Abstract
Prolonged ouabain administration to normal rats causes sustained blood pressure (BP) elevation. This ouabain-induced hypertension (OH) has been attributed, in part, to the narrowing of third-order resistance arteries (∼320 μm internal diameter) as a result of collagen deposition in the artery media (see Ref. 6). Here we describe the structural and functional properties of fourth-order mesenteric small arteries from control and OH rats, including the effect of low-dose ouabain on myogenic tone in these arteries. Systolic BP in OH rats was 138 ± 3 versus 124 ± 4 mmHg in controls (P < 0.01). Pressurized (70 mmHg) control and OH arteries, with only a single layer of myocytes, both had ∼165-μm internal diameters and ∼20-μm wall thicknesses. Even after fixation, despite vasoconstriction, the diameters and wall thicknesses did not differ between control and OH fourth-order arteries, whereas in third-order arteries, both parameters were significantly smaller in OH than in controls. Myogenic reactivity was significantly augmented in OH fourth-order arteries. Nevertheless, phenylephrine- (1 μM) and high K+-induced vasoconstrictions and acetylcholine-induced vasodilation were comparable in control and OH arteries. Vasoconstrictions induced by 5 μM phenylephrine and by 10 mM caffeine in Ca2+-free media indicated that releasable sarcoplasmic reticulum Ca2+ stores were normal in OH arteries. Importantly, 100 nM ouabain constricted both control and OH arteries by ∼26 μm, indicating that this response was not downregulated in OH rats. This maximal ouabain-induced constriction corresponds to a ∼90% increase in resistance to flow in these small arteries; thus ouabain at EC50 of ∼0.66 nM should raise resistance by ∼35%. We conclude that dynamic constriction in response to circulating nanomolar ouabain in small arteries likely makes a major contribution to the increased vascular tone and BP in OH rats.
Keywords: myogenic reactivity, resistance artery
ouabain, a mammalian adrenocortical hormone (4, 20, 21, 28), apparently plays an important role in the pathogenesis of hypertension in humans and rodents. About 50% of patients with essential hypertension and a majority of patients with adrenocortical adenomas and hypertension have elevated plasma endogenous ouabain (EO) (42, 45). In normal humans, ouabain infusion increases peripheral vascular resistance, reduces blood flow, and elevates blood pressure (BP) (35, 47). Moreover, high-dietary salt increases plasma EO levels in normal men (32).
Elevated EO levels are also observed in rats with DOCA-salt hypertension (20), reduced renal mass hypertension (24), and Milan strain hypertension (14). Additionally, the chronic treatment of normal rats and mice with ouabain elevates BP, i.e., ouabain-induced hypertension (OH) (12, 33, 50). Although digoxin, like ouabain, inhibits Na+ pumps (17), chronic digoxin administration to rats not only fails to elevate BP but even blocks the effect of ouabain on BP (23, 33). This might suggest that the effect of ouabain on BP is not the result of a reduced Na+ pump activity. However, the genetically reduced expression and activity of the ouabain-sensitive Na+ pump α2-catalytic subunit also causes hypertension (4, 51). Furthermore, the mutation of the α2-Na+ pump ouabain binding site abolishes the hypertensinogenic effect of ouabain (12).
In addition to its influence on ion transport, ouabain is a growth factor that activates signaling cascades via a Src kinase pathway linked to the Na+,K+-ATPase but independent of the influence of ouabain on ion distribution (31, 46). Consequently, ouabain may have multiple and complex effects in vivo, possibly including vascular growth and remodeling. Indeed, a number of studies indicate that vascular growth and remodeling may play a central role in the pathogenesis of hypertension (15, 22, 36). In at least several forms of hypertension, however, the remodeling appears to be an adaptive response rather than the cause of the elevated BP (7, 22, 34).
Many investigators have compared the acute effects of ouabain on arteries isolated from normotensive and hypertensive rats, but most have studied large arteries (e.g., aorta) and high concentrations (>10 μM) of ouabain (9, 40). A few studies on arteries from OH rats may provide information about the pathogenesis of the hypertension, but the data are inconsistent. Contractile responses to high K+, but not phenylephrine (PE), were augmented in the main renal arteries from OH rats, whereas acetylcholine (ACh)-induced relaxation was normal (25). In the mesenteric arteries from OH rats, however, nitric oxide (NO) and prostanoid production were increased, but endothelium-derived hyperpolarizing factor was decreased (41, 49). On the other hand, endothelial NO production was impaired in renal-descending vasa recta from OH rats (8). In isolated, pressurized (80 mmHg) third-order mesenteric arteries from OH rats, “internal diameters were significantly reduced...compared with controls” (∼320 μm); nevertheless, norepinephrine-induced vasoconstriction and ACh-induced vasodilation were normal (6). These authors also observed an increased collagen deposition in the media (containing 3 to 4 layers of myocytes), as well as a narrowing of the OH rat artery lumen, but no change in media or adventitia thickness. They suggested that the lumen narrowing might be involved in the genesis of the elevated BP in OH rats, but they ignored the possibility that the circulating ouabain itself might play a dynamic role in the sustained BP elevation.
To explore further the question of how the elevated BP in OH rats is sustained, we focused on fourth-order mesenteric arteries (∼165-μm internal diameter, with only a single layer of myocytes) from control and OH rats. Specifically, we asked whether structural changes or the intrinsic contractile properties of the pressurized small arteries or the influence of low-dose ouabain could account for the elevated BP in OH rats. The data indicate that, in contrast to third-order arteries (Ref. 6 and this report), the fourth-order arteries apparently are not structurally altered or narrowed. The normal contractile properties of fourth-order arteries are, however, consistent with similar findings in third-order arteries (6). An analysis of the distribution of resistances indicates that the smaller arteries account for much more of the peripheral resistance than do the third-order arteries. This, plus data on myogenic reactivity and the effect of low-dose ouabain, leads to the conclusion that the vasoconstrictor effect of the circulating ouabain in small arteries, and not structural changes, makes a major contribution to the elevated BP in OH rats.
METHODS
Experimental Animals
Ethical approval.
All rat protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Induction of ouabain hypertension.
Normal male Sprague-Dawley rats (Charles River, Wilmington, MA) were used for this study; body weights were 500–600 g at the time of euthanasia. Under halothane anesthesia, hypertension was induced by implanting 1.5-mg slow-release ouabain pellets (Innovative Research of America, Sarasota, FL) subcutaneously between the scapulae; control rats received vehicle pellet implants. BP rose to an elevated plateau in 2 to 3 wk in the ouabain-treated rats [e.g., see Fig. 6A in Cao et al. (8)]. Arteries from four groups of 10–12 rats were studied; in each group, half were ouabain-treated and half were controls.
BP Measurements
Systolic arterial BP (SBP) was measured in conscious, restrained rats using tail-cuff plethysmography (25). Three to five reproducible (within 15%) determinations were averaged to obtain a single BP value. The SBP data collection was performed “double blind;” i.e., the individual measuring the SBP was unaware of the status of the rats (OH vs. control) at the time of the measurements.
Arterial Structure
Arterial diameter and wall thickness.
The rats were euthanized by CO2 overdose followed by decapitation. Small (4th order) arteries from the superior mesenteric artery arcade were isolated. The arteries were cannulated at both ends, pressurized to 70 mmHg (with no internal flow), and superfused with physiological salt solution (PSS) at 35–37°C to permit myogenic tone (MT) to develop (51, 52). In most experiments, the artery external diameters were continuously monitored with a Nikon TMS microscope (Melville, NY) with a ×10 objective and with a monochrome CCD camera operated by LabView software (National Instruments, Austin, TX). Unless specifically noted, the reported diameters are external diameters because the electronic edge detector was often unable to discriminate clearly the inner edge of the artery wall in the low-magnification images required for the diameter measurements. Some studies were therefore performed on an Olympus IX81 inverted microscope equipped with a ×40 water immersion objective (Olympus America, Center Valley, PA); images were processed with Slidebook 4.2 software (Intelligent Imaging Innovations, Denver, CO). This enabled us to measure directly the wall thickness in pressurized arteries in various states of dilation and constriction to calculate the internal diameters.
