Abstract
We have recently reported that transgenic (mRen2)27 rats (Ren2 rats) exhibit pulmonary arterial hypertension (PAH), which is, in part, mediated by oxidative stress. Since 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors (statins) exhibit beneficial vascular effects independent of cholesterol synthesis, we hypothesized that rosuvastatin (RSV) treatment ameliorates PAH and pulmonary vascular remodeling in Ren2 rats, in part, by reducing oxidative stress. Six-week-old male Ren2 and Sprague-Dawley rats received RSV (10 mg·kg−1·day−1 ip) or vehicle for 3 wk. After treatment, right ventricular systolic pressure (RVSP) and mean arterial pressure (MAP) were measured. To evaluate treatment effects on pulmonary arteriole remodeling, morphometric analyses were performed to quantitate medial thickening and cell proliferation, whereas whole lung samples were used to quantitate the levels of 3-nitrotyrosine, superoxide, stable nitric oxide (NO) metabolites [nitrates and nitrites (NOx)], and expression of NO synthase isoforms. In the Ren2 rat, RVSP is normal at 5 wk of age, PAH develops between 5 and 7 wk of age, and the elevated pressure is maintained with little variation through 13 wk. At 8 wk of age, left ventricular function and blood gases were normal in the Ren2 rat. Ren2 rats exhibited elevations in medial hypertrophy due to smooth muscle cell proliferation, 3-nitrotyrosine, NOx, NADPH oxidase activity, and endothelial NO synthase expression compared with Sprague-Dawley rats. RSV significantly blunted the increase in RVSP but did not reduce MAP in the Ren2 rat; additionally, RSV significantly attenuated the elevated parameters examined in the Ren2 rat. These data suggest that statins may be a clinically viable adjunct treatment of PAH through reducing peroxynitrite formation.
Keywords: oxidative stress, NADPH oxidase
3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors (statins), originally developed for their cholesterol-lowering effects, exhibit diverse beneficial effects in the vascular wall independent of effects on cholesterol synthesis. Statins improve cardiovascular outcomes/risk by restoring endothelial and smooth muscle cell (SMC) function, inhibiting SMC proliferation, reducing oxidative stress and inflammation in the vascular wall, and decreasing platelet thrombogenic activity (43, 47).
Although statins display only minimal effects on systemic hypertension (11, 41), recent evidence has suggested that these agents may be efficacious in treating pulmonary arterial hypertension (PAH). For instance, in rat models of PAH, statins attenuated the development of pulmonary hypertension (PH), pulmonary vascular remodeling, and right ventricular (RV) hypertrophy (RVH) (10, 34, 42), and, in some reports, reversed established PH (35). In rodents, statins may improve endothelial function by reversing lung endothelial nitric oxide (NO) synthase (eNOS) dysfunction during hypoxia-induced PAH (10, 33) or increasing lung eNOS expression during monocrotaline-induced PAH (15, 34). A recent observational study (23) of adjunctive simvastatin therapy in patients with PH suggested functional improvements in symptoms.
We (6) recently reported a new model of PAH and pulmonary vascular remodeling: the male transgenic (mRen2)27 rat (the Ren2 rat). The Ren2 rat is a derivative of the Sprague-Dawley (SD) rat that expresses the mouse renin gene in extrarenal tissues, resulting in the increased local synthesis of ANG II via the local renin-angiotensin system (RAS) and ANG II-dependent hypertension and end organ damage. ANG II induces oxidative stress by stimulating ROS formation, which, in turn, promotes vasoconstriction, hypertrophy, fibrosis, and remodeling (12). Multiple molecular complexes are known to generate superoxide, including NADPH oxidase (13), xanthine oxidase (26), the mitochondrial electron transport chain, and uncoupled NOS (3, 14); however, in the vasculature, the ANG II-induced activation of NADPH oxidase is the major source of superoxide (12, 13). In the Ren2 rat, we (6) reported that the lung expresses mouse renin and showed increases in intrapulmonary NADPH oxidase activity, superoxide, RV systolic pressure (RVSP), and pulmonary vascular remodeling. These data from experiments in the Ren2 rat support the concept that PAH can occur as a consequence of oxidative stress induced by the activation of the local RAS within the pulmonary vasculature and lung parenchyma. Whether other risk factors, such as left ventricular (LV) dysfunction or hypoxia, contribute to the development of PAH in the Ren2 rat are unknown. Given the multiple favorable effects of statins on the vasculature, including the potential to reduce oxidative stress, we sought to test whether treatment with a clinically available statin [e.g., rosuvastatin (RSV)] improves PAH and pulmonary vascular remodeling in the Ren2 rat.
MATERIALS AND METHODS
Animal care.
Five-week-old male heterozygous transgenic Ren2 (n = 26) and SD (n = 26) rats were obtained from Carlos M. Ferrario (Wake Forest University School of Medicine, Winston-Salem, NC). In most instances, we received cohorts of SD and Ren2 rats that were littermates; however, to measure RVSP in recently weaned SD rats, we purchased five 4.5-wk-old SD rats from Harlan Laboratories. For the RSV experiments, animals were randomly distributed into the following groups: SD control (SDC; n = 9), RSV-treated SD (SDR; n = 8), Ren2 control (R2C; n = 7), and RSV-treated Ren2 (R2R; n = 7). Beginning at 6 wk of age, treated rats received daily RSV (10 mg/kg) by an intraperitoneal injection for a total of 21 days. As RSV was dissolved in saline, control rats received an intraperitoneal saline solution for 21 days. All procedures were approved in advance by the University of Missouri Institutional Animal Care and Use Committee, and animals were cared for in accordance with National Institutes of Health (NIH) guidelines.
In vivo cine MRI of LV function.
To further characterize the Ren2 model of PAH and explore whether PAH is secondary to LV dysfunction, we examined LV diastolic and systolic function using in vivo cine MRI technique. Scans were performed on anesthetized (1–3.5% isoflurane) Ren2 and SD littermate controls at 8–9 wk of age using a Varian 7T horizontal bore MRI (Varian, Palo Alto, CA). LV volume was determined with a modified ellipsoid equation using 14 images that were acquired at equal time intervals over the course of 1 cardiac cycle beginning at end diastole (27). At each time point, the endocardial borders were traced using Image J software (NIH, Bethesda, MD). The individual tracing the borders was unaware of the treatment group. Volumes were calculated using the area/length method as per the American Society of Echocardiography (27). The volumes were plotted, and systolic function [specifically, the ejection fraction (EF)] was measured as follows: EF = (EDV − ESV)/EDV × 100%, where EDV and ESV are end-diastolic and end-systolic volume, respectively. As an index of diastolic function, we calculated the rate of diastolic filling (slope of the early diastolic curve) normalized to EDV.
Surgical preparation and hemodynamic experiments.
Hemodynamic experiments were performed on anesthetized and ventilated Ren2 and SD rats as previously described (6). Briefly, a Millar Mikro-tip catheter was inserted into the jugular and passed into the RV to monitor RVSP, a surrogate marker of pulmonary artery systolic pressure. A fluid-filled catheter was placed in the right carotid artery to monitor systolic, diastolic, and mean arterial pressure (MAP) and heart rate. RVSP and MAP were recorded every 10 min for a period of 90 min to calculate an average for each variable.
Tissue preparation, morphometric analysis, and immunohistochemistry.
