Abstract
Neuropeptide signaling at the cell surface is regulated by metalloendopeptidases, which degrade peptides in the extracellular fluid, and β-arrestins, which interact with G protein-coupled receptors (GPCRs) to mediate desensitization. β-Arrestins also recruit GPCRs and mitogen-activated protein kinases to endosomes to allow internalized receptors to continue signaling, but the mechanisms regulating endosomal signaling are unknown. We report that endothelin-converting enzyme-1 (ECE-1) degrades substance P (SP) in early endosomes of epithelial cells and neurons to destabilize the endosomal mitogen-activated protein kinase signalosome and terminate signaling. ECE-1 inhibition caused endosomal retention of the SP neurokinin 1 receptor, β-arrestins, and Src, resulting in markedly sustained ERK2 activation in the cytosol and nucleus, whereas ECE-1 overexpression attenuated ERK2 activation. ECE-1 inhibition also enhanced SP-induced expression and phosphorylation of the nuclear death receptor Nur77, resulting in cell death. Thus, endosomal ECE-1 attenuates ERK2-mediated SP signaling in the nucleus to prevent cell death. We propose that agonist availability in endosomes, here regulated by ECE-1, controls β-arrestin-dependent signaling of endocytosed GPCRs.
Signal transduction at the plasma membrane is tightly regulated by well characterized mechanisms. For the large family of G protein-coupled receptors (GPCRs),2 regulation of extracellular agonist concentration and receptor coupling to heterotrimeric G proteins control signal transduction. Cell-surface metalloendopeptidases, exemplified by neprilysin, degrade neuropeptides in the extracellular fluid to regulate receptor activation (1, 2). G protein receptor kinases phosphorylate activated GPCRs to promote their interaction with β-arrestins, which uncouple receptors from G proteins and mediate desensitization (3). However, activated GPCRs internalize, and endocytosed receptors continue to signal by G protein-independent mechanisms. β-Arrestins mediate endocytosis and intracellular signaling of GPCRs. β-Arrestins couple GPCRs to clathrin and AP2 to mediate endocytosis (4, 5) and are scaffolds that recruit Src, Raf-1, and mitogen-activated protein kinases (MAPKs) to GPCRs in endosomes forming a MAPK signalosome, which determines the location and function of activated extracellular signal-regulated kinases (ERKs) (6–9). Compared with our understanding of receptor regulation at the plasma membrane, little is known about the mechanisms that regulate GPCR signaling and trafficking at the endosomal membrane. Specifically, it is not known whether agonist interaction with GPCRs or GPCR interaction with β-arrestins is necessary for receptor signaling in endosomes. Regulation of these interactions could determine the duration of β-arrestin-mediated ERK activation, with important functional implications (10, 11).
We hypothesized that mechanisms that regulate GPCRs at the plasma membrane also control receptor signaling and trafficking at the endosomal membrane. We recently reported that the metalloendopeptidase endothelin-converting enzyme-1 (ECE-1) is present at the endosomal membrane and that ECE-1 degrades internalized neuropeptides within acidified early endosomes (12–14). This degradation disrupts the peptide-GPCR-β-arrestin complex to promote β-arrestin translocation back to the cytosol and receptor recycling to the cell-surface, which mediates resensitization. Whether this process regulates signaling of receptors at the endosomal membrane and subsequent translocation of signals to the nucleus and throughout the cytosol is completely unknown.
We report that ECE-1, by degrading neuropeptides in endosomes, regulates the stability and activity of peptide-GPCR-β-arrestin-MAPK signalosomes to control the duration of ERK activation and subsequent activation of transcription factors, one of which mediates programmed cell death. We examined the role of ECE-1 in regulating signaling by substance P (SP). SP is expressed in the nervous and immune systems and interacts with the neurokinin 1 receptor (NK1R) to control neurogenic inflammation, pain, neurodegeneration, smooth muscle contraction, and exocrine secretions (15). SP induces NK1R interaction with β-arrestins at the plasma membrane, which mediates desensitization and causes β-arrestin-dependent endocytosis of the SP-NK1R-β-arrestin complex to early endosomes containing ECE-1 (13, 16). β-Arrestins recruit Src, MEK, and ERKs to the NK1R at the endosomal membrane, which mediates the effects of SP on gene expression and cell survival (6). However, nothing is known about the regulation of the stability and activity of the SP-NK1R-β-arrestin-MAPK signalosome in endosomes.
EXPERIMENTAL PROCEDURES
Reagents
Anti-pERK1/2 (E-4), anti-ERK2 (C-14), anti-Rab5a (S-19), anti-Nur77 (M-210), and mouse or rabbit nonspecific IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-pERK1/2 (phospho-p44/42 MAPK, E10 #9106), anti-ERK1/2 (p44/42 MAPK, #9102), anti-Src (L4A1), anti-phosphothreonine, and anti-c-Fos (9F6) were from Cell Signaling Technology (Danvers, MA). Anti-early endosomal antigen-1 (EEA1) was from BD Transduction Laboratories. Anti-Na+-K+-ATPase was from Novus Biologicals (Littleton, CO). Anti-lysosomal-associated glycomembrane protein 1 (LAMP1, H4A3) was from Developmental Studies Hybridoma Bank (Iowa City, IA). Anti-H3 histone was from Sigma. Anti-ECE-1 (AF1784) was from R&D System, Inc., (Minneapolis, MN). For anti-NK1R (94168), see Ref. 17. Anti-β-arrestin2 was a gift from R. Lefkowitz (Duke University, see Ref. 18). Mouse and rabbit anti-ubiquitin carboxyl-terminal hydrolase isozyme L1 (PGP9.5) was from Biogenesis (Sandown, NH). Goat anti-mouse light chain, mouse anti-rabbit light chain, and goat or donkey anti-mouse or rabbit IgG coupled to fluorescein isothiocyanate, Rhodamine Red-X, or Cy5 were from Jackson ImmunoResearch (West Grove, PA). Goat or donkey anti-mouse, rabbit, or goat IgG or IgM coupled to AlexaFluor488, AlexaFluor568, or AlexaFluor680 (Invitrogen) and coupled to IRDye800 were from Rockland Immunochemicals (Gilbertsville, PA). Human epidermal growth factor was from Invitrogen. SP and Sar9,Met(O2)11-SP (SMSP) was from Bachem (Torrance, CA). SM-19712, anti-β-actin (A-5441), and anti-FLAG (F7425) were from Sigma. AG1478 was from Calbiochem. PD-069185 (19) was a gift (Pfizer). Other reagents were from Sigma or have been described (12, 13, 20).
Animals
C57BL6 mice (male, 6–8 weeks) and Sprague-Dawley rats (male, 175–200 g; neonates, 4–8 days) were from Charles River Laboratories (Hollister, CA). The University of California, San Francisco Institutional Animal Care and Use Committee approved all experiments.
Transfected Cells and Cell Lines
Generation and maintenance of human embryonic kidney 293 (HEK) FLP cells (Invitrogen) and HEKFLP stably expressing rat NK1R have been described (12, 13, 20). In some experiments cells were transiently transfected with rat NK1R, rat FLAG-NK1RΔ325–407, rat β-arrestin1 V53D, rat β-arrestin1319–418, human β-arrestin2-GFP, human ECE-1c-GFP, or empty vector (control) using Lipofectamine2000 (Invitrogen). With the exception of experiments to examine Nur77 expression, cells were deprived of serum (Dulbecco's modified Eagle's medium, 0.1% bovine serum albumin) overnight before and during the experiments.
Agonist and Drug Treatments
Cells were preincubated for 1 h with vehicle (control, 0.1% DMSO), AG1478 (1 μm), SM-19712 (10 μm), bafilomycin A1 (1 μm), or UO126 (10 μm). Inhibitors were included throughout the experiments. Cells were stimulated with vehicle (0.9% NaCl) or SP, SMSP, Alexa-SP (10–1000 nm, 0–30 min), or EGF (100 ng/ml, 2 min), washed, and incubated in SP-free medium (0–24 h).
Subcellular Fractionation
For whole cell lysates, cells were suspended in 50 mm Tris/HCl, pH 7.4, 10 mm NaF, 0.1 mm Na3VO4, 0.1% Triton-X-100 and sonicated, and debris was cleared by centrifugation (16,000 × g, 10 min). Nuclear and cytosolic fractions were prepared as described (21).
Endosomal Isolation
Cells were suspended in 10 mm HEPES, pH 7.2, 100 mm KCl, 1 mm EDTA, 25 mm sucrose, passed through a 22-gauge syringe needle 15 times, and centrifuged (3000 g, 10 min, 4 °C). Supernatants were rotated with anti-EEA1 antibody (2.5 μg, overnight, 4 °C). Immune complexes were captured with goat anti-mouse IgG magnetic microbeads (2 h, 4 °C) and purified using MACS MS separation columns (Miltenyi Biotec, Auburn, CA) (22).
Preparation of Membranes from Tissues
Tissues were homogenized in 50 mm Tris/HCl, pH 7.4, containing a protease inhibitor mixture (Roche Applied Science) and sonicated, and cell debris was removed by centrifugation (1000 × g, 4 °C, 10 min). Supernatants were centrifuged (90,000 × g, 4 °C, 1 h), and the resulting membrane pellet was solubilized in 50 mm Tris/HCl, pH 7.4, containing 0.1% Triton-X-100 and a protease inhibitor mixture.
