Abstract
We examine the potential to treat unstable ventilatory control (seen in periodic breathing, Cheyne-Stokes respiration, and central sleep apnea) with carefully controlled dynamic administration of supplementary CO2, aiming to reduce ventilatory oscillations with minimum increment in mean CO2. We used a standard mathematical model to explore the consequences of phasic CO2 administration, with different timing and dosing algorithms. We found an optimal time window within the ventilation cycle (covering ∼1/6 of the cycle) during which CO2 delivery reduces ventilatory fluctuations by >95%. Outside that time, therapy is dramatically less effective: indeed, for more than two-thirds of the cycle, therapy increases ventilatory fluctuations >30%. Efficiency of stabilizing ventilation improved when the algorithm gave a graded increase in CO2 dose (by controlling its duration or concentration) for more severe periodic breathing. Combining gradations of duration and concentration further increased efficiency of therapy by 22%. The (undesirable) increment in mean end-tidal CO2 caused was 300 times smaller with dynamic therapy than with static therapy, to achieve the same degree of ventilatory stabilization (0.0005 vs. 0.1710 kPa). The increase in average ventilation was also much smaller with dynamic than static therapy (0.005 vs. 2.015 l/min). We conclude that, if administered dynamically, dramatically smaller quantities of CO2 could be used to reduce periodic breathing, with minimal adverse effects. Algorithms adjusting both duration and concentration in real time would achieve this most efficiently. If developed clinically as a therapy for periodic breathing, this would minimize excess acidosis, hyperventilation, and sympathetic overactivation, compared with static treatment.
Keywords: heart failure, Cheyne-Stokes respiration, inhaled carbon dioxide
the presence of oscillations of respiratory and cardiac parameters with an approximate period of 1 min in patients with congestive heart failure (CHF) indicates adverse prognosis (6, 8, 16, 23, 25, 30). This periodic breathing (PB) is primarily associated with oscillations of arterial CO2 (7, 10), where the troughs in CO2 engender apneas and/or hypopneas (40). These respiratory oscillations are accompanied by cardiovascular fluctuations (9, 11, 12), which may lead to adverse effects in patients with CHF (15, 17, 18).
Although PB in CHF patients is associated with increased mortality (8, 16), there is no clinical evidence suggesting that abolition or reduction of PB would improve this outcome in CHF patients. However, the potential improvement of quality of life alone, from improved sleep, may make the development and investigation of novel therapies worthwhile. Modeling studies can facilitate such developments and inform subsequent clinical investigations to test their relevance.
The use of inhaled CO2 to stabilize PB has been investigated since the early 1980s (5). Several other papers since then have reported that the administration of constant concentrations of inhaled CO2 elevates the troughs in CO2 oscillations (2, 24, 32, 39), preventing apneas, provided that sufficient CO2 is administered. However, the systemic consequences of the resulting hyperventilation and sympathetic overactivation have made this a less attractive treatment option (1, 24). While this form of static CO2 delivery reduces the oscillations in ventilatory parameters, the associated arousals are not reduced, with no overall improvement in sleep quality (33). This may be due to the excess CO2 directly stimulating the cortex or reducing the threshold for cortical arousal.
A fixed increase in inspired CO2 [inspired CO2 fraction (FiCO2)] mandates an increase in ventilation and, therefore, increases the work of breathing. This is often commented on in previous studies (1, 2, 5, 24, 32, 33, 35, 38, 39), but not always quantified. However, it can be easily calculated because, for constant net exhaled volume of CO2, the narrowed gap between expired and inspired CO2 must be compensated for by a reciprocal increase in alveoli ventilation (Eq. 1).
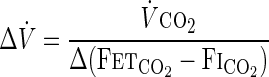 |
(1) |
where ΔV̇ is the change in alveolar ventilation; V̇co2 is a constant volume of body production of CO2; and Δ(FetCO2-FiCO2) is the change in difference of end-tidal CO2 fraction (FetCO2) to FiCO2. Using this calculation, which provides only a lower limit on the increment in ventilation, we see that ventilation is raised by up to 96% (24–96%). We have summarized calculated values of increase in ventilation due to inspired CO2 in previous studies (Supplementary Table S2; the online version of this article contains supplementary data). In fact, alveolar ventilation would have to have increased by even more than this, because the increase in ventilatory work is likely to increase V̇co2. Lorenzi et al. concluded: “Although CO2 inhalation abolishes CSR [Cheyne-Stokes respiration]-CSA [central sleep apnea] and reduces the frequency of arousals, it also increases ventilation and would therefore augment the energy and blood flow demands of the respiratory muscles in the face of low cardiac output. Therefore, it is unlikely that CO2 inhalation would be a useful long-term therapy for CSR-CSA in patients with CHF” (24).
In this study, we use mathematical modeling to investigate treating PB using optimally graded doses of inspired CO2 through dynamic delivery.
We aim to deliver targeted CO2 to counteract the troughs in end-tidal CO2 oscillations, thereby limiting the total quantity of CO2 administered. This might minimize the oscillations in the respiratory gases without producing the observed large increases in end-tidal CO2 and ventilation. We hypothesized that a small dose of inspired CO2 would reduce PB, if administered dynamically at an optimal phase and amplitude of ventilatory oscillation. This modeling approach allows a study of the consequences of different algorithms on the resultant control patterns of reproducibly unstable ventilatory control system.
METHODS
Regulation of Ventilation
Ventilation can be considered to be regulated through a feedback control loop of two physiological mechanisms: the chemoreflex and pulmonary gas exchange, which produce the controller and plant gain, respectively. The controller gain is the change in ventilation due to a change in end-tidal CO2, and plant gain is a change in end-tidal CO2 as a result of a change in ventilation (20).
Potential Steady State of the Feedback Control System
The dependence of FetCO2 on steady-state alveolar ventilation (plant gain) is an inversely proportional relationship, because the product of their means of ventilation and FetCO2 must equal the mean metabolic production of CO2 (provided that the total body production of CO2 remains constant). This relationship (Eq. 2) produces a hyperbolic curve (the isometabolic curve) when CO2 is plotted against ventilation.
 |
(2) |
The dependence of ventilation on end-tidal CO2 (controller gain) is determined by the chemoreflex response curve. This is shown as a linear relationship (Eq. 3) in Fig. 1, implying a constant value of chemoreflex gain, but can also be nonlinear (26).
