Abstract
Intermedin (IMD) is a newly discovered peptide closely related to adrenomedullin. We recently reported that IMD gene delivery prevented kidney damage and capillary loss in a rat model of chronic renal injury. In this study, we evaluated the role of IMD in angiogenesis in the ischemic hindlimb. Adenovirus containing human IMD or control adenovirus (Ad.Null) was injected into the adductor muscles of rats immediately after femoral artery ligation. The expression of human IMD was detected in the skeletal muscle 5 days after the viral injection. Blood perfusion in the ischemic hindlimb was monitored by laser-Doppler imaging from 1 to 3 wk after gene delivery. When compared with animals receiving Ad.Null, those with IMD gene transfer resulted in a time-dependent increase in blood perfusion. IMD gene delivery also increased capillary and arteriole density in ischemic hindlimb, identified by anti-CD-31 and α-smooth muscle actin immunostaining. Angiogenesis promoted by IMD was confirmed by increased capillary formation and hemoglobin content in Matrigel implants containing IMD peptide in mice. In cultured endothelial cells, IMD induced cell migration and tube formation, and these effects were blocked by the inhibition of extracellular signal-regulated kinase (ERK), Akt, nitric oxide (NO) synthase (NOS), vascular endothelial growth factor receptor-2 (VEGFR-2), and anti-IMD-neutralizing antibody. IMD was found to increase the phosphorylation of ERK, Akt, and endothelial NOS, as well as to augment NO formation, VEGF, and VEGFR-2 synthesis. Taken together, these results indicate that IMD enhances angiogenesis through ERK, Akt/NOS/NO, and VEGF/VEGFR-2 signaling pathways and raises the potential of IMD gene or peptide administration in the modulation of endothelial dysfunction.
Keywords: adenovirus, gene transfer, ischemia
peripheral artery disease (PAD) is a consequence of multiple diseases, such as hypertension, diabetes, and hyperlipidemia, and may lead to insufficient blood flow in the distal limbs (25). Although several options for PAD treatment are available (e.g., pharmacological intervention or surgical revascularization), leg amputation in cases of severe limb ischemia may be required if conventional approaches are unsuccessful. In the last decade and a half, this realization has prompted the investigation of therapeutic angiogenesis as a means to restore blood supply to the ischemic limb. Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor are just two of the several cytokines that participate in the angiogenic process. Accordingly, numerous studies have demonstrated the efficacy of angiogenic growth factor administration in restoring perfusion in patients with PAD and in animal models of tissue ischemia (18, 25).
Intermedin [IMD, also known as adrenomedullin-2 (AM-2)] is a newly discovered peptide that belongs to the calcitonin/calcitonin gene-related peptide family and shares ∼30% amino acid sequence homology with AM (26, 30). Like AM, IMD is expressed in many organs of the body, including the gastrointestinal tract, pancreas, and lung (3, 26, 30). Both AM and IMD are also localized in the tissues of the cardiovascular and renal systems and exert their biological actions by stimulation of calcitonin receptor-like receptor/receptor activity-modifying protein complexes (2, 22, 26). Reports have indicated that IMD peptide administration in rats induces blood pressure reduction, renal blood flow, and cardiac output (7–9). Moreover, ischemia-induced cardiac injury is reduced upon IMD peptide treatment (12, 32). Our recent study demonstrated that IMD gene transfer prevented endothelial cell loss, kidney damage, inflammation, and fibrosis in salt-induced hypertensive rats (10). Comparable hemodynamic, cardiac, and renal effects have been observed with AM (3, 4). AM has recently emerged as a powerful angiogenic growth factor (23). Added to the fact that AM and IMD share similar structural, chemical, and biological characteristics and mediate their effects through the same receptor complexes, we hypothesized that IMD may also be angiogenic. Therefore, the aim of this study was to determine the role of IMD on neovascularization of the rat ischemic hindlimb and in cultured endothelial cells.
MATERIALS AND METHODS
Construction of the IMD adenovirus.
