Abstract
Recently, we found that the translocation of inhaled nanoparticles from the air space to secondary organs is age dependent and substantially greater in neonates than in adults (J Respir Crit Care Med 177: A48, 2008). One reason for this difference might be age-dependent differences in alveolar barrier integrity. Because the neonate lung is undergoing morphogenetic and fluid balance changes, we hypothesize that the alveolar barrier of developing lungs is more easily compromised and susceptible to foreign material influx than that of adult lungs. On the basis of these hypotheses, we predict that the postnatally developing lung is also more likely to allow the translocation of some materials from the air space to the lymphatic lumens. To test this idea, we intratracheally instilled methyl methacrylate into immature and adult lungs and compared lymphatic filling between these two age groups. Scanning electron microscopy of the resultant corrosion casts revealed peribronchial saccular and conduit lymphatic architecture. Deep pulmonary lymphatic casts were present on the majority (58.5%) of airways in immature lungs, but lymphatic casting in adult lungs, as anticipated, was much more infrequent (21.6%). Thus the neonate lung appears to be more susceptible than the adult lung to the passage of instilled methyl methacrylate from the air space into the lymphatics. We speculate that this could imply greater probability of translocation of other materials, such as nanoparticles, from the immature lung as well.
Keywords: methyl methacrylate, nanoparticles, morphology, age groups
the pulmonary lymphatics contribute to the removal of fluid and small particles from the lung (10, 11, 13, 21, 23, 24). In normal adult lungs, methyl methacrylate resin rarely penetrates the alveolar epithelium, but in lungs with compromised alveolar barrier integrity due to hyperoxia, resin can move from the air space into the pulmonary lymphatic vessels (7), creating lymphatic casts. Our recent work (20) indicates that the translocation of inhaled nanoparticles from the air space to secondary organs is greater during the early postnatal period than in adulthood. Hypothesizing that the alveolar barrier of the postnatally developing lung can also be breached by other substances, similar to mature lungs with induced alveolar damage, we tested whether the translocation of resin from the air space to the pulmonary lymphatics would be greater in immature lungs than in adult lungs, resulting in more abundant lymphatic filling in neonates.
Scanning electron microscopy (SEM) of the resultant corrosion casts revealed abundant conduit and saccular lymphatic casts in all immature lungs. Lymphatic casting in mature lungs, as anticipated, was much less (∼3-fold) frequent. The results suggest that the neonate lung is more permissive than the adult lung to the passage of methyl methacrylate into the lymphatic vessels. We postulate that this could imply heightened ability of the neonate lung to allow the translocation of other materials, including nanoparticles, from the air space as well.
METHODS
Specific pathogen-free outbred Wistar rats were obtained from Charles River Labs (Wilmington, MA). Lactating females were housed with their litters, and adult rats were provided food pellets and water ad libitum. The data represent eight neonate (2- to 5-day-old) rats [9.8 ± 1.8 (SD) g body mass] and four adult (>100-day-old) male rats (391 ± 28 g body mass). Animals were treated in accordance with protocols approved by the Animal Care and Use Committee overseeing the Harvard School of Public Health.
Rats were euthanized with pentobarbital sodium (200 mg/kg ip; Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI) and weighed using a Mettler balance. To estimate the volume of resin required for instillation, we used the equation of Gunnison et al. (6) to calculate total lung capacity for each rat on the basis of body mass. The chest cavity was opened, and the trachea was intubated using polyethylene tubing that varied in size from PE-20 to PE-205, depending on the age of the rat. Dilution ratios, time after accelerant, and temperature were held constant for all resin instillations. To achieve an appropriate viscosity and cure time, we used a 4:1 ratio of Mercox to methacrylate monomer diluent plus 1% accelerator (benzoyl peroxide; Mercox kit, Ladd Research Industries, Burlington, VT) as the casting resin. This ratio produced a hardening of the resin within ∼5 min. Methods for casting via the air space were modified from Hainis et al. (7) and Peao et al. (14). Cooled (4°C) resin was instilled via the tracheal tube immediately (within seconds) after addition of the cure.
