Abstract
It is well established that exercise training results in increased muscle oxidative capacity. Less is known about how oxidative capacities in distinct muscles, in the same individual, are affected by different levels of physical activity. We hypothesized that 1) trained individuals would have higher oxidative capacity than untrained individuals in both tibialis anterior (TA) and vastus lateralis (VL) and 2) oxidative capacity would be higher in TA than VL in untrained, but not in trained, individuals. Phosphorus magnetic resonance spectroscopy was used to measure the rate of phosphocreatine recovery (kPCr), which reflects the rate of oxidative phosphorylation, following a maximal voluntary isometric contraction of the TA and VL in healthy untrained (7 women, 7 men, 25.7 ± 3.6 yr; mean ± SD) and trained (5 women, 7 men, 27.5 ± 3.4 yr) adults. Daily physical activity levels were measured using accelerometry. The trained group spent threefold more time (∼90 vs. ∼30 min/day; P < 0.001) in moderate to vigorous physical activity (MVPA). Overall, kPCr was higher in VL than in TA (P = 0.01) and higher in trained than in untrained participants (P < 0.001). The relationship between kPCr and MVPA was more robust in VL (r = 0.64, P = 0.001, n = 25) than in TA (r = 0.38, P = 0.06, n = 25). These results indicate greater oxidative capacity in vivo in trained compared with untrained individuals in two distinct muscles of the lower limb and provide novel evidence of higher oxidative capacity in VL compared with TA in young humans, irrespective of training status. The basis for this difference is not known at this time but likely reflects a difference in usage patterns between the muscles.
Keywords: mitochondrial capacity, magnetic resonance spectroscopy, training status, phosphocreatine recovery, gender
in addition to improved overall oxygen transport capacity, a principle adaptation to high levels of physical activity in skeletal muscle is an enhanced capacity for oxidative phosphorylation, which has positive implications for muscle function and human performance. In vitro studies have shown that physical activity, in the form of endurance training, results in increased mitochondrial content, respiration rates, and oxidative enzyme activities (18, 34). Similarly, in vivo studies have used phosphorus magnetic resonance spectroscopy (31P-MRS) to show higher oxidative capacity in a variety of muscles of trained compared with untrained humans (25, 37, 47) and in response to endurance training of specific muscles (7, 26, 27). It is thus well established that endurance training induces metabolic adaptations that increase muscle oxidative capacity.
Some studies of oxidative enzyme activities suggest that oxidative capacity is similar among different muscle groups within the same subjects (10, 11). For example, in young athletes, oxidative capacities did not differ between gastrocnemius and vastus lateralis (VL) (11). In contrast, other investigators have reported differences in oxidative capacities among different muscles in both untrained (9, 12, 19) and trained young adults (9, 23). Gollnick et al. (9) reported 25% greater succinate dehydrogenase (SDH) activity in VL compared with the deltoid muscle in untrained young adults. Although trained male athletes had greater SDH activity in both of these muscles compared with untrained male subjects, the differences between muscles were more pronounced in the trained athletes (9). Notably, whereas cyclists and runners had greater SDH activity in VL, canoeists and swimmers had greater SDH activity in the deltoid muscle (9). These results suggest a highly localized effect of training on muscle oxidative capacity, as reflected by mitochondrial enzyme activity.
Although training status and use of muscle play pivotal roles in determining muscle oxidative capacity, fiber-type composition may also interact to influence mitochondrial capacity. In untrained (5, 23) and recreationally active young individuals (12), oxidative enzyme activity was greater in type I than in type II fibers. In elite orienteers, Jansson and Kaijser (23) reported reduced SDH activity in type II fibers compared with type I fibers in the VL and deltoid muscle, whereas SDH activities of type I and II fibers were similar in the gastrocnemius. Together, these results indicate that, although type I fibers in an untrained muscle may exhibit a higher capacity for oxidative phosphorylation, type II fibers possess the ability to adapt to high oxidative demands and match the oxidative capacity of type I fibers in response to exercise training.
Although several in vitro studies suggest variations in oxidative capacity across different muscles (9, 12, 19), to our knowledge, no prior studies have examined the effects of training status on in vivo oxidative capacities in multiple human skeletal muscles within the same subjects. We studied two muscles with differing function and fiber-type composition, the tibialis anterior (TA) and VL, in untrained and trained young men and women. In young adults, the TA is composed of ∼68% type I fiber area (24), whereas the VL has ∼40% type I fiber area (43). Although both muscles are used for locomotion, the nature of different locomotor activities (e.g., walking and running) may load these muscles differently in terms of duration, intensity, and mode of contraction (29). Consequently, these muscles may be chronically exposed to different oxidative demands.