Adenovirus was generated using plasmids generously supplied by Dr. Mark Kay (Stanford University), according to a method previously described (21). The plasmid DNA pShuttle was linearized by enzyme digestion at the SmaI site. A blunt-end cytomegalovirus (CMV)-IMD-polyA sequence (pA) fragment was obtained by excising the DNA from the pcDNA3.CMV-IMD-pA plasmid (graciously provided by Sheau-Yu Teddy Hsu, Stanford University) and ligated to the shuttle vector. The CMV-IMD-pA transcription unit was released from the shuttle vector by I-CeuI and PI-SceI sequentially. The shuttle insert was then ligated to the adenoviral genome backbone plasmid pAdHM4 cut with the same enzymes. The final construct, pAdHM4.CMV-IMD-4F2-pA, was digested by PacI to release the complete linear adenoviral DNA. The DNA was transiently transfected into HEK-293 cells using Effectene reagent (Qiagen, Valencia, CA) according to the manufacturer's protocol. The resulting virus, adenovirus containing human IMD (Ad.IMD), was serially passaged five times.
Femoral artery ligation and adenovirus administration.
All procedures complied with the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Resources, National Academy of Sciences, Bethesda, MD). The protocol for our animal studies was approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. Male Wistar rats (Sprague-Dawley, Harlan, Indianapolis, IN) weighing ∼200 g were anesthetized by an intraperitoneal injection of 90 mg/kg ketamine and 10 mg/kg xylazine mixture in phosphate-buffered saline (PBS). For femoral artery ligation, an incision was made along the inner left hindlimb along the line of the femoral artery and vein, and the proximal end of the femoral artery was tied with 4.0 silk suture (Ethicon, Piscataway, NJ) and electrocauterized. The femoral artery was dissected free from the limb and its peripheral branches. The distal end was severed, and the artery was removed. Each rat received a local injection of Ad.IMD or empty virus (Ad.Null) (1 × 1010 plaque-forming units/rat in 100 μl of 10 mM Tris·HCl, pH 8.0), divided among four to five injection sites in the adductor and surrounding muscles. All virus injections were administered immediately after surgery. Three weeks after viral delivery, the rats were euthanized with the ketamine-xylazine cocktail. Rat hindlimb skeletal muscle was perfused with PBS followed by formalin and then embedded in paraffin for immunohistochemical staining.
Laser-Doppler imaging.
A laser-Doppler blood flow meter (Laser-Doppler Perfusion Imager System, Perimed) was used to evaluate the perfusion of both left (ischemic) and right (nonischemic) rat hindlimbs. The perfusion signal is subdivided into six different intervals, each displayed as a separate color. Low or no perfusion is displayed as dark blue, whereas the highest perfusion interval is displayed as red. The stored perfusion values behind the color-coded pixels remain available for data analysis. Excess hair was removed from the hindlimbs using a shaver. Before and during scanning, the animals were placed on a heating pad at 37°C to minimize variations in temperature. After the laser-Doppler images were recorded, the average perfusion values of the ischemic and nonischemic limbs were calculated on the basis of colored histogram pixels. To minimize variables such as ambient light and temperature, the calculated perfusion was expressed as the ratio of ischemic to nonischemic hindlimb perfusion in each animal. Perfusion analyses were performed for three consecutive weeks after surgery.
Immunostaining of capillaries and arterioles.
Skeletal muscle tissue sections (4 μm) were cut and stained with antibody to CD-31 (PharMingen, San Jose, CA) or α-smooth muscle actin (Sigma, St. Louis, MO) using a Vectastain ABC kit (Vector, Burlingame, CA). Capillaries and arterioles were counted in 10 random fields.
Matrigel implants.
Male mice (25 g body wt, Harlan) were subcutaneously injected with 0.5 ml growth factor-reduced Matrigel (BD Biosciences, San Jose, CA) containing 75 ng of human IMD8-47 (Phoenix Pharmaceuticals, Burlingame, CA) or VEGF (Sigma) in the abdomen as previously described (24). After 7 days, the Matrigel plugs were removed from mice and separated from the skin. The plugs were processed for hemoglobin content using a hemoglobin diagnostic kit (Sigma) or were embedded in paraffin and sectioned (4 μm) for Masson's trichrome staining. The blood vessels in the Matrigel implants were counted in 10 random fields.
Cultured endothelial cell migration.
Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (San Diego, CA) and cultured in endothelial growth medium-2 (Lonza, Walkersville, MD). HUVECs were seeded into 12-well plates and scratched with a 200-μl pipette tip. After washing with PBS and replacing serum-free medium with 0.1% FBS, IMD or VEGF (20 ng/ml each) was added to the wells. Twenty-four hours later, the cells were visualized and photographed. Modified Boyden chambers (Sigma) were also used to measure endothelial cell migration. The lower surface of the chambers was coated with a solution of collagen I (10 μg/ml) in PBS for 1 h at 37°C and blocked with 1% bovine serum albumin (BSA). The cells were harvested with trypsin-EDTA and washed once with serum-free cultured medium containing 20 μg/ml of trypsin inhibitor. The cells (2 × 105) were added in 200 μl of endothelial basal medium-2 (EBM-2; Lonza) containing 0.1% BSA to the upper chamber. IMD (10, 20, or 100 ng/ml) was added to the bottom chamber in the presence or absence of inhibitors (Calbiochem, La Jolla, CA) against nitric oxide (NO) synthase (NOS) isoforms [Nω-nitro-l-arginine methyl ester (l-NAME), 100 μM], MEK1 (PD-98059, 5 μM), phosphatidylinositol 3-kinase (PI3K; LY-294002, 10 μM), or VEGF receptor (VEGFR) tyrosine kinases (TKi, 10 μM) in 0.5 ml of EBM-2 with 0.1% BSA. The cells were incubated at 37°C for 16 h. In an additional experiment, IMD (20 ng/ml) was preincubated with anti-IMD antibody (50 μg/ml; Phoenix Pharmaceuticals) at 37°C for 1 h before the migration assay. The upper surface of the membrane was wiped with a cotton tip to mechanically remove nonmigratory cells. The migrant cells attached to the lower surface were fixed in 4% formalin at room temperature for 30 min and then stained for 20 min with a solution containing 1% crystal violet and 2% ethanol in 100 mM borate buffer (pH 9.0). After being washed, the cells on the lower surface of the filter were counted from six random fields. The migration rate is indicated by the total number of cells per field (400×). Each group was analyzed in duplicate wells in three separate experiments.
Cultured endothelial cell tube formation.
Growth factor-reduced Matrigel (BD Biosciences) was added to 24-well culture plates (200 μl per well). After polymerization at 37°C for 30 min, the gels were overlaid with a total of 4 × 104 HUVECs in 500 μl of EBM-2 supplemented with 0.1% fetal bovine serum. The cells were incubated with PBS or 20 ng/ml of IMD in the presence or absence of l-NAME (100 μM), PD-98059 (5 μM), LY-294002 (10 μM), or TKi (10 μM) for 24 h. In an additional experiment, IMD was preincubated with anti-IMD antibody at 37°C for 1 h before tube formation assay. Tube images were obtained using an inverted microscope.
Western blot analyses, nitrate/nitrite, and VEGF measurements.
The cell lysates of HUVECs were subjected to Western blot analysis for the detection of total and phosphorylated forms of ERK, Akt, and endothelial NOS (eNOS) (Cell Signaling). The nitrocellulose membranes were incubated with secondary antibody conjugated to horseradish peroxidase. Chemiluminescence was detected using an ECL-Plus kit (Perkin Elmer Life Science, Boston, MA) and visualized by Kodak X-ray film. The protein concentration was determined by Bio-Rad DC Protein Assay kit (Bio-Rad, Hercules, CA). Nitrate/nitrite levels (indicative of NO formation) in the culture medium were determined by fluorometric assay as previously described (19). Human VEGF levels were determined in cell culture medium 24 h after cell stimulation and were measured using a commercial ELISA kit (R&D Systems, Minneapolis, MN).
RT-PCR and real-time PCR.
