Abstract
In mammals, the two enzymes in the trans-sulfuration pathway, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), are believed to be chiefly responsible for hydrogen sulfide (H2S) biogenesis. In this study, we report a detailed kinetic analysis of the human and yeast CBS-catalyzed reactions that result in H2S generation. CBS from both organisms shows a marked preference for H2S generation by β-replacement of cysteine by homocysteine. The alternative H2S-generating reactions, i.e. β-elimination of cysteine to generate serine or condensation of 2 mol of cysteine to generate lanthionine, are quantitatively less significant. The kinetic data were employed to simulate the turnover numbers of the various CBS-catalyzed reactions at physiologically relevant substrate concentrations. At equimolar concentrations of CBS and CSE, the simulations predict that H2S production by CBS would account for ∼25–70% of the total H2S generated via the trans-sulfuration pathway depending on the extent of allosteric activation of CBS by S-adenosylmethionine. The relative contribution of CBS to H2S genesis is expected to decrease under hyperhomocysteinemic conditions. CBS is predicted to be virtually the sole source of lanthionine, and CSE, but not CBS, efficiently cleaves lanthionine. The insensitivity of the CBS-catalyzed H2S-generating reactions to the grade of hyperhomocysteinemia is in stark contrast to the responsiveness of CSE and suggests a previously unrecognized role for CSE in intracellular homocysteine management. Finally, our studies reveal that the profligacy of the trans-sulfuration pathway results not only in a multiplicity of H2S-yielding reactions but also yields novel thioether metabolites, thus increasing the complexity of the sulfur metabolome.
Hydrogen sulfide (H2S)2 elicits an array of physiological effects, including modulation of blood pressure and reduction of ischemia reperfusion injury (1, 2). Other novel effects of H2S include induction of a state of suspended animation in mouse by decreasing oxygen consumption and drastically reducing the metabolic rate (3) and synchronizing ultradian metabolic oscillation in yeast (4). Under conditions of metabolic cycling in yeast, H2S production is catalyzed by sulfite reductase in the sulfur assimilation pathway (4). Inhibition of sulfite reductase reduces H2S production and in turn perturbs metabolic oscillations. H2S is a specific and potent inhibitor of cytochrome c oxidase in the electron transport chain (3).
Although concentrations of H2S have been reported to range from 50 to 160 μm in brain (5–7) and 30–50 μm in the peripheral system (8), these appear to be grossly overestimated (9). Significantly lower H2S concentrations of 17 and 14 nm in liver and brain, respectively, have been reported recently (9). The very significant discrepancy between these and the previous estimates of H2S levels presumably derives from the earlier use of acidic conditions that led to the release of acid-labile sulfur from iron-sulfur centers.
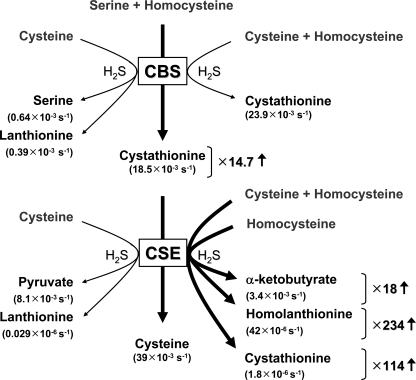
In mammals, the primary catalysts for H2S generation are reported to be the two pyridoxal phosphate (PLP)-dependent enzymes involved in the trans-sulfuration pathway, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) (10, 11). The trans-sulfuration pathway operates in the reverse direction in mammals serving to convert homocysteine to cysteine (Fig. 1), although in yeast and bacteria the pathway is involved in sulfur assimilation from sulfate to cysteine. CBS is widely assumed to be the major contributor to H2S production in the brain because of its relatively high expression in this organ (10). However, a recent study reported that 3-mercaptopyruvate sulfurtransferase together with cysteine aminotransferase might also generate H2S in brain (12). The relative contributions of these enzymes and of CSE, which is also present in brain (13, 14), to H2S production remain to be assessed. Genetic disruption of CSE in mouse leads to cardiac deficits, including pronounced hypertension and reduced endothelium-dependent vasorelaxation, consistent with a major role for CSE in the peripheral system (1). However, brain H2S levels are reportedly unchanged in CSE−/− mice.
FIGURE 1.
Diversity of reactions catalyzed by the trans-sulfuration pathway. The turnover numbers (v/[E]) estimated at physiological substrate concentrations, i.e. 10 μm homocysteine, 100 μm cysteine, 560 μm serine, and 5 μm cystathionine, are shown in parentheses for each reaction. The thick arrows highlight reactions that are sensitive to elevated levels of homocysteine. The fold change represents the fold increase in the turnover number of a given reaction under conditions of severe hyperhomocysteinemia (200 μm homocysteine).
Despite the growing recognition of the varied physiological effects of H2S, our understanding of its regulation and mechanism of its biosynthesis is poor. We have recently reported on the complex kinetics of H2S generation by human CSE (15). The profligacy of the human enzyme affords H2S generation by a multiplicity of routes involving cysteine and/or homocysteine as substrates. Kinetic simulations predict an increasingly important contribution of homocysteine to H2S generation with increasing grade of hyperhomocysteinemia, a risk factor for cardiovascular and neurodegenerative diseases (16–18). In addition to H2S, a variety of products is generated in these reactions, including two novel sulfur metabolites, lanthionine and homolanthionine, which represent the condensation products between 2 mol of cysteine and homocysteine, respectively. Although the steady-state kinetic parameters for H2S generation from cysteine and homocysteine have been reported for human CBS (hCBS) (19), a comparable detailed kinetic analysis of H2S generation by CBS by multiple pathways and their sensitivity to the grade of hyperhomocysteinemia is not known. Furthermore, the relative contributions of CBS and CSE to H2S and lanthionine generation at physiologically relevant concentrations of substrate are not known.
Human CBS is a unique heme containing PLP-dependent enzyme (20) that catalyzes the β-replacement of serine by homocysteine to produce cystathionine. The latter is further metabolized by CSE in an α,γ-elimination reaction to produce cysteine. Although yeast and human CBS are highly homologous and catalyze the same chemical reaction with similar kinetic parameters, the yeast enzyme lacks heme and is not allosterically regulated by S-adenosylmethionine (AdoMet) (21).
In this study, we have elucidated the kinetics of H2S biogenesis by yeast and human CBS and used simulations to estimate the relative contributions of CBS and CSE to H2S production at physiologically relevant concentrations of substrate. We find that CBS and CSE share a common feature, i.e. catalytic promiscuity. However, in contrast to CSE, which is proficient at catalyzing reactions at the β- and γ-carbons of substrates (15), CBS activity is confined to chemical transformations at the β-position. Our studies provide new insights into the existence of alternative trans-sulfuration reactions that can be a source of diverse sulfur metabolites, viz. H2S, lanthionine, and homolanthionine increasing the diversity of the sulfur metabolome.
EXPERIMENTAL PROCEDURES
Chemicals
l-Cysteine, dl-homocysteine, dl-lanthionine, l-serine, kanamycin, and ampicillin were purchased from Sigma.