Visualization of myocytes and endothelial cells.
To visualize individual myocytes or endothelial cells, a few arteries were first loaded with fluo-4 in PSS containing 15 μM fluo-4 AM (3 h, room temperature). For myocytes, the lumen was not perfused with this dye solution to avoid the confusion of simultaneous signals from myocytes and endothelial cells. Conversely, to visualize only the endothelial cells, the fluo-4 solution was introduced into the lumen and was omitted from the superfusion fluid.
Following washout of extracellular fluorochrome, the stained myocytes or endothelial cells within the arteries were visualized in a Zeiss LSM 5 Live inverted microscope equipped with a ×40 water immersion objective (Carl Zeiss, Stuttgart, Germany). This enabled us to obtain z-axis stacks of x-y plane images, 1 μm apart, at a rapid rate (up to two 30-plane z-stacks/s with 512 × 100 pixels/plane). Zeiss Efficient Navigation (Carl Zeiss) software was then used to construct three-dimensional (3-D) images of small artery segments as a function of time. Approximately 1% of the flurochrome was bleached during the accumulation of each z-stack. This and numerous spontaneous, local fluctuations of fluo-4 fluorescence, and thus Ca2+ concentration, precluded the detection of the anticipated small ouabain-induced increase in myocyte Ca2+ concentration (51). Also, the diameters of the arteries employed in this study were too large to obtain high-resolution x-y images or z-stacks through the entire artery cross section. Nevertheless, we were able to obtain high-resolution images of individual myocytes within small, crescent-shaped segments of the rat artery wall during vasoconstriction and dilation.
The internal elastic lamina (between the endothelial cell layer and the tunica media) and the numerous neuronal processes coursing through the adventitia exhibit substantial intrinsic tissue fluorescence. We took advantage of this property to visualize these structures in unstained or poorly stained arteries in which the endothelial cells and myocytes were essentially invisible.
Histology.
Small segments of the mesentery, including the fourth-order mesenteric artery arcade and the two third-order feeder arteries that feed the ends of the arcade (10), were isolated from control and OH rats. These mesentery segments were incubated in Ca2+-free PSS (2 × 10 min) and Ca2+-free PSS with 10 μM Na+-nitroprusside (1 × 10 min), fixed in 10% formalin (48 h), dehydrated in ethanol, and embedded in paraffin. Thin sections of the third- and fourth-order arteries were stained with Masson's trichrome.
Measurements of Arterial Function
Myogenic tone.
The magnitude of the myogenic constriction is expressed as a percentage of the passive external diameter (PD), measured in Ca2+-free PSS at the end of each experiment; i.e., MT = (myogenic constriction/PD) × 100.
PE- and K+-evoked vasoconstriction.
Vasoconstrictions evoked by PE or an elevated external K+ concentration are expressed as the evoked vasoconstriction relative to PD: Vasoconstriction = [(PD − constricted diameter)/PD] × 100. Note that in this method of expressing the constriction (43), the vasoconstrictor-induced constriction is superimposed on MT.
ACh-induced vasodilation.
Arteries were constricted with 5 μM PE and then treated with 10−9-10−4 M ACh in the absence and presence of 1 μM NG-nitro-l-arginine methyl ester (l-NAME), a NO synthase inhibitor. Relaxation is expressed in terms of the decline in the PE-evoked constriction (=100% in the absence of ACh).
Estimation of sarcoplasmic reticulum Ca2+ store content.
The relative size of the inositol trisphosphate-releasable and caffeine-releasable (ryanodine sensitive) sarcoplasmic reticulum (SR) Ca2+ stores were estimated from, respectively, the 5 μM PE- or 10 mM caffeine-evoked vasoconstriction in the absence of external Ca2+. The Ca2+-free medium was introduced 2 min before either the PE or caffeine was added; thus, the arteries were dilated to PD at the time the PE or caffeine was added. The PE was washed out after 2 min and the caffeine after 30 s, and normal Ca2+-replete PSS was restored 2 min later.
Reagents and Solutions
Artery dissection solution contained (in mM) 145 NaCl, 4.7 KCl, 1.2 MgSO4·7H2O, 2.0 MOPS, 0.02 EDTA, 1.2 NaH2PO4, 2.0 CaCl2·2H2O, 5.0 glucose, and 2.0 pyruvate and 1% albumin (pH 7.4 at 5°C). The PSS perfusion solution contained (in mM) 112 NaCl, 25.7 NaHCO3, 4.9 KCl, 2.5 CaCl2, 1.2 MgSO4·7H2O, 1.2 KH2PO4, 11.5 glucose, and 10 HEPES (adjusted pH to 7.3–7.4 with NaOH). High (10–75 mM) K+ PSS was made by replacing NaCl with equimolar KCl. Ca2+-free PSS was made by omitting Ca2+ and adding 0.5 mM EGTA. Solutions were gassed with 5% O2-5% CO2-90% N2 at 35–37°C; the measured O2 level in the open artery chamber was ∼12%.
The reagents and sources were as follows: ouabain, caffeine, PE, ACh, Na+-nitroprusside, and l-NAME were from Sigma-Aldrich, St. Louis, MO, and fluo-4 was from Molecular Probes, Eugene, OR. Other reagents were reagent grade or the highest grade available.
Data Analysis and Statistics
The data are expressed as means ± SE; n denotes the number of animals or the number of arteries studied (1 artery per animal). Comparisons of data were made using Student's paired or unpaired t-test, as appropriate; two-way ANOVA was used where indicated (see figure legends). Differences were considered significant at P < 0.05. Images were analyzed with customized Interactive Data Language software (IDL, Research Systems, Boulder, CO).
RESULTS
Blood Pressure
On average, the rats in the four control groups used here had a mean SBP of 124 ± 4 mmHg (n = 20). The mean SBP of the OH rats was 138 ± 3 mmHg (n = 23) at 4 to 5 wk after implanting the ouabain pellets (P < 0.01 vs. controls). The time course of the changes in SBP in one of the four groups of rats used in this study is shown in Fig. 6A of Cao and colleagues (8).
Small Artery Structure
Live arteries.
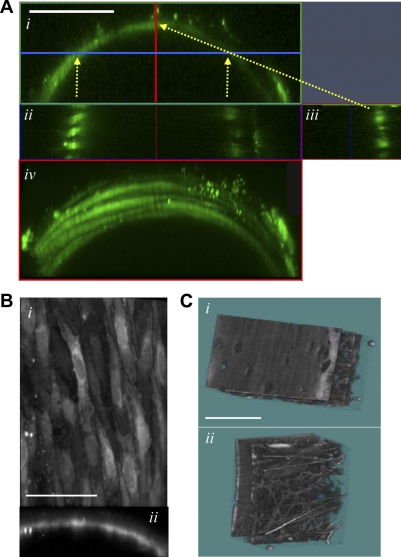
The fluorescent images in Fig. 1 are views of small portions of the wall from control rat mesenteric small (4th order) arteries pressurized to 70 mmHg. In Fig. 1A, all the myocytes, but no endothelial cells, are stained because the fluo-4 was loaded from the superfusion fluid and not the lumen. Artery wall longitudinal cross sections reveal that there is only a single layer of myocytes in the wall (Fig. 1A, ii and iii). The individual myocytes are best seen, however, in rotated views of the 3-D reconstructions (Fig. 1A,iv and supplemental video clip VC1; note: all supplemental material may be found posted with the online version of this article). The structure of the control rat artery segments was indistinguishable from that of OH rat arteries (not shown).