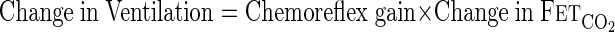 |
(3) |
Fig. 1.
The potential equilibrium point. It is at the intercept of the chemoreflex response and the curve of metabolic production of CO2 (26). FetCO2, end-tidal CO2 fraction.
The intercept of these two response curves is where the ventilation control system is potentially in equilibrium (Fig. 1).
The stability of this feedback control system depends on how it responds to small perturbations from the equilibrium state. If, after a small perturbation away from the equilibrium point, the system responses cause it to diverge further away from the equilibrium point, the system is unstable. In contrast, if the system returns to equilibrium, it is stable (26).
The Dynamic Treatment Model
We modified a previously published iterative model (26). In its original form, the model maps system behavior following a small, transient perturbation in CO2 from a steady-state condition and hence predicts whether the cardiorespiratory control system will be stable or unstable for a wide range of both linear and nonlinear chemoreflex response curves.
The development of the model described here allows the introduction of inspired CO2 at any desired time and dose during the simulation and allows the effects of this on the ventilation pattern to be quantified. Hence we were able to identify those treatment strategies that are likely to be of clinical benefit.
The Modified Model
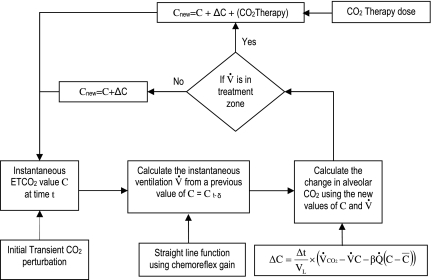
The iteration of the model begins with the instantaneous CO2 value at the steady-state position at time to followed by an introduction of a small, transient, positive perturbation on the CO2 (Fig. 2). The instantaneous ventilation value V̇ at time t (i.e., to + Δt, where Δt is 1 s in the model) is then calculated from a previous value of CO2 (Ct-δ) using a linear function of the chemoreflex gain. The new value of CO2 for time t is calculated using a difference equation below (Eq. 4), derived from a previously published analytic equation (13).
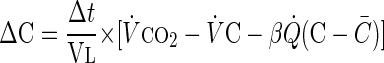 |
(4) |
where ΔC is the incremental change in end-tidal CO2 concentration during the small time increment Δt, Vl is a constant lung volume, V̇ and C are instantaneous ventilation and end-tidal CO2 variables, respectively, at time t, C̄ is the mean alveolar CO2 fraction, Q̇ is a constant cardiac output, and β is a constant of solubility of CO2 in blood.
Fig. 2.
The model. The simulation runs in a loop, calculating instantaneous values of end-tidal CO2 and ventilation for each simulation time-steps (second). It starts with the steady-state end-tidal CO2 and ventilation values as initial instantaneous values. A single, initial, transient, positive CO2 perturbation is introduced 10 simulation time-steps after the start of simulation, to determine whether the configuration of the model causes the system to stabilize or not. The model checks whether a new instantaneous value of ventilation is in the configured treatment zone. If the ventilation value is in the treatment zone, then it adds the programmed treatment CO2 to give the next instantaneous value of end-tidal CO2 (ETCO2). The derivation of the model was discussed in detail in Manisty et al. (26). See text for definition of acronyms.
The new value of V̇ is then tested according to a configured treatment regime for whether the model should administer CO2 therapy or not. If ventilation is unstable and the value of V̇ (both in amplitude and phase of oscillation) is in the configured treatment zone (as described in The Treatment Regime section below), then a corresponding programmed CO2 treatment dose is added to the new value of the end-tidal CO2.
In the treatment model, the instantaneous CO2 at time t (Ct) is given by,
 |
(5) |
Here C(t-Δt) is the instantaneous CO2 at the previous time point (t-Δt). ΔC is the component of change in CO2 that is caused by normal gas exchange processes, but assuming zero inspired CO2, and is obtained from Eq. 4. CO2 therapy is the component of change in CO2 that is caused by inspired CO2 and is calculated from the configured values of “peak concentration” and treatment phase window “duration”. This value is dependent on the amplitude and phase of the ventilation cycle at time t, by an algorithm discussed in more detail below, in The Treatment Regime section.
The new end-tidal CO2 value is then used to calculate the new value of ventilation.
In the model, the following cardiorespiratory parameters were held constant to standard values for all of our simulations: cardiac output, 3.5 l/min; metabolic production of CO2, 0.2 l/min; lung volume, 5 liters; linear chemoreflex response curve with slope of 1,200 l·min−1·atm−1; chemoreflex delay, 20 s; solubility of CO2 in blood, 5 l·l−1·atm−1; start point of CO2 equal to steady-state CO2, 4%; start point of ventilation equal to steady-state ventilation, 5 l/min; and positive CO2 perturbation, 0.001% 10 s after start. It has been shown that these parameters can be assumed constant near the equilibrium point, since they are not critical in the genesis of ventilatory instability (13). The constant values are based on previously published figures that would be typical for a patient with heart failure and PB (4, 14, 22, 26, 28, 36, 37).
The CO2 therapy is configured to automatically adjust to the amplitude of oscillation and the phase of ventilation during a treatment episode. This allowed us to dynamically configure the therapy (in concentration, duration, and delivery phase) to test the most effective treatment profile.
Severity of Ventilatory Oscillations
In this study, the severity of ventilatory oscillations was measured by calculating the relative standard deviation of ventilation over a 15-min simulation period following a 5-min initial transient interval. The smaller the relative standard deviation of ventilation, the more stable the breathing.
The Treatment Regime
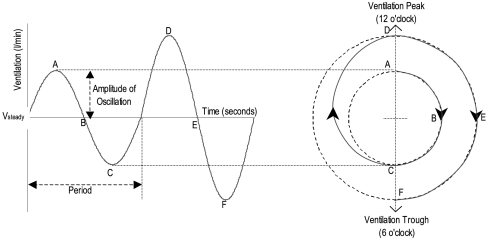
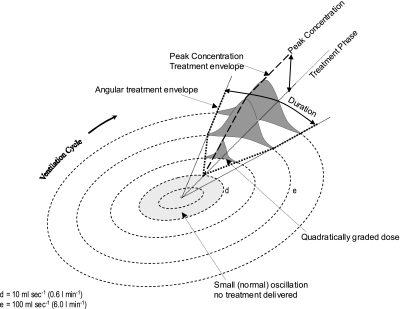
Visually, a cycle of ventilation can be represented as a clock face, with peak ventilation (hyperpnea) at 12 o'clock and trough ventilation (hypopnea) at 6 o'clock (Fig. 3). Increasing amplitude of oscillation is represented by an increase in distance away from the center of the clock. The treatment envelope is represented as a sector of the clock, where a longer duration of therapy is a proportionally larger sector, and where the concentration of therapy can be represented by the degree of shading: the darker the treatment area, the greater the concentration of CO2 delivered (Fig. 4).