Expression and Purification of hCBS, yCBS, and hCSE
Recombinant full-length hCBS and yeast CBS (yCBS) were purified as described previously (22, 23). The expression plasmids encoding hCBS and yCBS were provided by Drs. Warren Kruger (Fox Chase Cancer Center) and Edith Miles (National Institutes of Health, Bethesda) respectively. The expression plasmid for human CSE was provided by Dr. Markus Wahl (Max Planck Institute, Martinsried, Germany). Recombinant human CSE was purified as described previously (15).
Enzyme Activity Assays
The following assays were used to determine CBS activity. In all assays, the concentration of the variable substrate ranged from 0.2 × Km1 to 5–20 × Km1. One unit of activity is defined as 1 μmol of product formed min−1 mg−1 of protein. For assays with hCBS, the allosteric activator, AdoMet (360 μm), was added to the reaction mixture.
Radiolabeled Assay
The activities of hCBS and yCBS were measured in the radioactive assay using [14C]serine as described previously (24).
Detection of H2S
H2S generation from cysteine or from cysteine plus homocysteine was measured in a spectrophotometric assay as described previously (15). The reaction mixture containing 100 mm Hepes buffer, pH 7.4, 0.4 mm lead acetate, and varying concentrations of substrate (cysteine ± homocysteine) was preincubated at 37 °C for 2 min, and the reaction was initiated by addition of yCBS. For hCBS, the enzyme was preincubated with 360 μm AdoMet (final concentration) at 37 °C for 2 min, and the reaction was started by addition of substrate. Lead acetate (0.4 mm) did not inhibit CBS activity as determined in the radioactive assay (data not shown). A molar extinction coefficient of 5,500 m−1cm−1 was used for lead sulfide as described previously (15).
HPLC Analysis of Serine and Lanthionine
HPLC was used to estimate the rate of serine and lanthionine formation at varying concentrations of cysteine. The concentration of serine or lanthionine was determined following o-phthaldialdehyde derivatization as described previously (15). Under these conditions, lanthionine and serine eluted with retention times of 3.2 and 4.2 min, respectively. This HPLC method was also used to determine whether homoserine (5.26 min retention time) was produced by the CBS-catalyzed γ-elimination of homocysteine.
Detection of α-Ketoacid Products
CBS-catalyzed generation of H2S from cysteine or homocysteine could potentially generate the α-ketoacids, pyruvate and α-ketobutyrate. The colorimetric assay for detection of α-ketoacids was employed as described previously (15).
Detection of Cysteine
The 5,5′-dithiobis(nitrobenzoic acid) assay was used to measure the CBS- and CSE-catalyzed production of cysteine from lanthionine as described previously (15).
Estimation of Dissociation Constant for Cysteine and Serine
Fluorescence titrations were performed at 25 °C on a Shimadzu RF-5301PC spectrofluorimeter. Human CBS (0.2 mg/ml) in 100 mm Hepes buffer, pH 7.4, containing 360 μm AdoMet was incubated with varying concentrations of l-cysteine or l-serine. After 3 min of incubation, spectra were acquired with an excitation wavelength of 400 nm. Data were analyzed by plotting ΔF495 nm as a function of cysteine or serine concentration, and the Kd value was determined by fitting the data with the Michaelis-Menten equation.
Mass Spectrometric Analysis of Reaction Products
Mass spectrometric analysis of lanthionine was performed as described previously (15).
Kinetic Analysis
H2S-generating reactions catalyzed by CBS (reactions 2–4) are either unimolecular (reaction 2) or bimolecular (reactions 3 and 4). Thus Km1 and Vmax1 refer to the kinetic parameters associated with the unimolecular reaction, whereas Km2 and Vmax2 refer to substrate binding at the second site and the reaction velocity of the bimolecular reaction, respectively.
Serine and Lanthionine Generation
The Km and Vmax values for CBS-catalyzed serine (reaction 2) or lanthionine (reaction 3) production from cysteine were determined using the HPLC assay as described above, and data were fitted using Equations 1 and 2–4, respectively.
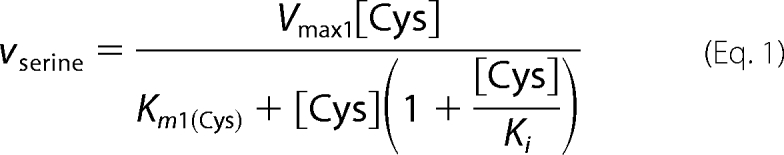 |
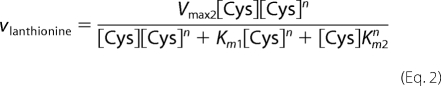 |
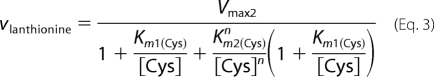 |
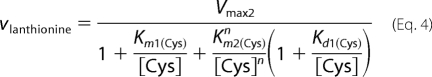 |
As binding of cysteine to the second binding site leads to depletion of substrate for the unimolecular reaction, a substrate inhibition term [Cys] × (1 + [Cys]/Ki) was included in Equation 1. In principle, the bimolecular reaction catalyzed by CBS can follow a binary (ping-pong) or sequential/ternary (random or ordered) mechanism. Hence, the experimental data for lanthionine production from cysteine (reaction 3) were fitted using Equations 2–4 that describe a ping-pong, random sequential, and ordered sequential mechanism, respectively. As cooperativity was clearly observed with yCBS for binding of substrate to site 2, a Hill coefficient (n) was included in these equations.
H2S Production from Cysteine
H2S production from cysteine by CBS is the sum of a unimolecular (reaction 2) and a bimolecular (reaction 3) reaction. The latter can proceed via a ping-pong or sequential (ordered or random) mechanism as discussed above. Hence, the experimental data for H2S production from cysteine (i.e. reactions 2 and 3) monitored by the continuous lead acetate assay described above were fitted using Equation 5 with vserine described by Equation 6 and vlanthionine described in Equations 2–4 to account for the alternative mechanisms.
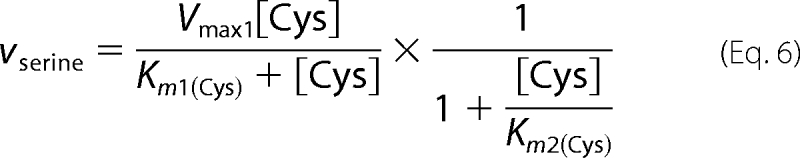 |
Binding of cysteine to the second binding site (reaction 3) will affect the Vmax1 values for H2S formation in the unimolecular reaction 2. Hence, an inhibition term, where Ki is assumed to be equal to Km2, was introduced in Equation 6. This assumption is consistent with the high Ki(Cys) (11 mm) reported for cysteine with respect to homocysteine for the CBS-catalyzed reaction by rat CBS (25).
H2S Production from Cysteine and Homocysteine
CBS does not catalyze detectable H2S formation from homocysteine alone as monitored in the lead acetate assay. However, in the presence of cysteine, CBS catalyzes a condensation reaction between these two amino acids (reaction 4). Hence, the kinetic parameters for reaction 4 were determined by fitting the experimental data for H2S formation obtained in the presence of 20 mm cysteine for hCBS or 15 mm cysteine for yCBS and varying the concentration of homocysteine. In this set of experiments, the observed rate of H2S production represents the sum of reactions 2–4, as described by Equation 7.
The values for v2–v4 corresponding to the reaction velocities for reactions 2–4, respectively, were obtained using Equations 8–10 for the ping-pong mechanism.
