Abstract
Although increased intracellular concentrations of hydrogen peroxide (H2O2) are associated with inhibition of 26 S proteasomal activity, the mechanisms responsible for such effects have not been well delineated. In the present studies, we found that direct exposure of purified 26 S proteasomes to H2O2 had negligible effects on their activity, whereas incubation with glutathione and H2O2 produced >80% decrease in chymotrypsin-like and trypsin-like activities. Rpn1 and Rpn2, which are subunits of the 19 S regulatory particle, undergo S-glutathionylation after exposure of purified 26 S proteasomes to glutathione and H2O2, as well as in HEK 293 cells and neutrophils incubated with H2O2. Increased oxidation of Rpn1 and Rpn2 cysteine thiols was also found in lung extracts from mice in which catalase was inactivated, a condition associated with augmented intracellular concentrations of H2O2 and diminished 26 S proteasomal activity. Although unoxidized Rpn2 enhanced 20 S proteolytic function in vitro, such potentiation was not found when the 20 S core particle was incubated with oxidized Rpn2. The composition of 26 S proteasomes was not altered after exposure to glutathione and H2O2, with similar amounts of Rpn1 and Rpn2 in control or oxidized 26 S proteasomal complexes. These findings identify S-glutathionylation of Rpn2 as a contributory mechanism for H2O2-induced inhibition of 26 S proteasomal function.
The ubiquitin-proteasomal system is a major pathway for protein degradation and is involved in multiple cellular processes, including cell cycle control, expression of cell surface receptors, and regulation of intracellular levels of structural and regulatory proteins and transcription factors (1–6). The 26 S proteasome is composed of two main components, a proteolytic 20 S core particle (CP)2 and an ATPase containing 19 S regulatory particle (RP) (7–10). Ubiquitinated proteins are unfolded in the 19 S RP and then translocated into the 20 S CP where they are degraded. The 20 S CP is composed of four heptameric rings of α and β subunits, with the two inner β rings having proteolytic activity. The 19 S RP consists of a lid and base subcomplex, with the base subcomplex, including the nonenzymatic Rpn1 and Rpn2 toroids, surrounded by six AAA-type ATPases. Rpn1 and Rpn2 appear to play a major regulatory role by modulating the translocation and subsequent degradation of ubiquitinated substrates in the proteolytic core of the 20 S CP (7, 11–15).
Decreased 26 S proteasomal activity, resulting in the accumulation of intracellular proteins, has been associated with aging (16, 17) and with pathophysiologic processes, including heart failure, neurodegenerative diseases, and alcohol induced liver injury (18–20). A number of recent studies have shown that time-limited inhibition of the 26 S proteasome is beneficial as a therapeutic intervention for malignancies, such as multiple myeloma, and in reducing inflammation and cell injury associated with ischemia-reperfusion injury and transplantation rejection (21–24).
Reactive oxygen species (ROS), including hydrogen peroxide (H2O2), are products of as well as participants in the regulation of cellular aerobic metabolism. Increased production of ROS accompanies inflammatory conditions, such as ischemia-reperfusion injury and sepsis, and appears to contribute to organ system dysfunction in these settings (25, 26). H2O2 rapidly transits across cell membranes, and incubation of neutrophils and other cell populations with H2O2 or the H2O2 generating combination of glucose and glucose oxidase results in increased intracellular concentrations of H2O2, diminished 26 S proteasomal activity, and stabilization of cytoplasmic levels of proteins, such as IκB-α, that are normally degraded by the 26 S proteasome (27–29). H2O2 appears to exert a differential effect on 20 S and 26 S proteasomal activities both in vitro and in vivo in H2O2-exposed neutrophils and other cell populations, with 26 S proteasomal function being much more sensitive to H2O2 than is that of the 20 S CP (27–29). Such findings suggest that the mechanism by which H2O2 reduces activity of the 26 S proteasome may be through oxidative modulation of the 19 S RP. In the present studies, we investigated this hypothesis to determine whether H2O2 exposure facilitates oxidative modification of 19 S regulatory subunits and, if so, how such effects affect the structure and function of the 26 S proteasome.
EXPERIMENTAL PROCEDURES
Mice
Male C57BL/6, acatalasemic C3Ga.Cg-Cat b/J, and control C3HeB/FeJ mice, 8–12 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were kept on a 12:12-h light-dark cycle with free access to food and water. Treatment of mice with aminotriazole (ATZ) was performed as previously described (30). Briefly, C57BL/6 mice were anesthetized with isoflurane and ATZ, 500 mg/kg in saline, or saline alone was given intraperitoneally. This dose of ATZ was previously used in murine models examining efficacy or toxicity of ATZ (31, 32). Lungs were harvested 12 and 24 h after ATZ administration. There were no deaths associated with ATZ administration. All experiments were conducted in accordance with institutional review board-approved protocols (Institutional Animal Care and Use Committee, University of Alabama at Birmingham).
Neutrophil Isolation
Human neutrophils were isolated as previously described (33).
Culture of Human Embryonic Kidney Cells
HEK 293 cells were maintained at 37 °C in 5% CO2 in RPMI 1640 growth medium (Amersham Biosciences) that contained 8% fetal bovine serum (Atlanta Biologicals, Norcross, GA), l-glutamine (2 mm), penicillin (100 units/ml), and streptomycin (100 ng/ml, Sigma). Prior to use in experiments, the cells were washed twice and incubated with RPMI 1640 medium (fetal bovine serum, 0.5%) for 2 h and then treated as described in the figure legends.
Reagents and Antibodies
Purified human 20 S and 26 S proteasomes, antibodies to human Rpn2 and 20 S α subunit, and Boc-Leu-Arg-Arg-AMC were purchased from BIOMOL (Plymouth Meeting, PA). Rpn1 antibody was from Boston Biochem (Boston, MA). The antibodies to murine Rpn1 and Rpn2 as well as anti-rabbit horseradish peroxidase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). AMC (7-amido-4-methyl-coumarin), Suc-Leu-Leu-Val-Tyr-AMC, MG132, and streptavidin-HRP were from Calbiochem. Biotinylated glutathione ethyl ester and streptavidin-agarose were purchased from Invitrogen, whereas hydrogen peroxide, glutathione, and ATZ were obtained from Sigma. Bio-Gel P10 was purchased from Bio-Rad.
Western Blot Analysis of Rpn1 and Rpn2
Lung homogenates or neutrophils (3.6 × 106/well) were prepared using lysis buffer (Tris, pH 7.4 (50 mm), NaCl (150 mm), Nonidet P-40 (0.5% v/v), EDTA (1 mm), EGTA (1 mm), Na3VO4 (1 mm), NaF (50 mm), and protease inhibitors), and then sonicated and centrifuged at 10,000 × g for 15 min at 4 °C. The protein concentration in supernatants was determined using Bradford reagent (Bio-Rad) with bovine serum albumin as a standard (34, 35). Samples were mixed with Laemmli sample buffer and boiled for 5 min. Equal amounts of protein were resolved by 8% SDS-PAGE, and transferred onto polyvinylidene difluoride membranes (Immobilon P, Millipore, Billerica, MA). The membranes were probed with specific antibodies to Rpn1 or Rpn2, followed by detection with horseradish peroxidase-conjugated anti-mouse or goat anti-rabbit IgG. Bands were visualized by enhanced chemiluminescence (SuperSignal, Pierce). Each experiment was carried out two or more times using HEK 293 cells, isolated neutrophils, or lung homogenates obtained from separate groups of mice.