Fig. 3.
Cyclic representation of ventilatory oscillations. The sinusoidal ventilatory oscillations of periodic breathing can be represented with amplitude as the radial distance from a central point and phase as an angular rotation.
Fig. 4.
Two-dimensional representation of the treatment envelope. The treatment envelope can be represented as a slice of the clock, where a longer duration of therapy is a proportionally larger slice, and where the concentration of therapy can be represented by the degree of shading: the darker the treatment area, the greater the concentration of CO2 delivered.
The angular distribution of the concentration follows a profile of [(1 − cosine)/2] curve, where it rises from zero at the beginning of the treatment episode, peaking in the midangle of the total duration, and then falls back to zero at the end of the treatment episode (Fig. 4). We reasoned that this profile would be best suited to counteract the sinusoidal-like shapes of the troughs of the CO2 oscillations.
During the dynamic therapy, the treatment is potentially available during the whole 20 min of the simulation. However, a dose of inspired CO2 is delivered during a cycle of ventilation, only if the amplitude of the oscillatory ventilation is >0.6 l/min during the configured treatment phase window. (This allows situations of only trivial oscillation to be left untreated, automatically). In a single treatment episode of a ventilation cycle, the actual dose of the delivered inspired CO2 is dependent on the amplitude of ventilatory oscillation during that cycle and the length of the treatment duration in the cycle.
For oscillations between 0.6 and 6.0 l/min, the dose of treatment increases from zero quadratically up to the programmed peak concentration and duration. The quadratic increase in dose is achieved by simultaneously increasing both the concentration and the treatment duration (per cycle) linearly, in response to increasing amplitude of ventilation. PB with oscillation >6 l/min is treated with the programmed maximum peak concentration and duration, as shown in Fig. 4. A three-dimensional representation of the treatment envelope is shown in Fig. 5. The configuration of the model allows the amount of CO2 treatment to vary from cycle to cycle, according to the pattern of ventilatory oscillations that develops.
Fig. 5.
Three-dimensional representation of the treatment envelope. The treatment envelope can be altered by changing the phase, duration, and peak concentration of the treatment, either separately or simultaneously. In this figure, the peak concentration and duration both increase linearly from d to e, resulting in a quadratic grading in the administered dose of CO2.
In general, when oscillations are more prominent, the algorithm delivers CO2, either at a higher peak concentration, or for a longer proportion of the PB cycle, or both. The relationship between oscillation amplitude and configured peak concentration and duration can be programmed at the start of each simulation and can be altered at any stage of the treatment simulation, if dose adjustment is required.
Use of Real-Time Fourier Analysis to Detect Phase Within the PB Cycle
PB sometimes has a sinusoidal envelope of ventilation, but more often has a nonsinusoidal shape, with the lower part flat, representing apnea.
In our model, we monitor the ratio of instantaneous to mean ventilation. The model uses a Fourier transform method to provide a sinusoidal fit to a wide range of oscillatory ventilation waveforms. The software determines the phase in the cycle of PB. This is achieved by performing a sliding Fourier transform on the preceding ventilation data, acquired during an approximately 1-min period, that can be adjusted to match the observed PB cycle length. The sinusoidal fit to the ventilation is then derived by constructing a sine wave with the amplitude and phase of the lowest frequency oscillatory component in the Fourier transform (i.e., that which is closest in cycle time to the period of ventilation signal transformed). This fitting technique has the advantage that it is not adversely affected by oscillatory cycles containing periods of apnea. It will also be unaffected by breath-to-breath fluctuations of the respiratory signal at frequencies much higher than that of PB.
Figure 6 shows an example of the sinusoidal fitting process satisfactorily applied to simulated noisy PB with periods of apnea. The phase of PB is detected validly.
Fig. 6.
The sinusoidal fit using the real-time Fourier transform allows the phase to be identified, even when there is a simulated pronounced apnea. The phase and amplitude of the fitted sinusoid continue to have useful meaning, even when the underlying signal is a markedly truncated sinusoid.
RESULTS
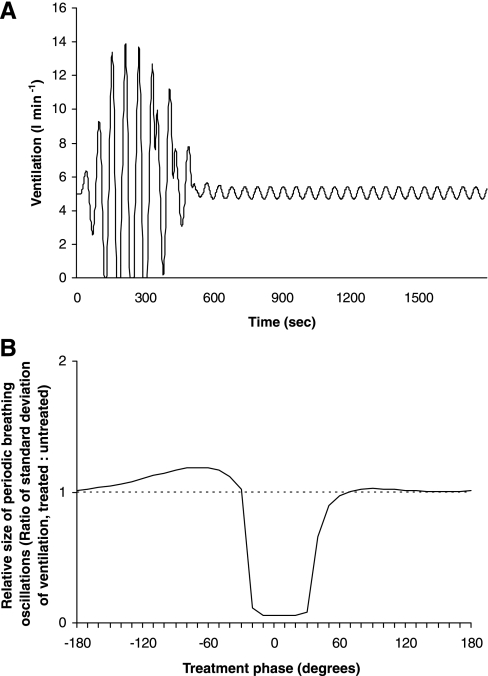
This model allowed the effect of dynamically timed therapy to be simulated, and the effect of changes in the therapy protocol to be studied. With no treatment programmed, when the model was run, it developed a pattern of PB, as shown in Fig. 7. The period of oscillations was ∼60 s.
Fig. 7.
Development of periodic breathing in the baseline, untreated simulation of the model.
We then implemented one of a range of treatment regimes and repeated the simulation, from the same starting point as the no-treatment simulation. The resulting ventilatory pattern was documented and compared with the pattern resulting from no-treatment simulation.