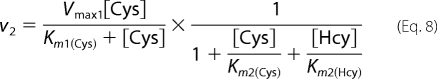 |
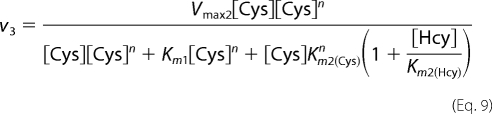 |
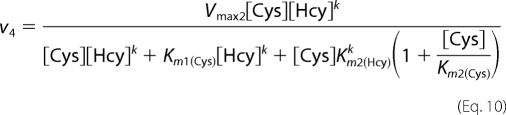 |
In these equations, n and k represent the Hill coefficient for the binding of the second substrate in reactions 3 and 4, respectively. The kinetic parameters obtained for reactions 2 and 3 as described above were constrained to obtain the kinetic parameters for reaction 4. A term for noncompetitive inhibition was introduced in Equation 8, assuming that Ki = Km2, because the simultaneous presence of both substrates will lead to inhibition of the unimolecular reaction. Also, a competitive inhibition term for the binding of the second substrate was included because cysteine and homocysteine will compete for binding at the second binding site to catalyze different bimolecular reactions. We assumed that Ki = Km2.
RESULTS
Initial Analysis of H2S Production by hCBS and yCBS
The specific activity of recombinant hCBS used in these studies was 5.1 ± 0.3 μmol of cystathionine formed min−1 mg−1 of protein at 37 °C and pH 7.4 when homocysteine and serine were employed as substrates and is comparable with the value published previously (22). The specific activity of yCBS was 24 ± 2 μmol of cystathionine formed min−1 mg−1 and is similar to the value reported previously (23).
The kinetics of H2S production by yeast or human CBS were characterized using the continuous spectrophotometric assay. Under Vmax conditions, the condensation of cysteine and homocysteine represents the preferred pathway for H2S production, as reported previously for hCBS (19), and specific activities of 60 ± 2.4 units/mg for yCBS and 18.7 ± 2.6 units/mg for hCBS were obtained (Table 1). These specific activities are ∼7- and 23-fold higher than those observed for the bimolecular reaction with cysteine as substrate for yeast (9.0 ± 1.3 units/mg) and human CBS (0.82 ± 0.08), respectively.
TABLE 1.
Kinetic parameters determined for reactions catalyzed by yeast or human CBS
All values reported in the table were obtained from the fit of a plot containing data from at least three independent experiments and are represented ±S.E.
| Reaction | No. | Vmax | Km | Ki | n | kcat |
|---|---|---|---|---|---|---|
| unitsa/mg | mm | mm | s−1 | |||
| Yeast CBS | ||||||
| Cystathionine generation from Ser + Hcysb | 1 | 24.3 ± 2.4 | 3.4 ± 0.2c | 25.5 ± 2.5 | ||
| H2S generation from Cys | 2 | 3.7 ± 1.1 | 3.6 ± 1.7 | 33 ± 3.7 | 2.4 ± 0.5 | 3.4 ± 1.0 |
| 3 | 9.0 ± 1.3 | 33 ± 3.7c | 8.2 ± 1.2 | |||
| H2S generation from Cys + Hcys | 4 | 60 ± 2.4 | 3.6 ± 1.7d | 0.9 ± 0.1 | 55 ± 2.2 | |
| 0.13 ± 0.02c | ||||||
| Human CBS | ||||||
| Cystathionine generation from Ser + Hcysb | 1 | 5.05 ± 0.3 | 2.76 ± 0.5c | 5.3 ± 0.3 | ||
| Serine generation from Cys | 2 | 0.34 ± 0.04 | 6.8 ± 1.7 | 42 ± 10.5 | 0.36 ± 0.04 | |
| Lanthionine generation from Cys | 3 | 0.77 ± 0.07 | 6.8 ± 1.7d | 2.1 ± 0.4 | 0.81 ± 0.07 | |
| 45.6 ± 5.6c | ||||||
| H2S generation from Cys | 2 | 0.46 ± 0.08 | 6.8 ± 1.7d | 27 ± 3.7 | 1.3 ± 0.3 | 0.48 ± 0.08 |
| 3 | 0.82 ± 0.08 | 27.3 ± 3.7c | 0.86 ± 0.08 | |||
| H2S generation from Cys + Hcys | 4 | 18.7 ± 2.6 | 6.8 ± 1.7d | 0.74 ± 0.1 | 19.6 ± 2.7 | |
| 3.2 ± 1.3c |
a One unit of activity is defined as 1 μmol of product generated min−1 at 37 °C.
b The activity was determined in the radiolabeled assay.
c The Km value corresponds to Km2, i.e. for substrate binding to site 2.
d The value of Km1 was fixed to determine the kinetic parameters Vmax2, Km2, and n.
Product Analysis of H2S Producing Reactions
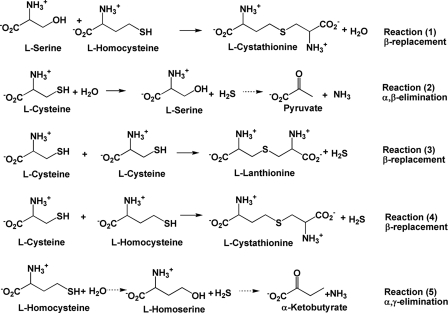
To distinguish between the multiple routes for H2S generation (Fig. 2, reactions 2–5), reaction products were analyzed by UV-visible spectroscopy, mass spectrometry, and HPLC. When cysteine was used as a substrate, serine and lanthionine formation were detected by HPLC, and the presence of the latter was confirmed by mass spectrometry. Two daughter ion peaks obtained by tandem mass spectrometry analysis of the parent ion peak (m/z = 209) were assigned as follows: m/z = 120 (corresponding to SCH2CH(NH2)COOH) and m/z = 192 (corresponding to loss of NH3 from lanthionine) (data not shown). Detection of serine and lanthionine is consistent with β-elimination (reaction 2) and β-replacement reactions (reaction 3) with cysteine as a substrate. In principle, serine formed in the unimolecular reaction (reaction 2) could be further converted to pyruvate and NH3 as observed with CSE (15). However, the α-ketoacid assay failed to reveal the breakdown product of serine, i.e. pyruvate. This is consistent with the earlier report (26) that neither chicken nor rat liver CBS catalyzes α,β-elimination reactions. Unlike CSE, CBS does not appear to use homocysteine by itself for H2S production because none of the expected reaction products, i.e. H2S, homoserine, or α-ketobutyrate, were detected. This is also consistent with fluorescence titration experiments, which failed to reveal evidence for binding of homocysteine to hCBS (data not shown).
FIGURE 2.
Reactions catalyzed by CBS leading to cystathionine, lanthionine, and H2S generation that were characterized in this study. The dotted arrows in reactions 2 and 5 denote reactions for which our study did not find evidence.