Because gravity-based head pressure alone is insufficient to overcome the high resistance of the small-diameter tubing required for neonatal intubation, the neonate and adult lungs were instead inflated using slow, gentle instillation of a calculated volume of resin (equivalent to the lung volume at 23-cm head pressure) by hand-held syringe. The justification for this hand-held technique is as follows. The majority of the pressure is lost before entry into the lung because of frictional loss within the small-diameter tubing. The important pressure for lymphatic filling is static pressure inside the lung, which is determined by lung volume and specific lung compliance. Because the specific compliances of neonate and adult lungs are similar (3), neonate lung volume equivalent to adult lung volume at 23-cm head pressure results in static lung pressure similar to that in the adult lung. To confirm that syringe-driven instillation of a preset volume produced results similar to head pressure-driven instillation, resin was gravity instilled at a height of 23 cm above the lung in three additional adult rats. There was no significant difference (P < 0.05, t-test) in the percentage of airways with cast lymphatics between adult lungs filled at 23-cm head pressure (n = 3) and those filled using a syringe [n = 4, 16.9 ± 1.1% and 21.6 ± 16.9% (SD), respectively], nor did cast morphologies differ, thus supporting our use of the hand-held syringe-based method.
Lung lobes inflated without observable leakage of resin. Because the curing of Mercox is exothermic, carcasses were packed in ice to offset the heat generated during the 30 min we allowed the resin to polymerize before lung harvest. The lungs were then left overnight in a 60°C water bath to complete resin polymerization. Slabs were cut from the left lung perpendicular to the long axis using a Teflon-coated razor blade (EMS, Hatfield, PA), with systematic sampling at fixed intervals (>5 mm apart) and with the position of the first cut chosen randomly within the interval (i.e., random start).
Connective tissue and other biological materials surrounding the methyl methacrylate casts were digested away using decreasing (10–60%) concentrations of potassium hydroxide alternating with distilled water washes for ∼3 wk. The resulting casts were then cleaned using 1% formic acid. After dehydration with ethanol and hexamethyldisilazane (EMS), the casts were sputter coated with palladium-gold and imaged using a scanning electron microscope (Quanta 200, FEI). Within a slab, there was no further sampling: the entire exposed surface of the slice was examined, and every detectable airway was included (mean number of airways examined per rat = 17, a total of >80 airways in the immature rats and >70 airways in the adult rats). Airways were carefully inspected over a range of magnifications (approximately ×70 to ×1,000) for the presence of lymphatics. For presentation purposes, in some cases, a median filter (radius = 1 pixel) was applied to reduce noise, or the entire image was globally adjusted for brightness and contrast. Types of lymphatics were identified on the basis of previously defined morphological criteria (15). Each airway was categorized as possessing no lymphatics, saccular lymphatics only, conduit lymphatics only, or conduit and saccular lymphatics. Mean values were plotted with error bars equivalent to ±1 SD. To compare the mean values of immature vs. mature lungs, a t-test was used. Statistics were calculated using Intercooled Stata (College Station, TX) software, with P < 0.05 taken to indicate a significant difference.
RESULTS
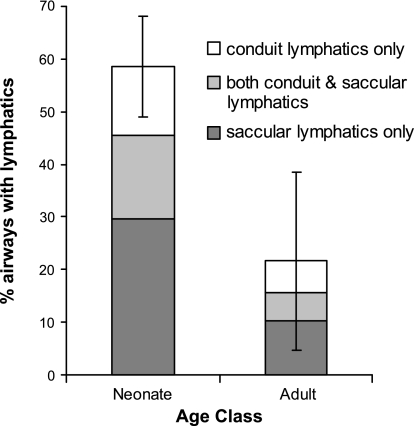
At all ages, the lumens of airways and alveoli were cast by the resin. Alveolar rupture and significant extravasation, which could indicate the creation of gross artifactual communications between the air space and the lymphatic vessels, were not observed. Exposed airways ranged from ∼50 to 700 μm, with the majority ∼100–250 μm. Lymphatic replicas were significantly (P < 0.05) more abundant on the airways of immature than mature lungs (Fig. 1), present on average on 58.5% (95% CI = 40.8–76.2) of all neonate airways but only 21.6% (CI = 5.1–38.2) of mature airways. Interindividual variation was considerable, but further binning within the neonate age class (2–5 days old) did not reveal additional age-dependent trends in lymphatic abundance or composition.
