Abstract
Prior studies indicate that cholinergic receptor (ChR) activation is linked to beating rate reduction (BRR) in sinoatrial nodal cells (SANC) via 1) a Gi-coupled reduction in adenylyl cyclase (AC) activity, leading to a reduction of cAMP or protein kinase A (PKA) modulation of hyperpolarization-activated current (If) or L-type Ca2+ currents (ICa,L), respectively; and 2) direct Gi-coupled activation of ACh-activated potassium current (IKACh). More recent studies, however, have indicated that Ca2+ cycling by the sarcoplasmic reticulum within SANC (referred to as a Ca2+ clock) generates rhythmic, spontaneous local Ca2+ releases (LCR) that are AC-PKA dependent. LCRs activate Na+-Ca2+ exchange (NCX) current, which ignites the surface membrane ion channels to effect an AP. The purpose of the present study was to determine how ChR signaling initiated by a cholinergic agonist, carbachol (CCh), affects AC, cAMP, and PKA or sarcolemmal ion channels and LCRs and how these effects become integrated to generate the net response to a given intensity of ChR stimulation in single, isolated rabbit SANC. The threshold CCh concentration ([CCh]) for BRR was ∼10 nM, half maximal inhibition (IC50) was achieved at 100 nM, and 1,000 nM stopped spontaneous beating. Gi inhibition by pertussis toxin blocked all CCh effects on BRR. Using specific ion channel blockers, we established that If blockade did not affect BRR at any [CCh] and that IKACh activation, evidenced by hyperpolarization, first became apparent at [CCh] > 30 nM. At IC50, CCh reduced cAMP and reduced PKA-dependent phospholamban (PLB) phosphorylation by ∼50%. The dose response of BRR to CCh in the presence of IKACh blockade by a specific inhibitor, tertiapin Q, mirrored that of CCh to reduced PLB phosphorylation. At IC50, CCh caused a time-dependent reduction in the number and size of LCRs and a time dependent increase in LCR period that paralleled coincident BRR. The phosphatase inhibitor calyculin A reversed the effect of IC50 CCh on SANC LCRs and BRR. Numerical model simulations demonstrated that Ca2+ cycling is integrated into the cholinergic modulation of BRR via LCR-induced activation of NCX current, providing theoretical support for the experimental findings. Thus ChR stimulation-induced BRR is entirely dependent on Gi activation and the extent of Gi coupling to Ca2+ cycling via PKA signaling or to IKACh: at low [CCh], IKACh activation is not evident and BRR is attributable to a suppression of cAMP-mediated, PKA-dependent Ca2+ signaling; as [CCh] increases beyond 30 nM, a tight coupling between suppression of PKA-dependent Ca2+ signaling and IKACh activation underlies a more pronounced BRR.
Keywords: submembrane Ca2+ release, ion channels, protein kinase A phosphorylation, signal transduction
nature has devised a potent “brake” on the rate at which the heart's pacemaker fires: signaling via cholinergic receptors (ChR) (6, 9). In situ, the ChR signaling cascade activated by vagal tone produces the most potent physiological suppression of the rate of spontaneous electrical impulses generated within the sinoatrial node, the heart's primary pacemaker. Spontaneous beating rate reduction (BRR) of sinoatrial node (SAN) cells (SANC) via ChR signaling involves Gi protein coupling (15) to several downstream targets: signaling via Giβγ activates ACh-activated potassium current (IKACh), leading to membrane hyperpolarization (8, 9); signaling via Giα inhibits adenylyl cyclase (AC) activity (10), which results in 1) in a reduction in cAMP, leading to a reduction of hyperpolarization-activated current (If) (via a shift of its voltage-dependent activation) and to changes in the early diastolic depolarization (DD) (11, 12, 14) and 2) a reduction of cAMP-mediated, PKA-dependent activity and phosphorylation of its downstream targets. Since L-type Ca2+ currents (ICa,L) activation is controlled by PKA-dependent signaling, e.g., during β-adrenoreceptor (β-AR) stimulation (17, 20), or in its absence (34, 39), reduction of ICa,L has been suggested as an additional mechanism in BRR by ChR stimulation (39). Thus it is currently believed that ChR signaling slows heart rate entirely by effects on the surface membrane ion channels, including IKACh, If, and ICa,L (9, 53).
On the other hand, recent studies demonstrate that PKA-dependent phosphorylation also controls Ca2+ cycling (47, 48) (referred as Ca2+ clock in SANC), manifested as rhythmic spontaneous subsarcolemmal Ca2+ releases (LCRs) (49) that affect the late DD via the electrogenic Na+/Ca2+ exchanger (3, 4). A high basal PKA-dependent phosphorylation of Ca2+ cycling proteins [phospholamban (PLB), ryanodine receptors (RyR), and L-type Ca2+ channels] is a crucial component of LCR occurrence and is required for SANC spontaneous beating (47) and for the increase in the beating rate in response to β-AR stimulation. Although the concept of the crucial importance of changes in sarcoplasmic reticulum (SR) Ca2+ cycling in normal automaticity and its response to β-AR stimulation, based on observations of ryanodine effects, had been challenged (21), this challenge has been comprehensively rebutted (27, 29). Whereas inhibition of basal PKA activity by PKI, a specific peptide inhibitor, damps LCRs, resulting in BRR (47), there has been no prior investigation of how activation of ChR signaling impacts on basal AC-dependent cAMP production or PKA signaling and functioning of the Ca2+ clock within the physiological BRR mechanism.
The purpose of the present study is to determine how the aforementioned ChR-Gi-coupled signaling mechanisms (targeting both sarcolemmal ion channels and LCRs) become integrated to generate the net response to a given intensity of ChR stimulation by carbachol (CCh). Specifically, we examined the integrated effects of ChR stimulation on action potential (AP) characteristics; IKACh, If, ICa,L; AC and guanylyl cyclase (GC) activities in the absence and presence of phosphodiesterase (PDE) inhibition; PKA-dependent PLB phosphorylation; SR Ca2+ cycling; and BRR. We also determined whether basal signaling via the ChR pathway in the absence of ChR-ligand activation is present within SANC, as in the case of high basal β-AR-independent cAMP/PKA-dependent signaling (47, 48). Finally, we employed numerical modeling to explore the coupling of SR Ca2+ cycling to membrane currents that affects BRR.
METHODS
Methods for SANC preparation, confocal Ca2+ imaging and electrophysiological recordings (48), Western blotting of PLB (24), and cAMP and cGMP measurements (52) have been described in detail previously, and additional details are provided in the Online Supplement.1 The animal protocols for this study were approved by the Animal Care and Use Committee of the Gerontology Research Center, National Institute on Aging (protocol no. 034LCS2010).
Numerical modeling.
Our previous SANC model (3, 47), featuring individual stochastic LCRs, was modified to include CCh modulation of IKACh, If, ICa,L, and LCRs (see Online Supplement).
Experimental protocols and drugs.
