded pMVs were washed with ice-cold 1× phosphate-buffered saline and lysed in a cell lysis buffer (50 mm Tris·HCl, 150 mm NaCl, 1% Igepal CA-630, pH 7.5) containing a mixture of protease inhibitors (Sigma) followed by sonication for further homogenization. Equal amounts of lysates (determined by the protein concentration) were separated by SDS-PAGE and subjected to Western blotting as described (17). Proteins of interest were visualized by ECL reagent (Pierce).
Kinetic Flow Cytometric Analysis of Cytosolic [Ca2+]
Kinetic analysis of changes in cytosolic [Ca2+] was performed as described (18). Briefly, 700 μl of purified CD8+ 2C T cells (2 × 106 cells/ml) were loaded with 3 μm Indo-1/Am (Invitrogen) for 50 min at 37 °C followed by thorough washing with ice-cold 1× phosphate-buffered saline and resuspended in normal Dulbecco's modified Eagle's medium (catalog number 21041, Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum or Ca2+-free Dulbecco's modified Eagle's medium (catalog number 21068, Invitrogen) supplemented with 10% heat-inactivated dialyzed fetal bovine serum. Seven hundred microliters of Indo-1-loaded 2C T cells (2 × 106/ml) were pre-warmed for 15 min at 37 °C and mixed with 70 μl of peptide-loaded pMVs (1 mg/ml). Flow cytometric analysis began 60 s before addition of pMVs and continued for 720 s afterward; flow cytometric analysis was carried out with an LSR II flow cytometer equipped with a temperature control device. Changes in the concentration of intracellular Ca2+ were determined measuring changes in the ratio of fluorescence intensities of Indo-1-loaded T cells detected by two separate photomultiplier tubes with bandpath filters of 440/40 nm (violet) and 530/30 nm (blue), respectively; Indo-1 was excited by a 351-nm UV laser.
RESULTS
Effects of CD28/B7-1 and LFA-1/ICAM-1 Interactions on Activation of PLC-γ1 and PI3K/Akt Signaling Cascades
We had shown that culture of 2C T cells with QL9 (or P2Ca) peptide-loaded LdB7-1ICAM-1 Drosophila pMVs resulted in activation of membrane-proximal signaling cascades involving protein-tyrosine kinases (PTKs) and phosphoinositide 3-kinase (PI3K) exemplified by tyrosine phosphorylation of PLC-γ1 (19) and threonine phosphorylation of Akt (14, 20). In this study, we examined the contributing roles of TCR/pMHC, CD28/B7-1, and LFA-1/ICAM-1 interactions in the phosphorylation of PLC-γ1 and Akt.
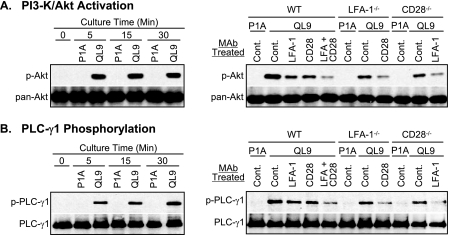
Phosphorylation of Akt at Thr308 occurred rapidly upon culture of 2C T cells with the pMVs loaded with QL9 (1 μm) peptide. The level of the Akt phosphorylation reached near maximum within 5 min of culture and was sustained through 30 min (Fig. 1A, left). Phosphorylation of PLC-γ1 at Tyr783 was also detected within 5 min of the culture, but the level of the phosphorylation increased gradually as the culture time extended (Fig. 1B, left).
FIGURE 1.