Fig. 1.
Lungs of immature rats had a greater proportion of airways with cast lymphatics than lungs of mature rats (P < 0.05). Error bars, ±1 SD for all cast lymphatics (conduit + saccular).
When lymphatics were present, two main types of morphology were observable: saccular and conduit lymphatics (Fig. 2). Decreases in the abundance of both lymphatic morphologies contributed to the lower proportion of casts in the mature lungs (Fig. 1). Representative adult airways lacking lymphatic casts are shown in Fig. 3. Saccular lymphatics decreased roughly threefold, from 45.5% (CI = 29.6–61.4) on neonate airways to 15.6% (CI = 2.4–28.7) on adult airways (Fig. 1). The percentage of airways with saccular lymphatics only was similarly higher in neonates than adults (29.7% vs. 10.2%), as was the percentage of airways with both saccular and conduit lymphatics (15.8% vs. 5.4%). Conduit lymphatics (alone or in association with saccular lymphatics; Fig. 1) were present on 28.8% of neonate airways and only 11.5% of adult airways. The percentage of airways that possessed only conduit lymphatics was 13.0% in neonates and 6.1% in adults (Fig. 1). The interindividual variability in the proportions of each type of lymphatic was considerable. A preliminary survey of lymphatic morphology in neonate and adult lungs did not reveal age-dependent structural differences in either type of lymphatic. Comparison of larger and smaller airways within an age class showed some tendency for the cast lymphatics, particularly conduit lymphatics, to be found on smaller, presumably more distal airways than on larger, more proximal airways.
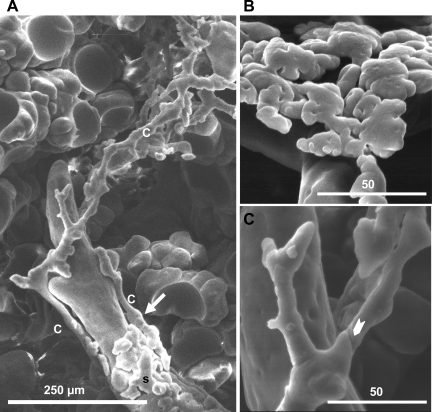
Fig. 2.
Immature lungs possessed abundant lymphatic casts of different morphologies. A: peribronchial saccular lymphatics (s) and peribronchial conduit lymphatics (c) cast from the lung of a 4-day-old rat. Connection is visible between conduit and saccular lymphatics (arrow). B: peribronchial saccular lymphatic from the lung of a 3-day-old rat at higher magnification. C: peribronchial conduit lymphatic from the lung of a 4-day-old rat at higher magnification. Arrowhead points to typical indentation.
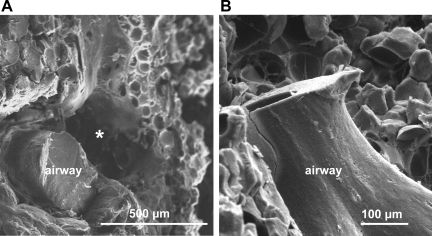
Fig. 3.
Airways of mature lungs typically lacked lymphatic casts. Scanning electron micrographs of intratracheally cast adult rat lungs show cross-sectional (A) and lateral (B) views of representative airways lacking lymphatic casting. Empty cylindrical space (*) beside the airway in A likely represents digestion of the blood vessel that accompanied the airway.
