Abstract
Flow-mediated dilatation (%FMD), an index of nitric oxide (NO)-mediated vasodilator function, is regarded as a surrogate marker of cardiovascular disease. Aging is associated with endothelial dysfunction, but underlying sex-related differences may exist and the effects of fitness and exercise on endothelial dysfunction in men (M) and women (W) are poorly understood. We compared %FMD of the brachial artery in 18 young [Y, 26 ± 1 yr; 9 M and 9 W], 12 older fit (OF, 57 ± 2 yr; 6 M and 6 W), and 16 older sedentary (OS, 59 ± 2 yr; 8 M and 8 W) subjects. Glyceryl trinitrate (GTN) administration was used to assess endothelium-independent vasodilatation, and the FMD-to-GTN ratio was calculated to characterize NO dilator function in the context of smooth muscle cell sensitivity. Brachial %FMD in Y (7.1 ± 0.8%) was significantly higher compared with OS (4.8 ± 0.7%, P < 0.05), but not OF (6.4 ± 0.7%). Differences between Y and OS subjects were due primarily to lower FMD in the OS women (4.3 ± 0.6%). OS women exhibited significantly lower FMD-to-GTN ratios compared with Y (P < 0.05) and OF women (P < 0.05), whereas these differences were not apparent in men. Exercise training improved brachial artery NO dilator function (FMD-to-GTN ratio) after 24 wk (P < 0.05) in OS women, but not men. These findings indicate that maintaining a high level of fitness, or undertaking exercise training, prevents the age-related decline in the brachial artery vasodilator function evident in women. In OS men, who had relatively preserved NO dilator function, no training adaptations were observed. This study has potential implications for the prevention of conduit artery endothelial dysfunction in men and women.
Keywords: fitness, exercise training, upper-limb nitric oxide dilator function
flow-mediated dilatation (FMD) serves as an index of nitric oxide (NO)-mediated endothelium-dependent vasodilator function in humans (19, 31) and is regarded as a surrogate marker of cardiovascular disease (49). Experimental and clinical studies suggest that endothelial dysfunction is an important feature of a number of cardiovascular disorders, including atherosclerosis (37), hypertension (43), and coronary and peripheral artery disease (4, 54, 55), and endothelial dysfunction predicts cardiovascular events in these groups (51).
Advancing age is associated with a generalized endothelial dysfunction (5, 14–16, 25, 33, 42) and an increased incidence of cardiovascular disease (39). Recent studies that have used a more contemporary methodology (35) indicate that brachial artery function may be preserved in older men, compared with younger men (53), whereas older women exhibit attenuated brachial responses relative to younger women (34). These findings are reinforced by Jensen-Urstad and Johannsson (26) who directly compared the brachial artery FMD in younger and older men as well as in women. Overall, these studies suggest that sex-related differences may exist in endothelial function as humans age, with older women demonstrating a more pronounced change in endothelial function. In this study we examined the potential sex differences without the confounding effects of differences in ultrasound equipment and testing protocols that markedly vary within the literature (28).
Exercise training typically improves arterial function in subjects with cardiovascular risk factors or diseases (20), including resistance vessel function in subjects of advanced age (11, 41). However, previous investigations of training-induced adaptations of conduit arteries in older healthy populations have proven inconsistent. For example, 6 wk of knee extensor exercise training improved brachial artery FMD in six older men (53), whereas another study in older sedentary men (n = 8) found that 8 wk of cycle exercise induced no improvements in FMD (47). This is despite evidence from cross-sectional studies indicating that both older fit men and women have greater FMD compared with older sedentary subjects (21, 36, 38). The impact of both fitness and exercise training on conduit artery function in older humans and the potential sex-related differences upon exercise training have not been previously documented in a single study.
We hypothesized that the magnitude of age-related attenuation in vascular function would be greater in older sedentary women than in older sedentary men, whereas the maintenance of cardiorespiratory fitness in older men and women would preserve brachial artery FMD. We also hypothesized that 6 mo of exercise training in sedentary older subjects would enhance brachial artery endothelial function, primarily in those subjects with a larger age-related attenuation in vascular function (e.g., women).
METHODS
Subjects
Eighteen healthy, young, recreationally active volunteers [Y: 9 men (M), 26.1 ± 1.1; and 9 women (W), 26.1 ± 1.2 yr], 16 healthy older sedentary (OS: 8 M, 58.5 ± 1.6; and 8 W, 60.0 ± 1.8 yr), and 12 age-matched older fit (OF) subjects (6 M, 57.3 ± 1.8; and 6 W, 57.2 ± 1.5 yr) (Table 1) were recruited from the community. All older women were postmenopausal, whereas premenopausal females were tested during the early follicular phase of their menstrual cycle. The study was approved by the Liverpool John Moores University Ethics Committee, and subjects provided written informed consent before participation. This conformed to the standards set out by the declaration of Helsinki.