Measurement of Proteasome Activity
Proteasome activity was measured as previously described (19, 36–38). Briefly, purified 20 S or 26 S proteasomes (0.3 μg) or cell extracts from neutrophil (40 μg) or HEK 293 cells (40 μg) were incubated in buffer (Tris, pH 7.5 (10 mm), EDTA (1 mm), glycerol (20% v/v), MgCl2 (5 mm), and protease inhibitors phenylmethylsulfonyl fluoride (50 μm), define as l-1-tosylamido-2-phenylethyl chloromethyl ketone (50 μm), aprotinin (2 μg/ml), and leupeptin (2 μg/ml)). The reaction was initiated by addition of ATP (1 mm) or SDS (0.6%) and the fluorogenic peptide substrates Suc-Leu-Leu-Val-Tyr-AMC (100 μm) or Boc-Leu-Arg-Arg-AMC (100 μm) to determine proteasomal chymotrypsin-like or trypsin-like activity, respectively. AMC fluorescence was measured in a microtiter plate fluorometer (Bio-Rad) at 1-min intervals over a 30- to 60-min period at 37 °C with an excitation filter of 380 nm and an emission filter of 460 nm. Proteasomal independent activity was determined by performing the assay in the presence of the proteasome inhibitor MG132 (10 μm). Proteasomal activity was determined using rate (fluorescence units/min) and standard AMC concentration curves, with calculated values expressed as -fold rates of control. The rates of chymotrypsin-like and trypsin-like activity (fluorescence units/min) were derived by subtracting the rates obtained in the presence of the proteasome inhibitor (MG132, 10 μm) from the values obtained in its absence. Assays were performed at least in triplicate, and results are presented from three independent experiments using isolated neutrophils or HEK 293 cells.
S-Glutathionylation of Purified 20 S and 26 S Proteasome
Purified 20 S and 26 S proteasomes (0.5 μg) in proteasomal reaction buffer (Tris, pH 7.5 (10 mm), EDTA (1 mm), glycerol (20% v/v), MgCl2 (5 mm), diethylenetriaminepentaacetic acid (100 μm)) were incubated with GSH-biotin (0 or 100 μm) for 15 min followed by exposure to H2O2 (200 μm) for an additional 5 min. Samples were then boiled in Laemmli sample buffer (without dithiothreitol) for 5 min, resolved in non-reducing SDS-PAGE, followed by Western blot analysis with streptavidin-HRP. Membranes were subsequently re-probed with antibodies specific to Rpn1 and Rpn2 subunits.
S-Glutathionylation of Rpn1 and Rpn2 in Cell Extracts
Cell extracts (500–1000 μg of protein/ml) obtained from HEK 293 cells were incubated with GSH-biotin (0 or 100 μm) for 10 min followed by exposure to H2O2 (0 or 200 μm) in the presence of diethylenetriaminepentaacetic acid (100 μm) for an additional 5 min. Samples were then passed through Bio-Gel P-10 to remove H2O2 and free GSH-biotin. Total biotin-GSS-protein conjugates were precipitated using streptavidin-agarose followed by SDS-PAGE and Western blot analysis with antibodies to Rpn1 and Rpn2.
Detection of GSS-Rpn1 and GSS-Rpn2 Adduct Formation in HEK 293 Cells and Isolated Neutrophils
HEK 293 cells (2 × 106/ml) or neutrophils (1 × 107/ml) were incubated with ethyl ester GSH-biotin (0.6 m) for 1.5 h. The cells were then washed twice with culture buffer to remove excess of GSH and treated with H2O2 (0 or 300 μm) for 15 or 30 min. The cells were lysed in the presence of N-ethylmaleimide (5 mm), and then cell extracts were passed through Bio-Gel P10 to remove free GSH-biotin and N-ethylmaleimide. The level of GSS-protein conjugates was determined using non-reducing Western blot analysis with streptavidin-HRP, whereas GSS-Rpn1 and GSS-Rpn2 levels were measured after pull down with streptavidin-agarose (60 min at 4 °C) followed by reducing SDS-PAGE and Western blot analysis with antibodies to Rpn1 or Rpn2.
Labeling of Rpn1 and Rpn2 Free Cysteine Thiols
The extent of free (un-oxidized) cysteine residues within Rpn1 and Rpn2 was determined using the BIAM labeling assay (39–42). Briefly, lung extracts (1 mg/sample) obtained from control, acatalasemic-treated, or ATZ-treated mice were incubated with BIAM (200 μm) for 30 min at room temperature, and then excess BIAM was removed by passing the extracts through Bio-Gel P10. Next, BIAM-protein conjugates were precipitated with streptavidin-agarose for 1 h at 4 °C. Samples were washed four times with lysis buffer containing 0.05% SDS and then subjected to reducing SDS-PAGE and Western blot analysis with antibodies to Rpn1 and Rpn2.
Clear Native PAGE and Measurement of Proteasomal Activity in Gel
The native PAGE gel resolution of purified proteasomes was performed as previously described (43). Briefly, 20 S and 26 S proteasomes (5 μg/sample) were resolved in 3.5% acrylamide gel containing a 2.5% stacker region. Electrophoresis was run at 100–110 mV (∼23–25 mA) for 4 h at 4 °C in a cold room, and then the gel was incubated in developing buffer (50 mm Tris (pH 7.4), MgCl2 (5 mm), and ATP (1 mm)) and Suc-Leu-Leu-Val-Tyr-AMC (50 μm) for 30 min. The AMC fluorescence was detected using UV light, and then the gel was stained with Coomassie Brilliant Blue to determine the amounts of proteasomal subunits. In experiments that examined Rpn1 and Rpn2 binding to 20 S core particles, the proteins resolved in native gel were transferred onto polyvinylidene difluoride membranes followed by Western blot analysis with antibodies specific to Rpn1, Rpn2, or the proteasomal α-subunit.
Purification of Rpn1 and Rpn2
The cDNA of Rpn1 and Rpn2 (Open Biosystems, Rockford, IL) was cloned into pET28b+ (6 × His) vector (EMD Biosciences, Madison, WI) and expressed in BL21(DE3)pLysS Escherichia coli. Rpn1-His or Rpn2-His was purified using nickel-nitrilotriacetic acid-agarose (Qiagen) as previously described (7, 44). The amounts of purified proteins were determined using SDS-PAGE and Coomassie staining with bovine serum albumin as a standard. The Rpn1 and Rpn2 sequence was confirmed by DNA sequencing. Western blot analysis was performed with specific antibodies to Rpn1 and Rpn2.