SDS-PAGE and Western Blotting
Samples (5–30 μg of protein) were separated by SDS-PAGE (8 or 12% acrylamide). Membranes were incubated with antibodies to pERK2 (E-4; 1:1,000), ERK2 (C-14; 1:5,000), c-Fos (9F6, 1:2,000), ECE-1 (1:1,000), EEA1 (1:10,000), Rab5a (1:2,000), LAMP1 (1:2,000), Na+-K+-ATPase (1:5,000), histone H3 (1:20,000), NK1R (1:10,000), β-arrestin2 (1:5,000), Src (1:1,000), Nur77 (1:200), phosphothreonine (1:1,000), or β-actin (A-5441, 1:10,000) overnight at 4 °C. Membranes were treated with secondary or tertiary antibodies and analyzed with an Odyssey Infrared Imaging System (Li-COR Biosciences) (12, 13, 20).
Immunoprecipitation
Cells were plated on 100-mm dishes for 48 h. Cells were washed with PBS containing Ca2+ and Mg2+, lysed with 1 ml of buffer (50 mm Tris/HCl, pH 7.4, 150 mm NaCl, 5 mm MgCl2, 10 mm NaF, 0.1 mm Na3VO4, 10 mm Na4P2O7, 0.1 mm ethylene glycol tetraacetic acid, 0.5% Nonidet P-40), and centrifuged (30 min, 16,000 × g, 4 °C). Supernatants were rotated with immunoprecipitating antibody (Nur77 or nonspecific rabbit IgG, 2 μg/ml, overnight, 4 °C) and analyzed by Western blotting.
Measurement of [Ca2+]i
[Ca2+]i was measured in populations of cells using Fura2-AM (Invitrogen) as described (12, 20). To assess resensitization, cells were incubated with vehicle or SP (10 nm, 0 or 10 min, 37 °C), washed, and recovered in SP-free medium for 2 h. Cells were challenged with SP (10 nm), and [Ca2+]i was measured.
Peptide Degradation and Mass Spectrometry
Peptides (250 μm) were incubated with recombinant human ECE-1 (65 or 195 nm) at room temperature in 50 mm MES-KOH, pH 5.5, or 50 mm Tris-HCl, pH 7.4, in 20 μl final volume containing a final 50% H218O (Sigma-Aldrich). The reaction products were sampled over 48 h by spotting aliquots directly onto the MALDI target with α-cyano-4-hydroxycinnamic acid (5 mg/ml in 50% acetonitrile, 0.1% trifluoroacetic acid) in a 1:1 v/v ratio. Samples were prepared and analyzed in triplicate using a 4800 Proteomics Analyzer MALDI-TOF mass spectrometer (Applied Biosystems, Foster City, CA). Peptides were identified, and 18O incorporation ratios were determined (23).
Immunofluorescence of Cultured Cells
To localize pERK1/2 and ERK1/2, HEK cells were fixed, and proteins were localized using primary antibodies pERK1/2 (phospho-p44/42 MAPK, E10 #9106, 1:200) and ERK1/2 (anti-p44/42 MAPK, #9102, 1:200) according to the manufacturer's guidelines. To examine the interaction of NK1RΔ325–407 and β-arrestin2, NK1RΔ325–407 was labeled at the cell surface by incubating HEK-NK1RΔ325–407-β-arrestin2-GFP cells with anti-FLAG for 30 min at 37 °C (20). Cells were washed with PBS, stimulated with SP (10 nm, 10 min), washed, recovered in SP-free medium, and fixed in 4% paraformaldehyde, 100 mm PBS, pH 7.4 (20 min, 4 °C). NK1R was detected by indirect immunofluorescence and β-arrestin2 using GFP. To localize NK1R, ECE-1, and PGP9.5 in myenteric neurons, cultures were fixed in paraformaldehyde (20 min, 4 °C), washed in PBS containing 10% normal donkey serum and 0.3% Triton X-100, and incubated with primary antibodies anti-NK1R (1:500), anti-ECE-1 (1:200), and anti-PGP9.5 (1:500) overnight at 4 °C. Cells were washed, incubated with secondary antibodies conjugated to fluorescein isothiocyanate or Rhodamine Red X (1:500, room temperature, 2 h), washed, and mounted. To localize Alexa-SP with ECE-1, myenteric neurons were incubated with 100 nm Alexa-SP (2 h, 4 °C), washed, and incubated for 30 min at 37 °C (24). Cells were fixed in paraformaldehyde (20 min, 4 °C), incubated with anti-ECE-1 (1:100, room temperature, 2 h), and processed.
Immunofluorescence of Tissues
Whole mounts of myenteric plexus of the rat terminal ileum were prepared and processed as floating preparations (25). Mouse spinal cord was fixed in 4% paraformaldehyde, 100 mm PBS, pH 7.4 (2 h, 4 °C), incubated in 30% sucrose in PBS (24 h, 4 °C), embedded in OCT compound (Miles, Elkhart, IN), and sectioned at 12 μm. Sections and whole mounts were incubated in PBS, 5% normal donkey serum, or 10% normal horse serum and 0.1% Triton X-100 with primary antibodies anti-NK1R (1:200), anti-ECE-1 (1:100–200), anti-PGP9.5 (1:200) at 4 °C for 48 h. Tissues were washed and incubated with secondary antibodies conjugated to fluorescein isothiocyanate, AlexaFluor488, AlexaFluor568, Rhodamine Red X, or Cy5 (1:200–500, room temperature, 2 h). Slides were washed and mounted.
Confocal Microscopy
Cells and tissues were observed by using Zeiss Axiovert with a Zeiss 510 Meta confocal microscope with Zeiss objectives Plan Apochromat x100 (NA 1.4), Fluar Plan Apochromat ×63 (NA 1.4), ×40 Plan-Neofluar (NA 1.3), or ×20 Plan-Apochromat (NA 0.8) and collected and processed as described (12, 20).
ECE-1 Antibody Preabsorption
Membranes were prepared from HEK cells transiently transfected with empty vector (control) or ECE-1c-GFP (12), and membrane proteins were solubilized in 50 mm Tris/HCl containing 1% Triton X-100. ECE-1 antibody (1:200, PBS, 10% normal horse serum, 0.1% Triton X-100) was preincubated with membrane proteins (10 mg/ml, overnight, 4 °C) before use.
ERK Activation in Mouse Spinal Cord
Mice were anesthetized by isoflurane (2.5%) and SM-19712 (17.5 μg/5 μl) or saline (control, 5 μl) were injected into the subarachnoid space on the midline between the L4 and L5 vertebrae using a Hamilton syringe with a 30-gauge needle. After 30 min animals received intraplantar injections (5 μl) of vehicle (ethanol:Tween 80:saline (1:8:1)) or capsaicin (10 μg). After 10 and 60 min, spinal cord was frozen in liquid nitrogen, homogenized in Camiolo buffer containing 10 mm NaF and 0.1 mm Na3VO4, and processed for Western blotting.
Cultured Myenteric Neurons
Myenteric neurons were isolated from the small intestine of neonatal rats (26). Neurons were grown in Dulbecco's modified Eagle's medium containing heat-inactivated fetal bovine serum (2.5%), l-glutamine (2 mm), N1 medium supplement (Sigma; 1:100), nerve growth factor (10 ng/ml), penicillin (100 units/ml), streptomycin (0.1 mg/ml), and amphotericin B (25 μg/ml). After 24 h neurons were placed in medium without heat-inactivated fetal bovine serum and were cultured for >4 days before experimentation.
Cytotoxicity Assay
Cell death was assessed using a cytotoxicity detection kit (Roche Applied Science) to measure the lactate dehydrogenase (LDH) activity. Released and total cellular LDH activity were measured. Results are expressed as LDH release as a percentage of the total cellular LDH and as the -fold increase over control of LDH activity released in response to stimulation with SP.
Reverse Transcription-PCR
RNA from mouse spinal cord (L4-L6) and myenteric ganglia (∼50) of rat ileum was isolated using Trizol (Invitrogen) and treated with DNase I (Ambion, Austin, TX). RNA was reverse-transcribed using standard protocols with random hexamers and TaqMan reverse transcription reagents (Applied Biosystems). Subsequent PCR reactions used primers specific for rat or mouse ECE-1 isoforms (sequences available on request). Control reactions omitted reverse transcriptase. PCR products were separated by electrophoresis, stained with ethidium bromide, and sequenced to confirm identity.
Statistics
Data are presented as the SE. of n ≥ 3 experiments or animals. Results were compared by Student's t test, with p < 0.05 considered significant (*).