The treatment regimes all contained a zone of therapy (duration), which occurred at a certain phase in the PB cycle, and had a certain peak concentration of administered CO2. The regimes differed in their 1) timing of center point (midangle) of the treatment episode within the PB cycle (expressed as a “treatment phase”, with 0° representing the time of peak ventilation); 2) duration of treatment episode within the PB cycle (expressed as “duration” an angle within a circle, with, for example, 90° representing treatment for one-fourth of the cycle); and 3) peak concentration of administered CO2 [expressed as “peak concentration” a fraction between 0 and 1 (100% CO2)].
During a treatment episode (i.e., in the therapy zone), the concentration rises from zero to the peak concentration and back to zero again, following a smooth sigmoid distribution of a cosine curve, with peak at the treatment phase, which is the center point of the duration.
Study 1: Existence of an Optimum Phase Within PB Cycle for CO2 Therapy
We used the model to vary the phase of ventilation at which the CO2 was delivered, while keeping peak concentration and treatment duration constant. We set the duration of therapy to be one-half the PB cycle (180° out of the 360° cycle), and peak CO2 concentration to be 1%. We repeatedly ran the model from the same baseline starting conditions (Fig. 7) with treatment phases varying in steps of 10° from 180° before peak ventilation to 180° after peak ventilation. To assess efficacy, we measured the ratio of the size of ventilatory oscillations (standard deviation of ventilation) with and without treatment. Values < 1.0 indicate reduced amplitude of PB.
We found that the CO2 therapy is most effective when delivered at a specific range of treatment phases, in this case between −30° and +40° around peak ventilation (0°), as shown in Fig. 8. The severity of PB is sharply reduced (by >95%) when the treatment phase approaches the optimum range from either end. Outside this phase range, the CO2 therapy actually worsens the PB (increased by >30%).
Fig. 8.
Graph of relative magnitude of ventilatory oscillations against treatment phase. The optimum phase of therapy is in the range −30° to +40°, with the oscillations in ventilation increasing if therapy is delivered too early or too late in the ventilation cycle.
This particular range of optimum treatment phase is dependent on other parameters, and, if these other parameters are changed, the range of optimum treatment phase also changes.
Study 2: Effect of CO2 Dose on Ventilatory Stability
We investigated the effect, on the ventilatory pattern, of administering peak concentrations of CO2, ranging from 0 (no treatment) to 10%. The duration of CO2 therapy was kept the same for each simulation at 180°, as in study 1. The treatment phase was also kept at peak ventilation for each simulation; this was shown in study 1 to be within the optimum range of phases for reducing PB for the given baseline conditions.
Initially, we titrated the peak concentration value of administered CO2 from zero in small increments to assess when sufficient CO2 had been delivered to affect the ventilatory pattern. When the concentration of administered CO2 was <0.01%, there was little or no effect on the PB. Small increments of the peak concentration from 0.01% started to affect the ventilatory pattern. Thereafter, peak concentrations between 0.1 and 0.2% reduced the PB sharply. Beyond this point, further increases in concentration resulted in only minimal reductions in PB (Fig. 9).
Fig. 9.
Graph of relative magnitude of ventilatory oscillations against concentration of treatment.
For any concentration of 0.2% or above, there was a time window during which treatment could be administered and yield a >90% reduction in PB. The most effective abolition of PB was observed with the highest peak CO2 dose (oscillations reduced by >98%). However, this high concentration has disadvantages: high concentration treatments reduce the margin of error for the optimum treatment phase (−20° to +30° for 10% CO2 vs. −30° to +40° for 2% CO2) and would be associated with greater systemic side effects from the CO2. When higher peak concentration treatment (10% CO2) is delivered outside the optimum phase window, PB worsens by >300%, whereas at lower peak concentration treatment (2% CO2), PB worsens by <33%. We, therefore, used 2% CO2 for subsequent simulations, as it provided the best balance of attenuation of ventilatory oscillations with the widest margin of error. Figure 9 demonstrates how the magnitude of residual PB varies with increasing concentration of administered CO2 therapy per ventilation cycle (treatment phase = 0°, duration = 180°).
Study 3: Effect of Duration of CO2 Therapy on Ventilatory Pattern
We studied the effect of duration of treatment on the ventilatory pattern. The simulation was run for various treatment durations between 0° and 360° (where supplemental CO2 treatment continues throughout the PB cycle, following the cosine curve). For each simulation, the peak concentration and treatment phase of administered CO2 were kept the same (2% and 0°, respectively). These were shown to be within the optimal range for reducing PB.
We titrated the duration of CO2 therapy from 0° in small increments to assess when sufficient CO2 had been delivered to affect the ventilatory pattern. When the duration of CO2 delivery was <0.5°, there was little or no effect on the PB. Small increments of duration between 0.5° and 12° reduced the PB sharply. Further increase of the duration beyond this point had little further effect (Fig. 10).
Fig. 10.
Graph of relative magnitude of ventilatory oscillations against duration of treatment.
For any duration of treatment of 12° or above, there was a time window during which treatment could be administered and yield >90% reduction in PB. The most effective abolition of PB was observed with the longest treatment duration of 360° (oscillations reduced by >98%). However, this long duration has disadvantages: it allows a smaller margin of error for the optimum treatment phase (20° less for duration of 360° compared with 180°). Furthermore, treatment with 360° duration worsens PB by ∼50%, if delivered outside the optimum treatment phase, compared with <33% increase in degree of PB when applying at 180° duration. In view of these factors, we used a treatment duration of 180° for subsequent simulations, which we felt to be optimal.
Study 4: Interchangeability of CO2 Therapy Concentration and Duration
We found that a decrease in the concentration of CO2 administered could be compensated for by an increase in the duration of therapy. In studies 2 and 3, we showed that the effect of peak concentration on PB has a pattern similar to that of the effect of treatment duration. Therefore, it should be possible to use several combinations of peak concentration and duration to deliver a similar dose of CO2 therapy, to produce an equivalent effect.
We have presented a three-dimensional representation of efficacy of treatment in the online supplement (Supplementary Fig. S2). The relationship between the peak concentration and duration can be expressed as:
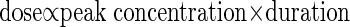 |
(6) |
Linear increase or decrease of peak concentration and duration simultaneously will change the resultant dose quadratically. This has its benefits in treating PB with moderate-to-severe amplitude oscillations, as we show below.