The majority of the lymphatics cast in the immature and adult rat lungs were of the saccular type, consisting of a dense mesh of lymphatic microvessels that created a plexiform layer partially or completely surrounding the bronchioles. In some cases, saccular lymphatic distribution was sparse and patchy; in others, the lymphatics formed complete dense cylinders encircling the airway. Although peribronchial lymphatics were easily observed, presumptive perivascular lymphatics, consisting of saccular lymphatic mesh surrounding an empty cylindrical space (the vestige of the artery that had accompanied the airway), were more difficult to identify because of the digestion of the blood vessels during maceration. For this reason, only peribronchial lymphatics were included in our quantification.
Tubular conduit lymphatics (Fig. 2A) ran down the long axis of some airways and could often be traced for hundreds of micrometers. The diameters of these lymphatics varied, and occasional surface bumps, indentations, and constrictions were observed. Larger proximal trunks produced bifurcating branches distally. When conduit and saccular lymphatics were present on a single airway, connections between these two types were sometimes visible (Fig. 2A, arrow). At high magnification, oval imprints representing lymphatic endothelial nuclei and subtle v-shaped imprints, possibly representing valves, were sometimes observed.
Lymphatic branching patterns, vessel size, and three-dimensional organization within the context of the airways and alveoli were revealed by SEM of the air space casts. The technique is well suited to visualization of conduit and saccular lymphatics, but prelymphatics and initial lymphatics were not detected in our examination of the alveolar region. These types of lymphatics, if cast, are likely lost during the maceration of the supporting interstitial tissue. Similarly, the pulmonary vasculature (which possesses a characteristic density, morphology, and branching pattern distinct from that of lymphatics) was not preserved.
DISCUSSION
In their flattened, undilated state, deep pulmonary lymphatics can be challenging to detect by any available method (15). Corrosion casting, the filling of lumens with resin followed by maceration of the surrounding tissue, produces near-perfect vascular replicas (2), but lymphatic replicas are only rarely produced in most healthy experimental models (15). The first demonstration of lung lymphatic casts by SEM did not occur until 1992 (15), and we are not aware of any previous studies describing the three-dimensional architecture of the lymphatic network in the postnatal developing lung. Hypothesizing that the developing lung, much like an injured adult lung, might be more susceptible to the passage of methyl methacrylate from the air space than the lung of a healthy adult, we attempted to cast pulmonary lymphatics via intratracheal instillation of resin into neonate rat lungs. This technique produced abundant lymphatic replicas in immature (2- to 5-day-old) animals (Fig. 1). In adult rats, far fewer lymphatic casts were produced, consistent with previous findings that lymphatic casting is infrequent in healthy adults (7). No obvious morphological differences were detected in the lymphatics among the different age groups or between the lymphatics on large vs. small airways. Two main types of lymphatic morphology were present: meshlike saccular lymphatics and elongated conduit lymphatics. Decreases in the abundance of both types of lymphatic morphology contributed to the overall decrease in the abundance of peribronchial lymphatics (Fig. 1). The results are consistent with age-dependent differences in the passage of methyl methacrylate from the air space into the lymphatics.
The casts represent a snapshot of lymphatic filling that occurred within a brief period, i.e., the 5-min period before the resin became rigid. Mercox, a relatively low-viscosity methyl methacrylate, polymerizes with little shrinkage and faithfully replicates structures down to 260 nm (26). It is known that this material rarely penetrates the alveolar epithelium of healthy mature lungs (7), but the details of its pathway across the alveolar barrier of compromised or immature lungs are unknown. The morphology of lymphatic replicas is similar whether casting is performed intratracheally via permeabilized alveolar epithelium or intravascularly via porous endothelium (7). Intravascular injection pressure of the resin appears to have little or no influence on whether lymphatics are cast (15); thus, in the case of lymphatic casting from the more permeable vasculature side, pressure does not appear to be a major factor in the formation of replicas. We are not aware of any literature assessing the impact of airway instillation pressure on lymphatic casting.
