Abstract
If normal, eupneic breathing fails, gasping is recruited. Serotonin was proposed as essential for gasping, based on findings using an in vitro mouse preparation. This preparation generates rhythmic activities of the hypoglossal nerve that are considered to be akin to both eupnea and gasping. In previous studies, gasping of in situ rat and mouse preparations continued unabated following blockers of receptors for serotonin. However, hypoglossal activity was not recorded in the mouse, and we hypothesized that its discharge during gasping might be dependent on serotonin. In the in situ mouse preparation, hypoglossal discharge had varying and inconsistent patterns during eupnea, discharging concomitant with the phrenic burst, at varying intervals between phrenic bursts, or was silent in some respiratory cycles. In eupnea, phrenic discharge was incrementing, whereas hypoglossal discharge was decrementing in 15 of 20 preparations. During ischemia-induced gasping, peak phrenic height was reached at 205 ± 17 ms, compared with 282 ± 27.9 ms after the start of the eupneic burst (P < 0.002). In contrast, rates of rise of hypoglossal discharge in gasping (peak at 233 ± 25 ms) and eupnea (peak at 199 ± 19.2 ms) were the same. The uncoupling of hypoglossal from phrenic discharge in eupnea was exacerbated by methysergide, an antagonist of serotonin receptors. These findings demonstrate that hypoglossal discharge alone cannot distinguish eupnea from gasping nor, in eupnea, can hypoglossal activity be used to differentiate neural inspiration from expiration. These findings have significant negative implications for conclusions drawn from the in vitro medullary slice of mouse.
Keywords: in vitro
eupnea and gasping are two patterns of automatic ventilatory activity. Eupnea is normal breathing. If eupnea fails, as in severe hypoxia or ischemia, gasping is recruited and can serve as a powerful mechanism of autoresuscitation to restart eupnea (19, 41, 43).
In studies using an in vitro slice of the medulla of mouse, gasping was reported to be dependent on endogenous serotonin (5-HT) to be generated (31, 53). This claim had potentially important implications in that an abnormality in the brain stem serotonergic system has been reported in victims of the sudden infant death syndrome (23). Sudden infant death syndrome as a failure of gasping has long been proposed (13, 15).
We could not confirm a link between endogenous 5-HT and gasping in two studies using an in situ preparation of the rat (46, 51) and in another study using an in situ preparation of the mouse (48). Both of these preparations have an intact pontomedullary brain stem. In all studies, gasping continued unabated following administration of blockers of multiple groups of receptors for 5-HT. In addition, the study with mice included a homozygous PET-1 strain in which neurons producing 5-HT are reduced by 80–90% compared with those without this genetic defect (48).
To explain this marked difference between results obtained in vitro and in situ, we proposed that the type and/or quantity of neurotransmitters would be greatly reduced in the medullary slice compared with the entire pontomedullary brain stem (46, 48, 51). Thus, in vitro, any remaining neurotransmitters, such as 5-HT, would assume a disproportionate importance. Another difference between studies in vitro and in situ concerns the age of the preparations, with neonates being used in the former and juvenile or adults in the latter. While maturational changes do occur in the brain stem respiratory control system, still eupnea and gasping are clearly distinguishable patterns from the day of birth in rats (55). Age-dependent changes in eupnea and gasping in mice have not been analyzed in detail (but see Ref. 4). A final difference between the in vitro and in situ preparations is that respiratory rhythms of the in vitro preparations are defined solely by the discharge of the hypoglossal nerve and/or of massed neuronal activities from the ventrolateral medulla (17, 29–32, 53, 56). Based on the rate of rise of these integrated discharges, respiratory rhythms have been characterized as “eupnea,” “gasping,” or “sighs” (17). While gasping persisted in both cranial and phrenic nerves following a blockade of 5-HT receptors in the rat in situ preparation, only phrenic discharge was recorded in the mouse in situ preparation. Thus we could not exclude the possibility that 5-HT might be critical for generation of gasps in the hypoglossal nerve of the mouse, as reported for in vitro studies. As few characterizations and comparisons of activities of cranial and phrenic nerves during various patterns of automatic ventilation have been performed in the mouse (4, 22, 25), the present study was undertaken. In this context, we have studied respiratory-related activity of the vagus nerve, in addition to that of the hypoglossal, to define whether any differences between hypoglossal and phrenic discharges were unique to the hypoglossal system or were general for activities of cranial vs. spinal nerves.
METHODS
Experimental Preparations and Procedures
All procedures used in these studies have been approved by the Institutional Animal Care and Use Committee of Dartmouth College and Dartmouth Medical School. The Animal Resource Center at Dartmouth Medical School is an American Association for Accreditation of Laboratory Animal Care approved facility.
PET-1 wild-type mice were used to allow comparison with our earlier study. Mice were studied 3–4 wk after birth. The preparation was as described in that previous study (48). Under deep anesthesia with enfluorane, mice were bisected caudal to the diaphragm and immersed in ice-cold mock cerebrospinal fluid. They were immediately decerebrated at a precollicular level, and the phrenic and hypoglossal or vagus nerves were sectioned. The left ventricle was cannulated, and the preparation perfused. The constituents of the perfusate were as described previously (48). The temperature of the perfusate was 31°C at the ventricle, and it was equilibrated with a gas mixture of 95% O2-5% CO2. Gallamine triethiodide was added to the perfusate to block neuromuscular transmission.
Activities of the various nerves were recorded with bipolar suction or hook electrodes, amplified, and filtered (0.6–6.0 kHz). Recordings were obtained continuously after rhythmic activities began. In some studies, methysergide was added to the perfusate to block multiple receptors for 5-HT. Methysergide is a mixed antagonist of 5-HT 1, 2, 4, 5, 6, and 7 receptors, as well as a weak agonist of 5-HT1 receptors (46, 48, 51). At variable periods after the commencement of recordings, but a minimum of 10 min after methysergide, perfusion was terminated for 40 s to produce ischemia and alter the pattern of ventilatory activity to gasping.
Variable of Neural Activities
Eupnea and gasping were distinguished, as in previous studies, from the rate of rise of integrated phrenic activity (11, 41–43, 49, 51). During both eupnea and gasping, integrated phrenic activity was analyzed as to the duration of the burst (neural inspiratory time), period between bursts (expiratory time), and peak height. Integrated hypoglossal and vagal discharges typically had a burst that approximated that of neural inspiration; the duration of this burst was defined, as were the peak heights during this phase. To compare the rates of rise of inspiratory activity for activities of the various nerves, we defined the time after onset for each burst to reach a peak integrated height.