Kinetics of H2S Generation by yCBS
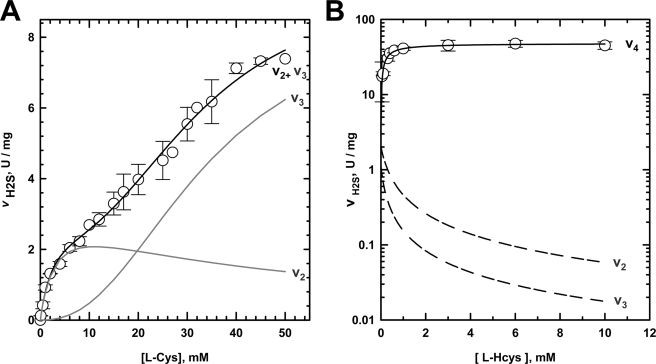
The dependence of the rate of H2S formation on cysteine concentration is markedly biphasic (Fig. 3A), which facilitated deconvolution of the kinetic parameters Vmax and Km associated with the two phases representing reactions 2 and 3, respectively (Table 1). The kinetic data obtained for the bimolecular reaction (reaction 3) were fitted for alternative mechanisms (i.e. binary versus ternary) (supplemental Table S1). Based on the quality of the fits, a distinction between a ping-pong and random sequential mechanism could not be made. In contrast, an ordered sequential mechanism provided a poor fit to the data. The active site of CBS can bind two amino acids, serine and homocysteine, to catalyze the canonical β-replacement reaction (reaction 1). These sites are referred to as site 1 (i.e. site at which the external aldimine with PLP is formed) and site 2, respectively. Yeast CBS exhibits an ∼10-fold lower Km value for cysteine at site 1 (3.6 ± 1.7 mm) compared with site 2 (33.0 ± 3.7 mm), and positive cooperativity for binding of the 2nd mol of cysteine is seen (n = 2.4 ± 0.5). Under Vmax conditions, the rate of the bimolecular reaction (reaction 3) is ∼2-fold higher than for the unimolecular reaction (reaction 2) (Table 1).
FIGURE 3.
Kinetics of H2S generation by yCBS. A, kinetics of H2S generation from cysteine and the contributions of reactions 2 and 3 catalyzed by yCBS. The kinetic data for H2S generation from cysteine (reactions 2 + 3) are shown by open circles. Each data point represents the mean (±S.D.) of at least three independent experiments. The data were analyzed as described under “Experimental Procedures,” and the kinetic parameters obtained from these plots are shown in Table 1. The contributions of the component reactions (v2 and v3) to the net rate of H2S generation are shown by gray lines. B, kinetics of H2S production (○) observed at 15 mm cysteine and varying concentrations of homocysteine. Each data point represents the mean (±S.D.) of three independent experiments. The relative contributions of the individual reactions (v2, v3, and v4) to net rate of H2S production were simulated using the kinetic parameters reported in Table 1 and as described under “Experimental Procedures.”
The kinetics of reaction 4, i.e. the condensation of cysteine and homocysteine to form cystathionine and H2S, is shown in Fig. 3B. The kinetic values for reaction 4 were determined by deconvolution of the three phases contributing to the net rate of H2S formation, using as input values the kinetic parameters determined in the presence of cysteine only as a substrate (Table 1). The kinetic data were fitted with alternative mechanisms, and a reasonable fit was only obtained for the ping-pong mechanism with cysteine binding first (supplemental Table S2). The Km,Hcys value for site 2 obtained from this analysis is ∼26-fold lower than the Km,Hcys value obtained for the condensation of serine and homocysteine (reaction 1) (Table 1). The reason for this difference is not known.
The turnover number under Vmax conditions (i.e. kcat) for the condensation of cysteine and homocysteine (reaction 4) is ∼2-fold higher than for the canonical condensation reaction of serine and homocysteine (reaction 1). Reaction 4 represents a significantly more efficient route for H2S generation than the competing reactions exhibiting an ∼16- and 6.7-fold higher kcat than reactions 2 and 3, respectively.
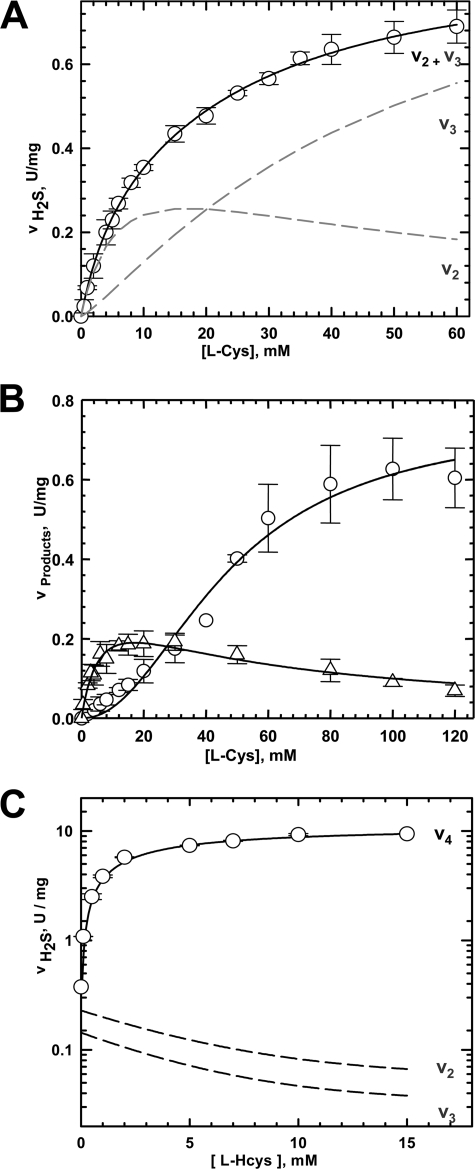
Kinetics of H2S Generation by hCBS
The dependence of the rate of H2S formation on cysteine concentration is not as markedly biphasic as observed with yCBS (Fig. 4A). Thus, the kinetic parameters for serine (reaction 2) and lanthionine (reaction 3) formation were experimentally determined at increasing concentrations of cysteine (Fig. 4B). The resulting Km1 value obtained for reaction 2 (Table 1) was then employed as an input parameter to fit the experimental data for H2S production (Fig. 4A). The Km value for cysteine binding to site 1 (6.8 ± 1.7 mm) is ∼4-fold lower than for site 2 (27.3 ± 3.7 mm) for reaction 3. The kinetic data for H2S production observed with cysteine and homocysteine as substrates are shown in Fig. 4C. As with yCBS, the kinetic data observed for the bimolecular reactions, i.e. reactions 3 and 4, were fitted with alternative mechanisms (supplemental Tables S2 and S3). The outcomes of the fits were similar to those observed with yCBS and ruled out an ordered sequential mechanism. We note that all the hCBS-catalyzed reactions were measured in the presence of the allosteric activator, AdoMet. As reported previously (19), H2S generation by hCBS exhibits a similar sensitivity to activation by AdoMet as does the canonical condensation reaction of serine and homocysteine (reaction 1) (data not shown).
FIGURE 4.
Kinetics of H2S generation by hCBS. A, dependence of H2S generation (reactions 2 + 3) on cysteine concentration. The experimental data are shown by open circles, and the lines represent the fits obtained as described under “Experimental Procedures” for the contributing reaction. B, kinetic dependence of serine (reaction 2, ▵) and lanthionine (reaction 3, ○) generation on cysteine concentration. The lines represent fits obtained as described under “Experimental Procedures.” C, kinetics of H2S production (○) observed at 20 mm cysteine and varying concentrations of homocysteine. Each data point represents the mean (±S.D.) of three independent experiments. The relative contributions of the individual reactions (v2, v3, and v4) to the net rate of H2S production were simulated using the kinetic parameters reported in Table 1 and as described under “Experimental Procedures.”
