Abstract
Intermittent hypoxia (IH) commonly occurs in patients with obstructive sleep apnea and can cause a wide range of pathology, including reduced left ventricular (LV) ejection fraction in rats as determined by echocardiography, in rodent models. We utilized echocardiography and pressure-volume (PV) loop analyses to determine whether LV contractility was decreased in inbred C57BL/6J mice exposed to IH and whether blockade of β-adrenergic receptors modified the response to hypoxia. Adult male 9- to 10-wk-old mice were exposed to 4 wk of IH (nadir inspired O2 5–6% at 60 cycles/h for 12 h during the light period) or intermittent air (IA) as control. A second group of animals were exposed to the same regimen of IH or IA, but in the presence of nonspecific β-blockade with propranolol. Cardiac function was assessed by echocardiography and PV loop analyses, and mRNA and protein expression in ventricular homogenates was determined. Contrary to our expectations, we found with PV loop analyses that LV ejection fraction (63.4 ± 3.5 vs. 50.5 ± 2.6%, P = 0.015) and other measures of LV contractility were increased in IH-exposed animals compared with IA controls. There were no changes in contractile proteins, atrial natriuretic peptide levels, LV posterior wall thickness, or heart weight with IH exposure. However, cAMP levels were elevated after IH, and propranolol administration attenuated the increase in LV contractility induced by IH exposure. We conclude that, contrary to our hypothesis, 4 wk of IH exposure in C57BL/6J mice causes an increase in LV contractility that occurs independent of ventricular hypertrophy and is, in part, mediated by activation of cardiac β-adrenergic pathways.
Keywords: blood pressure, cAMP, echocardiography, ejection fraction, pressure-volume loop, propranolol, sympathetic activation
the heart is highly dependent on oxidative metabolism to maintain normal function (38), and, consequently, cardiac tissue is susceptible to lack of O2. Despite innate defense systems that are induced by exposure to repeated and relatively brief episodes of hypoxia [i.e., ischemic preconditioning (25)], longer-term exposure to intermittent hypoxia (IH) may not be beneficial to cardiac function. Animal models of chronic IH are commonly used to simulate the hypoxic stress that occurs in obstructive sleep apnea (OSA). Rodent models of IH exposure lasting from days to weeks have exhibited multiple adverse outcomes, including hypertension (3, 9), insulin resistance (13, 31), hyperlipidemia (20), atherosclerosis (36), and increased size of experimentally induced infarct (16, 28). Two recent studies in rats concluded that cardiac contractility was compromised and pathways of hypertrophy and apoptosis were activated after 5 wk of exposure to chronic IH (4, 5). Taken together, these data suggest that chronic IH exposure is detrimental to many physiological processes, including cardiac function.
In the present study, we used a combination of in vivo (echocardiography), in situ (PV loop analyses), and biochemical approaches to carefully characterize cardiac function in C57BL/6J mice after 4 wk of IH exposure. We hypothesized, on the basis of the above-mentioned studies in rats and mice, that 4 wk of exposure to IH would impair cardiac function. Surprisingly, we found that cardiac contractility was increased in C57BL/6J after IH exposure, and we go on to show that this increased contractile response of the heart is attenuated by administration of the β-adrenergic antagonist propranolol.
MATERIALS AND METHODS
Animals.
Male C57BL/6J mice (20–25 g body wt, 9–12 wk old) were purchased from the Jackson Laboratory (Bar Harbor, ME) for use in the study. All studies were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh Medical Center and complied with the American Physiological Society guidelines for animal studies.
Protocol.
Mice were housed in regular cages that were customized, using previously described techniques (32), to deliver an IH stimulus or an intermittent room air (IA) stimulus as a control. Our approach in customizing the cages was to allow the animals to live in their normal environment continuously throughout the protocol. Briefly, a gas control delivery system regulated the flow of room air, N2, and O2 into the customized cages housing the mice. A series of programmable solenoids and flow regulators enabled the manipulation of inspired O2 from 20.9 to 5.0–6.0% over a 30-s period, with a rapid reoxygenation to room air levels using a burst of 100% O2 in the succeeding 30-s period. Hypoxic events occurred at a rate of one event per minute throughout the 12-h light period (8 AM–8 PM). During the 12-h dark period (8 PM–8 AM), the animals were maintained in a constant undisturbed room air environment. Control animals (sham exposure) were exposed to the same gas flow as the IH animals, but only room air was used. Animals were exposed to 28 consecutive days of IH or IA before the terminal experiment assessing PV loop function.