For recordings in eupnea, activities during 20 respiratory cycles were analyzed. These cycles were taken a minimum of 20 min after the rhythmic discharges commenced in the preparation. In preparations that received methysergide, a second group of 20 cycles was analyzed, commencing 10 min after the administration of the drug. Data for gasping were taken from a single trial with ischemia. Hence, in preparations that received methysergide, ischemia was only induced a minimum of 10 min after the drug had been administered. In addition to those same variables, noted above for eupnea, in gasping, we defined the time from the termination of perfusion, considered as the onset of ischemia, to the first gasp, and the time after the recommencement of perfusion to the first rhythmic burst of activity of each nerve. Finally, as an index of the variability of phrenic and hypoglossal discharge during the respiratory cycle, we computed the coefficient of variance. This computation was performed for the duration of phrenic burst and its rate of rise during approximately 20 respiratory cycles of eupnea and a minimum of four cycles of gasping. This coefficient of variance, which is the standard deviation of the measurement divided by the mean, was compared with the comparable coefficient of variance computed for hypoglossal discharge during the same respiratory cycles. The significance of the difference between the phrenic and hypoglossal values was assessed by a paired t-test.
Statistical Evaluations of Data
Comparisons were made by paired or unpaired t-tests. Probabilities less than 0.05 were considered as significant.
RESULTS
Comparison of Phrenic and Hypoglossal Activities in Eupnea and Gasping
Eupnea.
Activities of both nerves were recorded during eupnea in 20 preparations (Fig. 1). As described previously, integrated phrenic discharge had an incrementing rise to reach a peak height after most of the phrenic burst was finished (64.7 ± 2.1% of inspiratory time, equivalent to 282 ± 27.9 ms after the start of the burst). Phrenic discharge was compartmentalized to periodic bursts that defined neural inspiration.
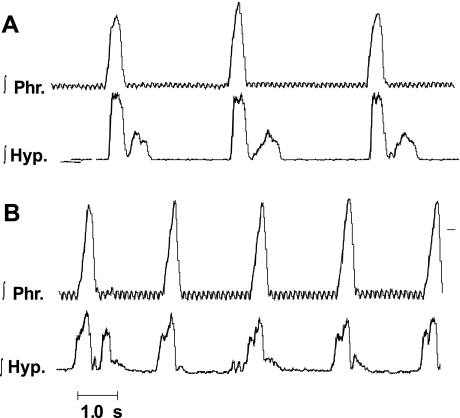
Fig. 1.
Patterns of discharge of the phrenic nerve and hypoglossal nerve during eupnea (E). A and B are from two different preparations and shown integrated activities of the nerves (∫Phr, phrenic; ∫Hyp, hypoglossal). Note the variability of hypoglossal discharge between preparations and between respiratory cycles in a single preparation. Small, high-frequency oscillations, which are visible on ∫Phr activity, represent the electrocardiogram.
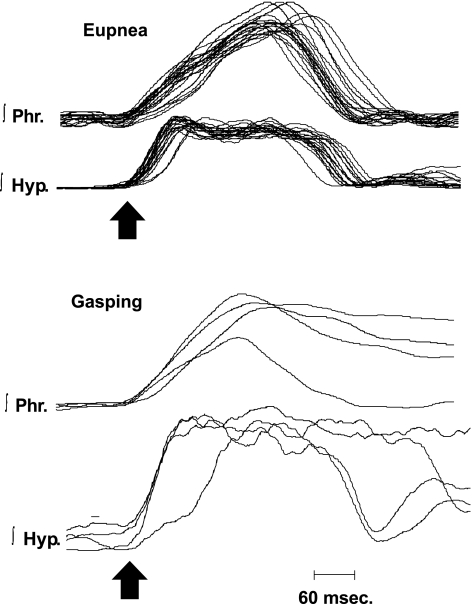
Hypoglossal discharge differed between preparations and was variable even within the same preparation (Fig. 1). In all 20 preparations, the hypoglossal nerve had a burst of activity that overlapped with that of the phrenic discharge in neural inspiration. These bursts of activity started at the same time (Fig. 1A) or before (Fig. 1B) the onset of the phrenic burst. In all preparations, the hypoglossal nerve also had bursts during neural expiration. These bursts could occur in early neural expiration (Fig. 1, A and B) or late neural expiration (Fig. 1B, second cycle), or large bursts could occur occasionally during neural expiration (Figs. 2 and 3). As is evident from Figs. 1–3, the presence and magnitude of expiratory bursts were unpredictable.
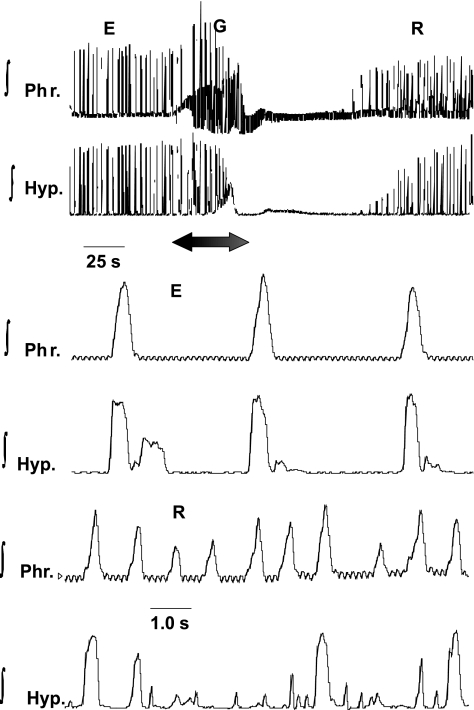
Fig. 2.
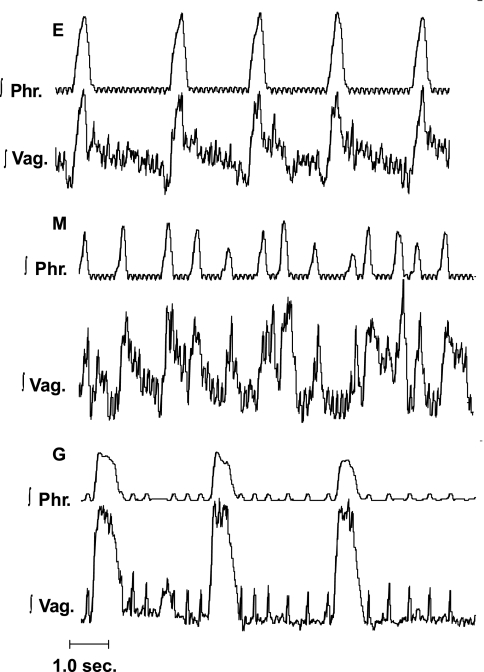
Alterations in ∫Phr and ∫Hyp nerves in E, ischemic-induced gasping (G), and recovery (R). Recordings in E are shown in top tracings and, on expanded time scale, on bottom tracings. Note greater rate of rise of ∫Hyp than ∫Phr discharge in E. During period designated by arrow, perfusion was terminated, and G was induced (see Fig. 3 for expanded recordings of E and G). Upon the recommencement of perfusion, a sustained apneic period intervened before neural activities recommenced (R). Note on the expanded tracings during this early R phase that the frequency of phrenic bursts had increased, and hypoglossal discharge was of low amplitude in some cycles, missing in others, and, finally, occurred in neural expiration. Small, high-frequency oscillations, which are visible on ∫Phr activity, represent the electrocardiogram.