Total RNA was isolated from rat skeletal muscle using TRIzol reagent (Invitrogen, Carlsbad, CA). Human IMD expression was determined by RT-PCR 5 days after femoral artery ligation. The sequences for the IMD primers were TGT TAT GGG TCA GCC TCT CG (forward) and TGG CTG AGA TTC TGC ACC TGG (reverse). The conditions for PCR and primer sequences were obtained from previously published data (16). For measuring VEGF and VEGFR-2 mRNA levels, the cells were serum starved overnight and were incubated with IMD (20 ng/ml) for 18 h. Total mRNA was prepared and cDNA was transcribed using a High cDNA Archive kit according to the manufacturer's protocol (Applied Biosystems, Foster, CA). Primers for VEGF and VEGFR-2 were purchased from Applied Biosystems. Real-time PCR analysis was then performed using the TaqMan Gene Expression Master Mix (Applied Biosystems).
Statistical analysis.
Data were analyzed by standard statistical methods and ANOVA followed by Fisher protected least significant difference and Bonferroni post hoc tests when appropriate. Group data were expressed as means ± SE. The values of all parameters were considered significantly different at a value of P < 0.05.
RESULTS
Human IMD expression after gene delivery.
Five days after femoral artery ligation and adenoviral injection, skeletal muscle RNA was extracted for RT-PCR analysis. Human IMD expression was detected in rat hindlimbs injected with Ad.IMD but not in rats receiving control virus (Fig. 1A).
Fig. 1.
Intermedin (IMD) gene delivery completely restores blood flow 3 wk after femoral artery ligation (FAL). A: amino acid sequence of the human mature peptide IMD8-47 (hIMD8-47). B: RT-PCR for hIMD was performed on skeletal muscle RNA 5 days after FAL and adenovirus injection. C: laser-Doppler imaging was performed weekly on rats subjected to hindlimb ischemia. D: results from Doppler images were quantified using Perimed software and expressed as percent perfusion of ischemic limb compared with normal limb. AD.IMD and Ad.Null, adenovirus containing hIMD or control adenovirus, respectively. Results are expressed as means ± SE (n = 8 animals).
Laser-Doppler perfusion imaging.
The ability of IMD gene transfer to augment blood perfusion in the ischemic hindlimb of rats was measured by laser-Doppler imaging (Fig. 1B). This noninvasive technique allows for multiple time points of blood flow to be assessed. Blood flow continued to increase in the ischemic limbs receiving IMD administration compared with the controls through week 3 in both groups (Fig. 1C). At week 1, a quantitative analysis showed a significant elevation in blood flow in rats receiving Ad.IMD compared with control rats. At week 3, blood perfusion was almost completely restored to levels of the normoperfused contralateral limb in rats injected with Ad.IMD (96.3 ± 1.9%, surgical vs. contralateral limb), whereas control rats receiving Ad.Null were restored to only 71.1 ± 1.0% of the normoperfused contralateral limb.
IMD gene transfer increases capillary and arteriole density in the ischemic hindlimb.
Capillaries and arterioles within skeletal muscle sections were visualized by immunostaining with antibody against CD-31 and α-smooth muscle actin, respectively, 3 wk after the induction of hindlimb ischemia. Representative images demonstrated that IMD gene delivery markedly increased both capillary and arteriole immunostaining (Fig. 2A). Quantitative analysis showed that IMD gene transfer significantly increased capillary density in the ischemic hindlimb compared with rats injected with Ad.Null (Fig. 2B; 513.3 ± 9.2 vs. 377.4 ± 9.5 capillaries/mm2, n = 8, P < 0.01). Arteriole density quantification also revealed a marked increase in arterioles by IMD gene transfer compared with control rats (Fig. 2C; 235.5 ± 7.2 vs. 119.4 ± 6.0 arterioles/mm2, n = 8, P < 0.01).
Fig. 2.
IMD gene delivery increases capillary and arteriole density in ischemic hindlimbs 3 wk after FAL. A: limb muscles were immunostained with antibodies against CD-31 and α-smooth muscle actin (α-SMA). Capillaries (B) and arterioles (C) were quantified in 10 random fields. Results are expressed as means ± SE (n = 8 animals).
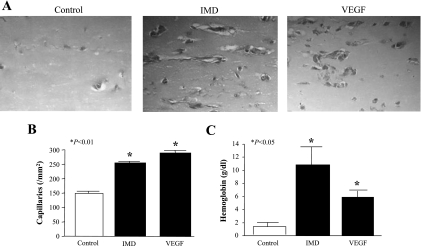
IMD increases neovascularization in Matrigel plugs in mice.