Table 1.
Characteristics of healthy young and healthy older fit and older sedentary subjects
|
Young |
Older Fit
|
Older Sedentary
|
||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| n | 9 | 9 | 6 | 6 | 8 | 8 |
| Age, yr | 26±1 | 26±1 | 57±2* | 57±2* | 59±2* | 60±2* |
| SBP, mmHg | 114±2 | 101±4 | 122±5 | 119±8* | 125±6* | 122±5* |
| DBP, mmHg | 72±3 | 56±3 | 74±6 | 69±3* | 73±3 | 67±3* |
| BMI, kg/m2 | 23±1 | 23±1 | 24±1 | 23±2 | 29±2*† | 31±2*† |
| %Body fat (DEXA) | 13±1 | 30±3 | 17±2 | 28±3 | 25±1*† | 39±2*† |
| Peak oxygen uptake, ml·min−1·kg−1 | 56±2 | 42±3 | 52±4 | 42±2 | 32±2*† | 24±1*† |
| Baseline diameter, mm | 4.2±0.2 | 3.3±0.02 | 4.7±0.3 | 3.3±0.3 | 4.8±0.2* | 3.6±0.2 |
| AUCSR, s × 103 | 16.9±3.1 | 19.0±3.7 | 15.4±3.2 | 25.8±3.4 | 16.3±2.3 | 24.1±3.9 |
Values are means ± SE; n, number of subjects. SBP, systolic blood pressure; DBP, distolic blood pressure; BMI, body mass index; DEXA, dual-energy X-ray absorptiometry; AUCSR, shear rate area under the curve.
P < 0.05, post hoc t-test significantly different from sex-matched young subjects (n = 18);
P < 0.05, post hoc t-test significantly different between sex-matched fit (n = 12) and sedentary older subjects (n = 16).
Experimental Design
Participants reported to the laboratory for an assessment of brachial artery FMD and endothelium-independent, glyceryl trinitrate (GTN)-mediated dilatation. All measurements were performed under standardized conditions following a 6-h fast and an 8-h abstinence from caffeine and/or alcohol and at least 24 h after strenuous physical activity. Healthy controls were defined as individuals undertaking <3 h of regular exercise per week at a recreational level, whereas the older sedentary group reported undertaking no regular exercise. The older fit group consisted of runners who regularly participated in half-marathon and marathon distances. All participants were screened for cardiac abnormalities and cardiovascular disease before entering the study. Subjects were specifically questioned regarding any history of hypercholesterolemia, hypertension, insulin resistance, and diabetes. Those who smoked, had a family history of premature coronary disease, or were on medications of any type, including oral contraceptives and hormone replacement therapy, were also excluded, as were individuals taking vitamin or any other supplements.
Experimental Procedures
Brachial artery vascular function measurements.
A rapid inflation/deflation pneumatic cuff was positioned on the imaged arm immediately distal to the olecranon process to provide a stimulus to forearm ischemia. A 10-MHz linear array transducer attached to a high-resolution ultrasound machine (Aspen, Acuson; Mountain View, CA) was used to image the brachial artery in the distal third of the upper arm, proximal to the cuff position.
Perpendicular incidence of the imaging ultrasound beam in relation to the orientation of the vessel provided clearly demarcated intimal-medial boundaries, which were optimized using contrast controls on the ultrasound machine. The insonation angle between the time-shared pulsed-wave Doppler beam and the vessel walls was adjusted by transducer manipulation, known as “heel toeing,” to allow the beam to be steered and the angle corrected in alignment with the vessel orientation and blood flow axis at a discrete segment of vessel ≤60°, as illustrated in a previous study (2).
After an initial 20-min resting period, baseline scans assessing resting vessel diameter and blood flow velocity estimates were recorded over 1 min. The cuff was then inflated to >200 mmHg for 5 min. Arterial diameter and blood flow velocity estimates resumed 30 s before cuff deflation and continued for 3 min thereafter. This allowed for the simultaneous calculation of shear rate using edge detection software. The determination of peak artery diameter, and the time taken to reach this peak following the release of the occlusion, was performed using post hoc edge detection software.
Endothelium-independent dilatation.