Fig. 1.
Images of live, pressurized 4th-order mesenteric small arteries from control (Ctrl) rats. A: smooth muscle cells in an artery loaded with fluo-4 contained in the superfusion fluid only. The fluorescent images come from 3-dimensional (3-D) reconstructions of a small segment of the artery wall. A,i: end-on view (x-z plane) of the wall segment. A, ii and iii: longitudinal cross sections of the wall at the arrowheads: Aii: this x-y plane cuts through the walls at an angle (at the blue line and two vertical arrows in A,i); A,iii: artery wall cross section (z-y plane) at the vertical red line (in A,i). A,iv: tilted 3-D view of the artery segment, looking down on the surface of the tunica media to visualize the myocytes. A, ii–iv, show that the small artery contains only a single layer of myocytes. Supplemental video clip VC1 (posted with the online version of this article) shows this arterial segment rotated around a horizontal line (A, i and iv, shows two views). Scale bar = 50 μm. B: this artery was loaded with fluo-4 from the lumen, so that only endothelial cells are stained. The en face view from the lumen (i) and end-on view of a portion of the artery cross section (ii) reveal that the endothelial cells form a continuous thin, flat sheet. These are two views from a supplemental video clip VC2 in which this artery segment is rotated around a horizontal line. Scale bar = 12.5 μm. C: intrinsic tissue fluorescent images of a segment of the wall of an unstained artery: lumenal view shows the surface of the internal elastic lamina that faces the ablumenal surface of the unstained (and, therefore, invisible) endothelial cell laye (i), and view from the external surface of the artery shows neuronal processes coursing through the adventitia (ii). The space (tunica media) between the internal elastic lamina and the adventitia, seen at the right in C,i and left in C,ii, is occupied by myocytes, which are unstained and therefore not visible here. Scale bar = 25 μm.
The stained endothelial cell layer is shown en face in Fig. 1B,i and in the cross section in Fig. 1B,ii. In this artery, fluo-4 was loaded via the lumen and was omitted from the superfusion fluid. The endothelial cells form a single, thin, virtually confluent, cobble stone-like layer lining the lumen. Rotated views of this figure are presented in supplemental video clip VC2. Note that in the pressurized artery, the endothelial cell layer does not exhibit the folding that would be expected in a markedly constricted artery (Fig. 2). This flat surface is mirrored in the adjacent smooth internal elastic lamina surface in the 3-D image of the artery wall intrinsic tissue fluorescence (Fig. 1C,i). The intrinsic tissue fluorescence, viewed from the external surface of the artery, reveals numerous neuronal processes coursing through the adventitia (Fig. 1C,ii). The endothelial cells and the myocytes situated between the internal elastic lamina and the adventitia are unstained and have very little intrinsic fluorescence; they are, therefore, invisible in these images.
Fig. 2.
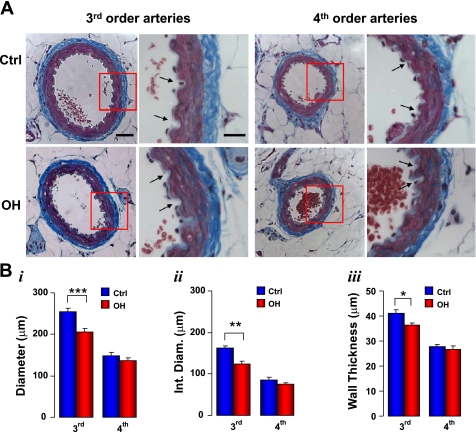
Histology of fixed Ctrl and ouabain-induced hypertension (OH) rat 3rd and 4th-order mesenteric arteries. A: images of artery cross sections stained with Masson's trichrome to visualize myocytes (red) and collagen (blue). Scale bars = 50 μm for the low-magnification images and 20 μm for the high-magnification images. Arrows in the high-magnification images point to some of the endothelial folds. B: summary data on the artery external diameter (i), internal diameter (Int Diam; ii), and wall thickness (iii). Data are means ± SE for 8 3rd-order and 11 4th-order Ctrl arteries (blue bars) and for 8 3rd and 12 4th-order OH arteries (red bars). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the respective Ctrl.
Fixed arteries.
The structure of fixed, stained mesenteric small arteries from control and OH rats was also examined; the arteries were stained with Masson's trichrome to distinguish between myocytes (red) and collagen (blue) (Fig. 2A). As illustrated, the third-order feeder arteries (10) used by Briones and colleagues (6) were considerably larger than the fourth-order arcade arteries (10) whose physiology is described in this report. The fixed arteries were markedly constricted despite the incubation in Ca2+-free PSS and exposure to Na+-nitroprusside before fixation. The internal diameters of the fixed fourth-order arteries (∼75–85 μm, Fig. 2B,ii) were much narrower than those of comparable fresh arteries with MT (∼165 μm at 70 mmHg; Fig. 3). The obvious endothelial folding in both third- and fourth-order arteries (arrows in the higher magnification images in Fig. 2A; and see Fig. 4A in Ref. 6) contrasts with the smooth endothelial cell layer and internal elastic lamina in isolated, pressurized arteries (Fig. 1, B and C). In the fixed, constricted fourth-order arteries, the myocytes overlap so that the arterial media appears to be, on average, about 2 myocytes thick (Fig. 2A). This clearly differs from the situation in the arteries with MT in which the myocytes are arrayed in only a single layer (Fig. 1A).
Fig. 3.
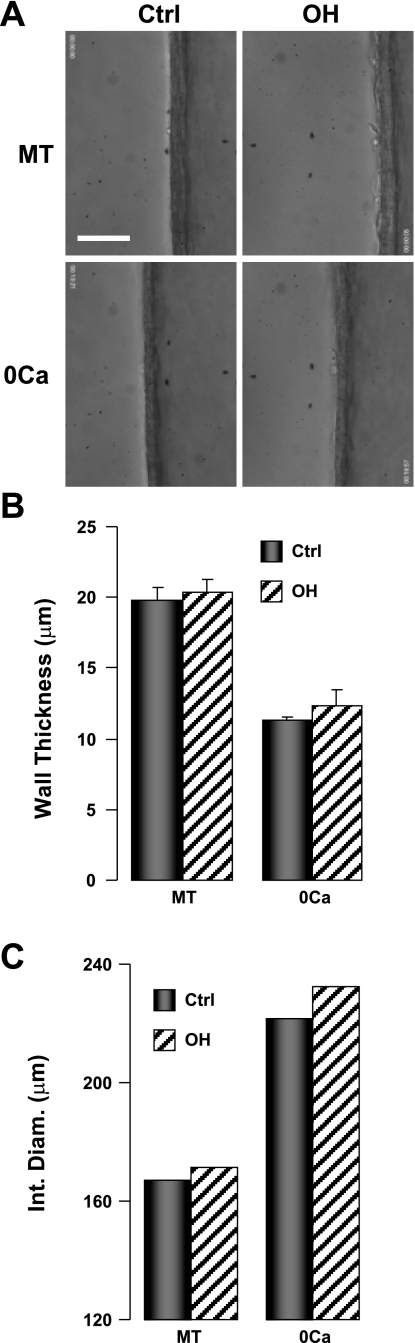
Wall thickness in Ctrl and OH rat 4th-order mesenteric arteries. A: transmitted light longitudinal cross-sectional images of the walls of a Ctrl artery (left) and an OH artery (right); the artery lumen is on the right in each panel. The arteries were pressurized to 70 mmHg (35°C). A, top: walls of arteries with myogenic tone (MT). A, bottom: walls of the same arteries, respectively, dilated to passive diameter (PD) in Ca2+-free physiological salt solution (PSS). Scale bar = 50 μm. Supplemental video clip VC3 shows the wall thickness decreasing as a Ctrl rat artery dilates to PD. B and C: summary data of wall thickness (B) and internal diameter (C). The data in B are from 6 Ctrl and 5 OH rat arteries. The data in C were calculated by subtracting twice the wall thickness (from B) from the external diameters of, respectively, Ctrl and OH arteries with MT at 70 mmHg (data from Fig. 4B).