To further investigate the angiogenic potential of IMD, we injected mice with Matrigel containing IMD or VEGF. Matrigel plugs were removed 1 wk after Matrigel implantation and stained with Masson's trichrome to visualize newly formed blood vessels (Fig. 3A). We found a significant increase in the number of capillaries in animals that received IMD and VEGF versus those that received Matrigel only (Fig. 3B; IMD, 252 ± 27, and VEGF, 268 ± 20 vs. control, 152 ± 25 capillaries/mm2, n = 6–7, P < 0.01). Hemoglobin content was also increased in Matrigel plugs containing IMD and VEGF compared with Matrigel only (Fig. 3C; IMD, 10.8 ± 2.4, and VEGF, 5.9 ± 1.0 vs. control, 1.4 ± 0.3 g/dl, n = 4, P < 0.05).
Fig. 3.
IMD increases blood vessel growth in Matrigel implants in mice. A: Matrigel, containing IMD or VEGF, was injected subcutaneously into mice. One week later, Matrigel plugs were removed and subjected to Masson's trichrome staining to visualize capillary growth. B: vessels were quantified in 10 random fields. Results are expressed as means ± SE (n = 6 animals). C: hemoglobin content was analyzed in Matrigel plugs. Results are expressed as means ± SE (n = 4 animals).
IMD increases HUVEC migration and tube formation.
HUVEC migration was stimulated by scratch injury in the presence or absence of IMD or VEGF. Twenty-four hours later, the cells showed a significant migration in response to both IMD and VEGF compared with that of control cells (Fig. 4A). Cell migration was also determined by the use of modified Boyden chambers, which indicated that IMD dose-dependently increased HUVEC migration (Fig. 4B). However, inhibitors of ERK, PI3K, NOS, and VEGFR-2 all blocked the migratory effect of IMD (Fig. 4C), denoting their involvement in mediating the actions of IMD on endothelial cell migration. Preincubation with anti-IMD antibody blocked the ability of IMD to induce HUVEC migration (Fig. 4D). In addition, endothelial cell tube formation was evaluated by seeding HUVECs onto Matrigel-coated cell culture plates. After 24 h, tube formation was observed in cells stimulated with IMD (Fig. 5A). Quantitation of tube length indicated that IMD increased tube formation by 50% compared with control, but the effect was abolished upon the inhibition of ERK, PI3K, NOS, and VEGFR-2 (Fig. 5B). Representative images and quantitative analysis indicated that anti-IMD antibody blocked IMD-induced tube formation (Fig. 5, C and D). [3H]thymidine incorporation assay for the measurement of HUVEC proliferation showed that IMD had no effect on the proliferation of endothelial cells (data not shown).
Fig. 4.
IMD stimulates endothelial cell migration. A: human umbilical vein endothelial cells (HUVECs) underwent scratch wound injury followed by stimulation with IMD or VEGF (20 ng/ml) for 24 h to visualize cell migration. B: IMD dose-dependently increased HUVEC migration in modified Boyden chambers after 16 h. C: induction of HUVEC migration by IMD (20 ng/ml) in modified Boyden chambers was blocked by Nω-nitro-l-arginine methyl ester (l-NAME), tyrosine kinase inhibitor (TKi), PD-98059 (PD), and LY-294002 (LY) after 16 h. D: neutralization of IMD by anti-IMD antibody (Ab) abrogated HUVEC migration. Results are expressed as means ± SE (n = 2 experiments/group, each experiment repeated twice).
Fig. 5.
IMD stimulates endothelial tube formation. A: HUVECs were incubated on Matrigel-coated plates with IMD (20 ng/ml) for 24 h to detect capillary tube formation. IMD-induced tube formation was blocked by l-NAME, TKi, PD, and LY. B: quantitation of tube length. C: neutralization of IMD by anti-IMD antibody abrogated tube formation of HUVECs. D: quantitation of tube length. Results are expressed as means ± SE (n = 2 experiments/group, each experiment repeated twice).
IMD signaling pathways in HUVECs.