After a resting period of 15 min, to allow the brachial diameter to return to baseline, a further 1-min recording was made. Subsequently, the endothelium-independent dilatation was examined using a single spray of sublingual GTN (400 μg), a NO donor, followed by a further 6-min recording of the diameter images.
Conduit Artery Diameter and Blood Flow Analysis
Posttest analysis of brachial artery diameters was performed using custom-designed edge-detection and wall-tracking software that is independent of investigator bias (52) and has been previously described in detail (2, 41).
In accordance with our recent findings (41), we quantified the shear rate stimulus responsible for endothelium-dependent FMD. The postdeflation shear rate data, derived from simultaneously acquired blood flow velocity estimates and diameter measures at 30 Hz, were exported to a spreadsheet, and the area under the shear rate curve was calculated based on the Riemann sum technique (29) for data up to the point of maximal postdeflation diameter (%FMD) for each individual. GTN responses were expressed as a percentage relative to baseline (%GTN). The ratio between FMD to GTN was also calculated, which provides a compound assessment of the NO dilator system, whereby endothelial NO function is presented in the context of smooth muscle cell sensitivity to NO (22, 23, 34, 36).
Assessment of Physical Fitness
Body composition and physical fitness.
A dual-energy X-ray absorptiometry (DEXA) scanner (Hologic QDR Series Discovery A, Bedford, MA) was used to determine body fat, bone mineral tissue, and residual tissue in each subject. Exercise testing was undertaken on a treadmill ergometer (H/P/Cosmos, Pulsar 4.0, Nussdorf-Traunstein, Germany), with the initial workload set at 4 km/h at 5% gradient and stepwise increments in speed and grade every 3 min until volitional exhaustion. Heart rate (HR) and rhythm were continuously recorded by a 12-lead ECG, and blood pressure was measured during the last 30 s of each 3-min stage. All tests performed in older subjects were medically supervised. The volume of oxygen consumed during exercise was directly calculated from minute ventilation, measured using a pneumotach and simultaneous breath-by-breath analysis of expired gas fractions (Medgraphics CPX/D and Ultima CardiO2 systems). Gas analyzers and flow probes were calibrated before each test. Oxygen consumption was recorded during the final 40 s of each stage of the test and expressed as maximal oxygen consumption (V̇o2 max) relative to body weight (in ml·kg−1·min−1). V̇o2 max was calculated as the highest consecutive 10-s period of gas exchange data occurring in the last minute before volitional exhaustion, which generally occurred due to leg fatigue or breathlessness.
Exercise Training Program
Following the assessments at study entry, 12 out of the 16 older sedentary subjects agreed to participate in a longitudinal exercise training study, initially involving three sessions of exercise per week at an intensity of 30% HR reserve (HRR) performed for 30 min per visit (treadmill walking and cycling). This 12-wk moderate intensity exercise training regimen has been demonstrated to improve cutaneous endothelial function (3). HRR was calculated using the following formula: [(maximal HR − resting HR) × intensity] + resting HR. The resting and maximal HR measures were derived from the maximal exercise test undertaken before and at the end of the initial and repeat V̇o2 max assessments. A Polar HR monitor (Polar Electro, Kempele, Finland) was used to continuously monitor HR. Two sessions were supervised in a dedicated training facility, with the other session performed at home or in regional gymnasia. Compliance with the home-based component was assessed by regional site visits and regular telephone checkups. After the initial 6 wk, the frequency of exercise increased to five sessions per week. Repeat assessments were performed following 12 wk of this regimen, after which the exercise intensity increased to 60% HRR. Further assessments were undertaken at 24 wk. A further four subjects (2 M and 2 W) acted as time controls, undergoing repeat testing at baseline and after 24 wk.
Statistics
Statistical analyses were performed using SPSS (SPSS, Chicago, IL) software. All data are reported as means ± SE, and statistical significance was assumed at P < 0.05. ANOVA and post hoc paired t-tests were used to assess the significance of difference between groups. Based on an anticipated difference in %FMD, which has a coefficient of variation (CV) of 6.7–10.5% (45), between sedentary and trained older subjects of 2.5%, a power of the study of 80%, and an α of 0.05, we needed eight subjects per individual group (52). We based our group-size calculation for the intervention part of our study on an anticipated change in %FMD of 1.5%, a power of 80%, and an α of 0.05. Based on these assumptions, we required eight subjects per group (52).
RESULTS
Subject characteristics for the cross-sectional and longitudinal exercise training designs are presented in Tables 1 and 2. In total, 1,183 of the 1,296 individual training sessions were attended (91%; 87% in M and 95% in W), and subjects were closely monitored such that they maintained their prescribed HR during the supervised sessions.