Fig. 3.
Activities of the phrenic and hypoglossal nerves during E and G in a single preparation. G was produced in ischemia. Note switch in pattern of ∫Phr discharge from incrementing in E to decrementing in G. ∫Hyp discharge was decrementing during both E and G. Preparation is a different mouse from that in Fig. 2.
For cycles in which hypoglossal discharge was present during neural inspiration, the duration of its burst (357 ± 31.3 ms) was not significantly different from that of the phrenic burst (388 ± 26.0 ms). However, the rate of rise of hypoglossal discharge was significantly greater than that of the phrenic. For all 20 preparations, integrated hypoglossal discharge reached a peak level of 199 ± 19.2 ms after the start of its burst, whereas the equivalent time for phrenic discharge was 282 ± 27.9 ms (P < 0.01 compared with hypoglossal discharge). Of the 20 preparations, in only 5 was the rate of rise of hypoglossal less than phrenic (e.g., Fig. 1B). For these five preparations, peak hypoglossal discharge was attained in 297 ± 29 ms and peak phrenic in 219 ± 23 ms.
The greater variability of hypoglossal compared with phrenic activity was confirmed by a greater coefficient of variance for the duration of the hypoglossal burst (0.18) compared with that of the phrenic (0.14; P < 0.01). Also significantly different was the coefficient of variance for the rate of rise of activity (hypoglossal = 0.38, phrenic = 0.20; P < 0.0001).
Records reported in Fig. 1 were obtained ∼20 min after rhythmic activity commenced in the perfused preparation. The hypoglossal discharge especially could change within a given preparation. The most consistent change was during recovery from ischemia-induced gasping, as shown in Fig. 2, in which hypoglossal discharge became exceedingly variable, with an absence of discharge in some respiratory cycles. Similar changes in hypoglossal discharge were found if activities were recorded for an extended period before exposure to ischemia. Thus, as noted above, in most preparations, hypoglossal discharge had a decrementing discharge when recordings were obtained 20 min after rhythmic discharge of the preparation commenced. Sixteen of twenty preparations were exposed to ischemia soon thereafter. The other four preparations were maintained for an additional 30 min, in hyperoxia. By the end of this additional 30-min period, the respiratory frequency had increased, and hypoglossal discharge had become irregular, with the pattern similar to that of Fig. 2 after recovery from ischemia. Because of this potential for time-dependent changes, control recordings of all preparations were those taken ∼20 min after the start of rhythmic activity. Hence, given this experimental design, no formal study of time-dependent changes in hypoglossal or phrenic discharges was performed.
Gasping.
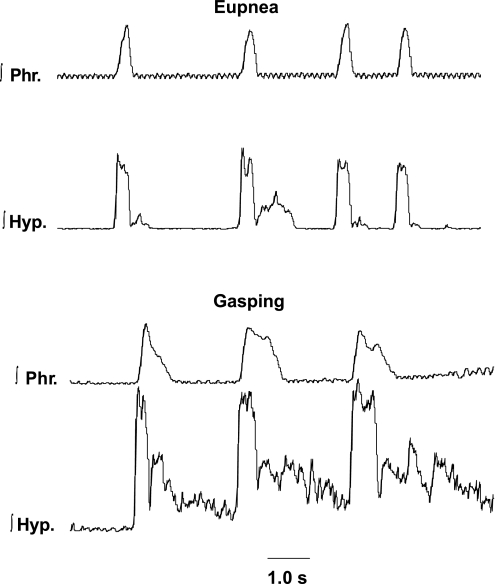
In an identical manner to that described in a previous paper in mice (48), the pattern of phrenic discharge was converted from the incrementing pattern of eupnea to the decrementing pattern of gasping within 13.5 ± 1.85 s. following the onset of ischemia (Figs. 2 and 3). Of the 20 preparations that were studied during eupnea, 5 received methysergide during eupnea, and the phrenic recording was lost in 1 other preparation. Hence, paired recordings in eupnea and gasping, without methysergide, were obtained in 14 preparations.
Regardless of the discharge pattern during eupnea, hypoglossal discharge had a decrementing pattern during neural inspiration of gasping (Fig. 3). If hypoglossal discharge was absent during some cycles of eupnea, it was always recruited in gasping. Although the peak level of this hypoglossal discharge during neural inspiration increased in 11 of 14 preparations (e.g., Fig. 3), this increase was not significant (115 ± 17.0% of value in eupnea, Fig. 4). Levels of activity in neural expiration were variably altered and fell as the duration of ischemia increased. Peak integrated phrenic discharge did increase significantly in gasping (195 ± 36.5% of control, Fig. 4). Durations of the burst of both phrenic and hypoglossal activities significantly increased with the change from eupnea to gasping. In gasping, the duration of hypoglossal discharge (668 ± 24 ms) was significantly longer than that of the phrenic (594 ± 25 ms). The duration of these two discharges had been similar in eupnea (phrenic = 388.5 ± 26 ms, hypoglossal = 357 ± 31.3 ms).
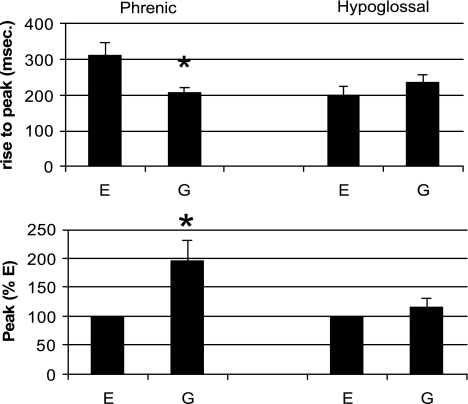
Fig. 4.
Comparison of rates of rise of inspiratory activity and peak of integrated inspiratory activity of phrenic and hypoglossal nerves during E and G. Note that peak height was greater for phrenic nerve in G than E, and time to reach this peak height was less in G. *P < 0.05. For hypoglossal activity, peak values and rates of rise were the same in E and G.