Contributing roles of CD28 and LFA-1 in phosphorylation of Akt and PLC-γ1. Cell extracts, prepared from 2C T cells cultured for different periods of time with LdB7-1ICAM-1 pMVs loaded with QL9 (1 μm) or P1A (1 μm) peptide, were subjected to Western blotting analysis for Akt phosphorylated at Thr308 (A, left) and PLC-γ1 phosphorylated at Tyr783 (B, left) along with pan-Akt and total PLC-γ1 as protein loading controls, respectively. Cell extracts, prepared from wild type (WT), LFA-1−/−, and CD28−/− 2C T cells pretreated with isotype control (Cont.), anti-LFA-1 (CD11a), or anti-CD28 mAb before culture with QL9- or P1A-loaded LdB7-1ICAM-1 pMVs for 15 min, were subjected to Western blot analysis for Akt (A, right) and PLC-γ1 (B, right). Note that P1A peptide forms a complex with Ld, but the Ld-P1A complex is not recognized by 2C TCR.
Both CD28/B7-1 and LFA-1/ICAM-1 interactions had positive effects on the PLC-γ1 and Akt phosphorylation (Fig. 1, A and B, right, and supplemental Fig. S1). Thus, wild type 2C T cells treated with either anti-CD28 or anti-LFA-1 mAb and mutant 2C T cells deficient in CD28 (2C.CD28−/−) or LFA-1 (2C.LFA-1−/−) expression showed significantly reduced levels of phosphorylation of both PLC-γ1 and Akt compared with wild type 2C T cells treated with control mAb. A further decrease in levels of phosphorylation was observed when the wild type T cells were co-treated with anti-CD28 plus anti-LFA-1 mAbs, and 2C.CD28−/− and 2C.LFA-1−/− T cells were treated with anti-LFA-1 and anti-CD28 mAbs, respectively.
Increase of Cytosolic [Ca2+] during Culture of 2C T Cells with the pMVs
Observation that the tyrosine phosphorylation of PLC-γ1 progressed quickly during culture of 2C T cells with the pMVs loaded with QL9 peptide prompted us to examine Ca2+ signaling during the culture.
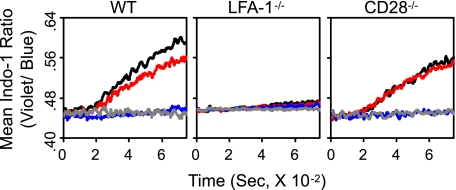
Wild type 2C T cells being cultured with the QL9 (1 μm)-loaded LdB7-1ICAM-1 pMVs showed a prominent increase in cytosolic [Ca2+] (Fig. 2). The increase became evident ∼2.5 min after mixing of 2C T cells with the pMVs and continued through the culture up to 12 min.
FIGURE 2.
Contributing roles of CD28 and LFA-1 in increase of cytosolic [Ca2+]. Kinetic flow cytometric analyses for changes in cytosolic [Ca2+] were performed with wild type (WT), LFA-1−/−, and CD28−/− 2C T cells being cultured with P1A (1 μm) or QL9 (1 μm) peptide-loaded LdB7-1ICAM-1 pMVs as indicated. 2C T cells were treated with isotype control (black and gray), anti-CD28 (red), or anti-LFA-1 (blue) mAb prior to culture with the pMVs. The flow analyses were initiated 60 s before addition of the pMVs and continued for 720 s afterward.
mAb blocking of CD28/B7-1 interaction slightly reduced the extent of Ca2+ increase (Fig. 2, left). In contrast, mAb blocking of LFA-1/ICAM-1 interaction almost completely abolished the Ca2+ increase. Importance of LFA-1/ICAM-1 interaction in the cytosolic Ca2+ increase was confirmed by experiments using 2C. LFA-1−/− T cells, in which no measurable Ca2+ increase was observed. Consistent with the result obtained with 2C T cells treated with anti-CD28 mAb, 2C.CD28−/− T cells showed conspicuous increase in cytosolic [Ca2+] during the culture (Fig. 2).