After rats had been killed, 2-mm-thick cross-sectional slices harvested from the lower portion of the left lower lung lobe were immersed in neutral buffered formalin. Tissue samples were embedded in paraffin, and 5-μm sections were mounted on glass slides and stained with Verhoeff vanGeison stain. Digital images of 20–30 small pulmonary arteries/section (<200 μm in diameter) were captured, and, with the aid of MetaVue image analysis software (Boyce Scientific, Gray Summit, MO), the areas of the media and lumen were calculated to determine the medial thickness of the small pulmonary arterioles. To evaluate the type of vascular remodeling that occurs in the Ren2 rat and the potential therapeutic responses to RSV, additional sections were immunolabeled for SMCs and endothelial cells using antibodies to α-smooth muscle actin antibody (MO851, Dako) and von Willibrand factor (sc-14014, Santa Cruz Biotechnology), respectively. To count cells in the medial layer of small pulmonary arterioles, sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI) to stain cell nuclei. Immunofluorescence microscopy was performed as previously described (16). Nuclei were counted on high-resolution images by a blinded observer. The levels of NADPH oxidase subunit proteins (NOX2 and Rac1) were also analyzed in pulmonary arterioles by semiquantitative immunohistochemistry as previously described (16).
RT-PCR.
Semiquantitative RT-PCR was used to evaluate the transcript expression of the angiotensin type 1 receptor (AT1R), angiotensinogen, angiotensin-converting enzyme (ACE), eNOS, inducible NOS (iNOS), neuronal NOS (nNOS), and GAPDH in lung RNA extracts. The tissue preparation, extraction, PCR conditions, and densitometric analysis were as previously described (6). Oligonucleotide primer sequences were designed in accordance with published rat DNA sequences and are shown in Table 1. Primers were purchased from Integrated DNA Technologies.
Table 1.
Oligonucleotide primer sequences for renin-angiotensin components and NOS isoforms
| Forward Primer | Reverse Primer | Base Pairs | References | |
|---|---|---|---|---|
| Angiotensin type 1 receptor | 5′-GCATCATCTTTGTGGTGGG-3′ | 5′-ATCAGCACATCCAGGAATG-3′ | 689 | 55 |
| Angiotensin-converting enzyme | 5′-GCAGTACAAAGACTTGCCTG-3′ | 5′-TGGCAGAGGCTGACATGTTA-3′ | 599 | 45 |
| Angiotensinogen | 5′-TTGTTGAGAGCTTGGGTCCCTTCA-3′ | 5′-CAGACACTGAGGTGCTGTTGTCCA-3′ | 280 | 25 |
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ | 5′-TCCACCACCCTGTTGCTGTA-3′ | 425 | 6 |
| Endothelial NOS | 5′-CCGGGACTTCATCAATCAGT-3′ | 5′-TGTTGTATCGGTGAGGGTCA-3′ | 724 | http://frodo.wi.mit.edu/ |
| Inducible NOS | 5′-CCAACAACACAGGATGACC-3′ | 5′-CCTGATGTTGCCACTGTTAG-3′ | 603 | 39 |
| Neuronal NOS | 5′-TGGAGGAGAGGAACACTGCT-3′ | 5′TCTCTGAAGACGCCCTTGTT-3′ | 743 | http://frodo.wi.mit.edu/ |
NOS, nitric oxide synthase. Sequences were obtained either from the indicated literature citations or generated using Primer 3 software (version 0.4.0) available at http://frodo.wi.mit.edu/.
Western and slot-blot analyses.
Primary antibodies to von Willibrand factor (sc-14014), NOX2 (sc-5827), eNOS (sc-654), and iNOS (sc-7271) were purchased from Santa Cruz Biotechnology. An antibody to α-smooth muscle actin was purchased from Dako (MO851). An antibody to Rac1 (05-389) was purchased from Upstate Biotechnology. A primary antibody to 3-nitrotyrosine was purchased from Chemicon (AB5411). Appropriate secondary antibodies were from Jackson ImmunoResearch. Whole lung protein extracts were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Membranes were subdivided so that each protein of interest and β-actin could be analyzed from a single transfer. Membranes were incubated in blocking buffer for 1 h [PBS-Tween 20 (PBST) containing 5% nonfat dry milk] and incubated in primary antibody overnight at 4°C in PBST containing 3% nonfat dry milk and sodium azide. Blots were washed in PBST and incubated for 1 h with the appropriate secondary antibody. After a thorough wash in PBST, signals were detected by chemiluminescence (Super Signal West Pico Chemiluminescent Kit, catalog no. 34080, Thermo Scientifice), and images were recorded using a Bio-Rad ChemiDoc XRS image-analysis system (Bio-Rad Laboratories, Hercules, CA). Quantitation of protein band density, normalized to β-actin band density, was performed using Quantity One software (Bio-Rad Laboratories). Data are reported as normalized protein band density (in arbitrary units).
Additionally, immunoblot analysis to detect protein nitration was performed using a slot blot rather than SDS-PAGE. This method allowed us to detect and quantify signals from one band rather than from an entire lane of separated protein. Whole lung protein extracts (50 μg/slot) were vacuum transferred to a nitrocellulose filter. After being incubated in blocking buffer, the membrane was incubated with an antibody to 3-nitrotyrosine overnight, washed, incubated with secondary antibody for 1 h, and washed again. Detection and quantitation of bands were as described above except that 3-nitrotyrosine band densities were normalized to band densities of amido black-stained protein.
Lucigenin-enhanced chemiluminescence.
Superoxide anions were measured via the lucigenin-enhanced chemiluminescence method as previously described (6). Briefly, the assay was performed in duplicate on homogenates of fresh lung samples from the right middle lung lobe (∼200 mg wet wt each). Superoxide production was calculated as counts per milligram of fresh tissue for each sample after subtraction of the background activity.
NADP(H) oxidase activity in lung plasma membrane extracts.
As previously described (6), aliquots of plasma membrane fractions containing 20 μg protein were incubated with NADPH (100 mM) at 37°C. NADPH activity was determined by measuring the conversion of Radical Detector (Cayman Chemical) in the absence and presence of the NADPH inhibitor diphenylene iodonium sulfate (500 μM) using spectrophotometric (450 nm) techniques. Initially, data were calculated as milli-optical density units per minute and normalized to the protein content of the sample. Because data were collected from several cohorts of rats and baseline NADPH oxidase activity in SDC rats varied between the cohorts, all data are expressed as a percentage of SDC within each cohort.
Measurement of nitrate and nitrite.
To estimate the level of NO present in whole lung homogenates, we measured the sum of stable NO metabolites [nitrate and nitrite (NOx)] using a colorimetric assay kit (Cayman Chemical) that involves the Griess reaction. Nitrate concentrations in tissue extracts were measured after reducing all nitrates into nitrite using nitrate reductase. The absorbances were read at a wavelength of 540 nm on a microplate reader (Powerwave X, Bio-Tek Instruments), and NOx levels are expressed as micromoles per milligram of protein.
Statisical analysis.
Results are reported as means ± SE; n indicates the number of rats per treatment group. Differences in outcomes between vehicle- and RSV-treated SD and Ren2 rats were determined using two-way ANOVA. Post hoc comparisons were made using the Tukey-Kramer test and considered significant when P < 0.05. However, when we compared only outcomes between SD to Ren2 rats (e.g., Figs. 2 and 3), Student's t-test was used. NCSS 2007 (NCSS, Kaysville, UT) was used for all other statistical analysis except when noninferiority tests were conducted using a reverse experiment tester (Boss Statistics) (30) to determine if there were significant similarities between groups.