We hypothesized that there would be an overall effect of training status such that trained compared with untrained subjects would show greater oxidative capacity in both muscles. We also hypothesized that oxidative capacity would be greater in the TA than in the VL in untrained but not in trained individuals. These results would suggest that, in untrained young adults, muscle oxidative capacity is, to some extent, dictated by the inherent fiber-type composition of the muscle, whereas in trained young adults, the higher metabolic demand experienced by the muscle is met by increased overall oxidative capacity, regardless of fiber-type composition.
METHODS
Subjects.
Twenty-six healthy, nonsmoking adults between the ages of 22 and 32 yr were recruited and divided into two groups, according to their training status. The untrained group (7 women, 7 men) consisted of sedentary individuals with a low level of habitual physical activity (no more than two bouts of 20 min of structured exercise per week). The trained group (5 women, 7 men) consisted of runners who averaged at least 30 miles of running per week. Participants provided written, informed consent in accordance with the procedures approved by the Human Subjects Review Boards at the University of Massachusetts, Amherst, and Yale University School of Medicine and conforming to the standards set by the Declaration of Helsinki.
Habituation session.
To familiarize the participants with the contraction protocols, all volunteers first completed a habituation session in the Muscle Physiology Laboratory at the University of Massachusetts, Amherst. During this session, height and body mass were also measured.
For the dorsiflexor (TA) protocol, subjects were positioned supine with the foot secured to a custom-built foot plate (ankle angle 120° relative to the tibia) interfaced with a strain gauge that allowed for measurement of an isometric contraction of the dorsiflexor muscles. The knee extensor (VL) protocol was performed with the subject in a supine position with the knee at ∼35° of flexion from straight and positioned over a custom-built apparatus with a built-in strain gauge. The foot was fixed with a strap, thus allowing for an isometric contraction of the knee extensor muscles. A minimum of three baseline MVICs (3- to 5-s duration, 2-min recovery between each) were practiced to ensure that subjects could perform these contractions consistently. Participants were verbally encouraged and received visual force feedback to ensure maximal effort during all contractions. At the end of this session, participants were instructed in the use of an accelerometer that was used to quantify habitual physical activity level.
Physical activity.
Participants wore a uniaxial accelerometer (Actigraph, Pensacola, FL) for 7 consecutive days during all waking hours, while maintaining their usual daily physical activities. For accuracy, subjects were instructed always to wear the accelerometer on the right hip and to keep a daily physical activity log detailing information about sleep patterns and description of activities performed during the day. Accelerometer data were sampled continuously and stored in 60-s bins. A custom-written MATLAB routine was used to classify different intensities of physical activity. In detail, time spent in low-intensity physical activity (LPA; 1–1,951 counts/min, ≤3 METS) and in moderate- to vigorous-intensity physical activity (MVPA; >1,952 counts/min, >3 METS) were calculated based on cutpoints developed for Actigraph accelerometers (8, 30). In addition, total physical activity (PAcounts; counts/day), which in part is dependent on intensity of activities, and total minutes of activities (PAmin; min/day), which is independent of intensity, were also calculated. An accelerometer “count” is a unitless measure representing the integration of gravitational accelerations measured during a certain time period, which, in this study, was 60-s bins. The MATLAB program was used to determine whether accelerometer data were included in the analysis based on two objective criteria: 1) the participant had to wear the monitor for at least 10 h on a particular day for that day to be included, and 2) for a participant's activity data to be included in the analysis, a minimum of 4 complete days (3 weekdays and 1 weekend day) were required. The day of muscle metabolic testing was excluded from the physical activity analysis.
Muscle metabolic tests.
At least 48 h after the habituation session, the participants were transported to the Magnetic Resonance Research Center at Yale University, where the muscle metabolic studies were performed in a 4.0-Tesla whole-body, superconducting magnet (Bruker Biospin, Rheinstetten, Germany), with simultaneous measures of intracellular energy metabolites and pH using 31P-MRS. During the 24 h preceding this testing session, subjects were instructed to avoid strenuous physical activity and alcohol, and they were asked to abstain from caffeine consumption for 6 h before testing. Approximately 4 h before testing, each subject consumed a standardized meal comprising 25% of their estimated daily energy expenditure, based on the Harris-Benedict equation (14).
Participants were positioned supine on the patient bed with one leg secured to the nonmagnetic equipment identical to that used for habituation. Volunteers performed two muscle contraction protocols: one protocol involving the dorsiflexor muscles and the other the knee extensor muscles. The tests were performed in two separate sessions of ∼75 min each, with at least 40 min of rest between tests. Between the two tests, subjects consumed a nutritional supplement bar (210 cal) to standardize caloric intake. Each protocol consisted of a brief, maximal voluntary isometric contraction (MVIC) that reduced phosphocreatine (PCr) to ∼50% of resting level without inducing acidosis. Based on pilot studies, these contraction durations were chosen to be 16 and 24 s for the dorsiflexor muscles and knee extensor muscles, respectively.