In a second series of experiments in a separate group of animals, mice were exposed to an identical 28-day period of IH or IA (see above), but in the presence of nonspecific β-blockade, with propranolol delivered at 10 mg·kg−1·day−1 (1, 30) throughout the 4-wk period via a miniosmotic pump (Alzet) implanted subcutaneously between the scapulae. The miniosmotic pump was loaded with propranolol and implanted 1 day before the start of IA or IH.
Echocardiography.
Transthoracic echocardiography was performed under light (∼1%) isoflurane inhalation anesthesia, with the animals breathing spontaneously. Measurements were obtained after 27 days of exposure to IH or IA, 1 day before PV loop analysis and subsequent death. Short-axis M- and B-mode images of the left ventricle (LV) were obtained using a VisualSonics 770 machine with a 25-MHz linear transducer to determine heart rate (HR), end-diastolic dimension (EDD), end-systolic dimension (ESD), diastolic anterior wall thickness, diastolic posterior wall thickness, and percent fractional shortening (%FS), which was calculated as follows: %FS = 100%*(EDD − ESD)/EDD. Using Teichholz's formula (39), end-diastolic volume [EDV; 7.0/(2.4 + LVEDD)*LVEDD3], end-systolic volume [ESV; 7.0/(2.4 + LVESD)*LVESD3], and ejection fraction [%; 100*(LVEDV − LVESV)/LVEDV] were calculated. These parameters were averaged over 10–20 cardiac cycles.
LV PV loop analyses.
Mice were removed from the IH or IA exposure chambers and anesthetized with 1–2% isoflurane in room air via facemask and placed on a heating pad with the temperature set to 37.5°C. The anesthetized animal was placed in a supine position, and a 10- to 15-mm incision was made in the anterior midline of the neck to expose the trachea. The left external jugular vein was dissected free and catheterized with PE-10 tubing. The trachea was cannulated, and the animal was attached to a positive-pressure volume-controlled rodent ventilator (MiniVent, Harvard Apparatus, Holliston, MA) that incorporated isoflurane inhalation anesthesia. Tidal volume was set at 200–250 μl and ventilator rate at 110–130 breaths/min. The ventilator setting and level of anesthesia were adjusted to maintain the animal in an anesthetized state without spontaneous breathing efforts.
The right carotid artery was dissected and exposed, and a Mikro-Tip (1F tip size) conductance catheter (model PVR-1045, Millar Instruments, Houston, TX) was introduced into the artery and advanced into the LV via the aortic valve (2). Once steady-state hemodynamics were achieved, PV loops were recorded and processed using an MVPS-400 system (Millar Instruments). For all animals, parallel conductance was determined individually using a 10- to 12-μl bolus of 15% saline given through the venous catheter (11). The cuvette calibration method (Millar Instruments) was used to calculate the absolute volume data. For the cuvette calibration, two separate groups of 9- to 10-wk-old male C57BL/6J mice (total n = 6) were exposed to IH or IA (see above), and the calibration data were subsequently applied to the corresponding groups. With use of PVAN 3.6 software (Millar Instruments), the PV loop data were processed to compute cardiac parameters as described previously (2, 11). The dP/dt at 40 mmHg LV pressure was determined using a macro imported to CHART software obtained from ADInstruments (Colorado Springs, CO). Where necessary (8 of 46 animals), the LV pressure was shifted to account for slight negative pressures at diastole, and in these eight animals, which were distributed across all four groups, the LV pressure shift was always <2.0 mmHg. At the end of the experiment, the animals were killed under anesthesia and mechanical ventilation to obtain a terminal blood draw, and the heart was excised and snap frozen with liquid nitrogen.
Quantitative real-time RT-PCR analysis.
The RNeasy mini-kit (Qiagen) was used according to the manufacturer's instructions to isolate total RNA from ventricular tissue homogenates of heart from IH- and IA-exposed mice. DNA was removed from the samples via a DNase step during RNA purification in the RNeasy kit. RT was performed on 1 μg of total RNA using the Reverse IT First Stand Synthesis Kit (Abgene). Real-time RT-PCR was subsequently performed using the DNA-binding dye SYBR Green3 and previously validated primers (Table 1) and conditions as described for mouse GAPDH, β1-, β2-, and β3-adrenergic receptors (6, 24), sarco(endo)plasmic reticulum Ca2+-ATPase, ryanodine receptor, α-myosin heavy chain (α-MHC), β-myosin heavy chain (β-MHC) (33), brain natriuretic peptide (BNP), and atrial natriuretic peptide (ANP) (10, 22).
Table 1.