Study 5: The Effect of Grading Peak Concentration and Duration Simultaneously When Treating Moderate to Severe (0.6–6.0 l/min) Amplitude Oscillation
Therapy is not administered for low-amplitude PB (<0.6 l/min), while, for high-amplitude PB (severe) (>6.0 l/min), it is more effective to deliver uniform duration and concentration to reduce the amplitude of PB. However, for moderate-to-severe amplitude (between 0.6 and 6.0 l/min) PB, the optimal dose of CO2 is no longer uniform but is quadratically related to the amplitude of the PB. The quadratic dose relation is achieved by simultaneously grading the duration and concentration delivery linearly (Fig. 11). This increased the efficacy of therapy by 22%, compared with linearly graded dosing. Grading either parameter linearly in isolation produced a narrower spectrum of optimum treatment range at these moderate-to-severe amplitudes, as shown in Fig. 11 diagrams.
Fig. 11.
Results comparison of linearly and quadratically graded dose therapy strategies. Both linearly graded dose therapies give narrow optimum treatment option (bottom left and middle) compared with quadratically graded dose treatment (bottom right). Top: duration, either uniform, above a threshold ventilation amplitude (left), or linearly increasing, to a maximum value, with amplitude of ventilatory oscillation (right). Middle: CO2 concentration, either uniform, above a threshold ventilation amplitude (left), or linearly increasing, to a maximum value, with amplitude of ventilatory oscillation (right). Bottom: results of three possible combinations of therapy strategies: linearly graded dose achieved by uniform duration and graded CO2 concentration ([CO2]) (left); linearly graded dose achieved by uniform [CO2] and graded duration (middle); quadratically graded dose achieved by increasing both duration and [CO2] with amplitude of ventilatory oscillation (right).
Study 6: Comparison of the Effectiveness of Static and Dynamic CO2 Delivery on PB
We programmed the model to deliver a static CO2 therapy at all times to the baseline configuration (Fig. 7) and hence established the constant CO2 concentration that optimally reduced PB (Fig. 12).
Fig. 12.
Response of the model to static therapy. The solid line shows the change in relative ventilatory oscillations, and the dashed line shows the change in average ventilation (V̇average) with increasing administered static [CO2]. PB, periodic breathing; PBrelative, relative PB.
We performed the simulation with small increments of CO2 concentration of 0.05% from 0 to 1% and in increments of 0.5% from 1 to 10%. We found that the optimum concentration for the given configuration with static therapy is ∼2%. This therapeutic concentration reduced the PB by >99%. However, this static treatment increased the average end-tidal CO2 from 4 to 4.3%, and the average ventilation from 5 to 8.4 l/min, over the 20-min treatment period. Therefore, abolishing ventilatory oscillations using static CO2 delivery is associated with significant increase in average ventilation (Fig. 12).
We studied the effect of using a dynamic treatment at a peak concentration of 2% (the same value as for this static treatment) on the average end-tidal CO2 and average ventilation. We also found the static concentration that gave the same reduction in PB (97%), as this dynamic treatment, and calculated the effect on the average end-tidal CO2 and average ventilation. The increases in mean end-tidal CO2 of the dynamic and static treatments were 0.0005 and 0.1710 kPa, respectively. The increases in mean ventilation of the dynamic and static treatments were 0.005 and 2.015 l/min, respectively (for the same 97% reduction in PB). The total CO2 delivered during the static treatments were >80% more than the amount delivered during dynamic treatment (Table 1).
Table 1.
Comparison of the effect of static and dynamic treatments on the average ventilation and the average end-tidal CO2
| Type of Treatment Regime | Concentration of Delivered CO2, % | Degree of Attenuation of Periodic Breathing, % | Average End-tidal CO2 Fraction, kPa | Average End-tidal CO2 Fraction Effect of Treatment | Average Ventilation, l/min | Average Ventilation Effect of Treatment |
|---|---|---|---|---|---|---|
| No treatment | 4.00 | 5.0 | ||||
| Dynamic treatment | 2 (peak) | 97 | 4.00 | +0.0005 | 5.0 | +0.005 |
| Static treatment with same efficacy as the above | 1.4 | 97 | 4.17 | +0.1700 | 7.0 | +2.000 |
| Optimum static treatment | 2 | 99 | 4.30 | +0.3000 | 8.4 | +3.400 |
Slow steady increase in inspired CO2.
We modeled the effect of a slow steady increase in FiCO2 on the ventilatory pattern, for comparison with our dynamic CO2 delivery method, by gradually increasing inspired CO2 from 0 to 5% over 2 h of simulated time.
We started with the baseline configuration, but, after the initial period, during which the PB had become fully developed and persistent, we introduced a series of 0.25% increments in inspired CO2 at 5-min intervals, from 0.00 to 5.00%.
We found that inspired concentrations >0.75% were large enough to affect the ventilatory pattern. By the time inspired CO2 had risen to 1.75%, the residual PB oscillations were <1/100 of the baseline size. However, this entailed a substantial increase in ventilation. At an optimum inspired CO2 value of 1.75%, average ventilation had already increased by >50%.
Study 7: The Effect of Other Parameters on the Optimum Treatment Phase of Ventilation
We studied the effect of other parameters on the optimal treatment window: cardiac output, chemoreflex gain, lung volume, metabolic production of CO2, and chemoreflex delay. Instead of the fixed values used in studies 1–6, in this study, we took values for these parameters lower and higher (cardiac output 2.5–4.5 l/min, chemoreflex gain 1,100–1,800 l·min−1·atm−1, lung volume 3–6 liters, metabolic production of CO2 100–400 ml/min and chemoreflex delay 17–40 s) (13).
For reasons of brevity, we have not shown here the results of every combination of these values, or of other values, but the findings can be summarized by observing that whenever the system configuration is unstable, there turns out to be an optimum window of therapy. The size and precise timing of the optimal window varies with system configuration, but is generally close to peak ventilation. For the extremes of the cardiac output range quoted above, the optimal delivery phase varied by 10° (0° at 2.5 l/min to 10° at 4.5 l/min). A similar variation was obtained for the extremes of chemoreflex gain, metabolic production of CO2, and lung volume.
Chemoreflex delay has a more dramatic effect, as might be expected. As chemoreflex delay ranges from 17 to 40 s, the period of oscillation increases proportionately (by a factor of ∼2.5), and the optimal treatment phase moves from −10 to +80° referenced to peak ventilation. This change in optimal treatment phase corresponds to a change in time of a magnitude similar to the increase in chemoreflex delay. Therefore, in a clinical device, it may be possible to predict the optimum treatment phase region by measuring the period of the ventilatory oscillation and inferring from this the approximate value of the chemoreflex delay.
