Abstract
Exercise training (EX) following percutaneous transluminal coronary angiography (PTCA) reduces progression to restenosis and increases event-free survival rates. Our aim was to determine whether EX inhibits lesion development and/or alters the extracellular matrix (ECM) composition of the neointima (NI) in a porcine PTCA model. Miniature Yucatan swine were assigned to cage confinement (SED) or EX for 20 wk. After 16 wk, all animals underwent a PTCA procedure of the left anterior descending artery (LAD) and left circumflex artery (LCX), with subsequent placement of an externalized jugular catheter. Animals recovered for 2 days and then resumed the previous protocol of SED or EX. Twelve days following PTCA, all animals received an intravenous bromodeoxyuridine (BrdU) injection to label proliferating cells. At 28 days following PTCA, the animals were euthanized, the LAD and LCX excised, and underwent standard histological processing for total collagen, type I collagen, fibronectin, BrdU, and Verhoeff-van Gieson stain. Our results demonstrate that EX significantly decreased lesion size and NI proliferation (−48%) in the LAD (P < 0.05) but not the LCX. Furthermore, EX attenuated type I collagen expression only in LAD, whereas total collagen was increased (5.9%) and fibronectin was decreased (−7.9%) in the NI of both vessels (P < 0.05). In conclusion, EX following PTCA may increase event-free survival rates following PTCA by decreasing lesion size and altering ECM composition.
Keywords: proliferation, extracellular matrix
exercise training (EX) has been shown to increase event-free survival rates and inhibit restenosis progression in humans (3) and inhibit neointimal formation in rodents (18, 24, 34). Neointimal formation following angioplasty, both with and without stent deployment, is primarily due to smooth muscle proliferation, migration, and smooth muscle-derived matrix synthesis. Thus proliferation following percutaneous transluminal coronary angioplasty (PTCA) is a critical therapeutic target for the prevention of restenosis. We have previously shown that coronary smooth muscle (CSM) from exercise-trained swine exhibit an attenuated proliferative response to mitogens, in vitro (40). Accordingly, EX inhibited smooth muscle cell (SMC) proliferation in balloon-injured rat carotid arteries (18). Thus exercise attenuation of CSM proliferation may be a primary mechanism for the salutatory effects of exercise on restenosis following PTCA in humans.
Recent experimental evidence has elucidated the role of proinflammatory cytokines as a key component in the proliferation of intimal cells, resulting in expansion of the neointima (NI) following balloon injury (23, 28). Conversely, the putative anti-inflammatory molecule TGF-β1 has been shown to reduce NI formation, suggesting that there is a proinflammatory and anti-inflammatory balance that regulates neointimal formation after vascular injury (21). Despite reductions in intimal hyperplasia with EX, no studies have assessed the effects of EX on postangioplasty neointimal size, composition, and the circulating anti-inflammatory molecule TGF-β1. However, several studies have examined the effects of EX on circulating TGF-β1 in healthy humans and animals. These investigations have demonstrated acute increases in plasma concentrations of TGF-β1 with aerobic EX (7, 15, 16). Furthermore, collagen type I and fibronectin, two extracellular matrix (ECM) proteins, are elevated in the NI following injury (6, 9, 10, 13, 37, 43) and have been shown to stimulate SMC proliferation in vitro (17, 20, 35, 36). In atherosclerotic animals, the TGF-β family enhances total collagen expression in vascular lesions (27, 30). Although total collagen is enhanced, it is unknown whether TGF-β1 suppresses the upregulation of collagen type I and fibronectin, thus attenuating intimal proliferation and NI formation.
On the basis of the aforementioned findings, the purpose of the present study was to determine whether EX 1) inhibits proliferation and the ensuing NI formation and/or 2) alters ECM expression in porcine coronary arteries following PTCA. We hypothesized that EX training would decrease NI proliferation and lesion size and alter ECM expression in the NI as reflected by increased total collagen and decreased fibronectin and type I collagen. Additionally, we hypothesized that these effects would be associated with acute increases in circulating concentrations of the anti-inflammatory molecule TGF-β1.
MATERIALS AND METHODS
Animals.
Intact male Yucatan miniature swine (∼40 kg) were obtained from the breeder (Sinclair Farms) and housed in the College of Veterinary Medicine, University of Missouri, Columbia, MO. Animals were on a 12-h light/dark cycle, fed a normal chow diet, and had water available ad libitum. The pigs were randomly assigned to cage confinement (SED = 7) or exercise training (EX = 8) for 20 wk.
All protocols were approved by the University of Missouri's Animal Care and Use Committee.
Exercise training.