Table 2.
Subject characteristics of the healthy older sedentary group who underwent 24 wk of exercise training
|
0 wk |
12 wk
|
24 wk
|
||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| n | 6 | 6 | 6 | 6 | 6 | 6 |
| Age, yr | 58±2 | 60±2 | 58±2 | 60±2 | 58±2 | 60±2 |
| SBP, mmHg | 125±7 | 124±7 | 120±7 | 117±5 | 120±7 | 117±6 |
| DBP, mmHg | 72±3 | 68±4 | 69±3 | 64±2 | 68±4 | 64±4 |
| BMI, kg/m2 | 29±2 | 30±2 | 29±2 | 29±2 | 28±2 | 28±1 |
| %Body Fat | 26±1 | 39±3 | 25±1 | 39±2 | 25±1 | 37±2 |
| V̇o2max, ml·kg−1·min−1 | 30±2 | 23±2 | 33±2 | 26±2 | 39±3* | 30±1* |
| Baseline diameter, cm | 4.7±0.2 | 3.4±0.2 | 4.7±0.1 | 3.5±0.2 | 4.6±0.1 | 3.5±0.3 |
| AUCSR, s × 103 | 17.1±3.1 | 25.7±5.2 | 15.9±1.7 | 26.5±7.6 | 21.8±2.9 | 27.1±7.1 |
Values are means ± SE; n = 6 men and 6 women. number of subjects. V̇o2max, maximal oxygen consumption.
P < 0.05, post hoc t-test significantly different from baseline.
Endothelium-Dependent NO-Mediated Vasodilator Function
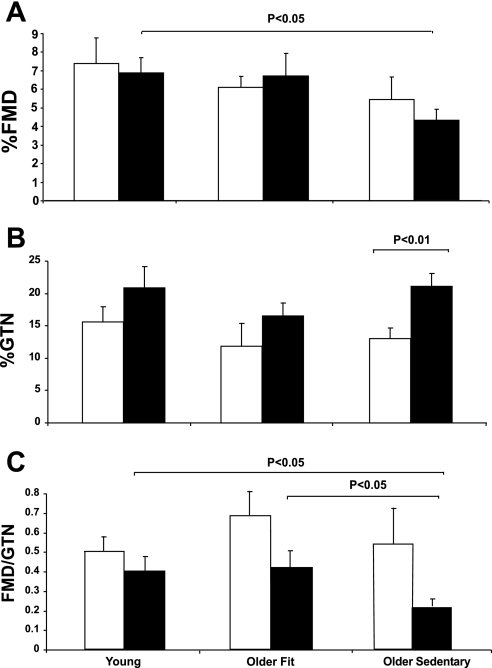
Brachial artery %FMD was significantly greater in younger subjects compared with older sedentary (P < 0.05) but not older fit subjects (P = 0.5). An analysis of the shear rate area under the curve revealed no significant differences between age or fitness groups (Table 1). In men, brachial artery %FMD did not significantly differ between young, older sedentary, and older fit men (ANOVA; P = 0.5, post hoc, Y vs. OS, P = 0.3; Y vs. OF, P = 0.7, Fig. 1A). In contrast, younger women demonstrated a greater brachial artery FMD than older sedentary women (P < 0.05), but not older fit women (P = 0.9).
Fig. 1.
Conduit artery function endothelium-dependent flow-mediated dilator function (FMD): %FMD (A), glyceryl trinitrate (%GTN) (B), FMD-to-GTN ratio (C) in young, older fit, and older sedentary subjects. Data are presented separately for men (white bars) and women (black bars).
Endothelium-Independent NO-Mediated Vasodilator Function
The administration of GTN resulted in a similar brachial artery dilator response in young, older sedentary, and older fit subjects (P = 0.8). Older women exhibited significantly higher brachial artery dilatation to GTN compared with men (P < 0.01, Fig. 1B). No differences in GTN responses were observed between young, older sedentary, or older fit men or between young, older sedentary, or older fit women.
FMD-to-GTN Ratio
No differences between young, older sedentary, and older fit subjects were observed when the ratio between FMD to GTN was calculated (P = 0.5). When the groups were subdivided by sex, the FMD-to-GTN ratio was generally greater in men than in women, but this did not achieve statistical significance (P = 0.09 ANOVA). Men exhibited no differences among the three groups (Fig. 1C). In contrast, older sedentary women had a significantly lower FMD-to-GTN ratio (P < 0.05) compared with both younger and older fit women (Fig. 1C).