We applied a broad-spectrum cholinergic agonist, CCh (Calbiochem), to activate ChRs during spontaneous beating or during voltage-clamp experiments. A ChR antagonist, atropine (10 μM, Sigma), was used in the presence of CCh to confirm that CCh-induced BRR occurred via ChR activation. To detect potential constitutive Gi/q protein activation in the absence of ChR activation, we pretreated a subset of SANC in Kraft-Brühe solution for 5–6 h with 1.5 mg/ml of pertussis toxin (PTX) (Calbiochem), and during CCh experiments these cells were continuously superfused with Tyrode buffer containing PTX to detect a possible presence of constitutive Gi activation (50). To disable IKACh, we incubated a subset of SANC for at least 1 h with 1 μM tertiapin-Q (TQ) (Sigma), a specific peptide inhibitor of IKACh (23, 51). Cells were subsequently studied in current-clamp or voltage-clamp experiments in which SANC were continuously superfused with TQ vs. control cells (no TQ treatment). We employed 2 mM CsCl to block If. cAMP or cGMP concentrations in SANC suspensions were measured in the absence and presence of IBMX, 100 μM (Sigma), to inhibit PDE activity. In some experiments the phosphatase inhibitor calyculin A (100 nM) was applied following CCh.
Statistical analysis and nonlinear regression method.
Data are presented as means ± SE unless otherwise stated. Statistical significance of differences between means was evaluated by Student's t-test or analysis of variance (ANOVA), when appropriate. A value of P < 0.05 was considered statistically significant. To determine the IC50 for the average dose-response curves for a given pharmacological intervention, and to compare IC50 of different pharmacological interventions, a standard nonlinear regression method (GraphPad Software, Prism 5, 2007) was used. A detailed description of applied method is provided in the Online Supplement.
RESULTS
Graded effects of ChR stimulation on BRR and AP characteristics.
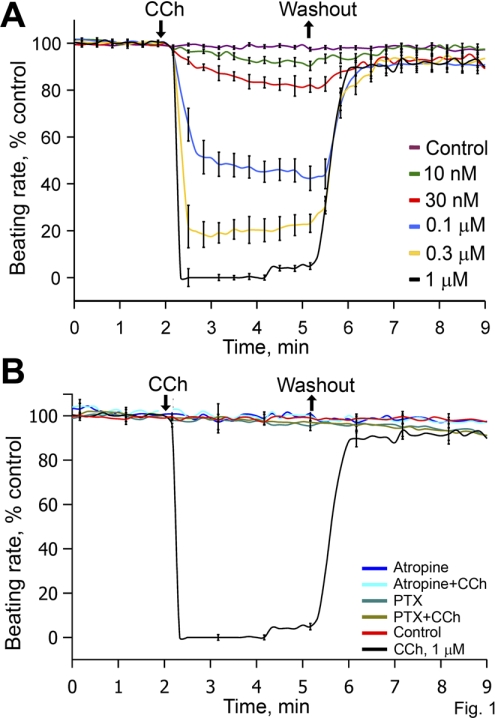
Figure 1A shows the average dose-time BRR response to graded CCh concentrations ([CCh]) (10 to 1,000 nM). Note the stability of the rhythmic beating rate in absence of drug and the completely reversible effects of CCh, regardless of its concentration. The threshold response of BRR to CCh occurs at ∼10 nM; the IC50 is ∼100 nM CCh; and 1,000 nM CCh stops spontaneous beating. Online Supplemental Fig. S1 illustrates representative examples of varying CCh concentration ([CCh]) (30, 100, and 1,000 nM) on action potentials (APs) of single isolated rabbit SANC. The average AP characteristics in control and during [CCh] exposure are listed in Supplemental Table S1. Note that the low control values for maximum rate of rise of action potential indicate that primary SANC are sampled in this study.
Fig. 1.
A: average beating rate reduction (BRR) time-dose effects of 5 different carbachol (CCh) concentrations ([CCh]; control, n = 39; 10 nM, n = 9; 30 nM, n = 6; 100 nM, n = 19; 300 nM, n = 17; 1,000 nM, n = 15). Each cell received only a single dose of CCh. B: average normalized BRR in control cells, cells exposed to CCh for 3 min, cells superfused with atropine alone or with atropine and CCh, and cells pretreated with and superfused with Tyrode's buffer containing 1.5 mg/ml of pertussis toxin (PTX), with and without CCh (control, n = 15; CCh 1 μM, n = 10; 10 μM atropine, n = 4; CCh+atropine, n = 4; PTX 1.5 mg/ml, n = 4; PTX+CCh, n = 4). The times of CCh addition and washout are indicated by arrows, which pertain only to protocols in which CCh was added and washed.
Absence of constitutive Gi activation in the absence of ChR ligand binding.
Atropine completely blocked the effect of a high [CCh] (1,000 nM) to stop spontaneous SANC beating (Fig. 1B) but had no effect on the beating rate in the absence of CCh. To explore the possibility of constitutive ChR activation that had been observed in other cell types in the absence of a receptor ligand (19, 42), we measured the spontaneous beating rate in SANC pretreated with PTX in the absence of CCh. PTX pretreatment did not affect the basal beating rate in the absence of the ChR ligand (182 ± 5 bpm in control, n = 39, 177 ± 27 bpm in PTX-treated cells, n = 6), but it completely blocked CCh effects on BRR (Fig. 1B). Thus constitutive Gi activity is not present in the absence of ligand, and the effect of ChR stimulation to affect BRR requires ChR-Gi coupling.
Effect of CsCl or TQ on SANC membrane currents and responses to ChR stimulation with CCh.
Figure 2A shows the average effect of CsCl on the total current-voltage relationship measured under the whole cell voltage-clamp configuration. Representative examples of raw currents measured across the voltage range in the presence or absence of Cs+ are illustrated in Supplemental Fig. S2A, i and ii. In main text Fig. 2A, note that CsCl markedly blocks the inward current at voltages negative to −60 mV. The inset in Fig. 2A shows the pre- and post-CsCl difference current-voltage relationship, i.e., the If I-V relationship. Note that as assessed by this method If becomes activated by hyperpolarization in SANC at around −60 mV.
Fig. 2.
A: average current-voltage (I-V) curves of total current density measured at 450 ms of the voltage pulse in the absence or presence of CsCl. Inset: difference current between the traces recorded in the absence and presence of 2 mM CsCl. This difference current is hyperpolarization-activated current (If). B: average effects of CCh on the I-V relationship of ACh-activated potassium current (IKACh) measured during If blockade (CsCl+CCh, n = 13) in control experiments (red line) and in experiments in which IKACh was blocked by 1 μM tertiapin Q (TQ) and If was blocked by 2 mM CsCl (TQ+CsCl+CCh, n = 9; blue line).
Since the I-V relationship of IKACh partially overlaps that of If, and because both If and IKACh are affected by CCh, it was necessary to measure IKACh in the presence of If blockade with 2 mM CsCl. Supplemental Fig. S2A, iii shows a representative example of the effect of CCh on IKACh across a wide voltage range. Supplemental Fig. S2B, i shows that the total current-voltage relationship measured following TQ application alone (i.e., in the absence of CCh) does not differ from control (Supplemental Fig. S2A, i). This indicates that IKACh is not activated in the absence of ChR ligand, and is consistent with a lack of an effects of TQ on the spontaneous beating rate or maximum diastolic potential (MDP) (Table S1). When CsCl is applied to TQ-treated cells (Supplemental Fig. S2B, ii), total inward current is markedly diminished, similar to the effect of CsCl in control cells (Supplemental Fig. S2A, i vs. ii). In the absence of TQ CCh activates IKACh (Supplemental Fig. S2A, iii), but in the presence of TQ CCh fails to do so (Supplemental Fig. S2B, iii). The average CCh effect in the presence of 2 mM CsCl on the steady-state IKACh I-V relationship in response to CCh is shown in Fig. 2B. Note that TQ completely abolishes steady IKACh current stimulated by CCh. The amplitude of the steady outward IKACh current ∼3.2 pA/pF, measured in the presence of Cs, within the voltage range relevant to diastolic depolarization (Fig. 2) is similar to the steady IKACh current measured in the absence of CsCl in prior studies, i.e., ∼2.3 pA/pF (12) and 4 pA/pF (6).