Statistical Analyses
Experiments with purified proteasomes or HEK 293 cells were each performed three or more times. Student's t test (for comparisons between two groups) was used, whereas the Bonferroni test was performed when more than two experimental groups were analyzed, with p < 0.05 considered to be statistically significant. Human neutrophils were isolated from 30 ml of blood from three individuals, whereas mouse lung homogenates were obtained from two separate groups of control or acatalasemic mice or mice treated with ATZ (n = 2 mice in each group).
RESULTS
Effects of H2O2 and Glutathione on 20 S and 26 S Proteasomal Activity
Previous studies, including those from our laboratory, have shown that exposure of diverse cell populations, including neutrophils, to H2O2 is associated with inhibition of 26 S proteasomal activity (27, 45, 46). However, it remains unclear if H2O2 directly affects the 26 S proteasome or if the inhibitory effects of increased cellular concentrations of H2O2 on 26 S proteasomal function are only indirect and due to upstream interactions between H2O2 and other intracellular components that then result in diminished 26 S activity. To examine this issue, we measured chymotrypsin-like and trypsin-like activity of purified human 26 S proteasomes after exposure to H2O2.
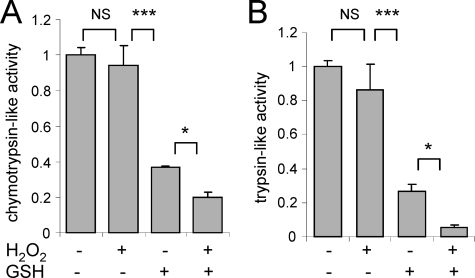
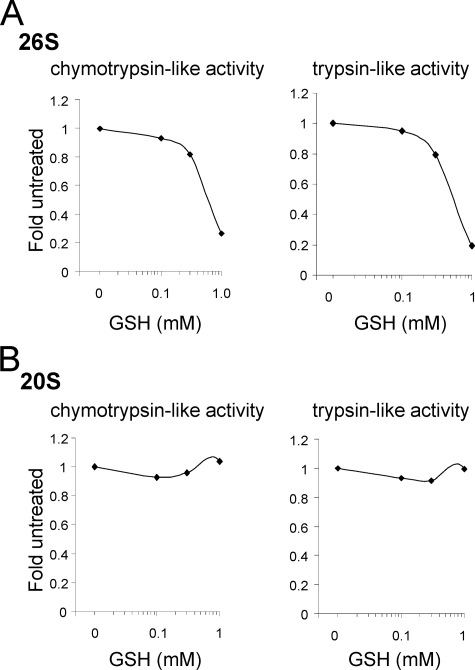
Incubation of purified human 26 S proteasomes with H2O2 (100 μm) had little or no effect on chymotrypsin-like or trypsin-like activity (Fig. 1, A and B). In contrast, incubation of purified 26 S proteasomes with l-glutathione (GSH, 1 mm) resulted in ∼60% decrease of both chymotrypsin-like and trypsin-like proteasomal activities. The inhibitory effects of GSH were even more pronounced (∼80%) in 26 S proteasomes that had been pre-treated with H2O2 (Fig. 1). The concentration of GSH used in these experiments, and that inhibited 26 S proteasome activity, is physiologically relevant because the basal levels of GSH found in the intracellular milieu are typically in the range of 1–10 mm (47). Although the activity of H2O2-treated 26 S proteasomes was dose dependently inhibited by incubation with glutathione, exposure to concentrations of 1 mm of GSH had no effect on the chymotrypsin-like or trypsin-like activity of the 20 S CP (Fig. 2, A and B). However, consistent with previous reports (48, 49), concentrations of GSH of 10 mm or higher induced inhibition of 20 S proteasomal activity (data not shown). These results suggest that S-glutathionylation of regulatory subunits within the 26 S proteasome, and particularly in the 19 S RP, may contribute to H2O2-dependent inhibition of 26 S proteasomal function in vivo.
FIGURE 1.
Effects of H2O2 and glutathione on 26 S proteasomal activity in vitro. The rates of 26 S proteasome (A) chymotrypsin-like or (B) trypsin-like activity were measured after treatment with H2O2 (0 or 100 μm), GSH (0 or 1 mm), or H2O2 (100 μm) and GSH (1 mm). The rates of AMC fluorescence were measured during the following 15 min. Means ± S.D. are shown, with n = 4 for all experiments. NS (non significant): *, p < 0.05, or ***, p < 0.001.
FIGURE 2.
Effects of glutathione exposure on the activity of pre-oxidized 20 S or 26 S proteasomes. 20 S CP or 26 S proteasomes were incubated with H2O2 (100 μm) for 15 min and then the rate of (A) chymotrypsin-like or (B) trypsin-like activity was measured after exposure to increasing concentrations of GSH (0–1 mm). Representative traces show the rates of AMC fluorescence obtained after incubation of purified 20 S CP or 26 S proteasomes with the fluorogenic substrates AMC-Suc-Leu-Leu-Val-Tyr-AMC or Boc-Leu-Arg-Arg-AMC.
Identification of the 26 S Proteasomal Subunits That Undergo S-Glutathionylation
As shown in Fig. 2, activity of the 26 S proteasome, but not of the 20 S CP, appeared to be sensitive to inhibition by a combination of GSH and H2O2, potentially as a result of oxidative modification of regulatory particles that are present in the 26 S complex, but not in the 20 S CP. To examine this possibility, S-glutathionylation of purified human 20 S CP and 26 S proteasomes was produced by incubation with H2O2 and biotin-tagged GSH. Non-reducing SDS-PAGE and Western blot analysis of biotin-GSS-proteasome adduct formation was then used to identify proteins that underwent S-glutathionylation (50, 51).
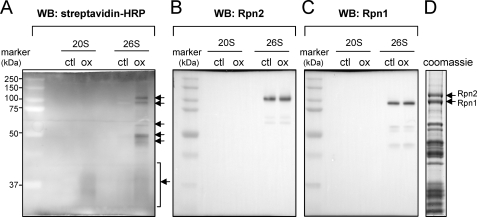
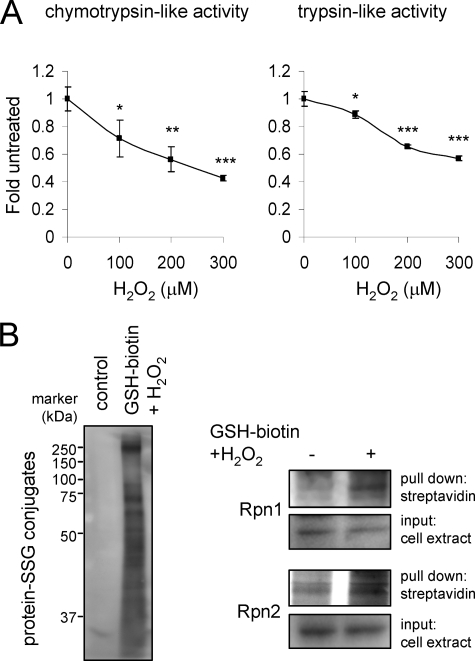
Among the 26 S proteasomal subunits, both Rpn1 and Rpn2 demonstrated GSS-adduct formation after exposure of the 26 S proteasome to H2O2 and GSH (Fig. 3). Mass spectrometry of the two top bands of ∼100 kDa (Fig. 3D) confirmed their identity as Rpn1 and Rpn2 (data not shown). The increase in S-glutathionylation of proteins found below ∼40 kDa is consistent with a recent report that components of the 20 S CP are also subject to GSS-protein adduct formation (48, 49).