Before PTCA, the exercise-trained animals (n = 8) underwent a progressive treadmill endurance training program (4, 5, 14, 25, 26) consisting of the following: week 1, a 5-min warm up at 2 mph, sprint run at 4–5 mph for 15 min, endurance run at 3 mph for 25 min, and cool down at 2 mph for 5 min, performed 5 days/wk. The intensity and duration of exercise increased over 11 wk in a manner that was dependent on each animal. At week 12, the swine were running for 85 min a session, 5 days/wk. The warm up was 2.5 mph for 5 min, sprint training at 6–7 mph for 15 min, endurance training at 4–5 mph for 60 min, and cool down at 2.5 mph for 5 min. This protocol was maintained until week 16. At week 16 all animals (SED and EX) underwent a PTCA with externalized jugular catheter placement (see Surgery). Following the surgical intervention, the exercise-trained animals recovered for 2 days in their pen. On the third day, the pigs were reintroduced to exercise training at an intensity and duration level dependent on the animal's ability. The intensity and duration was increased until the presurgery intensity was resumed (typically within 3–5 days). This protocol was maintained until week 20 when the animals were euthanized. A range of speeds are given because of the variable exercise capacities of the animals.
Surgery.
All swine were subject to a PTCA with subsequent externalized jugular catheter placement. Swine were sedated with an intramuscular injection (5 mg/kg telozol, 2.2 mg/kg xylazine, and 0.5 mg/kg atropine), intubated, and anesthetized with 2% isoflurane (AErrane, Baxter) and 1 l/min oxygen via mechanical ventilation (Mallard Medical) maintained anesthesia. Excede (5 mg/kg) was given intramuscularly. A catheter was placed in a vein of the ear to introduce fluids (lactated Ringers) and heparin. Heart rate, blood pressure, temperature, and oxygen saturation were monitored during the procedure. A right femoral artery cut down was performed, the artery exposed, and a 7F introducer inserted. Heparin (300 U/kg) was administered following femoral access and maintenance doses given every hour (100 U/kg). A guide catheter (6F) was directed up the aorta and engaged into the left ostium under fluoroscopic guidance. The left circumflex (LCX) artery was selectively engaged, and contrast dye (Visipaque 320 mgI/ml; Amersham Health) was injected to obtain angiograms. The artery was visualized with fluoroscopy, and arterial diameter was determined by quantitative angiography (Infimed) 2 min after intracoronary nitroglycerin. To induce injury, a 1.3–1.4:1.0 overinflation was performed using a standard balloon catheter (Maverick, Boston Scientific) directed into the selected site. The balloon catheter was inflated three times for 30 s at the target pressure, with a 1-min break between inflations. The angioplasty procedure was then repeated for the left anterior descending (LAD) artery.
Following PTCA and surgical closure of the leg, an externalized jugular catheter was placed to allow for blood draws and injections (see Bromodeoxyuridine injections). The right jugular vein was exposed, and an autoclaved catheter (Saint-Gobain Tygon tubing, formulation S-54-HL, Fisher Scientific; ID = 0.050 in., OD = 0.090 in.) was inserted and positioned just cranial to the heart in the vena cava. The catheter was externalized on the midline of the dorsal aspect of the neck with a custom-made trocar. Postsurgery animals received rimadyl (3 mg/kg) and buprenophine (0.01 ml/kg) injections. Aspirin (365 mg) was given the day before, the day of, and the day following surgery, with a maintenance dose (162 mg) given daily thereafter. Animals were euthanized on day 28 following surgery. The heart was surgically removed, under anesthesia, as approved by the Panel on Euthanasia of the American Veterinary Medical Association.
Bromodeoxyuridine injections.
To label proliferating cells in vivo bromodeoxyuridine (BrdU −30 mg/kg), dissolved in lactated Ringer's (30 mg/ml), was injected twice, 12 h apart, on day 12 postinjury. Injections were performed on day 12 to label proliferating cells within the expanding NI (29, 38).
Blood sampling.
Blood was sampled from both EX and SED animals on day 21 following PTCA. The EX animals were placed on a treadmill with blood sampled immediately before exercise, at peak exercise, and 1, 2, and 4 h postexercise. The SED animals were placed on the treadmill, had blood withdrawn, and were returned to their pen without exercising.
Plasma concentrations of anti-inflammatory molecules.
To assess plasma concentrations of TGF-β1, samples were spun down in tubes containing EDTA at 1,000 g for 20 min. The plasma was collected and stored at −80°C until it was assayed. Plasma was assayed according to the manufacturer's protocol for porcine-specific TGF-β1 ELISA kit (R & D Systems). A standard curve was plotted, and concentrations were calculated from the curve.
Morphology.
Following euthanasia, the LAD and LCX coronary arteries were dissected out and fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned (5 μm). Sections were stained with Verhoeff-van Gieson (VVG) elastic stain to quantify lesion size. After the staining with VVG, intimal/medial thickness (IMT) was determined by measuring the thickest point of the NI (intima) and the uninjured medial wall opposing the injury. The IMT was normalized to the rupture index (RI), where RI = medial dissection distance/internal elastic lamina distance, and the data were presented as IMT/RI.
Immunohistochemistry.
