Abstract
Aging contributes significantly to the development of cardiovascular disease and is associated with elevated production of reactive oxygen species (ROS). The beneficial effects of nitric oxide (NO)-mediated vasodilation are quickly abolished in the presence of ROS, and this effect may be augmented with aging. We previously demonstrated an age-induced impairment of flow-induced dilation in rat coronary arterioles. Therefore, the purpose of this study was to determine the effects of O2− scavenging, as well as removal of H2O2, the byproduct of O2− scavenging, on flow-mediated dilation in coronary resistance arterioles of young (4 mo) and old (24 mo) male Fischer 344 rats. Flow increased NO and H2O2 production as evidenced by enhanced diaminofluorescein and dichlorodihydrofluorescein fluorescence, respectively, whereas aging reduced flow-induced NO and H2O2 production. Endothelium-dependent vasodilation was evaluated by increasing intraluminal flow (5–60 nl/s) before and after treatment with the superoxide dismutase mimetic Tempol (100 μM), the H2O2 scavenger catalase (100 U/ml), or Tempol plus catalase. Catalase reduced flow-induced dilation in both groups, whereas Tempol and Tempol plus catalase diminished vasodilation in young but not old rats. Tempol plus deferoxamine (100 μM), an inhibitor of hydroxyl radical formation, reversed Tempol-mediated impairment of flow-induced vasodilation in young rats and improved flow-induced vasodilation in old rats compared with control. Immunoblot analysis revealed increases in endogenous superoxide dismutase, catalase, and nitrotyrosine protein levels with aging. Collectively, these data indicate that NO- and H2O2-mediated flow-induced signaling decline with age in coronary arterioles and that elevated hydroxyl radical formation contributes to the age-related impairment of flow-induced vasodilation.
Keywords: reactive oxygen species, superoxide dismutase, hydroxyl radical, deferoxamine, nitric oxide, hydrogen peroxide
increasing evidence indicates that aging contributes significantly to the development of ischemic heart disease. Vascular resistance increases, leading to impairments in coronary blood flow and flow reserve (15). Endothelial dysfunction similarly progresses with aging (4) and may be mediated by impaired nitric oxide (NO) activity (7, 21) or elevated oxidant stress (6, 10). Previous studies have demonstrated age-associated declines in flow-mediated dilation in coronary arterioles due to impaired phosphatidylinositol 3-kinase signaling (21) or elevated superoxide (O2−) production (7). Because flow-induced vasodilation is inextricably linked to NO-mediated signaling, it is possible that other factors directly involved in the NO pathway may be altered with advancing age.
In the presence of the O2− scavenger superoxide dismutase (SOD), O2− is converted to H2O2. Thus age-related increases in O2− point to examination of potential age-related changes in the contribution of H2O2 to vascular signaling. Recent evidence suggests a role for H2O2 in mediating endothelium-dependent vasodilation in human (24) and porcine (46) coronary arterioles. Miura et al. (24) demonstrated that endothelium-derived H2O2 contributes to flow-mediated dilation of coronary arterioles and that this vasodilation is inhibited by the H2O2 scavenger catalase. However, virtually nothing is known regarding age-induced changes in H2O2-mediated flow-induced signaling in the coronary vasculature.
Shear stress elicits NO (19), O2− (23), and H2O2 (24) release in isolated coronary vessels. However, reactive oxygen species (ROS) scavenging also has been shown to improve endothelial function in various vascular beds (6, 7, 12). Thus ROS production and scavenging are likely carefully modulated to produce appropriate flow-induced dilation. Therefore, the purpose of our study was 1) to investigate age-related changes in NO and H2O2 signaling during flow-induced vasodilation and 2) to examine the effects of ROS scavenging on flow-mediated dilation in coronary arterioles.
METHODS
Animals.
All procedures in this study were approved by the Institutional Animal Care and Use Committee at West Virginia University. All methods complied fully with guidelines set forth in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, revised 1996). Young (5–6 mo) and old (23–24 mo) male Fischer 344 rats were obtained from the National Institute of Aging colony. These rats are sexually mature adult animals at 4 mo, whereas 24-mo-old rats are senescent, with a colony mortality rate of ∼50%. Animals were housed at 23°C with a 12:12-h light-dark cycle and were provided water and rat chow ad libitum.
Microvessel preparation.
Rats were anesthetized (isoflurane 5%-O2 balance) and euthanized by excision of the heart, which was immediately placed in cold, filtered physiological saline solution (PSS; pH 7.4) containing (in mM) 145 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 3.0 MOPS buffer, and 1% bovine serum albumin. Resistance arterioles (<150 μm) were isolated from the left anterior descending coronary artery distribution as previously described (35). Arterioles were cannulated on pipettes matched for size and resistance in a Lucite chamber that contained PSS equilibrated with room air. In coronary arterioles <150 μm, intraluminal pressure is ∼40–50 mmHg (5) and mean arterial pressure and cardiac output are similar between young and old Fischer 344 male rats (8); therefore, mean intraluminal pressure was maintained at 45 mmHg in arterioles from both young and old rats for all experiments. Arterioles with leaks were discarded. Vessels determined to be free of leaks were warmed to 37°C and allowed to develop spontaneous tone. Only arterioles that developed and maintained >20% tone were utilized to assess vasodilatory responses.
Evaluation of vasodilator responses to intraluminal flow.