For the TA test, a probe assembly consisting of a 6-cm diameter 1H surface coil and a coplanar 3 × 4 cm elliptical 31P surface coil was secured over the belly of the TA muscle. For the VL test, a probe assembly consisting of a 9-cm diameter 1H surface coil and a coplanar 6 × 8 cm elliptical 31P surface coil was secured over the VL. For both muscles, series of T1-weighted scout images were obtained in the axial plan to ensure optimal positioning of the muscle volume in the isocenter of the magnet. Magnetic field homogeneity was optimized by localized shimming on the proton signal of tissue water. Phosphorous data were acquired (125-μs hard pulse; nominal 60° flip angle; 2-s repetition time; 2,048 data points; 8,000-Hz spectral width) for a 1-min baseline period before the contraction and then during each contraction and throughout a 10-min recovery period. The unfiltered PCr line widths (full width at half-maximal height) were 8.4 ± 0.8 Hz (mean ± SD) and 11.8 ± 1.7 Hz in the resting TA and VL (n = 26), respectively, indicating excellent homogeneity of the sample volume.
Spectral analyses.
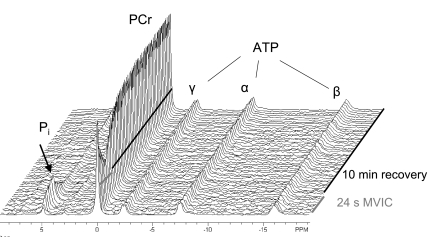
For both TA and VL, individual free-induction decays (FID) were averaged to yield 4-s resolution during the contraction, 8-s resolution during the first 5 min of recovery, and 30-s resolution during the last 5 min of recovery. The FIDs were zero-filled to 32k points and multiplied by an exponential corresponding to 10-Hz line-broadening before Fourier transformation was applied to create 31P spectra in the frequency domain (Fig. 1). The spectra were phased manually, and, to remove the underlying broad peak arising from phosphorous in bone, a fifth-order polynomial was fitted to the baseline. Subsequently, this baseline was subtracted from each spectrum. Peaks corresponding to PCr, Pi, and γ-ATP were then fit with Lorentzian-shaped curves (NUTS software, Acorn NMR, Livermore, CA) to quantify the area of each peak. Only the areas corresponding to the peaks of Pi and PCr were used for further analyses. Intramuscular pH was determined based on the chemical shift (σ) of Pi relative to PCr in parts per million:
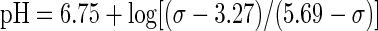 |
Fig. 1.
Phosphorus magnetic resonance spectroscopy (31P-MRS) spectra from the vastus lateralis of an untrained male subject. Representative stackplot of 31P spectra at rest, during 24-s maximal voluntary isometric contraction (MVIC), and 10-min recovery. As expected, during the contraction, PCr fell to 52% of resting level and recovered in a mono-exponential manner. Intracellular pH was 7.09 at the end of the contraction.
Because concentrations of free creatine and Pi in resting skeletal muscle, to a fair approximation, are equal, resting concentrations of 31P metabolites were calculated based on the assumption that [PCr] + [Pi] = 42.5 mM (15), where brackets denote concentration. In a subset of subjects (TA: n = 7; VL: n = 9), spectra were acquired under fully relaxed conditions (24 acquisitions, 30-s repetition time) to allow determination of saturation correction factors for Pi and PCr in TA (1.95, 1.92) and VL (1.95, 1.74), respectively. These were applied to all studies to provide accurate measures of resting 31P metabolites.
Calculation of mitochondrial capacity.
Since glycolysis quickly ceases when muscle contraction stops (38, 41), and since [ATP] does not change during the brief contractions used here, the resynthesis of PCr can be attributed to mitochondrial oxidative phosphorylation (1). As long as any change in pH is small, the resynthesis of PCr follows a monoexponential time course (32, 46). For each subject, data points from the end of the MVIC through 10 min of recovery were fitted with a monoexponential equation, accomplished using Sigmaplot software (Systat Software, San Jose, CA):
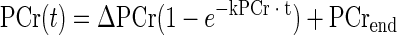 |
(1) |
where the rate constant of PCr recovery (kPCr) reflects the rate of oxidative phosphorylation, PCrend is the concentration of PCr at the end of the contraction, and ΔPCr is the difference in PCr between rest and end of contraction.
According to the linear model of oxidative phosphorylation described by Meyer and colleagues (33, 36), the theoretical maximal rate of oxidative phosphorylation is achieved when PCr is depleted. Therefore, in vivo oxidative capacity (Vmax) can be estimated as the product of kPCr and resting [PCr] using the following equation (33):
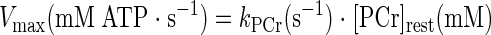 |
(2) |
Statistical analyses.