RESULTS
SP Activates ERKs by Epidermal Growth Factor Receptor (EGFR)- and β-Arrestin-dependent Pathways
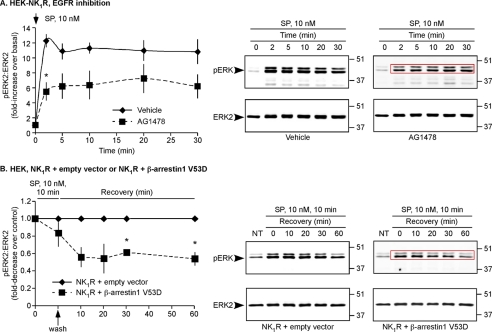
GPCRs activate ERKs by mechanisms operating at the plasma membrane and in endosomes (10, 11). One mechanism involves activation of cell-surface metalloendopeptidases, which release membrane-tethered ligands that activate the EGFR (27). To determine the contribution of the EGFR to SP-stimulated ERK activation, we examined the effects of the tyrosine kinase inhibitor AG1478 on SP-induced phosphorylation of ERK2 (pERK2). HEK-NK1R cells treated with vehicle or AG1478 were stimulated with SP (10 nm, 0–30 min), and pERK2 and ERK2 were quantified by Western blotting. In unstimulated cells, pERK2 was low (Fig. 1A). In vehicle- and AG1478-treated cells, SP stimulated a prompt increase in pERK2 within 2 min (Fig. 1A). However, the magnitude of the increase in pERK2 over basal was reduced in AG1478-treated cells (5.5 ± 1.2-fold increase, 2 min) compared with vehicle-treated cells (-fold increase, 2 min, 12 ± 0.9, Fig. 1A). Thus, SP rapidly activates ERK2 in part by transactivating the EGFR. To exclude this pathway, subsequent experiments used cells treated with AG1478 (unless indicated), which abolished EGF-stimulated ERK2 activation (supplemental Fig. 1A).
FIGURE 1.
SP induces EGFR- and β-arrestin-dependent ERK2 activation. A, HEK-NK1R cells were incubated with vehicle or AG1478 and stimulated with SP (10 nm, 0–30 min). Results are expressed as the -fold change of pERK2:ERK2 compared with basal. SP-induced pERK2:ERK2 was ∼2-fold higher in vehicle-treated cells. B, HEK cells expressing NK1R and empty vector (control) or β-arrestin1 V53D-GFP were incubated with AG1478, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–60 min). Results are expressed as -fold change of pERK2:ERK2 compared with control. SP challenge similarly increased pERK2:ERK2 in NK1R-control and NK1R-β-arrestin1 V53D-GFP cells. After removal of SP, β-arrestin1 V53D-GFP attenuated the response.
To determine whether SP also activates ERK2 in a β-arrestin-dependent manner, we expressed dominant negative β-arrestin1 V53D, which inhibits sequestration of receptors into clathrin-coated vesicles and disrupts activation of ERK2 by receptors in endosomes (6, 7, 28). To examine sustained ERK activation, HEK cells expressing NK1R and empty vector (control) or β-arrestin1 V53D-GFP were challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–60 min). AG1478 was included to suppress the EGFR pathway. SP challenge for 10 min similarly increased pERK2 in NK1R-control cells and in NK1R-β-arrestin1 V53D cells (Fig. 1B, supplemental Fig. 1B, upper panel). Expression of another dominant negative mutant, β-arrestin319–418, also did not affect the initial response to SP challenge (supplemental Fig. 1B, lower panel). However, after removal of SP, levels of pERK2 in NK1R-β-arrestin1 V53D cells were lower than NK1R control cells (-fold decrease, 30 min, 2.3 ± 0.1; 60 min 2.2 ± 0.1, Fig. 1B). Thus, SP induces sustained ERK2 activation in a β-arrestin-dependent manner, and this effect is independent of EGFR transactivation.
ECE-1 Regulates Assembly of the Endosomal MAPK Signalosome
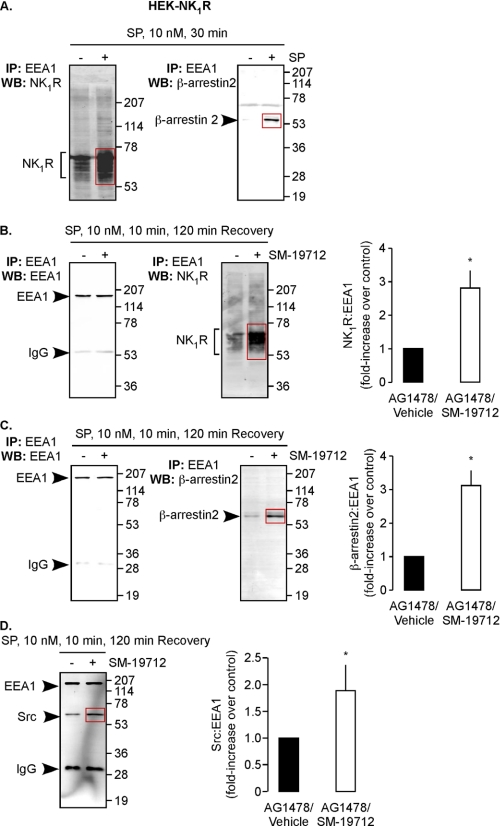
β-Arrestins recruit SP, NK1R, Src, MEK, and ERK1/2 to endosomes (6). To examine whether ECE-1 regulates the stability of this complex, we purified endosomes from HEK-NK1R cells by immunoprecipitation with antibodies to EEA1 (22). HEK cells naturally express all ECE-1 isoforms (12, 13). We confirmed the enrichment of endosomes by Western blotting for endosomal (EEA1, Rab5a), lysosomal (LAMP1), and cell-surface (Na+-K+-ATPase) markers. The presence of EEA1 and Rab5a and absence of LAMP1 and Na+-K+-ATPase indicate that endosomal fractions were highly enriched (supplemental Fig. 2A). NK1R and β-arrestin2 were low or undetectable in endosomes from unstimulated cells (Fig. 2A). SP (10 nm, 30 min) induced a marked increase in NK1R and β-arrestin2 in endosomes. To examine whether ECE-1 regulates stability of the MAPK signalosome in endosomes, we treated HEK-NK1R cells with vehicle or the specific ECE-1 inhibitor SM-19712 (29), stimulated with SP (10 nm, 10 min) and incubated in SP-free medium (120 min). Endosomes were analyzed for NK1R, β-arrestin2, and Src, normalized to EEA1. SM-19712 increased levels of endosomal NK1R (2.8 ± 0.5-fold), β-arrestin2 (3.2 ± 0.5-fold), and Src (1.9 ± 0.5-fold), compared with vehicle-treated cells (Fig. 2, B–D). Thus, ECE-1 regulates association of NK1R, β-arrestins, and Src with endosomes.
FIGURE 2.
ECE-1 regulates SP-induced recruitment of NK1R, β-arrestin2, and Src to endosomes. A, HEK-NK1R cells were stimulated with SP (10 nm, 0–30 min), and endosomes were isolated and analyzed for NK1R and β-arrestin2. SP increased levels of NK1R and β-arrestin2 in endosomes. B–D, HEK-NK1R cells were incubated with AG1478 and vehicle or SM-19712, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–120 min). Endosomes were isolated and analyzed for NK1R (B), β-arrestin2 (C), and Src (D), normalized to EEA1. SM-19712 caused retention of NK1R, β-arrestin2, and Src. IP, immunoprecipitation; WB, Western blot.
ECE-1 Regulates the Duration and Subcellular Location of SP-induced, β-Arrestin-dependent ERK Activation
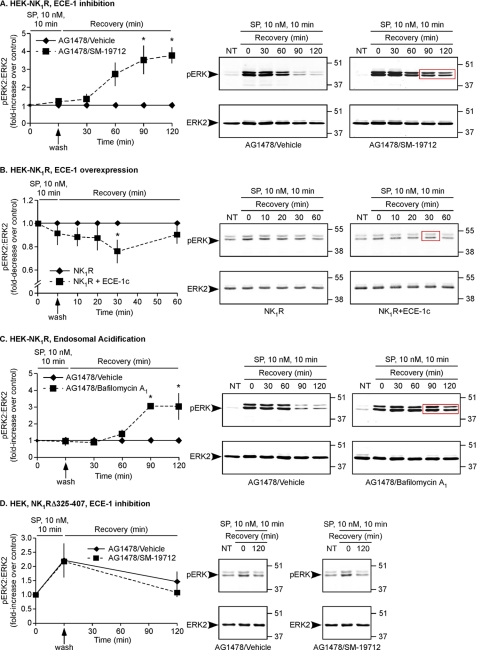
ECE-1 hydrolyzes SP at endosomal (5.5) but not extracellular (7.4) pH (13, 30). Thus, although ECE-1 is at the cell surface, it neither degrades SP in the extracellular fluid nor controls SP-induced activation of the NK1R at the plasma membrane (13). However, ECE-1 degrades SP in endosomes to destabilize the endosomal SP-NK1R-β-arrestin complex (13). Because ECE-1 inhibition/knockdown causes prolonged interaction of NK1R and β-arrestins in endosomes (13), we hypothesized that ECE-1 regulates sustained β-arrestin-dependent ERK2 activation but not rapid EGFR-dependent ERK2 activation. To test this hypothesis, HEK-NK1R cells were treated with vehicle or SM-19712, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–120 min). Although SM-19712 did not affect the ERK2 activation to the initial SP challenge (10 min), after removal of SP, ERK2 activation was markedly more sustained in cells treated with SM-19712 (3.5 ± 0.8-fold increase, 90 min; 3.8 ± 0.5-fold increase 120 min, Fig. 3A). We determined whether ECE-1 regulates ERK activation in the cytosol and nucleus by subcellular fractionation and immunofluorescence microscopy. Cytosolic fractions were enriched with β-actin and free of histone H3, whereas nuclear fractions were enriched with histone H3 and free of β-actin, which verifies purity (supplemental Fig. 2B). SM-19712 caused a markedly prolonged ERK2 activation in cytosolic (-fold increase, 90 min, 3.5 ± 0.3; 120 min, 4.3 ± 0.5-fold) and especially in nuclear (60 min, 5.6 ± 1.1; 90 min, 10 ± 2.6; 120 min, 9.4 ± 3.6) fractions compared with vehicle-treated cells (Fig. 4, A and B). In unstimulated cells pERK1/2 were undetectable in the cytosol and nucleus, and ERK1/2 were localized to the cytosol (Fig. 4, D and E). After SP challenge (10 min), pERK1/2 and ERK1/2 were detected in the cytosol and nucleus of both vehicle- and SM-19712-treated cells. After incubation in SP-free medium (120 min), pERK1/2 returned to prestimulation levels in the cytosol and nucleus of vehicle-treated cells but remained in the cytosol and nucleus of SM-19712-treated cells. This effect of SM-19712 was confirmed by quantification of the total pixel intensity of the cytosol and nucleus (-fold increase, 120 min, 1.5 ± 0.2, Fig. 4E).