FIGURE 3.
Identification of 19 S regulatory particles that undergo S-glutathionylation after H2O2 exposure. 20 S CP or 26 S proteasomes were incubated with GSH-biotin followed by exposure to H2O2. A, samples were resolved in non-reducing SDS-PAGE gels followed by Western blot analysis with streptavidin-HRP. The presence of GSS-proteasomal subunits is indicated with arrows. The membrane was re-probed with antibodies specific to human Rpn2 or Rpn1 as shown in B and C, respectively. D, subunits of purified 26 S proteasomes were resolved by SDS-PAGE followed by Coomassie staining. The positions of the Rpn1 and Rpn2 regulatory subunits are indicated with arrows.
Rpn1 and Rpn2 Undergo S-Glutathionylation under in Vivo Conditions Associated with Increased Intracellular H2O2 Concentration
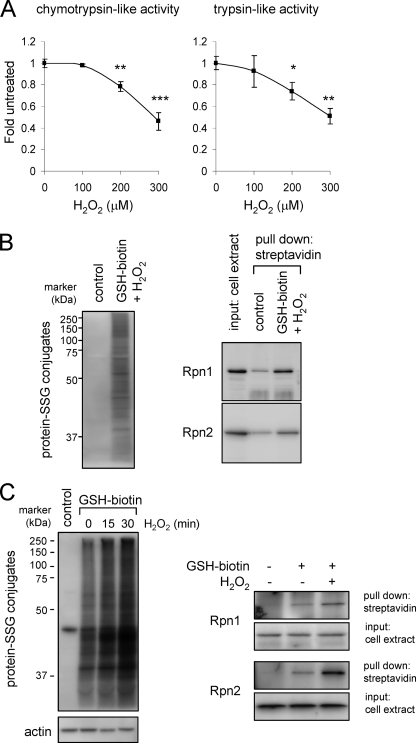
Incubation of cell extracts from HEK 293 cells with biotin-GSH and H2O2 resulted in S-glutathionylation of Rpn1 and Rpn2 (Fig. 4A). Glutathionylation of Rpn1 and Rpn2 was also detected in HEK 293 cells after incubation with H2O2 and ethyl ester GSH-biotin, which is membrane-permeable and from which GSH-biotin is generated intracellularly by interaction with cytoplasmic esterases (52) (Fig. 4B). Exposure of HEK 293 cells loaded with GSH-biotin to H2O2 produced time-dependent increase in the amounts of total proteins undergoing glutathionylation, including GSS-Rpn1 and GSS-Rpn2 adducts. Similar to what was found with HEK 293 cells, exposure of human neutrophils to H2O2 resulted in increased levels of total GSS-protein as well as GSS-Rpn1 and GSS-Rpn2 adducts (Fig. 5).
FIGURE 4.
Detection of GSS-Rpn1 and GSS-Rpn2 adduct formation in H2O2 exposed HEK 293 cells. A, cells were incubated without or with H2O2 for 60 min and then chymotrypsin-like (left panel) and trypsin-like (right panel) activities determined. Means ± S.D. are shown. Each experiment was repeated in triplicate. *, p < 0.05; **, p < 0.01; or ***, p < 0.001. B, cell extracts of HEK 293 cells were incubated with or without GSH-biotin and H2O2, and then biotin-GSS-protein conjugates precipitated using streptavidin-agarose followed by SDS-PAGE. Representative Western blots show GSS-protein adduct formation (left panel) and Rpn1 and Rpn2 levels before (input) and after precipitation with streptavidin agarose (right panel). Two additional experiments provided similar results. C, HEK 293 cells were loaded or not with GSH-biotin and then incubated with H2O2 for 15 or 30 min. The cell extracts were analyzed using non-reducing PAGE and Western blot analysis with streptavidin-HRP or after pull down of biotin-GSS-protein adducts with streptavidin-agarose. Representative Western blots show total GSS-protein conjugates (left panel) and GSS-Rpn1 and GSS-Rpn2 adducts (right panel). Two additional experiments provided similar results.
FIGURE 5.
Detection of GSS-Rpn1 and GSS-Rpn2 adduct formation in HEK 293 cells and neutrophils after incubation with H2O2. A, purified human neutrophils were cultured with H2O2 (0–300 μm) for 60 min and then 26 S proteasomal chymotrypsin and trypsin-like activities measured using fluorogenic substrates Means ± S.D. are shown. Each experiment was repeated in triplicate. *, p < 0.05; **, p < 0.01; or ***, p < 0.001. B, left panel shows the increase in total GSS-protein adduct formation obtained from human neutrophils loaded with ethyl ester GSH-biotin and treated with H2O2 for 30 min. GSS-Rpn1 and GSS-Rpn2 levels were detected after pull down with streptavidin-agarose. Representative Western blots are shown. A second experiment provided similar results.
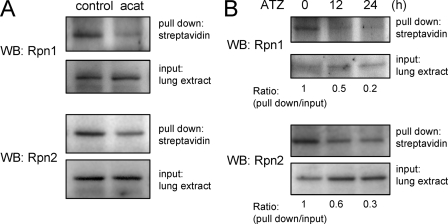
Inhibition or absence of catalase activity, such as occurs after aminotriazole treatment or in acatalasemic mice, results in elevated intracellular steady state levels of H2O2 and diminished 26 S proteasome activity in neutrophils and in the lungs (30). To determine if oxidation of Rpn1 and Rpn2 occurs under such conditions, lung extracts obtained for control or acatalasemic mice, or from mice treated with aminotriazole were labeled with BIAM to measure the amounts of free (i.e. un-oxidized) cysteine residues (39–42). As shown in Fig. 6, BIAM-adduct formation was deceased in Rpn1 or Rpn2 isolated from lung homogenates of acatalasemic or aminotriazole treated mice as compared with that found with Rpn1 and Rpn2 from control mice; these findings are consistent with increased oxidation of cysteine residues in Rpn1 and Rpn2 under in vivo conditions associated with increased intracellular H2O2 concentrations.
FIGURE 6.
Catalase inhibition or the absence of catalase activity is associated with increased oxidative modification of Rpn1 and Rpn2 in the lungs. Lung extracts from acatalasemic mice (A) or mice treated with aminotriazole (B) were incubated with BIAM followed by pull down with streptavidin-agarose. Representative Western blots show the levels of Rpn1 and Rpn2 before (input) and after streptavidin-agarose precipitation (pull down). The -fold changes were calculated as the ratio of the intensity of the precipitated Rpn1 or Rpn2 versus that found in the lung extracts before precipitation. A second experiment provided similar results.