Under Vmax conditions, the turnover number for reaction 4 is 3.7-fold higher than for the condensation reaction of serine and homocysteine (reaction 1). As with yCBS, reaction 4 is a much more significant contributor to H2S production being ∼41- and ∼23-fold more efficient than reactions 2 and 3, respectively. The observed increase in the specific activity of hCBS with cysteine versus cysteine and homocysteine as substrates is similar to the 30-fold difference reported by Braunstein et al. (27). The turnover number of H2S generation from reaction 4 is ∼2.8-fold higher for yeast versus human CBS (Table 1). The relative productivity of the three H2S-generating reactions follow the order 4 > 3 > 2 for both yeast and human enzymes.
Relative Contributions of the hCBS-catalyzed Reactions to H2S Generation
The primary role of CBS in the trans-sulfuration pathway as widely accepted is to catalyze the condensation of serine and homocysteine to generate cystathionine (Fig. 1). Therefore, the higher turnover number for cystathionine production via reaction 4 (from cysteine + homocysteine) versus reaction 1 (from serine + homocysteine) is surprising and suggests the existence of alternative trans-sulfuration routes, whose operation must be regulated by mechanisms that await elucidation. Because the intracellular substrate concentrations are low compared with their respective Km values (serine, cysteine, and homocysteine concentrations are assumed to be 560, 100, and 10 μm, respectively), the contribution of the various CBS-catalyzed reactions to the overall pool of H2S and cystathionine will be determined by substrate availability. Thus, to determine the partitioning between the three H2S-producing reactions (reactions 2–4) and the two cystathionine-producing reactions (reactions 1 and 4), simulations were performed at the relevant physiological substrate concentrations (Table 2) using the kinetic parameters reported in Table 1. Furthermore, the relative contributions of each of the reactions to total H2S or cystathionine production were estimated at three concentrations of homocysteine mimicking normal (10 μm) versus moderate (40 μm) and severe (200 μm) hyperhomocysteinemia (Tables 2 and 3). In this calculation, a competitive inhibition term (1+ [Ser]/Ki) was introduced in Equations 8–10, because serine with a Kd of 56 ± 6 μm will compete with cysteine to bind to the PLP site, i.e. site 1. Similarly, a competitive inhibition term (1 + [Cys]/Ki) was used for reaction 1 because cysteine exhibits a Kd of 400 ± 27 μm. We note that both Kd values were determined at 25 °C as described under “Experimental Procedures” and are likely to be higher at 37 °C where the enzymatic activity was determined. Unfortunately, attempts to obtain the Kd values at 37 °C resulted in poor data quality. Based on these values, the turnover number (v/[E]) for cystathionine production from serine and homocysteine (reaction 1) is predicted to be ∼1.3-fold lower at 10 μm homocysteine but ∼10.5-fold higher at 200 μm homocysteine than from cysteine and homocysteine (reaction 4). Thus, at low homocysteine concentrations, reactions 1 and 4 are predicted to contribute to cystathionine production at a ratio of ∼2:3.
TABLE 2.
Kinetic parameters for the hCBS-catalyzed reactions at varying homocysteine concentrations estimated by simulations
The reaction numbers correspond to those shown in Fig. 2.
| Substrates | No. | Vmaxa | Km1, Km2b | v/[E] 10 μm Hcys | v/[E] 40 μm Hcys | Fold-changed | v/[E] 200 μm Hcys | Fold-changed |
|---|---|---|---|---|---|---|---|---|
| units/mg | mm | s−1c | s−1 | s−1 | ||||
| Ser + Hcys | 1 | 5.1 ± 0.3 | 2.0 ± 0.3, 2.8 ± 0.5 | 185 × 10−4 | 700 × 10−4 | 3.8 | 2717 × 10−4 | 14.7 |
| Cys | 2 | 0.46 ± 0.08 | 6.8 ± 1.7 | 6.4 × 10−4 | 6.4 × 10−4 | 0.99 | 6.1 × 10−4 | 0.96 |
| Cys + Cys | 3 | 0.82 ± 0.08 | 6.8 ± 1.7, 27.3 ± 3.7 | 3.9 × 10−4 | 3.9 × 10−4 | 0.99 | 3.8 × 10−4 | 0.97 |
| Cys + Hcys | 4 | 18.7 ± 2.6 | 6.8 ± 1.7, 3.2 ± 1.3 | 239 × 10−4 | 253 × 10−4 | 1.06 | 259 × 10−4 | 1.09 |
a One unit corresponds to 1 μmol of product formed min−1. The Km and Vmax values were determined as described under “Experimental Procedures” and reported in Table 1.
b In reactions involving two substrates, the order of the Km values reflects the substrate order in the 1st column.
c The values for the turnover numbers at varying concentrations of homocysteine and physiological concentrations of serine (560 μm) and cysteine (100 μm) were obtained as described under “Experimental Procedures” considering a ping-pong mechanism for the bimolecular reaction and the Hill coefficients (n) reported in Table 1.
d Fold change refers to the change in v/[E] with respect to normal conditions, i.e. 10 μm homocysteine, which is assigned a value of 1 for each reaction.
TABLE 3.
The relative contributions of the H2S generating reactions at varying concentrations of homocysteine as predicted by kinetic simulations
The reaction numbers correspond to those shown in Fig. 2. % refers to the percent contribution of each reaction to net H2S production at each concentration of homocysteine in the presence of 560 μm serine and 100 μm cysteine.
| Substrates | Reaction no. | 10 μm Hcys | 40 μm Hcys | 200 μm Hcys |
|---|---|---|---|---|
| % | % | % | ||
| Cys | 2 | 2.6 | 2.4 | 2.3 |
| Cys + Cys | 3 | 1.6 | 1.5 | 1.4 |
| Cys + Hcys | 4 | 95.8 | 96.1 | 96.3 |
Next, we assessed the relative contributions of the hCBS-catalyzed H2S-generating reactions at three concentrations of homocysteine (Tables 2 and 3). Our simulations predict that the condensation of cysteine and homocysteine (reaction 4) is the major contributor, accounting for ∼96% of H2S production, which is largely invariant over a 20-fold range of homocysteine concentration. The combined contribution of reactions 2 and 3 is of negligible importance (Table 3). Importantly, the competition between serine and cysteine rather than homocysteine for site 1 on CBS makes H2S production by CBS relatively insensitive to homocysteine concentration, increasing only 8% as the concentration of homocysteine is increased from 10 to 200 μm (Table 2).