Lack of Intracellular Ca2+ Mobilization in 2C T Cells Cultured with LdB7-1ICAM-1 pMVs
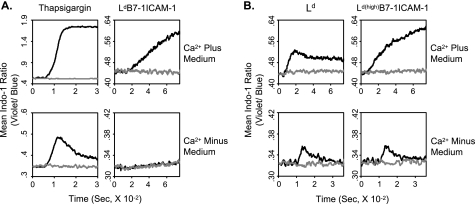
According to the mechanism of SOCE, entry of extracellular Ca2+ resulting in a surge in cytosolic [Ca2+] is preceded by mobilization of Ca2+ from intracellular Ca2+ store following PLC-γ1 activation by TCR triggering (2). We thus examined whether the increase in cytosolic [Ca2+] observed during culture of 2C T cells with QL9-loaded LdB7-1ICAM-1 pMVs was accompanied by mobilization of intracellular Ca2+ by culturing the cells in a Ca2+-free medium (Fig. 3). Surprisingly, no change in cytosolic [Ca2+] was detected during the culture (Fig. 3A, right). Note that strong mobilization of intracellular Ca2+ was detected after treatment of 2C T cells with thapsigargin, a well known intracellular Ca2+ mobilizer, both in the Ca2+-containing and -free media (Fig. 3A, left) (21).
FIGURE 3.
Extracellular Ca2+ entry accompanied with or without mobilization of intracellular Ca2+. Changes in cytosolic [Ca2+] of wild type 2C T cells and in Ca2+-containing or Ca2+-free medium were monitored after addition of thapsigargin (100 nm) (black) or DMSO alone (gray) (A, left) or QL9-loaded (1 μm) (black) or P1A-loaded (1 μm) (gray) LdB7-1ICAM-1 (A, right), Ld (B, left), or Ld(high)B7-1ICAM-1 (B, right) pMVs as indicated. Flow cytometric analyses were performed as in Fig. 2.
Detection of Intracellular Ca2+ Mobilization in 2C T Cells Cultured with pMVs Expressing Higher Level of Ld
The level of Ld expression in LdB7-1ICAM-1 Drosophila APCs is modest (supplemental Fig. S2). Thus, it was suggested that the level of Ld/QL9 expression in LdB7-1ICAM-1 pMVs was too low to trigger TCR signaling strong enough to induce mobilization of intracellular Ca2+ in primary resting T cells such as 2C transgenic T cells. To test the idea, we used pMVs prepared from Drosophila APCs expressing higher levels of Ld, namely pMVs prepared from two separate Drosophila APC clones expressing Ld alone (Ld) and higher level of Ld plus B7-1 plus ICAM-1 (Ld(high)B7-1ICAM-1) (supplemental Fig. S2).
2C T cells cultured with Ld pMVs loaded with QL9 peptide (1 μm) in Ca2+-containing medium showed a rapid increase in intracellular [Ca2+], which became evident less than a minute after addition of the pMVs (Fig. 3B, left). Different from the Ca2+ increase by LdB7-1ICAM-1 pMVS, the Ca2+ increase by Ld pMVs did not proceed progressively; instead, after an initial spike, the [Ca2+] decreased slightly and was sustained at a steady level throughout the culture. Consequently, at the end of the culture, the cytosolic [Ca2+] in 2C T cells cultured with QL9-loaded Ld pMVs was considerably lower than that in 2C T cells cultured with the QL9-loaded LdB7-1ICAM-1 pMVs (Fig. 3). Nevertheless, a transient but significant increase in cytosolic [Ca2+] was detected in 2C T cells cultured with QL9-loaded Ld pMVs in Ca2+-free medium (Fig. 3B, left).
2C T cells cultured with Ld(high)B7-1ICAM-1 pMVs loaded with QL9 (1 μm) peptide in Ca2+-containing medium showed a rapid and progressive increase in the concentration of cytosolic Ca2+ (Fig. 3B, right). The [Ca2+] measured after 12 min of culture with QL9-loaded Ld(high)B7-1ICAM-1 pMVs was higher than that measured after culture with LdB7-1ICAM-1 pMVs, but the difference was marginal. As with QL9-loaded Ld pMVs, rapid and transient increase in cytosolic [Ca2+] was detected when 2C T cells were cultured with QL9-loaded Ld(high)B7-1ICAM-1 pMVs in Ca2+-free medium (Fig. 3B, right).