Paraffin-embedded sections were deparaffinized and manually stained. Tris buffer and water wash were utilized after each step. Avidin/biotin blocking solution (Vector SP-2001) inhibited background staining, and endogenous peroxidase activity was quenched with 3% hydrogen peroxide. Nonspecific protein binding was inhibited with non-serum protein block (Dako). Primary antibodies, BrdU (1:200, Zymed), fibronectin (1:800, Dako), scavenger receptor A (SRA; 1:100, Transgenic), and collagen type I (1:200, Abcam) were incubated overnight at 4°C. After a 5-min exposure to diaminobenzidine (Dako), the staining was visualized. BrdU- and SRA-stained slides were counterstained with hematoxylin to visualize the nuclei.
Picrosirius red was used to stain for collagen fibers. Following deparaffinization, 0.2% phosphomolybdic acid was applied for 2 min to stabilize aqueous hydrogen peroxide. Slides were rinsed in distilled water, and filtered picrosirius red was added for 110 min and rinsed; slides were then dehydrated and coverslipped.
Quantification.
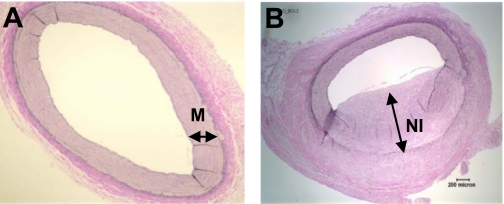
Digital photomicrographs were obtained using an Olympus BX60 photomicroscope, and quantification was performed with ImagePro software. Representative images are presented in Fig. 1 to demonstrate where the quantitative analysis was performed on the uninjured media and the NI of the LAD and LCX. To quantify the BrdU and SRA stainings, three regions of interest were counted three times each with the data expressed as mean percentages (BrdU-positive cells/total cells × 100).
Fig. 1.
Representative regions where quantitative analysis was performed. Representative images of an uninjured coronary artery (A) and injured coronary artery 28 days following percutaneous transluminal coronary angiography (PTCA) (B) stained with Verhoeff-van Gieson. Uninjured media (M) and neointima (NI) where analysis was performed is demarcated by a double-headed arrow.
To determine total collagen (types I and III; nonpolarized) and fibronectin density, the entire region of interest (uninjured media or NI) was quantified. A pixel-by-pixel analysis was performed with a predetermined and optimized red-green-blue color model (ImagePro software). The density for each slide was normalized to a positive control (kidney or bone) stained on the same day.
Statistical analysis.
Data are presented as means ± SE. Statistical analysis was performed with Sigma Stat 3.5 software. A two-way ANOVA was used to analyze all histological data. Post hoc analysis was performed with the Bonferroni t-test.
RESULTS
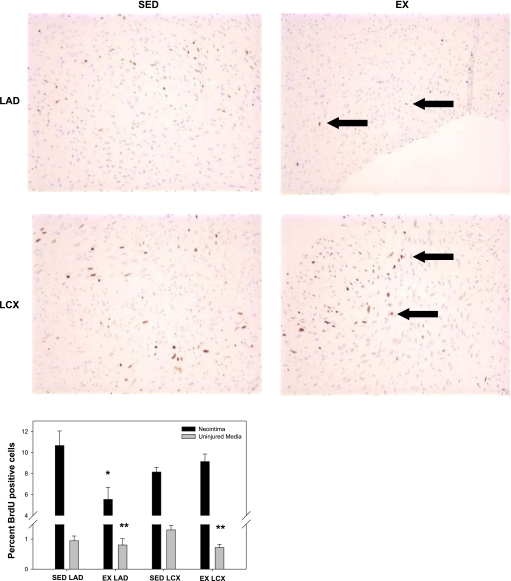
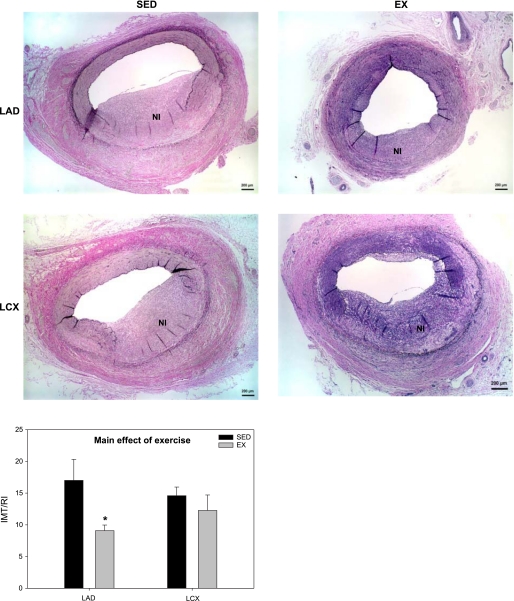
To determine the effects of exercise training on proliferation, BrdU was administered intravenously on day 12 to label mitotic cells in the NI and uninjured media. Neointimal formation following PTCA was associated with a large increase in cell proliferation (Fig. 2). EX inhibited NI proliferation in the LAD (5.5 ± 1.1% vs. 10.7 ± 1.4%; P < 0.05; Fig. 2) but not LCX (9.1 ± 0.7% vs. 8.1 ± 0.5%; Fig. 2). Furthermore, EX inhibited medial SMC proliferation in uninjured vessels (Fig. 2; main effect of EX; P < 0.05). A similar trend was observed with regard to NI formation. Morphological analysis revealed a main effect of EX to inhibit lesion size (Fig. 3; P < 0.05). However, the preponderance of the exercise effect occurred in the LAD (Fig. 3; P < 0.1). Taken collectively, these data demonstrate that EX attenuates NI proliferation, resulting in reduced lesion size in the LAD, not the LCX.