Impact of Exercise Training on Vascular Function
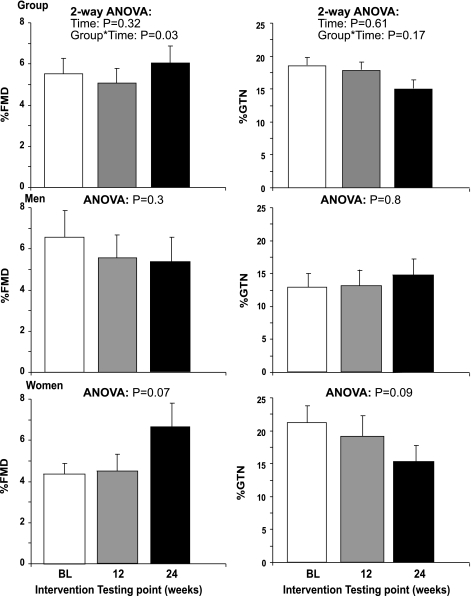
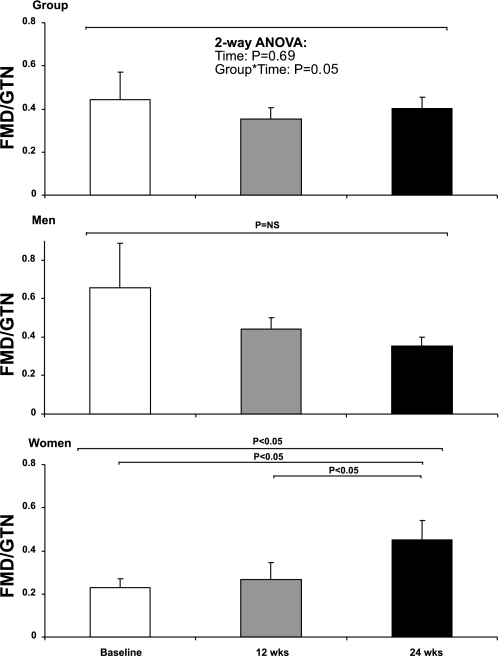
Our analysis revealed no change in %FMD across the 24 wk training intervention (P = 0.3), while a significant interaction between group and exercise training was observed (P = 0.03). Exercise training did not impact upon %GTN (2-way ANOVA; time, P = 0.6; interaction, 0.2) (Fig. 2). Furthermore, exercise training had no time effect on the FMD-to-GTN ratio (P = 0.7), although an interaction between group and exercise training was present (P = 0.05, Fig. 3). In the cohort of longitudinal time controls, we found no change in %FMD (3.7 ± 2.0 vs. 4.2 ± 0.4, P = 0.8), %GTN (16.3 ± 3.2 vs. 16.4 ± 2.6, P = 0.9), or FMD-to-GTN ratios (0.24 ± 0.1 vs. 0.29 ± 0.1, P = 0.7).
Fig. 2.
Brachial artery responses at pretraining baseline (white bars), 12 wk (grey bars), and 24 wk (black bars) of the exercise training program for %FMD (left) and %GTN (right). Group data (n = 12; top), men (n = 6; middle), and women (n = 6; bottom) are shown.
Fig. 3.
Brachial artery FMD-to-GTN ratios at pretraining baseline (white bars), 12 wk (grey bars), and 24 wk (black bars) of the exercise training program. Group data (n = 12; top), men (n = 6; middle), and women (n = 6; bottom) are shown. NS, not significant.
In men, exercise training did not change the %FMD (P = 0.3), %GTN (P = 0.8), or FMD-to-GTN ratio (P = 0.3). In contrast, the FMD-to-GTN ratio was significantly increased at 24 wk in women (P < 0.05, Fig. 3) who also demonstrated a tendency for improvement in FMD after 24 wk (P = 0.07) and a decrease in GTN (P = 0.09 Fig. 2). In addition, exercise training abolished the impaired FMD-to-GTN ratio between older previously sedentary women, compared with older fit and young women (0.45 ± 0.1, 0.4 ± 0.1, and 0.42 ± 0.1, respectively, ANOVA, P = 0.9).