Primers used for RT-PCR
| Gene | Accession No. | Primer Sequence (5′–3′) | Ref. | Size, bp |
|---|---|---|---|---|
| AdRβ1 | NM_007419 | 26 | ||
| Sense | CGCCTGCTACAACGACCCCAAG | 22 | ||
| Antisense | AGCCAGTTGAAGAAGACGAAGAGGCG | 26 | ||
| AdRβ2 | NM_007420 | 26 | ||
| Sense | GGTTATCGTCCTGGCCATCGTGTTTG | 26 | ||
| Antisense | TGGTTCGTGAAGAAGTCACAGCAAGTCTC | 29 | ||
| AdRβ3 | NM_013462 | 26 | ||
| Sense | TCTAGTTCCCAGCGGAGTTTTCATCG | 26 | ||
| Antisense | CGCGCACCTTCATAGCCATCAAACC | 25 | ||
| SERCA2a | NM_009722.2 | 33 | ||
| Sense | CATTTGCATTGCAGTCTGGAT | 21 | ||
| Antisense | CTTTGCCATCCTACGAGTTCC | 21 | ||
| Phospholamban | NM_023129.3 | 33 | ||
| Sense | AAGTGCAATACCTCACTCG | 19 | ||
| Antisense | GATCAGCAGCAGACATATC | 19 | ||
| α-MHC | NM_010856.3 | 10 | ||
| Sense | TGTGGTGCCTCGTTTCCA | 18 | ||
| Antisense | TTTCGGAGGTACTGGGCTG | 19 | ||
| β-MHC | NM_080728.2 | 10 | ||
| Sense | GCATTCTCCTGCTGTTTCCTT | 21 | ||
| Antisense | TGGATTCTCAAACGTGTCTAGTGA | 24 | ||
| BNP | NM_008726.3 | 33 | ||
| Sense | GAGGTCACTCCTATCCTCTGG | 21 | ||
| Antisense | GCCATTTCCTCCGACTTTTCT | 21 | ||
| Ryanodine receptor 2 | NM_023868.2 | 33 | ||
| Sense | TCAAACCACGAACACATTGAGG | 22 | ||
| Antisense | AGGCGGTAAAACATGATGTCAG | 22 | ||
| ANP | NM_008725.2 | 10 | ||
| Sense | GTGTACAGTGCGGTGTCCAA | 20 | ||
| Antisense | ACCTCATCTTCTACCGGCATC | 21 |
AdR, adrenergic receptor; SERCA2a, sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2a; MHC, myosin heavy chain; BNP and ANP, brain and atrial natriuretic peptide.
cAMP measurements.
Frozen ventricular tissue was cut, weighed, and homogenized on ice (1:10 vol/vol) in 0.1 M HCl. Samples were then spun at 10,000 rpm for 5 min, and the supernatant was collected for soluble protein and cAMP. Total protein in each sample was measured using the Bio-Rad protein assay kit. Equal amounts of protein were then assayed for cAMP using a cAMP Direct Immunoassay Kit (BioVision) as directed by the manufacturer.
Statistical analysis.
Statistical differences between IH and IA exposure on echocardiographic, PV loop, ELISA, and Western blot parameters were determined by two-way ANOVA with Newman-Keuls post hoc analyses where appropriate or unpaired Student's t-test. For RT-PCR measurement of mRNA, the comparative 2(−ΔΔCT) method was used (21). Differences were considered significant at P < 0.05. Values are means ± SE.
RESULTS
Body and organ weights.
Body and organ weight data at the end of 4 wk of exposure to IH or IA are shown in Table 2. As expected, mice exposed to IH did not exhibit weight increases over the 4-wk period comparable to those of the IA control group. There was no difference in absolute heart or lung weight, but in animals exposed to IH, we observed a reduced absolute liver weight that was not present when corrected for body weight.
Table 2.
Body and organ weights in animals exposed to IH or IA with or without β-adrenergic blockade
| IA (n = 13) | IH (n = 15) | IA + Propranolol (n = 9) | IH + Propranolol (n = 9) | |
|---|---|---|---|---|
| Body wt, g | ||||
| Day 0 | 23.9±0.5 | 23.5±0.7 | 25.7±0.7 | 26.8±0.3‡ |
| Day 28 | 26.4±0.6 | 23.4±0.6* | 28.2±0.7 | 27.4±0.6‡ |
| Heart wt, g | 0.119±0.003 | 0.110±0.004 | 0.133±0.006 | 0.126±0.004† |
| Lung wt, g | 0.142±0.004 | 0.138±0.006 | 0.149±0.005 | 0.157±0.005 |
| Liver wt, g | 1.211±0.020 | 1.039±0.029* | 1.273±0.045 | 1.182±0.06† |
| Heart wt/body wt, g/100 g | 0.45±0.01 | 0.47±0.01 | 0.47±0.01 | 0.46±0.01 |
| Lung wt/body wt, g/100 g | 0.54±0.01 | 0.59±0.01 | 0.53±0.03 | 0.57±0.02 |
| Liver wt/body wt, g/100 g | 4.61±0.13 | 4.45±0.12 | 4.49±0.08 | 4.32±0.21 |
Values are means ± SE; n, number of animals exposed to intermittent hypoxia (IH) or intermittent room air (IA) for 4 wk.