Further sensitivity analysis of the model is described in the online supplement.
Study 8: Peripheral and Central Chemoreceptors
In the previous sections, we have concentrated on a single chemoreceptor response for clarity in introducing the principles of the dynamic administration of CO2. To investigate the response of the algorithm on multiple chemoreceptors, we reconfigured the model with two notional groups of chemoreceptors with individual group chemoreflex gain and delay. The first set of chemoreceptors was programmed with a gain of 180 l·min−1·atm−1 and had one of three short time delays (15, 20, or 25 s). The second set of chemoreceptors was programmed with a gain of 1,020 l·min−1·atm−1 and had one of three long time delays (80, 100, or 120 s). This corresponds to a split into a ratio of 15 to 85% of the gains used in our simulations using a single chemoreflex gain. This ratio is typical of published values (34). We ran the simulation with various combinations of the individual set chemoreflex delay times. We incorporated a low-pass filter in line with the second set of receptors to account for the sluggish response of central chemoreceptors.
In all cases, we found that, whenever the system is unstable, there turns out to be a window of optimum CO2 administration to effect a reduction in PB. Similar findings were obtained with more equal distribution and when three receptor groups were simulated.
Study 9: Effect on System Stability of Large-amplitude, Long-period Initial Driving Oscillations
To establish whether the results above are peculiar to the particular transient, small-amplitude, initial perturbation that we tried, we repeated the simulations with large-amplitude, long-period initial driving oscillations. The driving oscillations lasted for 5 min, had cycle time of 1 min, and were of a size sufficient to generate apnea at the nadir of ventilation. The treatment was then applied as above, and, after a further 10 min, the resulting size of oscillation was calculated over a 15-min period. An example is shown in Fig. 13A.
Fig. 13.
The response of the model for large-amplitude and long-period initial driving oscillations. A: time domain example of optimum treatment of a large-amplitude, long-period oscillation. B: the optimum treatment phase window following large-amplitude and long-period oscillatory perturbation.
The results for optimum treatment phase are shown in Fig. 13B. In general, they are the same as those found with single, transient, small-amplitude, initial perturbations. Figure 13B also shows that the treatment delivered during the apneic phase (close to 180°) did not affect the PB.
DISCUSSION
We have devised and studied a novel mathematical model to deliver optimally timed dynamic CO2 therapy to ameliorate PB. Using our model, we were able to control the timing, concentration, and duration of CO2 delivered, as a function of the phase and amplitude of ventilatory oscillations. We have consistently shown that there is an optimum phase window in which a small dose of CO2 therapy can improve PB. If therapy is delivered outside the optimum range of treatment phase, PB worsens. Importantly, the model allows the prediction of this margin of error.
We considered several factors to determine the optimum treatment values, namely, the maximum PB reduction, with minimum dose delivered and maximum margin of error to remain in the optimum treatment window. For example, in Figs. 9 and 10, a treatment with concentration of 0.8% and duration of 40° may appear as effective as 2% concentration and 180° duration. However, these graphs show the response of the system only when treatment is delivered at the ideal time. In practice, it is useful to have an algorithm that works, even if treatment is not delivered at the ideal time, giving a wider window of opportunity to treat effectively. The window of optimum treatment phase is only one-half the size (−10 to +20°), with the former configuration than with the latter (−30 to +30°). The latter configuration is, therefore, preferable. Furthermore, we have identified that the ideal pattern for this algorithm to work would be with both the concentration and duration (length of treatment episode per ventilation cycle) increasing linearly (quadratic increase of dose), with increasing degree of oscillation.
Most relevant to potential clinical therapies, this study showed that the dynamic treatment increased neither the average end-tidal CO2, nor the average ventilation, from the baseline value. This is in contrast to the traditional static treatment, where our model shows these parameters to be increased by physiologically significant amounts. Our investigation, in study 6, demonstrates that the dynamic treatment requires a much smaller dose of CO2, compared with static treatment that reduces PB by the same level.
Previous clinical studies have used static CO2 to treat PB (1, 5, 24, 32, 33, 35, 39), based on the rationale that patients with PB have arterial CO2 levels below their apneic threshold. With static supplementary CO2 administration, the apneas are eliminated, as corroborated by our study 6. However, the mean ventilation increases, as demonstrated in Supplemental Table S2 of the online supplement. In contrast, our dynamic therapy aims to target only the troughs in end-tidal CO2 that drive hypoventilation. The targeted inspired CO2 will potentially counteract a transient reduction in arterial Pco2, preventing it from falling below the apneic threshold, without grossly increasing its mean value. Our proposed system aims not only to prevent apneas, but also to attenuate hypopneas. By minimizing end-tidal CO2 oscillations, ventilatory oscillations may potentially be reduced or abolished without significantly increasing mean ventilation.
There are some patients in whom mean ventilation is low: in these, our concern to minimize the increment in mean ventilation is not justified, and so the proposed therapeutic model need not be considered particularly advantageous.
We believe that the reason why PB worsens when treatment timing is outside the optimum region is that the inspired CO2 contributes to augmenting hypercapnia instead of counteracting the transient reduction of CO2.
One potential advantage of algorithms such as the one we present here is that it has a relatively short “memory”. Sleep is known to be characterized by discontinuous changes in system characteristics (20, 21, 22), which could be seriously problematic for a therapy strategy that relied on static therapy, or therapy aimed at a particular CO2 or ventilation target. Our dynamic cycle-detecting approach, however, automatically adjusts to new system characteristics as they arise. Moreover, if system characteristics become stable, therapy automatically ceases.
Previous static CO2 administration regimes have improved breathing in CSR, but not sleep quality, as reported by Steens et al. (32), Andreas et al. (1) and Szollosi et al. (33). The latter two groups suggested that the main cause for this lack of improvement in sleep quality is elevated sympathetic activity, due to hypercapnia. If this is so, the dynamic treatment suggested by our model may potentially enable reduction of PB without such arousals.