The integrity of vascular endothelial and alveolar epithelial barriers is crucial for the maintenance of lung fluid balance (15), with the alveolar epithelium presenting the more robust barrier. When these barriers are compromised, filling of the lymphatic vessels with resin is enhanced. In mature animals, pulmonary lymphatics can be cast via the airway if the alveolar epithelium has been damaged, as is the case with hyperoxic exposure and edema; lymphatic casting in control animals is rare (7). Lymphatic replicas are also rarely observed in healthy adults subject to intravascular casting, but they can be produced with interventions that induce vascular permeability, i.e., angiogenesis (15), edema (17), and increased lymph production and engorged, dilated lymphatics (18). The proportions of adult airways with saccular and conduit lymphatics that we found were just slightly higher than those found on the bronchioles of the control rats described by Hainis et al. (7). The percentage of airways with saccular and conduit lymphatics in neonate lungs was comparable to that in adult lungs injured by hypoxia during the early recovery period (7). In our study, the lymphatic casting via the air space in normal neonate lungs likely reflects vulnerability of the immature air-tissue barrier at the level of the alveolus. In contrast to our air space casting, careful descriptions of microvascular replicas produced by vascular casting of normal immature rat lungs do not mention lymphatic filling (4).
It is prudent to use care in the analysis of quantitative data derived from casting techniques. The variables that may be impacting the observed age-dependent differential lymphatic casting, in particular, have not been characterized and are deserving of additional study. A number of factors could potentially influence casting efficiency (8). The lymphatics of neonates and adults could differ in their response to resin toxicity, for example, or in their response to other details of the conditions of the casting procedure. Among many factors, the integrity of the alveolar wall barrier must play a crucial role in lymphatic casting from the alveolar side. Early postnatal structural changes, such as alveologenesis and vascular remodeling, could influence the integrity of the alveolar barrier and contribute to the translocation of resin and lymphatic filling at this stage. The deep pulmonary lymphatics drain to the hilar tracheobronchial lymph nodes, contributing to perinatal drainage (3), and lymphatic filling may also have been facilitated by dilated lymphatic vessels in neonates. Needless to say, resin infusion and translocation into the lymphatics of euthanized animals is likely quite different from translocation in vivo. The greater production of lymphatic casts in neonates than in adults is not likely an indication of genuine lymphatic decrease during maturation but, more likely, a result of differential casting efficiency in developing and mature lungs. We speculate that the increased movement of resin from the air space to the lymphatics could reflect greater permeability of immature lung parenchyma to some substances and the potential for heightened vulnerability of the neonate lung to other inhaled materials.
GRANTS
This research was supported in part by National Heart, Lung, and Blood Institute Grants HL-074022, HL-070542, and HL-054885 and was performed in part at the Harvard Center for Nanoscale Systems, a member of the National Nanotechnology Infrastructure Network supported by National Science Foundation Grant ECS-0335765.
REFERENCES
- 1.Aharinejad S, MacDonald IC, MacKay CE, Mason-Savas A. New aspects of microvascular corrosion casting: a scanning, transmission electron, and high-resolution intravital video microscopic study. Microsc Res Tech 26: 473–488, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Bland RD, Hansen TA, Hazinski TA, Haberkern CM, Bressack MA. Studies of lung fluid balance in newborn lambs. Ann NY Acad Sci 384: 126–145, 1982. [DOI] [PubMed] [Google Scholar]
- 3.Bolle I, Eder G, Takenaka S, Ganguly K, Zeller C, Neuner M, Kreyling WG, Tsuda A, Schulz H. Lung function in the developing rat lung. J Appl Physiol 104: 1167–1176, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Caduff JH, Fischer LC, Burri PH. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat Rec 216: 154–164, 1986. [DOI] [PubMed] [Google Scholar]
- 5.Grande NR, de Sa CM, Aguas AP, Carvalho E, Soares M. Time course and distribution of tungsten-laden macrophages in the hilar lymph nodes of the dog lung after experimental instillation of calcium tungstate into the left apical bronchus. Lymphology 23: 171–182, 1990. [PubMed] [Google Scholar]