All statistical analyses were performed using SAS software (SAS Institute, Cary, NC). Two-factor (group, sex) ANOVAs using the MIXED procedure were used to compare age, height, body mass, and physical activity data across groups. Three-factor (group, sex, muscle) ANOVAs using the MIXED procedure were used to compare kPCr, Vmax, [PCr], and pH in TA and VL across groups. Sex was used as a cofactor to ascertain whether there were gender-based differences in any variables between our untrained and trained participants. The slice option in SAS was used to partition significant group-by-muscle interactions. Relationships between physical activity and kPCr were evaluated by linear regression. Values are presented as means ± SD. Statistical significance was accepted when P < 0.05.
RESULTS
Subject characteristics.
Subject characteristics are summarized in Table 1. Although the men were taller than the women (P < 0.001), there were no other effects of group or sex on the subject characteristics.
Table 1.
Subject characteristics and physical activity variables
| Untrained |
Trained | |||
|---|---|---|---|---|
| Women (n = 7) | Men (n = 7) | Women (n = 5) | Men (n = 7) | |
| Age, yr | 26.9±3.4 | 24.6±3.7 | 27.0±4.3 | 27.9±2.9 |
| Height, cm* | 169.8±5.7 | 177.2±2.7 | 166.9±3.9 | 181.6±7.6 |
| Mass, kg | 75.0±20.1 | 81.5±16.6 | 62.3±6.2 | 77.2±6.1 |
| PAcounts, counts·day−1·1,000−1† | 192±55 | 255±89 | 617±155 | 685±129 |
| PAmin, min/day† | 418±86 | 460±94 | 516±49 | 549±90 |
| LPA, min/day | 393±87 | 423±92 | 413±58 | 466±79 |
| MVPA, min/day† | 24±13 | 37±13 | 104±24 | 83±21 |
Values are means ± SD. Men were taller than women. Daily physical activity, expressed as total accelerometer counts (PAcounts), total minutes (PAmin), and minutes of moderate-to-vigorous PA (MVPA), was higher in the trained group than in the untrained group. Significant (P < 0.05) main effects of sex (*) and group (†) are indicated. There was no difference between groups in low-intensity physical activity (LPA).
Physical activity.
Physical activity data are presented in Table 1. Total daily activity, expressed as PAcounts or PAmin (P < 0.001), was higher in the trained compared with the untrained group, as designed. Time spent in LPA was similar between groups, whereas the trained group spent a greater amount of time in MVPA (∼90 vs. ∼30 min; P < 0.001). Physical activity data from one female runner were not included due to an injury that changed her activity routine. The injury occurred after completion of the habituation visit and the muscle metabolic testing session.
Muscle metabolites and pH.
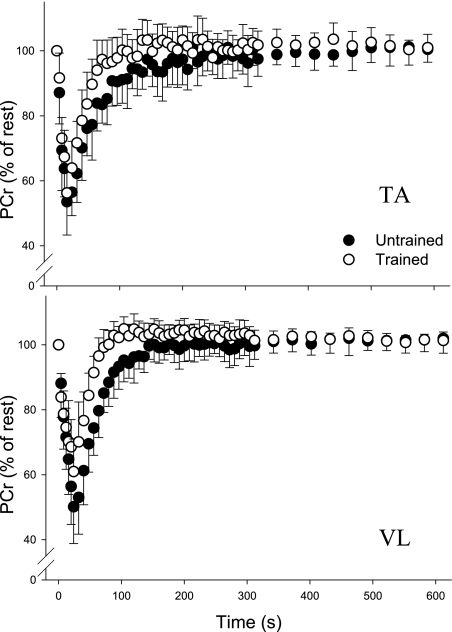
The changes in PCr for TA and VL during and after the contraction protocols are shown in Fig. 2, and metabolite concentrations and pH values are presented in Table 2. There were no significant effects of sex or any group-by-sex or muscle-by-sex interactions for kPCr, Vmax, [PCr], or pH, so these data were collapsed by gender for simplicity of presentation. Resting [PCr] was not different between muscles or between groups, although there was a group-by-muscle interaction such that resting [PCr] in VL was greater in untrained than in trained (P = 0.03). Intramuscular pH at rest was higher in VL than in TA (P < 0.001) and higher in untrained than in trained (P = 0.03).
Fig. 2.
Changes in PCr during and after brief maximal contractions in tibialis anterior (TA) and vastus lateralis (VL). PCr recovery after both MVICs was faster in trained than in untrained in TA (top) and VL (bottom) (P < 0.001). In both untrained and trained groups, PCr recovery was faster in VL than in TA (P = 0.01). Data are means ± SD.
Table 2.