Supplemental Fig. S4 shows the effect of CCh on ICa,L peak amplitude. On average, ICa,L was reduced by only 20% even in response to 10 μM CCh, i.e., a concentration tenfold higher than that to 1 μM, which stopped spontaneous beating (Figs. 1, 3, 4).
Fig. 3.
A: average dose-dependent peak effects of CCh on maximum diastolic potential (MDP) and BRR in spontaneously beating sinoatrial nodal cells (SANC) and in SANC with blocked If or blocked IKACh, or block of both currents simultaneously (CCh, n = 43; TQ+CCh, n = 22; CsCl+CCh, n = 22; TQ+CsCl+CCh, n = 12). Each cell received only a single dose of CCh. B: the average change in BRR at each [CCh] for CCh alone and for CCh+TQ has been normalized to the respective average maximum effect. Since each cell received only a single concentration of CCh, individual cells do not have a dose response. Therefore an average dose-response curve was calculated from the average of all cells at all concentrations. This yields a unique normalized dose-response curve, but without x-axis variation. The average normalized curves are the best fit with a nonlinear regression model for the experimental data points. The log[IC50] calculated from this regression model is indicated in the inset. TQ shifts the CCh IC50 toward the lower concentrations of CCh by 2.6-fold.
Fig. 4.
Average dose-time BRR effects in SANC produced by CCh alone (n = 43), with block of If by 2 mM CsCl (n = 22), with IKACh by 1 μM TQ (n = 22), or with block of both If and IKACh (n = 12). Each cell received only a single CCh dose.
Roles of IKACh activation or If suppression in ChR-induced suppression of BRR.
Figure 3 shows the complete BRR and MDP dose response to CCh in the absence or presence of If blockade, or IKACh blockade, or blockade of both If and IKACh (by CsCl and TQ, together). Note in Fig. 3A that, on average, MDP hyperpolarization, which indirectly reflects the level of IKACh activation in response to CCh, begins to occur at [CCh] > 30 nM. At [CCh] > 100 nM, the hyperpolarizing effects of IKACh activation increased and parallel increased BRR. Importantly, TQ completely abolishes the hyperpolarization of MDP caused by any [CCh], either in the presence of If blockade (green circles vs. blue circles) or in the absence of If blockade (black circles vs. red circles in Fig. 3; see also Supplemental Fig. S3). Note in Fig. 3A the presence of an IKACh-independent effect of CCh on BRR (red diamonds). Specifically, this IKACh-independent BRR by CCh begins to occur at 10 nM and plateaus at 100 nM, accounting for 25–30% of the maximum BRR (Fig. 3). Note also that even in the absence of IKACh blockade (Figs. 1A, red line, and 3A, black diamonds) BRR by CCh becomes evident at a [CCh] as low as 30 nM, at which no MDP hyperpolarization is evident (black circles). Figure 3B shows that the IC50 [log(IC50,CCh) = −6.94 M, Fig. 3B CCh] estimated from the best fit of the average normalized dose-response curve for BRR to CCh shifts to lower (2.6-fold) [CCh] when the CCh effect was partially blocked by the IKACh blocker TQ (Fig. 3B CCh+TQ, log[IC50,CCh] = −7.36 M).
Figure 3A also shows that CsCl, which markedly blocks If (Fig. 2A), does not affect BRR at any [CCh] (green vs. black diamonds). Prior to CCh, CsCl caused a 15% BRR, as was reported earlier (6, 45). Furthermore, the CCh effect on BRR in the absence of hyperpolarization when both IKACh and If are blocked (blue diamonds, Fig. 3A) is identical to that when IKACh alone is blocked (red diamonds, Fig. 3A), indicating that If does not have a major role in the average BRR by CCh.
Figure 4 illustrates the time course of the BRR response to four different [CCh] in the presence or absence of If and/or IKACh blockade. At 10 or 30 nM CCh, IKACh has no apparent effect on either the magnitude of BRR or the evolution of BRR kinetics, because at these [CCh] BRR by CCh and CCh+TQ do not differ. At [CCh] > 30 nM, however, IKACh blockade markedly slows the kinetics of BRR response and this blunting parallels the reduction in the magnitude of BRR. CsCl had no effect on the magnitude or kinetics of the BRR response to CCh at any [CCh].
The results presented thus far show that over the entire [CCh] range there is a mechanism in addition to IKACh and other than If that has an appreciable effect on BRR and that the relative magnitude of this inhibitory component plateaus at the IC50 CCh (Fig. 3 and 4). Next we determined whether this IKACh-If-independent component of BRR by CCh is linked to suppression of AC/cAMP/PKA/Ca2+ signaling.
Effects of ChR stimulation on cAMP, cGMP, and phospholamban phosphorylation.
Figure 5 shows the effects of [CCh] on total cAMP (Fig. 5A) and cGMP (Fig. 5B) in the absence (left bars) and presence of PDE inhibition by IBMX (right bars). In the absence of CCh or PDE inhibition total cAMP levels are 20 times higher than cGMP levels (Fig. 5, A and B, left bars). CCh, in the absence of PDE inhibition, had no detectable effect on either cAMP or cGMP (A and B, left bars). This lack of effect on total cAMP or cGMP is not surprising, as changes in functionally relevant local concentrations of nucleotides are often not apparent in the measurement of total nucleotides when PDE activity is present. PDE inhibition increased cAMP by sevenfold and doubled cGMP (Fig. 5, A and B, left bars). During a 20-min period of PDE inhibition by IBMX, CCh reduces total cAMP by 21 and 34% at 100 nM and 1 μM, respectively (Fig. 5A, right bars), but does not change cGMP (Fig. 5B, right bars). Although AC activity is not measured directly in this experiment, the reduction of cAMP over the 5-min period of CCh treatment in the presence of PDE inhibition indicates an effect of CCh to reduce net AC activity over that period.
Fig. 5.
CCh effects on total cAMP (A; n = 5) and cGMP (B; n = 4) levels in spontaneously beating SANC suspensions in the absence or presence of phosphodiesterase (PDE) inhibition by IBMX. Each cell suspension from a given sinoatrial node was first divided into 2 major groups: no IBMX prior to CCh, or IBMX (100 μM, 20 min pretreatment before CCh). Each group was further subdivided into 3 groups: control, 100 nM CCh, and 1 μM CCh. Total incubation time in all cell groups was 25 min. In PDE-treated groups IBMX was added immediately at the beginning of incubation. In groups with CCh treatment, CCh was added at the 20th minute of incubation. In other words, the duration of exposure to CCh was 5 min. PDE inhibition in the absence of CCh increases both cAMP and cGMP (*P < 0.05). In the presence of PDE inhibition CCh significantly reduces cAMP (#P < 0.05) but does not affect cGMP.