Level of Cognate pMHC Expression Versus LFA-1 Dependence of Ca2+ Signaling
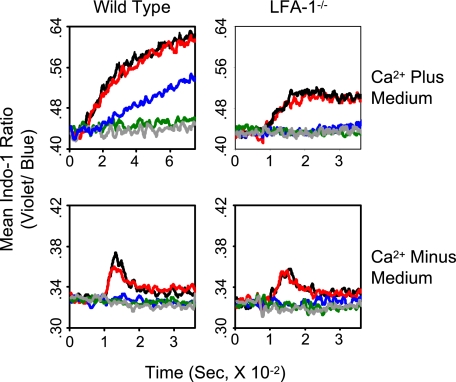
Next, we examined an effect of concentration of QL9 peptide loaded to Ld(high)B7-1ICAM-1 pMVs on both entry of extracellular Ca2+ and mobilization of intracellular Ca2+ observed in Ca2+-containing and Ca2+-free media, respectively (Fig. 4). When wild type 2C T cells were cultured with the pMVs loaded with QL9 peptide at 200 and 40 nm, respectively, both mobilization of intracellular Ca2+ and influx of extracellular Ca2+ were readily detected. When the pMVs were loaded with the peptide at 8 nm, a considerable increase in cytosolic [Ca2+] was detected when the T cells were cultured in Ca2+-containing medium, although no measurable change was monitored when cultured in Ca2+-free medium. When the pMVs were loaded with the peptide at a further lower concentration (1.6 nm), no Ca2+ increase was detected in either medium (Fig. 4, left).
FIGURE 4.
Relationship among levels of pMHC expression, mobilization of intracellular Ca2+, and LFA-1 dependence of extracellular Ca2+ entry. Wild type and LFA-1−/− 2C T cells in Ca2+-containing or Ca2+-free medium were mixed with Ld(high)B7-1ICAM-1 pMVs loaded with P1A (1 μm) (gray) or titrated concentrations of QL9 peptide (black, 200 nm; red, 40 nm; blue, 8 nm; green, 1.6 nm), and changes in cytosolic [Ca2+] were analyzed as in Fig. 2.
When 2C.LFA-1−/− T cells were used in the experiments (Fig. 4, right), different results were obtained. As with wild type 2C T cells, the mutant T cells being cultured with Ld(high)B7-1ICAM-1 pMVs loaded with QL9 peptide at higher concentrations (200 and 40 nm, respectively) displayed both influx of extracellular Ca2+ and mobilization of intracellular Ca2+. The pattern of Ca2+ increase in the mutant 2C T cells cultured in Ca2+-containing medium was, however, different from that in wild type 2C T cells and rather resembled that in wild type 2C T cells cultured with QL9-loaded Ld pMVs (Fig. 3B). When the same mutant T cells were cultured with Ld(high)B7-1ICAM-1 pMVs loaded with the peptide at lower concentrations (8 and 1.6 nm, respectively), they showed no Ca2+ increase in either medium.
Role of PLC-γ1 in LFA-1-dependent Extracellular Ca2+ Entry
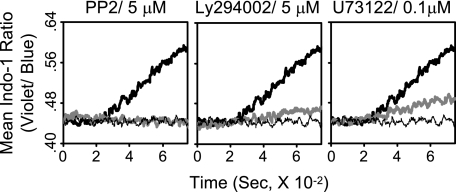
We also examined the involvement of key signaling molecules engaged in membrane-proximal TCR signaling, i.e. PTKs, PI3K, and PLC-γ1, in LFA-1-dependent Ca2+ entry using pharmacological agents specifically targeting those molecules. Treatment of wild type 2C T cells with PP2, Ly294002, or U73122, which target PTKs, PI3K, and PLC-γ1, respectively, before culture with LdB7-1ICAM-1 pMVs loaded with QL9 (1 μm) peptide strongly inhibited the Ca2+ entry (Fig. 5), indicating a critical role of those signaling molecules in the LFA-1-dependent Ca2+ entry. The importance of PI3K was further supported by comparable inhibition of the Ca2+ entry by wortmannin (500 nm), another well known PI3K inhibitor.3
FIGURE 5.