DISCUSSION
This study examined the effects of age, fitness, and sex on brachial artery endothelium-dependent and -independent responses using cross-sectional and longitudinal study designs. We found an age-related decline in brachial artery FMD when older sedentary subjects were compared with younger subjects. This was principally due to a larger effect of age on brachial artery endothelial function in women than in men, as the latter group did not exhibit significant differences between groups or in response to exercise training. In addition, older sedentary women had preserved GTN responses, while the FMD-to-GTN ratio, an aggregate measure of NO vasodilator system function, was also significantly lower in older sedentary women. The cross-sectional comparison of sedentary and fit older women indicated that the maintenance of cardiopulmonary fitness attenuated the age-related decline in vascular dysfunction. These differences appear to be associated with endothelium-dependent mechanisms, since endothelium-independent responses to GTN were similar between groups. Consistent with this effect of chronic exercise training, our longitudinal training data indicate that 24 wk of lower-limb exercise reversed the impaired FMD-to-GTN ratio in previously sedentary older women. This effect was primarily due to enhanced FMD with training. These data suggest that older sedentary women have an impaired endothelial function and that the maintenance of fitness or the adoption of an exercise training program can prevent or reverse this decline.
Previous studies have described an age-related decline in FMD (5, 14–16). Postmenopausal women appear to have a more rapid decline in FMD compared with age-matched men (5). More recent studies using normalization methods (35) have allowed for further insight into this observation, with older men having preserved conduit artery function in both the brachial and femoral arteries (53), whereas older women have attenuated brachial and popliteal artery FMD (34). However, the reported mean brachial artery FMD in young men is highly variable with values ranging between 5–7.5% in some studies (12, 13, 17, 18), which is considerably higher than the 2.0 to 4.5% demonstrated in other studies (1, 7, 9, 53), and likely to have an impact for determining age-related endothelial dysfunction. This highlights the lack of normative FMD reference values that exist for comparison between studies, due mainly to the absence of a standardized protocol, despite recent unsuccessful attempts to create one (8, 10). However, when comparisons are made within the same study, the relative changes between groups, such as young versus old, allow for valid conclusions to be made.
No previous study has directly compared men and women of different age and fitness levels within a single study. However, the results from the current study are broadly in agreement with previous data from various independent sources, in that we observed a greater attenuation in conduit artery endothelial function in older sedentary women compared with their younger counterparts (38%). Although not significantly different, the 26% difference in younger versus older men is in keeping with previous reports of an age-related decline in men (5, 13–15). This finding also supports previous studies that reported a more rapid decline in aging women than across different age groups within men (5). It is possible that, had we recruited more subjects, age might have been associated with significant decreases in FMD in both sedentary men as well as women; however, the germane finding from our study is that there appears to be a more pronounced effect in women than men with age and that this finding would likely be reinforced or perhaps exaggerated by a larger sample size.
The similar GTN responses between young, older fit, and older sedentary subjects indicate that vascular smooth muscle cell function was similar between groups and did not account for the differences that were observed. This is consistent with previous findings from other studies using the brachial artery model in men (36, 38). However, when the group data were subdivided by sex, women generally had greater vascular smooth muscle cell sensitivity to NO than men, especially in the older sedentary groups. The differences between men and women in the endothelial versus smooth muscle components of the NO dilator system reveal a sex difference in vascular wall function with sedentary aging.
Previous cross-sectional studies, which have reported that fit older men and women demonstrate greater brachial artery FMD compared with older sedentary peers (21, 36, 38), support the suggestion that the maintenance of cardiopulmonary fitness is an effective intervention for reversing endothelial dysfunction. Evidence from the current study endorses this, since our findings showed that fitness offsets the attenuation in endothelial dysfunction observed in older sedentary subjects. Specifically, this is the first study to demonstrate that FMD was similar between younger and older fit women, despite significant differences between young and older sedentary women. This confirms the purported beneficial effects that long-term exercise has on the vascular endothelium, perhaps through increased NO bioactivity (16, 40).
Aging is accompanied by an increase in muscle sympathetic nerve activity (MSNA), which may be greater in women than in men. Interestingly, this larger increase in MSNA in women is also associated with a larger increase in blood pressure over time (32). Previous studies have demonstrated an inverse relation between MSNA and FMD in younger (24) and older subjects (46). Therefore, the lower FMD in older women than men in our study may relate to sex differences in MSNA. Moreover, a study from Charkoudian et al. (6) found that subjects with a high resting MSNA have larger blood pressure responses to NO blockade than those with a low MSNA. This suggests that subjects with higher resting MSNA demonstrate a larger increase in blood pressure than those with a similar impairment of the NO function but lower MSNA. This may help to explain the link between the age-related increase in MSNA and blood pressure in older women.