Fig. 4.
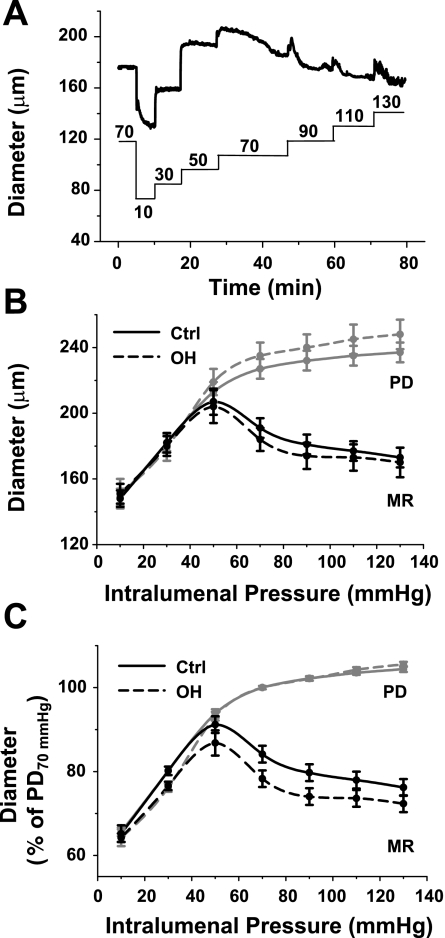
Myogenic reactivity (MR) in Ctrl and OH rat mesenteric small arteries. A: representative original vasoconstriction record from a Ctrl artery illustrates the diameter changes (top thick line) in response to the step pressure changes (in mmHg) indicated by the bottom line. B: mean external diameter of the Ctrl and OH rat arteries is graphed as a function of translumenal pressure in response to 20-mmHg step increases in pressure starting at 10 mmHg (see A). The MR curves were obtained in normal PSS. The arteries were then superfused with Ca2+-free PSS, and the sequence of pressure changes was repeated to obtain the PD curves. The PD curve for OH arteries is shifted slightly, but not significantly, upward relative to the Ctrl artery PD curve (ANOVA, P = 0.152). C: the data from B are normalized to PD of Ctrl arteries at 70 mmHg (48). The normalized MR curve for the OH arteries is shifted downward, relative to the Ctrl curve (ANOVA, P < 0.01); n = 12 and 11 for Ctrl and OH groups, respectively.
The summarized data in Fig. 2B show that the external diameter, internal diameter, and wall thickness are all significantly smaller in OH third-order arteries than in controls. The narrower diameter of these OH arteries is consistent with the results of Briones and colleagues (6). In striking contrast, the diameter and wall thickness of OH fourth-order arteries do not differ significantly from the respective measurements in control rat arteries (Fig. 2B).
Myogenic Responses
When pressurized to 70 mmHg and warmed to 35–37°C, rat small arteries spontaneously constrict; i.e., they develop MT (52). The mean MT in arteries from control rats in this study corresponded to a constriction of 19 ± 1% of PD (n = 16). Mean MT in arteries from OH rats was 21 ± 2% of PD (n = 17), slightly, but not significantly, greater than that in controls.
The transmitted light images in Fig. 3A illustrate the measurement of wall thickness in isolated, cannulated arteries. Mean wall thickness in control arteries with MT at 70 mmHg was 19.8 μm and declined to 11.3 μm at PD (Fig. 3B) in part because the myocytes presumably elongated without changing cell volume. Very similar results were obtained in OH rat arteries: wall thickness was 20.4 μm in arteries with MT and declined to 12.3 μm at PD (Fig. 3B). Supplemental video clip VC3 shows the dynamic change in wall thickness as a representative artery dilates from a constricted state (MT) to PD during the washout of Ca2+. The calculated internal diameters shown in Fig. 3C are based on the wall thickness data in Fig. 3B and the external diameters in Fig. 4B.
Myogenic reactivity to progressive increases in intralumenal pressure was measured in arteries isolated from control and OH rats. The intralumenal pressure was lowered from 70 to 10 mmHg and then raised to 130 mmHg in 20-mmHg increments; Fig. 4A depicts a set of original data from a control rat artery. The diameter versus pressure curves in Fig. 4B show that PD was slightly, but not significantly, larger in the OH rat arteries than the control arteries at all pressures. The respective myogenic reactivity curves from the OH and control arteries incubated in normal Ca2+-containing PSS were, however, nearly superimposable; thus internal diameters at 70 mmHg were nearly identical in control and OH arteries (∼165 μm; Fig. 3C). Nevertheless, normalization of the data in Fig. 2B to PD at 70 mmHg [i.e., classic myogenic reactivity curves (48)] reveals that the OH rat arteries exhibited significantly augmented myogenic constriction (Fig. 4C).
Effect of Ouabain
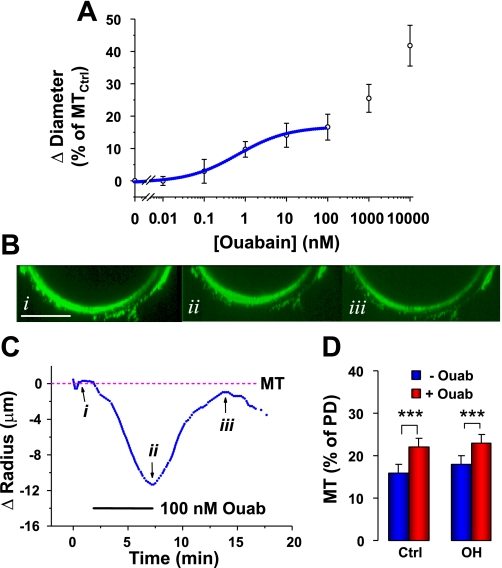
The circulating ouabain level is elevated in OH rats, but ouabain is washed out when the arteries are isolated. To understand better the in vivo situation, we tested the effect of an acute ouabain application on pressurized arteries from control and OH rats. Figure 5A shows that the ouabain dose-response curve in normal rat arteries is biphasic, with a plateau between ∼10 and ∼300 nM, as in mouse arteries (51). Ouabain concentrations between 0.01 and 100 nM, which selectively inhibit α2-Na+ pumps in rodents (39), augment MT with a half-maximal effect at 0.66 nM (EC50). This is close to the EC50 in mouse arteries (1.3 nM) (51). In most experiments, 100 nM ouabain was used because this dose rapidly and reversibly inhibits all Na+ pumps with an α2-subunit but has a negligible effect on rodent Na+ pumps with an α1 (low ouabain affinity)-subunit (51). Ouabain concentrations ≥ 1,000 nM inhibit α1-Na+ pumps (39), depolarize arterial myocytes (51), and induce profound vasoconstriction (Fig. 5A).
Fig. 5.