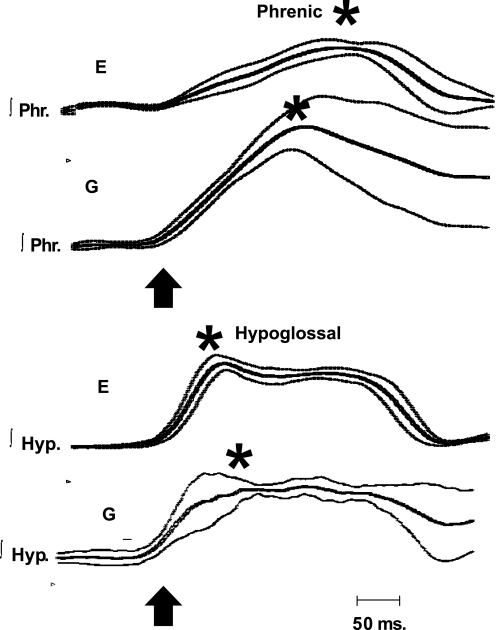
As would be expected from the definition of gasping, per se, the rate of rise of integrated phrenic discharge was significantly greater in gasping than eupnea (Fig. 4). Peak height was attained 308 ± 37 ms after the start of the eupneic burst and 205 ± 17 ms after the start of the gasp (P < 0.002, Fig. 4). In contrast to phrenic discharge, rates of rise of hypoglossal discharge were the same in eupnea (198 ± 25 ms) and gasping (233 ± 25 ms). The similarity of hypoglossal discharge in eupnea and gasping is shown graphically for multiple respiratory cycles in Figs. 5 and 6. Note, in these figures, that the rate of rise of phrenic discharge was significantly faster in gasping than eupnea.
Fig. 5.
Multiple plots of patterns of ∫Phr and ∫Hyp activities in E and G. The start of activities in 20 cycles of E and 4 cycles of G have been aligned (arrows), and the resulting integrated activity shown. Note the switch from incrementing to decrementing activity of phrenic discharge with the change from E to G, and the same decrementing pattern for hypoglossal activity during both E and G.
Fig. 6.
Average tracings of ∫Phr and ∫Hyp activities during E and G. Tracings of Fig. 5 have been averaged, and results are shown by thick line, with standard errors shown by thinner lines. Arrow designates the start of the burst, and asterisk designates peaks. Note that peak of ∫Phr discharge occurred much earlier in the gasp than eupneic inspiration, whereas peak of hypoglossal discharge was slightly later in the gasp than in E.
The greater similarity of hypoglossal and phrenic discharges in gasping than eupnea is further demonstrated by the coefficient of variance. These coefficients of variance were not significantly different for both durations of the burst and rates of rise of activity. Both had differed significantly in eupnea, as noted above.
A final difference between phrenic and hypoglossal discharges was in the recovery of rhythmic bursts from ischemia-induced gasping (see, e.g., Fig. 2). Phrenic bursts returned within 53 ± 8.6 s after the restoration of perfusion. For hypoglossal discharge, the comparable period before activity recommenced was 72± 12 s (P < 0.005).
Influence of Blockade of Receptors for 5-HT by Methysergide Upon Activities of the Phrenic and Hypoglossal Nerves in Eupnea and Gasping
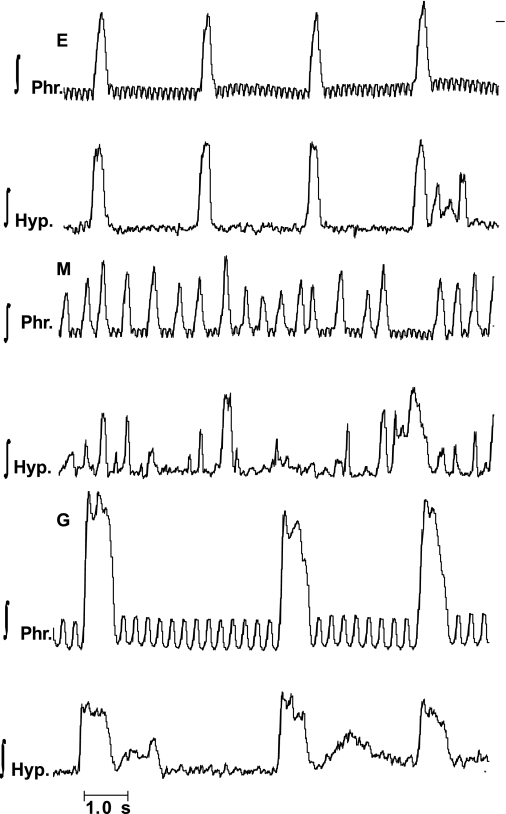
Methysergide, at a concentration of 3.0 μM, was administered to five mice. This concentration of methysergide was chosen based on results of our previous studies (46, 51) in which this concentration produced consistent changes in eupneic ventilatory activity. Indeed, as reported previously (46, 51), the frequency of the phrenic burst increased greatly, and the peak height fell following methysergide. Strikingly, in each preparation, hypoglossal discharge became completely uncoupled from that of the phrenic, with numerous cycles having no phasic hypoglossal discharge, and others in which hypoglossal bursts were recorded solely during neural expiration (Fig. 7). Thus the dissociation of hypoglossal and phrenic discharges, which was evident in control preparations, became profound following administrations of methysergide.
Fig. 7.
Influence of blockade of receptors for serotonin with methysergide (M) upon activities of the phrenic nerve (∫Phr) and hypoglossal nerve (∫Hyp) in E and G in the mouse. Note that in E, hypoglossal discharge was linked to the phrenic burst, but periodical bursts of hypoglossal activity were recorded in neural expiration. Following administration of 3.0 μM of M, phrenic and hypoglossal discharges were largely uncoupled. In G, phrenic and hypoglossal discharges were linked, although burst of hypoglossal discharge in neural expiration was seen. Small, high-frequency oscillations, which are visible on ∫Phr activity, represent the electrocardiogram.
Upon exposure to ischemia, gasping activities commenced at the same time for activities of both nerves. The first gasp occurred at 13.5 ± 0.67 s after the onset of ischemia. As is evident from Fig. 7, the rate of rise of inspiratory activity was much faster for phrenic discharge in gasping, after methysergide, than eupnea, before the drug was given (P < 0.001). Peak integrated phrenic discharge was reached in 326 ± 106 ms in eupnea and 228 ± 54 ms in gasping. Rates of rise of hypoglossal discharge in eupnea and gasping were very similar, with peak discharge being reached 237 ± 23 ms after the start of the eupneic burst and 246 ± 28 ms after the start of the gasp.