We also hypothesized that a 24-wk longitudinal exercise training program would improve FMD in older sedentary men and women. However, we observed modest change in FMD or GTN data in men, a finding that is in keeping with some previous experiments (27, 47). Men and women undertook similar training programs, so differences cannot be explained on the basis of different exercise modalities (44). It is possible that the relative lack of improvement in men may be related to the absence of vasodilator dysfunction a priori. This is supported by the observations in older sedentary women, who possessed impaired FMD at entry relative to their younger counterparts, but significant improvement in the FMD-to-GTN ratio following exercise training (P < 0.05). This improvement in the FMD-to-GTN ratio was primarily related to an enhanced FMD. Since the GTN response encompasses smooth muscle sensitivity to NO, the increase in the FMD-to-GTN ratio in the presence of a diminished GTN response is suggestive of a marked improvement in endothelial function. In contrast to the data in women, training did not have a significant impact on FMD or GTN in men. These divergent sex differences reinforce the suggestion made above, that changes in the vessel wall in response to interventions such as aging and exercise vary importantly between men and women. The cross-sectional and longitudinal evidence from the current study therefore indicate that exercise prevents and reverses the age-related decline in endothelial function evident in older sedentary women.
The strengths of this study include the cross-sectional and longitudinal study designs and the combination of endothelial and vascular smooth muscle measures of NO function. However, there are also several limitations. As intended, the older fit subjects were well matched for age compared with their sedentary peers but possessed a significantly higher level of cardiovascular fitness. As may be expected, the sedentary subjects exhibited significantly higher percent body fat and body weight than the fit cohort, which may have confounded our findings. However, the enhanced endothelial function following exercise training in unfit women was accompanied by an improved cardiopulmonary fitness but no changes in body composition. This is in line with previous studies that reported no change in body composition but a significant improvement in endothelial function in other groups (30, 50). This suggests that, at least for exercise-training intervention studies, potential differences in body composition are unlikely to explain the exercise-induced improvements in endothelial function. In addition, we examined the cross-sectional group difference by performing correlations between percent body fat (DEXA) with %FMD and with FMD-to-GTN ratios. A similar analysis was performed between mean arterial pressure and these key outcome variables. There were no significant correlations between changes in percent body fat (DEXA) or mean arterial pressure with %FMD (r = −0.198, P = 0.2; and r = −0.043, P = 0.8) or with FMD-to-GTN ratios (r = −0.258, P = 0.1; and r = 0.291, P = 0.06). Nonetheless, we cannot exclude the possibility that the significant differences in percent body fat between the three groups, in men as well as in women, may have impacted on our cross-sectional comparisons in %FMD and FMD-to-GTN ratios.
As stated in methods, subjects were excluded if hypercholesterolemia, hypertension, insulin resistance syndrome, or diabetes had been diagnosed. Furthermore, no subject was taking any medications. All subjects were asymptomatic and healthy and completed a 12-lead ECG stress test to maximal exertion on entry to the study under clinical supervision. We are therefore confident that the potential confounding influences on vascular function did not substantively impact on the results of the present study. Nonetheless, we acknowledge that there may have been occult or subclinical differences in lipid or blood glucose levels, particularly in the older groups that we recruited. A final potential limitation of the current study was the low number of subjects recruited within each subcategory and the potential for encountering type II errors. However, based on a %CV of 14.7%, a 1.5–2.0% change in FMD can be detected with a power of 80% using 6–8 subjects (48, 52). Because of recent advances in software analysis, we recently reported a %CV of 6.7–10.5 (45, 52), enabling us to detect smaller differences using the same group size. Furthermore, previous studies using similar study designs for investigating the effects of exercise training and/or aging on endothelial function have employed similar or smaller sample sizes (47, 53). While it is possible that a larger sample size might have, for example, uncovered a significant decrement in FMD in older sedentary compared with younger men, we believe our principal findings of differences between men and women with age, fitness, and training would be reinforced in a larger sample population.
In conclusion, the maintenance of cardiopulmonary fitness in older individuals attenuated the age-related decline in brachial artery endothelial function that we particularly observed in older sedentary women. Exercise training also improved endothelial function in this subgroup. In contrast, the impacts of aging, fitness, and exercise training on NO-mediated vasodilator function were less evident in men. This study highlights sex differences in the impact of aging and exercise in humans and also important differences in the relative impact of these factors on endothelial and vascular smooth muscle components of the vessel wall.
GRANTS
M. A. Black was supported by a British Heart Foundation Grant FS/05/117/19971. D. H. J. Thijssen is supported by a Netherlands Organization for Scientific Research Grant NWO-grant 82507010. D. J. Green was supported by the National Heart Foundation of Australia.