Fig. 2.
Exercise training (EX) inhibits proliferation in NI and uninjured media. Bromodeoxyuridine (BrdU) was administered intravenously on day 12 to assess proliferation in the vascular wall. EX attenuated proliferation in the NI of the left anterior descending artery (LAD) but not in the left circumflex artery (LCX). EX also had a main effect to inhibit proliferation in the uninjured medial sections vs. cage-confined (SED) sections. *P < 0.05 vs. SED LAD in NI, **P < 0.05, main effect of exercise in uninjured media (n = 5–8 for all conditions). Black arrows are pointing to the brown stained cells positive for BrdU.
Fig. 3.
Exercise training attenuates lesion size following PTCA. EX demonstrated a significant reduction in lesion size intimal/medial thickness/rupture index (IMT/RI; P < 0.05, main effect of EX), with an interaction within the LAD (*P < 0.1, EX LAD vs. SED LAD) (n = 5–8 for all conditions).
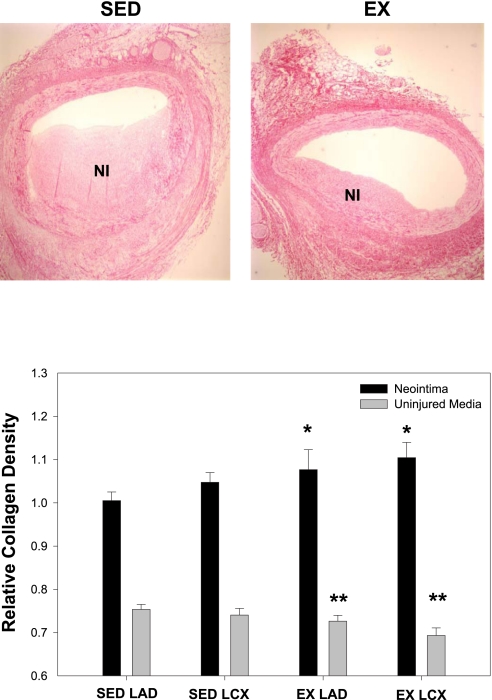
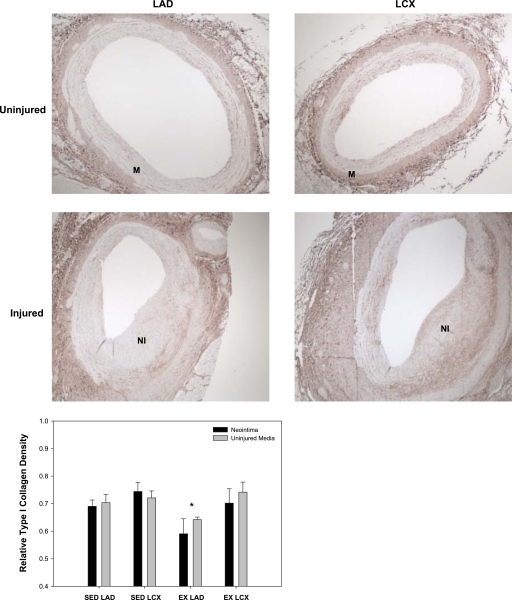
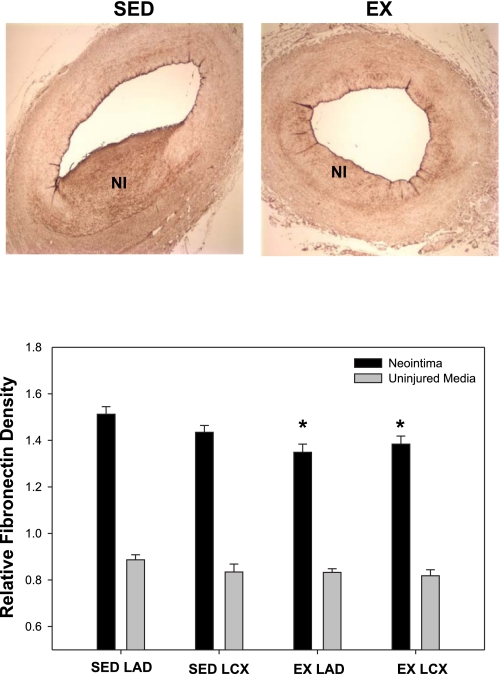
To investigate the effects of exercise training on ECM expression, total collagen, type I collagen, and fibronectin densities were assessed in the NI and uninjured media. Total collagen density was significantly increased in the NI of the EX animals compared with the SED group (Fig. 4; main effect; P < 0.05). Conversely, EX attenuated collagen density in the uninjured media compared with the SED (Fig. 4; main effect; P < 0.05). Total collagen density was unaffected by vessel type. Type I collagen was assessed to ascertain whether decreases in this ECM protein correlates with decreased proliferation and lesion size. Interestingly, no significant differences were observed between the NI and uninjured media for all conditions, indicating that type I collagen was unaffected by injury. Therefore, the NI and uninjured media data were pooled for each condition, and statistical analysis was performed on the pooled data. Our results demonstrate an effect of EX to decrease type I collagen in the combined NI and uninjured media in the LAD compared with the LCX (Fig. 5; P < 0.05), indirectly supporting a role for type I collagen in SMC proliferation (17). Furthermore, EX modestly decreased fibronectin expression in the NI with no change in the uninjured media (Fig. 6; main effect; P < 0.05). Collectively, these data demonstrate that EX alters ECM composition in both the normal vascular wall and NI formation following PTCA.