Predicted Relative Contributions of CBS Versus CSE to H2S Generation
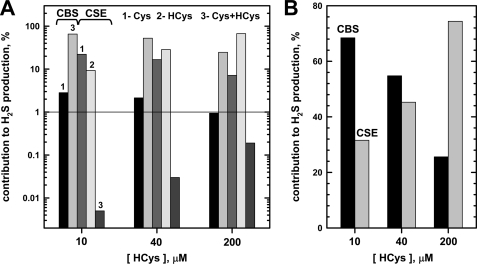
In contrast to CBS, the CSE-catalyzed generation of H2S is responsive to the grade of hyperhomocysteinemia (15). Comparison of the turnover numbers of these two enzymes indicates that at equimolar concentrations, CBS could be the major source of H2S production, if fully activated by the allosteric regulator AdoMet (Fig. 5 and Table 4). At low homocysteine concentrations (10 μm), the value of v/[E] for H2S generation is predicted to be 0.0249 and 0.0115 s−1 for CBS and CSE, respectively, corresponding to an ∼7:3 ratio (CBS/CSE) for H2S production (Table 4). The contribution of CBS to H2S production would be ∼2–3-fold lower in the absence of allosteric activation by AdoMet, which increases the reaction velocity of the CBS-catalyzed reactions by this factor. Also, in the absence of AdoMet, the Kd value for serine at the PLP site decreases ∼4-fold. Thus, the relative contributions of CBS and CSE to H2S production at low homocysteine concentrations might vary from ∼7:3 (full allosteric activation of CBS by AdoMet) to ∼2:8 (no activation).
FIGURE 5.
Relative contributions of fully activated hCBS and CSE to H2S generated via the trans-sulfuration pathway determined by simulations. A, relative proportions of fully activated CBS- and CSE-derived H2S at three concentrations of homocysteine and physiological concentrations of serine, cysteine, and cystathionine. B, relative contributions of fully activated CBS and CSE to net H2S production via the trans-sulfuration pathway at three concentrations of homocysteine mimicking normal (10 μm), moderate (40 μm), and severe (200 μm) hyperhomocysteinemia.
TABLE 4.
The relative contributions of CBS and CSE to net H2S production via the trans-sulfuration pathway at varying homocysteine concentrations predicted by kinetic simulations
The % contributions for CBS and CSE were calculated using the turnover numbers reported in Table 2 for CBS and the previously reported values for CSE (15) as described under “Experimental Procedures.”
| Enzyme | Substrate | 10 μm Hcys |
40 μm Hcys |
200 μm Hcys |
|||
|---|---|---|---|---|---|---|---|
| % | % total | % | % total | % | % total | ||
| CBS | Cys | 2.8 | 68 | 2.1 | 55 | 0.94 | 26 |
| Cys + Hcys | 65.6 | 52.6 | 24.6 | ||||
| CSE | Cys | 22.2 | 32 | 16.70 | 45 | 7.1 | 74 |
| Hcys | 9.3 | 28.5 | 67.1 | ||||
| Cys + Hcys | 0.005 | 0.03 | 0.19 | ||||
The value of v/[E] for H2S generation by CSE is predicted to increase to 0.0217 and 0.0781 s−1 at 40 and 200 μm homocysteine (15), respectively, whereas the comparable values for CBS under conditions of full allosteric activation are largely unaffected (0.0263 and 0.0269 s−1 at 40 and 200 μm homocysteine, respectively). Therefore, under conditions of moderate and severe hyperhomocysteinemia, CSE is predicted to become an increasingly important contributor to the H2S pool, with the proportion increasing to 45 and 74%, respectively, at mild and moderate hyperhomocysteinemia. These values represent a lower estimate for the overall contribution of CSE and would be higher if CBS is not fully activated by AdoMet.
Predicted Relative Contributions of CBS Versus CSE to Lanthionine Generation as a Side Product in H2S-producing Reactions
Lanthionine is a thioether that is produced as a side product in the H2S generating β-replacement reactions catalyzed by CBS and CSE in which cysteine serves as the substrate (reaction 3). At low homocysteine concentrations (10 μm) and equimolar concentrations of both enzymes, the v/[E] value for reaction 3 is estimated to be 2.9 × 10−8 and 3.9×10−4 s−1 for CSE and fully AdoMet-activated hCBS, respectively (Fig. 1). Thus, hCBS is predicted to be virtually the sole source (99.99%) of lanthionine formed by condensation of 2 mol of cysteine. We note that an alternative lanthionine-generating reaction (in which H2O rather than H2S is eliminated), namely β-replacement of serine by cysteine, might be catalyzed by CSE and CBS. The contribution of this reaction to the total lanthionine pool has not been addressed in this study.
Lanthionine Cleavage by CSE
Although lanthionine is a poor substrate for hCBS (Vmax < 0.05 unit/mg), human CSE exhibits substantial lanthionine cleavage activity with a Vmax of 1.03 ± 0.05 unit/mg (kcat = 0.8 s−1) and a Km,lan = 0.75 ± 0.17 mm. In comparison, CSE exhibits a Vmax value of 3.1 ± 0.1 units/mg (kcat = 2.3 s−1) and a Km value of 0.28 ± 0.03 mm for cystathionine.
DISCUSSION
Despite the pharmacological interest in modulating H2S production (28, 29), there has been little attention paid to the mechanism of its generation and regulation. In this study, we have investigated the various reactions catalyzed by yeast and human CBS that lead to H2S production. We have also examined the relative importance of hCBS versus human CSE to H2S production and the sensitivity of these reactions to the grade of hyperhomocysteinemia.
H2S Production by yCBS
In yeast, ultradian cycling is regulated by chemical signals, including H2S (30), to achieve population synchronization. Interestingly, the H2S producing sulfate assimilation pathway and respiratory oscillations have been linked to the trans-sulfuration pathway with intracellular cysteine levels and CSE playing a regulatory role (31).
Yeast CBS catalyzes the production of cystathionine by a β-replacement reaction involving serine and homocysteine (reaction 1). The turnover number of this reaction under Vmax conditions (25.5 s−1) is ∼2-fold lower than for the condensation of cysteine and homocysteine (reaction 4, 55.0 s−1), leading to H2S production (Table 1). Yeast maintains very low cysteine concentration (0.25–0.45 μm), which is ∼1–10% of the intracellular concentration of other amino acids in this organism (4, 31). Hence, at intracellular levels of cysteine and serine, reaction 1 is expected to dominate over reaction 4. Of the three H2S-generating reactions catalyzed by yCBS, the β-replacement of cysteine by homocysteine (reaction 4) exhibits a higher kcat value than for reactions 2 and 3 (Table 1). For the bimolecular reactions, the kinetic fits support a ping-pong mechanism, which is consistent with the mechanism previously reported for reaction 1 catalyzed by yCBS (21).
Because of its toxicity to cells at elevated concentrations (32), intracellular cysteine levels are tightly regulated. H2S production by yCBS from cysteine and homocysteine will be sensitive to levels of these amino acids. However, at intracellular concentrations of substrates, the H2S-generating reactions are likely to be competitively inhibited by serine, which is abundant, binding to site 1. The mechanisms by which yCBS is regulated so that it serves either in the trans-sulfuration pathway or is diverted for H2S production are not known and merit investigation.
Our studies reveal that yCBS is capable of generating lanthionine (reaction 3), a novel thioether product that is a component of the class of lantibiotics (33). Although cyclic lanthionine ketimine has been reported in bovine and human brain (34, 35), the presence and fate of this compound in yeast await elucidation. Lanthionine, like cysteine or cystine, can support the organic sulfur need for growth during the shift from the mycelial to the parasitic yeast form in the dimorphic pathogenic fungus, Histoplasma capsulatum (36). This suggests that lanthionine is transported into yeast cells and metabolized, probably by the action of CSE to cysteine, as discussed below.