FIGURE 3.
ECE-1 and endosomal acidification regulate the duration of SP-induced ERK2 activation. A, HEK-NK1R cells were incubated with AG1478 and vehicle or SM-19712, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–120 min). SP caused similar initial ERK2 activation in vehicle- and SM-19712-treated cells, but ERK2 activation was sustained in SM-19712-treated cells. B, HEK cells expressing NK1R and empty vector (control) or ECE-1c-GFP were incubated with AG1478 and vehicle, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–120 min). SP caused similar increases in ERK2 activation in NK1R-control and NK1R-ECE-1c-GFP cells. After removal of SP, the magnitude of the response in NK1R-ECE-1c-GFP cells was significantly lower than in NK1R-control cells. C, HEK-NK1R cells were incubated with AG1478 and vehicle or bafilomycin A1, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–120 min). SP caused similar ERK2 activation in vehicle- and bafilomycin A1-treated cells, but ERK2 activation was sustained in bafilomycin A1-treated cells. D, HEK cells expressing NK1RΔ325–407 were incubated with AG1478 and vehicle or SM-19712, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–120 min). The magnitude and duration of ERK2 activation were unaffected by SM-19712 (n ≥ 3).
FIGURE 4.
ECE-1 regulates β-arrestin-dependent ERK2 activation in cytosolic and nuclear fractions. A and B, HEK-NK1R cells were incubated with AG1478 and vehicle or SM-19712, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–120 min), and pERK2 and ERK2 were analyzed in cytosolic (A) and nuclear fractions (B). SM-191712 caused sustained ERK2 activation in cytosolic and nuclear fractions after SP removal. C–E, cells were treated as in A, and pERK1/2 and ERK1/2 were localized by immunofluorescence and confocal microscopy. SP caused similar ERK1/2 activation in vehicle (C)- and SM-191712 (D)-treated cells. SM-19712 caused sustained ERK1/2 activation in the cytosol (arrows) and nucleus (arrowheads) at 120 min of recovery. Scale bar, 10 μm. E, quantification of ERK1/2 activation (n = 60 cells).
To confirm the effects of SM-19712, we used another ECE-1 inhibitor, PD-069185 (19). First, we examined whether PD-069185, like SM-19712 (13), inhibits resensitization of NK1R signaling, which is because of endosomal retention of NK1R. HEK-NK1R cells were treated with vehicle or PD-069185, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (120 min). Changes in [Ca2+]i to a second challenge with SP were measured. After 120 min in SP-free medium, responses in vehicle-treated cells were fully resensitized (101 ± 6%, supplemental Fig. 3A). In contrast, responses in PD-069185-treated cells were strongly inhibited (55 ± 9%). Thus, inhibition of ECE-1 with PD-069185 prevents resensitization of SP-induced Ca2+ signaling. Next, we examined the effect of PD-069185 on SP-induced ERK2 activation. Challenge with SP (10 min) increased pERK2 to similar levels in cells treated with vehicle or PD-069185. However, levels of pERK2 were more sustained in PD-069185-treated cells (-fold increase, 120 min, 1.9 ± 0.3, supplemental Fig. 3B).
Because ECE-1 overexpression promotes NK1R and β-arrestin dissociation in endosomes to induce NK1R recycling and β-arrestin translocation to the cytosol (13), we hypothesized that ECE-1 overexpression would attenuate SP-induced ERK activation. To examine this possibility, HEK cells expressing NK1R and empty vector (control) or ECE-1c-GFP were challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–60 min). Although SP caused similar initial increases in pERK2 (10 min) in NK1R-control cells and NK1R-ECE-1c-GFP cells, overexpression of ECE-1c attenuated ERK2 activation at 30 min (-fold decrease, 30 min, 2.2 ± 0.5, Fig. 3B). After 60 min pERK levels were similar in both cell types. We verified overexpression of ECE-1c-GFP by Western blotting (supplemental Fig. 2C). Despite the large overexpression of ECE-1c-GFP, the effects on ERK2 activation were modest, possibly because HEK cells already express abundant ECE-1 (12, 13).
To confirm that ECE-1 regulates SP-induced activation of ERK1 in a similar manner to ERK2, we also examined the effects of ECE-1 inhibition and ECE-1 overexpression on SP-stimulated ERK1 activation. Whereas ECE-1 inhibition was markedly sustained in cells treated with SM-19712, ECE-1 overexpression attenuated SP-induced activation of ERK1 (supplemental Fig. 4, A and B). Thus, ECE-1 regulates the duration of ERK1 and ERK2 signals.
Bafilomycin A1, by inhibiting vacuolar H+-ATPase and preventing endosomal acidification, suppresses ECE-1-dependent SP degradation in endosomes and thereby causes endosomal retention of β-arrestin and NK1R (13). We hypothesized that bafilomycin A1 causes sustained SP-induced ERK activation. SP challenge (10 nm, 10 min) increased pERK2 to similar levels in vehicle- and bafilomycin A1-treated cells. However, after washing and incubation in SP-free medium, the levels of pERK2 in bafilomycin A1-treated cells were markedly higher than in control cells (-fold increase, 90 min, 3.0 ± 0.8; 120 min, 3.0 ± 0.8, Fig. 3C). Thus, endosomal acidification attenuates SP-induced ERK2 activation.
NK1R Affinity for β-Arrestins Determines Whether ECE-1 Regulates SP-induced ERK Activation
Full-length NK1R is a “class B” GPCR that interacts with β-arrestin1 and -2 for prolonged periods in endosomes (16). By degrading SP, ECE-1 promotes the dissociation of NK1R and β-arrestins in endosomes (13). In contrast, the bradykinin B2 receptor is a “class A” GPCR that interacts transiently with β-arrestin2 (31). Although ECE-1 can degrade bradykinin, ECE-1 does not regulate the brief interaction between the bradykinin B2 receptor and β-arrestins in endosomes (12). A truncated NK1R (NK1RΔ325–407) lacking the carboxyl-terminal tail containing multiple phosphorylation sites corresponds to a naturally occurring variant and resembles a class A GPCR that interacts with β-arrestins only weakly and transiently (6, 32). We hypothesized that ECE-1 would control neither NK1RΔ325–407 interaction with β-arrestins in endosomes nor NK1RΔ325–407-dependent ERK activation. To determine whether ECE-1 regulates trafficking of NK1RΔ325–407 and β-arrestins, we treated HEK cells expressing NK1RΔ325–407 and β-arrestin2-GFP with SM-19712 or vehicle, challenged with SP (10 nm, 0–10 min), washed, and incubated cells in SP-free medium (120 min). In unstimulated cells with active ECE-1, NK1RΔ325–407 was at the cell surface, and β-arrestin2-GFP was cytosolic (supplemental Fig. 5, A and B). SP (10 min) induced translocation of β-arrestin2-GFP to the cell surface and to endosomes containing NK1RΔ325–407. After incubation in SP-free medium (120 min), β-arrestin2-GFP returned to the cytosol. SM-19712 did not cause endosomal retention of NK1RΔ325–407 and β-arrestin2-GFP. In contrast, SM-19712 causes prolonged retention of full-length NK1R and β-arrestin2 in endosomes (13). Thus, ECE-1 does not regulate the interaction between NK1RΔ325–407 and β-arrestins. To determine whether ECE-1 controls NK1RΔ325–407-dependent ERK activation, we examined the effects of SM-19712 on SP-induced ERK2 activation in cells expressing NK1RΔ325–407. In HEK-NK1RΔ325–407 cells with active ECE-1, SP challenge increased pERK2, which returned to basal after 120 min (Fig. 3D). This stimulation was completely unaffected by SM-19712. In contrast, SM-19712 caused a 4–9-fold-increase in pERK2 in cells expressing full-length NK1R (Fig. 3A, Fig. 4, A and B). The results show that ECE-1 does not regulate the transient interaction of NK1RΔ325–407 with β-arrestins and does not control the coupling of this receptor to ERK.
Thus, ECE-1 controls the duration of SP-induced ERK activation by destabilizing the SP-NK1R-β-arrestin-Src complex in endosomes. ECE-1 does not control rapid SP-induced ERK activation, which depends on EGFR transactivation at the plasma membrane. SM-19712 did not affect immediate SP-induced ERK activation in HEK-NK1R cells (-fold increase, 2 min, SM-19712, 15 ± 0.9; vehicle, 12 ± 2.6, supplemental Fig. 1C).