Because of the role of ERK, PI3K, and NOS in IMD-induced endothelial cell migration, the signaling mechanisms of IMD on endothelial cells were elucidated by stimulating HUVECs with IMD at various doses and for various times. Western blot analysis showed that increasing concentrations of IMD elevated the phosphorylation of ERK and Akt after 5 min of incubation (Fig. 6A). Moreover, IMD caused a peak stimulation of ERK phosphorylation around 5 min, whereas eNOS phosphorylation levels were highest around 30 min (Fig. 6B). In addition, IMD increased nitrate/nitrite levels secreted in the culture medium (Fig. 6C). These results demonstrate that IMD stimulates the activation/phosphorylation of ERK, Akt, and eNOS and that these signaling molecules facilitate the effects of IMD on endothelial cell migration and tube formation. Since VEGFR-2 inhibition repressed the ability of IMD to promote endothelial cell migration and tube formation, we investigated whether IMD induces VEGF synthesis. Indeed, IMD upregulated VEGF protein levels as well as the mRNA levels of VEGF and VEGFR-2 (Fig. 6, D–F), indicating that IMD may also indirectly elicit its angiogenic effects through VEGF/VEGFR-2 signaling pathway.
Fig. 6.
Phosphorylation of ERK (p-ERK), Akt (p-Akt), and endothelial nitric oxide (NO) synthase (eNOS) (p-eNOS) is increased by IMD, as determined by Western blot analysis. A: IMD increased ERK and Akt phosphorylation in HUVECs after incubation for 5 min. B: IMD (20 ng/ml) elevated the phosphorylation of ERK and eNOS at different time points. IMD increased nitrate/nitrite (NOx) levels (C) and VEGF (D) protein levels in culture medium. VEGF (E) and VEGF receptor-2 (VEGFR-2) mRNA expression (F) was determined by real-time PCR.
DISCUSSION
In the current study, we demonstrate for the first time that IMD is a novel and potent angiogenic peptide. Human IMD gene transfer in the rat ischemic hindlimb caused a marked increase in capillary and arteriole density compared with the contralateral normoperfused limb, indicating that IMD is not only proangiogenic but augments arteriogenesis as well. This enhancement of neovascularization by IMD was accompanied by a complete restoration of blood flow. The implantation of Matrigel plugs containing IMD in mice underscored the ability of IMD to induce blood vessel growth. In support of our in vivo findings, IMD promoted the migration and tube formation of cultured endothelial cells. These results indicate that IMD enhances blood perfusion and neovascularization in limb ischemia and may, therefore, be a potential therapeutic agent for the treatment of peripheral vascular disease. It should be noted, however, that unchecked angiogenesis would be disastrous in patients with cancer. In order for IMD or any other angiogenic factor to be used in a clinical setting, the duration and location of protein expression (such as in the case of vector delivery) would need to be tightly monitored and regulated.
The PI3K-Akt-eNOS signaling cascade is the central pathway for the induction of angiogenesis in response to proangiogenic stimuli (5, 13, 14, 29). ERK has also been shown to play a role in endothelial cell proliferation induced by VEGF, though in a manner independent of PI3K (31). Since angiogenesis appears to rely on both ERK activation and the PI3K-Akt-eNOS pathway, we evaluated the effects of IMD on these signaling molecules in cultured endothelial cells. IMD was able to upregulate Akt and eNOS phosphorylation and NO levels of endothelial cells in vitro. However, blockade of PI3K and eNOS abolished the migratory effect of IMD as well as the ability of IMD to promote tube formation. As with other angiogenic factors, this suggests that eNOS is critical in the stimulation of endothelial cell mobility by IMD. In addition, inhibition of ERK activity suppressed IMD-induced endothelial migration and tube formation. Kim et al. (15) observed similar results with AM and the PI3K-Akt and ERK signaling pathways in regard to endothelial cell migration and tube formation. However, Miyashita and coworkers (20) reported that AM promotes endothelial migration via PI3K but not NOS activation. However, this discrepancy may be due to their use of a tenfold lower concentration of l-NAME as well as a higher molar amount of AM compared with that used in our study for l-NAME and IMD. Nevertheless, these data collectively support the notion that IMD activates the PI3K-Akt-eNOS pathway and stimulates ERK signaling in endothelial cells.