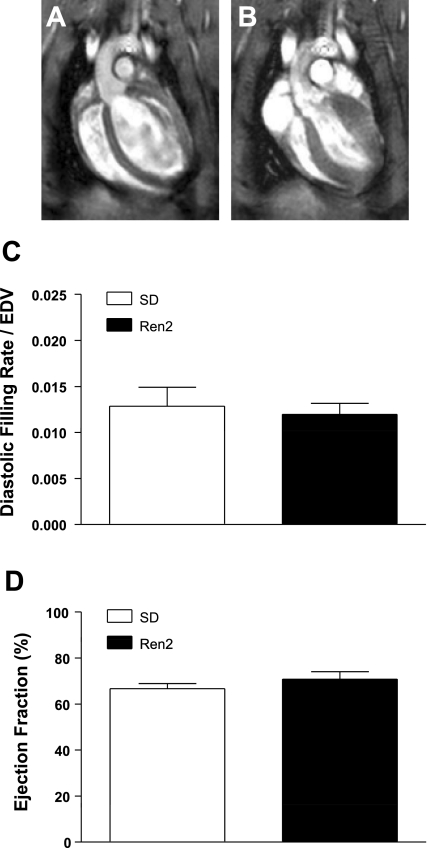
Fig. 2.
In vivo cine MRI analysis of left ventricular (LV) function in SD and Ren2 rats. Nine-week-old male Ren2 and SD rats were imaged by in vivo cine MRI. A and B: representative images of end systole (A) and end diastole (B) of a representative SD rat are shown. Movies for a representative SD and Ren2 rat are shown in Supplemental Fig. 1. C: no differences or similarities were found in the diastolic filling rate/end-diastolic volume (EDV). D: the ejection fraction between SD and Ren2 rats was significantly similar (power = 0.842) via a noninferiority test.
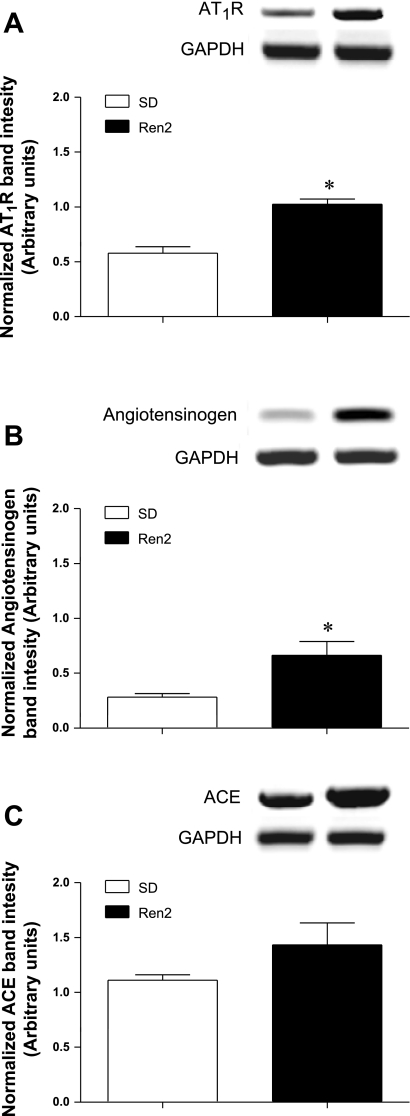
Fig. 3.
Lung expression of RNA transcripts of renin-angiotensin system components in SD and Ren2 rats. Angiotensin type 1 receptor (AT1R; A), angiotensinogen (B), and angiotensin-converting enzyme (ACE; C) mRNA were examined via RT-PCR in 9-wk-old male SD and Ren2 rats. Bar graphs show band densities (means ± SE) for each transcript normalized to GAPDH (n = 5). Bands displayed above each bar graph are representative of each transcript and corresponding GAPDH. *Statistically significant difference between the two groups.
RESULTS
Characterization of PAH in Ren2 rats.
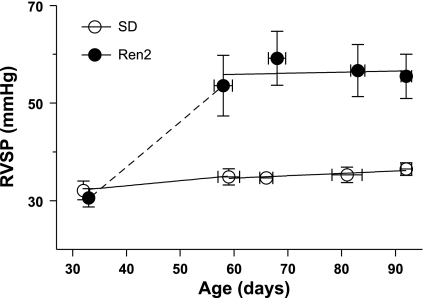
RVSP was measured in 5- to 13-wk-old male SD and heterozygous Ren2 rats (Fig. 1). The mean RVSP of 4.7-wk-old Ren2 rats was equal to SDC rats, but in Ren2 rats between 7 and 13 wk of age RVSP was 56 ± 3 mmHg, indicating that the pressure progressed from a normal range to sustained PAH between 5 and 7 wk of age. Regression lines for both strains indicated little change in RVSP between 7 and 13 wk of age.
Fig. 1.
Time course for the development of pulmonary hypertension in male transgenic (Ren2)27 rats (Ren2 rats). Right ventricular systolic pressures (RVSPs) of Sprague-Dawley (SD) and Ren2 rats are plotted versus age (n = 2–8 rats/age group). Values are means ± SE. Solid lines indicate best-fit regressions for 7- to 13-wk-old rats; dotted lines are estimates of RVSPs in Ren2 rats.
To exlude the possibility that PAH in the Ren2 rat is secondary to either LV dysfunction or hypoxia, we measured LV diastolic and systolic function using in vivo cine MRI and blood gases. No differences in LV diastolic (diastolic filling rate/EDV) or systolic (EF) function were apparent between 8-wk-old Ren2 and SD rats (Fig. 2). A noninferiority test indicated that EFs of SD and Ren2 rats were significantly similar (power = 0.842). Similarity could not be confirmed for diastolic filling rate/EDV. Representative video clips of cine MRI from a SD rat and a Ren2 rat are available online (Supplemental Fig. 1).1 Although no differences were found in ventricular function, the LV had already begun to hypertrophy by 8 wk of age in the Ren2 rat, and this was visable in the cine MRI. Blood gases of 12-wk-old Ren2 and SD rats were measured on arterial blood samples withdrawn from the carotid catheter using an iSTAT blood gas analyzer. The arterial Po2 was similar between Ren2 and SD rats (121 and 125 mmHg, respectively), suggesting that Ren2 rats are not hypoxemic.
mRNA transcript levels of the RAS were generally increased in the Ren2 rat compared with the SD rat (Fig. 3). AT1R and angiotensinogen were significantly increased in the Ren2 rat (P < 0.01 and 0.05, respectively), whereas ACE was not. These data support our previous results (6) showing that the Ren2 rat has increased ANG II signaling within the lungs and indicate that the Ren2 rat has an intrapulmonary RAS.
Effects of RSV on PAH.
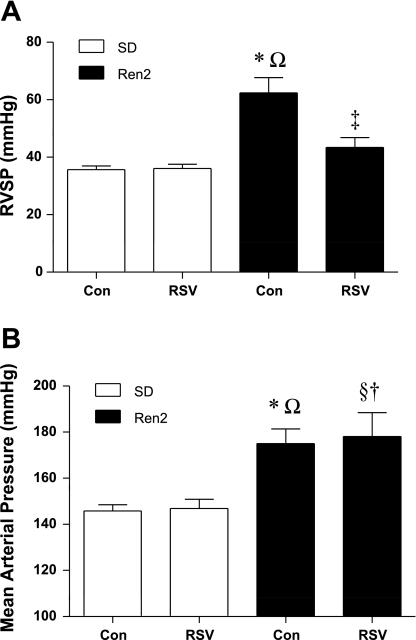
To test the hypothesis that RSV reduces PAH, we measured RVSP in anesthetized and ventilated 9-wk-old male SD and Ren2 rats. Figure 4 shows the RVSP (means ± SE) and MAP of control and RSV-treated Ren2 and SD rats. The Tukey-Kramer post hoc comparison indicated that the RVSP of R2C rats was elevated compared with SDC rats (P < 0.001). RVSP in R2R rats was significantly lower compared with R2C rats (P < 0.001) but was not different from SDC rats (P > 0.05). RVSP in SDR rats (n = 8) was significantly similar to SDC rats (36 ± 2 vs. 36 ± 1 mmHg, power = 0.93). Maximum RV dP/dt, a measure of the maximum rate of increase in RV pressure over time and an indicator of RV contractility, was elevated in Ren2 rats versus SD rats, and RV dP/dtmax decreased with RSV treatment in Ren2 rats (P < 0.05; Table 2). Similar trends were observed for RV dP/dtmin, an indicator of RV relaxation (Table 2). Two-way ANOVA could only detect a significant strain effect with regard to MAP (P < 0.05). Consistent with previous reports, MAP was elevated in male R2C rats compared with SDC rats (P < 0.001). RSV had no effect on MAP (P > 0.05): SDC rats compared with SDR rats were significantly similar (power = 0.99), and R2C rats were similar to R2R rats (power = 0.753).