Arterioles were exposed to graded increases in intraluminal flow by adjusting the height of fluid reservoirs in equal and opposite directions, thereby creating a pressure difference across the arterioles without altering intraluminal pressure within the arterioles (20). Diameter measurements were determined in response to stepwise pressure differences of 2, 4, 10, 20, 40, and 60 cmH2O for 2-min stages, corresponding to physiologically significant flow rates from 5 to 50 nl/s (26). Volumetric flow (Q) was calculated as previously described (26).
To examine the effects of free radical scavenging on flow-mediated dilation in coronary arterioles, we also assessed responses in the presence of the cell-permeable O2− dismutase mimetic Tempol [100 μM (51)], the H2O2 scavenger catalase [100 U/ml (11)], Tempol plus catalase, deferoxamine [an iron chelator and inhibitor of hydroxyl radical production; 100 μM (45)], or Tempol plus deferoxamine. Each pharmacological agent (Sigma) was applied for 20 min before evaluation of the flow response. The order of interventions was randomized except that a control flow response was always obtained before any pharmacological intervention was performed to verify endothelial responsiveness. In some experiments, flow responses in the presence of pharmacological agents were performed after completion of a complete control response. In instances where a complete control response was not obtained, verification of endothelial responsiveness was assessed by exposing the vessel to a brief period of flow (5–13 nl/s). Vessels that did not respond to flow under control conditions (whether in response to a complete or abbreviated flow response) were discarded. All drugs were prepared in distilled H2O, and both vehicle and time controls were performed to verify the absence of nonspecific effects on vascular reactivity.
Evaluation of vasodilator responses to H2O2.
To examine the effects of aging on arteriolar responsiveness to H2O2, we determined concentration-response curves. Isolated microvessels were equilibrated and allowed to develop spontaneous tone. Vasoreactivity to cumulative additions of extraluminal H2O2 (100 nM-10 mM, 3-min stages) were recorded.
Fluorescence detection of NO and H2O2.
Flow-induced increases in NO were detected with the cell-permeable fluorescent reagent 4-amino-5-methylamino-2′2′-difluorofluorescein diacetate (DAF; 5 μM) at an excitation wavelength of 490 nm and an emission wavelength of 515 nm. To examine flow-induced production of H2O2, we treated vessels with 5(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF; 5 μM). DCF fluorescence was visualized at 475- and 515-nm excitation and emission wavelengths, respectively, with a 0.5 neutral density filter (Zeiss) to minimize photooxidation. Because albumin may interfere with DAF and DCF fluorescence detection, albumin-free solutions were used for all fluorescence experiments (29, 40). Intraluminal loading was performed by introducing either DAF or DCF into one perfusion pipette and establishing 20 nl/s flow through the arteriole for 2 min. Flow through the arteriole was then arrested, and the dye was incubated in the arteriole for 5 min. After incubation, reverse flow was initiated (20 nl/s) and maintained through the vessel for 10 min to wash all dye from the vessel lumen and perfusion pipettes. The arteriole was then allowed to equilibrate for an additional 5 min before a 20-min incubation with vehicle, catalase (100 U/ml), or NG-nitro-l-arginine methyl ester (l-NAME; 10 μM). Fluorescent images were recorded using an Axiovert 40CFL inverted microscope (Zeiss) equipped with an HBO 50 super-pressure mercury lamp (Zeiss). At the beginning of each experiment, a baseline image was recorded in the absence of flow. Camera (Axiocam; Zeiss) settings were adjusted to this image and maintained throughout the course of the experiment (28). Fluorescent images were captured every 30 s during a 2-min exposure to flow at 7.5, 35, and 50 nl/s for a total of 6 min. At the conclusion of each experiment, vessels were exposed to the NO donor DEA-NONOate (1 μM) or H2O2 (10 mM) to verify that DAF or DCF, respectively, was sufficiently loaded through the duration of the experiment.
Fluorescence analysis.
A user-defined segment of the central portion of each vessel (region of interest; ROI) was analyzed for fluorescence intensity using ImageJ software (National Institutes of Health). Total fluorescence intensity was calculated as average fluorescence intensity per pixel × surface area. Basal fluorescence levels varied among ROIs and in different experiments. Therefore, changes in fluorescence intensity were expressed as (F − F0)/F0, where F is fluorescence intensity during flow and F0 is baseline fluorescence intensity. Both baseline and flow fluorescence intensity values were measured within the same ROIs in the same focal plane of the vessel.
Immunoblot analysis.
Coronary arterioles (5 pooled vessels per sample) were dissected, immediately snap frozen, and stored at −80°C until use. Vessels were lysed in 1× sample buffer (62.5 mM Tris, pH 6.8, 2% SDS, 6 M urea, 160 mM DTT, 0.1% bromophenol blue), and protein concentration was determined using the NanoOrange protein quantification kit (Invitrogen). Samples (10 μg of total protein) were subjected to SDS-polyacrylamide gel electrophoresis (10%) and then transferred to polyvinylidene difluoride membranes. Membranes were blocked for 1 h at room temperature (5% nonfat dry milk in Tris-buffered saline + 0.1% Tween 20) and then incubated overnight at 4°C with catalase (1:6,000; Chemicon), Cu,Zn-SOD (1:1,000; Stressgen), nitrotyrosine (NT; 1:500; Abcam) or β-actin (1:4,000, Cell Signaling) antibody. After being washed, membranes were incubated with respective horseradish peroxide-conjugated secondary antibody (Cell Signaling) for 1 h at room temperature. Peroxidase activity was determined using SuperSignal West Femto (10), with image analysis performed using ImageJ (17). Control analysis indicated that β-actin increased linearly in the range of 4–16 μg of total protein and did not change with age. Therefore, loading differences were normalized by expressing all data as relative densitometric units of the protein of interest vs. β-actin.
mRNA analysis.