Ouabain-induced constriction of Ctrl and OH rat mesenteric small arteries with myogenic tone. A: ouabain dose-response curve determined in pressurized (70 mmHg) Ctrl arteries with MT; each point is the mean value from 5–9 arteries. The ouabain was superfused until a steady effect was attained (5–10 min) and was washed out after each test dose to confirm reversibility. The 0–100 nM ouabain data are fitted to the saturation curve for a single binding site with an EC50 of 0.66 nM ouabain. The data for ≥1,000 nM ouabain, which were not curve fitted, correspond to a second (low affinity) binding site. B: end-on cross-sectional images from 3-D reconstructions of a portion of a fluo-4 stained Ctrl rat artery wall before (i), during treatment with 100 nM ouabain (ii), and after a 15-min recovery (iii). The images were corrected for bleaching (1% per z-stack). Scale bar = 50 μm. C: time course of displacement (Δradius, in μm) of the artery surface closest to the bottom of the tissue chamber (same artery as in A); the original position of the artery wall is 0. The bar indicates the period of application of 100 nM ouabain (Ouab). Arrows i, ii, and iii indicate the times when images in A, i–iii, were captured. D: summary data (diameters measured with an edge detector) illustrate the effects of 100 nM ouabain on MT (percentage of PD) at 70 mmHg in arteries from Ctrl and OH rats. Ouabain (100 nM; red bars) constricted both Ctrl and OH rat arteries (***P < 0.001). The ouabain-induced vasoconstriction (difference between red and blue bars) in OH rat arteries was not significantly different from that in Ctrl rat arteries (P > 0.05; n = 9 Ctrl and 7 OH arteries).
Figure 5B, i–iii, show a portion of a control rat mesenteric small artery cross section before, during, and after the application of 100 nM ouabain. These artery cross sections (see Fig. 1A) were reconstructed from z-stacks of x-y plane images parallel to the long axis of the artery. The images in Fig. 5B, i–iii, were captured at the time points indicated on the time-course graph in Fig. 5C. The 11 μm upward displacement of the wall, from “i” to “ii”, is the ouabain-induced decrease in artery radius (assuming concentric constriction); the downward displacement from “ii” to “iii” illustrates the recovery.
Summarized data on the 100 nM ouabain-induced vasoconstriction, reported as changes in MT in control and OH rat arteries, are shown in Fig. 5D. This constriction was accompanied by a 2.8 ± 0.3 μm (n = 6) increase in wall thickness (see Fig. 3A for method); thus ouabain decreased the internal diameter by ∼26 μm. Note that ouabain had a comparable effect on OH and control arteries: on average, it narrowed the lumen from 165 μm (Fig. 3C) to ∼140 μm at 70 mmHg. According to Poiseuille's law (29), this constriction should increase the resistance to flow by about 90%.
Mobilization of Sarcoplasmic Reticulum Ca2+ Stores
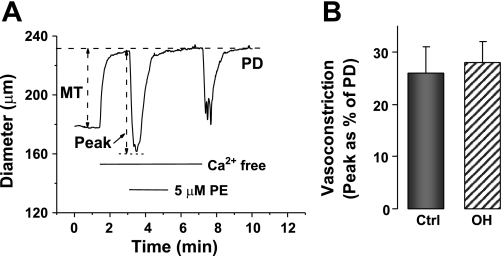
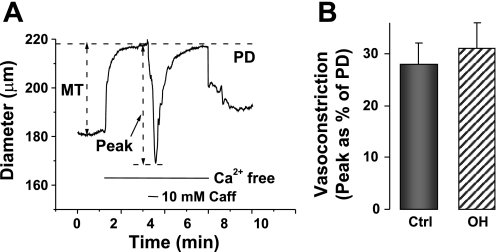
To estimate whether the inositol trisphosphate-releasable and ryanodine-sensitive SR Ca2+ stores were altered in the OH rats, we measured the maximal vasoconstrictions evoked by 5 μM PE and by 10 mM caffeine, respectively, in Ca2+-free media (Figs. 6 and 7). Figure 6A shows the protocol for the PE experiments. The results, summarized in Fig. 6B, reveal that the evoked vasoconstriction was marginally, but not significantly, greater in isolated OH rat arteries than in controls. The caffeine-induced vasoconstriction, too (see protocol in Fig. 7A), was slightly, but not significantly, greater in OH rat arteries than in controls (Fig. 7B).
Fig. 6.
Vasoconstrictions evoked by 5 μM phenylephrine (PE)-induced release of Ca2+ from sarcoplasmic reticulum stores in Ctrl and OH rat mesenteric small arteries. A: PE responses were evoked after the arteries had been exposed to Ca2+-free PSS for 2 min. B: summary data. The evoked vasoconstriction, expressed as a function of PD, in OH rat arteries is not significantly different from that in Ctrls (P > 0.05; n = 11 for both groups).
Fig. 7.
Vasoconstrictions due to release of Ca2+ from sarcoplasmic reticulum stores evoked by 10 mM caffeine (Caff) in Ctrl and OH rat mesenteric small arteries. A: Caff response was evoked 2 min after the Ca2+-free PSS was introduced. B: summary data. The evoked vasoconstriction, expressed as a function of PD, in OH rat arteries is not significantly different from that in Ctrls (P > 0.05; n = 4 for both groups).
PE and High-K+ Constriction Dose-Response Curves
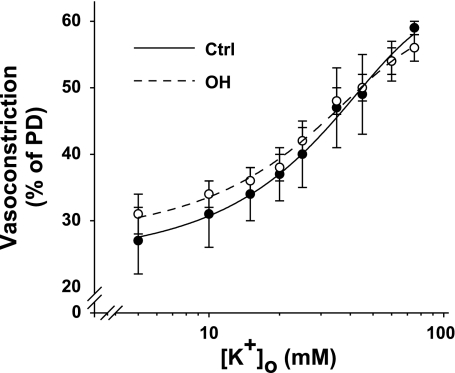
To determine whether vasoconstrictor responses were altered in the isolated OH rat arteries, we compared the K+ and PE cumulative dose-response curves in control and OH rat arteries. High K+-evoked vasoconstrictions were virtually identical in the arteries from the two groups of rats (Fig. 8). Likewise, except at very high nonphysiological PE concentrations, the two PE dose-response curves were indistinguishable (Fig. 9). In control arteries, 100 nM ouabain did not significantly augment the contractile responses to 0.1–1 μM PE (not shown).
Fig. 8.
Effect of elevated external K+ concentration ([K+]o) on the external diameters of Ctrl and OH rat mesenteric small arteries with MT. The vasoconstriction (expressed as a percentage of PD; see methods) is graphed as a function of log [K+]o. The vasoconstriction at [K+]o = 5 mM is the MT at 70 mmHg transmural pressure in normal PSS. Mean PD for the Ctrl and OH rat arteries was 242 ± 7 and 271 ± 10 μm, respectively. The 2 curves are not significantly different (ANOVA, P > 0.05; n = 4 Ctrl and 7 OH arteries).
Fig. 9.
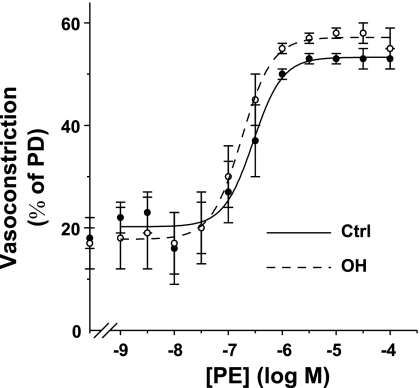
PE dose-response curves for Ctrl and OH rat mesenteric small arteries with MT. The vasoconstriction is shown as a percentage of PD (see methods). The vasoconstriction at [PE] = 0 is the MT at 70 mmHg transmural pressure in normal Krebs. Mean PD for the Ctrl and OH rat arteries was 242 ± 7 and 277 ± 10 μm, respectively. The PE-evoked vasoconstrictions were significantly greater in OH than in Ctrl arteries at [PE] ≥ 1 μM, a maximal concentration (ANOVA, P < 0.001); the two curves are, however, not significantly different (ANOVA, P > 0.05; n = 4 Ctrl and 8 OH arteries).