Fig. 4.
Effects of rosuvastatin (RSV) on pulmonary and systemic blood pressure in SD and Ren2 rats. RSV reduced RVSP (A) but not mean arterial pressure (MAP; B) in Ren2 rats. Con, control. Rats were divided into the following groups: SD control (SDC), RSV-treated SD (SDR), Ren2 control (R2C), and RSV-treated Ren2 (R2R). Significant differences were determined via ANOVA with a Tukey-Kramer post hoc test. *P < 0.05, SDC vs. R2C; §P < 0.05, SDC vs. R2R; †P < 0.05, SDR vs. R2R; ΩP < 0.05, SDR vs. R2C; ‡P < 0.05, R2C vs. R2R.
Table 2.
Age, body weight, ventricular weights normalized to body weight, RV dP/dtmax, RV dP/dtmin, and heart rate in SDC, SDR, RC2, and R2R rats
| SDC | SDR | R2C | R2R | |
|---|---|---|---|---|
| Sample size | 9 | 9 | 7 | 7 |
| Age, days | 69.0±1.3 | 70.0±1.2 | 65.0±1.7a,e | 69.0±2.2f |
| Final weight, g | 312±18 | 264±6b,e | 312±18 | 280±10 |
| Left ventricle + septum/body weight, mg/g | 2.47±0.04 | 2.54±0.05 | 3.36±0.04a,e | 3.56±0.16c,d |
| Right ventricle/body weight, mg/g | 0.65±0.01 | 0.66±0.02 | 0.64±0.03 | 0.69±0.04 |
| RV dP/dtmax, mmHg/s | 861±35 | 799±35 | 1,379±122a,e | 1,024±81f |
| RV dP/dtmin, mmHg/s | −902±47 | −825±43 | −1,608±179a,e | −1,188±143f |
| Heart rate, beats/min | 358±6 | 335±14 | 377±9e | 381±15d |
Values are means ± SE. BW, body weight; RV, right ventricular. Rats were divided into the following groups: Sprague-Dawley control rats (SDC), rosuvastatin-treated Sprague-Dawley rats (SDR), transgenic (Ren2)27 control rats (R2C), and rosuvastatin-treated transgenic (Ren2)27 rats (R2R). Data were analyzed by two-factor ANOVA with Tukey-Kramer post hoc tests.
P < 0.05, SDC vs. R2C;
P < 0.05, SDC vs. SDR;
P < 0.05, SDC vs. R2R;
P < 0.05, SDR vs. R2R;
P < 0.05, SDR vs. R2C;
P < 0.05, R2C vs. R2R.
Effects of RSV on LV hypertrophy, RVH, and heart rate.
We (6) have previously reported LV hypertrophy (LVH), but not RVH, in 9-wk-old male Ren2 rats. In this study, we report similar findings in R2C rats compared with SDC rats (Table 2). Two-way ANOVA demonstrated significant strain and treatment effects for the LV + septum-to-body weight ratio (P < 0.05), indicating LVH in the Ren2 rats, as well as slightly greater LVH with RSV treatment. It should be noted that because the Ren2 rat exhibits LVH, the conventional use of the Fulton index to measure RVH [RV/(LV + septum)] would be misleading. Two-way ANOVA indicated that Ren2 rats had an elevated heart rate, as indicated by a significant strain effect (Table 2). Post hoc tests confirmed that the heart rate was only elevated in the R2C and R2R groups compared with the SDR group but not compared with the SDC group (Table 2).
Effects of RSV on pulmonary vascular remodeling.
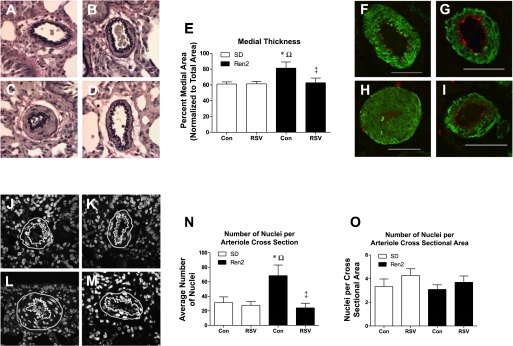
We (6) have previously reported medial thickening with concomitant luminal occlusion in the small pulmonary arteries of the Ren2 rat. Herein, we report similar findings. Representative sections of pulmonary arterioles indicated medial thickening in R2C rats compared with SDC, SDR, and R2R rats (Fig. 5, A–D). Post hoc tests confirmed medial layer thickening in R2C rats compared with SDC rats and normalization with RSV treatment (P < 0.05; Fig. 5E). Immunostaining with an antibody to α-smooth muscle actin (Fig. 5, F–I) indicated that the thickened medial layer of the pulmonary arterioles of Ren2 rats is comprised predominantly of SMCs (Fig. 5H). Also, the staining of the endothelial layer with an antibody to vonWillibrand Factor (Fig. 5, F–I) did not indicate that arteriolar wall thickening involved intimal hyperplasia of Ren2 arterioles (Fig. 5H). To test whether there was SMC proliferation or hypertrophy in the Ren2 rat, we counted the number of nuclei within cross sections of the pulmonary arteriolar medial layer via DAPI staining. Representative images are shown in Fig. 5, J–M; the nuclei count for each of these images was SDC = 29 (J), SDR = 28 (K), R2C = 65 (L), and R2R = 35 (M). The average total number of cells per cross section of small pulmonary arterioles of the R2C group was greater (P < 0.05) than in the SDC, SDR, and R2R groups (Fig. 5N); however, the cell number per unit cross-sectional area of arteriolar wall did not change among treated and untreated SD and Ren2 rats (Fig. 5O). This result suggests proliferation rather than hypertrophy as a mechanism for medial thickening. The normalization of cell number in the R2R group suggests that RSV inhibits cell proliferation.
Fig. 5.
Microscopic analysis of small pulmonary arterioles from control and RSV-treated SD and Ren2 rats. A–E: Verhoeff vanGeison-stained SDC (A), SDR (B), R2C (C), and R2R (D) small pulmonary arterioles were quantified for medial (E) and lumen thickness; the medial area was plotted as a percentage of the total arteriole area (media + lumen, n ≥ 4). F–I: representative small pulmonary arterioles from SDC (F), SDR (G), R2C (H), and R2R (I) were imaged by confocal microscopy for α-smooth muscle actin (green) and endothelial cell von Willibrand factor (red). Scale bars = 50 μm. J–M: confocal imaging was also used to count 4′,6-diamidino-2-phenylindole-stained nuclei; representative images of small pulmonary arterioles from SDC (J), SDR (K), R2C (L), and R2R (M) are shown. The white lines in J–M trace the outer and inner medial boundaries. N: average total number of nuclei in a cross section of the medial layer, that is, between the white lines. O: average number of nuclei divided by the cross-sectional area of the medial layer (n = 5). Significant differences were determined via ANOVA with a Tukey-Kramer post hoc test. *P < 0.05, SDC vs. R2C; ΩP < 0.05, SDR vs. R2C; ‡P < 0.05, R2C vs. R2R.