Figure 6A demonstrates a high basal PLB phosphorylation at the cAMP-mediated, PKA-dependent serine 16 phosphorylation site in SANC suspensions, shown previously (47, 52) to be due to a high basal AC activity within spontaneously firing SANC. CCh causes a dose-dependent reduction in the PLB phosphorylation. A representative example is shown in Fig. 6A, top, and the average data in bottom of Fig. 6A. The number of total cell suspensions treated at each [CCh] is shown in parentheses. Note that at the IC50 [CCh] for BRR (100 nM) the average reduction in PLB phosphorylation is also ∼50%. The best fit IC50 for PLB dephosphorylation [log(IC50) = −7.23] is similar to that for BRR by CCh when IKACh is blocked [log(IC50) = −7.36] and lies within the variance of the average IC50 for PLB dephosphorylation (large gray circle ± SD in Fig. 6B) measured directly in three cell suspensions in which a full dose-response to all [CCh] was determined.
Fig. 6.
A, top: representative Western blots of phosphorylated phospholamban (PLB) at the PKA-dependent phosphorylation site Ser-16 and total PLB immunolabeling in control SANC suspensions and cell suspensions exposed to a wide range of [CCh]. Bottom: average immunoblot data of phosphorylated PLB normalized to total PLB in response to CCh. The number of experiments at each [CCh] is indicated in parentheses. B: the average response at each [CCh] has been normalized to the maximum effect of CCh. The curves represent the best fit nonlinear regression model for the experimental data points. The curve for BRR in the presence of IKACh inhibition is the same as that was illustrated in Fig. 3B. The log[IC50] calculated from the nonlinear regression for each curve is shown in the inset. In 3 PLB phosphorylation experiments, cell suspensions received the entire range of [CCh]. Thus a final normalized dose-response curve could be calculated for each of these experiments, permitting direct calculation of the average IC50 and the variance around the average IC50. This is indicated by large solid gray point with ±SD on the PLB dose-response curve. Note that the IC50 for BRR by CCh in the presence of IKACh inhibition is within the SD of the IC50 for PLB dephosphorylation.
Effects of ChR stimulation on Ca2+ cycling.
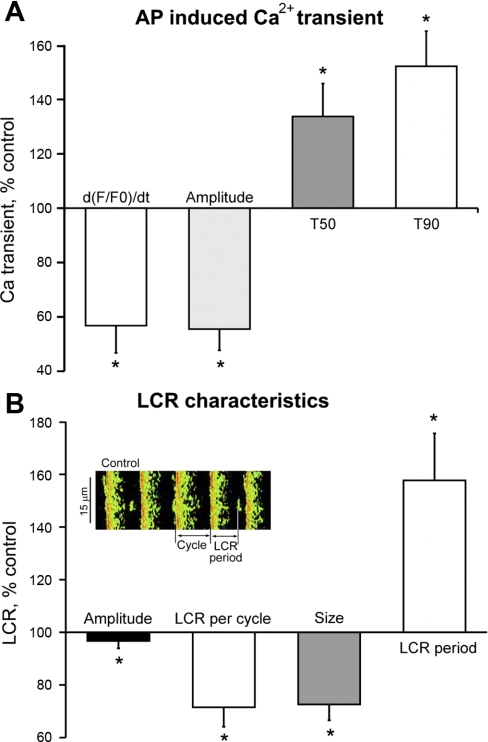
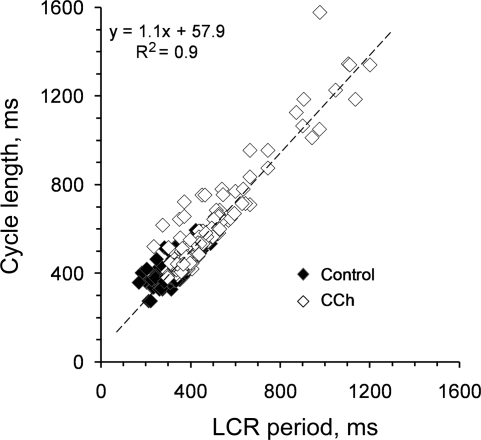
It has been shown previously that reduction of PLB phosphorylation reduces the kinetics of SR Ca2+ cycling, which markedly affects the timing and amplitude of spontaneous LCRs (47). Figure 7, left, depicts line scan images of Ca2+ beneath the cell surface membrane. The AP triggers a global SANC Ca2+ transient. The AP is preceded by LCRs. At 100 nM, i.e., the IC50 for BRR; CCh markedly affects both the AP-induced Ca2+ transient and diastolic LCRs. The average effects of CCh on the AP-induced Ca2+ transient and spontaneous LCRs are illustrated in Fig. 8. CCh decreases the maximum rate of rise of the AP-triggered Ca2+ transient [d(F/F0)/dt], an index of the SR Ca2+ release flux (44) (Fig. 8A), reduces the amplitude of AP-triggered Ca2+ transient and increases the times to 50 and 90% decay of cytosolic Ca2+ transient. Figure 8B illustrates the average effects of CCh on LCR characteristics: LCR frequency of occurrence and size are reduced by 28 and 27%, respectively; the LCR period, i.e., the time between the maximum rate of the upstroke of the Ca2+ transient triggered by the prior AP to the occurrence of maximum rate of upstroke of a subsequent LCR (as indicated in Fig. 8B inset), increased by ∼43%. Figure 9 depicts the relationship of concomitantly measured cycle length to the LCR period during the evolution of the BRR, i.e., the prolongation of spontaneous AP cycle length with time (1–3 min) following CCh application. Note that the time-dependent prolongation of cycle length closely tracks the concomitant prolongation of LCR period.
Fig. 7.
Confocal Ca2+ images (left) and analog Ca2+ transients (right), in a representative SANC prior to and during CCh exposure at the IC50 CCh. Both action potential (AP)-triggered Ca2+ transients and local Ca2+ release (LCR) characteristics are substantially reduced by CCh in spontaneously beating SANC. F, fluorescence during excitation; F0, background fluorescence.
Fig. 8.
A: average (n = 10) changes in AP-induced Ca2+ transient characteristics in the presence of CCh. d(F/F0)/dt, maximum rate of rise of the Ca2+ transient measured as F/F0 fluo 3-AM fluorescence; T90, transient duration at 90% of transient amplitude; T50, transient duration at 50% of transient amplitude. In control: d(F/F0)/dt = 0.032 ± 0.002 s−1; amplitude = 1.46 ± 0.08 F/F0; T90 = 264.4 ± 11 ms; T50 = 100.2 ± 3 ms. B: average (n = 10) change in LCR characteristics induced by CCh. In control: LCR amplitude = 1.1 ± 0.18 F/F0; LCR number per cycle = 1.02 ± 0.1; LCR size = 6.9 ± 0.7 μm; LCR period = 351 ± 22 ms (*P < 0.05). The method used to define LCR period and LCR cycle length is illustrated in the inset of B. The cycle length is the time between 2 successive AP-induced Ca2+ transients, and LCR period is measured as time from the preceding Ca2+ transient upstroke to the maximum rate of rise of subsequent LCR (*P < 0.05).
Fig. 9.
Least squares linear regression of concomitantly measured LCR periods and cycle lengths in 10 SANC in control and during a 1- to 3-min exposure to 100 nM (IC50) CCh.