A final difference between activities of the phrenic and hypoglossal nerves was in the recommencement of phasic activity after the restoration of perfusion. In four of five preparations, periodic phrenic discharge began 10–47 s before that of the hypoglossal. For the final mouse, phasic phrenic activity began at 16 s; phasic hypoglossal discharge never returned. The delay in the restoration of eupneic hypoglossal activity compared with phrenic activity was similar to the recovery of eupnea without methysergide (see previous section).
Comparison of Phrenic and Vagal Activities in Eupnea and Gasping: Influence of Methysergide
Activities of the phrenic and vagus nerve were recorded in six preparations. In three of these, methysergide (3.0 μM) was added to the perfusate after recordings in eupnea. Gasping was induced in ischemia in all six preparations, three of which had received methysergide.
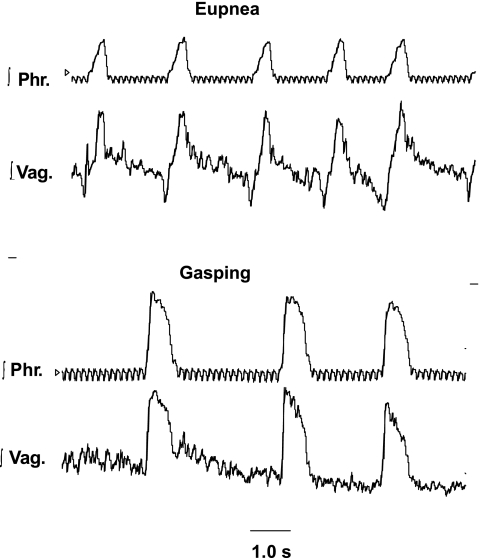
During eupnea, the vagus nerve had discharges during both neural inspiration and expiration (Figs. 8 and 9). For all six preparations, the durations of the phrenic (405 ± 19 ms) and vagal discharges (462 ± 21 ms) were not significantly different, nor was the time to reach peak integrated height after the onset of the burst different (phrenic = 274 ± 19 ms; vagus = 327 ± 21 ms).
Fig. 8.
Integrated activities of the phrenic and vagus nerves in E and G in the mouse. Top: note that integrated vagal discharge (∫Vag) had a phasic burst during the period of phrenic discharge (∫Phr) and also activity during neural expiration. Bottom: in G, both nerves had decrementing discharges, and expiratory vagal activity declined with continuing ischemia. Small, high-frequency oscillations, which are visible on ∫Phr activity represent the electrocardiogram.
Fig. 9.
Activities of the phrenic and vagus nerves in the mouse during E, after administration of M, and in G. Note that, following administration of 3.0 μM of M, the frequency of neural bursts increased, but ∫Vag remained linked to the ∫Phr. Small, high-frequency oscillations on records of ∫Phr activity and higher amplitude spies on records of vagal activity both represent electrocardiograms.
Following administration of methysergide, the frequency of phrenic and vagal bursts increased greatly. However, vagal discharge continued to be linked to that of the phrenic with a burst during each neural inspiration; some bursts in neural expiration were also observed (Fig. 9).
On exposure to ischemia, gasping activities were induced in each preparation (Figs. 8 and 9). The delay before the first gasp varied from 12 to 22 s in preparations that received no methysergide, and 13 to 23 s in those that had received the drug. Gasping in both groups of preparations was similar (Figs. 8 and 9). The rate of rise of phrenic activity increased greatly, with peak activity being reached after 67 ± 0.06% of the duration of the burst in eupnea and 38 ± 0.02% of the gasp (P < 0.001). This corresponded to a mean time to reach a peak integrated level of 274 ± 19 ms in eupnea and 232 ± 31 ms in gasping. The time to reach peak vagal activity was less in gasping than eupnea in five of six preparations (mean for all preparations, eupnea = 327 ± 21 ms, gasping = 266 ± 30 ms). Following the recommencement of perfusion, phrenic and vagal discharges appeared in the same respiratory cycle in five of six preparations; in the other, vagal discharge appeared earlier. Mean times for recovery of rhythmic activity were 57.8 ± 21 s for phrenic and 54.5 ± 22 s for vagal discharge.
DISCUSSION
Since first characterized by Lumsden in 1923, the primary distinction between eupnea and gasping has been a more “sudden beginning” of inspiration during gasping than eupnea (18, 19). This more sudden beginning of inspiration has been documented in multiple species, including mice, as shown herein, by a significant increase in the rate of rise of phrenic discharge. Stated differently, phrenic discharge, which has an incrementing pattern in most respiratory cycles of eupnea, changes to a decrementing pattern in gasping (41–43).
Hypoglossal Discharge During Neural Inspiration is Indistinguishable Between Eupnea and Gasping in the Mouse
In the in situ preparation of the rat during eupnea, the relationship between rates of rise of hypoglossal and phrenic discharges is variable. In some preparations, activities of both nerves are incrementing, whereas, in others, hypoglossal discharge is decrementing (16, 24, 51). In the in situ preparation of the mouse, the greater rate of rise of integrated hypoglossal, compared with phrenic activity, is extreme. This extremely rapid rate of rise of hypoglossal discharge in eupnea is such that this rate of rise does not increase further in gasping and, indeed, is greater in eupnea than gasping in most preparations. Hence, based on hypoglossal discharge alone, the primary factor that distinguishes eupnea from gasping, namely, the rate of rise of inspiratory activity, is indistinguishable.
In addition to hypoglossal discharge being the same in eupnea and gasping, the variable characteristics of hypoglossal discharge during eupnea would prevent designation of the type or phase of the respiratory rhythm. As opposed to phrenic discharge, discharge of the hypoglossal nerve occurs during neural inspiration, and, also, in an unpredictable fashion, bursts are recorded during neural expiration. Some of these bursts during neural expiration are incrementing, whereas, as noted above, many during neural inspiration are decrementing. Thus, from examining hypoglossal discharge alone during eupnea, it might be concluded that eupnea (incrementing discharge) and gasping (decrementing discharge) are occurring together. Of course, from hypoglossal discharge alone, the erroneous conclusion would be drawn that neural inspiration corresponds to periods of hypoglossal discharge and neural expiration to the absence of this discharge, when, in fact, hypoglossal discharges during neural expiration are frequent. At the other extreme, since hypoglossal discharge can be totally absent during some respiratory cycles in eupnea, no accurate calculation of respiratory frequency is possible from evaluation of this neural discharge alone (see also Ref. 40).