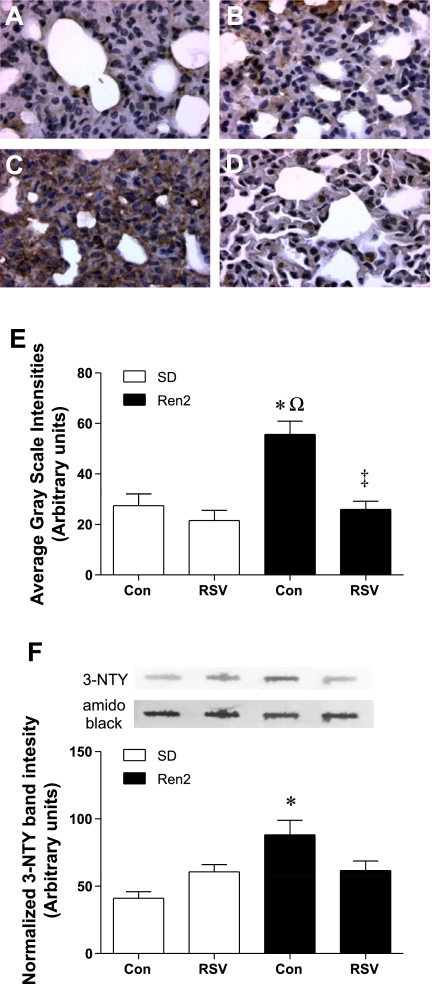
Effects of RSV on lung 3-nitrotyrosine content.
Lung 3-nitrotyrosine content, a marker of peroxynitrite, was evaluated by immunohistochemistry (Fig. 6, A–D), and the images were analyzed for staining intensity (Fig. 6E). Lung 3-nitrotyrosine content was significantly higher in R2C rats compared with SDC rats (P < 0.001), and treatment with RSV attenuated the increase in R2R rats (P < 0.001; Fig. 6E). To validate the immunostaining result, an immunoblot assay was performed using a slot blot (Fig. 6F). Two-way ANOVA demonstrated significant strain and interaction effects for 3-nitrotyrosine content (P < 0.05). Nitrotyrosine levels in the R2R lung did not differ from either SDC or R2C lungs, indicating a partial reduction in 3-nitrotyrosine with RSV treatment.
Fig. 6.
Effects of RSV on pulmonary 3-nitrotyrosine (3-NTY) in SD and Ren2 rats. A–D: representative micrographs of lung sections from SDC (A), SDR (B), R2C (C), and R2R (D) rats stained for 3-NTY. E: average grayscale intensity (±SE) of the 3-NTY immunostaining (n = 4). F: representative lanes from a slot blot of whole lung homogenates probed for 3-NTY and normalized to total protein by amido black staining are shown over a histogram quantifying 3-NTY (n ≥ 4). Significant differences were determined via ANOVA with a Tukey-Kramer post hoc test. *P < 0.05, SDC vs. R2C; ΩP < 0.05, SDR vs. R2C; ‡P < 0.05, R2C vs. R2R.
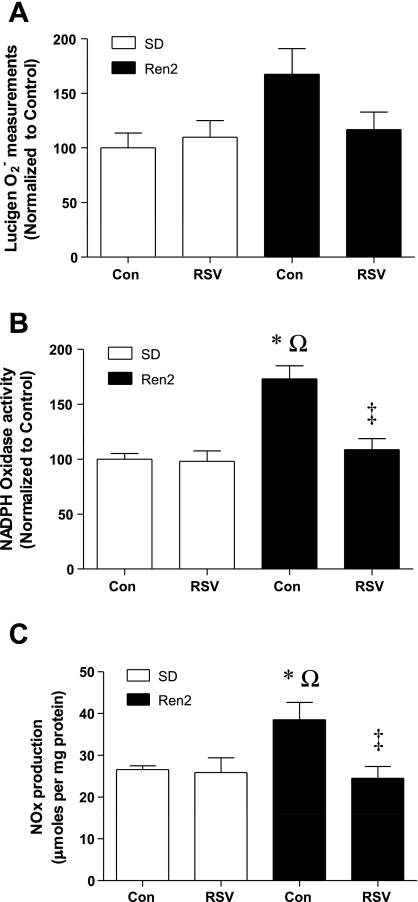
Effects of RSV on superoxide, NADPH oxidase activity, and NOx.
Estimates of superoxide levels were evaluated by the lucigenin chemiluminescence assay (Fig. 7A). Although main effects were not significant using two-way ANOVA, lung superoxide levels tended to be higher in the R2C group compared with the SDC group (168 ± 23% vs. 100 ± 14%, P = 0.0505). Adjustment of the significance cutoff of the two-way ANOVA to α = 0.051 yielded a significant strain effect, indicating greater superoxide levels in Ren2 lungs compared with SD lungs. RSV tended to blunt superoxide in the R2R group compared with the R2C group (117 ± 16% vs. 168 ± 23%). To confirm these results, NADPH oxidase activity was measured. NADPH oxidase activity was significantly higher in lung plasma membrane extracts of the R2C group compared with the SDC group (P < 0.001; Fig. 7B), and treatment with RSV attenuated the increased enzyme activity in the R2R group (P < 0.05).
Fig. 7.
Effects of RSV on ROS and nitric oxide (NO) production in the lungs of SD and Ren2 rats. A: lucigenin was used to measure superoxide (O2−) from fresh lung samples. B: NADPH oxidase-specific assays were also preformed (n ≥ 7). C: the sum of stable NO metabolites [nitrate and nitrite (NOx)] was measured in lung homogenates via the Griess reaction (n ≥ 5). Significant differences were determined via ANOVA with a Tukey-Kramer post hoc test. *P < 0.05, SDC vs. R2C; ΩP < 0.05, SDR vs. R2C; ‡P < 0.05, R2C vs. R2R.
NO levels were evaluated by the Griess assay, which detects NOx (Fig. 7C). NOx was greater in the R2C group than the SDC, SDR, and R2R groups (P < 0.05 for each paired comparison; Fig. 7C).
Effects of RSV on intrapulmonary expression of NOX2 and Rac1.
To examine the effects of RSV on the intrapulmonary expression of NADPH oxidase subunits NOX2 and Rac1, we performed semiquantitative immunohistochemical analyses targeted to small pulmonary arteries. Signal intensities of Rac1 and NOX2 (P < 0.05), normalized to the surface area of the vascular wall, i.e., the area between the outer elastic lamina and luminal interface, were increased in small pulmonary arteries of the R2C group compared with the SDC group (Supplemental Fig. 2). RSV had no effect on the expression of these two proteins in either rat strain. In contrast, an immunoblot analysis of whole lung homogenates did not detect differences in the protein expression of NOX2 or Rac1 among all groups (Supplemental Fig. 2).
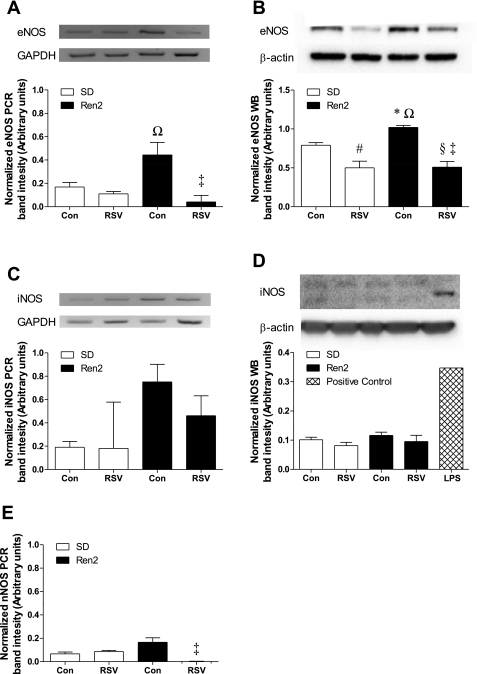
Effects of RSV on lung NOS expression.