Arterioles were snap frozen and stored at −80°C. Arterioles were later pulverized in lysis buffer, and total RNA was extracted using the RNAqueous isolation kit (Ambion). cDNA was made using the High-Capacity cDNA Archive kit (Applied Biosystems). Real-Time PCR was performed as previously described (39). Briefly, real-time PCR was performed in triplicate, with two no-template control samples and two reverse transcriptase negative samples (GeneAmp 384-well optical reaction plates). Cu,Zn-SOD gene expression was quantified with predesigned TaqMan primers (Applied Biosystems) using the ABI Prism 7900HT fast real-time PCR system. Quantification of relative gene expression was performed using the comparative threshold cycle method.
Data presentation and analysis.
All data are means ± SE. Development of spontaneous tone is expressed as the percent constriction relative to maximal diameter and was calculated as spontaneous tone (%) = (IDmax − IDb)/IDmax × 100, where IDmax is the maximal inner diameter recorded at a pressure of 45 mmHg and IDb is the steady-state baseline diameter. The vasodilator responses to flow and H2O2 are expressed using the equation relaxation (%) = [(IDa − IDb)/(IDmax − IDb)] × 100, where IDa is the arteriolar diameter during flow, IDb is the diameter recorded immediately before initiation of the flow- or concentration-diameter curves, and IDmax is the maximal diameter for the arteriole.
Responses to flow and H2O2 were evaluated using two-way repeated-measures ANOVA to detect differences between (young vs. old) and within (flow rate or concentration) groups. Post hoc analyses were performed using Bonferroni's test for pairwise comparisons. Group differences in animal and vessel characteristics were compared using Student's t-test. Differences in fluorescence intensity and protein content were assessed using one-way ANOVA. Statistical significance was defined as P ≤ 0.05.
RESULTS
Animal and vessel characteristics.
Body weight, left ventricular weight, and left ventricular weight-to-body weight ratios increased with age (Table 1). Advancing age did not alter spontaneous tone in coronary arterioles from the left descending artery, and maximal diameter was not different between vessels from young and old rats (Table 1).
Table 1.
Animal and vessel characteristics
| Young Rats | Old Rats | |
|---|---|---|
| Animal characteristics | ||
| Body weight, g | 373±5 | 433±5* |
| LV weight, mg | 676±12 | 845±25* |
| LV weight/body weight, mg/g | 1.80±0.03 | 1.95±0.06* |
| Vessel characteristics | ||
| Maximal diameter, μm | 145±4 | 158±7 |
| Spontaneous tone, % | ||
| Baseline | 31±2 (14) | 30±3 (9) |
| Post-Tempol | 35±3 (11) | 33±5 (8) |
| Post-Catalase | 35±4 (5) | 33±4 (5) |
| Post-Tempol + catalase | 36±3 (8) | 28±2 (5) |
| Post-Deferoxamine | 26±2 (5) | 29±1 (6) |
| Post-Tempol + deferoxamine | 23±2 (5) | 36±7 (5) |
Values are means ± SE for n = 39 young and 31 old rats; LV, left ventricular. For spontaneous tone subgroups, n = value shown in parentheses.
P < 0.05 vs. young rats.
Effect of age on vasodilator responses to intraluminal flow.
Flow-induced vasodilation was significantly impaired in coronary arterioles from old compared with young rats (Fig. 1). These data confirm previous results from our laboratory demonstrating an age-related reduction in NO-mediated endothelium-dependent dilation (21).
Fig. 1.
Flow-induced dilation in coronary arterioles from young (Y) and old (O) male rats. Aging reduces flow-mediated vasodilation in coronary arterioles from Fischer 344 rats. *P < 0.05, significant difference from young rats.
Flow-induced NO production.
DAF fluorescence microscopy was used to examine flow-induced NO production in coronary arterioles isolated from young and old rats. At 7.5, 35, and 50 nl/s flow, DAF fluorescence was significantly reduced in vessels from old rats compared with those from young rats, indicating that flow-induced NO production is diminished with age (Fig. 2). The contribution of NO in mediating flow-induced vasodilation was confirmed by the elimination of flow-induced increases in DAF fluorescence in arterioles from both age groups by l-NAME, a NO synthase inhibitor (Fig. 3, A and C). To examine the effect of H2O2 scavenging on flow-induced NO production, we assessed DAF fluorescence in vessels treated with catalase. Similarly to l-NAME, catalase eliminated flow-induced increases in DAF fluorescence in vessels from both young and old rats (Fig. 3, B and D), indicating that H2O2 is obligatory to flow-induced increases of NO in rat coronary arterioles.
Fig. 2.
Flow-induced nitric oxide (NO) production in coronary arterioles as measured by 4-amino-5-methylamino-2′2′-difluorofluorescein diacetate (DAF) fluorescence. A: representative images of vessels from young and old rats at flow rates of 0 (flow 0), 35, and 50 nl/s. B: increasing flow rates enhanced DAF fluorescence in coronary arterioles from both young (n = 11) and old (n = 9) rats. NO production in response to flow declined with age, as evidenced by reduced DAF intensity. Values are means ± SE. *P < 0.05, significant difference from flow 0. #P < 0.05, significant difference from young rats at respective flow rate.