The concomitant CCh induced inhibition of the spontaneous SANC beating rate, of Ca2+ cycling characteristics and of PLB phosphorylation suggests that dephosphorylation mediates the effects of the IC50CCh on Ca2+ cycling and BRR. Thus we determined whether phosphatase inhibition could reverse the effects of CCh on LCR and BRR (Figs. 10 and 11). In Fig. 10 note that the phosphatase inhibitor calyculin A (100 nM) reverses the effects of IC50 CCh on both cycle length and LCR characteristics. The relationship between cycle length and LCR period in control during exposure to CCh and following the addition of calyculin A in the continued presence of CCh is shown in Fig. 11. Note the close correlation between LCR period and cycle length, and, importantly, that the LCR period is always shorter than the cycle length.
Fig. 10.
A: representative confocal line scan images of the effects of the phosphatase inhibitor calyculin A (100 nM) to reverse the effects of a 3-min 100 nM CCh exposure on cycle length and LCRs. Calyculin A was added for 3 min in the continued presence of CCh. B: average data on cycle length and LCR characteristics in control, during CCh, and during CCh+calyculin A (n = 5), *P < 0.05 vs. control, ‡P < 0.05 vs. CCh.
Fig. 11.
Least square linear regression of LCR period and cycle length in 5 SANC in control, measured during a 3-min exposure to IC50 [CCh], and following a 3-min exposure to the phosphatase inhibitor calyculin A in the continued presence of 100 nM CCh.
Numerical modeling of CCh effect and continuity of the negative and positive chronotropic regulation by PKA.
Ion channel-related mechanisms of BRR in SANC by ChR modulation, previously numerically modeled in 1999 by Demir et al. (9) and in 2002 by Zhang et al. (53), have formulated important ChR-induced changes of IKACh, If, and ICa,L. Recent studies have shown that the signaling sequence, PKA → PLB → LCRs → NCX, underlies the late DD acceleration, providing a major contribution to the spontaneous beating rate in rabbit SANC (3, 47). The extent of PLB phosphorylation is high in the basal state (47, 48) but decreases under ChR stimulation with CCh (Fig. 6). This suggests that CCh also mediates BRR, at least in part, via the same signaling sequence.
In the present study, using our previous SANC model featuring individual stochastic LCRs in submembrane space (3, 47) (see Online Supplement for details), we explored the integration of classical BRR membrane mechanisms with the BRR produced by LCRs via NCX current (INCX) in response to the IC50 CCh. Thus, in addition to the ion channel changes described by classical models, we also introduced experimentally measured changes of LCR characteristics (LCR period increase and LCR signal mass decrease). Our model simulations closely predict our experimental results for BRR produced by IC50 CCh alone or combined with blockade of If (Cs) and/or IKACh (TQ), or with phosphatase inhibition by calyculin A (Fig. 12). Our simulations show that the late diastolic INCX (blue traces in Fig. 12A) mediates effects of LCRs in all perturbations and these LCR-induced (INCX-mediated) effects on the late DD substantially contribute to the BRR, which is in agreement with experimental results (Fig. 12).
Fig. 12.
Experimental data and numerical modeling (see Online Supplement for details) of the component mechanisms for BRR induced by 100 nM CCh. A: model simulations show LCR signaling to membrane potential (Vm, red) via Na+-Ca2+ exchange (NCX) current (INCX, blue) under variety of perturbations (gray labels). Eight arbitrary LCRs are shown by multiple colors (above INCX and Vm) in each subpanel (i–vii). B: the numerical model simulations closely predict negative chronotropic effects of IC50 CCh observed experimentally.
When the emergence of LCRs is delayed, the diastolic INCX is also delayed, resulting in a later DD acceleration and a longer cycle length (Fig. 12A, ii vs. i). When CCh-induced dephosphorylation is prevented by calyculin A, the diastolic INCX also remains almost unchanged (Fig. 12A,iv), resulting in a rather limited BRR (12.9% in the model and 12.5% in experiment) produced by phosphorylation-independent IKACh and If. On the other hand, blockade of IKACh or If, or both If and IKACh, still accounts for less than half of the BRR compared with the BRR induced by 100 nM CCh alone in the model simulation, which is in agreement with the experimental results. These model simulations provide a theoretical basis for the LCR dependence of the BRR response to CCh.
DISCUSSION
The present study dissected the complex ChR-linked signaling cascade (Supplemental Fig. S5) to discover which mechanisms are recruited in the BRR in response to a given intensity of ChR stimulation in rabbit SANC. Graded ChR stimulation by CCh (10 to 1,000 nM) produced graded BRR: the threshold [CCh] for BRR was ∼10 nM; the IC50 was 100 nM CCh; and in response to 1,000 nM CCh spontaneous APs ceased (Fig. 1, A and B). The entire BRR dose response to CCh requires Gi protein signaling, since pretreatment with PTX to prevent Gi activation abolished all effects of CCh (Fig. 1B). PTX effects on If and ICa,L suppression by ChR activation have also been reported previously (14, 17). The present results show that, in the absence of CCh, PTX does not affect the basal beating rate, which indicates an absence of any significant constitutive Gi activity linked to spontaneous beating in rabbit SANC. The Gi protein activation responsible for the ChR induced BRR can be mediated by either Giα subunit or Gβγ complex. The Gβγ complex directly activates IKACh, which affects BRR by hyperpolarizing the MDP (9, 12, 14). We observed that the CCh-induced MDP hyperpolarization only at a [CCh] > 30 nM, and this effect continued to increase with increasing [CCh]. Beginning at 100 nM CCh, graded hyperpolarization of MDP paralleled BRR as the [CCh] increased. At 1,000 nM CCh, just prior to spontaneous AP cessation, the hyperpolarization resulted in the MDP shift to −68.5 ± 1.2 mV (Supplemental Fig. S1, bottom). Thus IKACh is a highly effective mediator of BRR in response to ChR stimulation, but this effect occurs at higher intensities of ChR stimulation, as also observed in some prior studies that employed barium to block IKACh, or reported that the IC50 acetylcholine (ACh) to suppress If was 20 times less than the IC50 for IKACh activation (1, 6, 12).
Our results (Figs. 1, 3, and 4) also show that in response to lower [CCh] (up to 30 nM), whereas hyperpolarization of the MDP did not occur, BRR (15%) did occur; and, in the presence of full inhibition of IKACh, when no MDP hyperpolarization occurred at any [CCh] the BRR increased to 25–30% at 100 nM [CCh] (Figs. 3 and 4). In addition to IKACh desensitization or fading (7), BRR induced by low [CCh] or ACh concentration ([ACh]) has previously been attributed to suppression of If or ICa,L, which are modulated by cAMP or cAMP mediated PKA-dependent signaling, respectively (Supplemental Fig. S5).