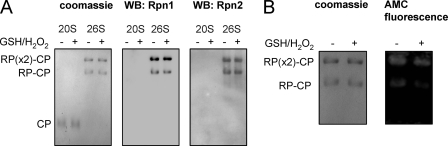
Oxidation and S-Glutathionylation of the 20 S CP and 26 S Proteasomes Do Not Affect Their Composition
Oxidation and glutathionylation of Rpn1 and Rpn2 can potentially affect 26 S proteasomal function through several mechanisms, including by altering their association with the 20 S CP and thereby diminishing the increase in 20 S CP proteolytic activity that normally occurs after interaction with the Rpn1/Rpn2 complex (7). To examine this possibility, purified human 20 S and 26 S proteasomes were incubated with GSH and H2O2 followed by resolution of the proteasomal complexes in clear native PAGE gels (43). No apparent differences were detected between control and oxidized 20 S CP or 26 S proteasomes in terms of their composition of 19 S RP and 20 S CP complexes, and similar amounts of Rpn1 or Rpn2 were found in untreated or GSH/H2O2-treated 26 S proteasomes (Fig. 7A). However, gel assays of proteasomal activity demonstrated that 26 S proteasomes treated with GSH and H2O2 had less chymotrypsin-like activity compared with that present under control conditions (Fig. 7B). These results indicate that exposure of the 26 S proteasome to H2O2 and GSH inhibits proteasome activity without any apparent structural changes.
FIGURE 7.
Lack of effect of exposure to H2O2 and GSH on the composition of 20 S CP or 26 S proteasomes. A, purified 20 S CP or 26 S proteasomes were incubated with or without H2O2 and GSH and then resolved in Clear Native PAGE gels. Panels show the Coomassie-stained gel and Western blots stained for Rpn1 or Rpn2. B, 26 S proteasomal activity was determined after resolution of proteasomes in Clear Native PAGE followed by measuring proteasomal activity in the gel using Suc-Leu-Leu-Val-Tyr-AMC as a substrate. The left panel shows that equal amounts of the 26 S proteasome complexes were present in each sample as determined by staining with Coomassie Blue, whereas the right panel presents a representative AMC fluorescence image. RP, regulatory particle; CP, core particle. RP-CP single or RP(2x)-CP double regulatory particles are in complex with a single core particle. Representative gels are shown. A second experiment provided similar results.
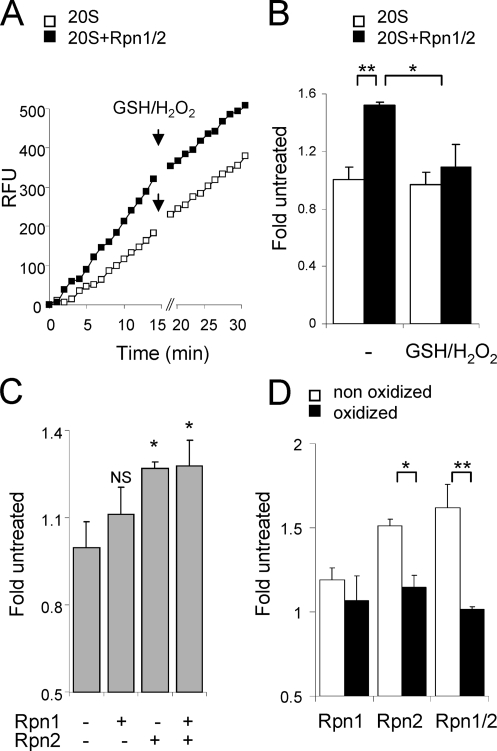
Effects of Glutathionylation of Rpn1 and Rpn2 on 20 S Proteasomal Function
Association of the Rpn1/Rpn2 complex with the 20 S CP results in enhanced 20 S proteasomal activity (7). We therefore determined if S-glutathionylation of Rpn1, Rpn2, or both regulatory subunits affects their ability to increase 20 S proteasomal activity. As shown in Fig. 8(A and B), whereas incubation of untreated Rpn1 and Rpn2 with the 20 S CP enhanced proteasomal activity, such an effect was not found when the Rpn1/2 complex was exposed to GSH and H2O2 before incubation with the 20 S CP. Addition of untreated Rpn2 alone to the 20 S CP increased proteasomal activity to a greater extent than did incubation of the 20 S CP with untreated Rpn1, whereas incubation of the 20 S CP with both Rpn1 and Rpn2 did not result in any further increase in activity beyond that found with Rpn2 alone (Fig. 8C). Such results indicate that the enhancement of 20 S proteasomal activity after exposure to Rpn1 and Rpn2 is primarily due to association with Rpn2. However, no increase in 20 S activity was found after incubation of purified 20 S proteasomes with oxidized Rpn2 or with oxidized Rpn2 and control, unoxidized Rpn1 (Fig. 8C).
FIGURE 8.
Effects of oxidized Rpn1 and Rpn2 on 20 S activity. Representative trace (A) and mean of AMC fluorescence (B) showing 20 S chymotrypsin-like activity measured in the presence or absence of Rpn1/Rpn2 (0.5 μg; 1:1 ratio) for 15 min and then for an additional 10 min after addition of H2O2 and GSH to the cultures. C, mean rates of 20 S proteasome chymotrypsin-like activity in the presence or absence of Rpn1, Rpn2, or both Rpn1 and Rpn2. D, 20 S CP (0.5 μg) was incubated for 10 min with or without unoxidized Rpn1 (0.5 μg), Rpn2 (0.5 μg), or Rpn1/Rpn2 (1:1 ratio) or oxidized Rpn1, Rpn2, or Rpn1/Rpn2 that had been pre-treated with GSH (100 μm) and H2O2 (200 μm). Chymotrypsin-like activity was then measured using AMC fluorogenic substrate. Means ± S.D. are shown. Each experiment was repeated in triplicate. *, p < 0.05 or **, p < 0.01.
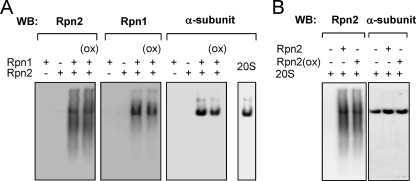
Although it is possible that decreased association of the 20 S CP with oxidized Rpn2 may be responsible for the lack of ability of oxidized Rpn2 to enhance 20 S proteasomal activity, this did not appear to be the case. Incubation of 20 S CP with oxidized Rpn1 and oxidized Rpn2 resulted in similar binding of Rpn1 and Rpn2 with the 20 S CP as was found when the 20 S CP was incubated with control, unoxidized Rpn1 and Rpn2 (Fig. 9A). Further, similar amounts of control unoxidized Rpn2 and oxidized Rpn2 were found to be associated with the 20 S CP after incubation (Fig. 9B).
FIGURE 9.