Role of PLC-γ1 in LFA-1-dependent Ca2+ entry. Wild type 2C T cells in Ca2+-containing medium were pretreated with a signaling inhibitor (bold gray line) or DMSO (bold and thin black line) as indicated for 30 min before culture with P1A (1 μm) (thin black line) or QL9 (1 μm) (bold black and gray line) peptide-loaded LdB7-1ICAM-1 pMVs. Changes in cytosolic [Ca2+] were analyzed as in Fig. 2.
DISCUSSION
Microvesicles prepared from plasma membrane of Drosophila APCs expressing defined mouse immunomolecules of interest (i.e. pMVs) (13, 14, 22) have several advantageous features in investigation of membrane-proximal signaling mechanisms primed by TCR stimulation. Given that physiological ligands expressed in biological membrane are used for stimulating T cells, experimental results obtained with pMVs may have superior physiological relevance compared with those obtained with receptor-specific mAbs or ligands immobilized on the surface of plastic or magnetic beads. Because of the huge difference in their sizes, separation of T cells from pMVs after culture is simple, and thus instant biochemical analysis of molecular alterations occurring during the culture becomes feasible; when intact cells are used for stimulating T cells, a complicated method is needed to separate T cells from stimulating cells. In addition, kinetic flow cytometric analysis of physiological changes occurring in the early stage of T cell activation, e.g. influx of extracellular Ca2+, is facilitated.
We initiated this study for investigating contributing roles of CD28/B7-1 and LFA-1/ICAM-1 interactions in a series of molecular changes in the early stage of T cell activation. We have shown before that the sole interaction of 2C TCR with Ld-QL9 complex expressed in LdB7-1ICAM-1 pMVs is necessary and sufficient to induce a near maximum level of F-actin polymerization; in that process, CD28 and LFA-1 played little role (14). Different from in F-actin polymerization, both CD28 and LFA-1 took a significant and comparable part in phosphorylation of Akt (Fig. 1A and supplemental Fig. S1), conforming to their roles in activation of PI3K signaling cascades reported by others (23, 24). They also exerted comparable effects on phosphorylation of PLC-γ1 (Fig. 1B and supplemental Fig. S1). Considering that PLC-γ1 phosphorylation at Tyr783 is mediated by the Syk family PTK, i.e. ZAP-70 in T cells, one may reason that CD28 and LFA-1 may take part in activation of ZAP-70 (25). Alternatively, it is also plausible that CD28/B7-1 and LFA-1/ICAM-1 interactions make contributions to the phosphorylation of PLC-γ1 via up-regulation of PI3K activity through which more phosphatidylinositol 3,4,5-trisphosphate becomes available. As a result, functionality of signaling molecules containing the PH domain is escalated facilitating formation of signalsome centered by PLC-γ1 and phosphorylation of PLC-γ1 by ZAP-70 (26, 27).
In striking contrast to roles in the PLC-γ1 phosphorylation, roles of CD28 and LFA-1 in the increase of cytosolic [Ca2+] were clearly distinct (Fig. 2). Considering that phosphorylation of PLC-γ1 at Tyr783 is reportedly critical for activation of its enzymatic activity (19), those results were intriguing and suggested a novel Ca2+ entry mechanism exerted by LFA-1 other than SOCE. Supporting that notion, the LFA-1-dependent Ca2+ entry proceeded without mobilization of measurable levels of intracellular Ca2+ (Fig. 3A). The lack of intracellular Ca2+ mobilization during culture of 2C T cells with LdB7-1ICAM-1 pMVs was ensured by the result that pre-culture of 2C T cells with the QL9-loaded pMVs in the calcium-free medium did not alter either onset or magnitude of intracellular Ca2+ mobilization by thapsigargin (supplemental Fig. S3).