In the context of the shape of the hypoglossal discharge, the decrementing discharge, seen in most preparations, could become incrementing if the preparation were maintained for relatively longer periods and/or during recovery following ischemic-induced gasping. However, this switch to an incrementing pattern was accompanied by a complete absence of hypoglossal discharge during some respiratory cycles and bursts of discharge in a seemingly random fashion during neural expiration. We have no indication as to factors responsible for the switch from incrementing to decrementing discharge of the hypoglossal nerve. However, regardless of the pattern, hypoglossal discharge was poorly correlated with the neu ral inspiration or expiration of eupnea, as defined by the incrementing phrenic discharge or respiratory pattern, be it eupnea or gasping, again, as defined by incrementing or decrementing integrated phrenic activity.
The lack of correspondence between hypoglossal and phrenic discharges of the in situ preparation is not a generalized phenomenon for respiratory-modulated activities of all cranial nerves. As opposed to hypoglossal discharge, respiratory-modulated activity of the vagus nerve remained tightly coupled to phrenic discharge during eupnea, although vagal discharge during early neural expiration was variable.
In summary, results of the present study demonstrate that, in the in situ preparation of the mouse, hypoglossal discharge alone cannot be used either to distinguish eupnea from gasping or to define the phases of the respiratory cycle. These results raise significant doubts as to the accuracy of the model of the thick medullary slice of the mouse for which it is claimed that eupnea, gasping, and sighs can be characterized, based on recordings of hypoglossal discharge alone and/or massed neuronal activities from the ventrolateral medulla (17).
Neuroanatomical and Pharmacological Basis for Lack of Correspondence Between Respiratory-Modulated Activities of Hypoglossal and Phrenic Nerves and Responses to Drugs
An extensive number of studies have demonstrated differences between respiratory-modulated activities of the hypoglossal nerve compared with the bulbospinal-phrenic system. These differences include premotor innervation and responses to pharmacological agents (e.g., Refs. 5–8, 14, 27, 28, 34, 37, 39). Concerning the former, the vast majority of pontile and medullary neurons that project upon hypoglossal motoneurons differ from those that project upon the bulbospinal-phrenic system. In addition to respiration, the hypoglossal system is involved in other rhythmic activities, such as mastication and deglutition (34, 36). Given these differences in premotor innervation and in multiple rhythmic physiological functions, it is not surprising that rhythmic hypoglossal discharge can become uncoupled from that of the phrenic nerve in a number of rat preparations (16, 47). Moreover, even the respiratory-related component of the hypoglossal discharge can be altered, independent of changes in phrenic activity. A most striking example of this independence is a separation of the preinspiratory and inspiratory components of hypoglossal discharge, which appear as a single continuous burst in eupnea, into two completely separate bursts (47).
In the in situ mouse preparation, different premotor innervations without doubt contributed to the total uncoupling of hypoglossal and phrenic discharges following a blockade of multiple types of receptors for 5-HT with methysergide. The 5-HT system is widespread in the brain stem, and influences on activities of premotor and both cranial and spinal motoneurons are well described (9, 20, 23, 27, 39, 48). Receptors for 5-HT are differentially distributed on hypoglossal compared with phrenic motoneurons. These differential alterations of the hypoglossal and phrenic discharges following administrations of methysergide are in keeping with previous data that document a different response of these systems to a range of sedatives and anesthetics (5, 6, 14, 51).
Many of the differences between the hypoglossal and bulbospinal-phrenic systems, considered above, could also be applied to comparisons with the vagal motor system. As the hypoglossal system, both inspiratory and expiratory vagal discharges are more sensitive to alterations by multiple drugs than the phrenic system (5, 6, 9, 20). Thus the tighter coupling of vagal discharges to phrenic activity than those of the hypoglossal nerve to phrenic activity is without a firm explanation. However, vagal motoneurons and premotor neurons are in close anatomical and functional proximity to bulbospinal respiratory neurons in the ventral medullary respiratory nucleus (1, 21, 43). This is in contrast to premotor hypoglossal motoneurons, which are, in general, far removed from the bulbospinal system (28, 52). Moreover, while the vagal motor system, as the hypoglossal system, is involved in other rhythmic behaviors, such as cough and swallow, the same respiratory-related premotor vagal neurons may be used to generate these behaviors (see discussions in Refs. 1, 36).
Comparison of Findings From In Vitro Slice Preparation and In Situ Preparation of Mouse
In 2000, a preparation of a thick medullary slice of the neonatal mouse was introduced with the claim that this preparation could generate respiratory patterns akin to eupnea, gasping, and sighing in vivo (17). These patterns were judged based on activities recorded from the hypoglossal nerve and/or from multiple neurons of the ventrolateral medulla. The “eupneic” pattern was considered as augmenting or bell shaped, whereas the rate of rise of activity was significantly increased in “gasping,” and the pattern became decrementing. The sigh was a mixed pattern, which started as “eupnea,” and then transformed to a “gasp.” Parenthetically, the designation of these different patterns from the in vitro slice of mouse appeared in conflict with results from other in vitro slice and en bloc preparations, in which only a single, decrementing pattern was recorded. Despite the decrementing pattern, this rhythm was also considered to be akin to eupnea (2, 10, 33, 42).
Until recently, few recordings of hypoglossal discharge have been made in the mouse, except in vitro. Hence, the designation of these various patterns recorded in vitro was based on patterns of integrated phrenic activity that were obtained in other species in vivo and in situ. Implicit for acceptance that these in vitro rhythms were accurate models for in vivo rhythms would be very similar discharges of the phrenic and hypoglossal nerves and of massed medullary neurons.
Results herein show that hypoglossal and phrenic discharges are not identical in the mouse during eupnea. One study reports that both hypoglossal and phrenic discharges in the anesthetized mouse in vivo are augmenting, but only a single respiratory cycle is shown (22). Hence, the consistency of the incrementing pattern of hypoglossal discharge cannot be judged. Moreover, even in the thick medullary in vitro slice of the neonatal mouse, activities recorded from the hypoglossal nerve and from massed neurons of the ventrolateral medulla may differ, with the former being absent in some cycles in which rhythmic bursts are recorded from the ventrolateral medulla (29, 35). This elimination of hypoglossal discharge may reflect a failure of synaptic transmission due to the presence of a hypoxic/anoxic core in the thick slice. Indeed, this failure becomes more prevalent with additional hypoxia of the slice (see discussion in Ref. 29). After such a failure, the assumption is made that massed neuronal activities represent the overall respiratory rhythm and that these activities are purely inspiratory. Evidence in support of this assumption is lacking as, when hypoglossal activity is present, neurons that fire in the period between hypoglossal bursts are recorded and designated as expiratory (17).