The Stability of SP in Endosomes Determines the Duration of SP-induced β-Arrestin-dependent ERK Activation
At endosomal pH 5.5, ECE-1 cleaves SP (RPKPQQFFGLM-NH2) at Gln6–Phe7, Phe7–Phe8, and Gly9–Leu10 (13, 30). The SP analogue SMSP, a full and selective NK1R agonist (33), has a substitution (Gly9 → Sar9) at an ECE-1 cleavage site. We hypothesized that SMSP is resistant to ECE-1 degradation and that ECE-1 does not regulate SMSP-stimulated ERK activation. To compare degradation rates, we incubated SP and SMSP with ECE-1 at pH 5.5 and 7., and used mass spectrometry to determine the rate of peptide degradation, product formation, and metabolism. ECE-1 degraded SP at pH 5.5 but not at pH 7.4 (Fig. 5, A and B). The first identified product was 1RPKPQQFFG9, which did not accumulate and was rapidly metabolized to 1RPKPQQ6 and 7FFG9. The mechanism of the generation of another major product, 1RPKPQQF7, is unclear. Thus, ECE-1 first degrades SP at Gly9–Leu10, and 1RPKPQQFFG9 is also rapidly degraded at Gln6–Phe7. Although ECE-1 degraded SMSP at pH 5.5 but not pH 7.4, the Sar9Met(O2)11-SP modification dramatically decreased the rate at which ECE-1 degraded SMSP (Fig. 5B). A 3-fold higher ECE-1 concentration was required to give similar degradation rates for SMSP (195 nm) compared with SP (65 nm). 1RPKPQQFFSar9 was the first product, which was rapidly metabolized to 1RPKPQQ6. Surprisingly, 1RPKPQQF7 was not formed from SMSP. SM-19712 prevented degradation of both SP and SMSP (not shown). Consistent with the diminished susceptibility of SMSP to degradation by ECE-1, SM-19712 enhanced SMSP-induced ERK2 activation by only 1.9 ± 0.1-fold at 120 min (Fig. 5C), compared with a 4–9-fold increase in SP-induced ERK2 activation at the same time (Figs. 3A and 4, A and B). Thus, agonist susceptibility to degradation by ECE-1 in acidified endosomes determines the duration of ERK activation.
FIGURE 5.
ECE-1 degrades SMSP with reduced efficiency and only partially regulates SMSP-induced ERK2 activation. A, kinetics of ECE-1 degradation of SP and SMSP. Peptides were incubated with ECE-1 at pH 5.5 or pH 7.4. Abundance of substrate and products was measured as the relative contribution of their respective ions to the total ion current in the MALDI-TOF MS spectrum as a function of time. Tripling the ECE-1 concentration gave similar rates of degradation of SMSP compared with SP. B, kinetics of ECE-1 degradation of SP and generation and metabolism of products. Degradation at pH 5.5 was monitored as in B. Displayed are the mass-to-charge ratio (m/z) of the detected peptide ions and their ion intensity during the reaction. Sequences of substrates and products and their (m/z) values are indicated in red. Sequence assignments were confirmed by MS/MS. C, HEK-NK1R cells were incubated with AG1478 and vehicle or SM-19712, challenged with SMSP (10 nm, 0–10 min), washed, and incubated in SMSP-free medium (0–120 min). SMSP caused similar initial ERK2 activation in vehicle- and SM-19712-treated cells, and the magnitude of the sustained response to SMSP was only slightly increased by SM-19712 (n ≥ 3).
ECE-1 Regulates ERK Activation in Spinal Neurons
We sought to determine whether ECE-1 regulates SP-induced ERK activation in vivo. The NK1R is prominently expressed in nociceptive neurons in the spinal cord. SP, released from central projections of primary spinal afferent neurons in the dorsal horn of the spinal cord, causes NK1R endocytosis (34) and activates ERK in nociceptive neurons (35), which results in inflammatory hyperalgesia (36). We determined if ECE-1 is also expressed in mouse spinal cord using reverse transcription-PCR, Western blotting, and immunofluorescence. Reverse transcription-PCR amplified mRNA transcripts for ECE-1a-d, and ECE-1 expression was confirmed by Western blotting (∼120 kDa) (supplemental Fig. 6, A and B). ECE-1-like immunoreactivity (LI) was prominently colocalized with NK1R-LI in the soma and fibers of neurons of the dorsal horn of the spinal cord (Fig. 6A). Preabsorption of the ECE-1 antibody with membranes from HEK-ECE-1c-GFP cells inhibited staining compared with membranes from cells expressing empty vector (supplemental Fig. 6C), confirming specificity. Thus, ECE-1 is appropriately localized to regulate SP-induced ERK activation in spinal neurons.
FIGURE 6.
ECE-1 regulates the duration of SP-induced ERK2 activation in spinal cord. A, colocalization of ECE-1-LI and NK1R-LI in neurons of the dorsal horn of the mouse spinal cord (arrows). Scale bar, 50 μm. B and C, vehicle (Veh) or SM-19712 was injected intrathecally followed 30 min later by intraplantar injection of vehicle or capsaicin. Spinal cord (L4-L6) was removed 10 or 60 min later and analyzed for pERK2 and ERK2. Capsaicin induced similar ERK2 activation in vehicle- and SM-19712-treated animals at 10 min (B). However, after 60 min ERK2 activation was sustained in SM-19712-treated animals (C) (n ≥ 3).
Intraplantar injection of capsaicin stimulates SP release in the dorsal horn, which causes NK1R endocytosis (34) and ERK activation (35). To determine whether ECE-1 regulates SP-induced ERK activation in nociceptive neurons, vehicle or SM-19712 were administered intrathecally to mice followed by intraplantar injection of vehicle or capsaicin. We examined ERK2 activation in the spinal cord (L4-L6) by Western blotting. Capsaicin similarly stimulated ERK2 activation in vehicle- and SM-19712-treated mice (1.3-fold increase over basal at 10 min, Fig. 6B). In vehicle-treated mice, pERK2 returned to sub-basal levels after 60 min (0.7-fold of basal at 60 min, Fig. 6C). In contrast, pERK2 was sustained in SM-19712-treated mice (1.3-fold increase over basal at 60 min, Fig. 6C). Thus, ECE-1 attenuates the effects of endogenous SP on ERK2 activation in spinal nociceptive neurons.
ECE-1 Regulates SP-induced Activation of Transcription Factors and Nur77-mediated Cell Death
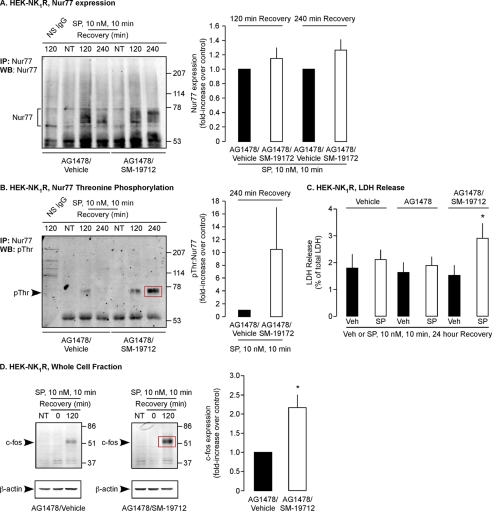
SP and the NK1R cause neurodegeneration in the central nervous system and induce non-apoptotic programmed cell death of HEK cells and striatal neurons (37–39). The transcription factor and nuclear receptor Nur77 mediates cell death, and ERK2 (not ERK1) induces threonine phosphorylation (Thr(P)) of Nur77 (40). SP up-regulates and phosphorylates Nur77, and SP-induced cell death requires β-arrestin-dependent activation of MEK2 and ERK2 and ERK2-mediated phosphorylation Nur77 (37). We hypothesized that ECE-1, by regulating the duration of β-arrestin-mediated ERK2 signaling, controls SP-induced Nur77 phosphorylation and cell death. To determine whether ECE-1 regulates expression and phosphorylation of Nur77, we treated HEK-NK1R cells with vehicle, AG1478, or AG1478 and SM-19712, challenged with SP (10 nm, 0–10 min), and washed and incubated cells in SP-free medium (2–4 h). Nur77 was immunoprecipitated, and Western blots were analyzed for Nur77 and Thr(P). Nur77 and Thr(P) were not detectable in unstimulated cells (Fig. 7, A and B). In vehicle-treated cells, SP stimulated expression and phosphorylation of Nur77 after 120 and 240 min. SM-19712 induced a minor up-regulation of Nur77 but markedly enhanced Nur77 phosphorylation (-fold increase, 240 min, 10.5 ± 6.6, Fig. 7, A and B).
FIGURE 7.
ECE-1 regulates phosphorylation of Nur77, prevents SP-induced cell death, and attenuates c-Fos expression. A and B, HEK-NK1R cells were incubated with AG1478 and vehicle or SM-19712, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (0–240 min). Nur77 was immunoprecipitated (IP), and levels of expression and threonine phosphorylation were analyzed by Western blotting (WB). SP caused similar Nur77 expression in vehicle- and SM-19712-treated cells, but threonine phosphorylation of Nur77 was greatly enhanced in SM-19712-treated cells. C, HEK-NK1R cells were incubated with vehicle, AG1478 and vehicle, or AG1478 and SM-19712, challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (24 h), and LDH was determined. Results are expressed LDH release as a percentage of the total cellular LDH measured at 24 h. SM-19712-treated cells released ∼2-fold more LDH compared with control. D, HEK-NK1R cells were treated as in A. In unstimulated cells and cells stimulated with SP (10 min), no c-Fos expression could be detected. In control cells expression of c-Fos was detected at 120 min after stimulation with SP, and SM-19712 enhanced c-Fos expression (n ≥ 3).