Fig. 4.
Total collagen density is altered with EX. Total collagen density within the NI was significantly increased with EX, whereas EX attenuated collagen expression in the uninjured media. *P < 0.05, main effect of exercise in NI; **P < 0.05, main effect of exercise in uninjured media (n = 5–8 for all conditions).
Fig. 5.
EX inhibits type I collagen expression in the LAD. Type I collagen density was significantly reduced with EX in the LAD, but no effect was observed in the LCX of EX animals. *P < 0.05 vs. EX LCX (n = 13–16 for all conditions; NI and uninjured data pooled for each vessel and condition).
Fig. 6.
Neointimal fibronectin is reduced with EX. EX attenuated fibronectin density within the NI, with no effect on the uninjured media. *P < 0.05, main effect of exercise (n = 5–8 for all conditions).
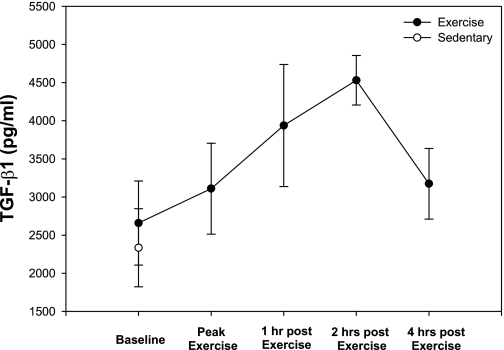
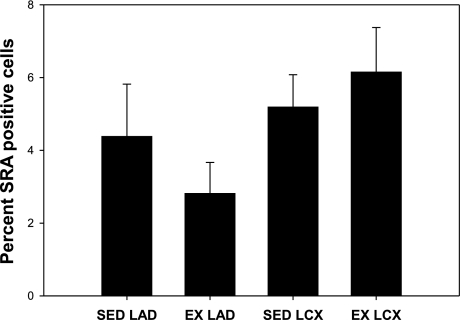
To assess the potential role of circulating anti-inflammatory cytokines in mediating the EX effects on coronary NI and ECM changes, blood was sampled before, immediately after, and 1, 2, and 4 h postexercise. Our results demonstrate a peak in circulating TGF-β1 concentrations 2 h postexercise that returned to near preexercise concentrations by hour 4 (Fig. 7). To determine whether EX had an effect on inflammation in the vascular wall, macrophage infiltration was determined histologically as SRA-positive cells. Our data demonstrate that EX tended to decrease macrophage accumulation in the LAD but not the LCX (Fig. 8). These results demonstrate that EX acutely increases circulating concentrations of TGF-β1 and may affect macrophage accumulation in the NI.
Fig. 7.
Acute increases of plasma TGF-β1 with exercise training. EX increased circulating concentrations of TGF-β1 2 h postexercise and returned to baseline concentrations by 4 h (n ≥ 4 at each time point).
Fig. 8.
EX tends to decrease macrophage accumulation in the NI of the LAD, not the LCX. Neointimal scavenger receptor A (SRA)-positive cells tend to be reduced in the LAD, but not the LCX of EX animals (n = 5–8 for all conditions).
DISCUSSION
Exercise training following PTCA attenuates restenosis progression and hospital readmissions and increases event-free survival (3). EX also has been shown to inhibit endothelin-1-induced coronary SMC proliferation (42), representing a potential mechanism for reduced NI growth following PTCA. Therefore, we hypothesized that EX would decrease lesion proliferation and size as well as favorably alter NI ECM composition, in association with acute increases in circulating anti-inflammatory molecule TGF-β1, as a potential mechanism for increased event-free survival following PTCA. The present investigation demonstrated for the first time in the coronary vasculature, the effects of EX to 1) inhibit neointimal proliferation and subsequent lesion development in the LAD but not the LCX, 2) inhibit SMC proliferation in the uninjured LAD and LCX, 3) increase total collagen in the NI of the LAD and LCX, 4) decrease total collagen expression in the uninjured media, 5) attenuate fibronectin expression in the NI of both coronary arteries, and 6) acutely elevate plasma concentrations of TGF-β1.