Muscle metabolic variables
| TA |
VL | |||
|---|---|---|---|---|
| Untrained (n = 14) | Trained (n = 12) | Untrained (n = 14) | Trained (n = 12) | |
| PCrrest, mM* | 37.4±1.3 | 37.9±1.3 | 38.0±1.2 | 36.9±1.0 |
| pHrest†,‡ | 7.02±0.03 | 7.01±0.02 | 7.07±0.03 | 7.05±0.02 |
| PCrend, % of rest† | 50.7±9.0 | 56.2±7.4 | 48.8±10.7 | 61.0±9.8 |
| pHend†,‡ | 7.01±0.05 | 7.06±0.05 | 7.04±0.07 | 7.10±0.04 |
| kPCr, s−1†,‡ | 0.023±0.006 | 0.038±0.014 | 0.028±0.007 | 0.046±0.009 |
| Vmax, mM/s†,‡ | 0.84±0.20 | 1.43±0.56 | 1.08±0.28 | 1.70±0.36 |
Data are means ± SD. Overall, muscle oxidative capacities were higher in the trained group than in the untrained group and in vastus lateralis (VL) compared with tibialis anterior (TA). Vmax, maximal rate of oxidative phosphorylation. kPCr, rate constant of PCr recovery. Significant (P < 0.05) main effects of muscle (†), group (‡), and muscle-by-group interactions (*) are indicated.
Both MVICs induced greater depletion of PCr in the untrained compared with the trained (P = 0.003). Specifically, relative to resting level, PCr was reduced to 51 and 49% in untrained and to 56 and 61% in trained in TA and VL, respectively. At the end of the MVICs, pH was not different from resting levels in either muscle (P ≥ 0.24), although it was lower in the untrained than in the trained muscles (P < 0.001) and in TA compared with VL (P = 0.01).
Muscle oxidative capacity.
The rate constant for PCr recovery (kPCr) was ∼64% higher in trained compared with untrained subjects (P < 0.001; Fig. 3). There was also a main effect of muscle, such that kPCr was higher in VL than in TA (P = 0.01). The differences in kPCr between muscles were ∼26% in the untrained group and ∼22% in the trained group. There was a positive correlation between kPCr in TA and VL (r = 0.56; P = 0.003). As with kPCr, the maximal rate of oxidative phosphorylation (Vmax), was higher in trained than in untrained (P < 0.001) and in VL compared with TA (P = 0.02) (Table 2).
Fig. 3.
Rate constant of PCr recovery (kPCr) in trained and untrained groups. The trained group had higher kPCr than untrained in both TA and VL (P < 0.001), and kPCr was higher in VL than in TA (P = 0.01). Data are means ± SD.
Relationship between physical activity and muscle oxidative capacity.
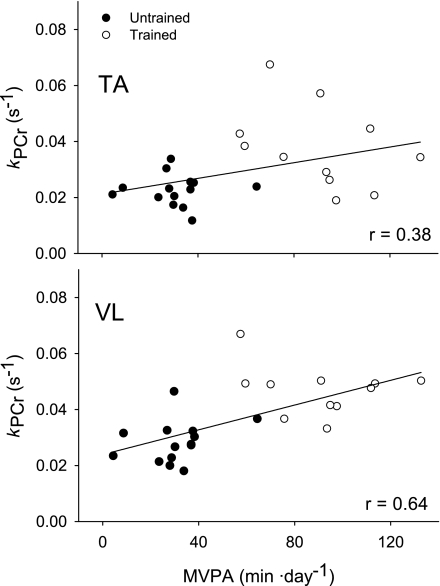
There were significant associations between physical activity level and muscle oxidative capacity that were more pronounced in VL than in TA (Fig. 4). In VL, kPCr was correlated with PAcounts (r = 0.67, P < 0.001, n = 25) and minutes of MVPA (r = 0.64, P = 0.001, n = 25). In the TA, the associations between kPCr and PAcounts (r = 0.44, P = 0.03, n = 25) and minutes of MVPA (r = 0.38, P = 0.06, n = 25) were not as robust as in the VL. Notably, there were no significant correlations between kPCr in TA or VL and total PAmin (r ≤ 0.37, P ≥ 0.07) or minutes of LPA (r ≤ 0.26, P ≥ 0.20).
Fig. 4.
Associations between minutes of moderate to vigorous physical activity (MVPA) and kPCr. Linear regressions (n = 25) revealed a more robust association between MVPA and kPCr in VL (y = 0.0239 + 0.00022x; P < 0.001; bottom) than in TA (y = 0.0212 + 0.00014x; P = 0.06; top).
DISCUSSION
The primary results of this study were that 1) in vivo oxidative capacity in two muscles of the lower limb was greater in trained compared with untrained young adults, 2) regardless of training status, oxidative capacity was higher in VL than in TA in young adults, and 3) oxidative capacity of VL, more so than TA, was associated with minutes of MVPA. These data provide novel evidence of differences in oxidative capacity in morphologically distinct muscles of healthy young adults. These differences likely reflect varying functional demands on the muscles.
Physical activity.