By terminating ERK2 activation, ECE-1 may prevent the cytotoxic actions of SP. To examine this possibility, HEK-NK1R cells were treated with vehicle, AG1478, or AG1478 and SM-19712, challenged with SP (10 nm, 0–10 min), and washed and incubated in SP-free medium (24 h). LDH release was measured to assess cell death. In cells not treated with SP, AG1478 or SM-19712 or both AG1478 and SM-19712 had no effect on LDH release measured at 24 h (Fig. 7C). Brief exposure to SP alone (with or without AG1478) also had no effect on LDH release measured 24 h later (Fig. 7C). However, in cells treated with SM-19712, SP caused a marked increase in LDH release (2 ± 0.2-fold). Thus, transient exposure to SP induces cell death only when ECE-1 is inhibited. ECE-1, by attenuating ERK2 activation, protects against SP-induced cell death.
SP stimulates NK1R-dependent expression of the transcription factor c-Fos in many cells, including nociceptive spinal neurons (41). To examine the contribution of ERK to SP-induced c-Fos expression, we treated HEK-NK1R cells with the MEK inhibitor UO126 or vehicle. Cells were challenged with SP (10 nm, 0–10 min), washed, and incubated in SP-free medium (120 min). c-Fos and β-actin were analyzed by Western blotting. c-Fos was not detected in unstimulated cells or after brief (10 min) stimulation with SP (Fig. 7D). c-Fos was readily detected in vehicle-treated cells stimulated with SP and then incubated in SP-free medium (120 min). UO126 strongly inhibited c-Fos (-fold decrease, 120 min, 5.6 ± 0.5, p < 0.05), which is, thus, dependent on the MEK pathway (not shown). To determine the role of ECE-1 in SP-induced c-Fos expression, we treated cells with SM-19712 or vehicle. SM-19712 markedly enhanced SP-induced expression of c-Fos (-fold increase, 120 min, 2.2 ± 0.3, Fig. 7D). Thus, ECE-1 regulates SP-induced c-Fos expression.
ECE-1 Regulates SP-induced Death of Myenteric Neurons
SP and the NK1R are expressed in myenteric neurons (17). SP cointernalizes with the NK1R and β-arrestins to endosomes of myenteric neurons (24), which may induce ERK activation. Transcripts corresponding to ECE-1a-d were amplified from rat myenteric plexus, and ECE-1 expression was confirmed by Western blotting (∼120 kDa) (supplemental Fig. 6, B and D). In whole mounts of myenteric plexus from rat ileum, ECE-1-LI was detected in endosomes, and NK1R-LI was at the plasma membrane of the same neurons, identified using the neuronal marker PGP9.5 (Fig. 8A). Preabsorption with membranes from HEK-ECE-1c-GFP cells inhibited ECE-1 staining (supplemental Fig. 6E), confirming specificity. Myenteric neurons in culture maintained coexpression of ECE-1-LI, NK1R-LI, and PGP9.5-LI (Fig. 8B). Alexa564-SP (100 nm) bound to the plasma membrane of these neurons after 120 min at 4 °C (not shown) and were prominently colocalized with ECE-1-LI in endosomes after 37 °C for 30 min (Fig. 8B). Thus, ECE-1 is appropriately localized to regulate β-arrestin-dependent NK1R signaling in endosomes of myenteric neurons.
FIGURE 8.
ECE-1 prevents SP-induced death of myenteric neurons. A, colocalization of ECE-1-LI, NK1R-LI and PGP9.5-LI in whole mounts (A) and cultures (B, upper and middle panels) of rat myenteric plexus. ECE-1-LI was in endosomes and NK1R-LI was at the plasma membrane. B, lower panel, cultured myenteric neurons were incubated with Alexa-SP (100 nm, 2 h, 4 °C), washed, and warmed to 37 °C for 30 min, and Alexa-SP and ECE-1-LI were localized. ECE-1-LI colocalized with Alexa-SP (arrows) in endosomes. C, cultured myenteric preparations were incubated with vehicle or SM-19712, challenged with SP (1 μm, 0–10 min), washed, and incubated in SP-free medium for 24 h, and LDH was determined. The left panel shows LDH release after 24 h expressed as a percentage of the total cellular LDH in neurons not treated with SP. Note that SM-19712 did not affect the basal release. The right panel shows the -fold increase of LDH release compared with controls in the left panel, normalized to 1. Note that SM-19712-treated neurons released ∼1.2-fold more LDH than control neurons (n ≥ 3).
SP can induce NK1R-mediated neurodegeneration (37–39), and myenteric neurons from the distal intestine are absent from mice lacking ECE-1 (42). To determine whether ECE-1 regulates death of myenteric neurons, cultured neurons were treated with vehicle or SM-19712, challenged with SP (1 μm, 0–10 min), and washed and incubated in SP-free medium (24 h). In neurons not treated with SP, there was considerable basal LDH release after 24 h, which is probably related to serum deprivation (Fig. 8C, left panel). This basal LDH release was unaffected by SM-19712. Brief challenge with SP alone did not further increase basal LDH release measured 24 h later (Fig. 8C, right panel). However, in neurons treated with SM-19712, SP caused a significant increase in LDH release (1.2 ± 0.04-fold). The small size of this effect may be related to the fact that only a small proportion of myenteric neurons expressed the NK1R. Moreover, the cultures contained glial cells, myocytes, and fibroblasts, which also do not express NK1R. However, transient exposure to SP induces neuronal death only when ECE-1 is inhibited. These results suggest ECE-1, by attenuating ERK2 activation, can protect against SP-induced neuronal death.
DISCUSSION
Although internalized GPCRs continue to signal in endosomes by β-arrestin-dependent ERK activation, it is not known whether agonist occupation of receptors or receptor interaction with β-arrestins is required for this sustained signaling and whether agonist degradation attenuates these signals. Our results show that ECE-1 degrades SP in acidified early endosomes to disrupt the endosomal SP-NK1R-β-arrestin-Src MAPK signalosome. SP degradation attenuates ERK2 activation in the cytosol and nucleus and terminates the nuclear actions of ERK2, which include activation of Nur77 death receptor (Fig. 9). Thus, SP-NK1R interaction is necessary for sustained β-arrestin-dependent ERK2 signaling from endosomes.
FIGURE 9.
Proposed mechanism by which endosomal ECE-1 regulates SP-stimulated ERK activation and cell death. 1, SP activation of the NK1R induces receptor phosphorylation by G protein receptor kinases (GRK) and membrane translocation of β-arrestins (β-ARR), which mediate NK1R desensitization and endocytosis. 2, β-arrestins recruit Src, MEK, and ERK1/2 to form a mitogen-activated protein kinase signaling module in endosomes. 3, ECE-1 degrades SP in acidified endosomes, causing disassembly of the SP-NK1R-β-arrestin-Src-MEK-ERK1/2 complex. 4, the NK1R, freed of SP and β-arrestins, recycles to mediate resensitization. 5, sustained ERK2 activation, which occurs in the absence of ECE-1, causes threonine phosphorylation and activation of Nur77, resulting in cell death.
Mechanisms of SP-induced ERK Activation
Because the consequences of ERK1/2 activation depend on subcellular location and duration of activation (10, 11), it is important to understand the mechanisms controlling the location and duration of the ERK1/2 signal. SP causes a rapid and sustained activation of ERK1/2 by temporally and spatially distinct mechanisms. Rapid activation depends on transactivation of EGFR, as the tyrosine kinase inhibitor AG1478 reduced ERK2 activation by 2-fold at early times. Transactivation probably occurs at or close to the plasma membrane, as it was observed within minutes of stimulation when NK1R is mostly at the cell surface (16, 24). Sustained activation depends on β-arrestins, as expression of dominant-negative β-arrestin1 V53D inhibited sustained ERK2 activation by 2-fold but did not affect rapid ERK2 activation. The sustained activation probably requires β-arrestin-dependent recruitment of NK1R and Src to endosomes, as it occurred at a time when the NK1R is associated with β-arrestins and Src in endosomes (6). We have previously shown that SP induces recruitment of β-arrestins, Src, MEK, and ERK1/2 to internalized NK1R in endosomes (6). The NK1R activates ERK1/2 by EGFR- and β-arrestin-dependent mechanisms in colonocytes (43), glioma cells (44), and epithelial cells (6, 37). Thus, NK1R antagonism at plasma and endosomal membranes is necessary to inhibit SP signaling.