Thomas et al. (35) showed that CO2 concentrations as low as 0.5% were an effective adjunct to continuous positive airway pressure in the treatment of severe complex sleep apneas, improving sleep quality as well as respiratory disturbances. Although our model corroborates the effectiveness of low-concentration CO2 therapy, it is not possible, from their work, to determine which proportion of the synergistic effects of CO2 and continuous positive airway pressure resulted in these beneficial effects. Our baseline simulation differs from this study in that we used levels of cardiac output compatible with those found in CHF patients (as in the clinical studies cited above), whereas they treated patients without heart failure.
Although our simulations showed that the approach of gradual increase in inspired CO2 abolished PB at low inspired CO2 level, it also showed that this increased the average ventilation hugely. This demonstrates that we still face similar problems of elevated ventilation with gradual CO2 increase as we would with static CO2 administration.
Added dead space has been shown to stabilize PB (19). However, this is achieved at the expense of increased ventilation similar to static CO2 treatment.
Two previous groups have produced ingenious and simple devices, which arrange to deliver added inspired CO2 during the patient's hyperventilation phase to increase end-tidal CO2 (3, 31). With these systems, therapy always centers around a phase near peak ventilation with a fixed time delay due to (circuit and physiological) dead space. As our model suggests, the period of PB can vary such that significant changes in the phase of optimal CO2 delivery away from peak ventilation are required for varying chemoreflex delays. Such variation cannot be achieved with these systems, but is obtainable with a model-mediated approach.
In both cited methods, once the fresh gas flow is set in its application to control breathing, the circuit could interpret increases in baseline ventilation as hyperventilation and would deliver CO2. Our proposed method would treat only periodic fluctuations in ventilations and not changes in the baseline value.
Study Limitations
This study is a mathematical simulation, and not a clinical study. Although this can be considered a limitation, it has the advantage of our being able to guarantee a constant underlying control system instability. This ensures that differences between one run of the simulation and another can be attributed to differences in the therapy algorithm and are not due to random environmental fluctuations. Clinical studies paralleling this one in terms of variety of algorithms tested would be difficult, because biological variability would necessitate large numbers of replicate experiments to give reasonable certainty that observed differences were due to differences between algorithms.
Another limitation of this study is that, to limit the number of simulations to show the aims of the study, we concentrated on the simple linear response of the chemoreflex gain, although the model can be configured to different curve responses, as shown in Manisty et al. (26). In a linear response chemoreflex gain, its slope near the steady-state CO2 is the predominant factor to determine system stability (27).
In this study, only a single example of an unstable respiratory control system was studied in great depth, i.e., a single set of constant values describing chemoreflex response characteristics, chemoreflex delay, lung volume, cardiac output, and metabolic production of CO2. However, in study 7, we varied each parameter between its extreme ranges, while maintaining our standard set of values for the others.
We have presented in an online supplement a further sensitivity analysis of the model, in which we have shown how it reacts to combinations of extreme changes to the various physiological parameters that are likely to be found in practice. These sensitivity analyses enable us to appreciate the relative importance of the various physiological parameters as sources of variations to the model outputs. Although these analyses demonstrate that the extreme range of unstable system configurations gives qualitatively similar responses to simulated treatments as those using our standard physiological parameter set, they do not give the detailed behavior of the system for the full spectrum of the input states.
One other limitation of this study is that, for simplicity and brevity, we have not included the possible effect of upper airway resistance in the model. However, in reality, a fall in CO2 may well result in the rise of upper airway resistance, which will reduce ventilation in a synergistic manner with the same direction of effect as the chemoreceptor-mediated fall in ventilatory effort. Therefore, we generally expect the overall behavior of the system will be similar, although the instability of a system with upper airway resistance sensitive to CO2 will be more enhanced (29).
In this study, we have used only a limited number of chemoreceptors for simplicity and brevity. Future development of the model should include a large number of chemoreceptors with individual gain and delay times.
This is a “basic science” study, applying respiratory and control system modeling. Previous mathematical models have increased our understanding of the pathophysiology of PB. Similarly, mathematical modeling provides insights into the construction of therapeutic algorithms. This study demonstrates that it is feasible to develop an algorithm that could work in real time (since our CO2-admininstering software module uses only real-time input of modeled ventilation and outputs only a desired inspired CO2 concentration). We recognize that the next stage of this research is to apply this modeling system to some real cases (with various unpredictable apnea-hyperpnea patterns) to further tune the timing and dosage of CO2 delivery and design an experimental study. However, this study gives a good background on the desirable characteristics of the affecting parameters (optimum dose and treatment timing) that an experimental setup would need.
Conclusions
Although static concentrations of CO2 administration can ameliorate PB, very much smaller quantities of CO2 can achieve the same degree of amelioration if it can be administered dynamically with careful timing and dosing. Algorithms adjusting both duration and concentration in real time appear to have the greatest potential to do this with minimal unwanted elevation in average CO2 levels.
With appropriate development of real-time delivery technology, it might be possible to develop clinical therapies for PB diseases, which use such a dynamic approach, with only very minor increments in systemic CO2 levels and, therefore, essentially avoiding the undesirable physiological effects of CO2 administration, such as hyperventilation or sympathetic overactivation.
GRANTS
Y. Mebrate was supported by the Royal Brompton and Harefield National Health Service Trust and St. Mary's Coronary Flow Trust (P11209). C. H. Manisty was supported by the Wellcome Trust (077049/Z/05/Z). K. Willson was supported by Foundation for Circulatory Health and the Coronary Flow trust. D. P. Francis was supported by the British Heart Foundation (FS/04/079).