As expected, the untrained group had less total physical activity, expressed both as PAcounts and PAmin, compared with the trained group (Table 2). Analysis of the physical activity data indicated that there was no difference in minutes spent in LPA each day between the untrained and trained groups, whereas the trained group spent approximately threefold more time in MVPA. This result suggests that the difference in physical activity pattern between the two groups was largely a result of the habitual exercise bouts (runs) of the trained individuals.
Muscle oxidative capacity.
As measured here, the rate of PCr recovery is directly proportional to the rate of oxidative phosphorylation (31, 36) and has been used as a noninvasive measure of mitochondrial function in vivo in a wide range of populations, including untrained and trained adults (16, 17, 25, 47). Using the rate constant kPCr, Vmax for oxidative phosphorylation can also be estimated, and the results of the present study indicate excellent agreement between these two variables. To our knowledge, this is the first study to determine the effects of training status on in vivo oxidative capacity in two distinct muscles in the same subjects.
As hypothesized, the trained group showed greater oxidative capacity than the untrained group in both TA and VL. In the untrained group, kPCr in TA and VL was similar to values reported for the TA (0.027 s−1) (28), VL (0.026 s−1) (25), plantarflexors (0.033 s−1) (17), and triceps surae (0.032 s−1) (6) in similar subject populations. The kPCr values in the trained group were also comparable to values previously reported in the plantarflexors (0.040 s−1) (16), VL (0.057 s−1) (25), and TA (0.050 s−1) (3) of endurance-trained young adults.
Several investigators have reported higher kPCr in trained compared with untrained young adults (25, 47). In a study by Yoshida (47), young distance runners had a 63% faster rate of PCr recovery in the biceps femoris than sedentary young adults, which is similar to the magnitude of difference in kPCr between trained and untrained groups in the present study. This result is also in agreement with a study by Mogensen et al. (34) who reported ∼41% higher rates of mitochondrial respiration and citrate synthase activity in the VL of trained compared with untrained young men. It should be noted that most of the aforementioned studies were conducted in men or have only included a few female participants, which limits the ability to examine potential differences in muscle oxidative capacity between genders. Although we did not control for the menstrual cycle phase, which is a limitation, to our knowledge there is no evidence to suggest that the menstrual cycle affects muscle oxidative capacity. Our results suggest that, in both untrained and trained men and women, there are no apparent gender-based differences in muscle oxidative capacities in vivo.
In contrast to our second hypothesis, we found higher oxidative capacity in VL than in TA, regardless of training status. Likewise, the observation that only ∼31% of the variance in kPCr between VL and TA was accounted for suggests some dissociation between the two muscles in terms of oxidative capacity. Assuming a greater proportion of type I fibers in TA than in VL (24, 43), our results suggest that the proportion of type I fibers is not the primary factor determining muscle oxidative capacity in vivo. This interpretation is consistent with studies that have failed to show correlations between muscle oxidative capacity and the proportion of type I fibers (21, 45, 48).
Further support of this conclusion is provided by Gregory et al. (12), who showed differences in enzyme activities across four muscles that were independent of fiber-type composition. In fact, single-fiber analyses indicated higher succinate dehydrogenase activities in all fiber types of VL compared with TA (Table 2; Ref. 12). Based on their results, the authors suggested that muscle enzyme profiles develop as a result of the functional demands experienced by the muscle and not merely as a result of fiber-type composition (12). In the present study, the daily activities of our subjects, which ranged from leisurely walking to strenuous running, may not have provided mechanical loading of the TA that was sufficient to match the loading experienced by the VL. Consequently, the VL may be exposed chronically to greater oxidative demands, thereby providing the stimulus for metabolic adaptations that promotes enhanced oxidative capacity, as observed here. In addition, it is apparent that, during locomotor activities, peak activity of the TA occurs transiently during heel strike, while the muscle most often is undergoing eccentric contractions (22, 29, 35). Because eccentric contractions are less metabolically demanding than concentric contractions (40), they likely provide a smaller stimulus for muscle metabolic adaptations.
Physical activity levels and muscle oxidative capacity.
Although improved muscle oxidative capacity as a result of endurance training is a well-known concept in physiology, recent studies have highlighted the critical effect of the intensity of exercise on training-induced changes in oxidative capacity. In a 6-wk training study conducted in young adults, sprint interval training induced similar improvements in markers of oxidative capacity in VL as traditional endurance training, despite a significantly lower training volume (225 vs. 2,250 kJ/wk) (2). Forbes and colleagues (7) also used a sprint interval protocol and demonstrated improved kPCr in the quadriceps of young adults after only 2 wk of training.
A recent cross-sectional study of young men and women showed that activities of daily living were associated with mitochondrial enzyme activities in VL (4). Notably, the association between citrate synthase activity and daily physical activity counts, measured using accelerometry, was only apparent in a subgroup of adults who spent at least 9 min/day in high-intensity (>4.5 METS) activity (4). Our results likewise suggest the importance of physical activity intensity in modulating muscle mitochondrial capacity. Furthermore, the absence of associations in the TA or VL between kPCr and either total minutes of physical activity or minutes of low-intensity activity reinforces the concept that the intensity of activity, moreso than just duration, is a critical determinant of muscle oxidative capacity.