Attenuation of lesion size with swimming in the rat carotid has been ascribed to decreased medial and intimal SMC proliferation (18). In this present investigation, we directly assessed the in vivo effect of treadmill EX on coronary NI proliferation at a time when the NI was actively expanding. BrdU, a thymidine analog that incorporates into newly synthesized DNA (i.e., proliferating cells), was injected intravenously 12 days postinjury to label proliferating cells of the NI. Our results demonstrate inhibition of intimal proliferation by decreased incorporation of BrdU in the developing NI of LAD but not the LCX with EX. Furthermore, our data demonstrate a main effect of EX to inhibit lesion size with the preponderance of the EX inhibition in the LAD (46%) compared with the LAD (16%). These data collectively demonstrate an effect of EX training to inhibit intimal proliferation and lesion size in the LAD but not the LCX. Furthermore, EX significantly decreased proliferation in the uninjured vessels of both the LAD and LCX, supporting our previous findings of reduced SMC proliferation with EX (42). Taken together, these results demonstrate that EX exerts an overall inhibition of coronary SMC proliferation throughout the coronary vascular wall and inhibits NI growth; however, these effects are predominant in the LAD compared with the LCX.
Collagen provides tensile strength and is elevated in carotid lesions of EX-trained rodents following chemical injury (34). We observed a significant increase in total collagen in both the LAD and LCX arteries, supporting our hypothesis that EX increases total collagen in our coronary balloon injury model. We further hypothesized that EX would decrease type I collagen expression in the NI, thus attenuating intimal proliferation and the ensuing NI. In support of this, we found a significant attenuation of type I collagen in the LAD of EX animals, but not in the LCX, consistent with a role for type I collagen in SMC proliferation (17, 20, 35). Type I collagen, which contributes to arterial stiffness (2), also was attenuated in the uninjured medial sections in the LAD of EX animals, consistent with previous findings of an increase in arterial compliance with exercise (33). Recent evidence has demonstrated that normal physiological cyclic stretch decreases proliferation and matrix metalloproteinase (MMP) activation (12). Our data suggest that the EX-induced decrease in type I collagen could enhance elasticity of the LAD, potentially contributing to inhibition of proliferation and lesion formation.
Fibronectin is upregulated following vascular injury (6, 9) and has been shown to enhance SMC proliferation in vitro (36). Interestingly, EX inhibited the upregulation of fibronectin in both the LAD and LCX. However, whereas fibronectin was significantly reduced by EX in both vessels, proliferation was inhibited only in the LAD. These data indicate that either fibronectin synthesis does not play a major role in regulating NI SMC proliferation in vivo or that there are other contributing factor(s) unique to the LAD. Fibronectin may also alter lesion composition by attenuating the inflammatory response following PTCA. Inflammatory cells, including macrophages, infiltrate the vascular wall in response to PTCA (22, 32) and release cytokines, stimulating proliferation and migration of SMC. Fibronectin is associated with macrophage accumulation in atherosclerotic lesions (1, 8, 31, 40), suggesting that the EX-induced decrease in NI fibronectin may attenuate macrophage infiltration into the NI. However, we found no conclusive evidence that EX altered macrophage accumulation although a nonsignificant trend was observed for decreased macrophages within the NI of the LAD in the EX animals (Fig. 8).
TGF-β1 inhibits lesion size following balloon injury in animal models of PTCA (21). Moreover, EX is known to increase circulating levels of the anti-inflammatory molecule TGF-β1 in humans (7, 15, 16) and represents a potential mechanism by which EX could inhibit lesion size and alter ECM composition. Therefore, we assessed the effects of EX on the circulating concentrations of TGF-β1 following PTCA. Plasma levels of TGF-β1 increased 2 h postexercise, returning to near baseline levels at 4 h postexercise, indirectly supporting a role for acute increases of TGF-β1 with EX in altering ECM composition.
Although the reason(s) for the observed coronary artery specific effects of EX are currently unknown, a potential explanation for the inhibition of collagen type I in the LAD, but not the LCX, is nitric oxide (NO). Rodents undergoing chronic NO inhibition with nitro-l-arginine methyl ester have demonstrated elevated total collagen and type I mRNA in resistance vessels in the kidney (41), indicating that NO suppresses type I collagen expression. Although shear-induced NO release should increase during EX thereby inhibiting collagen type I expression, potentially lower laminar shear in the more torturous LCX may produce less NO compared with the relatively straight LAD, thus contributing to decreased type I collagen and attenuating NI proliferation and size in the LAD.
A second potential explanation for the differential effects of EX on NI formation in the coronary arteries is differential macrophage accumulation between the LAD and LCX. A trend was observed for EX to decrease macrophage accumulation in the LAD compared with the LCX (Fig. 8). It has been shown that attenuating macrophage infiltration with a neutralizing antibody for monocyte chemoattractant protein-1 following balloon injury attenuates lesion formation. Therefore, attenuation of macrophage accumulation in the NI is a potential mechanism by which EX mediates its artery-specific effects on lesion development. However, future studies designed to test this directly will need to be performed to provide a definitive mechanism for the vessel-specific effects of EX.