- 6.Gunnison AF, Weideman PA, Sobo M, Koenig KL, Chen LC. Age-dependence of responses to acute ozone exposure in rats. Fundam Appl Toxicol 18: 360–369, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Hainis KD, Sznajder JI, Schraufnagel DE. Lung lymphatics cast from the airspace. Am J Physiol Lung Cell Mol Physiol 267: L199–L205, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Hossler FE, Douglas JE. Vascular corrosion casting: review of advantages and limitations in the application of some simple quantitative methods. Microsc Microanal 7: 253–264, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Kodavanti UP, Schladweiler MC, Ledbetter AD, Hauser R, Christiani DC, Samet JM, McGee J, Richards JH, Costa DL. Pulmonary and systemic effects of zinc-containing emission particles in three rat strains: multiple exposure scenarios. Toxicol Sci 70: 73–85, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Lauweryns JM, Baert JH. The role of the pulmonary lymphatics in the defenses of the distal lung: morphological and experimental studies of the transport mechanisms of intratracheally instillated particles. Ann NY Acad Sci 221: 244–275, 1974. [DOI] [PubMed] [Google Scholar]
- 11.Leak LV Lymphatic removal of fluids and particles in the mammalian lung. Environ Health Perspect 35: 55–76, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberdorster G, Gibb FR, Beiter H, Lu ST, Morrow PE. Studies of the lymphatic drainage of dog lungs. J Toxicol Environ Health 4: 571–586, 1978. [DOI] [PubMed] [Google Scholar]
- 13.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113: 823–839, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peao MN, Aguas AP, de Sa CM, Pereira AS, Grande NR. Scanning electron microscopy of the deep lymphatic network of the murine lung as viewed in corrosion casts. Lymphology 26: 42–48, 1993. [PubMed] [Google Scholar]
- 15.Schraufnagel DE Forms of lung lymphatics: a scanning electron microscopic study of casts. Anat Rec 233: 547–554, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Schraufnagel DE, Agaram NP, Faruqui A, Jain S, Jain L, Ridge KM, Sznajder JI. Pulmonary lymphatics and edema accumulation after brief lung injury. Am J Physiol Lung Cell Mol Physiol 284: L891–L897, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Schraufnagel DE, Basterra JL, Hainis K, Sznajder JI. Lung lymphatics increase after hyperoxic injury. An ultrastructural study of casts. Am J Pathol 144: 1393–1402, 1994. [PMC free article] [PubMed] [Google Scholar]
- 18.Schraufnagel DE, Schmid A. Microvascular casting of the lung: effects of various fixation protocols. J Electron Microsc Tech 8: 185–191, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Schraufnagel DE, Schmid A. Microvascular casting of the lung: vascular lavage. Scanning Microsc 2: 1017–1020, 1988. [PubMed] [Google Scholar]
- 20.Semmler-Behnke M, Tsuda A, Schulz H, Takenaka S, Kreyling WG. Age-dependent translocation of nanoparticles to secondary target organs in the developing lung (Abstract). J Respir Crit Care Med 177: A48, 2008. [Google Scholar]
- 21.Shwe TT, Yamamoto S, Kakeyama M, Kobayashi T, Fujimaki H. Effect of intratracheal instillation of ultrafine carbon black on proinflammatory cytokine and chemokine release and mRNA expression in lung and lymph nodes of mice. Toxicol Appl Pharmacol 209: 51–61, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Snipes MB, Muggenburg BA, Bice DE. Translocation of particles from lung lobes or the peritoneal cavity to regional lymph nodes in beagle dogs. J Toxicol Environ Health 11: 703–712, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Strom KA, Johnson JT, Chan TL. Retention and clearance of inhaled submicron carbon black particles. J Toxicol Environ Health 26: 183–202, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Videira MA, Botelho MF, Santos AC, Gouveia LF, de Lima JJ, Almeida AJ. Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. J Drug Target 10: 607–613, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Videira MA, Gano L, Santos C, Neves M, Almeida AJ. Lymphatic uptake of lipid nanoparticles following endotracheal administration. J Microencapsul 23: 855–862, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Weiger T, Lametschwandtner A, Stockmayer P. Technical parameters of plastics (Mercox CL-2B and various methylmethacrylates) used in scanning electron microscopy of vascular corrosion casts. Scan Electron Microsc 243–252, 1986. [PubMed]