Studies for SOCE have been routinely conducted following Ab cross-linking of TCRs, which elicits strong Signal 1; the TCR-mediated signaling mechanisms are collectively termed as Signal 1 (28). Reflecting the importance of Signal 1 in mobilization of intracellular Ca2+, a transient but distinct level of intracellular Ca2+ mobilization was readily detected when 2C T cells were cultured with pMVs expressing an elevated level of Ld class I MHC (Fig. 3B). The mobilization of intracellular Ca2+ occurred almost instantly after mixing of 2C T cells with the pMVs and peaked within a minute of the culture. In line with this, an increase in cytosolic [Ca2+] in 2C T cells cultured with the same pMVs in Ca2+-containing medium progressed much more promptly than with LdB7-1ICAM-1 pMVs.
Experiments using wild type and LFA-1−/− 2C T cells cultured with Ld(high)B7-1ICAM-1 pMVs loaded with titrated concentrations of QL9 peptide confirmed that significance of the LFA-1/ICAM-1 interaction in extracellular Ca2+ entry was determined by the level of cognate pMHC expression in the pMVs (Fig. 4). Under conditions that mobilization of intracellular Ca2+ was detected, namely when 2C T cells were cultured with the pMVs loaded with high concentrations of QL9 peptide, extracellular Ca2+ entry occurred without involvement of LFA-1/ICAM-1 interaction even though the Ca2+ entry proceeded more progressively in the presence of LFA-1/ICAM-1 interaction. Extracellular Ca2+ entry could still occur under a condition that intracellular Ca2+ mobilization was not detected. In that condition, extracellular Ca2+ entry was totally dependent on LFA-1/ICAM-1 interaction.
It is of interest that LFA-1-dependent Ca2+ entry still relies on activity of PLC-γ1 (Fig. 5) because the level of PLC-γ1 phosphorylation at Tyr783 showed little correlation with the level of cytosolic Ca2+ increase (Figs. 1 and 2). PLC-γ1 plays multiple roles in T cell activation. Hydrolysis of phosphatidylinositol 4,5-bisphosphate catalyzed by PLC-γ1 results in production of diacylglycerol (DAG) as well as IP3. DAG is imperative for activation of a family of protein kinase C and small GTP-binding proteins such as Rap1 (29). Activation of Rap1 by DAG may hold significant implications in LFA-1-dependent Ca2+ entry because of its role in inside-out signaling for LFA-1 activation (7, 30). Thus, inhibition of LFA-1-dependent Ca2+ entry by the PLC-γ1 inhibitor (U73122) could be explained by inhibition of DAG production by which LFA-1 activation process is impeded, and consequently signaling cascades promoted by high affinity/avidity LFA-1/ICAM-1 interaction are silenced (31).
In addition to Ca2+ channels involved in SOCE (e.g. ORAI-1), other types of Ca2+ channels are also expressed in T cells, e.g. L-type Ca2+ channel and transient receptor potential channel (TRPC) (2, 3, 32). Here we hypothesize that TRPC-mediated Ca2+ entry mechanism may be of special relevance to LFA-1-dependent Ca2+ entry for the following reasons. First, TRPC-mediated TCR-dependent Ca2+ entry has been revealed in human T cells (33). Second, TRPC-mediated Ca2+ entry has been found to operate without mobilization of Ca2+ from the intracellular Ca2+ store (34). Third, a few TRPCs have been found to be activated by DAG (35). According to recent study by Mor et al. (36), interaction of LFA-1 with ICAM-1 induces strong activation of phospholipase D to produce DAG functioning in the plasma membrane. Together, it appears possible that TCR triggering leads to activation of LFA-1 via inside-out signaling, and consequently, high affinity/avidity interaction of LFA-1 with ICAM-1 results in amplification of DAG production via activation of phospholipase D. As a result, formation of TRPC is facilitated to allow entry of extracellular Ca2+.