Supplementary Material
REFERENCES
- 1.Andreas S, Weidel K, Hagenah G, Heindl S. Treatment of Cheyne-Stokes respiration with nasal carbon dioxide. Eur Respir J 12: 414–419, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Badr MS, Grossman JE, Weber SA. Treatment of refractory sleep apnea with supplemental carbon dioxide. Am J Respir Crit Care Med 150: 561–564, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Banzett RB, Garcia RT, Moosair SH. Simple contrivance “clamps” and tidal Pco2 and Po2 despite rapid changes in ventilation. J Appl Physiol 88: 1597–1600, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Beecroft J, Duffin J, Pierratos A, Chan CT, McFarlane P, Hanly PJ. Enhanced chemoresponsiveness in patients with sleep apnea and end-stage renal disease. Eur Respir J 28: 151–158, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Berssenbrugge A, Dempsey J, Iber C, Skatrud J, Wilson P. Mechanisms of hypoxia-induced periodic breathing during sleep in humans. J Physiol 343: 507–524, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley TD, Floras JS. Sleep apnea and heart failure. II. Central sleep apnea. Circulation 107: 1822–1826, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Cherniack NS, von Euler C, Homma I, Kao FF. Experimentally induced Cheyne-Stokes breathing. Respir Physiol 37: 185–200, 1979. [DOI] [PubMed] [Google Scholar]
- 8.Corra U, Pistono M, Mezzani A, Braghiroli A, Giordano A, Lanfranchi P, Bosimini E, Gnemmi M, Giannuzzi P. Sleep and exertional periodic breathing in chronic heart failure: prognostic importance and interdependence. Circulation 113: 44–50, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Davies LC, Francis DP, Crisafulli A, Concu A, Coats AJ, Piepoli M. Oscillations in stroke volume and cardiac output arising from oscillatory ventilation in humans. Exp Physiol 85: 857–862, 2000. [PubMed] [Google Scholar]
- 10.Douglas C, Haldane J. The causes of periodic or Cheyne-Stokes breathing. J Physiol 38: 401–419, 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faber J, Lorimier P, Sergysels R. Cyclic haemodynamic and arterial blood gas changes during Cheyne-Stokes breathing. Intensive Care Med 16: 208–209, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Francis DP, Davies LC, Willson K, Ponikowski P, Coats AJ, Piepoli M. Very-low-frequency oscillations in heart rate and blood pressure in periodic breathing: role of the cardiovascular limb of the hypoxic chemoreflex. Clin Sci (Lond) 99: 125–132, 2000. [PubMed] [Google Scholar]
- 13.Francis DP, Willson K, Davies LC, Coats AJS, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation 102: 2214–2221, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Gabrielsen A, Videbaek R, Schou M, Damgaard M, Kastrup J, Norsk P. Non-invasive measurement of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clin Sci (Lond) 102: 247–252, 2002. [PubMed] [Google Scholar]
- 15.Hanly PJ, Millar TW, Steljes DG, Baert R, Frais MA, Kryger MH. Respiration and abnormal sleep in patients with congestive heart failure. Chest 96: 480–488, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med 153: 272–276, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Hanly P, Zuberi N, Gray R. Pathogenesis of Cheyne-Stokes respiration in patients with congestive heart failure: relationship to arterial Pco2. Chest 104: 1079–1084, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Javaheri S, Parker TJ, Wexler L, Michaels SE, Stanberry E, Nishyama H, Roselle GA. Occult sleep-disordered breathing in stable congestive heart failure. Ann Intern Med 122: 487–492, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Khayat RN, Xie A, Patel AK, Kaminski A, Skatrud JB. Cardiorespiratory effects of added dead space in patients with heart failure and central sleep apnea. Chest 123: 1551–1560, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Khoo MC, Gottschalk A, Pack AI. Sleep-induced periodic breathing and apnea: a theoretical study. J Appl Physiol 70: 2014–2024, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol 53: 664–659, 1982. [DOI] [PubMed] [Google Scholar]
- 22.Khoo MC, Yang F, Shin JJ, Westbrook PR. Estimation of dynamic chemoresponsiveness in wakefulness and non-rapid-eye-movement sleep. J Appl Physiol 78: 1052–1064, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation 99: 1435–1440, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzi-Filho G, Rankin F, Bies I, Bradley T. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 159: 1490–1498, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzi-Filho G, Genta PR, Figueiredo AC, Inoue D. Cheyne-Stokes respiration in patients with congestive heart failure: causes and consequences. Clinics (Sao Paulo) 60: 333–344, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Manisty CH, Willson K, Wensel R, Whinnett ZI, Davies JE, Oldfield WL, Mayet J, Francis DP. Development of respiratory control instability in heart failure: a novel approach to dissect the pathophysiological mechanisms. J Physiol 577: 387–401, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manisty CH, Willson K, Wensel R, Whinnett ZI, Davies JE, Oldfield WLG, Mayet J, Francis DP. Apnoeic threshold: a true determinant of cardiorespiratory control stability or a marker of chemoreflex gain? Thorax 61: 48–49, 2006.16396953 [Google Scholar]
- 28.Mohan RM, Amara CE, Cunningham DA, Duffin J. Measuring central-chemoreflexsensitivity in man: rebreathing and steady-state methods compared. Respir Physiol 115: 23–33, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Series F, Cormier Y, Desmeules M, La Forge J. Influence of respiratory drive on upper airway resistance in normal men. J Appl Physiol 66: 1242–1249, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation 102: 61–66, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Sommer LZ, Iscoe S, Robicsek A, Kruger J, Silverman J, Rucker J, Dickstein J, Volgyesi GA, Fisher JA. A simple breathing circuit minimizing changes in alveolar ventilation during hyperpnoea. Eur Respir J 12: 698–701, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Steens RD, Millar TW, Su X, Biberdorf D, Buckle P, Ahmed M, Kryger MH. Effect of inhaled 3% CO2 on Cheyne-Stokes respiration in congestive heart failure. Sleep 17: 61–68, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Szollosi I, Jones M, Morrell MJ, Helfet K, Coats AJ, Simonds AK. Effect of CO2 inhalation on central sleep apnea and arousals on sleep. Respiration 71: 493–498, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Talbot NP, Dorrington KL. Mechanics and control of ventilation. Surgery 21.3: iii–vi, 2003. [Google Scholar]
- 35.Thomas RJ, Daly RW, Weiss JW. Low-concentration carbon dioxide is an effective adjunct to positive airway pressure in the treatment of refractory mixed central and obstructive sleep-disordered breathing. Sleep 28: 69–77, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Turner MS, Bleasdale RA, Mumford CE, Frenneaux MP, Morris-Thurgood JA. Left ventricular pacing improves haemodynamic variables in patients with heart failure with a normal QRS duration. Heart 90: 502–505, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van den Aardweg JG, Karemaker JM. Influence of chemoreflexes on respiratory variability in healthy subjects. Am J Respir Crit Care Med 165: 1041–1047, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Villiger PM, Hess CW. Beneficial effect of inhaled CO2 in a patient with non-obstructive sleep apnea. J Neurol 241: 45–48, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Xie A, Rankin F, Rutherford R, Bradley TD. Effects of inhaled CO2 and dead space on idiopathic central sleep apnea. J Appl Physiol 82: 918–926, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med 165: 1245–1250, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.