Differences between the in situ mouse and neonatal in vitro mouse could reflect the difference in age of the preparations and/or that most of the brain stem respiratory system is missing in the slice preparation. Stated differently, the bursts of hypoglossal discharge during expiration in the in situ preparation could reflect pontile influences. Yet examinations of hypoglossal discharge in the neonatal mouse in vivo further adds to the probability that the rhythm designated as eupnea of the medullary slice is different from eupnea of preparations having an intact pontomedullary brain stem. Rhythmic hypoglossal activity is recorded in vitro from medullary slices of 0- to 14-day-old neonatal mice (4, 17, 25, 30). Yet no rhythmic hypoglossal activity is recorded during eupnea of most anesthetized in vivo neonatal mice younger than 9 days (4). This absence of rhythmic hypoglossal discharge is not due to technical problems of recording as rhythmic gasping activity can be recruited in the hypoglossal discharge (4). However, since it is well recognized that anesthesia may differentially suppress hypoglossal, compared with phrenic activity (e.g., Ref. 14), it cannot be entirely excluded that the presence of anesthesia is responsible for elimination of the hypoglossal discharge. This explanation would require that this sensitivity to anesthesia-induced depression is only manifested during eupnea and not gasping.
Concerning removal of the pontile portion of the brain stem respiratory system in the medullary slice, this again raises the question as to whether eupnea can be generated by medullary mechanisms alone. From multiple studies over multiple years, it was evident that gasping, and not eupnea, was the one respiratory pattern that could be reproducibly obtained following the removal of pons in vivo (41–43, 54). Yet, in some preparations, gasping was not obtained (54, see Ref. 10 for review). This absence of gasping was proposed by some as evidence of the generation of eupnea by medullary mechanisms alone (10, 33). However, this proposal carries the assumption that all “nongasping” medullary rhythms must be eupnea, even though rhythms recorded following removal of pons were markedly different from those recorded before this pontomedullary transection (see discussion in Ref. 45). Interpretation of these varying results following brain stem transections was significantly clarified by the recent results of Smith et al. (38). These investigators performed brain stem transections in the perfused, in situ preparation. Thus the confounding influence of changes in arterial perfusion of regions of the brain stem that, without doubt, occurred in vivo, was removed. Smith et al. reported that the respiratory pattern following removal of pons was markedly altered with incrementing phrenic discharges changing to “square-wave” patterns. Activity during early expiratory was also eliminated. With a further transection so as to isolate the “pre-Botzinger” complex, which is the region strongly advanced as the “noeud vital” for eupnea, only gasping was recorded.
Gasping would appear to be the one respiratory pattern that is accurately represented in the thick slice of the neonatal mouse. This statement is dependent on designating the decrementing hypoglossal and massed neuronal activities recorded during anoxia as gasping. However, we believe it extremely probable that, due to the anoxic core of the preparation, many of the bursts of activity labeled as eupnea, and having rates of rise very similar to gasps, are, in fact, gasps.
The similarity of “gasping rhythms” in vitro and in situ is documented by the role of pacemaker mechanisms, involving conductance through persistent sodium channels, in generating gasping in both preparations (24, 26, 31, 32, 51). Riluzole, a blocker of persistent sodium channels, eliminates gasping, both in situ and in vitro. The marked difference between the nominally eupneic rhythm in vitro and eupnea in situ or in vivo is documented by the elimination of eupnea, at least in some in vitro preparations, following administrations of riluzole (32). However, riluzole, in concentrations many fold higher than those that eliminate gasping in situ or in vivo, does not eliminate eupnea in either the in situ preparation or in vivo (50). Finally, another group of pacemakers, dependent on calcium conductances, are also hypothesized to play a role in the neurogenesis of eupnea (31, 32, 53). However, these pacemakers have only been identified in the thick slice of mouse medulla, and not in thin in vitro slices or in situ preparations. This pacemaker discharge is blocked by flufanemic acid, and simultaneous administration of flufanemic acid and riluzole eliminates the eupneic rhythm in vitro (32). Again, however, such simultaneous administrations do not eliminate eupnea in situ (44).
The presence of the final rhythm reported in the slice, the sigh, is enigmatic. In vivo, sighs cannot be generated following sectioning of the carotid sinus nerves and vagi (3, 12). Neither afferent nerve is present in the slice. Moreover, while sighs are recorded frequently in the medullary slice, we have never observed such a pattern in even a single respiratory cycle in any in situ mouse or rat preparation. Thus, as for eupnea, the sigh of the in vitro preparation may be unique to that preparation and totally different from sighs recorded in vivo.
In summary, we believe that, of the putative fictive rhythms generated from the thick slice of medulla of the mouse, only gasping is similar to gasping in vivo or in situ. Yet, even with gasping, the marked differences in response of the hypoglossal and phrenic systems to drugs may have led to erroneous conclusions concerning the necessity of some neuromodulators, especially 5-HT, in generating the gasp. Concerning eupnea and sighs, verification of findings in vitro would appear to be impossible in vivo, as the main index of respiratory activity, the discharge of the hypoglossal nerve, is silent during eupnea in vivo in neonatal mice of the same age as those in which a rhythmic activity, designated as eupnea, has been recorded in vitro.
GRANTS
These studies were supported by National Institutes of Health National Heart, Lung, and Blood Institute Grant 26091. Mice were obtained from a colony supported by funds from NIH National Institute of Child Health and Human Development Grant P01-HD36379.
REFERENCES
- 1.Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J Physiol 534: 565–581, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol 59: 583–634, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett D Origin and regulation of spontaneous deep breaths. Respir Physiol 12: 230–238, 1971. [DOI] [PubMed] [Google Scholar]
- 4.Berger AJ, Sebe J. Developmental effects of ketamine on inspiratory hypoglossal nerve activity studied in vivo and in vitro. Respir Physiol Neurobiol 157: 206–214, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bonora M, Shields GI, Knuth SL, Bartlett D, St. John WM. Selective depression by ethanol of upper airway respiratory motor activity in cats. Am Rev Respir Dis 130: 156–161, 1984. [DOI] [PubMed] [Google Scholar]
- 6.Bonora M, St. John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis 131: 41–45, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Brandes IF, Zuperku EJ, Dean C, Hopp FA, Jakovcevic D, Stuth EA. Retrograde labeling reveals extensive distribution of genioglossal motoneurons possessing 5-HT2A receptors throughout the hypoglossal nucleus of adult dogs. Brain Res 1132: 110–119, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue. J Comp Neurol 357: 376–394, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol 418: 323–345, 2000. [PubMed] [Google Scholar]
- 10.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–242, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung ML, Wang W, St. John WM. Medullary loci critical for expression of gasping in adult rats. J Physiol 480: 597–611, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir Physiol 16: 179–196, 1972. [DOI] [PubMed] [Google Scholar]
- 13.Hunt CE The cardiorespiratory control hypothesis for sudden infant death syndrome. Clin Perinatol 19: 757–771, 1992. [PubMed] [Google Scholar]
- 14.Hwang JC, St. John WM, Bartlett D. Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol 55: 785–793, 1983. [DOI] [PubMed] [Google Scholar]
- 15.Leiter JC, Böhm I. Mechanisms of pathogenesis in the sudden infant death syndrome. Respir Physiol Neurobiol 159: 127–138, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Leiter JC, St. John WM. Phrenic, vagal and hypoglossal activities in rat: pre-inspiratory, inspiratory, expiratory components. Respir Physiol Neurobiol 142: 115–125, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, signs and gasps. Nat Neurosci 3: 600–607, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Lumsden T Observations on the respiratory centres in the cat. J Physiol 57: 153–160, 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumsden T Effects of bulbar anaemia on respiratory movements. J Physiol 59: lvii–lx, 1924.