ECE-1 Degrades SP in Endosomes and Terminates β-Arrestin-mediated ERK Activation
Metalloendopeptidases cleave SP at plasma and endosomal membranes to control NK1R activation. Neprilysin, which is confined to the plasma membrane, rapidly degrades SP in the extracellular fluid to prevent NK1R activation (1, 2). Thus, neprilysin can regulate NK1R coupling to heterotrimeric G proteins and transactivation of the EGFR. Although ECE-1 isoforms constitutively traffic between the plasma membrane and endosomes, the unusual pH sensitivity of ECE-1 determines whether ECE-1 can degrade neuropeptides at these locations (12, 13, 30). ECE-1 slowly degrades SP at the neutral pH of extracellular fluid but rapidly degrades SP at the acidic pH of early endosomes. Thus, ECE-1 does not control NK1R coupling to heterotrimeric G proteins, and ECE-1 inhibitors did not affect the rapid activation of ERK1/2, which depends on EGFR transactivation. However, several observations from the present study suggest that ECE-1 degrades SP in endosomes to control β-arrestin-dependent ERK1/2 activation. First, the ECE-1 inhibitor SM-19712 caused accumulation of NK1R, β-arrestins, and Src in purified early endosomes. This finding supports our report that ECE-1 inhibition/knockdown cause sustained colocalization of the NK1R and β-arrestins in early endosomes, whereas ECE-1 overexpression has the opposite effect, promoting NK1R recycling and resensitization and stimulating the return of β-arrestins to the cytosol (13). Second, SM-19712 caused markedly sustained ERK2 activation, whereas overexpression of ECE-1c attenuated ERK2 activation. A second ECE-1 inhibitor, PD-069185, also caused prolonged ERK2 activation, although the effect was less pronounced, probably because PD-069185 is not membrane permeant (19), whereas SM-19712 is a membrane permeable inhibitor (29). Third, the H+-ATPase inhibitor bafilomycin A1, which prevents endosomal acidification and inhibits ECE-1-dependent SP degradation in endosomes (13), also caused sustained ERK activation. Together, these results support the hypothesis that ECE-1 degrades SP in acidified endosomes to disrupt the SP-NK1R-β-arrestin-Src complex and terminate ERK1/2 activation. The report that an NK1R-β-arrestin fusion protein constitutively activates ERK when expressed in HEK cells (45) supports our suggestion that interaction of NK1R and β-arrestins, regulated by ECE-1, controls the duration of SP-induced ERK activation.
The ability of ECE-1 to regulate NK1R-dependent ERK2 activation requires that the NK1R agonist is an ECE-1 substrate and that the NK1R shows sustained interactions with β-arrestins. Although SMSP is a full and selective agonist of the NK1R (33), ECE-1 degraded SMSP more slowly than SP, and SM-19712 had only a small effect on SMSP-stimulated ERK2 activation. Supporting the importance of substrate susceptibility is our observation that ECE-1 does not regulate signaling of angiotensin II, which is not an ECE-1 substrate at acidic pH (12, 13). Other mechanisms can regulate the duration of MAPK signals transmitted by the angiotensin AT1A receptor, including the dual specificity phosphatase MKP7, which interacts with β-arrestins and dephosphorylates JNK3 bound to β-arrestin2 (46). NK1RΔ325–407 interacts with β-arrestins only transiently (6, 32). In contrast to the full-length NK1R, ECE-1 inhibition did not cause retention of NK1RΔ325–407 with β-arrestins in endosomes and failed to cause sustained NK1RΔ325–407-mediated ERK2 activation. Supporting the importance of sustained interactions with β-arrestins, ECE-1 does not regulate signaling of the bradykinin B2 receptor (12), which also transiently interacts with β-arrestins (31).
ECE-1 Prevents the Cytotoxic Consequences of SP-induced ERK2 Activation
ECE-1 inhibition caused sustained ERK2 activation in the cytosol and nucleus, suggesting that the β-arrestin MAPK signalosome is a hub for ERK2 activation throughout the cell. Although cytosolic ERK2 has many targets (11), the functional relevance of SP-induced activation of cytosolic ERK2 is unclear. Nuclear ERK2 has multiple effects, including phosphorylation of transcription factors and regulation of differentiation, proliferation, and survival (10). SP also controls transcription, proliferation, and survival by ERK-dependent mechanisms, suggesting that ECE-1 may also regulate these components of SP signaling (6, 43). Although ERK activation usually promotes differentiation and proliferation, ERK can also mediate cell death in the nervous system (10). We evaluated the role of ECE-1 in controlling SP-induced phosphorylation of the death receptor Nur77, as SP phosphorylates Nur77 and causes cell death by β-arrestin-mediated activation of ERK1/2 (37). Nur77 (TR3 or nerve growth factor I-B) is an immediate early gene and an orphan member of the steroid-thyroid-retinoid family of nuclear receptors, and Nur77 has been implicated in regulating growth and cell death (47). We observed that SP stimulated threonine phosphorylation of Nur77 and that SM-19712 increased this response by >10-fold. Moreover, whereas brief (10 min) exposure of HEK-NK1R cells to SP had no cytotoxic effects in cells with active ECE-1, ECE-1 inhibition revealed marked cytotoxic effects of SP. Thus, ECE-1 is critically important in regulating the actions of SP-NK1R signaling in the nucleus and probably also the cytosol. Further studies are required to determine how Nur77 mediates SP-induced cell death, although Nur77 can translocate from the nucleus to mitochondria, where Nur77 interacts with Bcl-2 to activate apoptosis (48).
Roles of Neuronal ECE-1
Proinflammatory and noxious stimuli that excite primary spinal afferent neurons in peripheral tissues stimulate SP release from central projections of these neurons in the dorsal horn of the spinal cord. SP activates the NK1R on nociceptive spinal neurons to cause receptor endocytosis (34), ERK1/2 activation (35), and c-Fos expression (41). ERK mediates up-regulation of the NK1R and prodynorphin and is responsible for persistent thermal and mechanical hyperalgesia (36). We observed that ECE-1 is coexpressed with the NK1R in spinal neurons and is, thus, suitably localized to regulate these effects of SP. Intraplantar injection of capsaicin, which excites primary spinal afferent neurons by activating transient receptor potential vanilloid 1, induced transient ERK activation in the dorsal horn, and intrathecal administration of SM-19712 prolonged this activation. These results suggest that ECE-1 attenuates SP-induced ERK activation in spinal nociceptive neurons and thereby suppresses NK1R-mediated inflammatory pain. Additional experiments are required to examine this possibility. It is also possible that ECE-1 regulates c-Fos expression and gene transcription in these neurons, as we observed in HEK cells. Neprilysin also regulates SP signaling in the dorsal horn by degrading SP in the extracellular fluid (49).
The NK1R is expressed in myenteric neurons where SP regulates peristalsis (17). We observed that Alexa-SP internalizes in myenteric neurons to colocalize with ECE-1 in endosomes, which are known to contain NK1R and β-arrestins (24). Thus, ECE-1 is appropriately localized to degrade endocytosed SP in neurons and regulate the stability of SP-NK1R-β-arrestin MAPK signalosome of neurons. Although we were unable to study SP-induced ERK activation in cultured neurons, which did not tolerate serum starvation that is necessary to obtain low basal activity, we did observe that brief exposure to SP resulted in neuronal death in neurons treated with an ECE-1 inhibitor, which supports our findings with HEK-NK1R cells. It is not known whether ECE-1 also regulates the neurotoxic actions of SP in the central nervous system (37–39), and further experimentation is required to define the mechanism of SP toxicity in myenteric neurons. However, SP causes non-apoptotic death of striatal neurons by a mechanism that depends on β-arrestin, ERK2, and Nur77 (37), which would be amenable to regulation by ECE-1. ECE-1 knock-out mice lack myenteric neurons in the distal intestine (42). This phenotype mimics that observed in mice lacking the endothelin B receptor and is attributed to defective colonization of the developing intestine by cells of the neural crest. Whether ECE-1 deficiency by promoting sustained ERK2 activation also predisposes neurotoxicity is an intriguing possibility that requires further investigation. However, given that ECE-1 can degrade multiple neuropeptides in acidified endosomes, the mechanism that we describe may be a general one for controlling ERK activation (12–14, 30).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants DK43207 and DK39957 (to N. W. B. and B. E. P.). This work was also supported by British Heart Foundation Grant FS/08/017/25027 (to G. S. C.) and CJ Martin Fellowship 454858 (to D. P. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
- GPCR
- G protein-coupled receptor
- ECE-1
- endothelin-converting enzyme-1
- ERK
- extracellular signal regulated kinase
- pERK
- phosphorylated ERK
- LDH
- lactate dehydrogenase
- MAPK
- mitogen-activated protein kinase
- NK1R
- neurokinin 1 receptor
- SP
- substance P
- MALDI-TOF
- matrix-assisted laser desorption ionization time-of-flight
- LI
- -like immunoreactivity
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- EEA1
- early endosomal antigen-1
- LAMP1
- lysosomal-associated glycomembrane protein 1
- SMSP
- Sar9,Met(O2)11-SP
- HEK
- human embryonic kidney
- GFP
- green fluorescent protein
- PBS
- phosphate-buffered saline
- MES
- 4-morpholineethanesulfonic acid
- EGFR
- epidermal growth factor receptor
- Sar
- N-methylglycine or sarcosine
- NT
- not treated.