Since our results demonstrate that Gi protein activation is required for the overall BRR by ChR stimulation, the hyperpolarization-independent BRR effect must be attributable to activation of the Giα subunit, sometimes referred to as an indirect inhibitory signaling mediated through the cAMP-PKA pathway (9) (Supplemental Fig. S5): the active GTP-Giα protein complex directly inhibits AC activity, and this is followed by a reduction in cAMP-mediated, PKA-dependent protein phosphorylation (8, 10, 22, 35). Indeed, in the present study, CCh reduced the AC activity (inferred from a 21 and 34% cAMP reduction over a 5-min period of CCh treatment in IBMX-pretreated cells, Fig. 6A, right bars) and was accompanied by 25–30% BRR when IKACh or If or both are blocked (Fig. 3A, red and blue diamonds). Our experiments, however, cannot directly relate the cAMP reduction resulting from AC inhibition to the BRR in response to CCh, since the kinetics of former were not measured during 5 min of CCh incubation. Our results also show that in the absence of PDE inhibition total cGMP levels in SANC are only a fraction (1/20th) of cAMP levels, and whereas cGMP and cAMP both increase following PDE inhibition (Fig. 5B, right bars) cGMP does not change in response to ChR stimulation, even when PDEs are inhibited (Fig. 5B). Thus we find no evidence that ChR stimulation by CCh exerts its BRR effects via a change in guanylyl cyclase activity or cGMP.
If suppression has been interpreted to be an important mechanism in the BRR in response to ChR stimulation, particularly at low [CCh] or [ACh] (12, 14, 26). If is a prime candidate for BRR regulation by a ChR effect via suppression of AC activity because If channel subunits have a cAMP response element (Supplemental Fig. S5). The specific mechanism previously invoked for If suppression is that Gi protein activation by ChR stimulation inhibits AC activity (or reduces cAMP by activating PDE), which, in turn, reduces cAMP levels in SANC, shifting the If current activation curve toward more negative membrane voltages, rendering If less active (13). Earlier studies had extrapolated this effect of ACh (or CCh) in voltage-clamped SANC to spontaneously firing SANC to explain the ChR-induced BRR (12, 14). However, although ChR stimulation clearly affects If activation under voltage clamp, it does not necessarily follow that such an effect need be involved in BRR, as has previously been contended (9) because of the attendant reduction in PKA-dependent signaling that is evoked by ChR stimulation. The present study, in fact, shows (Figs. 3 and 4) that the full effect of CCh on BRR persists at all [CCh] in the presence of If blockade by 2 mM CsCl (Fig. 2) and that the effects of CCh at IC50 on both BRR and Ca2+ cycling are reversed by phosphatase inhibition (Figs. 10 and 11), an effect distal to cAMP, i.e., on PKA-dependent phosphorylation (see below). We interpret our result to indicate that modulation of If activation by ChR stimulation does not participate in (i.e., is neither sufficient nor necessary for) the ChR-induced BRR. The results of prior studies also have been interpreted to indicate the absence of a substantial participation of If in ChR-induced BRR (6, 53).
Prior reports of ChR stimulation in spontaneously beating intact hearts differ in their interpretation of the role of If in the BRR. With the advent of TQ, two studies were performed on the isolated Langendorff preparations to estimate the participation of IKACh and If currents in the bradycardic response to CCh. Yamada (51) did find a marked contribution of If current to the negative chronotropic response of isolated rabbit heart to CCh. On the other hand, in isolated guinea-pig heart, Bolter and English (5) showed that If current played little or no role in vagal slowing or in the pacemaker response to ACh. The difference between these studies cannot be explained by a species difference, since a significant contribution of If current to pacemaker activity has been convincingly demonstrated in both rabbit and guinea pig isolated SAN (28). Moreover, both studies used 300 nM TQ to block IKACh and 2 mM Cs to block If current. Thus results of our study are consistent with the findings of Bolter and English but not those of Yamada. Additional studies are required to define the contribution of the LCR mechanism to parasympathetic stimulation induced bradycardia of the intact, isolated heart.
We have previously demonstrated that, as a result of a high basal cAMP level, SANC have a high basal level of PKA-dependent phosphorylation of PLB and RyR (47). There is also indirect evidence for the importance of basal L-type Ca2+ channel phosphorylation (39). Graded dephosphorylation by a specific PKA inhibitor peptide, PKI, is highly correlated with graded reductions in the spontaneous basal beating rate (47). To determine whether ChR stimulation is indeed involved in reduction of basal PKA-dependent protein phosphorylation in SANC, we studied the effects of CCh on PLB phosphorylation as an index of PKA activity. Our results (Fig. 6A) show that at the IC50 CCh for BRR PLB phosphorylation is reduced by ∼50%, similar to the extent of estimated net reduction of AC activity [inferred from the CCh-induced cAMP reduction (Fig. 5A) over the 5 min of CCh treatment when PDEs are inhibited]. Furthermore, the IC50 for reduction in PLB phosphorylation by CCh mirrors that of BRR in the presence of IKACh blockade (Fig. 6B), and the IC50 CCh-mediated effects in BRR and Ca2+ cycling are reversed by phosphatase inhibition (Fig. 10, 11).
It is important to note although PDE inhibition was required to demonstrate a CCh-induced reduction in total cell cAMP levels, a local reduction in cAMP-mediated, PKA-dependent PLB phosphorylation and its effects on the kinetics of SR Ca2+ cycling, manifested by the changes in cell Ca2+ signaling (see below), can be detected even in the absence of PDE inhibition. Thus evidence for a functionally significant reduction of cAMP-mediated-PKA-dependent signaling by CCh emerges even in the absence of PDE inhibition, even though the demonstration of a CCh effect on total cAMP requires the presence of PDE inhibition. Nonetheless, the requirement for PDE inhibition to observe an effect of CCh to reduce cAMP may be considered as a limitation of our method.
Numerous studies have documented that ChR stimulation reduces ICa,L (17, 18, 34, 38, 39), and this effect has been ascribed in some studies (38, 39) to a PKA-dependent reduction in ambient L-type Ca2+ channel phosphorylation. The effect of phosphatase inhibition to reverse the effect of CCh on Ca2+ cycling and BRR is consistent with this idea. There is substantial disagreement, however, as to whether ChR inhibitory effects on ICa,L do occur in the absence of concurrent β-AR stimulation. Our findings show that in the absence of β-AR stimulation CCh reduced the ICa,L amplitude by 20% (Supplemental Fig. S4), but at a tenfold higher [CCh] than that required to stop spontaneous beating. An effect of ChR stimulation to suppress ICa,L has previously been attributed to activation of soluble GC, leading to elevation of cGMP and to a subsequent increase in PDE2 activity (18, 19). This mechanism is controversial, since ChR do not appear to directly link to GC (Supplemental Fig. S5). It has also been suggested that NO mediates its effects through G protein coupling (41), a result consistent with our observations. Although we did not directly investigate a role for NO signaling in the BRR effect of ChR stimulation, our results do indicate that if such an effect is present, it must be a Gi-coupled effect, since Gi inhibition abolishes all effects of ChR stimulation on spontaneous AP rate without affecting the basal beating rate (Fig. 1B).