Fig. 3.
Flow-induced increases in DAF fluorescence were abolished by both NG-nitro-l-arginine methyl ester (l-NAME) and catalase. Representative images of coronary arterioles from young and old rats are shown at flow rates of 0 and 50 nl/s after treatment with l-NAME (A) or catalase (B). In the presence of l-NAME (C) or catalase (D), DAF fluorescence intensity did not change with increasing flow over time. Arrows in C and D indicate stepwise increases in flow to 7.5, 35, and 50 nl/s, respectively. Values are means ± SE; n = 4 young and 4 old rats per group (8 total).
Flow-induced H2O2 production.
DCF fluorescence increased incrementally in response to increasing flow rates in arterioles from both young and old rats, indicating a rate-dependent production of H2O2 occurs in coronary arterioles exposed to intraluminal flow (Fig. 4A). Catalase treatment blunted flow-induced increases in DCF fluorescence in arterioles from both young and old rats (Fig. 4B), demonstrating specificity of increases in dye fluorescence to H2O2. DCF fluorescence intensity was dramatically reduced with aging at 50 nl/s flow (Fig. 4), suggesting that impaired H2O2 availability contributes to the age-related decline in flow-mediated vasodilation.
Fig. 4.
Flow-induced H2O2 production in coronary arterioles as measured by 5(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF) fluorescence. A: representative images of vessels from young and old rats at flow rates of 0 and 50 nl/s. B: DCF fluorescence increased in response to flow (50 nl/s) in coronary arterioles from both young and old rats; however, flow-induced H2O2 production declined with age. Catalase blunted DCF fluorescence in arterioles from both age groups. Values are means ± SE; n = 6 rats per group. *P < 0.05 significant difference from young rats. #P < 0.05, significant difference from control.
To determine whether aging alters dilation to authentic H2O2 in coronary arterioles, we assessed concentration-response curves to H2O2 (100 nM-10 mM). H2O2 at 10 mM elicited maximal dilation in arterioles from both young and old animals; however, the EC50 for H2O2 was significantly lower in arterioles from old rats (1.0 ± 0.1 mM) compared with the EC50 in those from young rats (2.5 ± 0.8 mM; Fig. 5), indicating that sensitivity to H2O2 increases with age.
Fig. 5.
Effect of H2O2 on coronary arterioles from young and old rats. H2O2 dilated coronary arterioles concentration dependently. EC50 values differed significantly between young (EC50 = 2.5 ± 0.8 mM) and old rats (EC50 = 1.0 ± 0.1 mM). Values are means ± SE. *P < 0.05.
Effect of ROS scavenging on age-specific vasodilator responses to intraluminal flow.
To determine the effects of O2− scavenging on flow-induced dilation, we incubated vessels from young and old rats with the SOD mimetic Tempol (100 μM) before evaluation of flow. Tempol significantly reduced flow-mediated dilation in coronary arterioles from young animals but did not alter the flow response in arterioles from old animals (Fig. 6A). As a result, age-related differences in flow-induced vasodilation were eliminated by treatment with Tempol.
Fig. 6.
Flow-induced dilation in coronary arterioles following treatment with reactive oxygen species (ROS) scavengers. A: Tempol significantly inhibited flow-mediated dilation in vessels from young rats but had no effect in vessels from old animals. B: treatment with catalase blunted flow to a greater extent in arterioles from young vs. old rats, consistent with direct H2O2 measures shown in Fig. 4. C: Tempol plus catalase (Tem + Cat) inhibited the flow response in vessels from young, but not old, rats. Similar to treatment with Tempol alone, the effect of additional Tem + Cat resulted in no net change in the flow response. Values are means ± SE. *P < 0.05, significant difference from control.
To assess the contribution of H2O2 to flow-mediated dilation, we treated vessels with the H2O2 scavenger catalase (100 U/ml). In coronary arterioles from both young and old rats, catalase severely blunted flow-induced dilation (Fig. 6B). These data are consistent with previous reports examining the flow response in isolated coronary arterioles (18, 24) and indicate a significant role for H2O2 in mediating flow-induced vasodilation. Consistent with our results showing that flow-induced H2O2 production declines with age (Fig. 4), reduction of flow-induced dilation by catalase was greater in arterioles from young rats compared with old rats. In addition, treatment with catalase eliminated age-associated differences between flow-mediated dilation in vessels from young and old rats.
To examine the combined effects of O2− and H2O2 scavenging, we incubated coronary arterioles with Tempol plus catalase before evaluation of flow. In arterioles from young rats, the inhibition of flow-induced dilation by Tempol plus catalase was comparable to the inhibition produced by catalase treatment alone (Fig. 6, B and C). By contrast, in arterioles from old rats, combined Tempol and catalase treatment did not diminish flow-mediated dilation, similar to the lack of inhibition produced by Tempol alone (Fig. 6, A and C).
Effect of downstream ROS scavenging.