In conclusion, previous evidence demonstrates a role for anti-inflammatory molecule TGF-β1 (21) and EX to inhibit proliferation and restenosis following PTCA (18, 24, 34). However, no studies have examined the relationship between anti-inflammatory molecules and coronary restenosis during exercise following PTCA. Our results demonstrate that EX inhibits postangioplasty neointimal size and composition and inhibits SMC proliferation in a coronary artery-specific manner, with the EX response being predominant in the LAD compared with the LCX. Type I collagen, which stimulates SMC proliferation in vitro (17, 20, 35), was decreased in the LAD, but not in the LCX, suggesting that EX inhibits type I collagen expression, resulting in inhibition of proliferation and lesion size. EX significantly increased total collagen and decreased fibronectin expression within the NI, implying that decreased expression of fibronectin may enhance collagen expression via inhibition of MMPs (19, 39). Collectively, these data provide novel mechanistic insight regarding the beneficial EX-induced effects on neointimal lesion size composition following PTCA.
Although the present data demonstrate an ability of EX to attenuate NI expansion following PTCA, there are, as with any study, limitations. Firstly, PTCA without stent placement is used infrequently compared with PTCA with stent deployment; therefore similar studies should be performed with stent placement to verify the artery-specific effects of EX on in-stent stenosis in the coronary arteries. Secondly, the commonly used balloon injury protocol in swine free of coronary disease, as utilized in this study, contrasts to clinical PTCA with stent placement performed in patients with preexisting atherosclerotic lesions. These data need to be interrupted with caution when extrapolating our findings to conditions with preexisting atherosclerotic lesions and stent deployment.
GRANTS
This work was funded by NIH HL052490.
Acknowledgments
We gratefully thank Jan Ivey and Dave Harrah for technical assistance.
REFERENCES
- 1.Babaev VR, Porro F, Linton MF, Fazio S, Baralle FE, Muro AF. Absence of regulated splicing of fibronectin EDA exon reduces atherosclerosis in mice. Atherosclerosis 197: 534–540, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol 34: 513–525, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol 37: 1891–1900, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bowles DK, Hu Q, Laughlin MH, Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol 275: H2159–H2169, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bowles DK, Laughlin MH, Sturek M. Exercise training increases K+-channel contribution to regulation of coronary arterial tone. J Appl Physiol 84: 1225–1233, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Clausell N, de Lima VC, Molossi S, Liu P, Turley E, Gotlieb AI, Adelman AG, Rabinovich M. Expression of tumour necrosis factor alpha and accumulation of fibronectin in coronary artery restenotic lesions retrieved by atherectomy. Br Heart J 73: 534–539, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czarkowska-Paczek B, Bartlomiejczyk I, Przybylski J. The serum levels of growth factors: PDGF, TGF-Beta, and VEGF are increased after strenuous physical exercise. J Physiol Pharmacol 57: 189–197, 2006. [PubMed] [Google Scholar]
- 8.Dietrich T, Perlitz C, Licha K, Stawowy P, Atrott K, Tachezy M, Meyborg H, Stocker C, Gräfe M, Fleck E, Schirner M, Graf K. ED-B fibronectin (ED-B) can be targeted using a novel single chain antibody conjugate and is associated with macrophage accumulation in atherosclerotic lesions. Basic Res Cardiol 102: 298–307, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Dubin D, Peters JH, Brown LF, Logan B, Kent KC, Berse B, Berven S, Cercek B, Sharifi BG, Pratt RE, Dzau VJ, Van De Water L. Balloon catheterization induces arterial expression of embryonic fibronectins. Arterioscler Thromb Vasc Biol 15: 1958–1967, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Farb A, Kolodgie FD, Hwang JY, Burke AP, Tefera K, Weber DK, Wight TN, Virmani R. Extracellular matrix changes in stented human coronary arteries. Circulation 110: 940–947, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, Matsushima K, Sasayama S. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res 84: 306–314, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Gambillara V, Thacher T, Silacci P, Stergiopulos N. Effects of reduced cyclic stretch on vascular smooth muscle cell function of pig carotids perfused ex vivo. Am J Hypertens 21: 425–431, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Glukhova MA, Frid MG, Shekhonin BV, Vasilevskaya TD, Grunwald J, Saginati M, Koteliansky VE. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol 109: 357–366, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heaps CL, Bowles DK, Sturek M, Laughlin MH, Parker, LJ. Enhanced L-type Ca2+ channel current density in coronary smooth muscle of exercise-trained pigs is compensated to limit myoplasmic free Ca2+ accumulation. J Physiol (Lond) 528: 435–445, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinemeier K, Langberg H, Kjaer M. Exercise-induced changes in circulating levels of transforming growth factor-Ãü-1 in humans: methodological considerations. Eur J Appl Physiol 90: 171–177, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of TGF-β1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol 95: 2390–2397, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Hollenbeck ST, Itoh H, Louie O, Faries PL, Liu B, Kent KC. Type I collagen synergistically enhances PDGF-induced smooth muscle cell proliferation through pp60src-dependent crosstalk between the α2β1 integrin and PDGF-β receptor. Biochem