P < 0.01 vs. IA.
P < 0.05 and
P < 0.01 vs. IH.
Echocardiographic and PV loop analyses.
Figure 1 shows representative echocardiograms from four animals exposed to 4 wk of IH or IA. There were no detectable differences in contractility, diastolic dimensions, or wall thickness of the LV between any of the animals. Group data also indicate no significant differences in fractional shortening, ejection fraction, LV EDD, EDV, or posterior wall thickness in mice exposed to 4 wk of IH compared with the IA control group (Table 3). HR was in the physiological range for light anesthesia and was comparable between the two groups during echocardiographic assessment.
Fig. 1.
Representative echocardiograms from 2 mice exposed to intermittent hypoxia (IH; right) or intermittent air (IA; left). M-mode short-axis images show no differences between IH- and IA-exposed mice.
Table 3.
Echocardiographic assessment of LV function in animals exposed IH or IA with or without β-adrenergic blockade
| IA (n = 13) | IH (n = 15) | IA + Propranolol (n = 9) | IH + Propranolol (n = 9) | |
|---|---|---|---|---|
| Heart rate, beats/min | 509±11 | 506±12 | 424±8* | 430±17† |
| EDD, mm | 0.385±0.01 | 0.378±0.014 | 0.383±0.01 | 0.381±0.003 |
| ESD, mm | 0.233±0.013 | 0.231±0.013 | 0.264±0.016 | 0.264±0.007 |
| EDV, μl | 0.143±0.011 | 0.143±0.015 | 0.143±0.01 | 0.139±0.003 |
| ESV, μl | 0.033±0.005 | 0.037±0.006 | 0.052±0.008 | 0.049±0.003 |
| Fractional shortening, % | 39.9±1.5 | 39.5±1.5 | 29.7±2.8* | 30.6±1.5† |
| Posterior wall thickness, mm | 0.076±0.01 | 0.078±0.01 | 0.074±0.004 | 0.072±0.002 |
| Ejection fraction, % | 76.4±1.8 | 76.1±1.7 | 64.6±4.0* | 64.7±2.1† |
Values are means ± SE; n, number of animals exposed to IH or IA for 4 wk. LV, left ventricular; EDD, end-diastolic dimension; ESD, end-systolic dimension; EDV, end-diastolic volume; ESV, end-systolic volume.
P < 0.01 vs. IA.
P < 0.01 vs. IH.
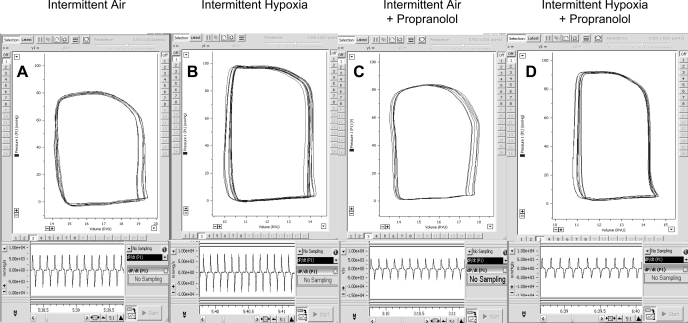
Representative traces of PV loops from animals exposed to IH and IA in the presence or absence of propranolol are shown in Figure 2. The steady-state PV loop shows a higher maximal LV systolic pressure and the pressure vs. time trace shows a greater maximum first derivative of ventricular pressure with respect to time (dP/dtmax) in the IH-exposed mouse without propranolol than in the IA-exposed mouse (Fig. 2, A and B).
Fig. 2.
Representative traces of steady-state pressure-volume loops (top) and maximum 1st derivative of ventricular pressure (dP/dtmax; bottom) in individual mice exposed to IA in the absence (A) and presence (C) of propranolol and in mice exposed to IH in the absence (B) and presence (D) of propranolol.