The SOD mimetic Tempol catalyzes the dismutation of O2− to H2O2, a known vasodilator (24, 30, 46). Thus treatment with Tempol could be expected to augment flow-induced dilation by both reducing the NO-scavenging effects of O2− and increasing the production of vasodilatory H2O2. Surprisingly, Tempol reduced flow-induced dilation in arterioles from young rats. Because H2O2 can be converted into the highly reactive hydroxyl radical (OH•) in the presence of ionic metals such as Fe2+, we evaluated flow in the presence of Tempol combined with deferoxamine (100 μM), an iron chelator (47). In Tempol-treated arterioles from young rats, deferoxamine improved flow-mediated vasodilation (Fig. 7A), restoring the response to control levels (Fig. 1). In coronary arterioles from old rats, deferoxamine significantly (P < 0.05) augmented the flow response to a level greater than that observed in arterioles from old rats under control conditions or treated with Tempol alone (Fig. 1). To determine the effects of hydroxyl scavenging in the absence of Tempol, we treated coronary arterioles with deferoxamine alone (100 μM). Deferoxamine had no effect on flow-induced dilation in vessels from young animals but enhanced vasodilation in vessels from old animals (Fig. 7B).
Fig. 7.
Effect of deferoxamine on flow-induced vasodilation in coronary arterioles from young and old rats. A: treatment with deferoxamine (Tem + Def) reversed Tempol-induced reduction of flow in vessels from young rats and augmented flow-induced dilation above control levels in vessels from old rats. B: deferoxamine alone similarly enhanced the flow response in old rats but did not alter flow-induced dilation in vessels from young animals. Values are means ± SE. *P < 0.05, significant difference from deferoxamine.
Immunoblot and real-time PCR analysis.
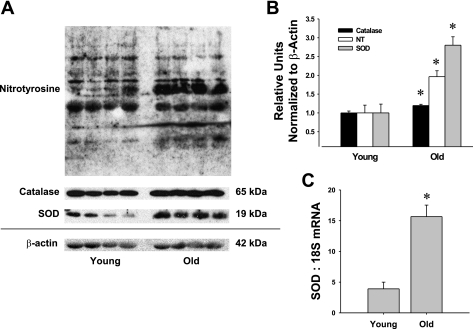
Protein levels for nitrotyrosine, an indicator of peroxynitrite-induced cell damage, increased with aging in vessels from old vs. young rats (Fig. 8A). SOD protein levels also were significantly higher in coronary arterioles of old rats compared with those from young rats (Fig. 8B). Endogenous catalase was also elevated with age (∼20%), but this increase was modest compared with the magnitude of increase (∼200%) in SOD protein (Fig. 8B). Real-time PCR analysis revealed a similar increase in SOD mRNA with age (Fig. 8C).
Fig. 8.
Immunoblot analysis of catalase, nitrotyrosine (NT), and superoxide dismutase (SOD) protein expression in coronary arterioles from young and old rats. A and B: aging increased protein levels of nitrotyrosine, a marker of oxidative stress. Catalase and SOD protein levels also increased with advancing age. C: real-time PCR analysis revealed an increase in SOD mRNA with aging in coronary arterioles. Values are means ± SE; n = 4 rats per group. *P < 0.05, significant difference from young rats.
DISCUSSION
The purpose of the current study was to determine the effects of aging and ROS scavenging on flow-mediated dilation in the coronary resistance arterioles of Fischer 344 rats. ROS are known to impart deleterious effects on the vasculature (9, 14, 38), and these effects may be heightened with aging (7, 27). Therefore, we hypothesized that ROS scavenging would improve flow-induced dilation, particularly in aged animals. Four major findings have emerged from this study. First, we confirmed previous reports (7, 21) that endothelium-dependent dilation is impaired with aging in coronary arterioles and extended these studies by demonstrating a concomitant decline in flow-induced NO production. Second, O2− scavenging impaired flow-mediated vasodilation in arterioles from young but not old rats, indicating that both the regulation and contribution of this ROS to flow-induced signaling change dramatically with age. Third, our results demonstrate that the contribution of H2O2 to flow-mediated dilation declines with age in coronary arterioles despite an increase in sensitivity to H2O2. Fourth, deferoxamine, an iron chelator that inhibits the conversion of H2O2 to OH−, ameliorated impairment of flow-induced dilation in an age-dependent manner, suggesting that aging increases the presence of non-heme iron in vascular tissue, leading to increased production of cytotoxic OH− and a decline in H2O2-dependent flow-induced signaling. These findings indicate that O2− and H2O2 are necessary components of flow-mediated vasodilation and that aging alters the balance between these ROS, contributing to the age-related impairment of flow-induced dilation in coronary arterioles.
Flow-mediated dilation is an important regulator of vascular control in the coronary resistance vasculature and requires an intact and functioning endothelium (20). Previous studies have indicated that NO-mediated endothelial function declines with advancing age (26, 43, 49), and recent reports have shown a similar impairment in endothelium-mediated vasodilation in the coronary microvasculature (7, 21). In the present study, we have demonstrated for the first time an age-induced decline in flow-induced NO production, as evidenced by diminished DAF fluorescence in isolated coronary arterioles of aged rats (Fig. 2). This decline in DAF fluorescence is NO specific, since treatment with l-NAME eliminated any increase in fluorescence intensity in response to flow in arterioles from both young and old rats (Fig. 3, A and C). These findings support a previous study in which Sun et al. (41) reported a decline in NO release in perfusate from mesenteric arteries of old rats.