Interestingly, IMD-induced endothelial cell migration was also blocked by the VEGFR-2 inhibitor. It is plausible that the blockade of VEGFR-2 prevents the cellular response of VEGF, which was upregulated by IMD. Indeed, the stimulation of eNOS phosphorylation and NO production by IMD may be responsible for the increase in VEGF expression, since it has been shown that the PI3K-NO pathway can promote VEGF synthesis in endothelial cells, vascular smooth muscle cells, and cardiomyocytes (6, 13, 17). Thus the effects of IMD may be mediated in part by VEGF.
Our study indicates that IMD is a new angiogenic growth factor by promoting endothelial cell migration, tube formation, and neovascularization through ERK, PI3K-Akt-eNOS-NO, and VEGF signaling pathways (Fig. 7). Endothelial progenitor cells (EPCs) have received much attention in recent years for their capacity in reendothelialization and vascular repair. These cells mobilize from the bone marrow to sites of tissue ischemia and differentiate into mature endothelial cells, thus contributing to neovascularization after vascular injury (27). eNOS-derived NO has been shown to serve as a physiological regulator of EPC mobilization (28). It is also known that VEGF is able to stimulate the migration of EPCs to areas of blood vessel growth (1). Since IMD was able to increase VEGF and VEGFR-2 expression and NO formation, it could potentially enhance EPC recruitment. Interestingly, AM was reported to augment the angiogenic potency of transplanted bone marrow-derived mononuclear cells (MNCs), which include EPCs, in a rat model of hindlimb ischemia (11). Furthermore, it was demonstrated in vitro that AM promoted MNC adhesion to a HUVEC monolayer and increased the number of MNC-derived EPCs (11). Because of the similar biological actions of AM and IMD, it is essential to further investigate the role of IMD in EPC mobilization and vascular repair in arterial diseases.
Fig. 7.
Proposed mechanism of the effects of IMD on endothelial cells. CRLR, calcitonin receptor-like receptor; RAMP, receptor activity-modifying protein; PI3K, phosphatidylinositol 3-kinase.
GRANTS
This work was supported by National Institutes of Health Grants HL-29397 and DK-066350 and by Extramural Research Facilities Program of the National Center for Research Resources Grant C06-RR015455.
REFERENCES
- 1.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18: 3964–3972, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell D, McDermott BJ. Intermedin (adrenomedullin-2): a novel counter-regulatory peptide in the cardiovascular and renal systems. Br J Pharmacol 153, Suppl 1: S247–S262, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunton DC, Petrie MC, Hillier C, Johnston F, McMurray JJ. The clinical relevance of adrenomedullin: a promising profile? Pharmacol Ther 103: 179–201, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Chao J, Kato K, Zhang JJ, Dobrzynski E, Wang C, Agata J, Chao L. Human adrenomedullin gene delivery protects against cardiovascular remodeling and renal injury. Peptides 22: 1731–1737, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res 86: 4–5, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Doronzo G, Russo I, Mattiello L, Anfossi G, Bosia A, Trovati M. Insulin activates vascular endothelial growth factor in vascular smooth muscle cells: influence of nitric oxide and of insulin resistance. Eur J Clin Invest 34: 664–673, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Fujisawa Y, Nagai Y, Miyatake A, Miura K, Nishiyama A, Kimura S, Abe Y. Effects of adrenomedullin 2 on regional hemodynamics in conscious rats. Eur J Pharmacol 558: 128–132, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Fujisawa Y, Nagai Y, Miyatake A, Miura K, Shokoji T, Nishiyama A, Kimura S, Abe Y. Roles of adrenomedullin 2 in regulating the cardiovascular and sympathetic nervous systems in conscious rats. Am J Physiol Heart Circ Physiol 290: H1120–H1127, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa Y, Nagai Y, Miyatake A, Takei Y, Miura K, Shoukouji T, Nishiyama A, Kimura S, Abe Y. Renal effects of a new member of adrenomedullin family, adrenomedullin2, in rats. Eur J Pharmacol 497: 75–80, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Hagiwara M, Bledsoe G, Yang ZR, Smith RS Jr, Chao L, Chao J. Intermedin ameliorates vascular and renal injury by inhibition of