ACh-Induced Vasodilation
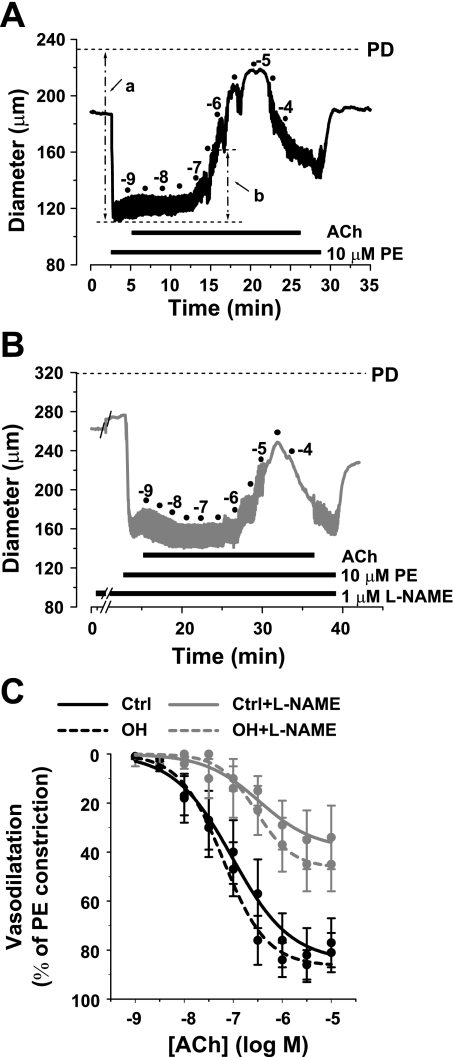
The effect of ACh on the constriction evoked by 10 μM PE (a near-maximal dose; Fig. 9) was also tested on isolated, pressurized small arteries from control and OH rats. The representative cumulative ACh concentration experiment from a control artery (Fig. 10A) shows that ACh antagonizes PE-induced vasoconstriction and dilates the artery. To determine how much of this vasodilation was due to NO, the dose-response to ACh was repeated after treatment with l-NAME. l-NAME concentrations of 1–5 μM block NO synthase (18, 26), whereas 100 μM l-NAME also constricts small pressurized arteries by an endothelium- and NO synthase-independent mechanism (37). We tested the effects of pretreatment with 0.1–100 μM l-NAME on the vasorelaxation induced by 10 μM ACh, a maximally effective concentration (Fig. 10A), in PE-constricted arteries. We, too, observed the vasoconstriction with 10–100 μM l-NAME; moreover, 1 μM was almost as effective as 100 μM l-NAME in inhibiting the response to ACh (not shown). Therefore, a 10-min exposure to 1 μM l-NAME was employed subsequently, as illustrated by the representative experiment in Fig. 10B. Indeed, the summary data (Fig. 10C) reveal that ACh-induced vasodilation is unimpaired in fourth-order mesenteric arteries from OH rats (vs. controls). About half of the ACh-induced relaxation was abrogated by this l-NAME pretreatment in both the control and the OH arteries.
Fig. 10.
Vasodilation evoked by acetylcholine (ACh) in Ctrl and OH rat mesenteric small arteries constricted with 10 μM PE in the absence and presence of 1 μM NG-nitro-l-arginine methyl ester (l-NAME). A: representative experiment illustrates the changes in arterial diameter in response to cumulative doses of ACh (10−9-10−4 M) in a pressurized, PE-constricted Ctrl artery. For example, a is the PE-induced vasoconstriction relative to PD and b indicates the vasodilation induced by 10−7 M ACh. B: representative experiment similar to that in A but in the presence of 1 μM l-NAME, which was added 10 min before PE. C: percentage of the maximal 10 μM PE-evoked constriction plotted as a function of the ACh concentration. PE constricted the Ctrl and OH arteries from 185 ± 8 to 116 ± 4 μm and from 213 ± 10 to 123 ± 4 μm, respectively. The curves for the OH arteries are not significantly different from those for Ctrl arteries (ANOVA, P > 0.05; n = 4 Ctrl and 8 OH arteries).
DISCUSSION
A central, but still unresolved, issue pertaining to most forms of hypertension is, What initiates and what sustains the elevated BP? In particular, in salt- and plasma volume-dependent hypertension, the initial rise in BP has usually been attributed to increased cardiac output (19, 38). The sustained elevation of BP was explained as a blood flow-induced compensatory shift (whole body autoregulation) to increased vasoconstriction and total peripheral vascular resistance (TPR) (11, 19). We have suggested that this shift to increased TPR is mediated by elevated circulating EO (3, 4). The ability of exogenously administered ouabain to induce hypertension in normal rodents (8, 12, 33, 50) follows from this idea. Ouabain-induced hypertension depends on the (normal) high-ouabain affinity binding site on Na+ pumps with an α2-catalytic subunit (12). Moreover, mice with a null mutation in one α2-allele have elevated BP, and their arteries exhibit increased MT and augmented myogenic reactivity (51).
Mechanism of Increased TPR in Hypertension: the Structure-Function Debate
Elevated TPR is the hallmark of chronic hypertension (11), but whether this has a structural or functional basis is still vigorously debated. Structural changes such as increased collagen deposition, media thickening, lumen narrowing, and myocyte hypertrophy or hyperplasia are documented in small resistance arteries, (22) as well as large arteries, in most forms of hypertension (2, 22, 36), including ouabain-induced hypertension (6). Nevertheless, small artery diameters appear to be normal, however, in some models of salt-sensitive hypertension (2).
At least two problems arise, however, in assessing the cause and effect of the increased TPR. One is that sustained functional increases in artery constriction and tone usually induce compensatory structural changes or remodeling (34). Secondly, when arteries are removed from hypertensive animals, the structural changes remain and usually can be readily detected (6, 22), whereas the in vivo function cannot necessarily be ascertained by studying the isolated arteries in vitro. This is especially important if the functional changes depend on circulating factors that are washed out in isolated artery studies, but such a possibility has generally been overlooked. Indeed, it has been noted that the evidence for altered arterial function is much weaker than the evidence that the structural changes are responsible for the increased TPR in hypertension (36). For this reason, in vivo augmentation of vasoconstrictor-evoked responses in hypertensive humans (13) and animals (5) might be attributed to the altered arterial structure.
Most of the peripheral vascular resistance to blood flow, and the regulation of flow and BP, occurs at the level of small resistance arteries (16). In the rat mesenteric vasculature, these are believed to be the arteries with in vivo internal diameters of 100–300 μm, but the locus of the main resistance to flow is uncertain (10).
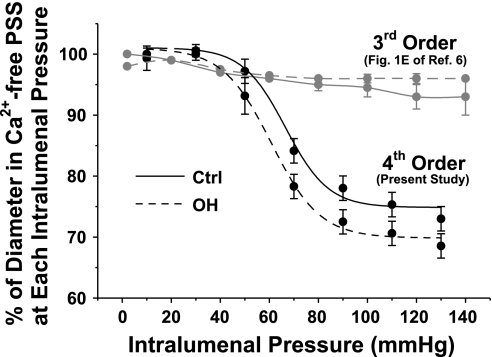
Recently, Briones and colleagues (6) studied isolated resistance arteries from control and OH rats. These third-order mesenteric feeder arteries (10), with internal diameters of ∼320 μm, had limited myogenic reactivity, especially when compared with the fourth-order arteries used here (Fig. 11). Chronic in vivo ouabain treatment increased collagen deposition and wall stiffness and narrowed the artery lumen from ∼320 to ∼280 μm (at 80 mmHg) without altering the adventitia or media thickness (6). Nevertheless, the physiological responses of the isolated OH rat third-order arteries to norepinephrine and ACh were normal. Briones and colleagues (6) therefore concluded that vessel narrowing “could play a role in the pathogenesis of [the] hypertension” in OH rats. The authors did not, however, address the question, Does circulating ouabain have a dynamic effect on the arteries?
Fig. 11.