Effects of oxidation of Rpn1 and Rpn2 on binding to the 20 S CP. Control unoxidized or oxidized Rpn1/Rpn2 (i.e. that had been preincubated with GSH (100 μm) and H2O2 (200 μm) for 15 min) (A) or oxidized Rpn2 or unoxidized Rpn2 (B) were incubated with purified 20 S CP (0.5 μg) for 10 min. Samples were then resolved in Clear Native PAGE followed by Western blot analysis with subsequent re-probing of the membrane with antibodies specific to Rpn1 or Rpn2, or to the 20 S α-subunit. Representative Western blots are shown. A second experiment provided similar results.
DISCUSSION
In the present study we examined mechanisms by which increased intracellular concentrations of H2O2 result in diminished 26 S proteasomal function and, in particular, explored the possibility that the inhibitory actions of H2O2 are through direct oxidative modification of proteasomal subunits. These experiments revealed that S-glutathionylation of Rpn2, a major regulatory particle of the 26 S proteasome, participates in H2O2-dependent inhibition of 26 S proteasomal activity in vitro and also that oxidative modification of Rpn2 was associated with diminished 26 S proteasome function in vivo.
A number of previous studies, including those from our laboratory, have shown that H2O2 participates in regulating proteasomal activity. For example, exposure of bone marrow neutrophils, cells of acute myelogenous leukemia origin, or endothelial cells to H2O2 or to the H2O2 generating combination of glucose/glucose oxidase resulted in inhibition of 26 S proteasomal function (27, 45, 46). Of note, these previous experiments also found a differential effect of H2O2 on the 20 S and 26 S proteasomes, with 26 S proteasomal activity being much more sensitive to inhibition by H2O2 than was that of the 20 S proteasome (28, 29, 53, 54). Similarly, increased intracellular concentrations of H2O2 are found in cells from acatalasemic mice or when catalase activity is blocked through treatment with aminotriazole, and both conditions result in greater inhibition of 26 S proteasomal activity as compared with that of the 20 S CP (30). However, despite the inhibitory actions of H2O2 on 26 S proteasomal function in many cell types, in the present experiments direct exposure of purified and functionally active 20 S CP or 26 S proteasomes to H2O2 had negligible effects on their chymotrypsin-like or trypsin-like activities. In contrast to the lack of effects of H2O2 alone, incubation with the combination of reduced glutathione or H2O2 and glutathione had potent inhibitory effects on 26 S, but not 20 S proteasomal activity, suggesting that subunits of the 19 S RP were the primary targets of oxidative modification and S-glutathionylation that then contributed to decreased 26 S proteasomal function.
Despite the presence of multiple cysteine residues among the components of the 19 S RP that are potentially vulnerable to oxidative modification (55, 56), only a limited number of subunits of the 19 S RP, including Rpn1 and Rpn2, demonstrated S-glutathionylation after in vitro treatment with glutathione and H2O2. Rpn1 and Rpn2 were found to undergo H2O2-mediated glutathionylation in HEK 293 cells and in neutrophils. Oxidation of Rpn1 and Rpn2 was also found in lung homogenates obtained from acatalasemic mice or mice treated with the catalase inhibitor aminotriazole, conditions associated with increased intracellular concentrations of H2O2 (30). Because of the critical role that the Rpn1/Rpn2 complex plays in directing ubiquitinated substrates into the catalytic core of the 20 S CP and thereby enhancing 26 S proteasomal activity, these findings suggest that glutathionylation of Rpn1 and Rpn2 might play an important role in diminishing both 20 S and 26 S proteasomal activity.
Similar to previous studies (29, 57), the present experiments found no alteration in the composition of the 20 S CP or 26 S proteasome after exposure to GSH/H2O2. Additionally, oxidation of Rpn1 and Rpn2 did not affect their association with the 20 S CP. However, in contrast to our study, diminished 26 S proteolytic activity has been found to be accompanied by a loss of 26 S proteasomal structure following exposure to metal-catalyzed oxidation as a result of carbonylation involving one of the S6 ATPase regulatory subunits (58). Strong oxidation of the 26 S proteasome under non-physiologic conditions can occur by exposure to hydroxyl radical generated in metal catalyzed reactions; in the present study, exposure of the 26 S proteasome to GSH and H2O2 was performed in the presence of metal chelator diethylenetriaminepentaacetic acid, eliminating the possibility of such hydroxyl radical-associated events.
Our findings indicate that the mechanism for H2O2-induced inhibition of 26 S proteasomal function is not simply due to reduced binding of Rpn1 or Rpn2 to the 20 S CP. Rather, as shown by the present experiments, incubation of the 20 S CP with oxidized Rpn2 results in diminished 20 S CP activity without any alteration in the amount of Rpn2 bound to the 20 S CP, suggesting that glutathionylation of Rpn2 affects 20 S CP and 26 S proteasomal function through inhibiting substrate processing in the 20 S catalytic core. Additional experiments are underway to examine this possibility.
Although the present experiments, as well as findings from previous studies (58), demonstrate that H2O2 can induce functionally relevant oxidative modifications of 26 S subunits that directly result in diminished proteasomal activity, it is possible that increased intracellular H2O2 concentrations may also modulate proteasomal function through other mechanisms. For example, decreased expression of Rpn11, a 19 S RP subunit, was found to be associated with aging (59), a condition known be accompanied by increased ROS formation (60). Additional indirect effects of H2O2 on proteasomal activity may be due to modulation of redox-sensitive signaling pathways, including those involving protein kinase A (61, 62), that participate in the phosphorylation of Rpt6, an AAA ATPase subunit, which contributes to 26 S proteasomal function (63).
Although the exact mechanisms by which the 19 S RP participates in substrate degradation remain to be completely elucidated (64), Rpn1 and Rpn2 are known to play a major role in processing and unfolding polyubiquitin chains and in transferring ubiquitinated proteins to the 20 S core where they are degraded (7, 11–15). Recent studies, as well as the present experiments, demonstrated that association of the 20 S CP with Rpn2 results in enhanced 20 S activity (7). Such findings suggest that glutathionylation of Rpn2 may directly affect 20 S activity. However, it is also possible that the inhibitory effects of glutathionylation of Rpn2 are associated with aberrant interaction of the Rpn1/Rpn2 complex with other proteasomal subunits, resulting in diminished substrate acquisition or processing within the 20 S proteolytic core.
Although prolonged exposure to H2O2 and other ROS, with associated inhibition of proteasomal activity, is thought to contribute to accelerating aging, DNA damage, and neurodegenerative disorders (36, 65), transient reduction of 26 S proteasomal activity may be beneficial in treating malignancies and acute life-threatening inflammatory conditions. For example, because the ubiquitin/proteasome pathway participates in cell cycle progression, inhibition of proteasomal function, such as that produced by the pharmacologic agent Bortezomib, has been shown to be useful as a chemotherapeutic approach for multiple myeloma (21, 22). Transiently increased intracellular concentrations of H2O2 are associated with diminished 26 S proteasomal activity and result in stabilization of cytoplasmic concentrations of the regulatory protein IκB-α thereby preventing activation of NF-κB signaling cascades and expression of pro-inflammatory mediators, including cytokines such as tumor necrosis factor-α, in lipopolysaccharide stimulated neutrophils (27). Similarly, blockade or absence of catalase activity is associated with increased intracellular H2O2 levels and diminished 26 S proteasomal activity, as well as diminished severity of lipopolysaccharide-mediated acute lung injury in mice (30).