For pooled data, HR and parallel conductance were comparable between the two groups during the PV loop analyses (Table 4). LV ejection fraction (P = 0.015), dP/dtmax (P = 0.035), and dP/dt at 40 mmHg LV pressure (P = 0.055; strong trend) were >10% higher in the IH group than in the IA control group (Table 4). Furthermore, even when LV function was examined independent of preload (LV preload-adjusted maximum power), contractility was greater in the IH group than the IA control group (Table 4; P = 0.040).
Table 4.
PV loop assessment of LV function in animals exposed IH or IA with or without β-adrenergic blockade
| IA (n = 13) | IH (n = 15) | IA + Propranolol (n = 9) | IH + Propranolol (n = 9) | |
|---|---|---|---|---|
| Heart rate, beats/min | 543±14 | 564±15 | 461±11c | 466±16e |
| Parallel conductance, μl | 34.9±1.0 | 35.2±1.1 | 31.8±1.1 | 32.5±1.1d |
| Maximal pressure, mmHg | 90.7±2.5 | 97.6±2.3 | 80.4±3.1 | 88.8±3.6 |
| dP/dtmax, mmHg/s | 6,951±524 | 8,698±484a | 4,843±285b | 6,072±448e |
| dP/dt@LVP40, mmHg/s | 6,556±579 | 8,398±627 | 4,600±223 | 5,772±430e |
| dP/dtmin, mmHg/s | −7,825±519 | −9,152±533 | −5,158±350c | −6,093±412e |
| End-diastolic pressure, mmHg | 4.35±0.45 | 5.74±0.90 | 3.34±0.17 | 4.78±0.99 |
| EDV, μl | 22.7±1.3 | 19.8±1.6 | 31.2±4.0 | 25.3±2.5 |
| ESV, μl | 13.0±1.2 | 8.8±1.3 | 18.2±2.9 | 13.3±1.5 |
| Stroke volume, μl | 11.6±0.5 | 12.7±0.6 | 15.3±1.5 | 13.6±1.7 |
| Cardiac output, μl/min | 6,294±213 | 7,169±384 | 7,107±771 | 6,246±687 |
| Stroke work, mmHg·μl | 829±48 | 951±54 | 982±74 | 985±122 |
| Arterial elastance, mmHg/μl | 7.63±0.34 | 7.56±0.43 | 6.12±0.74 | 7.21±1.0 |
| Ejection fraction, % | 50.5±2.6 | 63.4±3.5a | 48.5±2.2 | 51.2±3.6d |
| Preload-adjusted maximal power, mW/μl2 | 136±16 | 228±29a | 68.6±8.9 | 135±37 |
| τ, ms | 6.79±0.32 | 6.32±0.28 | 8.59±0.39c | 8.43±0.47e |
Values are means ± SE; n, number of animals exposed to IH or IA for 4 wk. PV, pressure-volume; dP/dt, 1st derivative of LV pressure (LVP) with respect to time; τ, time constant.
P < 0.05 vs. IA.
P < 0.05 and
P < 0.015 vs. IA.
P < 0.05 and
P < 0.015 vs. IH.
Ventricular β-adrenergic receptor mRNA and cAMP.
There was no statistically significant difference (n = 6 for each group) in the level of β1-, β2-, or β3-adrenergic receptor mRNA [CT = 9.27 ± 0.25 vs. 10.52 ± 0.77 (P = 0.17), 12.79 ± 0.18 vs. 14.03 ± 0.86 (P = 0.21), and 17.64 ± 0.33 vs. 18.24 ± 0.69 (P = 0.92), respectively] between the IH-exposed and the control IA-exposed mice. To further assess activation of the β-adrenergic signaling pathway, we measured cAMP level in the ventricular homogenate. We observed a significant increase in cAMP levels in mice exposed to 4 wk of IH compared with the IA control group (3.30 ± 0.39 vs. 1.98 ± 0.20 pmol/μg protein, n = 8 for each group, P = 0.013).
Cardiac gene expression level.
There were no significant differences (n = 3–6 for each group) in the mRNA levels of sarco(endo)plasmic reticulum Ca2+-ATPase (CT = 17.14 ± 1.32 vs. 17.20 ± 0.83, P = 0.97), ryanodine receptor (CT = 20.53 ± 0.73 vs. 22.47 ± 0.88, P = 0.17), α-MHC (CT = 8.94 ± 0.34 vs. 8.95 ± 0.20, P = 0.98), β-MHC (CT = 13.76 ± 2.28 vs. 13.65 ± 0.88, P = 0.36), brain natriuretic peptide (CT = 4.08 ± 0.30 vs. 3.60 ± 0.27, P = 0.26), or ANP (CT = 6.15 ± 0.75 vs. 6.32 ± 0.26. P = 0.72) between IH- and IA-exposed animals.