Comparison of myogenic responses of Ctrl and OH rat 3rd- and 4th-order mesenteric arteries. The data for the 3rd-order arteries are from Fig. 1E in Briones et al. (6); the MR data for the 4th-order arteries are from Fig. 4B, regraphed as a percentage of the PD at each pressure.
Arterial Diameter and Resistance to Flow
In this report, we described mesenteric arteries with internal diameters of ∼165 μm at 70 mmHg, and with only a single layer of smooth muscle cells. The isolated, pressurized fourth-order arteries from OH rats had diameters and wall thicknesses similar to control arteries. Even fixed, constricted fourth-order control and OH arteries had comparable diameters and comparable wall thicknesses (Fig. 2). In contrast, fixed, third-order OH rat arteries, like the isolated, pressurized OH arteries (6), had significantly smaller diameters than did the controls (Fig. 2).
It is instructive to consider the effects of these artery diameter differences on the resistance to blood flow. According to Poiseuille's law, the resistance to flow in the control fourth-order arteries will be about 14-fold greater per unit artery length than the resistance of third-order arteries. Moreover, even with the reduced diameter of third-order OH arteries, the resistance to flow will still be about 8.3-fold greater in fourth-order arteries, ignoring the possible dynamic influence of circulating ouabain. Because the third-order arteries are, on average, only about 20–25% longer than fourth-order arteries (15.5 ± 0.8 vs. 12.7 ± 1.4 mm, n = 10 for both), the implication is that these fourth-order arteries contribute much more to the peripheral vascular resistance than do the third-order arteries.
The Functional Consequences of Circulating Ouabain
Importantly, the isolated third-order (6), as well as the fourth-order, OH rat mesenteric arteries had normal responses to vasodilators and constrictors. For example, the OH rat fourth-order arteries exhibited normal vasoconstrictor responses to high K+ and moderate doses of PE, and normal vasodilator responses to ACh (Figs. 8–10).
Vasoconstriction to PE and to caffeine in Ca2+-free media, measures of the mobilization of SR-releasable Ca2+ stores, was also not significantly changed by the chronic ouabain treatment (Figs. 6 and 7). The similar MT in the control and OH rat arteries suggests that the baseline cytosolic Ca2+ concentration is similar in control and OH artery myocytes. Thus, even though the relationship between cytosolic Ca2+ concentration and vasoconstriction is nonlinear, the data in Figs. 6 and 7 imply that the releasable Ca2+ stores are not markedly altered in OH rat arterial myocytes.
We did observe augmented myogenic constriction in the smaller arteries from OH rats, however, as well as significantly greater constriction at high-dose (≥1 μM) PE. The latter may be the result of enhanced Ca2+ entry through receptor-operated channels. The diameters of the control and OH fourth-order arteries (Fig. 4, B and C), but not the larger arteries (Fig. 11) (6), were substantially reduced when intralumenal pressure was increased above ∼50 mmHg; this is the classical myogenic response (48). Furthermore, despite the increased MT of the fourth-order OH arteries, the diameters of the myogenically constricted control and OH arteries were virtually identical (Fig. 4B). Because wall thickness did not differ significantly between control and OH rat arteries (Figs. 2B and 3B), these data raise the possibility that the OH arteries may be more constricted to compensate for high pressure-induced stretch, so that the normal lumen diameter is maintained.
Most important is our observation that the myogenically constricted OH arteries responded normally to 100 nM ouabain (Fig. 5); thus the chronic in vivo exposure to ouabain did not downregulate the response. As noted in results, the 100 nM ouabain-induced constriction should increase resistance to blood flow by about 90%. With the assumption that ouabain also reduces the third-order artery diameter by the same amount (∼26 μm), the fourth-order arteries will still be about 11-fold more resistant to flow than the larger arteries. Furthermore, the still smaller branches that emanate from the fourth-order arcade and enter the intestinal wall (10) will have an even greater resistance. Even at a concentration close to EC50 of 0.66 nM (Fig. 5A), which is and within the range of EO concentrations observed in normal humans on a high-salt diet (32), ouabain should increase vascular resistance by ∼35%. The implication is that circulating ouabain in OH rats should augment MT and TPR. This finding, plus the absence of small vessel lumen narrowing, suggests that the dynamic effect of ouabain, rather than the remodeling of resistance arteries, may be a key contributor to the sustained elevation of BP in OH rats. Indeed, if most (or all) small and muscular arteries are similarly affected by low-dose ouabain, as seems likely (for example, mouse cremaster small arteries, <100 μm diameter) are also constricted by 100 nM ouabain (H. Raina and M. P. Blaustein, unpublished), this may be the dominant factor in the increased TPR in OH rats. Moreover, these same mechanisms may have a much broader implication, given the evidence summarized in the Introduction that EO is involved in the pathogenesis of hypertension (4). This could explain, for example, why Digibind, digoxin-specific antibody Fab fragments with high affinity for ouabain (44), rapidly (within minutes) lowers BP in several rat hypertension models (24, 27, 30) and in human pregnancy-induced hypertension (1).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant P01-HL-78870 Projects 1 (to M. P. Blaustein) and 3 (to W. G. Wier), and Core B (to J. M. Hamlyn) and an American Heart Association National Scientist Development grant; an International Society of Hypertension-Pfizer award (to J. Zhang), and a Japan Heart Foundation/Bayer Yakuhin Research grant (to E. Karashima).
Supplementary Material
Acknowledgments
We thank K. Frankel for assistance with the preparation of this manuscript.
Present address of R. Berra-Romani: Department of Biomedicine, School of Medicine, Benemèrita Universidad Autònoma de Puebla, Puebla, Mexico.
REFERENCES
- 1.Adair CD, Buckalew VM, Kipikasa J, Torres C, Stallings SP, Briery CM. Repeated dosing of digoxin-fragmented antibody in preterm eclampsia. J Perinatol 29: 163–165, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Ashen MD, Hamlyn JM. Smooth muscle hypertrophy and arterial remodelling in deoxycorticosterone acetate-salt hypertension. Clin Exp Hypertens 16: 261–282, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol Cell Physiol 232: C165–C173, 1977. [DOI] [PubMed] [Google Scholar]
- 4.Blaustein MP, Zhang J, Chen L, Song H, Raina H, Kinsey SP, Izuka M, Iwamoto T, Kotlikoff MI, Lingrel JB, Philipson KD, Wier WG, Hamlyn JM. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53: 291–298, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohr DF, Dominiczak AF, Webb RC. Pathophysiology of the vasculature in hypertension. Hypertension 18: III69–III75, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Briones AM, Xavier FE, Arribas SM, Gonzalez MC, Rossoni LV, Alonso MJ, Salaices M. Alterations in structure and mechanics of resistance arteries from ouabain-induced hypertensive rats. Am J Physiol Heart Circ Physiol 291: H193–H201, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bund SJ, West KP, Heagerty AM. Effects of protection from pressure on resistance artery morphology and reactivity in spontaneously hypertensive and Wistar-Kyoto rats. Circ Res 68: 1230–1240, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Cao C, Payne K, Lee-Kwon W, Zhang Z, Lim SW, Hamlyn J, Blaustein MP, Kwon HM, Pallone TL. Chronic ouabain treatment induces vasa recta endothelial dysfunction in the rat. Am J Physiol Renal Physiol 296: F98–F106, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceron PI, Bendhack LM. Increased contractile response induced with ouabain is abolished by thapsigargin in aorta of renal hypertensive rats. Gen Pharmacol 29: 707–712, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res 38: 1–12, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Cowley AW. Long-term regulation of arterial blood pressure. Physiol Rev 72: 231–300, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB. The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288: H477–H485, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Doyle AE, Black H. Reactivity to pressor agents in hypertension. Circulation 12: 974–980, 1955. [DOI] [PubMed] [Google Scholar]
- 14.Ferrandi M, Manunta P, Rivera R, Bianchi G, Ferrari P. Role of the ouabain-like factor and Na-K pump in rat and human genetic hypertension. Clin Exp Hypertens 20: 629–639, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Folkow B. The fourth Volhard lecture: cardiovascular structural adaptation; its role in the initiation and maintenance of primary hypertension. Clin Sci Mol Med Suppl 4: 3s–22s, 1978. [DOI] [PubMed] [Google Scholar]
- 16.Folkow B, Neil E. Circulation. New York: Oxford University Press, 1971, p. 593.