As discussed above, physical activity level is a potent regulator of muscle oxidative capacity. This effect may be particularly evident when adaptations in specific muscle fiber types are examined. Howald et al. (20) showed increased mitochondrial density in all muscle fiber types (type I, IIa, IIb) in VL following 6 wk of high-intensity endurance training in young men and women. The greatest increase occurred in type IIa fibers, which is consistent with a similar training study of the VL in young male participants that showed that the greatest increase in protein content of peroxisome proliferator-activator receptor coactivator-1 (PGC-1) occurred in type IIa fibers (39). Because PGC-1 is a critical regulator of mitochondrial biogenesis, this result indicates that all fiber types are responsive to training and that type IIa fibers may in fact undergo the greatest adaptations. A potential explanation for this notion is that muscle fibers innervated by higher threshold motoneurons (type IIa and type IIb) are recruited less frequently during habitual low-intensity activities (13, 22). Hence, if an adequate stimulus, here defined as physical activity intensity, is present, these fibers possess the greatest potential for improvement in oxidative capacity (7). Consequently, the relatively large proportion of type II fibers in the VL may provide the foundation for the stronger association between MVPA and kPCr observed in VL compared with TA (Fig. 4). The higher slope of this relationship in VL compared with TA reinforces this concept. Furthermore, the difference in patterns of use of TA and VL, as discussed above, may also influence the extent to which metabolic adaptations of TA and VL are affected by high-intensity physical activity.
Muscle metabolites and pH.
In both muscles, PCr fell somewhat more in the untrained group than in the trained group during the contractions (Table 2). However, the changes in PCr in all subjects were within the range of 30–70% of resting PCr that is desirable for the postcontraction PCr recovery measure of oxidative capacity (32, 36, 44, 46). During a MVIC, oxidative phosphorylation and glycolysis are activated and can contribute to some resynthesis of PCr, even as net [PCr] falls (42). Hence, a possible explanation for greater net PCr depletion in the untrained is that PCr resynthesis during the MVIC occurred at a faster rate in the muscles of the trained compared with untrained subjects and thus counteracted the depletion of PCr in the creatine kinase reaction.
At the end of the MVICs, the muscles of the trained group were slightly more alkalotic than in the untrained group, as was VL compared with TA. However, these differences in pH were minor and did not differ from resting values. Therefore, the differences in pH between groups and muscles likely did not affect the rate of PCr recovery (44, 46).
In summary, we show that human skeletal muscle oxidative capacity measured in vivo is higher in two lower limb muscles of trained compared with untrained young adults. We also provide novel evidence of higher muscle oxidative capacity in VL compared with TA muscles in vivo, irrespective of training status. Furthermore, we found a stronger association between physical activity intensity and oxidative capacity in VL than in TA. These data suggest a difference in oxidative capacity in vivo that may be due to differences in the patterns of use and energy demands in these two muscles.
GRANTS
This work was supported by National Institute on Aging Grants R01 AG-21094 and K02 AG-023582, and an American College of Sports Medicine Student Grant (R. G. Larsen).
Acknowledgments
The authors thank the participants in this study and the members of the Muscle Physiology Laboratory for helpful comments and critique at various stages of the project.
REFERENCES
- 1.Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial-function in vivo in human skeletal-muscle by means of P-31 NMR. Magn Reson Med 1: 307–315, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586: 151–160, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowther GJ, Jubrias SA, Gronka RK, Conley KE. A “functional biopsy” of muscle properties in sprinters and distance runners. Med Sci Sports Exerc 34: 1719–1724, 2002. [DOI] [PubMed] [Google Scholar]
- 4.den Hoed M, Hesselink MK, van Kranenburg GP, Westerterp KR. Habitual physical activity in daily life correlates positively with markers for mitochondrial capacity. J Appl Physiol 105: 561–568, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Essen B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand 95: 153–165, 1975. [DOI] [PubMed] [Google Scholar]
- 6.Forbes SC, Paganini AT, Slade JM, Towse TF, Meyer RA. Phosphocreatine recovery kinetics following low- and high-intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am J Physiol Regul Integr Comp Physiol 296: R161–R170, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes SC, Slade JM, Meyer RA. Short-term high-intensity interval training improves phosphocreatine recovery kinetics following moderate-intensity exercise in humans. Appl Physiol Nutr Metab 33: 1124–1131, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, accelerometer. Med Sci Sports Exerc 30: 777–781, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Gollnick PD, Armstrong RB, Saubert CW, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol 33: 312–319, 1972. [DOI] [PubMed] [Google Scholar]
- 10.Gollnick PD, Sjodin B, Karlsson J, Jansson E, Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflügers Arch 348: 247–255, 1974. [DOI] [PubMed] [Google Scholar]
- 11.Green HJ, Daub B, Houston ME, Thomson JA, Fraser I, Ranney D. Human vastus lateralis and gastrocnemius muscles. A comparative histochemical and biochemical analysis. J Neurol Sci 52: 201–210, 1981. [DOI] [PubMed] [Google Scholar]
- 12.Gregory CM, Vandenborne K, Dudley GA. Metabolic enzymes and phenotypic expression among human locomotor muscles. Muscle Nerve 24: 387–393, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Grimby L Firing properties of single human motor units during locomotion. J Physiol 346: 195–202, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris J, Benedict F. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie Inst. of Washington, 1919.