H2S Production by hCBS
In contrast to the profligacy of human CSE (15), hCBS has been reported to catalyze H2S formation solely by the β-replacement of the physiologically relevant substrates cysteine by homocysteine (19). We find that hCBS can generate H2S from cysteine, albeit with lower kcat values than from cysteine and homocysteine (Table 1). CBS has also been reported to catalyze H2S generation by β-replacement of cysteine by several compounds, e.g. β-mercaptoethanol, dithiothreitol, cysteamine, and methanethiol, with concomitant formation of the corresponding thioethers (26).
The canonical reaction catalyzed by hCBS in the trans-sulfuration pathway is the β-replacement of serine by homocysteine to generate cystathionine (Fig. 1). The turnover number under substrate saturation (kcat) of this reaction (reaction 1) is 3.7-fold lower than for cystathionine production from cysteine and homocysteine (4) (Table 1), and it could partly be explained by the better leaving group potential of H2S versus H2O. Consequently, at physiological substrate concentrations, the turnover number for reaction 1 is predicted to be 1.3-fold lower than for reaction 4 (Table 2). Thus, reaction 4 represents an alternative route for generating cystathionine at physiologically relevant substrate concentrations, accounting for ∼56% of its total synthesis (Fig. 1). However, unlike reaction 1, reaction 4 does not lead to net cysteine synthesis via the trans-sulfuration pathway because cysteine is consumed to produce cystathionine and then subsequently regenerated by cleavage of cystathionine catalyzed by CSE (Fig. 1). Because the other product of reaction 4 is the signaling molecule H2S, flux through this reaction must be regulated in the cell by mechanisms that await elucidation.
Of the three H2S-producing reactions catalyzed by CBS, reaction 4 is the most efficient, accounting for 96% of the net production (Table 3). In contrast, the β-elimination (reaction 2) and β-replacement reactions (reaction 3) involving cysteine are of minor importance, representing between 1.6 and 2.6% of the total H2S derived from hCBS (Table 3).
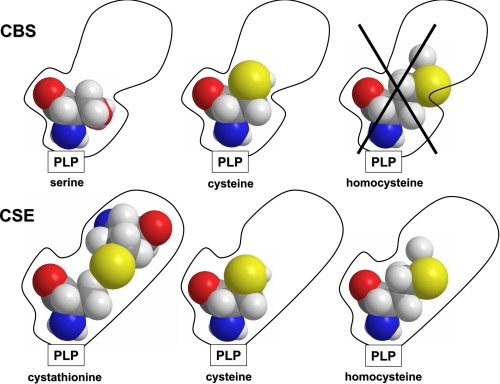
In contrast to the sensitivity of CSE-catalyzed H2S generation from homocysteine (15), our simulations reveal that H2S production by hCBS is insensitive to the grade of hyperhomocysteinemia (Table 2). Increasing homocysteine from normal to moderate to severely elevated levels has virtually no effect on the kcat value for reaction 4 for H2S production (Table 2). The insensitivity to homocysteine concentrations results from the relatively low Kd value for serine binding to site 1 (Kd ∼ 56 μm) compared with the physiological concentration of serine (560 μm). Thus, serine competes effectively with cysteine, which is present at lower concentrations and exhibits a higher Kd value, and thus limits the three H2S-generating reactions because they involve binding of cysteine to site 1 (Fig. 6). Unlike CSE, homocysteine appears to be unable to bind to site 1 in hCBS, and the enzyme does not catalyze reactions at the γ-carbon. Consequently, the only reaction rate that is boosted by an increase in homocysteine concentration is the β-replacement of serine by homocysteine (reaction 1) whose turnover number is predicted to increase 3.8- and 14.7-fold under moderate and severe hyperhomocysteinemia, respectively (Table 2). This enhancement would only be relevant in hyperhomocysteinemia conditions resulting from a deficiency in enzymes other than hCBS.
FIGURE 6.
Schematic representation for the sensitivity of the CSE- but not CBS-catalyzed H2S production to hyperhomocysteinemia. Unlike CSE, the PLP-binding pocket of CBS binds serine or cysteine but not homocysteine.
The insensitivity of H2S formation by CBS to homocysteine suggests that CSE will be primarily responsible for enhanced H2S production under conditions of hyperhomocysteinemia (Table 4 and Fig. 5). We have estimated the relative contribution of CBS and CSE to the net H2S pool, assuming equivalent amounts of both enzymes and full activation of CBS by AdoMet. Thus, under normal conditions (10 μm homocysteine), CSE accounts for ∼32% of the H2S produced by the trans-sulfuration pathway, and this increases to ∼45 and ∼74% under moderate and severe hyperhomocysteinemia conditions. However, in tissues where one or the other enzyme is absent or present at greatly reduced levels, their relative contributions to H2S biogenesis will clearly be different. Furthermore, depending on the extent of CBS activation by AdoMet, its relative contribution to the H2S pool could vary between ∼25 and 70% in cells containing equimolar CSE.
Mutations in CBS are the most common cause of severe hyperhomocysteinemia in comparison with defects elsewhere in the metabolic pathway (37). In homocystinurics with CBS deficiency, CSE will not only be the primary source of H2S but is predicted to generate higher H2S levels due to increased accumulation of homocysteine (15). Under conditions of elevated homocysteine due to deficiencies elsewhere in the methionine cycle, i.e. not in CBS, or a higher flux through the trans-sulfuration pathway (e.g. after a methionine-rich meal), cystathionine production is predicted to increase significantly (Fig. 1 and Table 2). This could lead in turn to enhanced production of cysteine and stimulate glutathione production, because cysteine is the limiting substrate for the synthesis of this antioxidant. The activity of γ-glutamylcysteine synthetase, the committing step in the synthesis of glutathione, is regulated by feedback inhibition by glutathione (38). Interestingly, H2S has been reported to enhance the activity of γ-glutamylcysteine synthetase (39).
Rat CBS has been reported to generate lanthionine from a mixture of cysteine and serine (25). Here we show that lanthionine (reaction 3) can also be produced by the condensation of 2 mol of cysteine (Table 1). The estimated turnover number for lanthionine production by hCBS is ∼13,000-fold higher than by human CSE. In contrast, CSE catalyzes the cleavage of lanthionine to cysteine and pyruvate more efficiently (kcat ∼ 0.8 s−1) than CBS (kcat < 0.05 s−1). Thus, lanthionine concentrations are expected to be higher in tissues in which CSE levels and/or activity are low. Our results demonstrate that lanthionine derived from cysteine is produced almost exclusively (99.99%) by CBS, thus linking the origin of this metabolite to cysteine metabolism and CBS.
Implications of Relaxed Substrate Specificity in the Trans-sulfuration Pathway
The profligacy of CBS and CSE leads to a much greater complexity of trans-sulfuration reactions catalyzed by these enzymes than previously appreciated and expand the sulfur metabolome. The relative insensitivity of CBS to the grade of hyperhomocysteinemia contrasts with that of CSE. These results imply that CSE plays a heretofore unrecognized role in intracellular homocysteine management both in the peripheral vasculature, where CBS levels are low or the enzyme is absent, and under hyperhomocysteinemic conditions. Evidence for the role of CSE in homocysteine homeostasis is provided by the ∼18-fold increase in homocysteine levels in aorta and heart in CSE−/− mice (1). The metabolic functions of lanthionine, which is predicted to be enriched in tissues with low CSE activity, and of homolanthionine, await further elucidation. The capacity of the trans-sulfuration pathway to catalyze a multitude of reactions raises important questions about how these reactions are regulated to meet cellular needs for the varied products.