Since the increased nitrotyrosine requires NO as well as superoxide, NOS isoform expression was examined by immunoblot analysis and RT-PCR on whole lung homogenates. eNOS transcript levels, normalized to GAPDH transcript levels, were significantly elevated in the R2C group compared with the SDR and R2R groups (P < 0.05; Fig. 8A) and tended to be higher in the R2C group compared with the SDC group. Lung eNOS protein, normalized to β-actin and expressed as arbitrary units (Fig. 8B), was significantly increased in the R2C group compared with the SDC group (P < 0.05). The R2R group had lower eNOS protein than the R2C group (P < 0.001) as well as lower eNOS protein compared with the SDC group (P < 0.05). iNOS transcript levels were more variable among the four treatment groups, and, as a consequence, we could not detect significant changes overall (Fig. 8C). However, iNOS protein levels were nearly undetectable in all groups (Fig. 8D). nNOS transcript levels were very low compared with eNOS and iNOS levels. nNOS was barely detectable in the R2R group and, as such, was significantly lower than in the R2C group (P < 0.05; Fig. 8E). Given the low transcript levels of nNOS, we did not probe for nNOS protein.
Fig. 8.
Effects of RSV on pulmonary NO synthase (NOS) isoform expression in SD and Ren2 rats. A–E: pulmonary mRNA and protein were examined via RT-PCR (A, C, and E) and Westen blot analysis (B and D), respectively, for endothelial NOS (eNOS; A and B), inducible NOS (iNOS; C and D), and neuronal NOS (nNOS; E). All analyses were conducted on the same gel/blot; representative blots are shown above each histogram except for nNOS, which was barely detectable, and aligned to match the labels for the histogram (n ≥ 3). Significant differences were determined via ANOVA with a Tukey-Kramer post hoc test. *P < 0.05, SDC vs. R2C; #P < 0.05, SDC vs. SDR; §P < 0.05, SDC vs. R2R; ΩP < 0.05, SDR vs. R2C; ‡P < 0.05, R2C vs. R2R.
DISCUSSION
There were three major findings of this study. First, treatment of heterozygous male transgenic Ren2 rats with RSV prevented the development of PAH. Second, Ren2 rats begin to develop PAH between 5 and 7 wk of age, and, although there were the beginnings of LVH, LV systolic and diastolic function as well as arterial blood gases were normal. Third, in addition to expressing the mouse renin gene (6), lungs of the Ren2 rat also have elevated expression of other RAS components, such as the AT1R and angiotensinogen. These findings suggests that the Ren2 model of PAH is not secondary to left heart disease or hypoxia and is likely due to an activated intrapulmonary RAS.
We and others have shown that the Ren2 rat is characterized by increased local synthesis of ANG II (2), which promotes NADPH oxidase-mediated oxidative stress (6, 16, 40, 50–54). We (6) have recently reported that the lungs of the Ren2 rat exhibit increased superoxide levels in concert with elevations in NADPH oxidase activity and the expression of NOX2 and Rac1. Thus, our previous work supports the hypothesis that oxidative stress plays a role in the development of PAH in the Ren2 model, similar to other models of PH (8, 14, 17, 19, 22). In this study, we report that the in vivo administration of RSV attenuates PAH and pulmonary vascular remodeling in the Ren2 rat. It is important to note that in this study and a previous report (16) we have shown that RSV fails to reduce systemic hypertension in the Ren2 rat. Additionally, RSV did not reduce LVH, suggesting that the early LVH is most likely due to blood pressure, which was not altered by RSV. This is consistent with a recent report (4) showing that RSV does not ameliorate hypertension-induced LVH and has limited value in the treatment of progressing heart failure. Thus, the beneficial effects of RSV seen in this study may result from direct actions within the lung and/or vascular wall of the pulmonary circulation. To that end, we show that RSV blunts protein nitration, and this is likely due to the suppression of peroxynitrite formation. In support of this, the lungs of RSV-treated rats have more normal levels of both NO and superoxide, which could directly result in lower levels of 3-nitrotyrosine, as observed.
ROS in animal models of PH.
The Ren2 rat exhibits the two principal pathological features in the pulmonary vasculature common to most forms of PH, which include excessive vasoconstriction and remodeling of the pulmonary arteriolar wall, primarily by a mechanism of SMC proliferation within the medial layer. ROS may promote vasoconstriction and vascular remodeling and, thus, may play a critical role in many forms of PH. In a mouse model of chronic hypoxia-induced PH (CH-PH), intrapulmonary artery superoxide levels were elevated (19, 32). Moreover, the pathological changes associated with exposure to chronic hypoxia, i.e., increased intrapulmonary artery superoxide, increased pulmonary artery pressure, RVH, and pulmonary vascular remodeling, are abolished in NOX2 knockout mice, demonstrating a critical role for superoxide generated by NOX2-containing NADPH oxidases (32). The rat monocrotaline model of PAH is also characterized by elevated intrapulmonary superoxide, which can be ameliorated by intratracheal gene transfer of the superoxide scavenger extracellular SOD (22). Whether the intrapulmonary oxidative stress in the monocrotaline model is due to NADPH oxidase-induced superoxide synthesis, other superoxide-generating systems, or deficiencies in antioxidant capacity, alone or in combination, is uncertain at this point. In two lamb models of persistent PH of the newborn caused by either prenatal placement of an aortopulmonary shunt (14) or ductal ligation (1), superoxide levels became elevated in pulmonary arterioles. Superoxide is the primary oxidant responsible for oxidative stress in these models and is derived mainly from NADPH oxidase and secondarily from uncoupled eNOS. Thus, it is increasingly apparent that the activation of intrapulmonary superoxide-generating systems, especially NADPH oxidases, play a key role in the development of diverse forms of PH, including the Ren2 rat.
Many of the therapeutic benefits of statins in the vasculature are the likely consequence of reduced synthesis of superoxide, which could result in more positive regulation of cell proliferation, apoptosis, growth, migration, inflammation, extracellular matrix synthesis and degradation, differentiation, and contraction (5). Statins are associated with the decreased expression of some of the NADPH oxidase subunit mRNAs and proteins as well as the increased expression of antioxidants, such as catalase (49) or heme oxygenase-1 (20). Indeed, most statins, including RSV, increase lung heme oxygenase activity in adult mice (20). We have recently reported that RSV decreases the expression of the NADPH oxidase subunit proteins NOX2, p22phox, p40phox, and Rac1 in the Ren2 myocardium. Given the potential of statins to reduce oxidative stress and the associated downstream consequences, this class of drugs would be expected to be beneficial for the treatment of PAH. In this study, we showed that the elevated activity of NADPH oxidase, the increased nitrotyrosine content, and increased superoxide and NO in the Ren2 lung are normalized after treatment with RSV. Unlike the Ren2 myocardium, the antioxidant properties of statins in the lung do not appear to result from decreases in the protein expression of NOX2 or Rac1. It is likely that the decrease in intrapulmonary NADPH oxidase activity observed in the Ren2 rat treated with RSV is due to blocking Rac1 geranylation and consequently reducing the ability of Rac1 to translocate to the membrane and signal properly (28, 44, 48). Further studies are required to support the role of Rac1 geranylation in this model.
Efficacy of statins in PH.