Effect of in vivo blockade of β-adrenergic receptors on response to IH.
Efficacy of the 4-wk administration of propranolol by miniosmotic pump to block cardiac β-adrenergic activity was evident from an ∼80 beat/min reduction in HR (P = 0.0028), a >2,000 mmHg/s decrease in dP/dtmax (P = 0.025), and strong trends for a 9-μl increase in EDV (P = 0.062) and a >3-μl increase in stroke volume (P = 0.067) in the IA-exposed mice treated with propranolol compared with untreated IA-exposed mice (Table 4). Echocardiography also showed a significant decrease in HR (P = 0.0003), fractional shortening (P = 0.002), and ejection fraction (P = 0.0017) in the IA-exposed mice treated with propranolol compared with untreated IA-exposed mice (Table 3). The effect of IH to increase cardiac contractility, as assessed by dP/dtmax, ejection fraction, and preload-adjusted maximal power, was attenuated by propranolol treatment in IH-exposed mice (Table 4). There was no significant difference in ejection fraction, LV preload-adjusted maximum power, dP/dtmax, or dP/dt at 40 mmHg LV pressure between IA- and IH-exposed mice treated with propranolol. There was also no evidence from echocardiography of a difference in contractility or in the presence of ventricular hypertrophy in the propranolol-treated IA- and IH-exposed mice (Table 3). The cAMP levels in mice treated with propranolol exposed to 4 wk of IH were not different from those in the IA control group (1.04 ± 0.05 vs. 1.11 ± 0.03 pmol/μg protein, n = 9 for each group).
DISCUSSION
In contrast to our original hypothesis, we determined by PV loop analyses that 4 wk of exposure to IH caused an increase in LV contractility in C57BL/6J mice. This chronic IH exposure was associated with a nonsignificant trend for an increase in LV maximal pressure that is comparable in magnitude to that described in our previous study showing systemic hypertension after 5 wk of IH exposure in C57BL/6J mice (3) and is consistent with an elevation of peripheral sympathetic nerve activity reported in patients with OSA (37) and in animal models of IH (18). The importance of a specific augmentation of cardiac sympathetic activity as a mechanism contributing to our observed increase in cardiac contractility in response to chronic IH exposure was demonstrated by 1) increased cardiac cAMP levels, indicating activation of β-adrenergic signaling pathways, and 2) attenuation of the IH-induced LV hypercontracility by long-term administration of the β-blocker propranolol. Thus, in a mouse model of IH, there is a compensatory increase in LV cardiac contractility that, at least in part, results from activation of cardiac β-adrenergic pathways.
The only previous research that has focused on the effects of IH exposure on cardiac function in a rodent model of IH are the rat studies of Chen et al. (4, 5). In the studies of Chen et al., 5 or 6 wk of exposure resulted in decreased LV fractional shortening and increased LV dilation determined by echocardiography and decreased LV dP/dtmax determined by a micromanometer-tipped catheter. The changes in fractional shortening were similar in control and IH mice at 3 wk and were only different at 5 wk. On the basis of these data from Chen et al., we expected similar compromise of LV function in mice exposed to IH for 4 wk. However, we observed an improved LV function in C57BL/6J mice, although no increase in ejection fraction was evident by echocardiography, leading us to speculate what differences between the studies may account for the disparate findings.
The first and most obvious explanation is a species-related difference. It is possible that, for a given regimen of IH, rats are more vulnerable to hypoxia-related pathology. For example, there are reports that exposure to a 10% nadir of inspired O2 can produce systemic hypertension in rats (40), but there are no comparable observations in mice. In general, we have found it difficult to induce pathology in mice with a 10% nadir (20) and routinely use 5–6% for the majority of our studies. In addition to any species-related hypoxic susceptibility, the studies of Chen et al. (4, 5) utilized a relatively severe IH regimen with 60-s periods of hypoxia that reached a nadir of 4–5% that was maintained for 15–20 s. Arterial Po2 was reported to be 44 ± 8 Torr during this 15- to 20-s nadir of inspired O2, although these data were collected in anesthetized animals. We reported recently (17) that arterial Po2 reached 47 ± 2 Torr during the transient nadir of 6% O2 in our murine model in conscious chronically instrumented animals. If both of these studies are taken on face value, it is apparent that the rats in the study of Chen et al. were exposed to longer periods of slightly more severe hypoxia than the mice in our study.
Mechanisms of increased LV contractility.