Biophys Res Commun 325: 328–337, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Indolfi C, Torella D, Coppola C, Curcio A, Rodriguez F, Bilancio A, Leccia A, Arcucci O, Falco M, Leosco D, Chiariello M. Physical training increases eNOS vascular expression and activity and reduces restenosis after balloon angioplasty or arterial stenting in rats. Circ Res 91: 1190–1197, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Jacob SS, Shastry P, Sudhakaran PR. Monocyte-macrophage differentiation in vitro: modulation by extracellular matrix protein substratum. Mol Cell Biochem 233: 9–17, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Kato S, Shanley JR, Fox JC. Serum stimulation, cell-cell interactions, and extracellular matrix independently influence smooth muscle cell phenotype in vitro. Am J Pathol 149: 687–697, 1996. [PMC free article] [PubMed] [Google Scholar]
- 21.Kingston PA, Sinha S, Appleby CE, David A, Verakis T, Castro MG, Lowenstein PR, Heagerty AM. Adenovirus-mediated gene transfer of transforming growth factor-β 3, but not transforming growth factor-β1, inhibits constrictive remodeling and reduces luminal loss after coronary angioplasty. Circulation 108: 2819–2825, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu R, Ueda M, Naruko T, Kojima A, Becker AE. Neointimal tissue response at sites of coronary stenting in humans : macroscopic, histological, and immunohistochemical analyses. Circulation 98: 224–233, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Kusaba K, Kai H, Koga M, Takayama N, Ikeda A, Yasukawa H, Seki Y, Egashira K, Imaizumi T. Inhibition of intrinsic interferon-γ function prevents neointima formation after balloon injury. Hypertension 49: 909–915, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109: 220–226, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Laughlin MH, Muller JM. Vasoconstrictor responses of coronary resistance arteries in exercise-trained pigs. J Appl Physiol 84: 884–889, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Laughlin MH, Welshons WV, Sturek M, Rush JWE, Turk JR, Taylor JA, Judy BM, Henderson KK, Ganjam VK. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J Appl Physiol 95: 250–264, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Lutgens E, Gijbels M, Smook M, Heeringa P, Gotwals P, Koteliansky VE, Daemen MJ. Transforming growth factor-β mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol 22: 975–982, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Maffia P, Grassia G, Di Meglio P, Carnuccio R, Berrino L, Garside P, Ianaro A, Ialenti A. Neutralization of interleukin-18 inhibits neointimal formation in a rat model of vascular injury. Circulation 114: 430–437, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Malik N, Francis SE, Holt CM, Gunn J, Thomas GL, Shepherd L, Chamberlain J, Newman CMH, Cumberland DC, Crossman DC. Apoptosis and cell proliferation after porcine coronary angioplasty. Circulation 98: 1657–1665, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-β signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res 89: 930–934, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Matter CM, Schuler PK, Alessi P, Meier P, Ricci R, Zhang D, Halin C, Castellani P, Zardi L, Hofer CK, Montani M, Neri D, Luscher TF. Molecular imaging of atherosclerotic plaques using a human antibody against the extra-domain B of fibronectin. Circ Res 95: 1225–1233, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation 104: 2228–2235, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T. Relationship between arterial stiffness and athletic training programs in young adult men. Am J Hypertens 20: 967–973, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Pynn M, Schafer K, Konstantinides S, Halle M. Exercise training reduces neointimal growth and stabilizes vascular lesions developing after injury in apolipoprotein E-deficient mice. Circulation 109: 386–392, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Raines EW, Koyama H, Carragher NO. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann NY Acad Sci 902: 39–52, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Roy J, Tran PK, Religa P, Kazi M, Henderson B, Lundmark K, Hedin U. Fibronectin promotes cell cycle entry in smooth muscle cells in primary culture. Exp Cell Res 273: 169–177, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt MR, Maeng M, Kristiansen SB, Andersen HR, Falk E. The natural history of collagen and α-actin expression after coronary angioplasty. Cardiovasc Pathol 13: 260–267, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation 94: 1655–1664, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Sudhakaran P, Radhika A, Jacob S. Monocyte macrophage differentiation in vitro: Fibronectin-dependent upregulation of certain macrophage-specific activities. Glycoconj J 24: 49–55, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood 104: 11–18, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Tharaux PL, Chatziantoniou C, Casellas D, Fouassier L, Ardaillou R, Dussaule JC. Vascular endothelin-1 gene expression and synthesis and effect on renal type I collagen synthesis and nephroangiosclerosis during nitric oxide synthase inhibition in rats. Circulation 99: 2185–2191, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Wamhoff BR, Bowles DK, Dietz NJ, Hu Q, Sturek M. Exercise training attenuates coronary smooth muscle phenotypic modulation and nuclear Ca2+ signaling. Am J Physiol Heart Circ Physiol 283: H2397–H2410, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Xiang D, He J, Yang C, Gong Z, Lai H, Fu R, Yi S, Qiu J. Dynamic changes in type I collagen, MMP-1 and TIMP-1 after angioplasty. Chin Med J 115: 352–354, 2002. [PubMed] [Google Scholar]