Acknowledgments
We acknowledge Dr. Stefanie Bracknell for medical supervision during all V̇o2 max treadmill tests.
REFERENCES
- 1.Betik AC, Luckham VB, Hughson RL. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol 286: H442–H448, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol 586: 3511–3524, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease. Circulation 108: 2093–2098, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol 291: H1378–H1383, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol 33: 1379–1385, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer DS, Charbonneau F, Creager MA, Deanfield J, Drexer H, Gerhard-Herman M, Herrington D, Vallance P, Vogel R. Guidelines for the ultrasound assessment of flow-mediated vasodilation of the brachial artery. J Am Coll Cardiol 39: 257–265, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Dawson EA, Whyte GP, Black MA, Jones H, Hopkins ND, Oxborough D, Gaze D, Shave RE, Wilson M, George KP, Green DJ. Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J Appl Physiol 105: 1562–1568, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens 23: 7–17, 2005. [DOI] [PubMed] [Google Scholar]
- 11.DeSouza CA, Shapiro LF, Clevenger C, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores the age-related decile in endothelium-dependent vasodilation. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001. [PubMed] [Google Scholar]
- 13.Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 290: H1446–H1453, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol 571: 661–668, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens 18: 510–516, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102: 63–71, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Green DJ. Point-Counterpoint: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1233–1234, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Green DJ, Maiorana AJ, O'Driscoll G, Taylor R. Topical Review: Effects of exercise training on vascular endothelial nitric oxide function in humans. J Physiol (Lond) 561: 1–25, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagmar M, Eriksson MJ, Lindholm C, Schenck-Gustafsson K, Hirschberg AL. Endothelial function in post-menopausal former elite athletes. Clin J Sport Med 16: 247–252, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 92: 3431–3435, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 45: 1441–1448, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39: 683–688, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Holowatz LA, Thompson-Torgerson CS, Kenney WL. Altered mechanisms of vasodilation in aged human skin. Exerc Sport Sci Rev 35: 119–125, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Jensen-Urstad K, Johansson J. Gender difference in age-related changes in vascular function. J Intern Med 250: 29–36, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Jodoin I, Bussieres LM, Tardif JC, Juneau M. Effect of a short-term primary prevention program on endothelium-dependent vasodilation in adults at risk for atherosclerosis. Can J Cardiol 15: 83–88, 1999. [PubMed] [Google Scholar]
- 28.Kasprzak JD, Klosinska M, Drozdz J. Clinical aspects of assessment of endothelial function. Pharmacol Rep 58, Suppl: 33–40, 2006. [PubMed] [Google Scholar]
- 29.Keisler H. Elementary Calculus: An Infinitesimal Approach. Boston: Prindle, Weber & Schmidt, 1986.
- 30.Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 38: 860–866, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo. Circ Res 88: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol 289: H308–H315, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol 291: H3043–H3049, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol 88: 761–766, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Rywik TM, Blackman MR, Yataco AR, Vaitkevicius PV, Zink RC, Cottrell EH, Wright JG, Katzel LI, Fleg JL. Enhanced endothelial vasoreactivity in endurance-trained older men. J Appl Physiol 87: 2136–2142, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol 41: 501–507, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol 79: 675–686, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Taddei S, Virdis A, Mattei P, Salvetti A. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension 21: 929–933, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sport Exerc 41: 1072–1079, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Thijssen DH, de Groot P, Kooijman M, Smits P, Hopman MT. Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. Am J Physiol Heart Circ Physiol 291: H3122–H3129, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Thijssen DH, de Groot PC, Smits P, Hopman MT. Vascular adaptations to 8-week cycling training in older men. Acta Physiol (Oxf) 190: 221–228, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Torgrimson BN, Meendering JR, Kaplan PF, Minson CT. Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am J Physiol Heart Circ Physiol 292: H2874–H2880, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Vita JA, Keaney JF. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Watts K, Beye P, Siafarikas A, Davis EA, Jones TW, O'Driscoll G, Green DJ. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol 43: 1823–1827, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Green D. Improved analysis of brachial artery ultrasound images using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Yataco AR, Corretti MC, Gardner AW, Womack CJ, Katzel LI. Endothelial reactivity and cardiac risk factors in older patients with peripheral arterial disease. Am J Cardiol 83: 754–758, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Zhao SP, Li XP, Gao M, Zhou QC. Endothelium-dependent and -independent functions are impaired in patients with coronary heart disease. Atherosclerosis 149: 19–24, 2000. [DOI] [PubMed] [Google Scholar]