The present experiments provide new insights into the mechanisms by which increased intracellular concentrations of H2O2 result in diminished function of the 26 S proteasome. In particular, in contrast to the enhanced catalytic activity of the 20 S CP that occurs after association with unoxidized Rpn2, there is no change in 20 S CP activity after binding to Rpn2 that has undergone S-glutathionylation. Because S-glutathionylation of cysteine residues is a reversible and physiologically relevant process (66, 67), the finding that oxidative modification of Rpn2 occurs under in vivo conditions associated with increased intracellular concentrations of H2O2, such as in H2O2-exposed neutrophils or in the lungs after catalase inhibition, may provide important insights into the anti-inflammatory actions induced by H2O2 (27, 30). These results also suggest that intracellular processes, such as those involving glutaredoxin 1 (50), that play a role in de-glutathionylation also may be important in affecting proteasomal function through modulating the degree of S-glutathionylation of Rpn2 and perhaps of other proteins associated with the 26 S proteasome.
Acknowledgment
We thank Dr. Jack Lancaster, Jr. for helpful advice.
This work was supported, in whole or in part, by National Institutes of Health Grants HL62221 and GM87748.
- CP
- core particle
- RP
- regulatory particle
- Rpn1
- 26 S proteasome regulatory subunit S2
- Rpn2
- 26 S proteasome regulatory subunit S1
- ROS
- reactive oxygen species
- NF-κB
- nuclear factor-κB
- IκB-α
- IκB kinase
- ATZ
- 3-amino-1,2,4-triazole
- AMC
- 7-amido-4-methyl-coumarin
- HRP
- horseradish peroxidase
- GSS-
- S-glutathionylation of
- BIAM
- biotinylated iodoacetamide.
REFERENCES
- 1.Ciechanover A., Orian A., Schwartz A. L. (2000) BioEssays 22, 442–451 [DOI] [PubMed] [Google Scholar]
- 2.Wang Q. E., Wani M. A., Chen J., Zhu Q., Wani G., El-Mahdy M. A., Wani A. A. (2005) Mol. Carcinog. 42, 53–64 [DOI] [PubMed] [Google Scholar]
- 3.Kornitzer D., Ciechanover A. (2000) J. Cell. Physiol. 182, 1–11 [DOI] [PubMed] [Google Scholar]
- 4.Hershko A. (2005) Cell Death Differ. 12, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 5.Chuang T. H., Ulevitch R. J. (2004) Nat. Immunol. 5, 495–502 [DOI] [PubMed] [Google Scholar]
- 6.Chen Z. J. (2005) Nat. Cell Biol. 7, 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenzweig R., Osmulski P. A., Gaczynska M., Glickman M. H. (2008) Nat. Struct. Mol. Biol. 15, 573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babbitt S. E., Kiss A., Deffenbaugh A. E., Chang Y. H., Bailly E., Erdjument-Bromage H., Tempst P., Buranda T., Sklar L. A., Baumler J., Gogol E., Skowyra D. (2005) Cell 121, 553–565 [DOI] [PubMed] [Google Scholar]
- 9.Verma R., Oania R., Graumann J., Deshaies R. J. (2004) Cell 118, 99–110 [DOI] [PubMed] [Google Scholar]
- 10.Verma R., Aravind L., Oania R., McDonald W. H., Yates J. R., 3rd, Koonin E. V., Deshaies R. J. (2002) Science 298, 611–615 [DOI] [PubMed] [Google Scholar]
- 11.Husnjak K., Elsasser S., Zhang N., Chen X., Randles L., Shi Y., Hofmann K., Walters K. J., Finley D., Dikic I. (2008) Nature 453, 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer C. T., Burdine L., Liu B., Ferdous A., Johnston S. A., Kodadek T. (2008) J. Biol. Chem. 283, 21789–21798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saeki Y., Sone T., Toh-e A., Yokosawa H. (2002) Biochem. Biophys. Res. Commun. 296, 813–819 [DOI] [PubMed] [Google Scholar]
- 14.Leggett D. S., Hanna J., Borodovsky A., Crosas B., Schmidt M., Baker R. T., Walz T., Ploegh H., Finley D. (2002) Mol. Cell 10, 495–507 [DOI] [PubMed] [Google Scholar]
- 15.Elsasser S., Gali R. R., Schwickart M., Larsen C. N., Leggett D. S., Müller B., Feng M. T., Tübing F., Dittmar G. A., Finley D. (2002) Nat. Cell Biol. 4, 725–730 [DOI] [PubMed] [Google Scholar]
- 16.Petropoulos I., Conconi M., Wang X., Hoenel B., Brégégère F., Milner Y., Friguet B. (2000) J. Gerontol A Biol. Sci. Med. Sci. 55, B220–227 [DOI] [PubMed] [Google Scholar]
- 17.Conconi M., Szweda L. I., Levine R. L., Stadtman E. R., Friguet B. (1996) Arch Biochem. Biophys. 331, 232–240 [DOI] [PubMed] [Google Scholar]
- 18.McNaught K. S., Belizaire R., Isacson O., Jenner P., Olanow C. W. (2003) Exp. Neurol. 179, 38–46 [DOI] [PubMed] [Google Scholar]
- 19.Bence N. F., Sampat R. M., Kopito R. R. (2001) Science 292, 1552–1555 [DOI] [PubMed] [Google Scholar]
- 20.Rubinsztein D. C. (2006) Nature 443, 780–786 [DOI] [PubMed] [Google Scholar]
- 21.Kane R. C., Bross P. F., Farrell A. T., Pazdur R. (2003) Oncologist 8, 508–513 [DOI] [PubMed] [Google Scholar]
- 22.Nalepa G., Rolfe M., Harper J. W. (2006) Nat. Rev. Drug Discov. 5, 596–613 [DOI] [PubMed] [Google Scholar]
- 23.Fissolo N., Kraus M., Reich M., Ayturan M., Overkleeft H., Driessen C., Weissert R. (2008) Eur. J. Immunol. 38, 2401–2411 [DOI] [PubMed] [Google Scholar]
- 24.Meiners S., Ludwig A., Stangl V., Stangl K. (2008) Med. Res. Rev. 28, 309–327 [DOI] [PubMed] [Google Scholar]
- 25.Macdonald J., Galley H. F., Webster N. R. (2003) Br. J. Anaesth. 90, 221–232 [DOI] [PubMed] [Google Scholar]
- 26.Fink M. P. (2002) Curr. Opin. Clin. Nutr. Metab. Care 5, 167–174 [DOI] [PubMed] [Google Scholar]