oxidative stress. Am J Physiol Renal Physiol 295: F1735–F1743, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwase T, Nagaya N, Fujii T, Itoh T, Ishibashi-Ueda H, Yamagishi M, Miyatake K, Matsumoto T, Kitamura S, Kangawa K. Adrenomedullin enhances angiogenic potency of bone marrow transplantation in a rat model of hindlimb ischemia. Circulation 111: 356–362, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Jia YX, Yang JH, Pan CS, Geng B, Zhang J, Xiao Y, Zhao J, Gerns H, Yang J, Chang JK, Wen JK, Tang CS, Qi YF. Intermedin1–53 protects the heart against isoproterenol-induced ischemic injury in rats. Eur J Pharmacol 549: 117–123, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta 1784: 150–158, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Katusic ZS. Therapeutic angiogenesis: new indication for endothelial NO synthase gene transfer. Arterioscler Thromb Vasc Biol 22: 1254–1255, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Kim W, Moon SO, Sung MJ, Kim SH, Lee S, So JN, Park SK. Angiogenic role of adrenomedullin through activation of Akt, mitogen-activated protein kinase, and focal adhesion kinase in endothelial cells. FASEB J 17: 1937–1939, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kindt F, Wiegand S, Loser C, Nilles M, Niemeier V, Hsu SY, Steinhoff M, Kummer W, Gieler U, Haberberger RV. Intermedin: a skin peptide that is downregulated in atopic dermatitis. J Invest Dermatol 127: 605–613, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Kuwabara M, Kakinuma Y, Ando M, Katare RG, Yamasaki F, Doi Y, Sato T. Nitric oxide stimulates vascular endothelial growth factor production in cardiomyocytes involved in angiogenesis. J Physiol Sci 56: 95–101, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Mikroulis D, Papanas N, Maltezos E, Bougioukas G. Angiogenic growth factors in the treatment of peripheral arterial disease. Curr Vasc Pharmacol 5: 195–209, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem 214: 11–16, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Miyashita K, Itoh H, Sawada N, Fukunaga Y, Sone M, Yamahara K, Yurugi T, Nakao K. Adrenomedullin promotes proliferation and migration of cultured endothelial cells. Hypertens Res 26, Suppl: S93–S98, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Mizuguchi H, Kay MA. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum Gene Ther 9: 2577–2583, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto R, Satoh F, Murakami O, Totsune K, Suzuki T, Sasano H, Ito S, Takahashi K. Expression of adrenomedullin2/intermedin in human brain, heart, and kidney. Peptides 28: 1095–1103, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Nagaya N, Mori H, Murakami S, Kangawa K, Kitamura S. Adrenomedullin: angiogenesis and gene therapy. Am J Physiol Regul Integr Comp Physiol 288: R1432–R1437, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 67: 519–528, 1992. [PubMed] [Google Scholar]
- 25.Rissanen TT, Vajanto I, Ylä-Herttuala S. Gene therapy for therapeutic angiogenesis in critically ischaemic lower limb—on the way to the clinic. Eur J Clin Invest 31: 651–666, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem 279: 7264–7274, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Rumpold H, Wolf D, Koeck R, Gunsilius E. Endothelial progenitor cells: a source for therapeutic vasculogenesis? J Cell Mol Med 8: 509–518, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki K, Heeschen C, Aicher A, Ziebart T, Honold J, Urbich C, Rossig L, Koehl U, Koyanagi M, Mohamed A, Brandes RP, Martin H, Zeiher AM, Dimmeler S. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA 103: 14537–14541, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith RS Jr, Lin KF, Agata J, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler Thromb Vasc Biol 22: 1279–1285, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett 556: 53–58, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Wu LW, Mayo LD, Dunbar JD, Kessler KM, Baerwald MR, Jaffe EA, Wang D, Warren RS, Donner DB. Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J Biol Chem 275: 5096–5103, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Yang JH, Qi YF, Jia YX, Pan CS, Zhao J, Yang J, Chang JK, Tang CS. Protective effects of intermedin/adrenomedullin2 on ischemia/reperfusion injury in isolated rat hearts. Peptides 26: 501–507, 2005. [DOI] [PubMed] [Google Scholar]