Supplementary Material
Acknowledgment
We thank Dr. Weidong Zhu (University of Nebraska, Lincoln) for assistance with performing the mass spectrometric analysis of our samples.
This work was supported, in whole or in part, by National Institutes of Health Grant HL58984.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3.
- H2S
- hydrogen sulfide
- CBS
- cystathionine β-synthase
- CSE
- cystathionine γ-lyase
- AdoMet
- S-adenosylmethionine
- Hcys
- homocysteine
- HPLC
- high performance liquid chromatography
- y
- yeast
- h
- human
- PLP
- pyridoxal phosphate.
REFERENCES
- 1.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A. K., Mu W., Zhang S., Snyder S. H., Wang R. (2008) Science 322, 587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elrod J. W., Calvert J. W., Morrison J., Doeller J. E., Kraus D. W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., Kimura H., Chow C. W., Lefer D. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 15560–15565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackstone E., Morrison M., Roth M. B. (2005) Science 308, 518. [DOI] [PubMed] [Google Scholar]
- 4.Sohn H., Kuriyama H. (2001) Yeast 18, 125–135 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell T. W., Savage J. C., Gould D. H. (1993) J. Appl. Toxicol. 13, 389–394 [DOI] [PubMed] [Google Scholar]
- 6.Savage J. C., Gould D. H. (1990) J. Chromatogr. 526, 540–545 [DOI] [PubMed] [Google Scholar]
- 7.Goodwin L. R., Francom D., Dieken F. P., Taylor J. D., Warenycia M. W., Reiffenstein R. J., Dowling G. (1989) J. Anal. Toxicol. 13, 105–109 [DOI] [PubMed] [Google Scholar]
- 8.Zhao W., Zhang J., Lu Y., Wang R. (2001) EMBO J. 20, 6008–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furne J., Saeed A., Levitt M. D. (2008) Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1479–R1485 [DOI] [PubMed] [Google Scholar]
- 10.Abe K., Kimura H. (1996) J. Neurosci. 16, 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao W., Wang R. (2002) Am. J. Physiol. Heart Circ. Physiol. 283, H474–H480 [DOI] [PubMed] [Google Scholar]
- 12.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., Kimura H. (2009) Antioxid. Redox. Signal. 11, 703–714 [DOI] [PubMed] [Google Scholar]
- 13.Vitvitsky V., Thomas M., Ghorpade A., Gendelman H. E., Banerjee R. (2006) J. Biol. Chem. 281, 35785–35793 [DOI] [PubMed] [Google Scholar]
- 14.Diwakar L., Ravindranath V. (2007) Neurochem. Int. 50, 418–426 [DOI] [PubMed] [Google Scholar]
- 15.Chiku T., Padovani D., Zhu W., Singh S., Vitvitsky V., Banerjee R. (2009) J. Biol. Chem. 284, 11601–11612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Refsum H., Ueland P. M., Nygård O., Vollset S. E. (1998) Annu. Rev. Med. 49, 31–62 [DOI] [PubMed] [Google Scholar]
- 17.Seshadri S., Beiser A., Selhub J., Jacques P. F., Rosenberg I. H., D'Agostino R. B., Wilson P. W., Wolf P. A. (2002) N. Engl. J. Med. 346, 476–483 [DOI] [PubMed] [Google Scholar]
- 18.Clarke R., Smith A. D., Jobst K. A., Refsum H., Sutton L., Ueland P. M. (1998) Arch. Neurol. 55, 1449–1455 [DOI] [PubMed] [Google Scholar]
- 19.Chen X., Jhee K. H., Kruger W. D. (2004) J. Biol. Chem. 279, 52082–52086 [DOI] [PubMed] [Google Scholar]
- 20.Kery V., Bukovska G., Kraus J. P. (1994) J. Biol. Chem. 269, 25283–25288 [PubMed] [Google Scholar]
- 21.Jhee K. H., McPhie P., Miles E. W. (2000) Biochemistry 39, 10548–10556 [DOI] [PubMed] [Google Scholar]
- 22.Singh S., Madzelan P., Stasser J., Weeks C. L., Becker D., Spiro T. G., Penner-Hahn J., Banerjee R. (2009) J. Inorg. Biochem. 103, 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taoka S., Banerjee R. (2002) J. Biol. Chem. 277, 22421–22425 [DOI] [PubMed] [Google Scholar]
- 24.Taoka S., Ohja S., Shan X., Kruger W. D., Banerjee R. (1998) J. Biol. Chem. 273, 25179–25184 [DOI] [PubMed] [Google Scholar]
- 25.Borcsok E., Abeles R. H. (1982) Arch. Biochem. Biophys. 213, 695–707 [DOI] [PubMed] [Google Scholar]
- 26.Braunstein A. E., Goryachenkova E. V., Tolosa E. A., Willhardt I. H., Yefremova L. L. (1971) Biochim. Biophys. Acta 242, 247–260 [DOI] [PubMed] [Google Scholar]
- 27.Braunstein A. E., Goryachenkova E. V., Lac N. D. (1969) Biochim. Biophys. Acta 171, 366–368 [DOI] [PubMed] [Google Scholar]
- 28.Szabó C. (2007) Nat. Rev. Drug Discov. 6, 917–935 [DOI] [PubMed] [Google Scholar]
- 29.Wallace J. L. (2007) Trends Pharmacol. Sci. 28, 501–505 [DOI] [PubMed] [Google Scholar]
- 30.Sohn H. Y., Murray D. B., Kuriyama H. (2000) Yeast 16, 1185–1190 [DOI] [PubMed] [Google Scholar]
- 31.Sohn H., Kuriyama H. (2001) Arch. Microbiol. 176, 69–78 [DOI] [PubMed] [Google Scholar]
- 32.Kumar A., John L., Alam M. M., Gupta A., Sharma G., Pillai B., Sengupta S. (2006) Biochem. J. 396, 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willey J. M., van der Donk W. A. (2007) Annu. Rev. Microbiol. 61, 477–501 [DOI] [PubMed] [Google Scholar]
- 34.Ricci G., Vesci L., Nardini M., Arduini A., Storto S., Rosato N., Cavallini D. (1989) Biochim. Biophys. Acta 990, 211–215 [DOI] [PubMed] [Google Scholar]
- 35.Fontana M., Brunori A., Costa M., Antonucci A. (1997) Neurochem. Res. 22, 821–824 [DOI] [PubMed] [Google Scholar]
- 36.McVeigh I., Houston W. E. (1972) Mycopathol. Mycol. Appl. 47, 135–151 [DOI] [PubMed] [Google Scholar]
- 37.Mudd S. H., Skovby F., Levy H. L., Pettigrew K. D., Wilcken B., Pyeritz R. E., Andria G., Boers G. H., Bromberg I. L., Cerone R., et al. (1985) Am. J. Hum. Genet. 37, 1–31 [PMC free article] [PubMed] [Google Scholar]
- 38.Richman P. G., Meister A. (1975) J. Biol. Chem. 250, 1422–1426 [PubMed] [Google Scholar]
- 39.Kimura Y., Kimura H. (2004) FASEB J. 18, 1165–1167 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.