Recent evidence from other rat models of PAH have also demonstrated the efficacy of statins for the treatment of PH. Simvastatin attenuates the development of PH, pulmonary vascular remodeling, and RVH in rats with CH-PH (10), and similar results were obtained with rosuvastatin in the rat monocrotaline model of PAH (42). Simvastatin also reverses a more severe form of PAH caused by the combination of monocrotaline plus pneumonectomy (35). Whether simvastatin or RSV reduced oxidative stress in these rodent models of PH was not examined. It would also be of value to test whether other commonly prescribed statins are as effective at reducing PH as simvastatin and RSV. A recent observational study (23) of adjunctive simvastatin therapy in patients with PH suggested functional improvements in symptoms. These encouraging animal and human studies warrant further clinical studies to test the efficacy of adjunctive statin therapy for the treatment of PH.
Statins exhibit multiple beneficial effects in the vasculature (for a review, see Ref. 43). One such effect is to improve endothelial function by improving the function of eNOS. The end product of eNOS activity, NO, protects the vasculature by promoting vasodilation and inhibiting platelet aggregation, leukocyte adhesion, and SMC proliferation. Thus, factors that limit the synthesis or bioavailabilty of the NO effect on endothelial function are of potential significance in the development of PH. Unlike the systemic vasculature, the basal synthesis of NO in the pulmonary vasculature of healthy rats is low and may be of little physiological importance in regulating the normally low tone in the pulmonary vasculature (for reviews, see Refs. 7 and 18). Multiple lines of evidence suggest that NO synthesis within the pulmonary vasculature increases with the development of pulmonary hypertensive states (9, 21, 37, 46) to offset the effects of increasing levels of potent vasoconstrictors such as endothelin-1 and ANG II. In fact, ANG II stimulates increased expression of eNOS and NO synthesis in pulmonary artery endothelial cells but not in coronary artery endothelial cells (38). These effects are mediated through angiotensin type 2 receptors and downstream activation of the ERK1/2 pathway (31). Thus, we speculate that in the Ren2 model, an activated intrapulmonary RAS promotes eNOS expression and NO synthesis. It should be noted that the observed increase in NOx in the Ren2 lung also reflects an increase in peroxynitrite, which is formed when superoxide combines with NO, because peroxynitrite decomposes into nitrite. Given the high level of oxidative stress in the Ren2 lung, it is likely that some of the increased NOx detected in the Ren2 lung represents peroxynitrite formation. The increased 3-nitrotyrosine immunostaining in the Ren2 lung supports the notion that peroxynitrite formation is increased in the Ren2 lung.
Interestingly, RSV therapy normalizes, rather than increases, eNOS expression and NO synthesis in the Ren2 rat, a result that may seem counterintuitive. Indeed, statins have been reported to enhance eNOS expression in monocrotaline-induced PH, where eNOS protein becomes deficient (15, 34). On the other hand, in established CH-PH, where eNOS expression increases, statin therapy reduces eNOS expression while enhancing NO synthesis (33). In CH-PH, statins enhance eNOS activity by decreasing eNOS coupling with caveolin-1, increasing interactions with heat shock protein 90, and increasing the phosphorylation of eNOS at Ser1177 (33). Despite decreases in eNOS protein after RSV treatment in both the SD and Ren2 lung, NO production is maintained at the same level as in the SDC lung. Thus, we speculate that the therapeutic effects of RSV in the Ren2 lung are related to reduced scavenging of NO by NADPH oxidase-generated superoxide as well as by the enhancement of posttranslational modifications that lead to balancing the reduced expression of eNOS protein with an increase in eNOS activity resulting in normal NO synthesis. This scenario would also result in less decoupling of eNOS, which is a potent source of superoxide. Further study is needed to determine whether statins regulate eNOS activity in the Ren2 lung through alterations in posttranslational modifications. It should be noted that the Ren2 rat is not hypoxemic, so any similarities in eNOS protein expression with the CH-PH rat are not due to hypoxia in the Ren2 rat.
It is intriguing that RSV reduced pulmonary but not systemic pressure in the Ren2 rat. In a previous study (16) using the same dose and duration of treatment of RSV, we also failed to detect a decrease in systemic blood pressure in 9-wk-old hypertensive male Ren2 rats. In humans, a meta-analysis of 20 clinical trials showed a small but statistically significant reduction in systolic pressure and a trend toward reduced diastolic blood pressure with statin treatment. Few studies have directly examined whether statins lower blood pressure in hypertensive rats. In one study (24), a high dose of atorvastatin (50 mg·kg−1·day−1 for 30 days) decreased systolic blood pressure in stroke-prone spontaneously hypertensive rats, in part, by decreasing sympathetic nervous system activity (24). Whether higher doses or a longer period of treatment with RSV could lower systemic blood pressure in Ren2 rats or in other rodent models of hypertension is unknown. Additionally, it is not clear that statins reduce blood pressure in all forms of hypertension. Thus, in the Ren2 rat, statins may be ineffective at lowering blood pressure.
In this study, we showed that pulmonary arterioles of Ren2 rats have a thickened medial layer due to an increase in number, but not density, of SMCs (Fig. 5, N and O). This raises the question of whether RSV directly inhibits SMC proliferation in the pulmonary vasculature. It has been established that statins inhibit SMC proliferation by inhibiting Rho (29) and that Rho kinase inhibitors ameliorate PH (36). Indeed, simvistatin reduces the proliferation and increases the apoptosis of neointimal and medial SMCs in pulmonary arteries or rats with PAH (35). Thus, it is possible that RSV prevented the medial thickening of pulmonary arterioles, in part, by reducing Rho-dependent SMC proliferation. More study is needed to determine the specific mechanism of RSV action on proliferation and apoptosis in SMCs in pulmonary arterioles.
In conclusion, this study extends the results of our recent report that proposed that an activated intrapulmonary RAS promotes ANG II-induced NADPH oxidase-mediated oxidative stress leading to PAH and pulmonary vascular remodeling in male heterozygous transgenic Ren2 rats. In the previous study, we verified the intrapulmonary expression of the murine renin transgene, and, here, we show that the Ren2 lung exhibits an increased expression of additional RAS genes, e.g., AT1R and angiotensinogen, important for local ANG II synthesis. Importantly, we confirm that the underlying cause of PAH in the Ren2 model cannot be attributed specifically to LV dysfunction or hypoxia, which are common risk factors for PH. Herein, we observed elevated levels of 3-nitrotyrosine in the lungs of Ren2 rats. We also observed elevated NADPH oxidase activity and superoxide levels as well as increased eNOS expression and total NOx, an estimate of NO levels. The increased levels of superoxide and NO could lead to the formation of pathological levels of peroxynitrite, which likely explains the observed high levels of 3-nitrotyrosine in the lung. Treatment with RSV ameliorated PAH and pulmonary vascular remodeling, and this was associated with the normalization of lung 3-nitrotyrosine, superoxide, and NO. Thus, the salutary effects of RSV may be related to the beneficial regulation of NADPH oxidase and eNOS, which results in reduced oxidative stress and protein nitration. This supports previous clinical data (23) showing that statins may be beneficial in the treatment of PAH.
GRANTS
This research was supported by the Children's Miracle Network (to V. G. DeMarco), the University of Missouri Research Council (to V. G. DeMarco), National Heart, Lung, and Blood Institute Grant R01-HL-73101-01A1 (to J. R. Sowers), Veterans Affairs Merit System Grant 0018 (to J. R. Sowers) and Veterans Affairs Grant VISN 15 (to A. T. Whaley-Connell and B. T. Andresen), the Missouri Kidney Program (to A. T. Whaley-Connell), and AstraZeneca Pharmaceuticals (to J. R. Sowers).