It has been perceived that enhancement of Ca2+ signaling by LFA-1 is attained via strengthening signaling cascades induced by the TCR/pMHC interaction, namely stable and prolonged T/APC contact established by the LFA-1/ICAM-1 interaction allows more sustainable TCR signaling to facilitate activation of the downstream Ca2+ signaling pathway (11). The results of this study appear to contradict that view and instead represent that LFA-1 activated by TCR triggering acts independently to drive extracellular Ca2+ entry via a mechanism separate from SOCE.
Supplementary Material
Acknowledgments
We thank Drs. Jonathan Sprent (Garvan Institute of Medical Research, Darlinghurst, Australia) and Dale Boger (Department of Chemistry and Chemical Biology at The Scripps Research Institute) for thoughtful advice. We also thank Alan Saluk, the director of Flow Cytometry Core Facility at The Scripps Research Institute, and staff for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant AI066146 (to I. H.). This is publication number 20067 from The Scripps Research Institute.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
K. Kim, L. Wang, and I. Hwang, unpublished data.
- APC
- antigen-presenting cell
- MHC
- major histocompatibility complex
- pMHC
- peptide-MHC complex
- pMV
- plasma membrane-derived membrane vesicles
- PTK
- protein-tyrosine kinase
- SOCE
- store-operated Ca2+ entry
- TRPC
- transient receptor potential channel
- PLC-γ1
- phospholipase C-γ1
- TCR
- T cell receptor
- ICAM-1
- intercellular adhesion molecule-1
- IP3
- inositol 1,4,5-trisphosphate
- Ab
- antibody
- mAb
- monoclonal Ab
- DAG
- diacylglycerol
- PI3K
- phosphoinositide 3-kinase.
REFERENCES
- 1.Lewis R. S. (2001) Annu. Rev. Immunol. 19, 497–521 [DOI] [PubMed] [Google Scholar]
- 2.Oh-hora M., Rao A. (2008) Curr. Opin. Immunol. 20, 250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintana A., Griesemer D., Schwarz E. C., Hoth M. (2005) Pflugers Arch. 450, 1–12 [DOI] [PubMed] [Google Scholar]
- 4.Feske S. (2007) Nat. Rev. Immunol. 7, 690–702 [DOI] [PubMed] [Google Scholar]
- 5.Evans R., Patzak I., Svensson L., De Filippo K., Jones K., McDowall A., Hogg N. (2009) J. Cell Sci. 122, 215–225 [DOI] [PubMed] [Google Scholar]
- 6.Pribila J. T., Quale A. C., Mueller K. L., Shimizu Y. (2004) Annu. Rev. Immunol. 22, 157–180 [DOI] [PubMed] [Google Scholar]
- 7.Mor A., Dustin M. L., Philips M. R. (2007) Immunol. Rev. 218, 114–125 [DOI] [PubMed] [Google Scholar]
- 8.Kanner S. B., Grosmaire L. S., Ledbetter J. A., Damle N. K. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 7099–7103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Seventer G. A., Bonvini E., Yamada H., Conti A., Stringfellow S., June C. H., Shaw S. (1992) J. Immunol. 149, 3872–3880 [PubMed] [Google Scholar]
- 10.Sirim P., Zeitlmann L., Kellersch B., Falk C. S., Schendel D. J., Kolanus W. (2001) J. Biol. Chem. 276, 42945–42956 [DOI] [PubMed] [Google Scholar]
- 11.Bachmann M. F., McKall-Faienza K., Schmits R., Bouchard D., Beach J., Speiser D. E., Mak T. W., Ohashi P. S. (1997) Immunity 7, 549–557 [DOI] [PubMed] [Google Scholar]