The increase in LV contractility that we observed over 4 wk of exposure to IH was unexpected. However, there is evidence that cardiac outcomes produced by 4 wk of IH exposure in mice can be different from those produced by a shorter 2-wk exposure. Park and Suzuki (28) demonstrated, in the same strain of mice used in our study, that the magnitude of myocardial injury in response to experimentally induced cardiac ischemia was significantly less in mice exposed to 4 wk of IH than in mice exposed to 1 or 2 wk of IH. Their data suggest that some form of cardiac compensatory response may be occurring as a result of IH exposure. However, the mechanisms proposed by Park and Suzuki to explain reduced ischemic injury after IH exposure, including activation of antiapoptotic and antioxidant pathways, are unlikely to account for the increased LV contractility that we report in IH-exposed mice with normal cardiac function.
Potentially, structural changes in the contractile proteins of cardiac myocytes or sustained activation of cardiac sympathetic adrenergic pathways in response to IH could lead to a compensatory increase in LV contractility. We assessed changes in protein isoform expression with respect to the myofilament lattice proteins, but we found no difference in α-MHC and β-MHC mRNA levels between heart muscle from mice exposed to IH and heart muscle from IA control mice. There was also no evidence based on ANP levels, echocardiographic assessment of LV posterior wall thickness, or heart weights for the presence of ventricular hypertrophy. In contrast, it is well known that, in OSA patients or rodents exposed to IH, there is a chronic increase in sympathetic nerve activity to the peripheral vasculature that can account for the development of sustained systemic hypertension (3, 9, 14, 15, 19, 37). It is possible that a similar hyperadrenergic response may also occur in the heart of OSA patients or animals exposed to IH. Indeed, in OSA patients, the increase in low-frequency HR variability and the ratio of low- to high-frequency HR variability suggest an increase in cardiac sympathetic drive (12, 35). These changes have been shown to correlate with the severity of OSA (29) and improve with nasal continuous positive-pressure therapy (34).
Our own data suggest that an increase in LV contractility is associated with an elevated level of cAMP in the ventricular homogenate, consistent with augmented adrenergic activity in cardiac tissue. Measurement of β-adrenergic receptor mRNA levels in mice exposed to 4 wk of IH only showed a weak trend toward an increase in β1-adrenergic receptor mRNA, the predominant adrenergic receptor in the heart modulating contractility. However, we did not measure the β-adrenergic receptor activity level, nor did we measure catecholamines in the circulation or the myocardium, which have been shown previously to be elevated in rodents exposed to IH (7, 8). Importantly, in vivo pharmacological blockade of β-adrenergic receptors effectively attenuated the increase in PV loop-assessed ejection fraction in untreated mice exposed to IH. Taken together, these data suggest that, at least in part, increased activation of cardiac β-adrenergic pathways contributes to a hypercontractile state of the LV after chronic exposure to IH.
Although β-adrenergic agonists increase cardiac contractility in the short term, clinical studies suggest that long-term use of medications such a dobutamine may increase cardiac mortality in patients with advanced heart failure (27). We, therefore, cannot exclude the possibility that, with a more prolonged exposure to IH and subsequent augmentation of β-adrenergic activity, LV function will ultimately deteriorate. Indeed, in the clinical setting, the increase in sympathetic activation that occurs with OSA can be present over many years and may contribute to the elevated cardiovascular mortality reported in this population (23).
In summary, contrary to our expectations, our model of chronic exposure to IH in C57BL/6J mice produced an increase in PV loop-assessed LV contractility. This hypercontractile state was associated with elevated cAMP levels and was attenuated with propranolol, suggesting a chronic activation of sympathetic adrenergic pathways to the heart, rather than the development of ventricular hypertrophy. However, further studies are needed to investigate whether the longer-term consequences of chronically augmented β-adrenergic activity to the heart may ultimately lead to pathology.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-077785.