- 15.Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33: 109–120, 1974. [PubMed] [Google Scholar]
- 16.Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol 86: 2013–2018, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol 97: 1077–1081, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Holloszy JO Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967. [PubMed] [Google Scholar]
- 19.Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol 85: 1337–1341, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflügers Arch 403: 369–376, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Hunter GR, Bamman MM, Larson-Meyer DE, Joanisse DR, McCarthy JP, Blaudeau TE, Newcomer BR. Inverse relationship between exercise economy and oxidative capacity in muscle. Eur J Appl Physiol 94: 558–568, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Jakobsson F, Borg K, Edstrom L, Grimby L. Use of motor units in relation to muscle fiber type and size in man. Muscle Nerve 11: 1211–1218, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Jansson E, Kaijser L. Muscle adaptation to extreme endurance training in man. Acta Physiol Scand 100: 315–324, 1977. [DOI] [PubMed] [Google Scholar]
- 24.Jaworowski A, Porter MM, Holmback AM, Downham D, Lexell J. Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand 176: 215–225, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Johansen L, Quistorff B. 31P-MRS characterization of sprint and endurance trained athletes. Int J Sports Med 24: 183–189, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol 90: 1663–1670, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Kent-Braun JA, McCully KK, Chance B. Metabolic effects of training in humans: a 31P-MRS study. J Appl Physiol 69: 1165–1170, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol 583: 1093–1105, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann RA, Hagy J. Biomechanics of walking, running, and sprinting. Am J Sports Med 8: 345–350, 1980. [DOI] [PubMed] [Google Scholar]
- 30.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol 167: 875–881, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCully KK, Fielding RA, Evans WJ, Leigh JS, Posner JD. Relationships between in-vivo and in-vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol 75: 813–819, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Meyer RA A linear-model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol 254: C548–C553, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Meyer RA Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol Cell Physiol 257: C1149–C1157, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Mogensen M, Bagger M, Pedersen PK, Fernstrom M, Sahlin K. Cycling efficiency in humans is related to low UCP3 content and to type I fibres but not to mitochondrial efficiency. J Physiol 571: 669–681, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novacheck TF The biomechanics of running. Gait Posture 7: 77–95, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol Cell Physiol 272: C501–C510, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Park JH, Brown RL, Park CR, Cohn M, Chance B. Energy metabolism of the untrained muscle of elite runners as observed by 31P magnetic resonance spectroscopy: evidence suggesting a genetic endowment for endurance exercise. Proc Natl Acad Sci USA 85: 8780–8784, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J 291: 681–686, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52: 2874–2881, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Ryschon TW, Fowler MD, Wysong RE, Anthony A, Balaban RS. Efficiency of human skeletal muscle in vivo: comparison of isometric, concentric, and eccentric muscle action. J Appl Physiol 83: 867–874, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Sahlin K, Harris RC, Hultman E. Resynthesis of creatine phosphate in human muscle after exercise in relation to intramuscular pH and availability of oxygen. Scand J Clin Lab Invest 39: 551–558, 1979. [DOI] [PubMed] [Google Scholar]
- 42.Shulman RG Glycogen turnover forms lactate during exercise. Exerc Sport Sci Rev 33: 157–162, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48: 623–629, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Thompson CH, Kemp GJ, Sanderson AL, Radda GK. Skeletal muscle mitochondrial function studied by kinetic analysis of postexercise phosphocreatine resynthesis. J Appl Physiol 78: 2131–2139, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Tonkonogi M, Sahlin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiol Scand 161: 345–353, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Walter G, Vandenborne K, McCully KK, Leigh JS. Noninvasive measurement of phosphocreatine recovery kinetics in single human muscles. Am J Physiol Cell Physiol 272: C525–C534, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida T The rate of phosphocreatine hydrolysis and resynthesis in exercising muscle in humans using 31P-MRS. J Physiol Anthropol Appl Human Sci 21: 247–255, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Zoll J, Sanchez H, N'Guessan B, Ribera F, Lampert E, Bigard X, Serrurier B, Fortin D, Geny B, Veksler V, Ventura-Clapier R, Mettauer B. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 543: 191–200, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]