The phosphorylation of “Ca2+ clock” proteins, i.e., L-type Ca2+ channel, PLB, and RyRs, markedly affects the amplitude and kinetics of Ca2+ releases in cardiac cells, including SANC (32). The present results demonstrate that 1) in addition to reducing PLB phosphorylation, CCh, at the IC50 for BRR, reduces the amplitude and slows the kinetics of both cytosolic Ca2+ transients triggered by the AP and of rhythmic submembrane LCRs (Figs. 7 and 8) that occur spontaneously later in the cycle, i.e., during the late DD; and 2) these effects are reversed by phosphatase inhibition. Prior studies have demonstrated that LCRs activate INCX that drives in acceleration of the late DD toward the ICa,L activation threshold, thus igniting the subsequent AP firing (3, 4, 31). When the LCR period is prolonged and LCR net signal mass (i.e., the combination of LCR amplitude, spatial size, and frequency of occurrence) is damped, as occurs in response to CCh (Fig. 8B), the diastolic cycle length becomes prolonged. A hypothetical biophysical mechanism for the prolongation could be that the LCR impact on the DD via INCX is expected to be reduced and to occur later in time following the prior AP, thereby resulting in a longer time for the membrane potential to achieve the threshold for ICa,L. Indeed, our results show that the time-dependent effect of CCh to prolong the LCR period is highly correlated to the concomitant time-dependent BRR (Fig. 9). The reduction in beating rate by CCh, per se, reduces Ca2+ influx and the net cell and SR Ca2+ load [via the Bowditch treppe effect (25)], thus further damping LCRs. This “feed-forward” effect further contributes to the extent of steady-state BRR by CCh.
We employed numerical modeling to provide theoretical support for interpretation of our experimental results, i.e., that a CCh-induced change in Ca2+ cycling and its specific coupling role to INCX is crucial in physiologically relevant regime of BRR. Our simulations at IC50 CCh for BRR clearly show that LCRs, indeed, critically influence membrane function via reducing and delaying (after prior AP) INCX (Fig. 12A). In contrast, CCh induced BRR becomes substantially blunted when INCX remains unchanged. Furthermore, when CCh-induced dephosphorylation is largely prevented, phosphorylation-independent IKACh and If produce a rather moderate BRR (12.9% in the model simulation and 12.5% in our experiment with calyculin A) (Fig. 12A, iv). Thus we conclude that sarcolemmal electrogenic mechanisms and Ca2+ cycling [i.e., the system Ca2+ clock as previously defined (30)] tightly cooperate to effect the strong and robust BRR at IC50 CCh.
It would be an oversimplification to assume that the intrinsic spontaneous cycle length or AP characteristics of individual rabbit SANC in isolation or their modulation by ChR stimulation are identical to those of all SANC within the intact SAN. The autonomic milieu is heterogeneous throughout the SAN, with the central area having the greatest density of nerve endings and autonomic receptors on SANC (2, 40). Further shifts of the impulse origin from the initial, primary site in SAN preparations occur in response to autonomic receptor stimulation or to different ion manipulations (16, 36, 37, 43, 46). The complexity of the intact SAN notwithstanding, the present findings, interpreted in the context of prior work (3, 4, 31, 32, 47, 48), support a novel general theoretical formulation for the interplay of both cholinergic and adrenergic autonomic regulation of spontaneous AP firing of isolated rabbit SANC across the full physiological range of spontaneous beating rates (Supplemental Fig. S5).
Since isolated SANC have no constitutive β-AR activity (47), or ChR or Gi protein activation (Fig. 1B), the intrinsic AP firing rate in SANC is determined by constitutive Ca2+ activation of AC (33, 52) that results in cAMP-mediated PKA-dependent phosphorylation of Ca2+ clock proteins (32, 47, 48, 52). A high constitutive PDE activity acts as a brake to control the effects of intrinsic Ca2+-activated AC activity by increasing cAMP hydrolysis (48). ChR stimulation in vivo by ACh release from the nerve endings “clamps” the basal PKA-dependent Ca2+ signaling (Ca2+ clock) that is intrinsic to SANC and that determines the AP firing rate in the absence of any receptor stimulation. But ChR stimulation, rather than increasing hydrolysis of cAMP to control the cAMP level, as do PDEs, affects cAMP production, via a Giα effect to reduce AC activity. Still, concerted cAMP reduction by both constitutively active PDEs and ChR stimulation regulates the AP firing rate via a common downstream signaling point: PKA-dependent phosphorylation of Ca2+ cycling proteins, and resultant effects on Ca2+ cycling (Supplemental Fig. S5). As the intensity of ChR increases, cycle length prolongation by AC-cAMP-PKA-LCR-NCX interactions is extended further by a waxing IKACh contribution, i.e., by an effect to reduce a tight integration of both mechanisms. A marked alteration of ChR signaling in AC Type 5 (a Gs-coupled AC) knockout mice in which IKACh is intact (35) is, in fact, consistent with this idea. During the flight or fight reflex, i.e., in response to stress, as persistent and more marked vagal tone withdrawal occurs, the PKA-dependent Ca2+ cycling mechanisms become further activated by β-AR stimulation, although the increase in cAMP to activate PKA may be effected by different ACs (Ca2+ inhibited) that link to β-ARs via Gs proteins (Supplemental Fig. S5), rather than by ACs (Ca2+ activated) that control the intrinsic AP firing rate (52).
GRANTS
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Supplementary Material
Footnotes
Supplemental data for this article are available online at the American Journal of Physiology Heart and Circulatory Physiology website.
REFERENCES
- 1.Accili EA, Redaelli G, DiFrancesco D. Differential control of the hyperpolarization-activated current (i(f)) by cAMP gating and phosphatase inhibition in rabbit sino-atrial node myocytes. J Physiol 500: 643–651, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beau SL, Hand DE, Schuessler RB, Bromberg BI, Kwon B, Boineau JP, Saffitz JE. Relative densities of muscarinic cholinergic and beta-adrenergic receptors in the canine sinoatrial node and their relation to sites of pacemaker activity. Circ Res 77: 957–963, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Bogdanov KY, Maltsev VA, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD, Lakatta EG. Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res 99: 979–987, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: molecular partners in pacemaker regulation. Circ Res 88: 1254–1258, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Bolter CP, English DJ. The effects of tertiapin-Q on responses of the sinoatrial pacemaker of the guinea-pig heart to vagal nerve stimulation and muscarinic agonists. Exp Physiol 93: 53–63, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Boyett MR, Kodama I, Honjo H, Arai A, Suzuki R, Toyama J. Ionic basis of the chronotropic effect of acetylcholine on the rabbit sinoatrial node. Cardiovasc Res 29: 867–878, 1995. [PubMed] [Google Scholar]
- 7.Boyett MR, Roberts A. The fade of the response to acetylcholine at the rabbit isolated sino-atrial node. J Physiol 393: 171–194, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev 24: 765–781, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Demir SS, Clark JW, Giles WR. Parasympathetic modulation of sinoatrial node pacemaker activity in rabbit heart: a unifying model. Am J Physiol Heart Circ Physiol 276: H2221–H2244, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Dessauer CW, Posner BA, Gilman AG. Visualizing signal transduction: receptors, G-proteins, and adenylate cyclases. Clin Sci (Lond) 91: 527–537, 1996. [DOI] [PubMed] [Google Scholar]
- 11.DiFrancesco D, Ducouret P, Robinson R. Differential effects of ACh on cardiac pacemaker cells. Trends Neurosci 15: 249–250, 1992. [DOI] [PubMed] [Google Scholar]
- 12.DiFrancesco D, Ducouret P, Robinson RB. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science 243: 669–671, 1989. [DOI] [PubMed] [Google Scholar]