REFERENCES
- 1.Boluyt MO, Long X, Eschenhagen T, Mende U, Schmitz W, Crow MT, Lakatta EG. Isoproterenol infusion induces alterations in expression of hypertrophy-associated genes in rat heart. Am J Physiol Heart Circ Physiol 269: H638–H647, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol 99: 2028–2035, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Zhang J, Gan TX, Chen-Izu Y, Hasday JD, Karmazyn M, Balke CW, Scharf SM. Left ventricular dysfunction and associated cellular injury in rats exposed to chronic intermittent hypoxia. J Appl Physiol 104: 218–223, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Chernogubova E, Hutchinson DS, Nedergaard J, Bengtsson T. Alpha1- and beta1-adrenoceptor signaling fully compensates for β3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology 146: 2271–2284, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Levy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med 177: 227–235, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 20: 612–619, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher EC, Lesske J, Qian W, Miller CC 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Gaussin V, Tomlinson JE, Depre C, Engelhardt S, Antos CL, Takagi G, Hein L, Topper JN, Liggett SB, Olson EN, Lohse MJ, Vatner SF, Vatner DE. Common genomic response in different mouse models of β-adrenergic-induced cardiomyopathy. Circulation 108: 2926–2933, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol Heart Circ Physiol 274: H1416–H1422, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Guilleminault C, Poyares D, Rosa A, Huang YS. Heart rate variability, sympathetic and vagal balance and EEG arousals in upper airway resistance and mild obstructive sleep apnea syndromes. Sleep Med 6: 451–457, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 175: 851–857, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imadojemu VA, Gleeson K, Gray KS, Sinoway LI, Leuenberger UA. Obstructive apnea during sleep is associated with peripheral vasoconstriction. Am J Respir Crit Care Med 165: 61–66, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest 131: 1406–1413, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Joyeux-Faure M, Stanke-Labesque F, Lefebvre B, Beguin P, Godin-Ribuot D, Ribuot C, Launois SH, Bessard G, Levy P. Chronic intermittent hypoxia increases infarction in the isolated rat heart. J Appl Physiol 98: 1691–1696, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Lee EJ, Woodske ME, Zou B, O'Donnell CP. Dynamic arterial blood gas analysis in conscious, unrestrained C57BL/6J mice during exposure to intermittent hypoxia. J Appl Physiol 107: 290–294, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia—influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol 79: 581–588, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol 102: 557–563, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Makikallio K, Rounioja S, Vuolteenaho O, Paakkari J, Hallman M, Rasanen J. Fetal cardiac natriuretic peptide expression and cardiovascular hemodynamics in endotoxin-induced acute cardiac dysfunction in mouse. Pediatr Res 59: 180–184, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005. [DOI] [PubMed] [Google Scholar]
- 24.McGaffin KR, Sun CK, Rager JJ, Romano LC, Zou B, Mathier MA, O'Doherty RM, McTiernan CF, O'Donnell CP. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc Res 77: 54–63, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Neidhold S, Eichhorn B, Kasper M, Ravens U, Kaumann AJ. The function of α- and β-adrenoceptors of the saphenous artery in caveolin-1 knockout and wild-type mice. Br J Pharmacol 150: 261–270, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Connor CM, Gattis WA, Uretsky BF, Adams KF Jr, McNulty SE, Grossman SH, McKenna WJ, Zannad F, Swedberg K, Gheorghiade M, Califf RM. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J 138: 78–86, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Park AM, Suzuki YJ. Effects of intermittent hypoxia on oxidative stress-induced myocardial damage in mice. J Appl Physiol 102: 1806–1814, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Park DH, Shin CJ, Hong SC, Yu J, Ryu SH, Kim EJ, Shin HB, Shin BH. Correlation between the severity of obstructive sleep apnea and heart rate variability indices. J Korean Med Sci 23: 226–231, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patrizio M, Musumeci M, Stati T, Fecchi K, Mattei E, Catalano L, Marano G. Propranolol promotes Egr1 gene expression in cardiomyocytes via β-adrenoceptors. Eur J Pharmacol 587: 85–89, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med 7: 7–16, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Rajan S, Ahmed RP, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, Arteaga GM, Wolska BM, Solaro RJ, Liggett SB, Wieczorek DF. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res 101: 205–214, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Roche F, Court-Fortune I, Pichot V, Duverney D, Costes F, Emonot A, Vergnon JM, Geyssant A, Lacour JR, Barthelemy JC. Reduced cardiac sympathetic autonomic tone after long-term nasal continuous positive airway pressure in obstructive sleep apnoea syndrome. Clin Physiol 19: 127–134, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Roche F, Gaspoz JM, Court-Fortune I, Minini P, Pichot V, Duverney D, Costes F, Lacour JR, Barthelemy JC. Screening of obstructive sleep apnea syndrome by heart rate variability analysis. Circulation 100: 1411–1415, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol 37: 7–11, 1976. [DOI] [PubMed] [Google Scholar]
- 40.Thongboonkerd V, Gozal E, Sachleben LR Jr, Arthur JM, Pierce WM, Cai J, Chao J, Bader M, Pesquero JB, Gozal D, Klein JB. Proteomic analysis reveals alterations in the renal kallikrein pathway during hypoxia-induced hypertension. J Biol Chem 277: 34708–34716, 2002. [DOI] [PubMed] [Google Scholar]