REFERENCES
- 1.Lu B., Figini M., Emanueli C., Geppetti P., Grady E. F., Gerard N. P., Ansell J., Payan D. G., Gerard C., Bunnett N. (1997) Nat. Med. 3, 904–907 [DOI] [PubMed] [Google Scholar]
- 2.Okamoto A., Lovett M., Payan D. G., Bunnett N. W. (1994) Biochem. J. 299, 683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. (1990) Science 248, 1547–1550 [DOI] [PubMed] [Google Scholar]
- 4.Ferguson S. S., Downey W. E., 3rd, Colapietro A. M., Barak L. S., Ménard L., Caron M. G. (1996) Science 271, 363–366 [DOI] [PubMed] [Google Scholar]
- 5.Goodman O. B., Jr., Krupnick J. G., Santini F., Gurevich V. V., Penn R. B., Gagnon A. W., Keen J. H., Benovic J. L. (1996) Nature 383, 447–450 [DOI] [PubMed] [Google Scholar]
- 6.DeFea K. A., Vaughn Z. D., O'Bryan E. M., Nishijima D., Déry O., Bunnett N. W. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFea K. A., Zalevsky J., Thoma M. S., Déry O., Mullins R. D., Bunnett N. W. (2000) J. Cell Biol. 148, 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luttrell L. M., Ferguson S. S., Daaka Y., Miller W. E., Maudsley S., Della Rocca G. J., Lin F., Kawakatsu H., Owada K., Luttrell D. K., Caron M. G., Lefkowitz R. J. (1999) Science 283, 655–661 [DOI] [PubMed] [Google Scholar]
- 9.Tohgo A., Pierce K. L., Choy E. W., Lefkowitz R. J., Luttrell L. M. (2002) J. Biol. Chem. 277, 9429–9436 [DOI] [PubMed] [Google Scholar]
- 10.Lu Z., Xu S. (2006) IUBMB Life 58, 621–631 [DOI] [PubMed] [Google Scholar]
- 11.Luttrell L. M. (2003) J. Mol. Endocrinol. 30, 117–126 [DOI] [PubMed] [Google Scholar]
- 12.Padilla B. E., Cottrell G. S., Roosterman D., Pikios S., Muller L., Steinhoff M., Bunnett N. W. (2007) J. Cell Biol. 179, 981–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roosterman D., Cottrell G. S., Padilla B. E., Muller L., Eckman C. B., Bunnett N. W., Steinhoff M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11838–11843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roosterman D., Kempkes C., Cottrell G. S., Padilla B. E., Bunnett N. W., Turck C. W., Steinhoff M. (2008) Endocrinology 149, 2200–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennefather J. N., Lecci A., Candenas M. L., Patak E., Pinto F. M., Maggi C. A. (2004) Life Sci. 74, 1445–1463 [DOI] [PubMed] [Google Scholar]
- 16.McConalogue K., Déry O., Lovett M., Wong H., Walsh J. H., Grady E. F., Bunnett N. W. (1999) J. Biol. Chem. 274, 16257–16268 [DOI] [PubMed] [Google Scholar]
- 17.Grady E. F., Baluk P., Böhm S., Gamp P. D., Wong H., Payan D. G., Ansel J., Portbury A. L., Furness J. B., McDonald D. M., Bunnett N. W. (1996) J. Neurosci. 16, 6975–6986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attramadal H., Arriza J. L., Aoki C., Dawson T. M., Codina J., Kwatra M. M., Snyder S. H., Caron M. G., Lefkowitz R. J. (1992) J. Biol. Chem. 267, 17882–17890 [PubMed] [Google Scholar]
- 19.Ahn K., Sisneros A. M., Herman S. B., Pan S. M., Hupe D., Lee C., Nikam S., Cheng X. M., Doherty A. M., Schroeder R. L., Haleen S. J., Kaw S., Emoto N., Yanagisawa M. (1998) Biochem. Biophys. Res. Commun. 243, 184–190 [DOI] [PubMed] [Google Scholar]
- 20.Cottrell G. S., Padilla B., Pikios S., Roosterman D., Steinhoff M., Grady E. F., Bunnett N. W. (2007) J. Biol. Chem. 282, 12260–12271 [DOI] [PubMed] [Google Scholar]
- 21.Andrews N. C., Faller D. V. (1991) Nucleic Acids Res. 19, 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H. S., Stolz D. B., Romero G. (2005) Traffic 6, 324–334 [DOI] [PubMed] [Google Scholar]
- 23.Robinson S., Niles R. K., Witkowska H. E., Rittenbach K. J., Nichols R. J., Sargent J. A., Dixon S. E., Prakobphol A., Hall S. C., Fisher S. J., Hardt M. (2008) Proteomics 8, 435–445 [DOI] [PubMed] [Google Scholar]
- 24.McConalogue K., Corvera C. U., Gamp P. D., Grady E. F., Bunnett N. W. (1998) Mol. Biol. Cell 9, 2305–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole D. P., Castelucci P., Robbins H. L., Chiocchetti R., Furness J. B. (2002) Auton. Neurosci. 101, 39–47 [DOI] [PubMed] [Google Scholar]
- 26.Anselmi L., Stella S. L., Jr., Lakhter A., Hirano A., Tonini M., Sternini C. (2005) Neuropeptides 39, 349–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daub H., Weiss F. U., Wallasch C., Ullrich A. (1996) Nature 379, 557–560 [DOI] [PubMed] [Google Scholar]
- 28.Krupnick J. G., Santini F., Gagnon A. W., Keen J. H., Benovic J. L. (1997) J. Biol. Chem. 272, 32507–32512 [DOI] [PubMed] [Google Scholar]
- 29.Umekawa K., Hasegawa H., Tsutsumi Y., Sato K., Matsumura Y., Ohashi N. (2000) Jpn. J. Pharmacol. 84, 7–15 [DOI] [PubMed] [Google Scholar]
- 30.Johnson G. D., Stevenson T., Ahn K. (1999) J. Biol. Chem. 274, 4053–4058 [DOI] [PubMed] [Google Scholar]
- 31.Simaan M., Bédard-Goulet S., Fessart D., Gratton J. P., Laporte S. A. (2005) Cell. Signal. 17, 1074–1083 [DOI] [PubMed] [Google Scholar]
- 32.Lai J. P., Lai S., Tuluc F., Tansky M. F., Kilpatrick L. E., Leeman S. E., Douglas S. D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12605–12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drapeau G., D'Orléans-Juste P., Dion S., Rhaleb N. E., Rouissi N. E., Regoli D. (1987) Neuropeptides 10, 43–54 [DOI] [PubMed] [Google Scholar]
- 34.Mantyh P. W., DeMaster E., Malhotra A., Ghilardi J. R., Rogers S. D., Mantyh C. R., Liu H., Basbaum A. I., Vigna S. R., Maggio J. E. (1995) Science 268, 1629–1632 [DOI] [PubMed] [Google Scholar]
- 35.Kawasaki Y., Kohno T., Zhuang Z. Y., Brenner G. J., Wang H., Van Der Meer C., Befort K., Woolf C. J., Ji R. R. (2004) J. Neurosci. 24, 8310–8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji R. R., Befort K., Brenner G. J., Woolf C. J. (2002) J. Neurosci. 22, 478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro-Obregón S., Rao R. V., del Rio G., Chen S. F., Poksay K. S., Rabizadeh S., Vesce S., Zhang X. K., Swanson R. A., Bredesen D. E. (2004) J. Biol. Chem. 279, 17543–17553 [DOI] [PubMed] [Google Scholar]
- 38.Liu H., Cao Y., Basbaum A. I., Mazarati A. M., Sankar R., Wasterlain C. G. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 12096–12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zachrisson O., Lindefors N., Brené S. (1998) Brain Res. Mol. Brain Res. 60, 291–295 [DOI] [PubMed] [Google Scholar]
- 40.Slagsvold H. H., Østvold A. C., Fallgren A. B., Paulsen R. E. (2002) Biochem. Biophys. Res. Commun. 291, 1146–1150 [DOI] [PubMed] [Google Scholar]
- 41.Doyle C. A., Hunt S. P. (1999) Neuroscience 89, 17–28 [DOI] [PubMed] [Google Scholar]
- 42.Yanagisawa H., Yanagisawa M., Kapur R. P., Richardson J. A., Williams S. C., Clouthier D. E., de Wit D., Emoto N., Hammer R. E. (1998) Development 125, 825–836 [DOI] [PubMed] [Google Scholar]
- 43.Koon H. W., Zhao D., Na X., Moyer M. P., Pothoulakis C. (2004) J. Biol. Chem. 279, 45519–45527 [DOI] [PubMed] [Google Scholar]
- 44.Al-Sarraj A., Thiel G. (2002) Neurosci. Lett. 332, 111–114 [DOI] [PubMed] [Google Scholar]
- 45.Jafri F., El-Shewy H. M., Lee M. H., Kelly M., Luttrell D. K., Luttrell L. M. (2006) J. Biol. Chem. 281, 19346–19357 [DOI] [PubMed] [Google Scholar]
- 46.Willoughby E. A., Collins M. K. (2005) J. Biol. Chem. 280, 25651–25658 [DOI] [PubMed] [Google Scholar]
- 47.Woronicz J. D., Calnan B., Ngo V., Winoto A. (1994) Nature 367, 277–281 [DOI] [PubMed] [Google Scholar]
- 48.Lin B., Kolluri S. K., Lin F., Liu W., Han Y. H., Cao X., Dawson M. I., Reed J. C., Zhang X. K. (2004) Cell 116, 527–540 [DOI] [PubMed] [Google Scholar]
- 49.Duggan A. W., Schaible H. G., Hope P. J., Lang C. W. (1992) Brain Res. 579, 261–269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.