ROS have been implicated in the development of age-related endothelial dysfunction (7, 10). Immunoblot analysis revealed that protein levels of NT, a marker of oxidant stress, increased significantly with aging in coronary microvessels (Fig. 8), similar to previous reports comparing young and middle-aged rats (7). Similarly, in situ examination of left ventricles from Sprague-Dawley rats revealed increased hydroethidine staining in old vs. young coronary vessels (7). In addition to heightened ROS production, the age-induced decline in endothelial function also may be associated with blunted antioxidant activity and, specifically, a reduction in SOD bioavailability. SOD activity in rat mesenteric arteries is suppressed with aging (41), and in old Mn-SOD-deficient mice, endothelium-dependent relaxation is dramatically reduced (48). Therefore, we hypothesized that the exogenous application of the SOD mimetic Tempol would improve flow-mediated dilation in isolated coronary vessels. We found that Tempol had no effect in arterioles from aged animals and, surprisingly, impaired the flow response in vessels from young rats (Fig. 6A). These results contrast with previous reports in the literature that describe the ameliorative effects of SOD and SOD analogs on vascular function (1, 6, 12, 14, 37, 41, 50). Our findings in old vessels coincide with those reported by Phillips et al. (28), in which treatment with polyethylene glycol-SOD, a cell-permeable SOD analog, had no effect on flow-mediated vasodilation in isolated human visceral adipose arterioles. Furthermore, recent findings from Herspring et al. (16) demonstrated a decline in skeletal muscle blood flow following treatment with Tempol in old rats. Our data contrast with findings of a previous study by Csiszar et al. (7), in which treatment with Tiron, a SOD mimetic, improved flow-induced dilation of septal arterioles from old Sprague-Dawley rats but had no effect in vessels from young rats. In addition, aging did not alter SOD protein levels in septal vessels (7), whereas our data indicate that both SOD protein and mRNA levels increase with aging (Fig. 8). It is notable that the septal artery is derived from a superficial portion of the right ventricular side of the septal wall, whereas, in the present study, vessels were obtained from within the left ventricular free wall. Previous studies have demonstrated increased glycolytic activity in left ventricle vs. septal tissue samples from bovine and rat hearts (36, 42). Therefore, it is possible that differences in metabolic demand of these vascular beds may contribute to the variant findings. These contrasting results also suggest that there may be strain-related differences in the response of coronary microvessels to SOD or its analogs.
SOD dismutates O2− into H2O2, which has known vasodilatory effects on the coronary vasculature (24, 46). It is possible that an increase in SOD might lead to increased H2O2 and, thus, enhanced vasodilation (25). Therefore, we investigated the effects of the H2O2 scavenger catalase on flow-mediated dilation in coronary arterioles from young and old rats. Catalase treatment blunted flow-induced vasodilation in vessels from both age groups, eliminating the age-related difference present under control conditions (Figs. 1 and 6B). Our findings support previous studies implicating H2O2 as a mediator of flow-induced dilation in coronary arterioles (24, 32). H2O2 has been proposed to act as a redox-sensitive dilator in canine coronary microvessels (31) and also has been identified as a critical mediator of endogenous vascular tone in arterioles from rat skeletal muscle (33) and porcine myocardium (46). DCF fluorescence intensity increased significantly in coronary arterioles exposed to flow, and authentic H2O2 induced marked dilation of coronary arterioles, indicating that H2O2 contributes to flow-mediated vasodilation in rat coronary arterioles (Fig. 4A).
To our knowledge, this is the first study to investigate the contribution of H2O2 to flow-mediated dilation in an aging model. Flow augmented DCF fluorescence intensity to a larger degree in vessels from young compared with old rats (Fig. 4B), indicating an age-induced impairment in flow-induced H2O2 release in coronary arterioles. Our data confirmed DCF specificity for H2O2, since catalase eliminated increases in flow-induced DCF fluorescence. Comparable to its effects on DCF fluorescence, catalase suppressed flow-induced increases in DAF fluorescence intensity in arterioles from both age groups (Fig. 3, B and D), suggesting a dependence of NO production on the presence of H2O2. Koller and Bagi (18) proposed that the reactive dilation of coronary arterioles involves an initial H2O2-dependent phase, followed by a later NO-dependent phase. This later NO phase is likely at least partly contingent on the early H2O2 phase, since intracellularly produced H2O2 has been shown to induce vasodilation through a guanylate cyclase-dependent pathway (34).
To determine whether age altered responsiveness of arterioles to H2O2, we administered increasing concentrations of exogenous H2O2 and found that sensitivity to H2O2 was enhanced with aging (Fig. 5). Aging similarly increased sensitivity to H2O2 in aortic rings from Wistar rats (44). Thus our data indicate that flow-induced H2O2-mediated signaling declines with advancing age (Fig. 4), despite increased sensitivity to authentic H2O2 with aging (Fig. 5). Previous studies by Sato et al. (34) suggest that exogenously and endogenously produced H2O2 may evoke differential vasodilatory mechanisms in coronary arterioles. Our data suggest that reduced endogenous H2O2 production is partially compensated by increased sensitivity to H2O2; however, an overall impairment of H2O2-mediated vasodilation appears to contribute to the age-associated decline in flow-mediated dilation.
Our experiments investigating the effects of Tempol and catalase on flow-mediated dilation suggest that mechanisms that regulate intracellular levels of O2− and H2O2 change with age. In coronary arterioles from young rats, treatment with either Tempol plus catalase or catalase alone produced nearly complete inhibition of flow-induced dilation. Contrarily, in vessels from aged rats, addition of Tempol to catalase-treated vessels reversed the catalase-induced impairment of flow-induced dilation (Fig. 6C). These data suggest that H2O2 is a major contributor to flow-induced dilation in coronary arterioles from young rats and that the contribution of H2O2 to flow-mediated dilation declines with age.