- 20.Morin D Compared effects of serotonin on the inspiratory activity of glossopharyngeal, vagal, hypoglossal and cervical motoneurons in neonatal rat brain stem-spinal cord preparations. Neurosci Lett 160: 61–64, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Nunez-Abades PA, Pasaro R, Bianchi AL. Localization of respiratory bulbospinal and propriobulbar neurons in the region of the nucleus ambiguus of the rat. Brain Res 568: 165–172, 1991. [DOI] [PubMed] [Google Scholar]
- 22.O'Neill MH, Spiegel ET, Chon KH, Solomon IC. Time-frequency representation of inspiratory motor output in anesthetized C57BL/6 mice in vivo. J Neurophysiol 93: 1762–1775, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Paterson DS, Trachtenberg FJ, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kenney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Paton JFR, Abdala APL, Koizumi H, Smith JC, St. John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci 9: 311–316, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Paton JFR, Richter DW. Maturational changes in the respiratory rhythm generator of the mouse. Pflügers Arch 430: 115–124, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Paton JFR, St. John WM. Medullary pacemakers are essential for gasping, but not eupnea in mammals. J Appl Physiol 103: 718–720, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Peever JH, Necakov A, Duffin J. Nucleus raphe obscurus modulates hypoglossal output of neonatal rat in vitro transverse brain stem slices. J Appl Physiol 90: 269–279, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience 110: 711–722, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Peña F, Meza-Andrade R, Páez-Zayas V, González-Marín MC. Gasping generation in developing Swiss-Webster mice in vitro and in vivo. Neurochem Res 33: 1512–1500, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Peña F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43: 105–117, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez JM, Viemari JC. Determinants of inspiratory activity. Respir Physiol Neurobiol 147: 145–157, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Rekling JC, Feldman JL. PreBotzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60: 385–405, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Roda F, Gestreau C, Bianchi AL. Discharge patterns of hypoglossal motoneurons during fictive breathing, couching and swallowing. J Neurophysiol 87: 1703–1711, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Ruangkittisakul A, Schwarzacher SW, Seechia L, Ma Y, Poon NBY, Funk GD, Ballanyi K. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci 28: 2447–2458, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito Y, Ezure K, Tanaka I. Difference between hypoglossal and phrenic activities during lung inflation and swallowing in the rat. J Physiol 544: 183–193, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol 100: 1751–1769, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JC, Abdala APL, Koizumi H, Rybak IA, Paton JFR. Spatial and functional architecture of mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sood S, Liu X, Liu H, Nolan P, Horner RL. 5-HT at hypoglossal motor nucleus and respiratory control of genioglossus muscle in anesthetized rats. Respir Physiol Neurobiol 138: 205–221, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Stettner GM, Zanella S, Huppke P, Gärtner J, Hilaire G, Dutschmann M. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respir Physiol Neurobiol 160: 21–27, 2008. [DOI] [PubMed] [Google Scholar]
- 41.St. John WM Neurogenesis, control, and functional significance of gasping. J Appl Physiol 68: 1305–1315, 1990. [DOI] [PubMed] [Google Scholar]
- 42.St. John WM Medullary regions for neurogenesis of gasping: noeud vital or noeuds vitals? J Appl Physiol 81: 1865–1877, 1996. [DOI] [PubMed] [Google Scholar]
- 43.St. John WM Neurogenesis of patterns of automatic ventilatory activity. Prog Neurobiol 56: 97–117, 1998. [DOI] [PubMed] [Google Scholar]
- 44.St. John WM Rhythmic respiratory activity of in situ rats persists following blockers of both types of in vitro burster activities. Respir Physiol Neurobiol 160: 353–356, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.St. John WM, Leiter JC. High frequency oscillations in phrenic activity during pontile and medullary respiratory rhythms in rats. Exp Physiol 92: 457–466, 2007. [DOI] [PubMed] [Google Scholar]
- 46.St. John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia-induced depression requires activation of receptors for norepinephrine and serotonin. J Appl Physiol 104: 665–673, 2008. [DOI] [PubMed] [Google Scholar]
- 47.St. John WM, Leiter JC, Paton JFR. Uncoupling of rhythmic hypoglossal from phrenic activity. Exp Physiol 89: 727–737, 2004. [DOI] [PubMed] [Google Scholar]
- 48.St. John WM, Li A, Leiter JC. Genesis of gasping is independent of levels of serotonin in the PET-1 knockout mouse. J Appl Physiol (February 12, 2009). doi: 10.1152/japplphysiol.91461.2008. [DOI] [PMC free article] [PubMed]
- 49.St. John WM, Paton JFR. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol 123: 201–213, 2000. [DOI] [PubMed] [Google Scholar]
- 50.St. John WM, Waki H, Dutschmann M, Paton JFR. Maintenance of eupnea of in situ and in vivo rats following riluzole, a blocker of persistent sodium channels. Respir Physiol Neurobiol 155: 97–100, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Toppin VAL, Harris MB, Kober AM, Leiter JC, St. John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol 103: 220–227, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Travers B, Rinaman L. Identification of lingual motor control circuits using two strains of pseudorabies virus. Neuroscience 115: 1139–1151, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci 26: 2623–2634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang SC, Ngai SH, Frumin MJ. Organization of central respiratory mechanisms in the brain stem of the cat: genesis of normal respiratory rhythmicity. Am J Physiol 190: 333–342, 1957. [DOI] [PubMed] [Google Scholar]
- 55.Wang W, Fung ML, Darnall RA, St. John WM. Characterizations and comparisons of eupnoea and gasping in neonatal rats. J Physiol 490: 277–292, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zavala-Tecuapella C, Aguileta MA, Lopez-Guerro JJ, Gonzales-Marin MC, Pena F. Calcium-activated potassium currents differentially modulate respiratory rhythm generation. Eur J Neurosci 27: 2871–2884, 2008. [DOI] [PubMed] [Google Scholar]