- 12.Wülfing C., Sjaastad M. D., Davis M. M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6302–6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang I., Shen X., Sprent J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 6670–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K., Wang L., Hwang I. (2009) PLoS One 4, e6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. (1988) Nature 335, 271–274 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg D. E., Kornfeld S. (1983) J. Biol. Chem. 258, 3159–3165 [PubMed] [Google Scholar]
- 17.Swaney J. S., Roth D. M., Olson E. R., Naugle J. E., Meszaros J. G., Insel P. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. (1986) J. Immunol. 137, 952–961 [PubMed] [Google Scholar]
- 19.Law C. L., Chandran K. A., Sidorenko S. P., Clark E. A. (1996) Mol. Cell. Biol. 16, 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke T. F., Yang S. I., Chan T. O., Datta K., Kazlauskas A., Morrison D. K., Kaplan D. R., Tsichlis P. N. (1995) Cell 81, 727–736 [DOI] [PubMed] [Google Scholar]
- 21.Lytton J., Westlin M., Hanley M. R. (1991) J. Biol. Chem. 266, 17067–17071 [PubMed] [Google Scholar]
- 22.Kovar M., Boyman O., Shen X., Hwang I., Kohler R., Sprent J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11671–11676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okkenhaug K., Bilancio A., Emery J. L., Vanhaesebroeck B. (2004) Biochem. Soc. Trans. 32, 332–335 [DOI] [PubMed] [Google Scholar]
- 24.Sánchez-Martín L., Sánchez-Sánchez N., Gutiérrez-López M. D., Rojo A. I., Vicente-Manzanares M., Pérez-Alvarez M. J., Sánchez-Mateos P., Bustelo X. R., Cuadrado A., Sánchez-Madrid F., Rodríguez-Fernández J. L., Cabañas C. (2004) J. Biol. Chem. 279, 16194–16205 [DOI] [PubMed] [Google Scholar]
- 25.Soede R. D., Wijnands Y. M., Van Kouteren-Cobzaru I., Roos E. (1998) J. Cell Biol. 142, 1371–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michel F., Attal-Bonnefoy G., Mangino G., Mise-Omata S., Acuto O. (2001) Immunity 15, 935–945 [DOI] [PubMed] [Google Scholar]
- 27.Wange R. L. (2000) Sci. STKE 2000, RE1. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz R. H. (2003) Annu. Rev. Immunol. 21, 305–334 [DOI] [PubMed] [Google Scholar]
- 29.Griner E. M., Kazanietz M. G. (2007) Nat. Rev. Cancer 7, 281–294 [DOI] [PubMed] [Google Scholar]
- 30.Kinashi T., Katagiri K. (2004) Immunol. Lett. 93, 1–5 [DOI] [PubMed] [Google Scholar]
- 31.Katagiri K., Shimonaka M., Kinashi T. (2004) J. Biol. Chem. 279, 11875–11881 [DOI] [PubMed] [Google Scholar]
- 32.Kotturi M. F., Hunt S. V., Jefferies W. A. (2006) Trends Pharmacol. Sci. 27, 360–367 [DOI] [PubMed] [Google Scholar]
- 33.Philipp S., Strauss B., Hirnet D., Wissenbach U., Mery L., Flockerzi V., Hoth M. (2003) J. Biol. Chem. 278, 26629–26638 [DOI] [PubMed] [Google Scholar]
- 34.Patterson R. L., van Rossum D. B., Nikolaidis N., Gill D. L., Snyder S. H. (2005) Trends Biochem. Sci. 30, 688–697 [DOI] [PubMed] [Google Scholar]
- 35.Hardie R. C. (2003) Annu. Rev. Physiol. 65, 735–759 [DOI] [PubMed] [Google Scholar]
- 36.Mor A., Campi G., Du G., Zheng Y., Foster D. A., Dustin M. L., Philips M. R. (2007) Nat. Cell Biol. 9, 713–719 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.