Acknowledgments
The authors thank Tammy Strawn, Matthew Morris, and Nathan Rehmer for technical assistance.
Footnotes
Supplemental Material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat. Hypertension 25: 1014–1020, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 102: 9056–9061, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang SA, Kim YJ, Lee HW, Kim DH, Kim HK, Chang HJ, Sohn DW, Oh BH, Park YB. Effect of rosuvastatin on cardiac remodeling, function, and progression to heart failure in hypertensive heart with established left ventricular hypertrophy. Hypertension. In press. [DOI] [PubMed]
- 5.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res 71: 216–225, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol 294: H2659–H2668, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Fagan KA, McMurtry I, Rodman DM. Nitric oxide synthase in pulmonary hypertension: lessons from knockout mice. Physiol Res 49: 539–548, 2000. [PubMed] [Google Scholar]
- 8.Farahmand F, Hill MF, Singal PK. Antioxidant and oxidative stress changes in experimental cor pulmonale. Mol Cell Biochem 260: 21–29, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Frasch HF, Marshall C, Marshall BE. Endothelin-1 is elevated in monocrotaline pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 276: L304–L310, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Girgis RE, Li D, Zhan X, Garcia JG, Tuder RM, Hassoun PM, Johns RA. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol 285: H938–H945, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH. Reduction in blood pressure with statins: results from the UCSD Statin study, a randomized trial. Arch Intern Med 168: 721–727, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept 91: 21–27, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 290: L1069–L1077, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Guerard P, Rakotoniaina Z, Goirand F, Rochette L, Dumas M, Lirussi F, Bardou M. The HMG-CoA reductase inhibitor, pravastatin, prevents the development of monocrotaline-induced pulmonary hypertension in the rat through reduction of endothelial cell apoptosis and overexpression of eNOS. Naunyn Schmiedebergs Arch Pharmacol 373: 401–414, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Habibi J, Whaley-Connell A, Qazi MA, Hayden MR, Cooper SA, Tramontano A, Thyfault J, Stump C, Ferrario C, Muniyappa R, Sowers JR. Rosuvastatin, a HMG-CoA reductase inhibitor, decreases cardiac oxidative stress and remodeling in Ren2 transgenic rats. Endocrinology 148: 2181–2188, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hampl V, Bibova J, Banasova A, Uhlik J, Mikova D, Hnilickova O, Lachmanova V, Herget J. Pulmonary vascular iNOS induction participates in the onset of chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 290: L11–L20, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev 80: 1337–1372, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Hoshikawa Y, Ono S, Suzuki S, Tanita T, Chida M, Song C, Noda M, Tabata T, Voelkel NF, Fujimura S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol 90: 1299–1306, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Hsu M, Muchova L, Morioka I, Wong RJ, Schroder H, Stevenson DK. Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem Biophys Res Commun 343: 738–744, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Igari H, Tatsumi K, Sugito K, Kasahara Y, Saito M, Tani T, Kimura H, Kuriyama T. Role of EDRF in pulmonary circulation during sustained hypoxia. J Cardiovasc Pharmacol 31: 299–305, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med 177: 219–226, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Kao PN. Simvastatin treatment of pulmonary hypertension: an observational case series. Chest 127: 1446–1452, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kishi T, Hirooka Y, Mukai Y, Shimokawa H, Takeshita A. Atorvastatin causes depressor and sympatho-inhibitory effects with upregulation of nitric oxide synthases in stroke-prone spontaneously hypertensive rats. J Hypertens 21: 379–386, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fischer D, Manes C, Mueller M, Drexler H. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol 27: 943–948, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Laufs U, Kilter H, Konkol C, Wassmann S, Bohm M, Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc Res 53: 911–920, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1). J Biol Chem 274: 21926–21931, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Lew MJ. Principles: when there should be no difference–how to fail to reject the null hypothesis. Trends Pharmacol Sci 27: 274–278, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Zhao X, Li X, Lerea KM, Olson SC. Angiotensin II type 2 receptor-dependent increases in nitric oxide synthase expression in the pulmonary endothelium is mediated via a Gαi3/Ras/Raf/MAPK pathway. Am J Physiol Cell Physiol 292: C2185–C2196, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Murata T, Kinoshita K, Hori M, Kuwahara M, Tsubone H, Karaki H, Ozaki H. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol 25: 2335–2342, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura T, Faul JL, Berry GJ, Vaszar LT, Qiu D, Pearl RG, Kao PN. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med 166: 1403–1408, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 108: 1640–1645, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol 155: 444–454, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oka M, Hasunuma K, Webb SA, Stelzner TJ, Rodman DM, McMurtry IF. EDRF suppresses an unidentified vasoconstrictor mechanism in hypertensive rat lungs. Am J Physiol Lung Cell Mol Physiol 264: L587–L597, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Olson SC, Dowds TA, Pino PA, Barry MT, Burke-Wolin T. ANG II stimulates endothelial nitric oxide synthase expression in bovine pulmonary artery endothelium. Am J Physiol Lung Cell Mol Physiol 273: L315–L321, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Skimming JW, Nasiroglu O, Huang CJ, Wood CE, Stevens BR, Haque IU, Scumpia PO, Sarcia PJ. Dexamethasone suppresses iNOS yet induces GTPCH and CAT-2 mRNA expression in rat lungs. Am J Physiol Lung Cell Mol Physiol 285: L484–L491, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of NADPH oxidase and cardiac remodeling. Endocrinology 148: 3773–3780, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Strazzullo P, Kerry SM, Barbato A, Versiero M, D'Elia L, Cappuccio FP. Do statins reduce blood pressure?: a meta-analysis of randomized, controlled trials. Hypertension 49: 792–798, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Ku DD. Rosuvastatin provides pleiotropic protection against pulmonary hypertension, right ventricular hypertrophy, and coronary endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol 294: H801–H809, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 21: 1712–1719, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, Kitakaze M, Liao JK. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest 108: 1429–1437, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toba H, Miki S, Shimizu T, Yoshimura A, Inoue R, Sawai N, Tsukamoto R, Murakami M, Morita Y, Nakayama Y, Kobara M, Nakata T. The direct antioxidative and anti-inflammatory effects of peroxisome proliferator-activated receptors ligands are associated with the inhibition of angiotensin converting enzyme expression in streptozotocin-induced diabetic rat aorta. Eur J Pharmacol 549: 124–132, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Tyler RC, Muramatsu M, Abman SH, Stelzner TJ, Rodman DM, Bloch KD, McMurtry IF. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 276: L297–L303, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Vaughan CJ, Gotto AM Jr, Basson CT. The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol 35: 1–10, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Wassmann S, Laufs U, Baumer AT, Muller K, Konkol C, Sauer H, Bohm M, Nickenig G. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol 59: 646–654, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, Linz W, Bohm M, Nickenig G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol 22: 300–305, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension 50: 384–391, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Whaley-Connell A, Chowdhury N, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, Gallagher PE, Tallant EA, Cooper SA, Link CD, Ferrario CM, Sowers JR. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Renal Physiol 291: F1308–F1314, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump CS, Ferrario CM, Sowers JR. Angiotensin-II mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355–E363, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Whaley-Connell A, Habibi J, Cooper SA, DeMarco VG, Hayden MR, Stump CS, Link D, Ferrario C, Sowers JR. Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab 295: E103–E109, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51: 474–480, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang MZ, Yao B, Cheng HF, Wang SW, Inagami T, Harris RC. Renal cortical cyclooxygenase 2 expression is differentially regulated by angiotensin II AT1 and AT2 receptors. Proc Natl Acad Sci USA 103: 16045–16050, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]