- 27.Zmijewski J. W., Zhao X., Xu Z., Abraham E. (2007) Am. J. Physiol. Cell Physiol. 293, C255–C266 [DOI] [PubMed] [Google Scholar]
- 28.Reinheckel T., Ullrich O., Sitte N., Grune T. (2000) Arch Biochem. Biophys. 377, 65–68 [DOI] [PubMed] [Google Scholar]
- 29.Reinheckel T., Sitte N., Ullrich O., Kuckelkorn U., Davies K. J., Grune T. (1998) Biochem. J. 335, 637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zmijewski J. W., Lorne E., Zhao X., Tsuruta Y., Sha Y., Liu G., Abraham E. (2009) Am. J. Respir. Crit Care Med. 179, 694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C., Hennig G. E., Whiteley H. E., Manautou J. E. (2002) J. Biochem. Mol Toxicol. 16, 227–234 [DOI] [PubMed] [Google Scholar]
- 32.Koechling U. M., Amit Z. (1994) Alcohol. 11, 235–239 [DOI] [PubMed] [Google Scholar]
- 33.Nauseef W. M. (2007) Methods Mol. Biol. 412, 15–20 [DOI] [PubMed] [Google Scholar]
- 34.Zmijewski J. W., Lorne E., Zhao X., Tsuruta Y., Sha Y., Liu G., Siegal G. P., Abraham E. (2008) Am. J. Respir. Crit. Care Med. 178, 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zmijewski J. W., Lorne E., Banerjee S., Abraham E. (2009) Am. J. Physiol. Lung Cell Mol. Physiol. 296, L624–L634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding Q., Lewis J. J., Strum K. M., Dimayuga E., Bruce-Keller A. J., Dunn J. C., Keller J. N. (2002) J. Biol. Chem. 277, 13935–13942 [DOI] [PubMed] [Google Scholar]
- 37.Nam S., Smith D. M., Dou Q. P. (2001) J. Biol. Chem. 276, 13322–13330 [DOI] [PubMed] [Google Scholar]
- 38.Pajonk F., Riess K., Sommer A., McBride W. H. (2002) Free Radic. Biol. Med. 32, 536–543 [DOI] [PubMed] [Google Scholar]
- 39.Kim J. R., Yoon H. W., Kwon K. S., Lee S. R., Rhee S. G. (2000) Anal. Biochem. 283, 214–221 [DOI] [PubMed] [Google Scholar]
- 40.Choi K. S., Park S. Y., Baek S. H., Dey-Rao R., Park Y. M., Zhang H., Ip C., Park E. M., Kim Y. H., Park J. H. (2006) Prep Biochem. Biotechnol. 36, 65–79 [DOI] [PubMed] [Google Scholar]
- 41.Cross J. V., Templeton D. J. (2006) Antioxid. Redox Signal. 8, 1819–1827 [DOI] [PubMed] [Google Scholar]
- 42.Ying J., Clavreul N., Sethuraman M., Adachi T., Cohen R. A. (2007) Free Radic. Biol. Med. 43, 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elsasser S., Schmidt M., Finley D. (2005) Methods Enzymol. 398, 353–363 [DOI] [PubMed] [Google Scholar]
- 44.Schmitt J., Hess H., Stunnenberg H. G. (1993) Mol. Biol. Rep 18, 223–230 [DOI] [PubMed] [Google Scholar]
- 45.Kotamraju S., Tampo Y., Keszler A., Chitambar C. R., Joseph J., Haas A. L., Kalyanaraman B. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10653–10658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotamraju S., Kalivendi S., Shang T., Kalyanaraman B. (2005) Methods Enzymol. 396, 526–534 [DOI] [PubMed] [Google Scholar]
- 47.Lu S. C. (2000) Curr. Top Cell Regul. 36, 95–116 [DOI] [PubMed] [Google Scholar]
- 48.Demasi M., Shringarpure R., Davies K. J. (2001) Arch Biochem. Biophys 389, 254–263 [DOI] [PubMed] [Google Scholar]
- 49.Demasi M., Silva G. M., Netto L. E. (2003) J. Biol. Chem. 278, 679–685 [DOI] [PubMed] [Google Scholar]
- 50.Shelton M. D., Chock P. B., Mieyal J. J. (2005) Antioxid. Redox Signal. 7, 348–366 [DOI] [PubMed] [Google Scholar]
- 51.Shelton M. D., Mieyal J. J. (2008) Mol. Cells 25, 332–346 [PMC free article] [PubMed] [Google Scholar]
- 52.Minhas H. S., Thornalley P. J. (1995) Biochem. Pharmacol 49, 1475–1482 [DOI] [PubMed] [Google Scholar]
- 53.Breusing N., Grune T. (2008) Biol. Chem. 389, 203–209 [DOI] [PubMed] [Google Scholar]
- 54.Davies K. J. (2001) Biochimie 83, 301–310 [DOI] [PubMed] [Google Scholar]
- 55.Coux O., Tanaka K., Goldberg A. L. (1996) Annu. Rev. Biochem. 65, 801–847 [DOI] [PubMed] [Google Scholar]
- 56.Murata S., Yashiroda H., Tanaka K. (2009) Nat. Rev. Mol. Cell Biol. 10, 104–115 [DOI] [PubMed] [Google Scholar]
- 57.Hosler M. R., Wang-Su S. T., Wagner B. J. (2003) Int. J. Biochem. Cell Biol. 35, 685–697 [DOI] [PubMed] [Google Scholar]
- 58.Ishii T., Sakurai T., Usami H., Uchida K. (2005) Biochemistry 44, 13893–13901 [DOI] [PubMed] [Google Scholar]
- 59.Tonoki A., Kuranaga E., Tomioka T., Hamazaki J., Murata S., Tanaka K., Miura M. (2009) Mol. Cell. Biol. 29, 1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Droge W. (2002) Physiol. Rev. 82, 47–95 [DOI] [PubMed] [Google Scholar]
- 61.Barlow C. A., Kitiphongspattana K., Siddiqui N., Roe M. W., Mossman B. T., Lounsbury K. M. (2008) Apoptosis 13, 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Pina M. Z., Vazquez-Meza H., Pardo J. P., Rendon J. L., Villalobos-Molina R., Riveros-Rosas H., Pina E. (2008) J. Biol. Chem. 283, 12373–12386 [DOI] [PubMed] [Google Scholar]
- 63.Zhang F., Hu Y., Huang P., Toleman C. A., Paterson A. J., Kudlow J. E. (2007) J. Biol. Chem. 282, 22460–22471 [DOI] [PubMed] [Google Scholar]
- 64.Tanaka K. (2009) Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 85, 12–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farout L., Friguet B. (2006) Antioxid. Redox Signal. 8, 205–216 [DOI] [PubMed] [Google Scholar]
- 66.Dalle-Donne I., Rossi R., Giustarini D., Colombo R., Milzani A. (2007) Free Radic. Biol. Med. 43, 883–898 [DOI] [PubMed] [Google Scholar]
- 67.Giustarini D., Rossi R., Milzani A., Colombo R., Dalle-Donne I. (2004) J. Cell Mol. Med. 8, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]