- 17.Godfraind T, Tona Lutete DN. Inhibition by digoxin and SC4453 of (Na+ + K+)-ATPase prepared from human heart, guinea-pig heart and guinea-pig brain. Eur J Pharmacol 60: 329–336, 1979. [DOI] [PubMed] [Google Scholar]
- 18.Gradin KA, Li JY, Zhu H, Simonsen U. Blunted pancreatic polypeptide-induced vasodilatation in mesenteric resistance vessels from spontaneously hypertensive rats. Eur J Pharmacol 601: 118–123, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Guyton AC, Granger HJ, Coleman TG. Autoregulation of the total systemic circulation and its relation to control of cardiac output and arterial pressure. Circ Res 28, Suppl 1: 93–97, 1971. [PubMed] [Google Scholar]
- 20.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci USA 88: 6259–6263, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamlyn JM, Lu ZR, Manunta P, Ludens JH, Kimura K, Shah JR, Laredo J, Hamilton JP, Hamilton MJ, Hamilton BP. Observations on the nature, biosynthesis, secretion and significance of endogenous ouabain. Clin Exp Hypertens 20: 523–533, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension 21: 391–397, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Huang BS, Kudlac M, Kumarathasan R, Leenen FH. Digoxin prevents ouabain and high salt intake-induced hypertension in rats with sinoaortic denervation. Hypertension 34: 733–738, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Kaide J, Ura N, Torii T, Nakagawa M, Takada T, Shimamoto K. Effects of digoxin-specific antibody Fab fragment (Digibind) on blood pressure and renal water-sodium metabolism in 5/6 reduced renal mass hypertensive rats. Am J Hypertens 12: 611–619, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Kimura K, Manunta P, Hamilton BP, Hamlyn JM. Different effects of in vivo ouabain and digoxin on renal artery function and blood pressure in the rat. Hypertens Res 23, Suppl: S67–S76, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Konishi C, Naito Y, Ohara N. Changes in the regulation by endothelium of norepinephrine response in isolated, perfused mesenteric vascular bed of rats at different ages. Mech Ageing Dev 106: 161–172, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Krep H, Price DA, Soszynski P, Tao QF, Graves SW, Hollenberg NK. Volume sensitive hypertension and the digoxin-like factor. Reversal by a Fab directed against digoxin in DOCA-salt hypertensive rats. Am J Hypertens 8: 921–927, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Laredo J, Hamilton BP, Hamlyn JM. Secretion of endogenous ouabain from bovine adrenocortical cells: role of the zona glomerulosa and zona fasciculata. Biochem Biophys Res Commun 212: 487–493, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Levy MN, Pappano AJ. Cardiovascular Physiology (9th ed.). Philadelphia: Mosby, 2007, p. 110–114.
- 30.Li M, Wen C, Whitworth JA. Hemodynamic effects of the Fab fragment of digoxin antibody (digibind) in corticotropin (ACTH)-induced hypertension. Am J Hypertens 10: 332–336, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Xie Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflügers Arch 457: 635–644, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Manunta P, Hamilton BP, Hamlyn JM. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. Am J Physiol Regul Integr Comp Physiol 290: R553–R559, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Manunta P, Hamilton J, Rogowski AC, Hamilton BP, Hamlyn JM. Chronic hypertension induced by ouabain but not digoxin in the rat: antihypertensive effect of digoxin and digitoxin. Hypertens Res 23, Suppl: S77–S85, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24: 45–57, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Mason DT, Braunwald E. Studies on Digitalis. X. Effects of ouabain on forearm vascular resistance and venous tone in normal subjects and in patients in heart failure. J Clin Invest 43: 532–543, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci 17: 105–109, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Murphy TV, Kotecha N, Hill MA. Endothelium-independent constriction of isolated, pressurized arterioles by Nω-nitro-l-arginine methyl ester (l-NAME). Br J Pharmacol 151: 602–609, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norman RA Jr, Coleman TG, Wiley TL Jr, Manning RD Jr, Guyton AC. Separate roles of sodium ion concentration and fluid volumes in salt-loading hypertension in sheep. Am J Physiol 229: 1068–1072, 1975. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien WJ, Lingrel JB, Wallick ET. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch Biochem Biophys 310: 32–39, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Orlov SN, Taurin S, Tremblay J, Hamet P. Inhibition of Na+,K+ pump affects nucleic acid synthesis and smooth muscle cell proliferation via elevation of the [Na+]i/[K+]i ratio: possible implication in vascular remodelling. J Hypertens 19: 1559–1565, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Padilha AS, Pecanha FM, Vassallo DV, Alonso MJ, Salaices M. Ouabain treatment changes the role of endothelial factors in rat resistance arteries. Eur J Pharmacol 600: 110–116, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Pierdomenico SD, Bucci A, Manunta P, Rivera R, Ferrandi M, Hamlyn JM, Lapenna D, Cuccurullo F, Mezzetti A. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am J Hypertens 14: 44–50, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Potocnik SJ, Murphy TV, Kotecha N, Hill MA. Effects of mibefradil and nifedipine on arteriolar myogenic responsiveness and intracellular Ca2+. Br J Pharmacol 131: 1065–1072, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pullen MA, Brooks DP, Edwards RM. Characterization of the neutralizing activity of digoxin-specific Fab toward ouabain-like steroids. J Pharmacol Exp Ther 310: 319–325, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Rossi G, Manunta P, Hamlyn JM, Pavan E, De Toni R, Semplicini A, Pessina AC. Immunoreactive endogenous ouabain in primary aldosteronism and essential hypertension: relationship with plasma renin, aldosterone and blood pressure levels. J Hypertens 13: 1181–1191, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol 293: C509–C536, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Schulte KL, van Gemmeren D, Thiede HM, Meyer-Sabellek W, Gotzen R, Distler A. Ouabain-induced elevation in forearm vascular resistance, calcium entry and alpha-adrenoceptor blockade, and release and removal of noradrenaline. J Hypertens Suppl 5: S215–S218, 1987. [PubMed] [Google Scholar]
- 48.Wang HX, Davis MJ, Rajanayagam MA, Potocnik SJ, Hill MA. Myogenic reactivity of rat epineurial arterioles: potential role in local vasoregulatory events. Am J Physiol Heart Circ Physiol 277: H144–H151, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Xavier FE, Rossoni LV, Alonso MJ, Balfagon G, Vassallo DV, Salaices M. Ouabain-induced hypertension alters the participation of endothelial factors in alpha-adrenergic responses differently in rat resistance and conductance mesenteric arteries. Br J Pharmacol 143: 215–225, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan CM, Manunta P, Hamlyn JM, Chen S, Bohen E, Yeun J, Haddy FJ, Pamnani MB. Long-term ouabain administration produces hypertension in rats. Hypertension 22: 178–187, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, Bianchi G, Ferrari P, Hamlyn JM, Iwamoto T, Lingrel JB, Matteson DR, Wier WG, Blaustein MP. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol 569: 243–256, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Wier WG, Blaustein MP. Mg2+ blocks myogenic tone but not K+-induced constriction: role for SOCs in small arteries. Am J Physiol Heart Circ Physiol 283: H2692–H2705, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.