- 13.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 351: 145–147, 1991. [DOI] [PubMed] [Google Scholar]
- 14.DiFrancesco D, Tromba C. Muscarinic control of the hyperpolarization-activated current (if) in rabbit sino-atrial node myocytes. J Physiol 405: 493–510, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrzynski H, Marples DD, Musa H, Yamanushi TT, Henderson Z, Takagishi Y, Honjo H, Kodama I, Boyett MR. Distribution of the muscarinic K+ channel proteins Kir3.1 and Kir34 in the ventricle, atrium, and sinoatrial node of heart. J Histochem Cytochem 49: 1221–1234, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Fedorov VV, Hucker WJ, Dobrzynski H, Rosenshtraukh LV, Efimov IR. Postganglionic nerve stimulation induces temporal inhibition of excitability in rabbit sinoatrial node. Am J Physiol Heart Circ Physiol 291: H612–H623, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Han X, Kobzik L, Severson D, Shimoni Y. Characteristics of nitric oxide-mediated cholinergic modulation of calcium current in rabbit sino-atrial node. J Physiol 509: 741–754, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han X, Shimoni Y, Giles WR. A cellular mechanism for nitric oxide-mediated cholinergic control of mammalian heart rate. J Gen Physiol 106: 45–65, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanf R, Li Y, Szabo G, Fischmeister R. Agonist-independent effects of muscarinic antagonists on Ca2+ and K+ currents in frog and rat cardiac cells. J Physiol 461: 743–765, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartzell HC, Fischmeister R. Effect of forskolin and acetylcholine on calcium current in single isolated cardiac myocytes. Mol Pharmacol 32: 639–645, 1987. [PubMed] [Google Scholar]
- 21.Honjo H, Inada S, Lancaster MK, Yamamoto M, Niwa R, Jones SA, Shibata N, Mitsui K, Horiuchi T, Kamiya K, Kodama I, Boyett MR. Sarcoplasmic reticulum Ca2+ release is not a dominating factor in sinoatrial node pacemaker activity. Circ Res 92: e41–e44, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Jurevicius J, Fischmeister R. Acetylcholine inhibits Ca2+ current by acting exclusively at a site proximal to adenylyl cyclase in frog cardiac myocytes. J Physiol 491: 669–675, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamura H, Yokoyama M, Akita H, Matsushita K, Kurachi Y, Yamada M. Tertiapin potently and selectively blocks muscarinic K(+) channels in rabbit cardiac myocytes. J Pharmacol Exp Ther 293: 196–205, 2000. [PubMed] [Google Scholar]
- 24.Kuschel M, Zhou YY, Spurgeon HA, Bartel S, Karczewski P, Zhang SJ, Krause EG, Lakatta EG, Xiao RP. beta2-Adrenergic cAMP signaling is uncoupled from phosphorylation of cytoplasmic proteins in canine heart. Circulation 99: 2458–2465, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Lakatta EG. Beyond Bowditch: the convergence of cardiac chronotropy and inotropy. Cell Calcium 35: 629–642, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Lakatta EG, Difrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol 47: 157–170, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakatta EG, Maltsev VA, Bogdanov KY, Stern MD, Vinogradova TM. Cyclic variation of intracellular calcium: a critical factor for cardiac pacemaker cell dominance. Circ Res 92: e45–e50, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Leitch SP, Sears CE, Brown HF, Paterson DJ. Effects of high potassium and the bradycardic agents ZD7288 and cesium on heart rate of rabbits and guinea pigs. J Cardiovasc Pharmacol 25: 300–306, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res 77: 274–284, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol 296: H594–H615, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maltsev VA, Vinogradova TM, Bogdanov KY, Lakatta EG, Stern MD. Diastolic calcium release controls the beating rate of rabbit sinoatrial node cells: numerical modeling of the coupling process. Biophys J 86: 2596–2605, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharm Sci 100: 338–369, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current. J Physiol 582: 1195–1203, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mery PF, Abi-Gerges N, Vandecasteele G, Jurevicius J, Eschenhagen T, Fischmeister R. Muscarinic regulation of the L-type calcium current in isolated cardiac myocytes. Life Sci 60: 1113–1120, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Okumura S, Kawabe J, Yatani A, Takagi G, Lee MC, Hong C, Liu J, Takagi I, Sadoshima J, Vatner DE, Vatner SF, Ishikawa Y. Type 5 adenylyl cyclase disruption alters not only sympathetic but also parasympathetic and calcium-mediated cardiac regulation. Circ Res 93: 364–371, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Opthof T. Embryological development of pacemaker hierarchy and membrane currents related to the function of the adult sinus node: implications for autonomic modulation of biopacemakers. Med Biol Eng Comput 45: 119–132, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Opthof T, de Jonge B, Jongsma HJ, Bouman LN. Functional morphology of the mammalian sinoatrial node. Eur Heart J 8: 1249–1259, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Petit-Jacques J, Bescond J, Bois P, Lenfant J. Particular sensitivity of the mammalian heart sinus node cells. News Physiol Sci 9: 77–79, 1994. [Google Scholar]
- 39.Petit-Jacques J, Bois P, Bescond J, Lenfant J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflügers Arch 423: 21–27, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Roberts LA, Slocum GR, Riley DA. Morphological study of the innervation pattern of the rabbit sinoatrial node. Am J Anat 185: 74–88, 1989. [DOI] [PubMed] [Google Scholar]
- 41.Sandirasegarane L, Diamond J. Pertussis toxin-sensitive G protein but not NO/cGMP pathway mediates the negative inotropic effect of carbachol in adult rat cardiomyocytes. Pharmacology 70: 46–56, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Scheer A, Cotecchia S. Constitutively active G protein-coupled receptors: potential mechanisms of receptor activation. J Recept Signal Transduct Res 17: 57–73, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Shibata N, Inada S, Mitsui K, Honjo H, Yamamoto M, Niwa R, Boyett MR, Kodama I. Pacemaker shift in the rabbit sinoatrial node in response to vagal nerve stimulation. Exp Physiol 86: 177–184, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Song LS, Sham JS, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking 'Ca2+ spikes' in rat cardiac myocytes. J Physiol 512: 677–691, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinogradova TM, Bogdanov KY, Lakatta EG. beta-Adrenergic stimulation modulates ryanodine receptor Ca(2+) release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res 90: 73–79, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Vinogradova TM, Fedorov VV, Yuzyuk TN, Zaitsev AV, Rosenshtraukh LV. Local cholinergic suppression of pacemaker activity in the rabbit sinoatrial node. J Cardiovasc Pharmacol 32: 413–424, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, Yang D, Ruknudin AM, Spurgeon H, Lakatta EG. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res 102: 761–769, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res 94: 802–809, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Xiao RP, Zhang SJ, Chakir K, Avdonin P, Zhu W, Bond RA, Balke CW, Lakatta EG, Cheng H. Enhanced G(i) signaling selectively negates beta2-adrenergic receptor (AR)—but not beta1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation 108: 1633–1639, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Yamada M. The role of muscarinic K(+) channels in the negative chronotropic effect of a muscarinic agonist. J Pharmacol Exp Ther 300: 681–687, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Younes A, Lyashkov A, Graham D, Sheydina A, Volkova M, Mitsak M, Vinogradova T, Lukyanenko Y, Li Y, Ruknudin A, Boheler K, van Eyk J, Lakatta EG. Ca2+-stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem 283: 14461–14469, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Holden AV, Noble D, Boyett MR. Analysis of the chronotropic effect of acetylcholine on sinoatrial node cells. J Cardiovasc Electrophysiol 13: 465–474, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.