In the presence of heavy metals such as iron, H2O2 is quickly converted to hydroxyl radical (OH•), which has known vasoconstrictor properties (22). Previous studies indicate the presence of increased ferritin protein in cardiac muscle of rat hearts with aging (2), and in elderly humans, serum ferritin is similarly augmented (13). Elevated levels of non-heme iron may increase conversion of H2O2 to OH•, contributing to an overall decline in flow-mediated dilation with aging. Furthermore, previous reports have suggested that hydroxyl may contribute to reduced NO-mediated vasodilation (45). We examined flow-mediated dilation in the presence of Tempol plus deferoxamine, an iron chelator and inhibitor of OH• formation, and found that addition of deferoxamine induced a robust improvement in flow-induced dilation in Tempol-treated arterioles from both young and old rats (Fig. 7A). In old rats, flow-induced dilation was enhanced vs. dilation under control conditions (Fig. 1 vs. Fig. 7A). Furthermore, deferoxamine alone similarly improved flow-induced dilation in coronary arterioles from aged rats but had no effect in arterioles from young rats (Fig. 7B). Previous studies indicate that Tempol also may scavenge peroxynitrite as well as its free radical decomposition products (3). However, the direct effects of deferoxamine on Tempol-induced changes in vasodilation support the predominance of the O2−-scavenging effects of Tempol during flow-induced dilation in coronary arterioles. Collectively, our findings strongly suggest that the age-related decline in flow-induced vasodilation is due at least in part to increased OH• formation resulting from an age-associated increase in vascular levels of non-heme iron.
A proposed mechanism for ROS handling during flow-mediated dilation in coronary microvessels of young and aged rats is described in Fig. 9. In general, SOD dismutates O2− into H2O2, which in turn is scavenged by catalase. Although additional Tempol would be expected to increase H2O2 production and, thus, dilation, we found that Tempol reduced flow-induced dilation in vessels from young rats (Fig. 6A). It is possible that excess Tempol overwhelms the endogenous catalase system, thereby shunting more H2O2 toward conversion to OH− and increasing vasoconstriction. Accordingly, addition of catalase also reduces flow-induced dilation as vasodilatory H2O2 is converted to H2O. By contrast, in arterioles from old rats, with addition of Tempol plus catalase, we found no net effect on the flow response. Our data suggest that Tempol, compounded with basally elevated SOD, produces sufficient H2O2 to replace the H2O2 scavenged by catalase alone. In addition, we found that a dramatic age-related increase in endogenous SOD protein was not matched by a similar rise in catalase protein, and this disproportionate increase in the expression of these antioxidant enzymes may contribute to a relative imbalance between H2O2 production and H2O2 scavenging in advanced age.
Fig. 9.
H2O2 and O2− regulatory pathways involved in flow-mediated vasodilation. With aging, elevated Fe3+ leads to increased OH− production and diminished vasodilation. A dramatic age-related increase in SOD also may contribute to reduced flow-induced dilation with aging as the endogenous catalase system is overwhelmed and more H2O2 is shunted to formation of OH−. GC, guanylate cyclase.
In conclusion, our data demonstrate that both NO- and H2O2-mediated flow-induced signaling decline with advancing age in coronary arterioles. We propose that elevated hydroxyl radical formation contributes to impairment of flow-induced vasodilation with aging and that maintenance of a balanced O2−/H2O2 system is required for flow-induced vasodilation to occur. The current study demonstrates that disruption of this system, whether due to addition of exogenous scavenging agents or age-induced changes in endogenous scavenging systems, leads to impairment of coronary arteriolar responses to flow. Thus therapeutic interventions that restore the H2O2 and O2− balance in coronary arterioles may mitigate age-related impairments in H2O2- and NO-mediated endothelial dilation.
GRANTS
This work was supported by American Heart Association, Texas Affiliate, Grant 0255956 (to J. Muller-Delp) and National Heart, Lung, and Blood Institute Grant HL077224-01 (to J. Muller-Delp).
REFERENCES
- 1.Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287: H2448–H2453, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bulvik B, Grinberg L, Eliashar R, Berenshtein E, Chevion MM. Iron, ferritin and proteins of the methionine-centered redox cycle in young and old rat hearts. Mech Ageing Dev 130: 139–144, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Carroll RT, Galatsis P, Borosky S, Kopec KK, Kumar V, Althaus JS, Hall ED. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) inhibits peroxynitrite-mediated phenol nitration. Chem Res Toxicol 13: 294–300, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Cooke CL, Davidge ST. Endothelial-dependent vasodilation is reduced in mesenteric arteries from superoxide dismutase knockout mice. Cardiovasc Res 60: 635–642, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Delp MD, Evans MV, Duan C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J Appl Physiol 85: 1813–1822, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Didion SP, Ryan MJ, Baumbach GL, Sigmund CD, Faraci FM. Superoxide contributes to vascular dysfunction in mice that express human renin and angiotensinogen. Am J Physiol Heart Circ Physiol 283: H1569–H1576, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Emsley AM, Jeremy JY, Gomes GN, Angelini GD, Plane F. Investigation of the inhibitory effects of homocysteine and copper on nitric oxide-mediated relaxation of rat isolated aorta. Br J Pharmacol 126: 1034–1040, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng MG, Dukacz SA, Kline RL. Selective effect of tempol on renal medullary hemodynamics in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 281: R1420–R1425, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Fleming DJ, Jacques PF, Tucker KL, Massaro JM, D'Agostino RB Sr, Wilson PW, Wood RJ. Iron status of the free-living, elderly Framingham Heart Study cohort: an iron-replete population with a high prevalence of elevated iron stores. Am J Clin Nutr 73: 638–646, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation 115: 245–254, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hachamovitch R, Wicker P, Capasso JM, Anversa P. Alterations of coronary blood flow and reserve with aging in Fischer 344 rats. Am J Physiol Heart Circ Physiol 256: H66–H73, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Herspring KF, Ferreira LF, Copp SW, Snyder BS, Poole DC, Musch TI. Effects of antioxidants on contracting spinotrapezius muscle microvascular oxygenation and blood flow in aged rats. J Appl Physiol 105: 1889–1896, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Horiuchi M, Tsutsui M, Tasaki H, Morishita T, Suda O, Nakata S, Nihei S, Miyamoto M, Kouzuma R, Okazaki M, Yanagihara N, Adachi T, Nakashima Y. Upregulation of vascular extracellular superoxide dismutase in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol 24: 106–111, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Koller A, Bagi Z. Nitric oxide and H2O2 contribute to reactive dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 287: H2461–H2467, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol Heart Circ Physiol 261: H1706–H1715, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 259: H1063–H1070, 1990. [DOI] [PubMed] [Google Scholar]
- 21.LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295: H2280–H2288, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Li W, Liu W, Altura BT, Altura BM. Mechanisms of hydroxyl radical-induced contraction of rat aorta. Eur J Pharmacol 499: 171–178, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Li H, Bubolz AH, Zhang DX, Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med Biol Eng Comput 46: 469–478, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Morikawa K, Fujiki T, Matoba T, Kubota H, Hatanaka M, Takahashi S, Shimokawa H. Important role of superoxide dismutase in EDHF-mediated responses of human mesenteric arteries. J Cardiovasc Pharmacol 44: 552–556, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Newaz MA, Yousefipour Z, Oyekan A. Oxidative stress-associated vascular aging is xanthine oxidase-dependent but not NAD(P)H oxidase-dependent. J Cardiovasc Pharmacol 48: 88–94, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol 292: H93–H100, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Pittner J, Wolgast M, Persson AE. Perfusate composition influences nitric oxide homeostasis in rat juxtamedullary afferent arterioles. Acta Physiol Scand 179: 85–91, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Rogers PA, Chilian WM, Bratz IN, Bryan RM Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol 292: H1404–H1411, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN Jr, Saitoh S, Tune JD, Chilian WM. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol 291: H2473–H2482, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614–2621, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Samora JB, Frisbee JC, Boegehold MA. Hydrogen peroxide emerges as a regulator of tone in skeletal muscle arterioles during juvenile growth. Microcirculation 15: 151–161, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato A, Sakuma I, Gutterman DD. Mechanism of dilation to reactive oxygen species in human coronary arterioles. Am J Physiol Heart Circ Physiol 285: H2345–H2354, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res 66: 374–383, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Smith SH, Kramer MF, Reis I, Bishop SP, Ingwall JS. Regional changes in creatine kinase and myocyte size in hypertensive and nonhypertensive cardiac hypertrophy. Circ Res 67: 1334–1344, 1990. [DOI] [PubMed] [Google Scholar]
- 37.Sorop O, Spaan JA, Sweeney TE, VanBavel E. Effect of steady versus oscillating flow on porcine coronary arterioles: involvement of NO and superoxide anion. Circ Res 92: 1344–1351, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation 107: 1383–1389, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramaniam R, Fan XJ, Scivittaro V, Yang J, Ha CE, Petersen CE, Surewicz WK, Bhagavan NV, Weiss MF, Monnier VM. Cellular oxidant stress and advanced glycation endproducts of albumin: caveats of the dichlorofluorescein assay. Arch Biochem Biophys 400: 15–25, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sylven C, Lin L, Kallner A, Jansson F. Regional distribution of citrate synthase and lactate dehydrogenase isoenzymes in the bovine heart. Acta Physiol Scand 136: 331–337, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Tanguy S, Boucher F, Toufektsian MC, Besse S, de Leiris J. Aging exacerbates hydrogen peroxide-induced alteration of vascular reactivity in rats. Antioxid Redox Signal 2: 363–368, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol 26: 2035–2042, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol 285: H2255–H2263, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Wei EP, Kontos HA. H2O2 and endothelium-dependent cerebral arteriolar dilation. Implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension 16: 162–169, 1990. [DOI] [PubMed] [Google Scholar]
- 48.Wenzel P, Schuhmacher S, Kienhofer J, Muller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Munzel T, Burkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res 80: 280–289, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Yada T, Shimokawa H, Morikawa K, Takaki A, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Role of Cu,Zn-SOD in the synthesis of endogenous vasodilator hydrogen peroxide during reactive hyperemia in mouse mesenteric microcirculation in vivo. Am J Physiol Heart Circ Physiol 294: H441–H448, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Zollner S, Haseloff RF, Kirilyuk IA, Blasig IE, Rubanyi GM. Nitroxides increase the detectable amount of nitric oxide released from endothelial cells. J Biol Chem 272: 23076–23080, 1997. [DOI] [PubMed] [Google Scholar]